Abstract
Objective
To examine prospectively the emergence of behavioral signs of autism in the first years of life in infants at low and high risk for autism.
Method
A prospective longitudinal design was used to compare 25 infants later diagnosed with an autism spectrum disorder (ASD) with 25 gender-matched low-risk children later determined to have typical development. Participants were evaluated at 6, 12, 18, 24, and 36 months of age. Frequencies of gaze to faces, social smiles, and directed vocalizations were coded from video and rated by examiners.
Results
The frequency of gaze to faces, shared smiles, and vocalizations to others were highly comparable between groups at 6 months of age, but significantly declining trajectories over time were apparent in the group later diagnosed with ASD. Group differences were significant by 12 months of age on most variables. Although repeated evaluation documented loss of skills in most infants with ASD, most parents did not report a regression in their child’s development.
Conclusions
These results suggest that behavioral signs of autism are not present at birth, as once suggested by Kanner, but emerge over time through a process of diminishment of key social communication behaviors. More children may present with a regressive course than previously thought, but parent report methods do not capture this phenomenon well. Implications for onset classification systems and clinical screening are also discussed.
Keywords: Autism, Onset, Infancy, Regression
This study examined when and how behavioral signs of autism spectrum disorders (ASD) emerge in the first years of life. Most previous investigations of this topic have been retrospective, relying on parent report of earlier development or analysis of home videotape of infants later diagnosed with ASD. The existing literature suggests that behavioral signs of autism emerge in two different patterns, an early onset and a regressive course. Retrospective studies have demonstrated that children with early-onset ASD differ from age-matched children with delayed and typical development in orienting to name, gaze to faces, joint attention, and affect sharing.1–6 Differences are most evident in the second year of life7 but some studies have detected signs of ASD before the first birthday.1,5,8 This early onset pattern is thought to occur in the majority of individuals with ASD.
In the regressive pattern of onset, children appear to be developing typically for a year or longer, but then lose communication and social skills that they had previously acquired. Retrospective studies using both parent report and home videotape analysis have documented losses in a wide range of social communicative behaviors.9–12 Regression is most often reported between the first and second birthdays, with mean ages across samples ranging from 16 to 20 months.9,11,13–15 The prevalence of regression varies across studies and is dependent upon both sampling methods and definitions of regression. Two large population-based studies found regression in 15.6%13 and 27% of samples.16
Recently, the adequacy of a dichotomous classification of onset has been questioned,17 based on emerging results from studies that provide evidence of other onset patterns. Some parents report that their child displayed neither early signs of autism nor regression, a phenomenon that has been called developmental plateau17,18 or stagnation.19 Other studies have found evidence of both early signs of ASD and regression simultaneously present in the retrospective histories of children with ASD.9,11,12,20
Questions about when and how behavioral signs of autism emerge may better be answered through prospective, rather than retrospective, studies. Prospective investigations are a powerful method of studying onset, because they reduce errors due to parental recall and biases introduced by selective home videotaping by sampling behaviors directly.7 In addition, they provide the opportunity to test specific hypotheses through experimental methods. In the past decade, several research groups have prospectively studied infants at elevated risk for ASD because they have one or more siblings with the condition. These infant sibling studies reliably find differences in the second year of life in the same social communicative domains as retrospective studies.21–25 However, few group differences predictive of ASD outcomes have been found before the first birthday in prospective studies.21,22,24,25 Bryson et al,26 in a consecutive case series of infant siblings followed prospectively from 6 months of age, describe several children whose symptoms were not present at their 6-month (and, for some, 12-month) visits, but emerged slowly during the second year of life. None of the children who developed autism displayed marked limitations in social reciprocity at 6 months. All nine infants were described as interested in social interactions and responsive to others, demonstrating sustained eye contact and social smiles. Thus, prospective studies suggest that social behavior may be grossly intact in the first 6 to 9 months of life in infants who are later diagnosed with autism.
The present prospective investigation examined the timing and course of onset of behavioral signs of autism in an infant sibling sample. Two prospective methods were used to examine symptom emergence: precise coding of the frequency of specific social communication behaviors exhibited during the session and examiner ratings of the frequency of infant social engagement. We also collected a traditional retrospective measure of symptom emergence using parent report, permitting us to examine how prospective and retrospective methods of data collection corresponded in terms of determining onset.
METHOD
Participants
The sample reported in this paper was drawn from a larger longitudinal study of infant siblings of children with ASD (High-Risk group) or typical development (Low-Risk group), recruited at two sites (UC Davis, n = 33; UCLA, n = 17). The sole inclusion criterion for the High-Risk group was status as a younger sibling of a child with ASD. Diagnosis of the affected older sibling was confirmed by meeting ASD criteria on both the Autism Diagnostic Observation Schedule (ADOS)27 and the Social Communication Questionnaire (SCQ).28 Of the 25 infants with ASD outcomes, 19 had older siblings with Autistic Disorder, 3 had older siblings with PDDNOS, and 3 were from the Low Risk group and did not have an older sibling with ASD. Exclusion criteria for the High-Risk group included birth before 36 weeks of gestation and a known genetic disorder (e.g., Fragile X syndrome) in the older affected sibling. The primary inclusion criteron for the Low-Risk group was status as a younger sibling of a child (or children) with typical development. Low-risk status of all older siblings was confirmed by an intake screening questionnaire and scores below the ASD range on the SCQ. Exclusion criteria for the Low-Risk group were birth before 36 weeks of gestation, developmental, learning, or medical conditions in any older sibling, and ASD in first-, second-, or third-degree relatives.
At the final study visit at 36 months of age, participants were classified into one of two outcome groups, using the following definitions developed by the Baby Siblings Research Consortium, a network of researchers studying very young children at risk for ASD.29
ASD outcome criteria were values above the ASD cutoff of the ADOS and meeting DSM-IV-TR criteria for Autistic Disorder or PDD-NOS according to expert clinician.
Typical development outcome criteria were as follows: Mullen Early Learning Composite score >78 and no more than one Mullen subtest ≤1.5 SD below mean and no Mullen subtest ≤2 SD below mean and two or more points below ASD cutoff of ADOS and does not meet DSM-IV-TR criteria for Autistic Disorder, PDD-NOS, or any other developmental delay according to expert clinician.
In this paper, we report on all infants at both sites who met criteria for an ASD outcome, as defined above, at 36 months of age (n = 25). They are compared to 25 infants who met criteria for Typical Development (TD) at 36 months, randomly selected from the Low-Risk group after matching the gender ratio to the ASD outcome group. Because of missing visits and/or unusable video, 6-month data was available for n = 28, 12-month data for n = 35, 18-month data for n = 41, 24-month data for n = 34, and 36-month data for n = 41. Table 1 contains descriptive characteristics of the two groups at the 36-month outcome visit.
TABLE 1.
Group Characteristics at 36 Months of Age
| ASD (n = 25) | Typical Development (n = 25) | |
|---|---|---|
| Gender | ||
| Female | 6 (24%) | 5 (20%) |
| Male | 19 (76%) | 20 (80%) |
| Racial or ethnic minoritya | 9 (41%) | 6 (24%) |
| Recruitment group | ||
| High-risk | 22 (88%) | 0 (0%) |
| Low-risk | 3 (12%) | 25 (100%) |
| Mean Visual Reception T Score (SD) | 40.7 (21.0) | 64.1 (11.0) |
| Mean Early Learning Composite (SD) | 74.5 (24.1) | 112.8 (12.0) |
| Mean ADOS Communication + Social Algorithm Score | 13.4 (3.3) | 1.4 (1.3) |
Note: ADOS = Autism Diagnostic Observation Schedule; ASD = autism spectrum disorder.
Missing for three subjects in ASD group.
Measures
Two types of measures were included, those used to characterize the sample and/or determine outcome and those used to track emergence of behavioral signs of autism.
Measures Used for Sample Characterization and Outcome Determination
Autism Diagnostic Observation Schedule
Autism Diagnostic Observation Schedule27 is a semistructured standardized interaction and observation that measures symptoms of autism. It has two empirically derived cutoffs, one for ASD, and one for Autistic Disorder. Psychometric studies report high inter-rater reliability and agreement in diagnostic classification (autism vs. nonspectrum) for individuals aged 24 months and older. The ADOS was used to confirm older sibling diagnosis and to determine infant outcome at 36 months of age.
Social Communication Questionnaire
The Social Communication Questionnaire28 parent report questionnaire is composed of 40 yes/no questions about behaviors characteristic of autism. Previous studies have shown good to excellent internal consistency reliability and discriminative validity of the SCQ across a wide age range.28,30 It was used to verify diagnosis of the older sibling.
Mullen Scales of Early Learning
The Mullen Scales of Early Learning (MSEL)31 is a standardized developmental test for children birth to 68 months. Four subscales were administered: Fine Motor, Visual Reception, Expressive Language, and Receptive Language. An overall score, the Early Learning Composite, is also obtained. The Mullen subscales have excellent internal consistency (median 0.91) and test-retest reliability (median 0.84). This test was used to measure cognitive functioning at each visit and help determine outcome status at 36 months. Ongoing administration and scoring fidelity procedures were implemented to insure that there were minimal cross-examiner and cross-site differences.
Measures Used to Track Behavioral Symptom Emergence
Social Communication Behavior Codes
Six social communicative behaviors were coded, adapted from a coding system developed by Werner and Dawson:12 gaze to faces, gaze to objects, smiles, nonverbal vocalizations, single word verbalizations, and phrase verbalizations (Table 2). DVD recordings were coded in real time using Noldus: The Observer 5.0 behavioral observation software with a time resolution of 0.5 seconds. Data were coded initially in duration to permit the derivation of additional second-order variables (discussed below), but then converted to frequency counts (e.g., all durations >0.5 seconds equal one occurrence).
TABLE 2.
Individual Social Communicative Behaviors Coded
| Code | Description |
|---|---|
| Gaze to Face | Infant’s gaze is directed toward the face of the examiner |
| Gaze to Objects | Infant’s gaze is directed toward an object that the examiner is presenting to the child or to another object visible in the frame. |
| Smile | Infant displays smile (at least one corner of mouth must be clearly upturned) and/or laughs. |
| Nonverbal Vocalizations | Infant vocalizes using a non-word sound (sighs, coos, whines, cries, open vowels [aaah, oooh], consonant-vowel combinations [bababa]) |
| Word Verbalizations | Infant vocalizes using a distinct word or word approximation. |
| Phrase Verbalizations | Infant vocalizes using a two or more word combination. |
Coding of all data from both sites was undertaken at UC Davis. Coders were initially trained to 90% agreement on all codes. In all, 15% of data were then double-coded to maintain ongoing reliability. Intraclass correlation coefficients (ICC) were in the good to excellent range for all codes: nonverbal vocalizations 0.93, verbalizations–words 0.77, verbalizations–phrases 0.72, gaze to face 0.95, gaze to objects 0.98, and smiles 0.96. Nonverbal vocalizations, word verbalizations, and phrase verbalizations were combined into one “vocalizations” variable because their individual frequencies were low at most ages.
Two second-order variables, social smiles and directed vocalizations, were then derived by examining the co-occurrence of individual codes. Co-occurrence was defined when the duration of events overlapped in time course by at least 0.5 second. Social smiles were defined as the co-occurrence of gaze to face and smiles, while directed vocalizations were defined as the co-occurrence of gaze to face and vocalizations. The ICC for social smile frequency was 0.98 and for directed vocalization frequency was 0.99.
The primary dependent variables analyzed were gaze to faces, social smiles, and directed vocalizations. Also of theoretical interest was gaze to objects, but there were no group differences in the duration of this variable at any time point, so it was not considered further in the models.
Behavior was coded during the Mullen Visual Reception (VR) subtest of each visit. This context for coding social communication behavior was selected because 1) it was assessed at each visit, 2) the tasks involved objects as well as an interactive partner, thus providing the opportunity to examine gaze to both people and objects, and 3) it used objects that are generally attractive to young children with autism, thus assuring cooperation and attention. Gaze, affect, and vocalizations that occurred during the first five VR items administered to the child were coded, along with the item score (pass/fail).
Examiner Ratings of Social Engagement
At the end of the session, examiners rated three behaviors using a three-point scale (1 = rare, 2 = occasional, 3 = frequent): 1) frequency of eye contact, 2) frequency of shared affect, and 3) overall social responsiveness. These three scores were summed to create a social engagement composite score (range, 3 to 9).
Autism Diagnostic Interview–Revised (ADI-R).32
The ADI-R is a structured, standardized parent interview developed to assess the presence and severity of symptoms of autism. Only the questions that pertain to onset (items 1 through 28) were used in the current study. All ADI-R raters were trained to research reliability by the instrument’s developers or authorized trainers.
Procedure
This study was conducted under the approval of both sites’ IRBs. Infants were assessed at 6, 12, 18, 24, and 36 months of age by examiners unaware of the child’s group membership.
Data Analysis
A Generalized Estimating Equations (GEE)33 approach was used to analyze developmental trajectories for dependent variables that were not normally distributed (e.g., coded social communication behaviors, examiner ratings of social engagement, and proportion of Mullen Visual Reception items failed). Random-effects regression models34 were used for dependent variables that were normally distributed (e.g., raw scores on Mullen subscales). Both methods take into account the correlated structure of the data due to repeated assessments over time and allow for missing observations. The goals of the statistical models were to estimate patterns of change in the outcome variables over the course of the study and to test whether outcome diagnosis was related to either initial level of or rate of change in the dependent variables. Preliminary analyses suggested that there may be a quadratic trend over time in some of the variables, especially in the ASD group. Thus, for each of the variables, we first fitted a model with a main effect for diagnosis, both linear and quadratic effects of time (measured in months, from the 6-month visit) and interactions between the linear and quadratic effects of time and diagnosis. After fitting this initial model, we examined the terms in the model and removed those that did not add significantly to the model. A variable was considered to be a significant predictor in the model if its significance level exceeded .05. All analyses were implemented using PROC GENMOD and MIXED in SAS Version 9.2.
RESULTS
Social Communication Behavior Codes
Since the behaviors were frequency counts, normal distribution was not applicable. Poisson and negative binomial distributions are commonly used to represent the distributions of count data. The mean and variance in a Poisson model are equal, and when this assumption is violated (a phenomenon called overdispersion), a negative binomial distribution is used instead. In models for count data, log of the mean (rather than the mean) is modeled as a linear combination of predictors. Because behaviors were coded over different time intervals for each child and each visit, the value of task duration (in minutes) was log transformed and entered into the model as an offset. This resulted in a rate parameterization, in which the outcomes of interest were the number of coded behaviors per minute and the regression coefficients represented the linear effect of the predictor variables on the log of the rate of behaviors. Because evidence of overdispersion was found in our data, the negative binomial distribution was used to model the number of behaviors in the GEE analyses. Separate models were fit for each of the three primary social communication codes: gaze to faces, social smiles, and directed vocalizations.
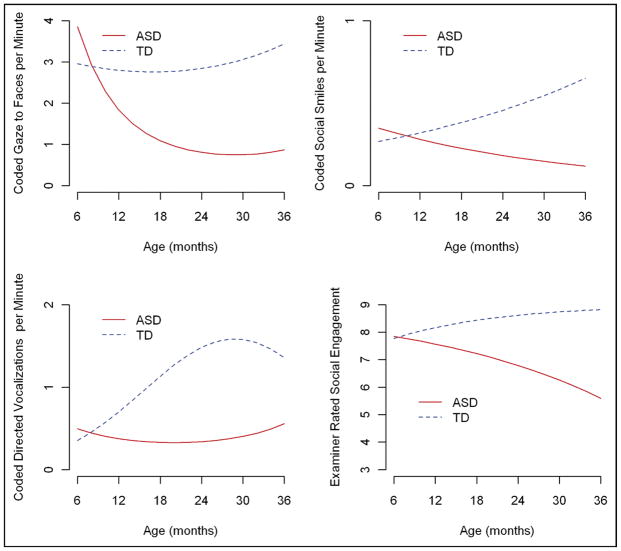
Table 3 summarizes the results of the GEEs for the three coded behaviors. The two groups behaved similarly at 6 months: none of the three codes differed between the groups and effect sizes were in the small range. Over time, the TD group had a significant increase in social smiles and directed vocalizations, while maintaining the same level of gaze to faces. In the ASD group, in contrast, all three behaviors dramatically decreased over time. Figure 1 displays the estimated trajectories for the three behaviors, showing comparable values at 6 months, group differences becoming significant by 12 months for gaze to faces and directed vocalizations, and by 18 months for social smiles. These group differences persisted and widened over time.
TABLE 3.
Estimated Trajectories for Typically Developing Children and Children with Autism Spectrum Disorders from the Generalized Estimating Equations Models
| Age | Coded Social Communication Behaviors per Minute Estimate (SE) |
Examiner Ratings Estimate (SE) |
||||||
|---|---|---|---|---|---|---|---|---|
| Gaze to Faces |
Social Smiles |
Directed Vocalizations |
Social Engagement |
|||||
| TD | ASD | TD | ASD | TD | ASD | TD | ASD | |
| 6 Mo | 2.96 (0.35) | 3.85 (0.64) | 0.27 (0.07) | 0.35 (13) | 0.35 (0.12) | 0.50 (0.18) | 7.77 (0.33) | 7.85 (0.29) |
| 12 Mo | 2.80 (0.25) | 1.83 (0.23)** | 0.32 (0.06) | 0.28 (0.07) | 0.70 (0.13) | 0.37 (0.06)* | 8.17 (0.18) | 7.57 (0.29) |
| 18 Mo | 2.76 (0.27) | 1.09 (0.15)*** | 0.38 (0.06) | 0.23 (0.04)* | 1.12 (0.17) | 0.33 (0.05)*** | 8.57 (0.13) | 7.22 (0.27)*** |
| 24 Mo | 2.84 (0.28) | 0.80 (0.12)*** | 0.46 (0.06) | 0.18 (0.04)*** | 1.46 (0.23) | 0.34 (0.07)*** | 8.62 (0.12) | 6.79 (0.25)*** |
| 36 Mo | 3.43 (0.35) | 0.86 (0.19)*** | 0.65 (0.13) | 0.12 (0.05)*** | 1.31 (0.28) | 0.55 (0.17)* | 8.82 (0.09) | 5.59 (0.37)*** |
Note: ASD = autism spectrum disorder; TD = typically developing children.
p < .05;
p < .01;
p < .001.
FIGURE 1.
Estimated Trajectories for Coded Social Communication Behaviors and Examiner Ratings of Social Engagement. ASD = autism spectrum disorders; TD = typically developing children.
The GEE estimates of within-cluster correlations were modest (ranging from 0.06 for directed vocalizations to 0.29 for gaze to faces). Thus, most of the variation was within child (e.g., over time) and not within group. Box-and-whisker plots displaying the distribution of the behaviors for each group over time and showing the average values and degree of variability within and between groups can be found in Figure S1.
Mullen Scales of Early Learning
Three analyses were conducted using scores from the Mullen scales. First, we tested whether differences in level of cognitive functioning between the two groups explained some of the group differences in social communication behaviors. We added the Mullen Early Learning Composite score as a time varying covariate into the GEE models predicting social communication behaviors. This variable was not significant, indicating that differences in cognitive ability between the groups over time could not explain the declines in social communication observed in the ASD group.
Next, we examined whether there were group differences in performance on the individual Mullen Visual Reception items administered during the segment of the session when social communication coding occurred. Cochran-Armitage trend tests were performed to investigate whether the groups differed in the item sets administered to them. At the 18 and 36 month visits, higher numbered (more difficult) items were more likely to be administered to the TD group (both p values < .001), but not at the 6-, 12-, and 24-month visits. Performance on the five coded items was treated as binary (pass/fail) and analyzed using GEEs for binomial data. This approach modeled the log-odds of failing an item. Terms for diagnosis, time, and their interaction were included, to test whether the proportion of failures at baseline and the rate of change in failures over time were different between the two groups. There were no significant group differences in either the proportion of failures at baseline or their progression over time. Thus, the group differences in social communication behavior coded during the VR subtest do not appear to be due to lower rates of successful performance in the ASD group.
Finally, we examined trajectories of performance on the four Mullen subscales. Separate random-effects models were fit for raw scores on the Mullen Visual Reception, Fine Motor, Receptive Language, and Expressive Language sub-scales. Figure 2 displays the estimated trajectories for the Expressive Language subtest. Mirroring the results of the social communication code analyses, the groups demonstrated comparable values at 6 months, with group differences becoming significant by 12 months, and continuing to widen over time. Unlike the social communication trajectories, both groups demonstrated an increasing trend over time, but children in the ASD group had a significantly slower rate of change than those in the TD group. Similar trajectories were seen on the other three Mullen subscales, with similar group performance at 6 months and then group differences becoming significant either by 12 months (Receptive Language) or 18 months (Visual Reception and Fine Motor) (Table S1 and Figure S2).
FIGURE 2.
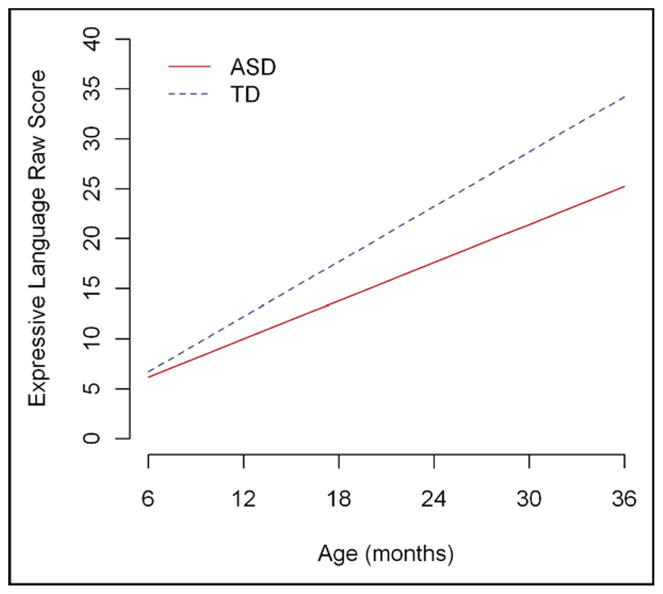
Estimated Trajectory for Mullen Expressive Language Raw Scores. ASD = autism spectrum disorders; TD = typically developing children.
Examiner Ratings of Social Behavior
GEE models for negative binomial data described previously were fitted to the examiner ratings of social engagement. The range of the composite social engagement rating was 3 to 9 (with higher scores indicating better social engagement); this was recoded to 0 to 6 for the purposes of the GEE models. Figure 1 also displays the estimated trajectories for the two groups, converted back to the original scale. As with the individual social communication behaviors, there were no group differences in the 6-month examiner ratings; however, whereas the TD group showed a significant trend to increase social engagement over time, reaching close to the maximum score of 9 (estimate 8.82, SE = 0.09) by 36 months, the children in the ASD group had a strong decline in social engagement over the same time period, with the mean 36-month rating less than “occasional” on the composite social engagement score (estimate = 5.59, SE = 0.37).
Spearman rank correlation coefficients were computed to examine the relationship between the social communication behaviors coded during the session and the social engagement ratings made by the examiner (Table 4). Starting with the 18-month visit, the rates of both gaze to faces and social smiles were strongly and positively related to examiner ratings of these behaviors.
TABLE 4.
Spearman Correlation Coefficients between Social Engagement Ratings and Social Communication Behavior Codes
| Gaze to Faces (rate) | Social Smiles (rate) | |
|---|---|---|
| 6 Mo | 0.34 | 0.25 |
| 12 Mo | 0.22 | 0.45* |
| 18 Mo | 0.49** | 0.55** |
| 24 Mo | 0.59** | 0.55** |
| 36 Mo | 0.64*** | 0.66*** |
p < .05,
p < .01,
p < .001
Prospective Classification of Onset vs. Retrospective Parent Report of Onset
The ADI-R was used to collect parent recall of symptom onset and possible regression. It was administered to parents when the participant was 36 months of age. Regression was considered to be present by parent report if a response of yes was given to either question 11 regarding language loss (meeting the standard ADI-R criteria of at least five words used spontaneously, meaningfully, and communicatively for at least three months before being lost for at least 3 months) or question 25 regarding diminishment of social engagement behaviors. The ADI-R was missing for 2 of the 25 participants with ASD. Of the remaining 23, three were reported by parents to have experienced typical development followed by a regression, one was reported to have shown both early signs of autism and a later regression, eight were reported by parents to display neither early signs nor a regression (e.g., developmental plateau), and 11 were reported to show clear signs of autism before the first birthday and no regression (e.g., early onset type). Of the four children reported to lose skills, parents of three noted regression in the social domain alone (eye contact, interest in people, social engagement), whereas one reported losses of both social (response to name, social interest) and communication (loss of 20 words, gestures, language comprehension) behaviors.
To examine the correspondence between parent report and prospectively collected data, we were also interested in how many ASD participants demonstrated declining trajectories similar to those depicted for the entire group in Figure 1. To accomplish this, first we computed 95% confidence intervals for the change from one visit to the next for gaze to faces for the TD sample. This variable was selected because we believe it is most sensitive to emerging signs of autism, as well as central to the definition of the second-older variables of social smiles and directed vocalizations. Then we counted how many of the participants with ASD outcomes displayed declines in social communication behaviors that were greater than the 95% confidence interval for visit-to-visit change in the TD group. This analysis found that 86.4% of infants in the ASD group (19 of 22 with sufficient data at multiple time points) showed declines in social communication that were outside the expected TD change. All four children whose parents reported a regression on the ADI-R showed declines of this magnitude, along with 15 additional ASD children whose parents had not reported skill loss on the ADI-R.
DISCUSSION
This study examined several key social communication behaviors in a group of infants who later developed autism and a typically developing, low-risk comparison sample. The primary findings were that there were no group differences at 6 months of age, but that over the next 12 months, most of the infants who were later diagnosed with ASD demonstrated declining trajectories of social communication behavior and loss of skills. These results were consistent across both exact coded frequencies of behavior and examiner frequency ratings of social engagement. The second important finding was that these changes in social communication development documented through repeated evaluations provided a very different picture of symptom onset than did parents’ retrospective report. Most parents (83%) did not report any loss of skills during this period. These findings lead us to two major conclusions. First, the behavioral symptoms of ASD appear to emerge over time, beginning in the second half of the first year of life and continuing to develop for several years. Second, our most widely used and recommended practice for gathering information about symptom onset, parent-provided developmental history, does not provide a valid assessment of the slow decline in social communication that can be observed prospectively.
The comparability of social behavior at 6 months of age in the ASD and TD groups replicates findings from other prospective studies.25,26,35 The lack of group differentiation at this age does not appear to result from low power, as the group means were very similar and effect sizes were very small. Most convincingly, the effects were in the opposite direction as predicted, with the ASD outcome group demonstrating (nonsignificantly) better social communication behavior at 6 months than the TD outcome group on all variables. After 6 months, the ASD group shows a rapid decline in eye contact, social smiling, and examiner-rated social responsiveness. Group differences were significant by 12 months in gaze to faces and social smiling and by 18 months on all other variables. Similar declining trajectories in the onset of autism symptoms have also been reported by others.35
Some have suggested that the decrease in social interest in this time period is related, at least in part, to an overly focused attention to objects or difficulty with attentional switches from objects to people.36 However, our data did not support this hypothesis; we found no group differences in looking at objects at any age and duration of looks to objects did not increase over time in the ASD group. Thus, the decline in looking at faces and social smiling could not be accounted for by an increase in interest in objects. Similarly, neither group differences in cognitive functioning, slowing of cognitive growth between 12 and 36 months in the ASD group, nor less successful performance on the Mullen VR subscale (which provided the context for coding) could explain the decrease in social communication behavior over time in the ASD group.
Collectively, the present investigation and recent prospective studies suggest that signs of autism emerge over the first year or so of life in many children with ASD, rather than being present from close to birth, as once suggested by Kanner.37 Although there are likely cases in which behavioral signs are indeed evident at or before 6 months of age, this pattern may be less common than originally thought.
Interestingly, cognitive and language skills, as measured by the Mullen, did not demonstrate the same declining trajectory in the ASD group as social communication skills. Mullen raw scores increased over time in both groups, but showed significantly slower growth in the ASD group starting at 12 months. This suggests that developmental loss of skills may occur specifically in the social communicative domain, rather than generally across all developmental domains. One implication of this finding is that the Mullen and other standardized developmental tests will not be good prospective measures for tracking regression, as they appear to be less sensitive to the kinds of behaviors that decline as autism emerges.
The results of the current prospective study suggest that the traditionally defined categories of early onset and regressive autism do not portray accurately how symptoms emerge, nor does the newer-onset category involving a developmental plateau. In the present study, specific social communicative behaviors clearly decreased rather than failing to progress. Losses were particularly dramatic between 6 and 18 months. This suggests that ASD onset marked by loss of social communication behaviors occurs much more often than has been recognized using parent report methods. However, rather than the rapid and marked losses typically reported in previous studies of regression onset, the declines that could be detected through specific probes of social development every 6 months were relatively subtle and gradual, were often preceded by earlier parental concerns, and were often followed by failures to progress in other areas.38 This may have made the losses difficult for families to identify, as demonstrated by the low rate of parental reports of regression on a standardized measure, the ADI-R, administered at 36 months. It might be expected that parents who have already experienced the developmental course of autism onset in an older child would be more sensitive to these signs than the general population, underscoring how difficult it will be for most parents to report about skill loss. For this reason, it is critical to explore other methods of detecting the earliest signs of autism.
In the present study, a second method of measuring social communication development was examiner ratings, which were conducted at the end of every visit, blind to the group assignment of the participant. We found that these global ratings of the frequency of social communication behaviors were highly correlated with coded rates of the same behaviors, validating both approaches to measurement. Although it is not practical to code behavior frequencies during medical office visits, these results suggest that it may be possible to develop measures that can be rated by physicians or nursing staff during well-child visits that capture “snapshots” of an infant’s current social repertoire. If such measures are found to be valid reflections of infant sociability, then they could be added to developmental monitoring exams and serially rated over time to identify infants at higher risk for ASD and in need of further specialized evaluation.
The present findings suggest that existing definitions of onset patterns will need to undergo further development. One possibility is that we need to expand the number of categories used to describe onset. For example, perhaps there are four rather than two categories of onset, including groups characterized by developmental plateaus and by mixed features of early symptoms plus later regression, as we have suggested elsewhere.17 The prospective data acquired in the present investigation suggest another possibility, however. We hypothesize that symptom emergence may better be considered dimensionally, as a continuum characterized by the amount and timing of regression. In this conceptualization, at one end of the continuum lie children who display loss of social interest so early that the regression is difficult to see and symptoms appear to have always been present. At the other end of the continuum lie children who experience losses of social interest and communication skills so late that the regression appears quite dramatic. Supporting the idea that there may not be two different processes of symptom onset, but rather one dimension, several recent studies have found few differences in later developmental, functional, and adaptive outcomes between children with traditionally defined early onset and regressive autism.18,39 Resolution of opposing viewpoints regarding onset (e.g., multiple categories v. a dimensional view that emphasizes timing) is urgently needed for etiologic studies, which have been hindered already by the tremendous heterogeneity of the autism phenotype.
Limitations of the present investigation include the relatively small sample size and the coding of behavior during only one segment of a longer visit. Future studies with more participants, longer sampling of behavior, and examination of cross-task consistency in behavior are necessary. Nonetheless, since the present results are consistent with other prospective infant studies,25,26,35 we believe that they have significant clinical implications for early autism screening, diagnosis, and intervention. Universal screening has been recommended by the American Academy of Pediatrics (AAP) twice by the second birthday, and many are hoping that identification may be possible even earlier.40 This study suggests that identification of autism by the first birthday may not be possible in the majority of affected children. Therefore, the AAP recommendation for screening at both 18 and 24 months is essential and may even need to be supplemented by screens after age 2 years, as many children will be missed at earlier time points. Finally, we urge professionals to refer to intervention any infant or toddler who displays a sustained reduction in social responsivity over time. Given the gradual course of symptom emergence and the paucity of diagnostic tools for infants and toddlers with suspected autism, the diagnostic process can be quite protracted and intervention may be needlessly delayed.
Supplementary Material
Box-plots for Coded Social-Communication Behaviors and Examiner Ratings of Social Engagement for typically developing children and children with autism spectrum disorders. In these box-and-whisker plots, the box gives the interquartile range (the third quartile minus the first quartile) and the median. The means are represented by dots. The whiskers are 3 halved the interquartile range rolled back to where there are data. For clarity, the outliers are not drawn (as points outside the whiskers). ASD = autism spectrum disorders; TD = typically developing children.
Estimated trajectories for Mullen Visual Reception, Fine Motor, and Receptive Language for typically developing children and children with autism spectrum disorders. ASD = autism spectrum disorders; TD = typically developing children.
Estimated Trajectories for Typically Developing Children and Children with Autism Spectrum Disorders from the Random-Effect Models for Mullen Raw Scores
Acknowledgments
This study was supported by grants from the National Institute of Mental Health: R01 MH068398 (Ozonoff) and U54 MH068172 (Sigman).
The authors thank the children and families who participated in this longitudinal study, as well as Geraldine Dawson, Ph.D., and Emily Werner, Ph.D., for sharing their coding system with us.
Footnotes
Disclosure: Drs. Ozonoff, Iosif, Cook, Hutman, Rogers, Rozga, Sigman, Steinfeld, and Young, and Mr. Baguio, Ms. Hill, and Ms. Sangha report no biomedical financial interests or potential conflicts of interest.
Editorial support for the preparation of this article was provided by Diane Larzelere, UC Davis.
Contributor Information
Dr. Sally Ozonoff, University of California–Davis
Dr. Ana-Maria Iosif, University of California–Davis
Ms. Fam Baguio, University of California–Davis
Dr. Ian C. Cook, University of California–Davis
Ms. Monique Moore Hill, University of California–Davis
Dr. Ted Hutman, University of California–Los Angeles
Dr. Sally J. Rogers, University of California–Davis
Dr. Agata Rozga, University of California–Los Angeles
Ms. Sarabjit Sangha, University of California–Davis
Dr. Marian Sigman, University of California–Los Angeles
Dr. Mary Beth Steinfeld, University of California–Davis
Dr. Gregory S. Young, University of California–Davis
References
- 1.Baranek GT. Autism during infancy: a retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. J Autism Dev Disord. 1999;29:213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- 2.Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord. 1994;24:247–257. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- 3.Stone WL, Hoffman EL, Lewis SE, Ousley OY. Early recognition of autism. Parental reports vs. clinical observation. Arch Pediatr Adolesc Med. 1994;148:174–179. doi: 10.1001/archpedi.1994.02170020060010. [DOI] [PubMed] [Google Scholar]
- 4.Stone WL, Lee EB, Ashford L, Brissie J, Hepburn SL, Coonrod EE, Weiss BH. Can autism be diagnosed accurately in children under 3 years? J Child Psychol Psychiatry. 1999;40:219–226. [PubMed] [Google Scholar]
- 5.Werner E, Dawson G, Osterling J, Dinno N. Brief report: recognition of autism spectrum disorder before one year of age: a retrospective study based on home videotapes. J Autism Dev Disord. 2000;30:157–162. doi: 10.1023/a:1005463707029. [DOI] [PubMed] [Google Scholar]
- 6.Wetherby AM, Woods J, Allen L, Cleary J, Dickinson H, Lord C. Early indicators of autism spectrum disorders in the second year of life. J Autism Dev Disord. 2004;35:473–493. doi: 10.1007/s10803-004-2544-y. [DOI] [PubMed] [Google Scholar]
- 7.Palomo R, Belinchon M, Ozonoff S. Autism and family home movies: a comprehensive review. J Dev Behav Pediatr. 2006;27:S59–S68. doi: 10.1097/00004703-200604002-00003. [DOI] [PubMed] [Google Scholar]
- 8.Clifford SM, Dissanayake C. The early development of joint attention in infants with autistic disorder using home video observations and parental interview. J Autism Dev Disord. 2008;38:791–805. doi: 10.1007/s10803-007-0444-7. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg WA, Osann K, Filipek PA, Laulhere T, Jarvis K, Modahl C, Flodman P, Spence AM. Language and other regression: assessment and timing. J Autism Dev Disord. 2003;33:607–616. doi: 10.1023/b:jadd.0000005998.47370.ef. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg WA, Thorsen KL, Osann K, Spence AM. Use of home videotapes to confirm parental reports of regression in autism. J Autism Dev Disord. 2008;38:1136–1146. doi: 10.1007/s10803-007-0498-6. [DOI] [PubMed] [Google Scholar]
- 11.Ozonoff S, Williams BJ, Landa R. Parental report of the early development of children with regressive autism: the delays-plus-regression phenotype. Autism. 2005;9:461–486. doi: 10.1177/1362361305057880. [DOI] [PubMed] [Google Scholar]
- 12.Werner E, Dawson G. Validation of the phenomenon of autistic regression using home videotapes. Arch Gen Psychiatry. 2005;62:889–895. doi: 10.1001/archpsyc.62.8.889. [DOI] [PubMed] [Google Scholar]
- 13.Fombonne E, Chakrabarti S. No evidence for a new variant of measles-mumps-rubella-induced autism. Pediatrics. 2001;108:E58. doi: 10.1542/peds.108.4.e58. [DOI] [PubMed] [Google Scholar]
- 14.Kurita H. Infantile autism with speech loss before the age of thirty months. J Am Acad Child Psychiatry. 1985;24:191–196. doi: 10.1016/s0002-7138(09)60447-7. [DOI] [PubMed] [Google Scholar]
- 15.Shinnar S, Rapin I, Arnold S, Tuchman RF, Shulman L, et al. Language regression in childhood. Pediatr Neurol. 2001;24:183–189. doi: 10.1016/s0887-8994(00)00266-6. [DOI] [PubMed] [Google Scholar]
- 16.Lingam R, Simmons A, Andrews N, Miller E, Stowe J, Taylor B. Prevalence of autism and parentally reported triggers in a north east London population. Arch Dis Child. 2003;88:666–670. doi: 10.1136/adc.88.8.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozonoff S, Heung K, Byrd R, Hansen R, Hertz-Picciotto I. The onset of autism: patterns of symptom emergence in the first years of life. Autism Res. 2008;1:320–328. doi: 10.1002/aur.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen RL, Ozonoff S, Krakowiak P, et al. Regression in autism: prevalence and associated factors in the CHARGE study. Ambul Pediatr. 2008;8:25–31. doi: 10.1016/j.ambp.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Siperstein R, Volkmar F. Parental reporting of regression in children with pervasive developmental disorders. J Autism Dev Disord. 2004;34:731–734. doi: 10.1007/s10803-004-5294-y. [DOI] [PubMed] [Google Scholar]
- 20.Richler J, Luyster R, Risi S, Hsu WL, Dawson G, Bernier R, et al. Is there a regressive phenotype of autism spectrum disorder associated with the measles-mumps-rubella vaccine? A CPEA study. J Autism Dev Disord. 2006;36:299–316. doi: 10.1007/s10803-005-0070-1. [DOI] [PubMed] [Google Scholar]
- 21.Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. J Child Psychol Psychiatry. 2006;47:629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- 22.Nadig AS, Ozonoff S, Young GS, Rozga A, Sigman M, Rogers SJ. Failure to respond to name is an indicator of possible autism spectrum disorder. Arch Pediatr Adolesc. 2007;161:378–383. doi: 10.1001/archpedi.161.4.378. [DOI] [PubMed] [Google Scholar]
- 23.Stone WL, McMahon CR, Yoder PJ, Walden TA. Early social communicative and cognitive development of younger siblings of children with autism spectrum disorders. Arch Pediatr Adolesc Med. 2007;161:384–390. doi: 10.1001/archpedi.161.4.384. [DOI] [PubMed] [Google Scholar]
- 24.Yirmiya N, Gamliel I, Pilowsky T, Feldman R, Baron-Cohen S, Sigman M. The development of siblings of children with autism at 4 and 14 months: social engagement, communication, and cognition. J Child Psychol Psychiatry. 2006;47:511–523. doi: 10.1111/j.1469-7610.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- 25.Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Bryson SE, Zwaigenbaum L, Brian J, Roberts W, Szatmari P, Rombough V, McDermott C. A prospective case series of high-risk infants who developed autism. J Autism Dev Disord. 2007;37:12–24. doi: 10.1007/s10803-006-0328-2. [DOI] [PubMed] [Google Scholar]
- 27.Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule–Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 28.Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. Br J Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- 29.Yirmiya N, Ozonoff S. The very early phenotype of autism. J Autism Dev Disord. 2007;37:1–11. [Google Scholar]
- 30.Corsello C, Hus V, Pickles A, Risi S, Cook EH, Leventhal B, Lord C. Between a ROC and a hard place: decision making and making decisions about using the SCQ. J Child Psychol Psychiatry. 2007;48:932–940. doi: 10.1111/j.1469-7610.2007.01762.x. [DOI] [PubMed] [Google Scholar]
- 31.Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- 32.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview–Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 33.Hardin JW, Hilbe JM. Generalized Estimating Equations. Boca Raton, FL: Chapman and Hall/CRC; 2003. [Google Scholar]
- 34.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 35.Landa R, Holman KC, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Arch Gen Psychiatry. 2007;64:853–864. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- 36.Landry R, Bryson SE. Impaired disengagement of attention in young children with autism. J Child Psychol Psychiatry. 2004;45:1115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- 37.Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 38.Ozonoff S, Young GS, Steinfeld MB, Hill MM, Cook I, Hutman T, Macari S, Rogers SJ, Sigman M. How early do parent concerns predict later autism diagnosis? J Dev Behav Pediatr. 2009;30:367–375. doi: 10.1097/dbp.0b013e3181ba0fcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heung K, Ozonoff S, Byrd R. The relationship between onset type and later functional outcomes in children with autism. Submitted for publication. [Google Scholar]
- 40.Johnson CP, Myers SM. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120:1183–1215. doi: 10.1542/peds.2007-2361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Box-plots for Coded Social-Communication Behaviors and Examiner Ratings of Social Engagement for typically developing children and children with autism spectrum disorders. In these box-and-whisker plots, the box gives the interquartile range (the third quartile minus the first quartile) and the median. The means are represented by dots. The whiskers are 3 halved the interquartile range rolled back to where there are data. For clarity, the outliers are not drawn (as points outside the whiskers). ASD = autism spectrum disorders; TD = typically developing children.
Estimated trajectories for Mullen Visual Reception, Fine Motor, and Receptive Language for typically developing children and children with autism spectrum disorders. ASD = autism spectrum disorders; TD = typically developing children.
Estimated Trajectories for Typically Developing Children and Children with Autism Spectrum Disorders from the Random-Effect Models for Mullen Raw Scores