Abstract
CONTEXT
The association between depression and inflammation is inconsistent across research samples.
OBJECTIVE
This study tested the hypothesis that a history of childhood maltreatment could identify a subgroup of depressed individuals with elevated inflammation levels, thus helping to explain previous inconsistencies.
DESIGN
Prospective longitudinal cohort study.
SETTING
New Zealand.
PARTICIPANTS
A representative birth cohort of 1,000 individuals was followed to age 32 years as part of the Dunedin Multidisciplinary Health and Development Study. Study members were assessed for history of childhood maltreatment and current depression.
MAIN OUTCOME MEASURES
Inflammation was assessed by a clinically-relevant categorical measure of high-sensitivity C-reactive protein (hsCRP >3mg/L), and a dimensional inflammation factor indexing the shared variance of continuous measures of hsCRP, fibrinogen, and white blood cells.
RESULTS
Although depression was associated with high hsCRP (RR=1.45; 95%CI=1.06;1.99), this association was significantly attenuated and no longer significant when the effect of childhood maltreatment was taken into account. Individuals with current depression and childhood maltreatment history were more likely to show high hsCRP levels than controls (N=27; RR=2.07; 95%CI=1.23;3.47). In contrast, individuals with current depression only showed a non-significant elevation in risk (N=109; RR=1.40; 95%CI=0.97;2.01). Results generalized to the inflammation factor. The elevated inflammation levels in depressed+maltreated individuals were not explained by correlated risk factors, such as depression recurrence, low socioeconomic status in childhood or adulthood, poor health, or smoking.
CONCLUSIONS
A history of childhood maltreatment contributes to the co-occurrence of depression and inflammation. Information about experiences of childhood maltreatment may help to identify depressed individuals with elevated inflammation levels and thus cardiovascular disease risk.
Major depression is a multi-systemic disorder affecting both brain and bodily functions1. Increasing evidence suggests that inflammation may contribute to the link between psychological and somatic symptoms in depressed individuals2. For example, depression and cardiovascular disease often co-occur3,4, and inflammation has been associated with both conditions5,6. Yet, not all individuals with depression show elevated inflammation levels5. Those who do, however, could be at highest risk for cardiovascular disease. Evidence that childhood maltreatment - a major risk factor for depression - predicts adult inflammation7 led us to test the hypothesis that a history of maltreatment could help to identify depressed individuals with elevated inflammation levels.
Inflammation may be related to depression through two-way neuro-immunoendocrine interactions. On the one hand, brain functioning regulates glucocorticoid hormones’ secretion, and glucocorticoids, in turn, can inhibit inflammation processes8. On the other hand, inflammation reduces glucocorticoid signaling, and reduced glucocorticoid signalling, in turn, may lead to abnormal brain functioning9. Depressed individuals often show impaired glucocorticoid signaling10, and may therefore be at higher risk for inflammation11.
Inflammation is also related to cardiovascular disease, through its involvement in the pathophysiology of atherosclerosis12. Even mild rises in inflammation levels appear to predict increased risk of cardiovascular disease in apparently healthy individuals6. Moreover, therapeutic reduction in inflammation levels decreases cardiovascular disease risk13. For these reasons, a marker of inflammation – the high-sensitivity C-reactive protein (hsCRP) - has been recently endorsed as an adjunct to traditional risk-factor screening for cardiovascular disease14.
Inflammation has therefore been hypothesized to partly explain the observed comorbidity between depression and cardiovascular disease2, two of the leading causes of disability worldwide. However, studies testing the association between depression and inflammation have reported inconsistent results5. This inconsistency could be due to heterogeneity in the etiological pathways leading to a diagnosis of depression. For instance, it is possible that inflammation could be influenced by etiological factors associated with depression, rather than by depression itself. Consistent with this hypothesis, inflammation persists in individuals with a history of depression even when their depressive symptoms are absent15. Similarly, poor prognosis of heart disease, which can be influenced by inflammation, persists in individuals with a history of depression even when depressive symptoms have been significantly reduced by treatment4.
Initial evidence suggests that depression, inflammation, and cardiovascular disease may share common origins in early-life stress, such as childhood maltreatment7,16,17. Experimental research also shows that depressed adults with increased early-life stress have greater inflammatory response to an acute psychosocial stress challenge than non-depressed controls, which has been interpreted to suggest that early-life stress might influence long-term inflammation processes in depressed individuals18. However, it is not clear whether this experimental finding may translate into clinically-relevant changes in inflammation levels.
In order to better characterize the heterogeneity of inflammation levels in depressed individuals, we tested the hypothesis that childhood maltreatment could predict which depressed adults have clinically-relevant inflammation levels. At age 32, study participants were too young to show cardiovascular outcomes. Yet, inflammation levels can be used to predict their risk for future cardiovascular disease14.
METHODS
Sample
Participants are members of the Dunedin Multidisciplinary Health and Development Study. Of infants born in Dunedin, New Zealand, between April 1972 and March 1973, 1,037 children (91% of eligible births; 52% male) participated in the first follow-up at age 3, constituting the base sample for the longitudinal study. Participants represent the full range of socioeconomic status in the general population of New Zealand’s South Island and are primarily white. Assessments have been carried out at ages 3, 5, 7, 9, 11, 13, 15, 18, 21, 26, and most recently at age 32 when we assessed 972 (96%) of the 1,015 Study members still alive in 2004-2005.
Childhood Maltreatment
As previously described19, evidence of childhood maltreatment during the first decade of life (ages 3-11) was ascertained using parental reports, prospective behavioral observations, and retrospective reports by Study members once they reached adulthood. First, exposure to maternal rejection (characterizing 14% of Study participants) was assessed at age 3 by observational ratings of mothers’ interaction with the Study children. Second, exposure to harsh discipline was assessed at ages 7 and 9 according to parental reports of disciplinary behaviors. Parents scoring in the top decile of the sample-wide distribution (10% of participants) were classified as unusually harsh for that time and culture. Third, exposure to disruptive caregiver changes was assessed through age 11 and defined by two or more changes of the child’s primary caregiver (6% of participants). Fourth, exposure to physical abuse through age 11 (4% of participants) was assessed retrospectively at age 26 on the basis of Study members’ reports of severe physical punishment resulting in lasting bruising or injury. Fifth, exposure to sexual abuse (12% of participants) was assessed retrospectively at age 26 on the basis of Study members’ reports of unwanted sexual contact through age 11. We derived a cumulative exposure index for each child by counting the number of maltreatment indicators during the first decade of life19: 64% of children experienced no maltreatment, 27% experienced 1 indicator of maltreatment, and 9% experienced 2 or more indicators of maltreatment. We previously reported that only the experience of two or more indicators of childhood maltreatment significantly predicted inflammation in adulthood7. We therefore compared individuals with two or more maltreatment indicators (hereafter “maltreated”) to individuals without such a maltreatment history (“non-maltreated”).
Current Major Depression at age 32
Clinical interviews for the diagnosis of depression at age 32 used the Diagnostic Interview Schedule (DIS)20, and followed DSM-IV21. At age 32, the past-year depression prevalence in the Dunedin cohort was 16% (62% female), which is comparable to the past-year prevalence of 12% for 15-34 year olds in the National Comorbidity Survey22. Study members who were depressed at age 32 self-reported mean impairment ratings of 3.57 (SD = 0.99) on a scale from 1 (some impairment) to 5 (severe impairment), reflecting how much depression interfered with their lives. Of age-32 depression cases, 62% said they sought mental-health services in the past year, and 31% said they took medication for their disorder.
Adult Inflammation
Physical examinations and venepuncture (always between 4:15-4:45 pm) were conducted at the age-32 assessment: 92% of the participants (N=892) provided blood samples. Pregnant women (N=26) were excluded from the reported analyses. We assessed three measures of inflammation.
High-sensitivity C-Reactive Protein (hsCRP, mg/L) was measured on a Hitachi 917 analyzer (Roche Diagnostics, GmbH, D-68298, Mannheim, Germany) using a particle enhanced immunoturbidimetric assay. We adopted the CDC/AHA definition of high cardiovascular risk (hsCRP>3mg/L) to identify our risk group14.
Fibrinogen (g/L) was measured on a Sysmex CA-1500 using a fully automated cap piercing coagulation analyzer (Mahberg, Germany).
White Blood Cells (WBC, x109/L) were measured on a Sysmex XE2100 automated hematology analyzer (Kobe, Japan) via flow-cytometry using a semiconductor laser.
We report results for the categorical measure of inflammation, the high hsCRP (hsCRP>3mg/L), because of its clinically-significant predictive value14. In addition, we found that the continuous measures of (log-transformed) hsCRP, fibrinogen and WBC at age 32 were positively correlated (range r= .20 to .63). A principal-component analysis identified a single inflammation factor accounting for 59% of the variance in the continuous measures of these three markers of inflammation7. Given that all three inflammatory measures index long-term risk for cardiovascular disease23, a common factor takes full advantage of their predictive values, while minimizing measurement errors of the single components. Thus, we also report results for this dimensional inflammation factor to ascertain whether findings transcend categorical versus continuous measurements of inflammation.
Co-occurring risk factors and potential mediating variables
It is possible that depressed individuals with a history of maltreatment are also characterized by factors other than maltreatment that could explain their inflammation risk. Therefore, we considered five alternative explanatory hypotheses.
Recurrent depression history
According to the “depression-history hypothesis,” depressed individuals with a history of maltreatment may experience earlier onset of depression and more depressive episodes across the lifespan16. In turn, repeated depressive episodes might cumulatively affect inflammation risk24. As such, we controlled for history of recurrent depression, defined as the number of study assessments when members met criteria for diagnosis of depression. As previously described25, diagnoses of depression at ages 11, 13, 15, 18, 21, 26, and 32 were made via the then age-appropriate version of the Diagnostic Interview Schedule20,26,27, and the then-current version of DSM21,28,29.
Low SES in childhood
According to the “childhood-risk hypothesis,” depressed individuals with a history of maltreatment may have experienced socioeconomic disadvantage in childhood30, and childhood socioeconomic disadvantage could affect adult inflammation31. As such, we controlled for socioeconomic status (SES) in childhood, as measured repeatedly from birth through age 15 using a scale that placed parents’ occupation into one of six categories based on education and income associated with that occupation in data from the New Zealand census32. The variable used in our analyses is the average across assessments of the highest SES level of either parent.
Low SES in adulthood
According to the “adulthood-risk hypothesis,” depressed individuals with a history of maltreatment may grow up to be exposed to more socioeconomic disadvantage in adulthood30, and adult socioeconomic disadvantage could lead to elevated inflammation levels33. As such, we controlled for SES at the age 32 assessment. Study members’ current or most recent occupation was coded using a 6-point scale for occupations in New Zealand; homemakers and those not working were rated on the basis of their educational status according to criteria included in the New Zealand Socio-economic Index34.
Cardiovascular risk cluster
According to the “health-risk hypothesis,” depressed individuals with a history of maltreatment may show poorer health in adulthood35, and the increase in inflammation levels could reflect a cluster of health-risks rather than an influence of maltreatment specifically on inflammation. As such, we controlled for a cluster of cardiovascular risk factors. As previously described7, health risk-factor clustering was assessed by measuring six biomarkers: (i) overweight, (ii) high blood pressure, (iii) high total cholesterol, (iv) low high-density cholesterol, (v) high glycated hemoglobin, (vi) low VO2max adjusted for body weight. Study members were “clustered” if they had at least three of these risk factors.
Smoking
According to the “health-behavior hypothesis,” depressed individuals with a history of maltreatment are more likely to engage in unhealthy lifestyles like smoking36, which may, in turn, affect their inflammatory risk. As such we controlled for smoking habits by dividing Study members into non-smokers, light smokers (up to 10 cigarettes per day), moderate smokers (11-20), and heavy smokers (>20) according to their self-reports at age 32.
Medications
On the day of the age-32 assessment Study members were assessed for their use of medications. Here we examined antidepressants and drugs with anti-inflammatory effect, including systemic steroids, respiratory steroids, non-steroidal anti-inflammatory drugs, prophylactic aspirin, anti-gout medications, anti-rheumatic medications, statins, estrogens.
Statistical analysis
To estimate the relative contribution of childhood maltreatment and depression to inflammation in adulthood, we assigned Study members to one of four groups: (i) current depression and history of maltreatment (hereafter ‘depressed+maltreated’), (ii) current depression and no history of maltreatment (‘depressed-only’), (iii) no current depression and history of maltreatment (‘maltreated-only’), (iv) no current depression and no history of maltreatment (‘controls’). Controls include people who were not depressed at age 32 when inflammation was measured, although they could have been depressed in the past.
We tested the association between the study groups and categorical high hsCRP with Cox regression analysis with constant time of follow-up and robust variance, and the association between the study groups and the continuous inflammation factor with ordinary-least-squares regression analysis. The regression models were expanded to include other covariates to test alternative explanations for the association between study groups and inflammation. Sex and medication use were controlled for in all adjusted analyses.
RESULTS
Is depression associated with inflammation?
Depression was associated with inflammation markers (high hsCRP: RR=1.45; 95%CI=1.06;1.99; inflammation factor: b=0.18; 95%CI=0.00;0.36). However, childhood maltreatment was more common in depressed than in non-depressed individuals (RR=2.40; 95%CI=1.58;3.63), and maltreated individuals had elevated levels of inflammation markers (high hsCRP: RR=1.71; 95%CI=1.22;2.41; inflammation factor: b=0.36; 95%CI=0.14;0.59). Therefore, we tested whether a history of childhood maltreatment could explain the co-occurrence of depression and inflammation. Once the effect of maltreatment history was taken into account, the association between depression and inflammation markers was attenuated and no longer significant (high hsCRP: RR=1.35; 95%CI=0.98,1.86; inflammation factor: b=0.14; 95%CI= -0.04,0.32). A Sobel-Goodman test confirmed a statistically significant attenuation of the association between depression and inflammation markers after adjustment for maltreatment history (reduction of 19.2% for hsCRP, z=2.277, p=0.023; reduction of 22.2% for the inflammation factor, z=2.451, p=0.014). As a further test of this observation, we hypothesized that if childhood maltreatment had an important role in explaining the heterogeneity of inflammation levels in depressed individuals, elevated inflammation levels would be present in depressed individuals with a history of maltreatment but not in those without maltreatment history.
Which depressed individuals show increased inflammation?
Table 1 reports the descriptive statistics for inflammatory markers stratified by the four study groups: controls, depressed-only, maltreated-only, depressed+maltreated. Depressed+maltreated and, to a lesser extent, maltreated-only individuals were more likely to show high hsCRP levels and higher mean levels of the inflammation factor than controls. In contrast, depressed-only individuals showed a non-significant elevation in inflammatory markers (see also Figure 1).
TABLE 1.
Descriptive statistics and association analysis for inflammation markers and potential intervening variables stratified by the four study groups
| Controls (N=673) |
Depressed- only (N=109) |
Maltreated- only (N=56) |
Depressed+ Maltreated (N=27) |
Differences
among the 4 groups |
|
|---|---|---|---|---|---|
| Inflammation markers | |||||
| C-Reactive Protein >3mg/L, % (n) |
17.9% (120) | 25.0% (27) | 30.4% (17) | 37% (10) |
X2=12.05,
p=0.007 |
|
Differences between study
groups and controls |
- |
X2=3.05,
p=0.08 |
X2=5.23,
p=0.02 |
X2=6.26,
p=0.01 |
|
| Inflammation Factor, mean (se) |
−0.06 (0.04) | 0.07 (0.10) | 0.25 (0.15) | 0.48 (0.24) |
F=4.17,
p=0.006 |
|
Differences between study
groups and controls |
- |
F=1.51,
p=0.22 |
F=5.33,
p=0.02 |
F=7.90,
p=0.005 |
|
|
Potential intervening
variables |
|||||
| Depression recurrence, % (n) | |||||
| 0 | 67.5% (454) | 0.0% (0) | 67.9% (38) | 0.0% (0) |
X2=259.2,
p<0.0001 |
| 1 | 21.7% (146) | 44.0% (48) | 16.1% (9) | 25.9% (7) | |
| 2+ | 10.9% (73) | 56.0% (61) | 16.1% (9) | 74.1% (20) | |
|
Differences between study
groups and controls |
- |
X2=203.84,
p<0.0001 |
X2=2.02,
p=0.36 |
X2=96.54,
<0.0001 |
|
| SES in Childhood, % (n) | |||||
| Low | 16.7% (112) | 24.8% (27) | 40.0% (22) | 33.3% (9) |
X2=24.91,
p<0.0001 |
| Medium | 67.1% (449) | 56.0% (61) | 45.5% (25) | 55.6% (15) | |
| High | 16.2% (108) | 19.3% (21) | 14.6% (8) | 11.1% (3) | |
|
Differences between study
groups and controls |
- |
X2=5.71,
p=0.06 |
X2=18.58,
p<0.0001 |
X2=5.04,
p=0.08 |
|
| SES in Adulthood, % (n) | |||||
| Low | 27.8% (187) | 41.7% (45) | 42.9% (24) | 37.0% (10) |
X2=20.20,
p=0.003 |
| Medium | 34.8% (234) | 34.3% (37) | 25.0% (14) | 48.2% (13) | |
| High | 37.4% (252) | 24.1% (26) | 32.1% (18) | 14.8% (4) | |
|
Differences between study
groups and controls |
- |
X2=10.72,
p=0.005 |
X2=5.90,
p=0.05 |
X2=5.74,
p=0.06 |
|
| Cardiovascular Risk Cluster, % (n) |
16.5% (111) | 16.5% (18) | 17.9% (10) | 18.5% (5) |
X2=0.14,
p=0.99 |
|
Differences between study
groups and controls |
- |
X2=0.00,
p=0.99 |
X2=0.07,
p=0.80 |
X2=0.08,
p=0.78 |
|
| Smoking, % (n) | |||||
| 0 | 60.9% (410) | 57.4% (62) | 37.5% (21) | 33.3% (9) |
X2=39.84,
p<0.0001 |
| ≤ 10 | 17.5% (118) | 20.4% (22) | 21.4% (12) | 11.1% (3) | |
| 11-20 | 17.4% (117) | 19.4% (21) | 28.6% (16) | 33.3% (9) | |
| >20 | 4.2% (28) | 2.8% (3) | 12.5% (7) | 22.2% (6) | |
|
Differences between study
groups and controls |
- |
X2=1.28,
p=0.73 |
X2=16.27,
p=0.001 |
X2=25.02,
p<0.0001 |
|
| Other variables | |||||
| Male sex, % (n) | 56.0% (377) | 39.5% (43) | 44.6% (25) | 44.4% (12) |
X2=12.84,
p=0.005 |
|
Differences between study
groups and controls |
- |
X2=10.36,
p=0.001 |
X2=2.70,
p=0.10 |
X2=1.41,
p=0.24 |
|
| Medications, % (n) | 32.7% (218) | 23.2% (25) | 20.4% (11) | 18.5% (5) |
X2=8.71,
p=0.03 |
|
Differences between study
groups and controls |
- |
X2=3.93,
p=0.05 |
X2=3.49,
p=0.06 |
X2=2.39,
p=0.12 |
FIGURE 1.
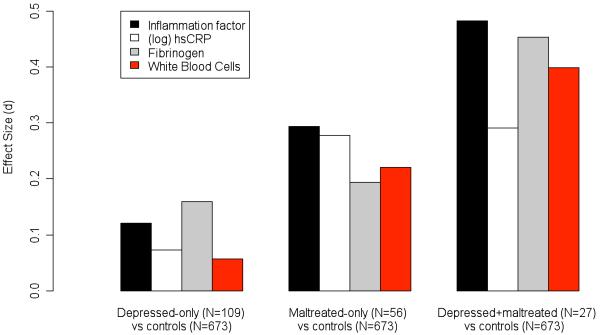
Differences in effect size (Cohen’s d) units resulting from a priori defined contrasts between controls and other study groups. Effect sizes are commonly interpreted as small when equal to 0.2, moderate when equal to 0.5, and large when equal to 0.8. The “depressed-only individuals versus controls” contrast showed effect sizes of d=0.12 for the inflammation factor, d=0.07 for (log) hsCRP, d=0.16 for fibrinogen, and d=0.06 for white blood cells (WBC). The “maltreated-only individuals versus controls” contrast showed effect sizes of d=0.29 for the inflammation factor, d=0.28 for (log) hsCRP, d=0.19 for fibrinogen, and d=0.22 for WBC. The “depressed+maltreated individuals versus controls” contrast showed effect sizes of d=0.48 for the inflammation factor, d=0.29 for (log) hsCRP, d=0.45 for fibrinogen, and d=0.40 for WBC.
Are depressed+maltreated individuals different from depressed-only individuals?
We tested whether, within depressed individuals, those with a history of maltreatment had higher inflammatory risk than individuals without maltreatment. This test was compromised by low power, as it involved comparing 27 depressed+maltreated individuals to 109 depressed-only individuals. Nevertheless, results are instructive. Individuals with depression+maltreatment were 1.48 times (95%CI=0.82;2.68) more likely to show high hsCRP levels and had higher mean levels of the inflammation factor (0.48 versus 0.07, F=2.88; p=0.09) than individuals with depression-only, with effect sizes of d=0.26 and d=0.35, respectively.
We also tested whether depressed+maltreated individuals differed from depressed-only individuals in their depressive symptoms’ profile. We found no systematic difference in symptom presentation (Table 2), suggesting it is not possible to infer maltreatment history from depressive symptoms’ profile alone.
TABLE 2.
Descriptive psychopathology of the current depressive episode in depressed-only and depressed+maltreated individuals
| Depressed- only (N=109) |
Depressed+ maltreated (N=27) |
||
|---|---|---|---|
| Depressive symptoms | |||
| 1. Depressed mood, % (n) | 94% (102) | 78% (21) | X2=6.25, p=0.01 |
| 2. Diminished interest, % (n) | 74% (81) | 89% (24) | X2=2.61, p=0.11 |
| 3. Appetite / weight changes: | |||
| 3a. less appetite / weight loss, % (n) | 69% (75) | 67% (18) | X2=0.05, p=0.83 |
| 3b. more appetite / weight gain, % (n) | 33% (36) | 33% (9) | X2=0.00, p=0.98 |
| 4. Sleep changes: | |||
| 4a. sleep deficit, % (n) | 76% (83) | 93% (25) | X2=3.58, p=0.06 |
| 4b. sleep excess, % (n) | 31% (34) | 30% (8) | X2=0.02, p=0.88 |
| 5. Psychomotor changes, % (n) | 80% (87) | 93% (25) | X2=2.43, p=0.12 |
| 6. Fatigue, % (n) | 93% (101) | 93% (25) | X2=0.00, p=0.99 |
| 7. Guilt / worthlessness, % (n) | 59% (64) | 89% (24) | X2=8.63, p=0.003 |
| 8. Concentration problems, % (n) | 95% (103) | 93% (25) | X2=0.14, p=0.71 |
| 9. Thoughts of death, % (n) | 45% (49) | 59% (16) | X2=1.77, p=0.18 |
What is the role of other known risk factors for inflammation?
Consistent with the “depression-history hypothesis,” depressed+maltreated participants were more likely to have experienced multiple depressive episodes (Table 1). In turn, individuals with recurrent depression showed non-significant elevation in risk for high hsCRP (RR=1.11; 95%CI=0.94,1.31) and significantly elevated mean levels of the inflammation factor (b=0.09; 95%CI=0.00,0.17). However, after controlling for recurrent depression history, the association between age-32 depression+maltreatment and inflammation markers remained significant (Table 3, Model 3).
TABLE 3.
Group differences in predicting high hsCRP risk and inflammation factor levels. Model 1 shows the unadjusted analysis. Model 2 shows the analysis adjusted for sex and use of anti-inflammatory medications. Model 3 indexes the “depression-history hypothesis”. Model 4 indexes the “childhood-risk hypothesis”. Model 5 indexes the “adulthood-risk hypothesis”. Model 6 indexes the “health-risk hypothesis”. Model 7 indexes the “health-behavior hypothesis”.
| Controls (N=673) |
Depressed-only (N=109) |
Maltreated-only (N=56) |
Depressed+ Maltreated (N=27) |
|
|---|---|---|---|---|
| C-Reactive Protein >3 mg/L † | ||||
| 1. Unadjusted | - | 1.40 [0.97;2.01] | 1.69 [1.10;2.60] | 2.07 [1.23;3.47] |
| 2. Sex, medications | - | 1.34 [0.93;1.92] | 1.61 [1.03;2.50] | 2.06 [1.21;3.51] |
| 3. Depression recurrence* | - | 1.52 [1.00;2.34] | 1.62 [1.04;2.53] | 2.41 [1.31;4.43] |
| 4. SES in Childhood* | - | 1.31 [0.91;1.87] | 1.52 [0.98;2.35] | 1.92 [1.12;3.29] |
| 5. SES in Adulthood* | - | 1.30 [0.90;1.87] | 1.57 [1.01;2.44] | 1.97 [1.16;3.37] |
| 6. CV Risk Cluster* | - | 1.32 [0.93;1.87] | 1.56 [1.02;2.38] | 2.00 [1.21;3.32] |
| 7. Smoking* | - | 1.35 [0.94;1.94] | 1.64 [1.05;2.55] | 2.13 [1.24;3.66] |
| Inflammation Factor § | ||||
| 1. Unadjusted | - | 0.12 [−0.08;0.33] | 0.31 [0.04;0.58] | 0.53 [0.15;0.91] |
| 2. Sex, medications | - | 0.10 [−0.10;0.30] | 0.27 [0.00;0.55] | 0.52 [0.14;0.90] |
| 3. Depression recurrence* | - | 0.10 [−0.13;0.33] | 0.27 [0.00;0.55] | 0.52 [0.12;0.92] |
| 4. SES in Childhood* | - | 0.09 [−0.11;0.29] | 0.22 [−0.05;0.49] | 0.47 [0.10;0.85] |
| 5. SES in Adulthood* | - | 0.07 [−0.13;0.27] | 0.26 [−0.02;0.53] | 0.49 [0.11;0.86] |
| 6. CV Risk Cluster* | - | 0.10 [−0.09;0.29] | 0.25 [0.00;0.51] | 0.50 [0.14;0.85] |
| 7. Smoking* | - | 0.09 [−0.11;0.29] | 0.21 [−0.06;0.48] | 0.42 [0.04;0.80] |
Cox regression analysis with constant time of follow-up and robust variance. Controls (i.e., non-depressed non-maltreated individuals) were considered the reference group.
Ordinary-least-squares regression analysis. Controls were considered the reference group.
Adjusted analyses also include sex and medications covariates.
Consistent with the “childhood-risk hypothesis,” depressed+maltreated participants were more likely to have grown up in low-SES families (Table 1). In turn, low childhood SES was associated with elevated inflammation in adulthood (high hsCRP: RR=1.36; 95%CI=1.09,1.68; inflammation factor: b=0.26; 95%CI=0.15,0.37). However, after controlling for the effect of childhood SES, the association between depression+maltreatment and inflammation markers remained significant (Table 3, Model 4).
Consistent with the “adulthood-risk hypothesis,” depressed+maltreated participants were more likely to have low adult SES (Table 1). In turn, low adult SES was associated with elevated inflammation in adulthood (high hsCRP: RR=1.17; 95%CI=1.00,1.38; inflammation factor: b=0.13; 95%CI=0.05,0.21). However, after controlling for adult SES, the association between depression+maltreatment and inflammation markers remained significant (Table 3, Model 5).
Turning to the “health-risk hypothesis,” we found that the prevalence of the cardiovascular risk cluster did not differ across study groups (Table 1). The cardiovascular risk cluster was associated with elevated inflammation in adulthood (high hsCRP: RR=2.39; 95%CI=1.84,3.10; inflammation factor: b=0.87; 95%CI=0.70,1.04). After controlling for cardiovascular risk clustering, the association between depression+maltreatment and inflammation markers remained significant (Table 3, Model 6).
Consistent with the “health-behavior hypothesis,” depressed+maltreated participants were more likely to smoke (Table 1). In turn, smoking was associated with elevated mean levels of the inflammation factor (b=0.13; 95%CI=0.06,0.20), but was not associated with the risk for high hsCRP levels (RR=0.98; 95%CI=0.84,1.13). After controlling for smoking, the association between depression+maltreatment and inflammation markers remained significant (Table 3, Model 7).
CONCLUSIONS
This study addressed the possible developmental origins of heterogeneity in inflammation markers’ levels among depressed individuals. The results suggest that a history of maltreatment has a significant role in explaining the co-occurrence of depression and inflammation in adulthood. Information about experiences of childhood maltreatment may help to identify depressed individuals with elevated inflammation levels and thus greater cardiovascular disease risk.
These new findings should be evaluated alongside several limitations. First, findings from this New Zealand cohort require replication in other studies and in different ethnic groups. However, given the consistent effect of childhood maltreatment in explaining depression heterogeneity with regard to other stress biomarkers37-40, there is reason to believe that our results may be replicated in other settings. Second, lacking measures of inflammation markers before the onset of depression, we are unable to test the direction of the effect. Future research should address this issue. Third, although childhood maltreatment appeared to predict elevated inflammation levels in depressed individuals, some depressed individuals with elevated inflammation had not been maltreated. Further studies are needed to uncover other factors contributing to the heterogeneity of inflammation markers’ levels in depression. Nevertheless, the current results may have implications for research, psychiatric nosology, and clinical practice.
With regard to research implications, the present findings help to reconcile previous puzzling evidence about the relationship between depression and inflammation, such as the inconsistency of this association across samples and the persistence of inflammation and cardiovascular disease risk in individuals with a history of depression but no current depression4,5,15. We showed that current depression diagnosis alone was less likely to be related to inflammation risk, but current depression and maltreatment history combined appeared to be good predictors of inflammation levels. Moreover, even in the absence of a current depression diagnosis, maltreatment history alone still conferred an elevated risk for clinically-relevant inflammation levels (see Table 1 and Figure 1). This suggests that previous inconsistencies across studies could be due to variation in the prevalence of childhood maltreatment in different samples. Results also suggest that elevated inflammation levels and cardiovascular disease risk observed in individuals with a history of depression but no current depression may be due to the long-term effect of childhood maltreatment7,17.
With regard to implications for psychiatric nosology, our study adds to a growing body of research suggesting that childhood maltreatment could identify a subgroup of depressed individuals characterized by multiple markers of abnormal stress response. Table 4 summarizes findings from three lines of research. First, reduced volume of the hippocampus, a brain region regulating the extinction of the stress response, has been shown in depressed+maltreated individuals compared to controls, but not in depressed-only individuals40. Second, greater neuroendocrine response to a psychosocial stress test and insufficient glucocorticoid signalling have been reported in depressed+maltreated individuals compared to controls, but not in depressed-only individuals37-39. Third, consistent with previous experimental evidence18, in the present study we report that depressed+maltreated individuals were twice as likely to show clinically-relevant levels of hsCRP compared to control individuals, while depressed-only individuals showed a non-significant increase in the risk for high hsCRP. It is possible that a subgroup of depressed individuals with stressful developmental experiences is at highest risk for future disease. Further studies are needed to better characterize individuals with this “developmental-stress” subtype of depression.
TABLE 4.
Summary of brain imaging, neuroendocrine, and immunological differences among study groups compared to controls (adapted from ref. 39). Biological differences between depressed+maltreated and depressed-only individuals suggest the existence of a “developmental-stress" subtype of depression with abnormal stress response and increased risk of medical comorbidity
| Depressed- only |
Maltreated- only |
Depressed+ maltreated |
|
|---|---|---|---|
| Brain Imaging | |||
| Hippocampus volume (ref.40) | = | ? | ↓ |
| Psychosocial stress challenge | |||
| ACTH (ref. 37) | = | ↑ | ↑ |
| Cortisol (ref. 37) | = | = | ↑ |
| CRF stimulation test | |||
| ACTH (ref. 38) | ↓ | ↑ | ↓ |
| Cortisol (ref. 38) | ↓ | ↓ | ↓ |
| ACTH stimulation test | |||
| Cortisol (ref. 38) | ↓ | ↓ | ↓ |
| DEX suppression test (0.5 mg) | |||
| ACTH (ref. 39) | = | = | ↓ |
| Cortisol (ref. 39) | = | = | ↓ |
| Inflammation | |||
| hsCRP >3 mg/L (present study) | = | ↑ | ↑ |
| Inflammation Factor (present study) | = | ↑ | ↑ |
CRF: corticotrophin releasing hormone. ACTH: adrenocorticotropic hormone. DEX: dexamethasone. hsCRP: high-sensitivity C-reactive protein.
Symbols in the table indicate whether values of different biological markers in study groups are equal (=), increased (↑), or decreased (↓) compared to controls, or whether comparisons are missing (?).
With regard to implications for clinical practice, our results support the importance of collecting information about childhood maltreatment from depressed individuals. Indeed, in our cohort the information conveyed by maltreatment history was not captured by information about symptom presentation during the depressive episode (see Table 2). Moreover, we showed that other commonly assessed factors with potential influence on inflammation processes do not account for the effect of childhood maltreatment on adult inflammation in depressed individuals (see Table 3). For these reasons, we suggest that routine assessment of maltreatment history could provide clinicians with necessary information to identify depressed individuals with elevated risk of inflammation and potentially poor health. In turn, the early recognition of the health risk associated with maltreatment history might help to address pressing needs for the care of depressed individuals, such as the reduction of the impact of depression on comorbid medical illness1.
Acknowledgements
We thank the Dunedin Study members and Study founder Dr. Phil Silva. The Dunedin Multidisciplinary Health and Development Research Unit is supported by the New Zealand Health Research Council. This research received support from Medical Research Council grant G0100527, NIMH grants MH45070, MH49414, and MH077874, and from the William T. Grant Foundation. Dr. Danese is a Wellcome Trust Research Training Fellow. Dr. Pariante is a Medical Research Council Research Fellow. Drs. Moffitt and Caspi are Royal Society-Wolfson Merit Award holders. The Study protocol was approved by the institutional review boards of the participating universities. Study members gave informed consent before participating.
Contributor Information
Andrea Danese, Social, Genetic, and Developmental Psychiatry Centre, Institute of Psychiatry, King’s College London, London SE5 8AF, United Kingdom
Terrie E. Moffitt, Social, Genetic, and Developmental Psychiatry Centre, Institute of Psychiatry, King’s College London, London SE5 8AF, United Kingdom and Departments of Psychology and Neuroscience, Psychiatry and Behavioral Sciences, and Institute for Genome Sciences and Policy, Duke University, Durham, NC 27708-0086
Carmine M. Pariante, Department of Psychological Medicine, Institute of Psychiatry, King’s College London, London SE5 8AF, United Kingdom
Antony Ambler, Social, Genetic, and Developmental Psychiatry Centre, Institute of Psychiatry, King’s College London, London SE5 8AF, United Kingdom
Richie Poulton, Dunedin School of Medicine, University of Otago, Dunedin 9015, New Zealand
Avshalom Caspi, Social, Genetic, and Developmental Psychiatry Centre, Institute of Psychiatry, King’s College London, London SE5 8AF, United Kingdom and Departments of Psychology and Neuroscience, Psychiatry and Behavioral Sciences, and Institute for Genome Sciences and Policy, Duke University, Durham, NC 27708-0086
REFERENCE LIST
- 1.Insel TR, Charney DS. Research on major depression: strategies and priorities. JAMA. 2003;289:3167–8. doi: 10.1001/jama.289.23.3167. [DOI] [PubMed] [Google Scholar]
- 2.Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O’Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Jr., Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–89. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA. 1993;270:1819–25. [PubMed] [Google Scholar]
- 4.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Norman J, Powell LH, Raczynski JM, Schneiderman N. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289:3106–16. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 5.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 7.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104:1319–24. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–28. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 11.Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. Am J Cardiol. 2002;90:1279–83. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- 12.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 14.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr., Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 15.Kling MA, Alesci S, Csako G, Costello R, Luckenbaugh DA, Bonne O, Duncko R, Drevets WC, Manji HK, Charney DS, Gold PW, Neumeister A. Sustained low-grade pro-inflammatory state in unmedicated, remitted women with major depressive disorder as evidenced by elevated serum levels of the acute phase proteins C-reactive protein and serum amyloid A. Biol Psychiatry. 2007;62:309–13. doi: 10.1016/j.biopsych.2006.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry. 2007;64:49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- 17.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–6. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 18.Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–3. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 19.Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–4. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 20.Robins LN, Cottler L, Bucholz KK, Compton W. The Diagnostic Interview Schedule for DSM-IV. Washington University; St. Louis, MO: 1995. [Google Scholar]
- 21.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th edn. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 22.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 23.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–82. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 24.Ford DE, Erlinger TP. Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2004;164:1010–4. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- 25.Moffitt TE, Harrington H, Caspi A, Kim-Cohen J, Goldberg D, Gregory AM, Poulton R. Depression and generalized anxiety disorder: cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Arch Gen Psychiatry. 2007;64:651–60. doi: 10.1001/archpsyc.64.6.651. [DOI] [PubMed] [Google Scholar]
- 26.Costello E, Edelbrock C, Kalas R, Kessler M, Klaric S. National Institute of Mental Health Diagnostic Interview Schedule for Children. National Institute of Mental Health; Rockville, MD: 1982. [Google Scholar]
- 27.Robins LN, Helzer JE, Cottler L, Goldring E. The Diagnostic Interview Schedule for DSM-III-R. Washington University School of Medicine; St.Louis, MO: 1989. [Google Scholar]
- 28.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3rd edn. American Psychiatric Association; Washington, DC: 1980. [Google Scholar]
- 29.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3rd edn. American Psychiatric Association; Washington, DC: 1987. revised. [Google Scholar]
- 30.Widom CS. The cycle of violence. Science. 1989;244:160–6. doi: 10.1126/science.2704995. [DOI] [PubMed] [Google Scholar]
- 31.Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biol Psychiatry. 2006;60:819–24. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Poulton R, Caspi A, Milne BJ, Thomson WM, Taylor A, Sears MR, Moffitt TE. Association between children’s experience of socioeconomic disadvantage and adult health: a life-course study. Lancet. 2002;360:1640–5. doi: 10.1016/S0140-6736(02)11602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steptoe A, Owen N, Kunz-Ebrecht S, Mohamed-Ali V. Inflammatory cytokines, socioeconomic status, and acute stress responsivity. Brain Behav Immun. 2002;16:774–84. doi: 10.1016/s0889-1591(02)00030-2. [DOI] [PubMed] [Google Scholar]
- 34.Davis P, Jenkin G, Coope P. An update and revision of the New Zealand Socio-economic index of Occupational Status. Statistics New Zealand; Wellington: 2003. New Zealand Socio-economic index 1996. [DOI] [PubMed] [Google Scholar]
- 35.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS, The Adverse Childhood Experiences (ACE) Study Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. Am J Prev Med. 1998;14:245–58. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 36.Anda RF, Croft JB, Felitti VJ, Nordenberg D, Giles WH, Williamson DF, Giovino GA. Adverse childhood experiences and smoking during adolescence and adulthood. JAMA. 1999;282:1652–8. doi: 10.1001/jama.282.17.1652. [DOI] [PubMed] [Google Scholar]
- 37.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–7. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 38.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158:575–81. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 39.Newport DJ, Heim C, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal responses to standard and low-dose dexamethasone suppression tests in adult survivors of child abuse. Biol Psychiatry. 2004;55:10–20. doi: 10.1016/s0006-3223(03)00692-9. [DOI] [PubMed] [Google Scholar]
- 40.Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, Brummer M, Staib L, Vermetten E, Charney DS, Nemeroff CB, Bremner JD. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–80. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]