Abstract
The quantitative contribution of the thymus to the maintenance of peripheral populations of naïve T cells is poorly understood. Several new lines of evidence indicate that thymic activity continues into adulthood, albeit at lower levels than in early life, and that this is important for a range of lymphopenic disorders. A measure of thymic activity that is often used is the quantification of T-cell receptor excision circles (TREC). It has been shown that TREC levels decline after infection with HIV-1, and that they recover to above normal levels after antiretroviral treatment. The reasons for the latter observation are unknown. Here we quantitatively explore different possible causes for supra-normal levels of TREC/cell, and show that the small total number of cells involved in reconstituting the TREC+ T-cell pool of HIV-1 infected patients suffices to explain the observation. Even the expected small thymic outputs into a strongly depleted naïve T-cell peripheral pool lead to a slow transient of elevated levels of TREC/cell. The main biological lesson from our quantitative modeling approach is that middle-aged human thymi continue to produce naïve T cells, and that this production can be demonstrated by tracking the increase of total TREC numbers (rather than the TREC content).
Keywords: HIV, thymus, TREC, mathematical models
Introduction
The quantitative contribution of the thymus to the maintenance of peripheral populations of naïve T cells is poorly understood, but is known to decline with age. Thymic activity into adulthood has recently been shown to be important for reconstituting the peripheral naïve T-cell pool and for restoring repertoire diversity after lymphopenic conditions, such as HIV infection and certain cancer therapies 1–4. New quantitative assays based on measuring T-cell receptor excision circles (TREC), which are formed during T cell ontogeny in the thymus, have been developed to identify recent thymic emigrant T cells in the periphery 5, 6. These assays may allow a better characterization of the role of thymic output in reconstituting naïve T cells with increasing age, after bone marrow transplantation, and during antiretroviral therapy for HIV infection.
Several studies have shown that T cells in HIV-infected subjects have a lower TREC content (i.e., TREC/cell) compared to age matched uninfected individuals 5, 7–9. TREC measurements between individuals are variable, however, and there is a substantial overlap between the TREC content of HIV patients and age-matched controls 9. Different studies have shown that antiretroviral treatment leads to an increase in TREC+ cells 5, 10–12, and at least one report indicated that after therapy with successful suppression of viremia, HIV-infected people can have higher TREC content in their naïve T cells than normal subjects (i.e., “supra-normal” levels of TREC), and this occurs before the naïve T-cell numbers have recovered 11. Several other studies suggest a similar pattern of supra-normal TREC contents, which is reviewed below in “Experimental Data”. However, there is no consensus on how to interpret the “supra-normal” TREC contents during HAART.
We and others have shown that the precise interpretation of TREC data depends heavily on the assumed dynamics of naïve T-cells and recent thymic emigrants (RTE) in particular 8, 13, 14. Notwithstanding these difficulties, several putative mechanisms have been put forth to explain the supra-normal levels of TREC content upon successful suppression of viremia by HAART 10, 11, 15. One possibility is that there are different subpopulations of naïve T cells, with different TREC content. For example, Kimmig et al. have shown that a subpopulation of naïve CD4+ T-cells expressing the CD31 marker is enriched for TREC, and suggested that these could be true recent thymic emigrants (RTE) 16. However, CD31 can not be a unique marker for recent thymic emigrants, because in human adults thymic output should be low, and a major fraction of naïve T cells is CD31+ 16–18. One study showed that the repertoire diversity of CD31− naïve T cells is much lower than what one would expect for a true naïve repertoire 18, suggesting that CD31− naïve T cells have been selected to clonally expand. Although, another study showed that the CD31− naïve T cell repertoire is diverse 17. Finally, the TREC contents of CD31+ and CD31− naïve T cells decline in parallel 17, demonstrating that CD31+ naïve T-cells also divide. Other experiments, measuring the regulation of thymic output after grafting extra thymii into mice, have indicated that RTE are a subpopulation of the naïve compartment with dynamics that are different from the established peripheral naïve T-cell pool 19, 20. It has been proposed 11, 15 that this RTE population is responsible for the rapid increase in TREC content mentioned above, if most of the TRECs reside in a relatively small sub-population of RTEs with distinct dynamics 21.
Another possibility is that the subpopulations of TREC+ and TREC− cells have different trafficking properties resulting in a different spatial distribution between blood and lymph nodes 22, 23. Since TREC+ naïve T cell should on average be less mature than TREC− naïve T cells, one could understand that TREC+ naïve T cells preferentially home to lymphoid tissues. Since HAART induces a redistribution of T-cells from lymph nodes to blood, suggested to be the main source of the early increase in memory CD4+ T-cells in the blood 24–29, HAART could affect TREC+ and TREC− cells in different ways, leading to the increase in TREC content in the periphery during therapy 11, 22. A third possibility has been implicated by Di Mascio et al. 10, who interpreted their longitudinal data using a mathematical model. In this way, they suggested that quantitative differences in the normalization of the model parameters (i.e., death, proliferation, and activation rates) during HAART explain the dynamics of TREC vs. naïve cells. Finally, other processes may be at play, including abnormal recovery of inflammation status, and compromised or altered signals, such as cytokine production or MHC class II interactions 11.
Our objective here is to systematically present a quantitative analysis of the hypotheses of redistribution and the putative two populations in explaining the evidence for “supra-normal” TREC/cell. We also analyze a new hypothesis that we believe explains most of the observations in a more parsimonious way. Our major finding is that even low thymic production, of a few new TREC+ naïve T cells, suffices to explain the observations, given that total naïve T cell numbers are severely depleted. The most important implications of this are that the size of the naïve T cell pool plays a major role in the TREC dynamics, that the thymus remains functional in these adult human patients, and that thymic output can be measured by the recovery of the peripheral TREC+ T cell populations.
Results
Experimental Data
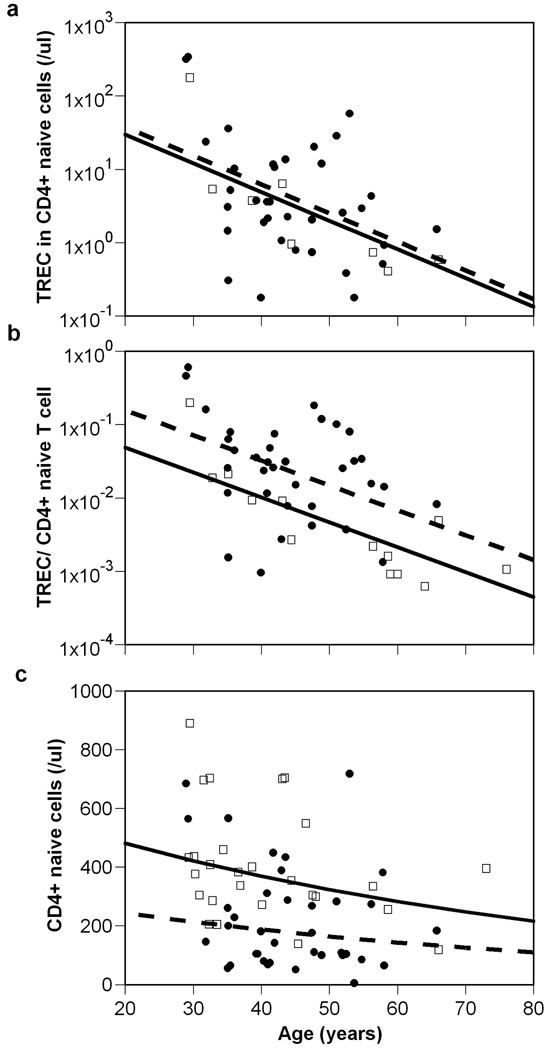
An important experimental observation that came to light recently is that in the periphery TRECs increase faster than naïve T cells do upon HAART treatment of HIV-1 infected individuals. Several groups have explicitly made this observation 10, 11. At least one detailed study has shown that not only TRECs in naïve cells increase with treatment, but that TREC/cell in successfully treated patients achieved levels higher than in age-matched uninfected controls 11. Adding the information on TREC totals (TREC/µl), we depict the data from this study in Figure 1. Taking age explicitly into consideration, naïve T cell numbers in treated patients remain significantly below normal (p=0.0001), whereas the TREC total normalizes (p=0.71). As a consequence the TREC/content is supra-normal in treated patients (p=0.016) after controlling for age. Indeed, in these well suppressed HIV-infected patients, who are on therapy for a median of 20 months, the median TREC content was 0.026 TREC/naïve T cell, whereas in age-matched uninfected controls the corresponding median was almost 10-fold lower, i.e., a frequency of 0.0027 TREC/naïve T cell.
Figure 1.
Change with age in (a) TREC total (/µl), (b) TREC content (per naïve T cell), and (c) naïve T cells in the CD4+ population. The ● and □ represent data from HIV-infected and uninfected subjects, respectively. The straight lines show the best regression through the data for uninfected (solid line) and infected (dashed) subjects. There are no significant differences between the slopes of the lines in any panel, but there is an increased TREC content in HIV-infected subjects, when corrected for age (p=0.016). Data from 11.
The Harris et al. 11 study had a cross-sectional population design. In a more recent longitudinal study 10, twenty-three HIV-infected patients were followed from the start of HAART up to 42 months post-treatment initiation. For each patient, naïve T cell numbers, total TREC and TREC content were measured at least on three occasions: baseline, 5–8 months post-treatment start (median 6 months), and 13–42 months after HAART (median 18 months). The total number of TRECs recovered faster than the naïve T cells, i.e., a ~4-fold increase vs. a ~2-fold increase, respectively, over the 18 month period. This implied a very fast increase in TREC content that reached a mean of more than 0.2/naïve T cell at the 6 month time point 10. This study did not include age-matched controls, but such a high TREC content is expected to be supra-normal 9, although their patients had unusually high number of TREC/cell before treatment.
It is important to note that several other studies indicate similar longitudinal trends. For example, in a study of TREC and HIV infection, longitudinal data was presented for a number of individual patients during treatment 5. In at least some patients studied, the TREC content in naïve T cells increases much faster than naïve T cell numbers (eg. patients A36, C25, D29, E29), while other patients show similar behavior for those two quantities. In another study where TREC content was analyzed in PBMC, chronically infected patients starting at low levels of TREC content (<0.0022 TREC per PBMC) had large increases in this variable, reaching apparent supra-normal values by 9–12 months after the start of treatment (see Figure 4 in Zhang et al. 9).
This “meta-analysis” of current data on TREC dynamics during treatment of HIV+ patients suggests that TREC contents often, but not always, become supra-normal. Analyzing different hypotheses to interpret these experimental data, we seek for an explanation for the phenomenon of supra-normal TREC levels, and for the fact that this phenomenon is not always observed.
Interpretation of the Experimental Data
Redistribution
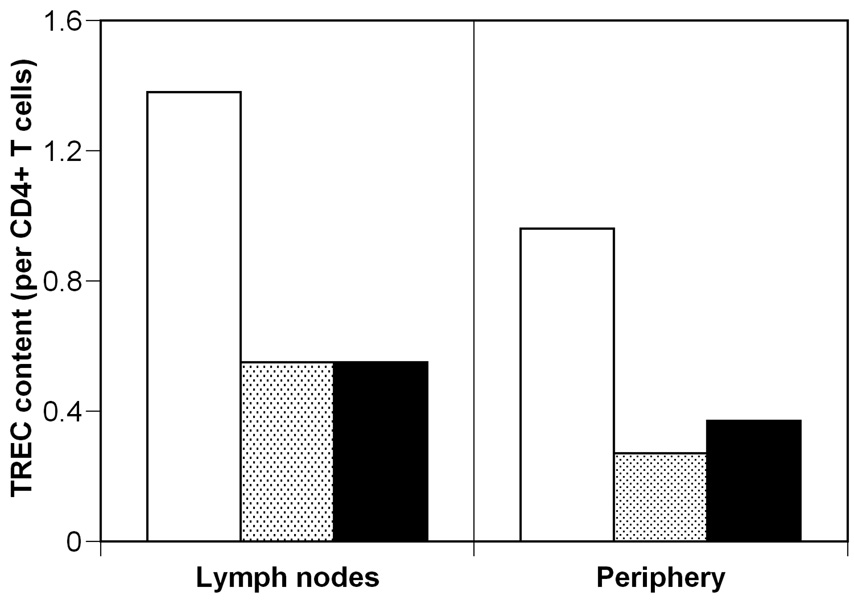
One possible explanation for the supra-normal TREC content is the preferential release of TREC+ cells (over TREC− cells) from the lymph nodes during treatment. Indeed, some evidence indicates that TREC+ cells are entrapped in lymphoid tissue during HIV infection 23; and that they “are rapidly and selectively released into circulation with antiretroviral treatment” 22. Experimental evidence indicates that the TREC content of CD4+ T-cells is higher in lymph tissue (LT) than in peripheral blood, both in healthy controls (1.38 cjTRECs per CD4+ T-cell in LT vs. 0.96 cjTRECs per CD4+ T-cell in the blood) and during HIV infection (0.55 cjTRECs per CD4+ T-cell vs. 0.27 cjTRECs per CD4+ T-cell, respectively) 23 (Figure 2). Note that this study reports cjTREC, rather than the more usual sjTREC, which explains the higher values, and that both types of TREC decline with age almost in parallel 5. During HIV infection there is a greater bias for retention of TREC+ cells in the lymph tissues because in uninfected individuals the ratio of TREC content in lymph tissue over that in PBMC is 1.38/0.96≈3/2, which does not reach statistical significance, and in infected subjects this ratio has increased to 0.55/0.27≈2/1, which is a significant difference between content in LT and blood 23.
Figure 2.
Comparison of TREC content in lymph nodes and periphery. Experimentally measured cjTREC per CD4+ T-cell for uninfected (white) and infected individuals (shaded bars) from 23. Expected cjTREC content for treated individuals (black bars), if treatment leads to re-establishment of the relative proportion of TREC content between lymph nodes and periphery, i.e., the ratio between the black bars is the same as between the white bars.
However, there are two problems with the “redistribution” explanation. First, one would expect that therapy induced redistribution would tend to re-establish the pre-infection relationship between the LT and PBMC TREC content. Thus, the TREC content in the periphery would go from 1/2 of that in the LT to the normal 2/3 of the TREC content in the LT. But because the TREC content in LT is lower than normal, the predicted TREC content in PBMC after therapy would also be lower than normal, i.e., 2/3×0.55 = 0.37 cjTRECs per CD4+ T-cell (Figure 2), even in the best case scenario where redistribution to the periphery does not lead to appreciable loss of TREC content in the LT. By redistribution, it is difficult to obtain supra-normal TREC content in the periphery when the source of these cells, i.e., the lymph tissue, has a lower than normal TREC content. It could be argued that TREC+ CD4+ T cells are released much earlier from the LT into the periphery than TREC− CD4+ T cells 22, but then a second problem becomes apparent. Redistribution acts on a short time scale of weeks, and this is why it has been invoked as the mechanism for the early increase in CD4+ T-cells after HAART 24–29. However, the increase to supra-normal levels of TREC content is observed even after 20 months of therapy 11, and it seems unlikely that the difference in the timescale at which the distribution of TREC+ and TREC− CD4 T cells normalizes is so large.
Two subpopulations of naïve T cells
Another hypothesis for explaining the transient supra-normal TREC content during HAART is that naïve T-cells are composed by two sub-populations, i.e., recent thymic emigrants (RTE) and truly naïve T-cells, and that most TREC reside in the RTE sub-population, which has much faster dynamics 11, 15, 16, 20. A rapid and increased recruitment of RTE into the naïve T cell repertoire after the start of antiretroviral treatment is thought to explain the observed supra-normal TREC content.
To analyze this possibility in more detail, we developed a population dynamical model for these subpopulations (see Appendix). We considered a RTE population (R) and a truly naïve population (N). Together these populations form the experimentally observed naïve-phenotype T-cell population (T) (either naïve CD4+ or CD8+ T-cells). We also considered the populations of TREC positive cells among the RTE and truly naïve T cells, TR and TN, respectively. RTE are produced in the thymus and, after their arrival in the periphery, they either die, or become recruited into the truly naïve T-cell pool. In this model, we deliberately do not include T-cell redistribution 12, because we analyzed the impact of redistribution by itself above and the present model serves to separately analyze the effect of two naïve populations. Thus we are studying each of these effects in turn.
Calculating the steady state of this model demonstrated an important counterintuitive result (see Appendix). Increasing the recruitment rate of RTE into the truly naïve population actually decreases the steady-state TREC content of the whole naïve T-cell pool. Although the total TREC numbers at steady state increased due to increased recruitment of RTE, the total naïve T cell population increased at least as much. Thus, increasing RTE recruitment reduces the average TREC content of naïve T cells.
One criticism of this model is that we expect that homeostatic mechanisms are in operation to keep the naïve T cell population at regulated levels. Indeed, earlier work has shown that predictions about TREC content may crucially depend on the inclusion of homeostasis in the model 8, 12. To test the robustness of our counterintuitive result, we included homeostasis on the truly naïve population in the model (see Appendix). Incorporation of RTE into the naïve T cell pool probably depends on the size of this pool, such that when truly naïve T cells are depleted, the fraction of RTE incorporated into the long-lived population increases. Current experimental evidence 19 suggests that the size of the RTE pool itself is independent of peripheral homeostatic mechanisms, and depends mostly on the magnitude of the thymic output. Accordingly, in our model the size of the RTE population is independent of the fraction of RTE recruited. Homeostasis could also regulate the proliferation and/or death rates of truly naïve T cells, which has similar effects on naïve T cell numbers and their TREC content 30.
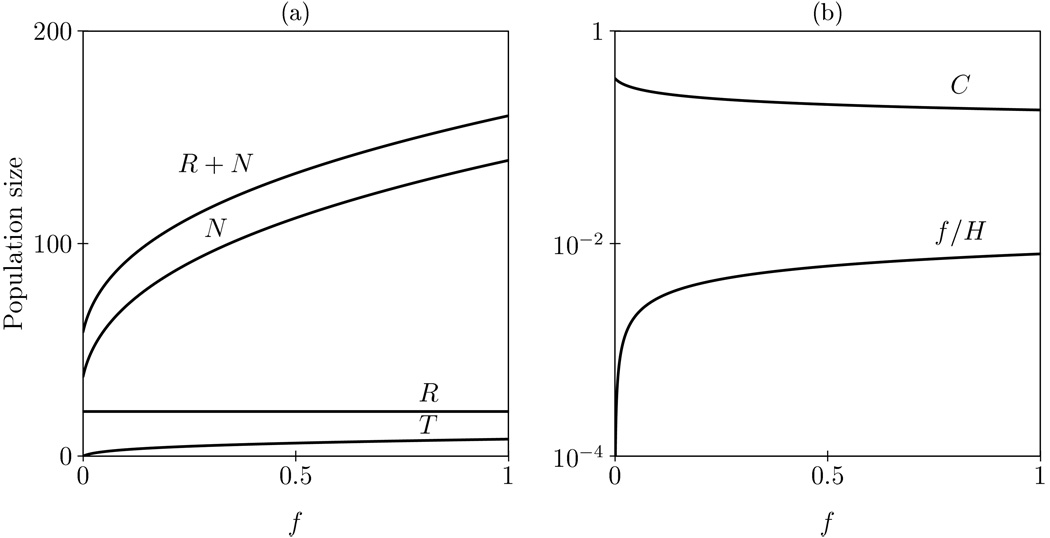
The steady states of this model where RTE recruitment and the rate of naïve T cell division are homeostatically regulated (i.e., both increase when naïve T cell numbers decrease) are shown as a function of the maximal rate of RTE recruitment in Figure 3. Importantly, increasing the maximal rate of RTE recruitment decreases the TREC content (Fig. 3b), which confirms the analytical result for the non-homeostatic version of the model. Note that the actual recruitment, f/H, does increase when the maximum rate of recruitment, f, increases (Fig. 3b). The explanation for this counterintuitive result is the same as before, even though increasing RTE recruitment leads to more TRECs, it leads to even more truly naïve T-cells. And indeed naïve T cells increase faster that total TREC (Fig. 3a), resulting in an overall decrease in TREC content. We have repeated these analyses for a model with homeostasis on RTE recruitment and naïve T cell death, dN, and a fixed naïve T cell division rate, p. The results obtained in terms of the effect of increased recruitment were very similar (not shown). Overall, these equilibrium results suggest that even in a homeostatic model, increased recruitment of RTE’s into the truly naïve T cell population can not explain the long-term supra-normal TREC content as seen in HIV-1 treated patients that are well suppressed for almost two years 11.
Figure 3.
Steady states of the RTE model (see Appendix) as a function of the RTE recruitment rate f. We allow for homeostasis on RTE recruitment, f, and naïve T cell renewal, p, by dividing these rates by H=1+(N/h)2. Panel (a) shows the effect of RTE recruitment on the total number of naive T cells (R+N), on truly naive T cells (N) and RTEs (R), and on the total number of TRECs (T). Panel (b) shows that the TREC content per naïve T cell, C, goes down when the actual RTE recruitment, f/H, goes up. Parameters: c=16, h=12.5 cells, p=0.01 day−1, f=0.1, dN=0.001 day−1, dR=1/21 day−1, σ=1 cell day−1.
Thymic output
Here we would like to propose a new hypothesis for the supra-normal TREC content based on the crucial aspect that recovery to supra-normal levels involves small numbers of TREC+ cells. First, let us consider an illustrative example of this phenomenon (Table 1): a typical 39-year old uninfected individual may have ~400 naïve T cells/µl and 0.0094 TREC per naïve T cell, corresponding to an average of only 3.8 TREC+ cells/µl (data from a real individual in 11). Assume that during infection, i.e., until the start of treatment, the patient has lost half of its naïve T cells (to ~200 /µl) and, to explain the lower TREC content, has lost most of its TREC+ cells (e.g., to 1/µl) leading to 0.005 TREC per naïve cell. Then, after ~20 months of successful treatment, this patient may have recovered to ~230 naïve T cells/µl and 0.0452 TRECs per naïve T cell (data taken from another, age-matched, real individual from 11). This means that during treatment, the patient’s TREC total has approached 10 TREC+ cells/µl. If all these new naïve T cells come from the thymus, this would imply that during the 20 months of treatment the thymus has had an output of (at least) 30 cells/µl, of which 9 (30%) where TREC+. These numbers correspond to a thymic output of 0.05 cell/µl per day (a total output of ~1.2×107 cells/day), which is perfectly consistent with the very slow turnover of human naïve T cells, and readily explains the supra-normal value of TREC content observed in this patient.
Table 1.
Illustrative example of changes in TREC content for a normal individual after infection with HIV-1, followed by treatment*.
| Naïve cells | TREC/cell | TREC/µl | |
|---|---|---|---|
| Uninfected | 400 | 0.0094 | 3.8 |
| HIV-1 infected | 200 | 0.005 | 1 |
| HIV-1 treated | 230 | 0.045 | 10.4 |
In this Table, the data for the uninfected and treated individuals are from reference 11, whereas the HIV-1 infection case is hypothetical.
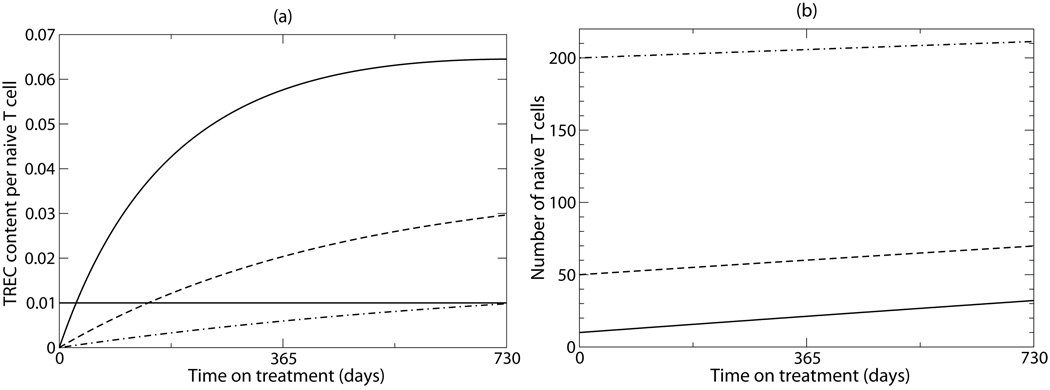
The calculations in the previous paragraph serve only to illustrate the point that a small output from the thymus, within the range of that expected for adults, suffices to explain supra-normal levels of TREC. To analyze this scenario in more detail, we developed a simple mathematical model, with just one population of naïve T cells, some of which are TREC+ and some are TREC− (Figure 4). (This model differs from the above, because here we do not consider RTE, again to study the different hypothesis separately.) In this model, new naïve T cells are produced by the thymus, and once in the periphery these cells may proliferate, or be lost due to activation and/or death. Choosing reasonable values for these parameters (see caption of Fig. 4), we demonstrate that starting from very low numbers of total TREC and naïve T cells in the periphery, it is easy to achieve supra-normal TREC content during therapy (Fig. 4a). Moreover, these supra-normal values are achieved relatively quickly and stay elevated for considerable periods of time. This happens because of the small absolute numbers involved (only ~3 TREC+ cells/µl are produced by the thymus over the period of two years, consistent with a thymic output of ~0.03 cells/µl per day as above), and occurs in spite of the fact that the majority of new cells produced by the thymus and by proliferation is TREC negative. In agreement with the data, the recovery of naïve T-cells is much slower than that of the TRECs, and naive T cell numbers remain well below normal levels after two years of successful therapy (Fig. 4b).
Figure 4.
Supra-normal TREC content during naive T cell recovery induced by the thymus. We consider the following model for TREC+ naive T cells, N+, and total naive T cells, N: . Thus, C=N+/N is the TREC content per naive T cell. Considering a human adult, the thymic output, σ, should be small. We assumed a thymic production of σ = 0.032 cell/µl/day corresponding to a fractional output of less than 10−4 /day (taking a count of 400 CD4+ T cells per µl for a normal individual 11). The parameter α was set to 1/8 to represent the TREC content of RTE (e.g., cord blood cells) 31. Since naive T cells are long-lived we give them a half-life of two years by setting dN=0.001 day−1, and if naive T cell numbers ultimately approach a normal steady state of N=400 cells after the transient recovery phase, one can solve that p=0.00092 day−1. At the steady state the normal TREC content is C=0.01 /cell (see the horizontal line in Panel (a)). For simplicity we start with no TREC+ cells (i.e., N+(0)=0). The three lines correspond to three initial populations sizes: N=10 (solid line), N=50 (dashed line), N=200 (dash-dotted line).
The parameters in Fig. 4 were tuned to obtain a steady state corresponding to the healthy controls in the Harris et al. 11 data. Similar results to those depicted in Fig. 4 are obtained in the much simpler case where we ignore proliferation and death during the recovery phase, i.e., setting p=dN=0 such that both the TREC+ cells and the total number of naïve T cells increase linearly with time. Indeed, our results are quite robust to the set of parameters used.
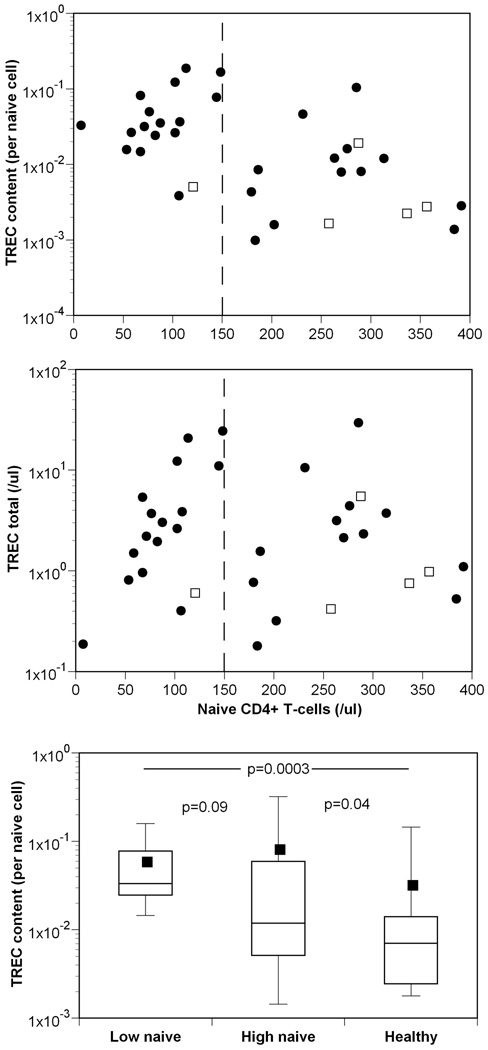
Another feature that is explained by this “low TREC numbers” phenomenon is that the TREC content reaches higher levels for those patients starting from a lower nadir of naïve CD4+ T-cells (see the different lines in Fig. 4a), which is a natural explanation for the corresponding experimental observation 11. We plot the experimental data 11, adding the information on TREC totals, for those individuals with naïve T cells counts <400 cells/µl in Figure 5 (patients with higher, i.e., normal, counts are either not expected to have experienced a large depletion during infection – e.g., a short untreated infections –, or to have recovered for longer to nearly normal levels of naïve T cells (see below)). The data in this figure are consistent with our simple mathematical model: patients with the lowest naïve T cell counts have the highest TREC content (Fig. 5a), but have similar TREC numbers (Fig. 5b). The TREC content of patients with medium to normal naïve CD4+ T cell counts overlap substantially with those of the healthy controls (Fig. 5c), and the difference between these two groups is marginally significant, largely due to a few patients with very high TREC content. Summarizing, this suggests that the supra-normal TREC content is just a consequence of the dynamics of low TREC and T cell numbers. Moreover, in patients treated early, with larger numbers of CD4+ T-cells before treatment, we do not expect to see supra-normal TREC content even after successful therapy (see dashed-dotted line in Fig. 4a).
Figure 5.
TREC levels versus naïve T-cell numbers. (a) For treated infected individuals (●) with low naïve T cell counts (<150 cell/µl, indicated by the vertical dashed line) the TREC content is higher than for individuals with higher naïve T-cell numbers or for uninfected individuals (□); whereas (b) TREC total are similar for a wide range of naïve T-cell counts, including both infected (●) and uninfected individuals (□). In panel (c) we present box plots of the TREC content for three groups, infected individuals with less than 150 cell/µl (n=16); infected individuals with more than 150 cell/µl (n=19); and uninfected individuals (n=8) (Data from 11.)
After a long period of successful treatment and viral load suppression, our model will approach a steady state with normal naïve T cell numbers, and a normal TREC content. This implies that after several years the TREC content will decrease from its supra-normal levels to approach its normal levels (not shown). How high TREC content gets and how long it stays elevated depend on the initial conditions and the specific parameters chosen for the model, which obviously differs from patient to patient. This would give rise to the wide range of TREC contents seen in the data.
Discussion
The supra-normal TREC content observed in treated HIV-1 infected individuals 10, 11 is readily explained by the very simple argument that the release of a few TREC+ naïve T cells from the thymus into a strongly depleted, largely TREC−, naïve T cell population transiently allows for a very high TREC content. Here we have shown that this transient of supra-normal TREC content easily extends over a period of more than two years, and that TRECs recover much faster than the naïve T cell count. The most important biological lesson that we learn from this is that these data do not provide evidence for, or against, a dynamically separate RTE population containing most of the TRECs, but do provide evidence for de novo production of new naïve T cells by the thymus in middle-aged HIV patients under antiretroviral treatment. Moreover, our simple quantitative reasoning also explains why the largest increases in TREC content are usually seen in patients with lower naïve CD4+ T-cell counts before therapy 9, 11, 12. The same thymic output into a smaller naïve T cell population naturally leads to higher TREC content.
The highest TREC content that our hypothesis can account for is that of RTE, which is estimated to be around 0.11 TREC/cell 31, and which is one or two orders of magnitude above the normal levels of 0.001 to 0.01 TRECs per naïve T cell 9. Interestingly, some individuals, both normal and HIV-1 infected subjects, have higher TREC content of up to 0.61 TREC/cell 11. Such a high TREC content could either be measurement noise, as TREC data tend to be variable, or might indicate an early exit of RTE from the thymus after only 1 or 2 divisions post the formation of the signal-joint TREC.
In order to test whether increased recruitment of RTEs during therapy could explain the supra-normal TREC content, we developed a model to analyze the dynamic of recent thymic emigrants, as a separate population from the truly naïve T-cells. We found that increased recruitment per se can not explain that observation, because it is expected to decrease the equilibrium TREC content. The fact that we do not need a small rapid subpopulation of RTE containing most of the TRECs to explain the supra-normal TREC contents in treated HIV patients 11, 15 should not be taken as evidence against the notion that RTE form a dynamically distinct subpopulation. Indeed, in our RTE model (Eqs. (1) & (2)), we can observe similar supra-normal TREC contents as depicted in Figure 4, if we consider similar cases of strongly depleted naïve T cell populations with small thymic outputs of TREC+ RTE (not shown). The mechanism underlying these increased TREC content is not the increased recruitment rate of RTE, because that would give the opposite results, but simply the incorporation of a limited number TREC+ RTE into a small pool of largely TREC− naïve T cells. In contrast, with our explanation of the supra-normal TREC levels, the previous suggestion that most of the TRECs in the peripheral blood reside in a small subpopulation of RTE 15 does loose most of its evidence. Moreover, TREC data obtained after thymectomy in monkeys argue against this, because one would expect a very fast early decline in total TREC after thymectomy, which is not seen in the data 31, 32.
In the longitudinal study of Di Mascio et al. 10 TREC totals per µL also increased faster than naïve T cells over approximately 18 months of treatment. Comparing naïve CD4+ with naive CD8+ T cells, the recovery rates were somewhat faster in the CD4+ T cells. This could be taken as evidence in favor of our notion that most of the recovery is due to thymic production, because CD4+ cells typically outnumber CD8+ cells in the single positive thymocyte population. In the same paper 10 the TREC content of the CD4+ naïve T cells increased from 0.1 to more than 0.2 TRECs/naïve T cell, whereas that of the CD8+ cells increased from 0.075 to 0.1 TRECs/naïve T cell after approximately 6 months of treatment. The higher TREC content in the CD4+ T cells is readily explained by the faster recovery of the TREC totals and the lower nadir in the naïve CD4+ T cells as compared to the CD8 T cells. Thus, these longitudinal data seem in agreement with our interpretation, and therefore do not require the more complicated interpretation of differences in the normalization of proliferation, death, and the recruitment rates of naïve T cells into memory cells 10.
Summarizing, the main biological lesson from our quantitative modeling approach is that middle-aged human thymi continue to produce naïve T cells, and that this production can be demonstrated by tracking the increase of total TREC numbers (rather than the TREC content). A true quantification of thymic output from TREC data remains difficult because TREC+ naïve T cells may die, and/or be recruited into clonal expansion and contraction by antigenic stimulation. Recovery rates of TREC+ naïve T cells can therefore only provide a lower bound on the thymic output.
Acknowledgements
We would like to thank Jeffrey Harris for sharing published data with us, and Michele Di Mascio for discussions in the initial phases of this project.
Grant Support: Part of the work was performed at the Santa Fe Institute. RdB would like to thank the Dutch NWO (VICI grant 016.048.603) and the HFSP (grant RGP0010/2004) for financial support. Portions of this work were done under the auspices of the U.S. Department of Energy under contract DE-AC52-06NA25396 and R.M.R was supported by grant P20-RR18754 from the National Center for Research Resources (NCRR), a component of the NIH.
Appendix
We consider a RTE population (R) and a truly naïve population (N), and together these populations form the experimentally observed naïve-phenotype T-cell population (T) (either CD4+ or CD8+ T-cells). We also consider the populations of TREC positive cells among the RTE and truly naïve, TR and TN, respectively. With these populations, we write the following model
| (1) |
where σ is the thymic output in cells/day, dR is the daily loss rate of RTE, either by incorporation into the truly naïve pool (of a fraction f≤1) or by death of the RTE, c is a term that allows for expansion of RTE as they incorporate into the naïve pool (there is no expansion if c=1), p and dN are the proliferation and loss rates of the truly naïve T cells. Here we assume that RTE only proliferate when they are recruited into the naïve pool. We now write the corresponding equations for the TREC positive cells in those two compartments. They are
| (2) |
where α is the proportion of thymic emigrants that are TREC positive. This is a simplified version of previous models developed by us and others 8, 10, 12. Here we do not include redistribution from tissue, because we are interested in analyzing the different hypotheses for supra-normal levels of TREC content separately: two populations of naïve cells vs. redistribution from tissues.
At steady state, equations (1) and (2) yield
| (3) |
for naïve total cells and TREC+ cells, respectively. The TREC content (TREC/cell) of the observable naïve population is (TR+TN)/(R+N), which is easily calculated to be
| (4) |
And the total TREC in the naïve T cell population is
| (5) |
One can see from equation (4) that increasing the recruitment of RTE, f, decreases the TREC content. This effect is observed both if we consider an expansion model (c>1, p=0) or a proliferation model (c=1, p>0). In fact, inspection of equation (4), for the equilibrium of TREC content, demonstrates that increasing f always leads to a decrease in C, as long as either p>0 or c>1. This occurs because, when f increases, the expression cfdR/(dN−p) in the denominator always increases more than the expression fdR/dN in the numerator. On the other hand, increases in f do lead to increases in TREC total, but these increases are only proportional to the amount of TREC residing in the truly naïve population. That is, if 90% of the naïve T cell TRECs reside in the RTE population and only 10% in the truly naïve subpopulation, then increasing recruitment, f, 10-fold would only approximately double the measured TREC total.
We also study similar models that include homeostatic mechanisms of maintenance of the total naïve pool. In these, we assume that homeostasis only works on the truly naïve T cells and not on the RTE population 19, 20. Homeostasis is implemented by dividing p and f, in the equations above, by 1+(N/h)2. That is, the larger N, the smaller the proliferation rate and/or the fraction of cells incorporated into the truly naïve pool.
The existence of these two populations makes a difference for the dynamics of TREC, only if there is an imbalance in the proportion of TREC residing in each, and/or there is a difference between the dynamics of these populations. This last property is reflected in the different loss rates for these two populations, dR and dN. Studies in mice 19, 20 suggested that RTE have a fairly rapid turnover compared to truly naïve T cells, i.e., an expected life span of approximately 3 weeks. We therefore assume that dR>>dN, and use dR=1/21 day−1. The dynamical importance of the RTE population depends on its size, i.e., what fraction it represents of the total naïve population, R̄/R̄(+N̄), and on the fraction of TREC residing in RTE, i.e., T̄R/T̄R(+T̄N). As an example, by choosing the following parameters: c=16, p=0.01 day−1, f=0.1, dN=0.001 day−1, dR=1/21 day−1, σ=1 cells day−1 and h=12.5, the fraction of RTE in the total naïve population at steady state is 23%, and the fraction of TREC in RTE is 87%. The former was tuned to have good agreement with the experimental data 19, 20, and the latter was chosen to maximize the effect of RTE on TREC 15.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Douek DC, Vescio RA, Betts MR, et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000 May 27;355(9218):1875–1881. doi: 10.1016/S0140-6736(00)02293-5. [DOI] [PubMed] [Google Scholar]
- 2.Hazenberg MD, Otto SA, de Pauw ES, et al. T-cell receptor excision circle and T-cell dynamics after allogeneic stem cell transplantation are related to clinical events. Blood. 2002 May 1;99(9):3449–3453. doi: 10.1182/blood.v99.9.3449. [DOI] [PubMed] [Google Scholar]
- 3.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–560. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 4.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004 Feb;4(2):123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 5.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396(6712):690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 6.Kong FK, Chen CL, Six A, Hockett RD, Cooper MD. T cell receptor gene deletion circles identify recent thymic emigrants in the peripheral T cell pool. Proc Natl Acad Sci U S A. 1999;96(4):1536–1540. doi: 10.1073/pnas.96.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatzakis A, Touloumi G, Karanicolas R, et al. Effect of recent thymic emigrants on progression of HIV-1 disease. Lancet. 2000 Feb 19;355(9204):599–604. doi: 10.1016/S0140-6736(99)10311-8. [DOI] [PubMed] [Google Scholar]
- 8.Hazenberg MD, Otto SA, Cohen Stuart JW, et al. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat Med. 2000 Sep;6(9):1036–1042. doi: 10.1038/79549. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Lewin S, Markowitz M, et al. Measuring recent thymic emigrants in blood of normal persons and HIV-1-infected patients before and after effective therapy. J Exp Med. 1999;190:725–732. doi: 10.1084/jem.190.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Mascio M, Sereti I, Matthews LT, et al. Naive T-cell dynamics in human immunodeficiency virus type 1 infection: effects of highly active antiretroviral therapy provide insights into the mechanisms of naive T-cell depletion. J Virol. 2006 Mar;80(6):2665–2674. doi: 10.1128/JVI.80.6.2665-2674.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris JM, Hazenberg MD, Poulin JF, et al. Multiparameter evaluation of human thymic function: interpretations and caveats. Clin Immunol. 2005 May;115(2):138–146. doi: 10.1016/j.clim.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Lewin SR, Ribeiro RM, Kaufmann GR, et al. Dynamics of T cells and TCR excision circles differ after treatment of acute and chronic HIV infection. J Immunol. 2002 Oct 15;169(8):4657–4666. doi: 10.4049/jimmunol.169.8.4657. [DOI] [PubMed] [Google Scholar]
- 13.Hazenberg MD, Borghans JA, de Boer RJ, Miedema F. Thymic output: a bad TREC record. Nat Immunol. 2003 Feb;4(2):97–99. doi: 10.1038/ni0203-97. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro RM, Perelson AS. Determining thymic output quantitatively: using models to interpret experimental T-cell receptor excision circle (TREC) data. Immunol Rev. 2007 Apr;216:21–34. doi: 10.1111/j.1600-065X.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- 15.Grossman Z. Immune reconstitution in HIV infection: how to measure thymic function? Clin Immunol. 2005 May;115(2):115–117. doi: 10.1016/j.clim.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Kimmig S, Przybylski GK, Schmidt CA, et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002 Mar 18;195(6):789–794. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilpatrick RD, Rickabaugh T, Hultin LE, et al. Homeostasis of the naive CD4+ T cell compartment during aging. Journal of immunology (Baltimore, Md. 2008 Feb 1;180(3):1499–1507. doi: 10.4049/jimmunol.180.3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohler S, Wagner U, Pierer M, et al. Post-thymic in vivo proliferation of naive CD4+ T cells constrains the TCR repertoire in healthy human adults. Eur J Immunol. 2005 Jun;35(6):1987–1994. doi: 10.1002/eji.200526181. [DOI] [PubMed] [Google Scholar]
- 19.Berzins SP, Boyd RL, Miller JF. The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. J Exp Med. 1998;187(11):1839–1848. doi: 10.1084/jem.187.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berzins SP, Godfrey DI, Miller JF, Boyd RL. A central role for thymic emigrants in peripheral T cell homeostasis. Proc Natl Acad Sci U S A. 1999 Aug 17;96(17):9787–9791. doi: 10.1073/pnas.96.17.9787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grossman Z, Min B, Meier-Schellersheim M, Paul WE. Concomitant regulation of T-cell activation and homeostasis. Nature Reviews Immunology. 2004 May;4(5):387–395. doi: 10.1038/nri1355. [DOI] [PubMed] [Google Scholar]
- 22.Diaz M, Douek DC, Valdez H, et al. T cells containing T cell receptor excision circles are inversely related to HIV replication and are selectively and rapidly released into circulation with antiretroviral treatment. AIDS. 2003 May 23;17(8):1145–1149. doi: 10.1097/00002030-200305230-00005. [DOI] [PubMed] [Google Scholar]
- 23.Nokta MA, Li XD, Al-Harthi L, et al. Entrapment of recent thymic emigrants in lymphoid tissues from HIV-infected patients: association with HIV cellular viral load. AIDS. 2002 Nov 8;16(16):2119–2127. doi: 10.1097/00002030-200211080-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pakker NG, Notermans DW, de Boer RJ, et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998 Feb;4(2):208–214. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 25.Dimitrov DS, Martin MA. CD4+ T-cell turnover. Nature. 1995;375:193. doi: 10.1038/375194b0. [DOI] [PubMed] [Google Scholar]
- 26.Mosier DE. CD4+ T-cell turnover. Nature. 1995;375:193. doi: 10.1038/375193b0. [DOI] [PubMed] [Google Scholar]
- 27.Phillips AN, Sabin CA, Mocroft A, Janossy G. CD4+ T-cell turnover. Nature. 1995;375:193. doi: 10.1038/375195a0. [DOI] [PubMed] [Google Scholar]
- 28.Sprent J, Tough DF. CD4+ T-cell turnover. Nature. 1995;375:193. doi: 10.1038/375194a0. [DOI] [PubMed] [Google Scholar]
- 29.Hazenberg MD, Stuart JW, Otto SA, et al. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000 Jan 1;95(1):249–255. [PubMed] [Google Scholar]
- 30.Dutilh BE, de Boer RJ. Decline in excision circles requires homeostatic renewal or homeostatic death of naive T cells. J Theor Biol. 2003 Oct 7;224(3):351–358. doi: 10.1016/s0022-5193(03)00172-3. [DOI] [PubMed] [Google Scholar]
- 31.Arron ST, Ribeiro RM, Gettie A, et al. Impact of thymectomy on the peripheral T cell pool in rhesus macaques before and after infection with simian immunodeficiency virus. Eur J Immunol. 2005 Jan;35(1):46–55. doi: 10.1002/eji.200424996. [DOI] [PubMed] [Google Scholar]
- 32.Sodora DL, Douek DC, Silvestri G, et al. Quantification of thymic function by measuring T cell receptor excision circles within peripheral blood and lymphoid tissues in monkeys. Eur J Immunol. 2000 Apr;30(4):1145–1153. doi: 10.1002/(SICI)1521-4141(200004)30:4<1145::AID-IMMU1145>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]