Abstract
Breast cancer related lymphoedema (BCRL), the chronically swollen arm of patients that have been treated for breast cancer, is no longer considered to be a result of lymphatic obstruction as recent studies have identified failing peripheral lymphatic function as a principal contributing factor. The aetiology and pathophysiology that results in this lymphatic failure is not clearly understood, but it can occur with minimal or even in some cases no damage to the axillary lymph nodes, and evidence suggests that some patients are pre-disposed to develop the disease, and have poor lymphatic function in their non-affected arms. It has been shown that interstitial forces such as hydrostatic pressure, and interstitial fluid velocity, can regulate both lymph flow, and lymph formation, and there is good evidence that interstitial forces are dysregulated in lymphoedema patients. Here I outline a hypothesis for how dysregulation of interstitial parameters could contribute to the generation of breast cancer related lymphoedema, by combining disparate strands of current evidence on the molecular and physiological control of interstitial and lymph flows. One mechanism by which lymphoedema could be generated is that a reduction in interstitial velocity results in increased VEGF-C production, which in low flow conditions, instead of acting on the lymphatics to increase pumping and lymphangiogenesis, acts on vasculature to increase fluid filtration. The resulting increase in interstitial pressure restores flow, but at the expense of increased volume and hence oedema. The evidence supporting the hypothesis and possible tests of it are presented and discussed.
Keywords: Breast cancer related lymphoedema, Pathophysiology, VEGF-C
1. Introduction
1.1. Breast cancer related lymphoedema (BCRL)
Following treatment for breast cancer 25% of patients develop a chronic, sustained swelling of the limb on the treated side, referred to as breast cancer related lymphoedema (BCRL), which is distinct from the acute swelling experienced by many patients immediately after surgery [1]. There is usually a substantial delay between the initial treatment and appearance of BCRL, with most cases developing within 3 years of treatment [2]. The onset of this chronic oedema is rapid but the chronically swollen limb may remain in a relatively steady state after the initial onset of lymphoedema [3]. There is evidence to suggest that the swelling may gradually worsen over time but there is only a weak positive correlation between the degree of swelling and the duration of swelling [4]. The pathophysiology of lymphoedema has been attributed to an obstruction of the lymphatics after surgery, resulting in an increased downstream pressure, which is transmitted to the interstitium [5]. In the last few years this view has been substantially challenged, by the concept of lymphatic pump failure as an underlying cause of the disease [6]. There is now substantial evidence that the lymphatic collecting vessels along the arm, often at a large distance away from the initial insult to the lymphatics fail in lymphoedema [7], and that this results in an inability to clear interstitial fluid from the arm, and hence increased arm volume. Moreover, lymphoedema is a chronic sustained condition, in which swelling, once it occurs is maintained over many years, despite treatment with compression hosiery, manual lymphatic drainage or other treatments [2]. There is therefore a clear and substantial alteration in the interstitial forces that control fluid movement into and out of the arm. This review will lay out an additional hypothesis for lymphoedema, that is complementary to and consistent with the view of lymphatic pump failure as a key driver of establishment and maintenance of BCRL, but which invokes an interstitial contribution mediated by lymphatic growth factor expression.
1.2. Interstitial components of lymphoedema
Tissue oedema results from an imbalance between fluid movement into the tissue, i.e. the filtration of fluid from the capillary into the tissue, and fluid movement out of the tissue, either re-absorption of fluid back into the blood or lymph flow [8]. Thus in a steady state (i.e. no active swelling), the net fluid filtration out of the capillaries into the tissue, must balance the net lymph flow out of the tissue [9]. Lymph flow can increase in response to increased filtration rate up to a point, and the difference between the normal filtration rate and the maximal lymph flow can be thought of as the lymphatic reserve. In chronic lymphoedema, therefore, when no swelling is actively occurring, but the arm is in a steady state, there must be a balance between this filtration and fluid efflux by lymph flow.
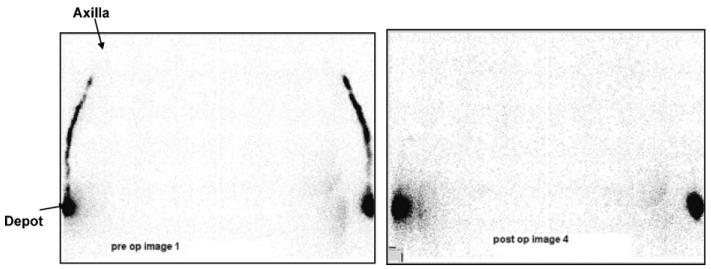
The classic view of secondary lymphoedema is that obstruction of the lymphatics results in reduced outflow of fluid, and reduces lymphatic reserve [10]. If that is the case, then in the steady state there must be a reduced filtration rate. One of the consequences of reduced filtration rate is an increase in interstitial protein concentration. However, the interstitial protein concentration in breast cancer related lymphoedema is inversely, not positively correlated with the increase in arm volume, indicating that the bigger the increase in arm volume, the lower the protein concentration, and hence the higher the filtration rate [11]. This is in contrast to the clearly demonstrated reduction in tracer removal from the arm in BCRL patients [12]. This reduction in the amount of time it takes a tracer to be removed from the arm is a result of reduced fluid velocity [13]. Fig. 1 shows that approximately 50 min after injection of radioactive tracer into the lower arm the tracer has moved from the injection site to the upper arm in the pre-operative state, but not the post operative state [14,15], indicating that lymph velocity (not necessarily lymph flow) is reduced in BCRL, a finding widely reported [16]. Interestingly however, the images shown in Fig. 1 come from a study that showed no relationship between reduced lymph velocity after treatment compared with before treatment in the whole cohort of patients who developed lymphoedema [15]. A reduction in lymphatic velocity may reflect a downstream reduction in interstitial fluid velocity.
Fig. 1.
Reduced transport of radiolabelled dye from periphery to axilla in BCRL. From [14].
Interstitial fluid velocity has been reported at rates of up to 0.3–1 μms−1 in normal tissues [17]. Recent evidence from the Swartz group indicates that cells can recognise small fluid velocities well below this magnitude [18]. Moreover, lymphatic regeneration (Fig. 2), and the lymphatic growth factor vascular endothelial growth factor C (VEGF-C) appear to be regulated by the presence or absence of interstitial flow through tissues [19]. It has been shown that VEGF-C expression is increased in the absence of fluid flow relative to normal flows. VEGF-C is required for lymphatic growth, but acts through two receptors, VEGFR3 on lymphatic endothelial cells (and on vascular endothelial cells in tumours and during development), and VEGFR2, restricted to vascular endothelial cells [20]. VEGF-C can act on lymphatic vessels and blood vessels. On lymphatic vessels, VEGF-C results in lymphangiogenesis [21], lymphatic contraction, and increased lymph flows [22]. On blood vessels, VEGF-C acts to increase vascular permeability [23,24] and to stimulate angiogenesis [25]. VEGF-C results in a significant increase in hydraulic conductivity in a similar manner to VEGF-A [23]. Interestingly, chronic exposure of vascular endothelial cells to VEGFs result, through VEGFR2 activation in increased hydraulic conductivity in the absence of altered oncotic reflection coefficient [26]. This latter property means that the filtration of fluid is increased without a concomitant increase in protein permeability, which would result in a reduced interstitial protein concentration [27], as seen in lymphoedema [11].
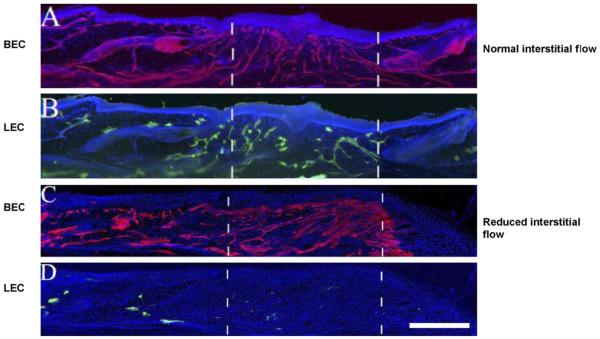
Fig. 2.
Low lymph flow velocity results in reduced lymphatic migration in animal models. The dotted lines indicate denuded tissue. When interstitial flow occurs (A and B) both lymphatics (green) or blood vessels (red) regenerate. In the absence of flow (C and D) there is no lymphatic regeneration, although blood vessel regeneration is unaffected. From [18]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
VEGF-C therefore has properties that would be consistent with both increasing fluid efflux, and lymph flow [22], and can be responsive to a reduction in fluid velocity [19]. However, there is as yet little direct evidence that VEGF-C is upregulated in extant, chronic lymphoedema in humans, although it is upregulated in animal models of chronic lymphoedema [28]. However, lymphography of patients with BCRL indicate that the area of lymphatics that took up a bolus of fluorescence, was significantly increased in the swollen arm compared to the non-swollen contralateral arm [29]. This can be interpreted in one of two ways, either there is more lymphatics, or there is the same number of lymphatics but they are taking up the dye more easily. Subsequent work on biopsies taken from patients with lymphoedema stained with LYVE 1 for lymphatics revealed that the number of lymphatics per unit area in the skin was not altered (Joory, in prep.).
It has recently been demonstrated that the response to VEGF-C is dependent on the local blood vessel density. Over-expression of VEGF-C by adenoviruses in the rat mesenteric fat pad resulted in lymphangiogenesis and angiogenesis in the mesenteric connective tissue [30]. However, in areas of low blood vessel density, VEGF-C is strongly lymphangiogenic, but in areas of high blood vessel density there is less increase in lymphatic density [30], indicating that the local bioavailability of VEGF-C determines its physiological outcome—acting more on blood vessels in the absence of lymphatics. Thus VEGF-C appears to act on the vessels it sees first. So, if VEGF-C was to be upregulated by a lack of flow then its action would depend upon whether it is close to a lymphatic or a blood vessel. Blood vessel density in human skin is of the order of 80–100 mm−2 [31], whereas lymphatic vessel density is 8–10 mm−2 [32]. Thus it is significantly more likely that VEGF-C molecules upregulated by lack of flow will act first on a blood vessel rather than a lymphatic. It is therefore possible that the target cell type for VEGF-C may depend on interstitial flow. Using a mouse model of a lymphatics in the tail, the Swartz group have shown that regeneration of lymphatics [33], but not blood vessels, is dependent upon flow [19]. Moreover, in the presence of VEGF-C, but absence of flow, there is no lymphatic growth but there is increased blood endothelial growth [19]. In this case the VEGF-C is acting on the blood endothelial cells, but not on the lymphatic vessels. So it appears there is evidence for the hypothesis that the response to VEGF-C is dependent on fluid flow.
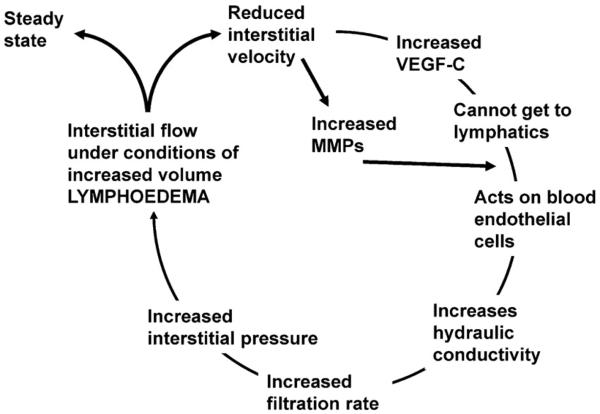
This leads us to a novel interstitial hypothesis for lymphoedema (Fig. 3). Lymphatic failure results in a reduction in velocity of fluid flow from the interstitium into the lymphatics. This decrease is detected in the cells of the interstitium (probably fibroblasts) and results in an increase in the production of VEGF-C. This secreted VEGF-C, in the absence of flow towards a draining lymphatic network, diffuses to reach the nearest VEGFR that it can act on, most likely on the blood vessels, where it increases hydraulic conductivity and hence fluid filtration, which thus increases interstitial fluid volume, and interstitial pressure, and increases the hydrostatic pressure difference between the interstitium and the lymphatics, so resulting in fluid flow towards those lymphatics, and hence reducing VEGF-C induction. So this increased pressure is required to induce sufficient fluid velocity, and therefore a requirement for increased hydrostatic pressure and hence increased volume in the interstitium of the arm in order to get a steady state. If the steady state begins to drop again due to further lymph failure, then VEGF-C is again produced and the cycle continues until a new steady state is reached.
Fig. 3.
Interstitial hypothesis for secondary lymphoedema.
This hypothesis raises a number of specifically testable predictions. These include:
- (A) During development of lymphoedema:
- There will be reduced interstitial fluid velocity.
- There will be increased VEGF-C.
- VEGF-C will act on VEGFR2 on blood endothelial cells.
- There will be an increase in hydraulic conductivity of the capillaries in lymphoedema.
- (B) During steady state:
- There will be increased interstitial hydrostatic pressure.
- Interstitial fluid velocity in established lymphoedema will compare with that before lymphoedema.
- There will be evidence of lymphatic pump failure.
Some of these predictions have already been tested, are being tested, or could be tested relatively easily. Predictions during the steady state have been tested. The interstitial pressure in the lymphoedematous arm is significantly higher than in the control arm [34]. The final point (evidence of lymph pump failure) has recently been clearly shown by Modi et al., who measured the pressure required to inhibit the lymphatic's ability to drain a radioactive tracer across a compression cuff inflated to specific pressures. They found that in lymphoedematous arms a much lower pressure was required to prevent lymph drainage indicating that the lymphatics were unable to generate as high pressures in lymphoedematous compared with the normal arm [6].
The capillary filtration coefficient of patients with established lympheodema has been measured by Stanton et al., and there was no significant difference in the filtration between the two arms indicating that filtration has re-established itself [4]. However, this was in established lymphoedema, not during the swelling process. In fact the vast majority of studies have been done on patients who are already in a steady state, and as the swelling can occur quite precipitously, it will be difficult to measure parameters during swelling. To do this prospective studies are required and it is this recent advance that has been quite informative. Of specific interest is the recent finding that a subset of patients that have lymphoedema have high filtration rates to start with (i.e. before they develop lymphoedema) [35]. This implies that some patients are susceptible to lymphoedema due to their normally higher filtration rates. There is evidence to suggest that there may be a subset of breast cancer patients who have a predisposition to developing BCRL. The basis of this evidence is that the prevalence of BCRL in patients treated for bilateral breast cancer is no higher than in those treated for unilateral breast cancer [2]. The probability of developing BCRL in one or both arms following bilateral breast cancer treatment can be calculated based on the prevalence in unilateral breast cancer patients, assuming that the development of swelling in one arm is independent of swelling in the other arm. The calculated theoretical probability (48%) was substantially higher than the reported prevalence in bilateral breast cancer patients (14.5–34.2%) suggesting a pre-existing congenitally determined lymphatic insufficiency as a putative predisposing factor. There is also evidence to suggest that variations in lymphatic function may affect the development of BCRL. Moreover, lymphatic function appears to be reduced in the contralateral non-swollen arm of patients with BCRL with hand swelling, compared with the contralateral non-swollen arm of BCRL patients who have no hand swelling [15].
1.3. Assumptions
The hypothesis is built on a number of assumptions, which need at this point to be questioned.
There is reduced interstitial fluid velocity in BCRL. A reduced lymphatic velocity does not necessarily translate to reduced interstitial velocity. This assumption has yet to be tested.
Human VEGF-C production is interstitial flow dependent. The work currently done showing VEGF-C induction in vivo has been done in mouse tissues. However, there is evidence from human cancer cells that human VEGF-C can be induced by flow through the matrix through which the cells are embedded.
In summary, if the hypothesis I have outlined is correct, then we would expect to see in patients that are going to go on to develop lymphoedema that the interstitial velocity is higher than normal, resulting in reduced lymphatic reserve, and lymphatic pump failure in the presence of increased resistance after axillary node interference during cancer surgery or radiotherapy, and/or increased filtration of fluid into the arm either as a result of a specific event (e.g. a trigger effect), or because the patient has a high filtration rate and therefore reduced lymphatic reserve compared to the general population. When the lymph pump fails, a reduction in the interstitial fluid velocity occurs, which results in an increase in VEGF-C, and increase in blood endothelial VEGF-R2 phosphorylation, increased hydraulic conductivity and increase in interstitial filtration rate, resulting in an increased interstitial pressure. That increase in interstitial pressure will then act to limit the increase in filtration rate and so terminate swelling at the point at which the pressure in the interstitium is enough to drive the fluid into the lymphatics. This interstitial flow returns towards normal at the expense of an increase in interstitial fluid volume.
If the above hypothesis can be demonstrated, this also outlines a new potential avenue for therapy, in that targeting the vasculature may aid BCRL. Inhibition of VEGFR2 in patients after their breast cancer treatment would be predicted to reduce the effect of VEGF-C on blood endothelial cells. It will therefore be of immense interest to determine whether current clinical trials of VEGFR kinase inhibitors in breast cancer [36] result in less lymphoedema than in patients undergoing therapy that results in either no VEGFR2 inhibition, or patients undergoing anti-angiogenic therapy with agents that do not affect VEGFR2 (e.g. bevacizumab) [37].
References
- 1.Clarke D, Martinez A, Cox RS. Analysis of cosmetic results and complications in patients with stage I and II breast cancer treated by biopsy and irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1983;9:1807–1813. doi: 10.1016/0360-3016(83)90348-6. [DOI] [PubMed] [Google Scholar]
- 2.Mortimer PS, Bates DO, Brassington HD, Stanton A, Strachan DP, Levick JR. The prevalence of arm oedema following treatment for breast cancer. Month. J. Assoc. Phys. 1996;89:377–380. [Google Scholar]
- 3.Mortimer PS. The pathophysiology of lymphedema. Cancer. 1998;83:2798–2802. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2798::aid-cncr28>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Stanton AW, Holroyd B, Mortimer PS, Levick JR. Comparison of microvascular filtration in human arms with and without postmastectomy oedema. Exp. Physiol. 1999;84:405–419. doi: 10.1111/j.1469-445x.1999.01810.x. [DOI] [PubMed] [Google Scholar]
- 5.Browse NL. Lymphoedema of the arm. Br. Med. J. (Clin. Res. Ed.) 1987;295:3–4. doi: 10.1136/bmj.295.6589.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modi S, Stanton AW, Svensson WE, Peters AM, Mortimer PS, Levick JR. Human lymphatic pumping measured in healthy and lymphoedematous arms by lymphatic congestion lymphoscintigraphy. J. Physiol. 2007;583:271–285. doi: 10.1113/jphysiol.2007.130401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanton AW, Mellor RH, Cook GJ, Svensson WE, Peters AM, Levick JR, Mortimer PS. Impairment of lymph drainage in subfascial compartment of forearm in breast cancer-related lymphedema. Lymphat. Res. Biol. 2003;1:121–132. doi: 10.1089/153968503321642615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starling E. On the absorption of fluids from the connective tissue spaces. J. Physiol. 1896;19:312–326. doi: 10.1113/jphysiol.1896.sp000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levick JR, Mortimer PS, Bates DO. Spatial filtration – reabsorption balance along the capillary axis – fact or fiction. Lymphology. 1994;27:460–463. [Google Scholar]
- 10.Olszewski W. On the pathomechanism of development of postsurgical lymphedema. Lymphology. 1973;6:35–51. [PubMed] [Google Scholar]
- 11.Bates DO, Levick JR, Mortimer PS. Change in macromolecular composition of interstitial fluid from swollen arms after breast cancer treatment, and its implications. Clin. Sci. 1993;85:737–746. doi: 10.1042/cs0850737. [DOI] [PubMed] [Google Scholar]
- 12.Richards TB, McBiles M, Collins PS. An easy method of diagnosis of lymphoedema. Ann. Vasc. Surg. 1990;4:255–259. doi: 10.1007/BF02009453. [DOI] [PubMed] [Google Scholar]
- 13.Levick JR, Mortimer PS. The interpretation of lymphoscintigraphy removal rates. Lymphology. 1994;27:123. [Google Scholar]
- 14.O'Mahony S, Britton TM, Solanki CK, Ballinger JR, Pain SJ, Mortimer PS, Purushotham AD, Peters AM. Lymphatic transfer studies with immunoglobulin scintigraphy after axillary surgery. Eur. J. Surg. Oncol. 2007;33:1052–1060. doi: 10.1016/j.ejso.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Stanton AW, Modi S, Mellor RH, Peters AM, Svensson WE, Levick JR, Mortimer PS. A quantitative lymphoscintigraphic evaluation of lymphatic function in the swollen hands of women with lymphoedema following breast cancer treatment. Clin. Sci. (Lond.) 2006;110:553–561. doi: 10.1042/CS20050277. [DOI] [PubMed] [Google Scholar]
- 16.Williams WH, Witte CL, Witte MH, McNeill GC. Radionuclide lymphangioscintigraphy in the evaluation of peripheral lymphedema. Clin. Nucl. Med. 2000;25:451–464. doi: 10.1097/00003072-200006000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Chary SR, Jain RK. Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc. Natl. Acad. Sci. U.S.A. 1989;86:5385–5389. doi: 10.1073/pnas.86.14.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleury ME, Boardman KC, Swartz MA. Autologous morphogen gradients by subtle interstitial flow and matrix interactions. Biophys. J. 2006;91:113–121. doi: 10.1529/biophysj.105.080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman J, Conley KA, Raehl A, Bondy DM, Pytowski B, Swartz MA, Rutkowski JM, Jaroch DB, Ongstad EL. Regulation of lymphatic capillary regeneration by interstitial flow in skin. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H2176–H2183. doi: 10.1152/ajpheart.01011.2006. [DOI] [PubMed] [Google Scholar]
- 20.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. Embo J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 21.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 22.Breslin JW, Gaudreault N, Watson KD, Reynoso R, Yuan SY, Wu MH. Vascular endothelial growth factor-C stimulates the lymphatic pump by a VEGF receptor-3-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H709–H718. doi: 10.1152/ajpheart.00102.2007. [DOI] [PubMed] [Google Scholar]
- 23.Hillman NJ, Whittles CE, Pocock TM, Williams B, Bates DO. Differential effects on microvascular hydraulic conductivity (Lp) of vascular endothelial growth factor C (VEGF-C) and placental growth factor-1 (PlGF-1) J. Vasc. Res. 2001;38:176–185. doi: 10.1159/000051044. [DOI] [PubMed] [Google Scholar]
- 24.Saaristo A, Veikkola T, Enholm B, Hytonen M, Arola J, Pajusola K, Turunen P, Jeltsch M, Karkkainen MJ, Kerjaschki D, Bueler H, Yla-Herttuala S, Alitalo K. Adenoviral VEGF-C overexpression induces blood vessel enlargement, tortuosity, and leakiness but no sprouting angiogenesis in the skin or mucous membranes. Faseb J. 2002;16:1041–1049. doi: 10.1096/fj.01-1042com. [DOI] [PubMed] [Google Scholar]
- 25.Oh SJ, Jeltsch MM, Birkenhager R, McCarthy JE, Weich HA, Christ B, Alitalo K, Wilting J. VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev. Biol. 1997;188:96–109. doi: 10.1006/dbio.1997.8639. [DOI] [PubMed] [Google Scholar]
- 26.Bates DO. The chronic effect of vascular endothelial growth factor on individually perfused frog mesenteric microvessels. J. Physiol. (Lond.) 1998;513:225–233. doi: 10.1111/j.1469-7793.1998.225by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renkin EM, Joyner WL, Sloop CH, Watson PD. Influence of venous pressure on plasma-lymph transport in the dog's paw: convective and dissipative mechanisms. Microvasc. Res. 1977;14:191–204. doi: 10.1016/0026-2862(77)90018-8. [DOI] [PubMed] [Google Scholar]
- 28.Rutkowski JM, Moya M, Johannes J, Goldman J, Swartz MA. Secondary lymphedema in the mouse tail: lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc. Res. 2006;72:161–171. doi: 10.1016/j.mvr.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mellor RH, Stanton AWB, Azarbod P, Sherman MD, Levick JR, Mortimer PS. Enhanced Cutaneous lymphatic network in the forearms of women with postmastectomy oedema. J. Vasc. Res. 2000;37(6):501–512. doi: 10.1159/000054083. [DOI] [PubMed] [Google Scholar]
- 30.Benest AV, Harper SJ, Herttuala SY, Alitalo K, Bates DO. VEGF-C induced angiogenesis preferentially occurs at a distance from lymphangiogenesis. Cardiovasc. Res. 2008;78:315–323. doi: 10.1093/cvr/cvm094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasyk KA, Thomas SV, Hassett CA, Cherry GW, Faller R. Regional differences in capillary density of the normal human dermis. Plast. Reconstr. Surg. 1989;83:939–945. discussion 946–947. [PubMed] [Google Scholar]
- 32.Shields JD, Borsetti M, Rigby H, Harper SJ, Mortimer PS, Levick JR, Orlando A, Bates DO. Lymphatic density and metastatic spread in human malignant melanoma. Br. J. Cancer. 2004;90:693–700. doi: 10.1038/sj.bjc.6601571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boardman KC, Swartz MA. Interstitial flow as a guide for lymphangiogenesis. Circ. Res. 2003;92:801–808. doi: 10.1161/01.RES.0000065621.69843.49. [DOI] [PubMed] [Google Scholar]
- 34.Bates DO, Levick JR, Mortimer PS. Subcutaneous interstitial fluid pressure and arm volume in lymphoedema. Int. J. Microcirc. Clin. Exp. 1992;11:359–373. [PubMed] [Google Scholar]
- 35.Stanton AW, Modi S, Bennett Britton TM, Purushotham AD, Peters AM, Levick JR, Mortimer PS. Lymphatic drainage in the muscle and subcutis of the arm after breast cancer treatment. Breast Cancer Res. Treat. 2008 doi: 10.1007/s10549-008-0259-z. [DOI] [PubMed] [Google Scholar]
- 36.Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, Lehman M, Adams BJ, Bello CL, DePrimo SE, Baum CM, Miller KD. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J. Clin. Oncol. 2008;26:1810–1816. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 37.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]