Abstract
Objective
To examine the strength and consistency of the evidence on the relationship between depression and adherence to antihypertensive medications.
Methods
The MEDLINE, CINAHL, PsycINFO, Embase, SCOPUS, and ISI databases were searched from inception until December 11, 2009 for published studies of original research that assessed adherence to antihypertensive medications and used a standardized interview, validated questionnaire or International Classification of Diseases Ninth Revision (ICD-9) code to assess depression or symptoms of depression in patients with hypertension. Manual searching was conducted on 22 selected journals. Citations of included articles were tracked using Web of Science and Google Scholar. Two investigators independently extracted data from the selected articles and discrepancies were resolved by consensus.
Results
Eight studies were identified that included a total of 42,790 patients. 95% of these patients were from one study. Only 4 of the studies had the assessment of this relationship as a primary objective. Adherence rates varied from 29% to 91%. There were widely varying results within and across studies. All 8 studies reported at least one significant bivariate or multivariate negative relationship between depression and adherence to antihypertensive medications. Insignificant findings in bivariate or multivariate analyses were reported in 6 of 8 studies.
Conclusions
All studies reported statistically significant relationships between depression and poor adherence to antihypertensive medications, but definitive conclusions cannot be drawn because of substantial heterogeneity between studies with respect to the assessment of depression and adherence, as well as inconsistencies in results both within and between studies. Additional studies would help clarify this relationship.
Keywords: Hypertension, depression, adherence, systematic review
Treatment of hypertension and its complications is one of the most common reasons for visits to physicians in the United States [1]. Hypertension affects approximately 72 million adults in the United States [2, 3]and nearly one in three adults has the condition [4]. Poorly-controlled hypertension results in end-organ damage and plays a major role in the development of myocardial infarction, stroke and end-stage kidney disease [5]. Although successful treatment of hypertension is possible with antihypertensive medications [6,7], optimal blood pressure control is achieved in less than two-thirds of patients who are prescribed antihypertensive treatment [6]. The major modifiable cause of poor control in patients on therapy is nonadherence to antihypertensive medications [8]. Since hypertension generally does not cause symptoms, cues to take medications are often absent. Indeed, “not remembering” to take antihypertensive medications has been shown to be the most common reason for treatment nonadherence [9]. Factors shown to be associated with adherence to antihypertensive treatment include age [10], medication side effects [11, 12], race/ethnicity [13, 14], and medication cost [15].
Depression is present in approximately 5–10% of patients in primary care [16] and is associated with a 3-fold greater risk of nonadherence to medical treatment [17]. Depression therefore represents a potentially important predictor of treatment nonadherence in patients with hypertension. The importance of understanding the relationship between depression and antihypertensive treatment nonadherence is underlined when one considers that depressed patients are at greater risk than those without depression to develop hypertension [18]. This systematic review of the literature was therefore conducted to assess the strength and consistency of evidence linking depression or depressive symptoms to treatment adherence to antihypertensive medications.
Methods
Search Strategy
Searches of multiple databases and hand searching provide more complete inclusion of existing studies for systematic reviews than other methodologies [19–22]. The MEDLINE, CINAHL, PsycINFO, Embase, SCOPUS, and ISI databases were searched from inception until December 11, 2009 (see Appendix A for search terms). In addition, 22 selected journals (Appendix B) were hand searched for eligible articles published from September 2007 through July 2009. Manual searching was also conducted on the references of eligible original articles, and citations of included articles were tracked using Web of Science and Google Scholar, since each of these tools has been shown to return unique citations [23–25]. Authors of studies included in the review were contacted to attempt to identify unpublished studies with data on depression and adherence in hypertension.
Organization and Tracking of Literature
Search results were downloaded into the citation management database RefWorks (RefWorks, RefWorks-COS, Bethesda, MD, USA), and the software’s duplication check was used to eliminate citations retrieved from multiple sources. The RefWorks software was also used to store and track search results and to track results of the review process.
Study Selection
Article eligibility criteria were established a priori. Published studies of original research in any language were included if they used a standardized interview, validated questionnaire or International Classification of Diseases Ninth Revision (ICD-9) code to assess depression or symptoms of depression in patients with hypertension. Hypertension was identified by self-report, information from medical records or blood pressure measurements during the study. Both the Sixth and Seventh Reports of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure define hypertension as systolic blood pressure greater than or equal to 140 mm Hg and/or diastolic pressure greater than or equal to 90 mm Hg [7,26]. Studies that defined their patient population as diagnosed with hypertension according to this definition or indicated that patients with hypertension were identified through medical or pharmacy records, use of antihypertensive medications, or by self-report were included. Studies that used any of the following modes of assessing adherence to antihypertensive medications were included: self-report interview or questionnaire; self-report diary; other report (e.g., parent, family member, spouse, or researcher); physician, nurse or allied health professional report; pill count; Medication Event Monitoring System (electronic pill monitor); pharmacy records; or medical records. To be eligible, a study had to report the association between depression or depressive symptoms and adherence to antihypertensive medications.
In the case of multiple articles published on the same cohort, only the publication with the most complete data was included. Studies with mixed patient populations were included only if data on patients with hypertension were reported separately. Articles were excluded if they consisted of case reports or if only a meeting abstract was provided. Two investigators independently evaluated studies for inclusion. Titles and abstracts were initially reviewed to identify potentially eligible articles. If either investigator selected an article for further consideration during title/abstract review, then a full-text review of the article was completed. Discrepancies between reviewers at the article selection stage were resolved by consensus.
Data Extraction and Assessment of Methodological Quality
Data extraction forms were developed from consensus by the investigative team to reflect items that were most important for describing the characteristics of each study and summarizing study results. For all eligible studies, extracted data included the first author, year of publication, the country or countries where the research was conducted, number of patients, mean age, percent male, ethnicity, education, key inclusion criteria, key exclusion criteria, method used to assess depression, co-morbidities, measure of adherence to antihypertensive medications, and the effect size of the relationship of adherence to antihypertensive medications and depression. Two investigators independently extracted data and entered results directly into Excel spreadsheets. Entries were compared for accuracy, and discrepancies were resolved by consensus. Authors of eligible articles were contacted as necessary to clarify information related to depression and adherence scales, cutoff scores used in the original studies and to clarify methodological and statistical issues Authors of all included studies were sent emails in an attempt to identify any relevant unpublished studies.
Methodological quality of studies was assessed according to a set of criteria used in previous systematic reviews of observational studies [27, 28 ] that was modified for the purposes of this review (Appendix C). Studies were rated “yes/no” according to the presence or absence of each quality criterion. Sample size, validated measures of depression and depressive symptoms, adherence to antihypertensive medications, diagnosis of hypertension, response rate and quality of statistical methods were among the items evaluated.
Data Analysis
Eligible studies were evaluated to determine whether data were sufficiently similar to warrant pooling of results. Substantial heterogeneity between studies was found with respect to assessment of depression or depressive symptoms, how adherence was defined and measured, and methods used to assess the relationship between depression or depressive symptoms and adherence. Thus, it was determined that pooling to generate a combined effect size of the relationship between depression and adherence to antihypertensive medications was not appropriate, and the methods and results of each study were reported individually.
Results
Search Results
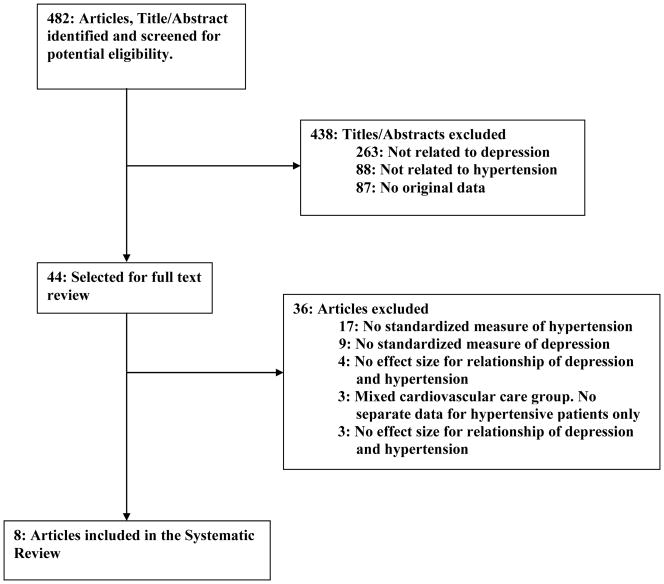
The search process identified 482 unique titles and abstracts that were screened for potential eligibility. During the title/abstract review, 438 citations were excluded leaving 44 articles for full-text review. Of these, 36 were excluded. Reasons for exclusion during both abstract and full-text reviews included lack of standardized measures of depression and adherence, no reported effect size of the association of depression or depressive symptoms with adherence to antihypertensive medications, or no separate data on hypertensive patients in a mixed cardiovascular care group. A total of 8 articles [29–36] were eventually included in this review. Details are provided in Figure 1.
Figure 1. Search and Selection of Eligible Articles.
General Description of the Studies
Details of the 8 studies included are shown in Table 1. All 8 studies were published between 2002 and 2009 and examined a total of 42,790 patients, including one study with 40,492 patients [33] and 7 studies with 190 to 496 patients each [29–32,34–36]. The study with the largest sample size [33] is described in detail in Table 2. Six studies were from the United States [29–31,33–35], one from the United Kingdom [36] and one from Pakistan [32]. Four studies reported data on patients from single centers [29,30,31,35] and four reported studies from multiple centers [32,33,35,36]. Mean patient age ranged from 44 to 69 years, and the percentage of males from 15% to 100%.
Table 1.
Summary of Studies Reviewed: Characteristics of Patients
| First Author Year |
Country | N | Mean Age | % Male | Race/Ethnicity |
|---|---|---|---|---|---|
|
Schoenthaler29 2009 |
United States | 167 | 54 | 15 | 100% AA |
|
Morris30 2006 |
United States | 469–475π | 57 | 27 | 68% AA |
|
Kim, Eun-Young31 2007 |
United States | 208 | 53 | 45 | 100% Korean American |
|
Hashmi32 2007 |
Pakistan | 438 | 52 | 45 | 100% |
|
Siegel33 2002 |
United States | 40,492 | 69 | 96 | 51% White, 33% Unstated, 8% Black, 4% Asian, 3% Hispanic |
|
Wang34 2002 |
United States | 496 | NR | 67 | 95% White, 5% non-White |
|
Kim, Miyong35 2003 |
United States | 190 | 44 | 100 | 100% AA |
|
Maguire36 2008 |
United Kingdom | 324 | NR | 46 | NR |
Abbreviations: NR, Not reported; AA, African Americans.
Between 469 and 475 patients were used in different analyses.
Table 2.
Study Characteristics (Siegel et al.)33
| Study Sites | 6 Veterans institutions in the United States |
| Study Subjects – number | 40,492 |
| Mean Age - Ω(SD), year | 68.6 (11.2) |
| Males – number (%) | 39,038 (96.4) |
| Race/Ethnicity – number (%) | |
| White | 20,495 (50.6) |
| Black | 3,330 (8.2) |
| Asian | 1,644 (4.1) |
| Hispanic | 1,364 (3.4) |
| Unstated | 13,502 (33.3) |
| Other | 157 (0.4) |
| Depression | |
| Diagnosis | ICD-9 Codes in medical records |
| Prevalence | Not mentioned |
| Adherent by ΩMTOT(% within drug class) | |
| Ω ACEI | 16,008 (81.1) |
| Beta-blocker | 12,360 (79.5) |
| Calcium antagonist | 10,163 (82.7) |
| Thiazide diuretics | 9,044 (78.3) |
| Alpha-blocker | 5,876 (78.8) |
| Ω ARB | 2,843 (83.6) |
| Predictors of antihypertensive medication adherence ♀Odds ratio (P value) | |
| Depression | 0.861 (<0.001) |
| ∞ Drug class | 0.991 (0.178) |
| Gender | 1.096 (0.090) |
| Race | 0.921 (<0.001) |
| Age | 1.01 ( <0.001) |
| Diabetes | 1.023 (0.309) |
| Psychosis | 0.978 (0.514) |
| Dementia | 1.036 (0.572) |
| Number of drug classes | 1.158 ( <0.001) |
| Number of all medications | 1.012 ( <0.001) |
| Facility | 1.018 (<0.001) |
SD, Standard Deviation; MTOT, Medication Possession Ratio, defined as the sum of days supply of medication dispensed except for last fill/number of days between first and last fill; ACEI, Angiotensin Converting Enzyme Inhibitor; ARB, Angiotensin Receptor Blocker.
From logistic regression analysis.
Classes of antihypertensive medications.
Description of Study Designs and Methods
Of the 8 studies in the review, 4 were conducted to examine the association of depression or depressive symptoms and adherence to antihypertensive medications [29,34–36] and 4 examined multiple factors related to adherence, but did not identify depression or depressive symptoms as the primary variable of interest [30–33]. Of the four studies designed to examine the relationship between depression or depressive symptoms and adherence to antihypertensive medications, the authors of one study [29] evaluated patients at baseline and 3 months for depressive symptoms, medication adherence, and self-efficacy. Another study [34], which was cross-sectional in design, reviewed the utilization of antihypertensive medications based on documentation in the electronic medical record in order to identify factors associated with poor adherence to antihypertensive medications. A third study [35] used data derived from interviews conducted 24 months after enrollment in a trial of a blood pressure control intervention among young urban Black men to assess the cross-sectional relationships among depressive symptoms, adherence to treatment recommendations and blood pressure. The fourth study [36] was conducted using prescription records from multiple pharmacies with the primary aim of exploring the association of concurrent depressive symptoms and medication beliefs with medication adherence.
Among studies that examined general factors related to adherence to antihypertensive medications, one [32] was a questionnaire-based cross-sectional analysis of selected patients from two tertiary care hospitals. Another study [30] was a cross-sectional analysis of the baseline data from an ongoing randomized controlled trial of a pharmacy-based intervention intended to improve drug adherence in hypertensive individuals. A third study was a cross-sectional analysis examining factors affecting adherence to antihypertensive medications in a trial of psychobehavioural education and telephone counseling [31]. The fourth study [33], also cross-sectional, examined the association of different classes of antihypertensive medication with adherence.
Diagnosis of Hypertension
The diagnosis of hypertension and use of antihypertensive medications were retrieved from electronic medical records in 5 studies [29,30,33,34,36]. Two studies documented hypertension with ICD-9 codes 401 to 401.9 [29,33]. Two studies used a chart note of hypertension as recorded in electronic medical records [30, 34] and one study [36] used pharmacy records documenting the prescription of at least one antihypertensive medication for at least one year.
Three studies [31,32,35] used multiple methods to classify patients with hypertension. All three studies included self-report of hypertension and/or antihypertensive medication use [31,32,35]and two of the three studies used the JNC-VI definition of hypertension as well [31,35].
The duration of hypertension was reported in only two studies [29,31]. The mean time since diagnosis of hypertension was 10 years in one of the studies [29] and 6 years in the other study [31].
Assessment and Prevalence of Depression
Methods used to assess depression or depressive symptoms varied across the 8 studies. Two studies [32,33] used a dichotomous or categorical depression variable to assess the association between depression and adherence; four studies [29,30,31,35] used continuous measurement of depressive symptoms; and two studies [34,36] presented results with both dichotomous/categorical and continuous assessment. Of the studies that used dichotomous/categorical assessments, two studies [33,34] identified depression based on medical records and another study [32] defined depression as a score of 20 or above on a depression screening instrument, the Aga Khan University Anxiety and Depression Scale (AKU-ADS). One study [36] separated patients into sub-threshold patients and categories of depression severity according to the Center for Epidemiologic Studies Depression Scale (CES-D). Six studies [29,30,31,34,35,36] used depressive symptoms as continuous predictors of adherence and measured symptoms with self-report questionnaires including the CES-D [29,35,36] an 8-item version of the Patient Health Questionnaire depression scale (PHQ-8) that did not include the item on suicide/self-harm from the PHQ-9 [30] the Kim Depression Scale for Korean Americans [31] and the Brief Symptom Inventory depression subscale [34].
In one study, the prevalence of depression based on chart notes was 10% [34]. Two studies used a cutoff score of ≥16 on the CES-D and reported rates of 33% [29]and 38% [36]. One study used a CES-D score of >16 and reported a rate of 27% [35]. One study reported that 38% of patients scored 10 or greater on the PHQ-8 [30] and one study that used the AKU-ADS reported a rate of 43% of patients above the cutoff threshold [32]. Two studies [31,33] did not report how many patients had depression or elevated symptoms of depression.
Assessment and Rates of Adherence to Antihypertensive Medication
Self-report was used to assess adherence in 5 studies [29,30,32,35,36] whereas 3 studies determined adherence based on a review of the electronic medical record, obtaining medication prescription, dispensing or refill records [30,33,34]. Of the 5 studies that used self-reported adherence measures, one of the studies reported that 39% of patients were adherent to antihypertensive medication based on responses to a 5-item questionnaire [30]. Another study [32] used 2 different methods to assess adherence. Adherence was calculated as the self-report of the number of pills taken in one week divided by the number of pills prescribed during that same period. Patients who took the medications at least 80% were considered adherent. A total of 77% of patients were adherent by this measure. This study also assessed adherence by self-report using the Morisky Medication Adherence Scale (MMAS), a 4-item validated questionnaire with scores ranging from 0 to 4, as a continuous measure with higher scores indicating better medication adherence [37]. Mean adherence score in this study was 2.5 ± 1.3. Another study [29] also used the MMAS as a continuous measure reporting an average adherence score of 0.7 ± 0.9 with 47% of the study participants’ classified as adherent at 3 months of follow-up.
Two studies [31,35] used the Hill-Bone Adherence Scale [38], a validated questionnaire for assessing adherence in hypertensive adults, as a continuous measure of adherence. However, one of these [31] used only 4 items from the original 14-item scale. The mean scores of adherence were not reported in either of these studies. The final study [36] assessed adherence with the Reported Adherence to Medications scale, which consists of 4 items with total scores ranging from 4 to 20. Using a cutoff score of ≥16, 90.7% of the patients studied were considered adherent.
Of the three studies that used electronic medical records to assess adherence [30,33,34], one study [30] estimated refill adherence by determining the Medication Possession Ratio, which is the number of days between the last refill date and the next needed refill date divided by the number of days between the last refill date and the date the patient actually received the refill. A total of 57.9% of patients were considered adherent, defined as a Medication Possession Ratio between 0.8 and 1.2. In another study [33], patients were considered “adherent” if their Medication Possession Ratio was > 0.8. The rate of adherence in this study varied between 78% and 84% according to the class of the prescribed antihypertensive medications. The third study [34] calculated the number and percent of days covered by antihypertensive therapy for each patient during the study year by noting the quantity of medication dispensed and the number of days covered by each filled prescription. Fewer than one third of the study population was covered for 80% of the days during the study year.
The Relationship of Depression and Adherence to Antihypertensive Medications
A study [33] which comprised of 95% of all patients included in this review found that patients with an ICD-9 diagnosis of depression in their medical record were less likely to be adherent in multivariate analysis (odds ratio [OR] =0.86, p<0.01; unadjusted OR not reported). Another study [35] of 190 urban Black males showed that depressive symptoms were associated with poor adherence in a bivariate analysis (r=0.30; p<0.01), but did not report multivariate results.
Each of the other six studies reported at least one negative result, but also at least one statistically insignificant result, depending on the type of analysis (bivariate versus multivariate) [29,31,34,36] and the measure of adherence or depression used in a given analysis [30,32,34]. Details are shown in Table 3.
Table 3.
Summary of Studies Reviewed: Assessment of Key Variables and Results
| First Author | Assessment of Depressive Symptoms |
Cutoff (Percent Above Cutoff) |
Adherence Measure/ Definition of Adherencex |
Continuous/ Dichotomous Assessment of Adherence |
Continuous/ Dichotomous Assessment of Depression |
Timing of Depression Assessment |
Variables in Multi- variate Analyses |
Rate of Adherence to Antihypertensive Medications |
Prevalence of Depression |
Bivariate Association Between Depression and Adherence to Antihypertensive Medications (95% CI, p value) |
Multivariate Association Between Depression and Adherence to Antihypertensive Medications (95% CI, p value) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Schoenthaler29 | CES-D | ≥16 Ω | MMAS/a | Continuous | Continuous | Prospective 3 months before adherence | Self-efficacy | 53% at 3 months post-baseline | 33%♀ | β≠=.013 (0.01 – .025, p=0.04) | β=.010 (p=0.09) |
| Morris30 | PHQ-8Ω | ≥10 | Refill adherenceb Self-Reportc |
Dichotomous Dichotomous |
Continuous | Cross-sectional | Age, marital status, race/ethnicity | a. 57% by refill adherence b. 38% by self reported adherence |
38% | a. OR=0.82 (0.56– 1.19, p=0.25) b. OR=0.41 (0.27–0.61, p< 0.01) |
a. OR= 0.94 (0.63–1.4, p=0.76) b. OR=0.48 (0.32–0.72, p=0.01) |
| Kim, Eun-Young31 | Kim Depression Scale for Korean Americans | NR | Hill-Bone Compliance Scaled | Continuous | Continuous | Cross-sectional | Age, gender, marriage, income, education, co-morbidities | a. NR for intentional adherence b. NR for unintentional adherence. |
NR | a. Hedges’g =0.48¥ (p=0.006) b. Hedges’g =0.17¥ (p=0.30) |
a. OR of intentional =1.012 (0.95–1.07) b. OR of unintentional =1.018 (0.97–1.07) |
| Hashmi32 | AKU-ADS | ≥20 | Self-Reporte MMASaA |
a. Continuous b. Continuous |
Dichotomous | Cross-sectional | Age, drugs prescribed, patients’ knowledge | a. 77% by self reportΩ b. NR by MMAS |
43% | a. OR=0.82 (0.5–1.3) ¥ b. βΩ=0.34(0.1–0.6, p=0.005) |
a. NR b. NR |
| Siegel33 | ICD-9 Code in medical record | NA | Medication Possession Ratiof | Dichotomous | Dichotomous | Cross-sectional | Antihypertensive drug class, gender♀ | 78% to 84% according to drug class. | NR | NR | OR= 0.86 (0.82–0.91, p<0.01)Ω |
| Wang34 | a. BSIϕ b. Chart diagnosis of depression |
a. NR b. NA |
% of days of medication coverage in 1 yr | Dichotomous | a. Continuous b. Dichotomous |
Cross-sectional | Other illnesses, demo-graphics, thiazides | 29% with >80% adherence | a. NR by BSI. b. 10%¥ by chart diagnosis |
a. OR = 0.97(0.91– 1.03) b. OR = 0.77 (0.44– 1.34) |
a. OR = 0.93 (0.87–0.99, p=0.03) b. NR |
| Kim, Miyong35 | CES-D | >16 Ω | Hill-Bone Adherence to Hypertension Therapy Scaleg | Continuous | Continuous | Cross-sectional | Education, income, jail time, illicit drugs, alcohol use, smoking | NR | 27% | r = 0.30 (p<0.01) | NR |
| Maguire36 | CES-D | ≥16 | Reported Adherence to Medications scaleh,∞. | Dichotomous | a. Dichotomous b. 4 categories c. Continuous€ |
Cross-sectional | Age, gender, self-efficacy, social support, beliefs about medication | 91% | 38% | a. NR, (p = .07) b. NR(p =0.02) |
c. OR=1.00 (0.96– 1.05, p= 0.92) |
Abbreviations: NR, Not reported; NA, Not applicable; BDI, Beck Depression Inventory; CES-D, Center for Epidemiologic Studies Depression Scale; ICD-9, International Classification of Diseases 9th Revision; OR, Odds Ratio; CI, Confidence Interval; r, regression coefficient; AA, African American; PHQ-8, Patient Health Questionnaire; IN, Intentional nonadherence; UN, Unintentional nonadherence; MMAS, Morisky Medication Adherence Scale; BSI, Brief Symptom Inventory Depression Subscale; r, correlation coefficient; HTN, hypertension; AKU-ADS, Aga Khan University Anxiety Depression Scale.
Definition of Adherence as follows:
“yes” response on ≥ 1 of 4 adherence items indicating poorer adherence,
4 item questionnaire with total score ranging from 0 (non adherent) to 4 (adherent)
80–120% overall medication possession ratio by pharmacy,
“yes” response on the first question and “no” on the next four questions,
responses rated 2 or higher on the 4-point medication subscale;
pills taken over the study period divided by pills prescribed for that period of time;
sum of days medication was dispensed compared with the number of days elapsed from index prescription date to finish date,
4-point scale with higher scores indicating lower levels of adherence.
A self report adherence 5 point scale with scores ranging from 4 to 20.
β=standardized regression coefficient from a path model.
Calculated from the article, Hashmi et al, OR for the relationship between depression assessed by the Aga Khan University Anxiety and Depression Scale (AKU-ADS) and adherence; Wang et al- Average percentage prevalence of depression in the two study sites; Kim E et al -Hedges’g for depression score and intentional nonadherence, Hedges’g for depression score andunintentional nonadherence.
Siegel et al - Other covariates include race, age, diabetes, psychosis, dementia, number of antihypertensive drug classes, number of all drugs, facility.
7 items from Brief Symptom Inventory depression subscale to create a total depression severity score ranging from 0–14.
Mean Reported Adherence to Medications Scale for CES-D<16 =18.54 and 18.06 for CES-D≥16 (p=0.071, Chi-square).
Verifications from authors: Schoenthaler et al - Depression defined as ≥ 16 score on the CES-D; Morris et al - 8 item version of PHQ did not include item on suicide/self-harm from the PHQ-9; Hashmi et al - self-reported adherence measured as pill intake over 7 days, Persons with 80% rate on self-reported adherence scale was considered adherent, Unstandardized β, no multivariate effect size calculated for the relationship between depression and adherence; Siegel et al – 95% CI for the effect size as documented, coefficient = − 0.150, SE= 0.0259, Wald Stat=33.471; Wang et al- multivariate analysis of relationship between depression as assessed from chart records and adherence was not conducted and primary data not available for further review; Kim Miyong et al – CES-D cut-off used is scores > 16 not ≥ 16.
Participants were classified as (i) not having symptoms indicative of depression if CES-D < 16; (ii) symptoms indicative of depression if CES-D ≥ 16. (b) Participants were classified into 4 categories of depression symptomatology; CES-D <16=normal, 16–20=mild, 21–30=moderate, 31–60=severe (c) CES-D scores were used as continuous measure.
One study [29] that used path models reported a small unstandardized regression coefficient of 0.01 (p=0.04) between depression and poor adherence to antihypertensive medications at 3 months of follow-up for 167 African American patients, but it was no longer statistically significant when a measure of self-efficacy was added to the model (unstandardized regression coefficient=0.01, p=0.09). Another study [36] with 324 patients reported a statistically significant association between dichotomized depressive symptom and adherence variables (p=0.02, no effect size reported), but found that continuous depressive symptom scores were not associated with adherence in multivariate analysis (OR=1.00, 95% confidence interval [CI 0.96–1.05, p=0.92]). A study of 208 Korean American patients [31] that assessed nonadherence as intentional and unintentional, reported significantly higher depressive symptom scores among patients who were intentionally nonadherent in bivariate analysis (Hedges’ g=0.48, p=0.01). However, dichotomized depression classifications did not predict intentional nonadherence in multivariate analysis (OR=1.012, 95% CI 0.95–1.07). Depressive symptoms were not significantly related to unintentional nonadherence in bivariate (Hedges’ g=0.17, p=0.30) or multivariate analyses (OR=1.018, 95% CI 95% 0.97–1.07). One study of just under 500 patients [30] found significant negative bivariate (OR=0.41, 95% CI 0.27–0.61, p<0.01) and multivariate (OR=0.48, 95% CI 0.32–0.72, p=0.01) associations between depression and adherence to antihypertensive medications based on self-report. The same study, however, did not find a significant relationship between depression and adherence when adherence was assessed objectively by prescription refills (OR=0.82, 95% CI 0.56–1.19, p=0.25). In another study of 438 patients from Pakistan [32], there was a significant association between continuous self-reported MMAS adherence scores and depressive symptoms (unstandardized β=0.34, 95% CI 0.1–0.6, p=0.005) but there was not a significant relationship when adherence was defined as self-report of taking 80% or more of prescribed medication (OR=0.82, 95% CI 0.5–1.3). Finally, one study of 496 treated hypertensive patients [34] reported that depression was not significantly associated with adherence either when the diagnosis of depression was based on a review of the medical record (OR=0.77, 95% CI 0.44–1.34) or when it was based on continuous Brief Symptom Inventory scores (OR=0.97, 95% CI 0.91–1.03). On the other hand, a small, statistically significant negative relationship between depression assessed by the Brief Symptom Inventory subscale and adherence was noted in adjusted analysis (OR=0.93, 95% CI 0.87–0.99, p=0.03).
Methodological Quality of Studies
Quality characteristics of individual studies are shown in Table 4. All the studies included more than 100 patients. Seven studies included between 167 and 496 patients, and one study reported data from 40,492 patients [33]. Only one study was prospective in design [29]. Only one study documented a patient participation rate of at least 70% [32]. Only three studies [29, 31, 35] defined hypertension by the JNC-6 criteria. All the studies assessed depression or depressive symptoms with a validated questionnaire except one [33] which used the ICD-9 code diagnosis in the medical record. All cut-offs for the depression scales were standard except for one study where a CES-D score >16 was used [35] rather than the standard ≥16. Adherence was also generally assessed with validated scales in all the studies. Only two studies [33, 34] included an assessment of adherence to medication that was not based on self-report. Appropriate multivariate statistical techniques were used in three studies [29, 31, 33].
Table 4.
Summary of Methodological Quality Characteristics of Studies Reviewed*
| Schoenthaler 2009 | Morris 2006 | Kim, E 2007 | Hashmi 2007 | Siegel 2007 | Wang 2002 | Kim, M 2003 | Maguire 2008 | |
|---|---|---|---|---|---|---|---|---|
| Sample Selection and Size | ||||||||
| 1. HTN as defined by JNC-6 and JNC-7 | YES | NO | YES/NOa | NO | NO | NO | YES | NO |
| 2. Multicenter | NO | NO | NO | YES | YES | YES | NO | YES |
| 3. Description of demographic characteristics and at least 1 socioeconomic indicator | YES | YES | YES | YES | NO | YES | YES | YES |
| 4. Participation rate ≥ 70% | NO | NR | NO | YES | NA | NO | NO | NO |
| 5. Description of medical characteristics | YES | YES | YES | YES | YES | NO | YES | YES |
| 6. Sample size ≥ 100 | YES | YES | YES | YES | YES | YES | YES | YES |
| Assessment of depression or symptoms of depression | ||||||||
| 7. Depression assessed by a validated questionnaire or structured interview | YES | YES | YES | YES | NO | YES | YES | YES |
| Assessment of Adherence to Antihypertensive Medications | ||||||||
| 8. Assessment by a validated scale | YES | NO | YES | YES | YES | YES | YES | YES |
| 9. Assessment by self-report | YES | YES | YES | YES | NO | NO | YES | YES |
| Statistical Analysis | ||||||||
| 10. Multivariate analysis of relationship of depression and adherence to antihypertensive medication | YES | YES | YES | NO | YES | YES | NO | YES |
| 11. Appropriate multivariate statistical techniques used | YES | NO | YES | NO | YES | NO | NO | NO |
N = No; Y = Yes. Detailed criteria are presented in Appendix C.
NA = Not Applicable.
YES/NO because a participant could have been included if he/she was on antihypertensive medications but not diagnosed by the JNC criteria.
Discussion
Although several studies have examined the relationship between depression and adherence to antihypertensive medications, this systematic review shows that definitive conclusions cannot be drawn about this topic because of the substantial heterogeneity between studies with respect to the assessment of both depression and adherence, as well as inconsistencies in results both within and between studies. The studies included in the review used a wide variety of measures of both depression and antihypertensive medication adherence, making it difficult to draw firm conclusions about the relationship of depression to treatment adherence. Although all 8 studies included in this systematic review reported statistically significant bivariate [29, 30, 31, 32, 35, 36] or multivariate relationships [30, 33, 34] between depression or depressive symptoms and poor adherence to antihypertensive medications, many of these same studies also reported insignificant findings in bivariate analysis [30,31,32,34,36] and multivariate analysis [29,30,31,36]. There were often different results when the analysis was adjusted and unadjusted [29, 31, 34, 36], when different measures of adherence were used [30, 32] and when depression symptoms were analyzed in a continuous or categorical fashion [36]. In some cases, a significant association between depressive symptoms and adherence in bivariate analysis was no longer significant after adjustment for certain variables. This may have been because of the relationship between depressive symptoms and these variables, rather than because depressive symptoms did not have an important effect on adherence. For example, in one study [29], both depressive symptoms and low self efficacy were associated with poor adherence to antihypertensive medications. The relationship between depressive symptoms and medication adherence was no longer significant when self efficacy was controlled for, but this may be because of the well-known relationship between depression and low self efficacy [39,40].
Only two studies [33,34] used an objective measure of adherence that was not based on self-report. Both reported statistically significant, but relatively small associations between depression or depressive symptoms and adherence, including an OR of 0.86 (95% CI 0.82–0.91) in a study that used an administrative database with over 40,000 patients [33] and an OR of 0.93 (95% CI 0.87–0.99) in a study of 496 patients [34].
There is evidence that depression adversely affects adherence to treatment recommendations in many different medical conditions [17]. It is known that adherence to medications and other treatment recommendations improve outcomes and decreases rates of readmission. Since the relationship between nonadherence to antihypertensive medications and poor outcomes is established [41, 42], specific evidence that hypertensive patients with depression adhere poorly to taking antihypertensive medications would have important public health implications. However, because of limitations in the existing evidence, this systematic review was not able to conclusively determine the degree to which depression is associated with poor adherence to antihypertensive medications. Given this limitation, and considering the high prevalence of hypertension and its disabling complications [43], additional studies that are designed to specifically address the role of depression in antihypertensive medication nonadherence seem warranted. These studies should incorporate prospective designs, include validated and consistent objective measures of adherence to antihypertensive medications, and use validated and consistent methods to assess depression or depressive symptoms. If depression is found to be an “upstream” cause of poor blood pressure control, additional studies may be warranted that address whether depression treatment improves adherence to antihypertensive medications and results in a greater percentage of patients achieving target blood pressure goals. Of relevance to this is the observation reported in two separate studies of depressed acute coronary syndrome survivors that improvement in depression is associated with greater medication adherence [44, 45]. Thus, with greater emphasis on the importance of multimodal approaches to improving patient adherence [46], it is possible that addressing depression may be a potentially useful intervention in improving medication adherence in a variety of medical conditions.
The primary limitation of this systematic review was the small number of studies included and the even smaller number of included studies that were designed specifically to assess the association between depression and adherence to antihypertensive medications. A second limitation is related to the number of different methods used by studies reviewed to define the key variables of interest, namely depression, adherence, and the diagnosis of hypertension. Some studies identified hypertensive patients based on their use of “antihypertensive medications,” but this may not be accurate because several of these medications have indications other than the treatment of hypertension. Additionally, there was only one prospective study [29], and it was limited by a small sample size, and the lack of control for important potentially confounding variables. It should also be noted that authors of included articles were contacted for inquires about unpublished works, although only 4 [29, 30, 32, 34] out of the 8 authors responded. Furthermore, the quality of the studies varied with one study having a sample size as small as 190 patients [29] and another consisting of more than 40,000 patients [33]. Another important limitation was that only 2 studies used objective measures of adherence, and recall bias may have been present in studies that used self-report assessment tools for adherence or diagnosis of hypertension. There are also limitations from the use of administrative databases such as coding inaccuracies, incomplete coding and differences in data quality across institutions.
Conclusion
All studies reported statistically significant relationships between depression and poor adherence to antihypertensive medications, but definitive conclusions cannot be drawn because of substantial heterogeneity between studies with respect to the assessment of depression and adherence, as well as inconsistencies in results both within and between studies. Additional studies in this area that specifically address the role of depression and adherence, each objectively assessed, would help clarify this relationship.
Unstructured Summary.
Depression is associated with treatment nonadherence in many medical conditions, but the strength and consistency of the evidence on the relationship between depression and adherence to antihypertensive medications has not been examined.
The MEDLINE, CINAHL, PsycINFO, Embase, SCOPUS, and ISI databases were searched from inception until December 11, 2009 for studies that assessed adherence to antihypertensive medications and depression or symptoms of depression in patients with hypertension.
Eight studies were identified that included a total of 42,790 patients. All 8 studies reported at least one significant bivariate or multivariate negative relationship between depression and adherence to antihypertensive medications.
All studies reported statistically significant relationships between depression and poor adherence to antihypertensive medications, but definitive conclusions cannot be drawn because of substantial heterogeneity between studies with respect to the assessment of depression and adherence, as well as inconsistencies in results both within and between studies. Additional studies in this area that specifically address the role of depression and adherence, each objectively assessed, would help clarify this relationship.
Acknowledgments
Dr. Thombs is supported by a New Investigator Award from the Canadian Institutes of Health Research and an Établissement de Jeunes Chercheurs award from the Fonds de la Recherche en Santé Québec. Drs. Eze-Nliam and Ziegelstein are supported by grant number R24AT004641 from the National Center for Complementary & Alternative Medicine, and Dr. Ziegelstein is also supported by the Miller Family Scholar Program of the Johns Hopkins Center for Innovative Medicine.
Appendix A. Search Strategies
MEDLINE (from 1950)
(complian* OR adheren* OR noncomplian* OR nonadheren*) AND (depress* AND (hypertens* OR “high blood pressure” OR hbp)) AND (“last 1 year”[PDat])
PsycINFO (from 1887); EMBASE (from 1974); CINAHL (from 1981)
((‘depression’/exp) AND (‘patient compliance’/exp/mj)) AND ((‘hypertension’/exp) OR (‘antihypertensive agent’/exp) OR (‘antihypertensive therapy’/exp))
SCOPUS (from 1869)
(TITLE-ABS-KEY(complian* OR adheren* OR noncomplian* OR nonadheren*) AND TITLE-ABS-KEY(depress* AND (hypertens* OR “high blood pressure” OR hbp))) AND SUBJAREA(mult OR agri OR bioc OR immu OR neur OR phar OR mult OR medi OR nurs OR vete OR dent OR heal OR mult OR arts OR busi OR deci OR econ OR psyc OR soci)
ISI (from 1900)
Topic=(complian* or adheren* or noncomplian* or nonadheren*) AND Topic=(depress* and (hypertens* or “high blood pressure” or hbp))
Appendix B: Journals Included in Manual Searching
American Heart Journal
American Journal of Cardiology
American Journal of Hypertension
American Journal of Medicine
Annals of Behavioral Medicine
Annals of Internal Medicine
Archives of Internal Medicine
British Medical Journal
Circulation
Current Hypertension Reports
European Heart Journal
Heart
Hypertension
JAMA
Journal of Behavioral Medicine
Journal of Clinical Hypertension
Journal of Human Hypertension
Journal of Hypertension
Journal of the American College of Cardiology
Journal of General Internal Medicine
Lancet
New England Journal of Medicine
Appendix C: Criteria for a “Yes” Rating on Items Assessing Methodological Quality
(1) Hypertension as defined by The Sixth and Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-6 and JNC-7): The average of two or more properly measured, seated blood pressure readings on each of two or more office visits with systolic blood pressure of 140 mm Hg and/or diastolic blood pressure of 90 mm Hg.
(2) Multi-center: Patients from two or more centers were included in data.
(3) Description of demographic characteristics included age, sex, and at least 1 socioeconomic indicator: Data included age, sex, and at least 1 socioeconomic indicator (e.g., income, education, work status).
(4) Participation rate ≥ 70%: At least 70% of eligible patients were successfully recruited and participated in the study.
(5) Description of medical characteristics includes disease duration, comorbidities, number of antihypertensive medications taken.
(6) Sample size ≥ 100: At least 100 patients were included in analyses.
(7) Major depressive disorder was assessed using a structured clinical interview (e.g., the Structured Clinical Interview for DSM [SCID], the Diagnostic Interview Schedule [DIS], or the Composite International Diagnostic Interview [CIDI]) or symptoms of depression were assessed with a self-report questionnaire or rating scale and using a cutoff score that enables comparison with other studies and patient groups (e.g., BDI ≥ 10, HADS ≥ 8, HADS ≥ 11, CES-D ≥ 16, CES-D ≥ 19).
(8) Assessment of adherence to antihypertensive medications by a validated scale which could be by self-reporting, medication counting, prescription refills, medical records. However, operational definition of adherence such as adherence being defined by filling prescriptions > 80% during study period should be employed.
(9) Assessment of adherence to antihypertensive medications by self-report: See above.
(10) Multivariate analysis of relationship of depression and adherence to antihypertensive medication: The effect size of this relationship as measured by predictor variables in multivariate models.
(11) Appropriate multivariate statistical techniques used: Descriptions of statistical methods adhered to published reporting guidelines (e.g., How to Report Statistics in Medicine, Lang and Secic, 1997; Publication Manual of the American Psychological Association, 5th edition, 2001), two-tailed significance tests were used, automated stepwise procedures were not used unless cross-validated (Freedland et al., Statistical Guidelines for Psychosomatic Medicine, 2005;67:167), and sample size was adequate in relation to the numbers of predictors (e.g., 10:1, Using Multivariate Statistics, 4th edition, Tabachnik and Fidell, 2001).
Footnotes
Disclosures: The authors declare no conflict of interest.
References
- 1.Schappert SM, Nelson C. National ambulatory medical care survey: 1995–96 summary. Vital Health Stat. 1999;13(142):i–vi. 1–122. [PubMed] [Google Scholar]
- 2.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in united states adults between 1988–1994 and 1999–2004. Hypertension. 2008;52:818–827. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 3.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics 2007 update. A report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 4.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the united states 1999 to 2000: A rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 5.Wolf-Maier K, Cooper RS, Banegas JR, Giampaoli S, Hense HW, Joffres M, Kastarinen M, et al. Hypertension prevalence and blood pressure levels in 6 european countries, canada, and the united states. JAMA. 2003;289:2363–2369. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- 6.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National High Blood Pressure Education Program Coordinating Committee. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 7.Psaty BM, Manolio TA, Smith NL, Heckbert SR, Gottdiener JS, Burke GL, Weissfeld J, et al. Time trends in high blood pressure control and the use of antihypertensive medications in older adults: The cardiovascular health study. Arch Intern Med. 2002;162:2325–2332. doi: 10.1001/archinte.162.20.2325. [DOI] [PubMed] [Google Scholar]
- 8.Elliott WJ. Improving outcomes in hypertensive patients: Focus on adherence and persistence with antihypertensive therapy. J Clin Hypertens. 2009;11:376–382. doi: 10.1111/j.1751-7176.2009.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vawter L, Tong X, Gemilyan M, Yoon PW. Barriers to antihypertensive medication adherence among adults--united states, 2005. J Clin Hypertens. 2008;10:922–929. doi: 10.1111/j.1751-7176.2008.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monane M, Bohn RL, Gurwitz JH, Glynn RJ, Levin R, Avorn J. The effects of initial drug choice and comorbidity on antihypertensive therapy compliance: Results from a population-based study in the elderly. Am J Hypertens. 1997;10:697–704. doi: 10.1016/s0895-7061(97)00056-3. [DOI] [PubMed] [Google Scholar]
- 11.Monane M, Bohn RL, Gurwitz JH, Glynn RJ, Levin R, Avorn J. Compliance with antihypertensive therapy among elderly medicaid enrollees: The roles of age, gender, and race. Am J Public Health. 1996;86:1805–1808. doi: 10.2105/ajph.86.12.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizzo JA, Simons WR. Variations in compliance among hypertensive patients by drug class: Implications for health care costs. Clin Ther. 1997;19:1446–1457. doi: 10.1016/s0149-2918(97)80018-5. [DOI] [PubMed] [Google Scholar]
- 13.Bosworth HB, Dudley T, Olsen MK, Voils CI, Powers B, Goldstein MK, Oddone EZ. Racial differences in blood pressure control: Potential explanatory factors. Am J Med. 2006;119:70.e9–70.e15. doi: 10.1016/j.amjmed.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Kramer H, Han C, Post W, Goff D, Diez-Roux A, Cooper R, Jinagouda S, et al. Racial/ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA) Am J Hypertens. 2004;17:963–970. doi: 10.1016/j.amjhyper.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Stuart B, Zacker C. Who bears the burden of medicaid drug copayment policies? Health Aff. 1999;18:201–212. doi: 10.1377/hlthaff.18.2.201. [DOI] [PubMed] [Google Scholar]
- 16.United States, Agency for Health Care Policy and Research, United States, Depression Guideline Panel. Depression in primary care: detection, diagnosis and treatment. U.S. Dept. of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; Rockville, MD: 1993. p. 21. [Google Scholar]
- 17.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–07. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 18.Patten SB. Long-term medical conditions and major depression in a canadian population study at waves 1 and 2. J Affect Disord. 2001;63:35–41. doi: 10.1016/s0165-0327(00)00186-5. [DOI] [PubMed] [Google Scholar]
- 19.Conn VS, Isaramalai SA, Rath S, Jantarakupt P, Wadhawan R, Dash Y. Beyond MEDLINE for literature searches. J Nurs Scholarsh. 2003;35:177–182. doi: 10.1111/j.1547-5069.2003.00177.x. [DOI] [PubMed] [Google Scholar]
- 20.Crumley ET, Wiebe N, Cramer K, Klassen TP, Hartling L. Which resources should be used to identify RCT/CCTs for systematic reviews: A systematic review. BMC Med Res Methodol. 2005;5:24. doi: 10.1186/1471-2288-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Betran AP, Say L, Gulmezoglu AM, Allen T, Hampson L. Effectiveness of different databases in identifying studies for systematic reviews: Experience from the WHO systematic review of maternal morbidity and mortality. BMC Med Res Methodol. 2005;5:6. doi: 10.1186/1471-2288-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whiting P, Westwood M, Burke M, Sterne J, Glanville J. Systematic reviews of test accuracy should search a range of databases to identify primary studies. J Clin Epidemiol. 2008;61:357–364. doi: 10.1016/j.jclinepi.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, scopus, web of science and google scholar: Strengths and weaknesses. FASEB J. 2008;22:338–342. doi: 10.1096/fj.07-9492LSF. [DOI] [PubMed] [Google Scholar]
- 24.Bakkalbasi N, Bauer K, Glover J, Wang L. Three options for citation tracking: Google scholar, scopus and web of science. Biomed Digit Libr. 2006;3:7. doi: 10.1186/1742-5581-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopewell S, Clarke M, Lefebvre C, Scherer R. Handsearching versus electronic searching to identify reports of randomized trials. Cochrane Database Syst Rev. 2007;2:MR000001. doi: 10.1002/14651858.MR000001.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Wang QJ. The prevalence of prehypertension and hypertension among US adults according to the new joint national committee guidelines: New challenges of the old problem. Arch Intern Med. 2004;164:2126–2134. doi: 10.1001/archinte.164.19.2126. [DOI] [PubMed] [Google Scholar]
- 27.Thombs BD, Bass EB, Ford DE, Stewart KJ, Tsilidis KK, Patel U, Fauerbach JA, et al. Prevalence of depression in survivors of acute myocardial infarction. J Gen Intern Med. 2006;21:30–38. doi: 10.1111/j.1525-1497.2005.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells GA, Shea B, O’Connell D, Petersen J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. [Assessed January 22, 2010]; < http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm>.
- 29.Schoenthaler A, Ogedegbe G, Allegrante JP. Self-efficacy mediates the relationship between depressive symptoms and medication adherence among hypertensive African Americans. Health Educ Behav. 2009;36:127–137. doi: 10.1177/1090198107309459. [DOI] [PubMed] [Google Scholar]
- 30.Morris AB, Li J, Kroenke K, Bruner-England TE, Young JM, Murray MD. Factors associated with drug adherence and blood pressure control in patients with hypertension. Pharmacotherapy. 2006;26:483–492. doi: 10.1592/phco.26.4.483. [DOI] [PubMed] [Google Scholar]
- 31.Kim EY, Han HR, Jeong S, Kim KB, Park H, Kang E, Shin HS, et al. Does knowledge matter?: Intentional medication nonadherence among middle-aged Korean Americans with high blood pressure. J Cardiovasc Nurs. 2007;22:397–404. doi: 10.1097/01.JCN.0000287038.23186.bd. [DOI] [PubMed] [Google Scholar]
- 32.Hashmi SK, Afridi MB, Abbas K, Sajwani RA, Saleheen D, Frossard PM, Ishaq M, et al. Factors associated with adherence to anti-hypertensive treatment in Pakistan. PLoS One. 2007;2:e280. doi: 10.1371/journal.pone.0000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siegel D, Lopez J, Meier J. Antihypertensive medication adherence in the department of veterans affairs. Am J Med. 2007;120:26–32. doi: 10.1016/j.amjmed.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 34.Wang PS, Bohn RL, Knight E, Glynn RJ, Mogun H, Avorn J. Noncompliance with antihypertensive medications: The impact of depressive symptoms and psychosocial factors. J Gen Intern Med. 2002;17:504–511. doi: 10.1046/j.1525-1497.2002.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim MT, Han HR, Hill MN, Rose L, Roary M. Depression, substance use, adherence behaviors, and blood pressure in urban hypertensive black men. Ann Behav Med. 2003;26:24–31. doi: 10.1207/S15324796ABM2601_04. [DOI] [PubMed] [Google Scholar]
- 36.Maguire LK, Hughes CM, McElnay JC. Exploring the impact of depressive symptoms and medication beliefs on medication adherence in hypertension-a primary care study. Patient Educ Couns. 2008;73:371–376. doi: 10.1016/j.pec.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self- reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Kim MT, Hill MN, Bone LR, Levine DM. Development and testing of the hill-bone compliance to high blood pressure therapy scale. Prog Cardiovasc Nurs. 2000;15:90–96. doi: 10.1111/j.1751-7117.2000.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 39.Robinson-Smith G, Johnston MV, Allen J. Self-care self-efficacy, quality of life, and depression after stroke. Arch Phys Med Rehabil. 2000;81:460–464. doi: 10.1053/mr.2000.3863. [DOI] [PubMed] [Google Scholar]
- 40.Haukkala A, Uutela A, Vartiainen E, McAlister A, Knekt P. Depression and smoking cessation: the role of motivation and self-efficacy. Addict Behav. 2000;25:311–6. doi: 10.1016/s0306-4603(98)00125-7. [DOI] [PubMed] [Google Scholar]
- 41.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 42.Mazzaglia G, Ambrosioni E, Alacqua M, Filippi A, Sessa E, Immordino V, Borghi C, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120:1598–1605. doi: 10.1161/CIRCULATIONAHA.108.830299. [DOI] [PubMed] [Google Scholar]
- 43.Macmahon S, Rodgers A. The Epidemiological Association between Blood Pressure and Stroke - Implications for Primary and Secondary Prevention. Hypertens Res. 1994;17:S23–S32. [Google Scholar]
- 44.Rieckmann N, Gerin W, Kronish IM, Burg MM, Chaplin WF, Kong G, Lesperance F, et al. Course of depressive symptoms and medication adherence after acute coronary syndromes: An electronic medication monitoring study. J Am Coll Cardiol. 2006;48:2218–2222. doi: 10.1016/j.jacc.2006.07.063. [DOI] [PubMed] [Google Scholar]
- 45.Glassman AH, Bigger JT, Gaffney M. Psychiatric characteristics associated with long-term mortality among 361 patients having an acute coronary syndrome and major depression: seven-year follow-up of SADHART participants. Arch Gen Psychiatry. 2009;66:1022–1029. doi: 10.1001/archgenpsychiatry.2009.121. [DOI] [PubMed] [Google Scholar]
- 46.Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA. 2006;296:2563–2571. doi: 10.1001/jama.296.21.joc60162. [DOI] [PubMed] [Google Scholar]