Abstract
Background
Inflammatory bowel disease (IBD) is hypothesized to result from stimulation of immune responses against resident intestinal bacteria within a genetically susceptible host. Mast cells may play a critical role in IBD pathogenesis, since they are typically located just beneath the intestinal mucosal barrier and can be activated by bacterial antigens.
Methodology/Principal Findings
This study investigated effects of mast cells on inflammation and associated neoplasia in IBD-susceptible interleukin (IL)-10-deficient mice with and without mast cells. IL-10-deficient mast cells produced more pro-inflammatory cytokines in vitro both constitutively and when triggered, compared with wild type mast cells. However despite this enhanced in vitro response, mast cell-sufficient Il10 −/− mice actually had decreased cecal expression of tumor necrosis factor (TNF) and interferon (IFN)-γ mRNA, suggesting that mast cells regulate inflammation in vivo. Mast cell deficiency predisposed Il10 −/− mice to the development of spontaneous colitis and resulted in increased intestinal permeability in vivo that preceded the development of colon inflammation. However, mast cell deficiency did not affect the severity of IBD triggered by non-steroidal anti-inflammatory agents (NSAID) exposure or helicobacter infection that also affect intestinal permeability.
Conclusions/Significance
Mast cells thus appear to have a primarily protective role within the colonic microenvironment by enhancing the efficacy of the mucosal barrier. In addition, although mast cells were previously implicated in progression of sporadic colon cancers, mast cells did not affect the incidence or severity of colonic neoplasia in this inflammation-associated model.
Introduction
Inflammatory bowel disease (IBD) is characterized by aberrant immune responses against microorganisms that are present in the intestine. Debilitating clinical symptoms of pain and diarrhea result from intestinal mucosal damage that is driven by the continuous activation of the mucosal immune system by enteric bacteria. A variety of genetic, microbial, and environmental factors have been identified that increase susceptibility to IBD in both animal models and humans. Based on this data, we have proposed that the development of IBD requires three factors [1]. First, bacterial antigens and adjuvants must be present within the intestine. This factor is not easily modifiable, since potentially colitogenic bacterial antigens and adjuvants are present within the intestine of all humans and all mice that are not kept in germ-free facilities. Second, the mucosal barrier must be defective so that the bacterial antigens and adjuvants present within the intestine can come in contact with the innate and adaptive immune cells to generate responses. And third, the host must have a defect in immune regulation that allows induction of sustained immune responses against these antigens. This three-factor model can potentially explain how the known susceptibility alleles and IBD-related triggers in existing murine models result in the development of chronic colitis. The model also predicts that colitis can potentially be prevented or treated by interventions that favor maintenance of appropriate immune regulation and/or enhancement of mucosal barrier function.
Most of the currently used IBD treatments target the immune regulatory pathways, but resulting immunosuppression can increase patient risk for developing opportunistic infections and/or treatment-related lymphomas. Mechanisms that govern the barrier function of the intestinal mucosa are thus of great interest to identify potential targets for novel IBD therapies that can add or synergize with existing therapies. A number of murine models of intestinal inflammation have been established [1], [2]. One very commonly used model uses mice deficient in interleukin (IL)-10. These mice have defects in immune regulation and develop chronic enterocolitis with loss of tolerance to bacterial stimuli when triggered by environmental exposures that decrease mucosal barrier function [3], [4], [5], [6].
Mast cells are innate immune cells that can potentially contribute to IBD through their pro- inflammatory activity and/or effects on immunoregulation. Their pattern recognition molecules allow them to readily recognize and rapidly respond to bacteria that breach the epithelium [7]. Upon activation, mast cells can immediately release large amounts of pro-inflammatory cytokines that are contained in pre-formed granules [8] and can continue to synthesize and release a wide array of pro-inflammatory mediators de novo. Mast cells thus rapidly and selectively produce appropriate mediators that enhance effector-cell recruitment and complement other effector components of the immune system [9]. For example, mast cell-derived mediators can contribute to colitis severity by enhancing neutrophil influx and thus perpetuating ongoing inflammation. However mast cells have also been documented to have anti-inflammatory or immunosuppressive functions, such that they can serve to either enhance or to limit innate or adaptive immune responses, depending on the context [10].
Mast cells are physically located adjacent to the intestinal epithelium, so their activation may also affect the function of the mucosal barrier. The presence of mast cells or the mast cell-produced proteinase Mcpt4 (chymase) was recently shown to enhance the permeability of jejunal segments studied ex vivo [8]. The same study showed that mast cell-deficient KitW-sh/W-sh (sash) mice have changes in small intestinal architecture, including increased crypt depth, decreased migration of epithelial cells up the villus, and decreased expression of the tight junction protein claudin-3 compared to wild type mice [8]. Claudin-3 is an important sealing protein and its loss from colon tissue has been correlated with lack of tight junction integrity [11]. Mast cells have also been shown to mediate increased intestinal permeability caused by exposure to stress neuropeptides (e.g. corticotropin-releasing factor or sauvagine) in vitro [12]. Several studies have shown increased numbers of mast cells or increased release of mast cell mediators from actively inflamed colon of IBD patients compared with non-inflamed colon or normal controls [13], [14], [15], [16], suggesting a potential role for mast cells in the pathogenesis of IBD. However, specific in vivo data relating to mechanisms by which mast cells may influence IBD pathogenesis remains limited.
In view of the the well known role of mast cells in exacerbating inflammatory diseases such as arthritis, allergy, and asthma [17], [18], [19] and the potential of mast cells to contribute to one or more of the three factors that affect development of IBD, we hypothesized that mast cells might play a prominent role in the pathogenesis of IBD. We used a well-established model of IBD, based on IL-10-deficient mice with and without added mast cell deficiency to test this hypothesis.
Results
Mediator response is elevated in Il10−/− mast cells, but absence of mast cells does not affect the severity of IBD triggered by piroxicam in Il10−/− mice
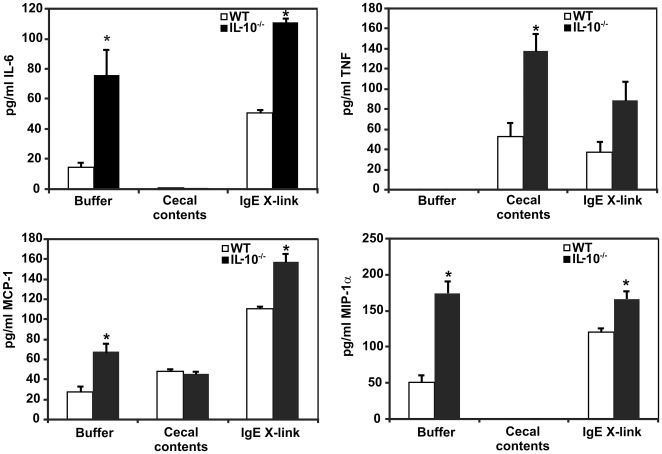
The purpose of these studies was to determine the role of mast cells in the pathogenesis of IBD. Il10 −/− mice were used, since they are highly susceptible to developing IBD when subjected to conditions that enhance mucosal permeability. Since IL-10 can also directly affect the function of immune cells including mast cells [20], bone marrow-derived mast cells (BMMC) from wild-type (WT) and Il10 −/− mice were first compared to determine how IL-10 deficiency affected the ability of BMMC to produce other inflammatory mediators. Similar mast cell survival and % degranulation in vitro were observed for WT vs. Il10 −/− BMMC after the treatment with IgE + cross-linking with anti-IgE. Exposure to enteric bacteria (cecal contents) did not cause significant degranulation of either wild type or Il10 −/− mast cells and also did not affect mast cell survival (data not shown). However, Il10 −/− BMMC produced higher baseline levels of IL-6, MCP-1, and MIP-1α in the absence of stimulation (buffer-treated cells) than did WT BMMC (Figure 1). Levels of IL-6 and MCP-1 secretion following IgE-induced degranulation were further increased in Il10 −/− BMMC compared with WT BMMC (Figure 1). Exposure of BMMC to enteric bacteria (cecal contents) triggered a pattern of cytokine production distinct from that triggered by IgE stimulation, with increased production of tumor necrosis factor (TNF) by Il10 −/− compared with WT BMMC (p<0.05), but decreased or unchanged production of MCP-1, IL-6, and MIP-1α (Figure 1). The increased baseline and stimulated production of pro-inflammatory cytokines by Il10 −/− BMMC to both classic (e.g. IgE) and IBD-relevant (e.g. enteric bacteria) activation stimuli in vitro suggested that Il10 −/− mast cells might be particularly potent in stimulating inflammatory reactions in the gut following breakdown of the mucosal barrier in vivo.
Figure 1. BMMC cytokine production after stimulation with IgE or enteric bacteria.
BMMC were stimulated for 4 hrs, then media was harvested for cytokine analysis via Luminex bead-based fluorescent immunoassays. Il10 −/− BMMC make more IL-6, MCP-1, and MIP-1α constitutively (buffer treatment) and following IgE stimulation than WT BMMC (p<0.05). Exposure to enteric bacteria in cecal contents triggers a different pattern of cytokine expression, with higher TNF production by Il10 −/− vs. WT BMMC. Data shown is the mean ± SEM for 3–5 independent experiments. * indicates p≤0.05 vs. WT.
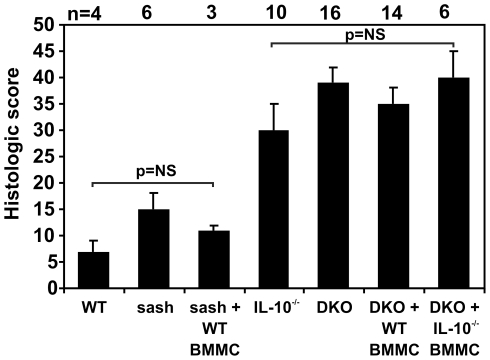
Exposure to the non-steroidal anti-inflammatory agent (NSAID) piroxicam uniformly triggers the development of IBD in Il10 −/− mice by enhancing apoptosis of mucosal epithelial cells, resulting in barrier breakdown that massively exposes immune cells in the lamina propria to bacterial antigens [5]. Mice variously deficient in IL-10 and mast cells received 200 ppm piroxicam for 7 days and then were observed for 16 additional days prior to assessment of colon inflammation. Wild type mice did not develop chronic colitis when exposed to piroxicam (mean histologic scores ± SEM = 7±2; Figure 2). Mast cell-deficient sash mice also did not develop colitis following piroxicam exposure, either with or without reconstitution with WT BMMC (Figure 2). As reported previously [5], IL-10-deficient mice developed moderate to severe colitis when exposed to piroxicam (mean histologic scores ± SEM = 30±5; Figure 2). Il10 −/− mice with mast cell deficiency due to the KitW-sh/KitW-sh mutation (DKO mice), DKO mice reconstituted with WT BMMC, and DKO mice reconstituted with Il10 −/− BMMC also developed moderate to severe colitis when exposed to piroxicam, with severity that statistically did not differ from that seen in mast cell-sufficient Il10 −/− mice (p = 0.14, 0.34, and 0.93, respectively; Figure 2). Thus, even though Il10 −/− mast cells produce elevated levels of inflammatory mediators when stimulated in vitro, the absence of mast cells does not affect the severity of IBD triggered by exposure of Il10 −/− mice to the mucosal barrier-damaging NSAID piroxicam.
Figure 2. Mast cells do not affect the severity of colitis triggered by piroxicam.
Mice of the indicated genotypes ± reconstitution with WT or Il10 −/− BMMC received 200 ppm piroxicam ×7 days to trigger the onset of colitis. Tissue was harvested for histologic analysis 16 d later. WT or sash mice ± reconstitution with WT BMMC did not develop colitis in this study. Mice deficient in IL-10 uniformly developed colitis, however the presence or absence of mast cells and whether or not the mast cells could produce IL-10 did not affect the severity of inflammation.
Mast cells modulate production of pro-inflammatory mediators in vivo, but absence of mast cells does not affect the severity of helicobacter-triggered IBD in Il10−/− mice
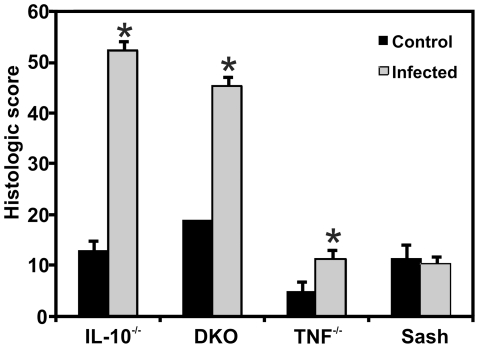
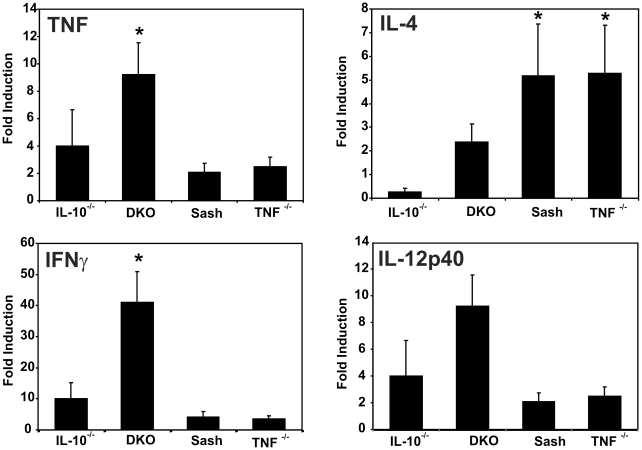
In vivo activation responses of mast cells may differ from their responses in vitro due to the complexities of the in vivo colonic microenvironment. To address this issue, we determined the effect of mast cells on the inflammatory milieu in IBD-susceptible and control mice with mucosal barrier compromise due to helicobacter infection. Colon tissues from Il10 −/−, DKO, sash, and TNF-deficient mice with and without co-infection by H. typhlonius and H. rodentium were analyzed for inflammation severity and for the production of selected cytokines by real time reverse transcriptase polymerase chain reaction (PCR) at 4–5 wks after infection. At this time point, Il10 −/− and DKO mice have severe chronic colitis, while Tnf −/− and mast cell-deficient sash mice do not (Figure 3). DKO mice had significantly increased production of mRNA encoding TNF and interferon (IFN)-γ, with strong trends toward increased IL-4 and IL-12/23p40 compared with mast cell-sufficient Il10 −/− mice (Figure 4). Elevated levels of IL-4 were also seen in sash mice singly deficient in mast cells and in TNF-deficient mice that do not develop colitis under these conditions, compared with Il10 −/− mice that develop severe colitis (Figure 4). Thus, tissue from DKO mice with colitis showed increased production of pro-inflammatory cytokines compared with mice singly deficient in IL-10, mast cells, or TNF. This demonstrates that mast cells play a role in down-regulating production of pro-inflammatory cytokines within the inflamed colon, at this relatively early time point in the course of chronic colitis.
Figure 3. Mast cells do not affect early colitis severity in Helicobacter-infected Il10 −/− mice.
Both IL-10-deficient and DKO mice exhibited severe colonic inflammation when infected with H. rodentium and H. typhlonius for 30 d (n = 3 for Ill0 −/− and n = 4 for DKO mice) compared to Tnf −/− and sash mice (n = 4 each) (p<0.001 for both Il10 −/− and DKO vs. Tnf −/− and sash). * indicates p<0.01, comparing control (non-infected) and infected mice of a given genotype.
Figure 4. Mast cells modulate pro-inflammatory cytokine mRNA expression in inflamed cecal tissues.
Cytokine mRNA expression in cecal tissue of mice of the indicated genotypes harvested after 5 weeks of infection with H. rodentium and H. typhlonius was measured by real-time PCR, normalized to β-actin and expressed as fold-induction relative to non-infected cecal tissue of the same genotype. Bars represent 5–6 mice per genotype. * indicates p<0.05 compared to Il10 −/− mice. Infected DKO mice have increased expression of Th1 cytokines (TNF, IFN-γ) and a trend towards increased Th2 (IL-4) and Th17-inducing cytokines (IL-12/23p40) compared with mast cell-sufficient Il10 −/− mice.
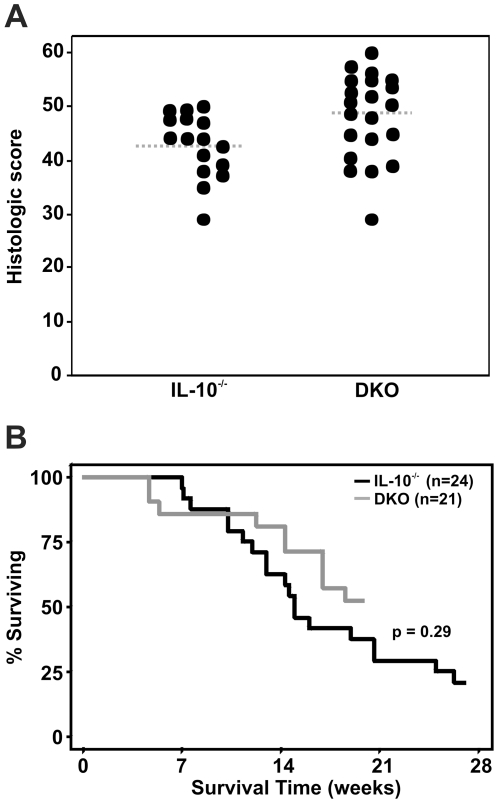
To determine how mast cells affected the long-term severity of IBD in helicobacter-infected mice that had sustained mucosal barrier dysfunction, Il10 −/− and DKO mice were infected with H. rodentium and H. typhlonius and monitored for up to 27 wks post-infection. Infected Il10 −/− and DKO mice both demonstrated severe colitis that was slightly increased in the absence of mast cells (mean histologic score = 43±1 in Il10 −/− mice vs. 48±2 in DKO mice; p = 0.03) (Figure 5A). However, this slight difference in colitis severity did not translate into a difference in survival based on presence or absence of mast cells (p = 0.29; Figure 5B).
Figure 5. Mast cells do not affect long-term colitis severity in Helicobacter-infected Il10 −/− mice.
Il10 −/− mice with (n = 21) and without (n = 24) genetic deficiency of mast cells were infected with H. rodentium and H. typhlonius and monitored for up to 27 wks. DKO mice had a small increase in histologic score compared with mice deficient in IL-10 alone (A; p = 0.03), but there was no difference in survival between DKO and mast cell-sufficient Il10 −/− mice (B).
Mast cells do not affect inflammation-associated colonic neoplasia in helicobacter-infected Il10−/− mice
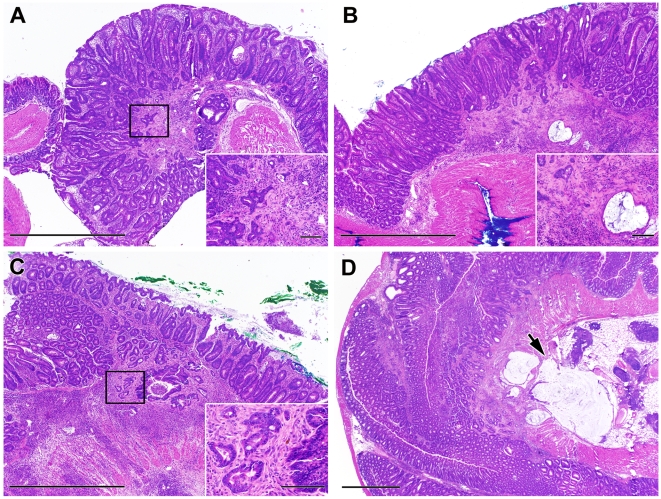
Colonic inflammation has previously been shown to predispose to the development of malignancy, both in humans and in murine models of IBD [6], [21]. Mast cells have also been shown to affect the incidence and severity of neoplasia in models of sporadic colon cancers [22], [23]. Therefore, the effect of mast cells on inflammation-associated neoplasia was also investigated in Il10 −/− mice with colitis triggered by helicobacter infection. Sixty three percent of Il10 −/− mice (n = 24) developed colorectal neoplasia by 18±1 weeks after IBD triggering, with an average of 3±2 neoplastic lesions per mouse (range = 1–6) (Figure 6A and B). Similarly, 62% (n = 21) of DKO mice had colorectal neoplasia 17±1 weeks after IBD triggering, with an average of 2±1 neoplastic lesions per mouse (range = 1–6) (Figure 6C and D). Half of these neoplastic lesions were invasive at the time of detection in both groups. Thus mast cells do not affect the incidence of inflammation-associated neoplasia in Il10 −/− mice with severe long-standing compromise of the mucosal barrier due to helicobacter infection.
Figure 6. Colon histology and neoplasia in Helicobacter-infected and non-infected Il10 −/− and DKO adult mice.
Helicobacter-infected Il10 −/− (A, B) and DKO mice (C, D) uniformly exhibited mucosal hyperplasia with prominent inflammatory infiltrates. Examples of neoplastic lesions seen in the long-term study are shown. The arrow in D indicates malignant glands that have invaded into the serosa. Bar represents 1 mm in the large panels and 100 µm in the insets.
Absence of mast cells predisposes to spontaneous development of IBD in Il10−/− mice
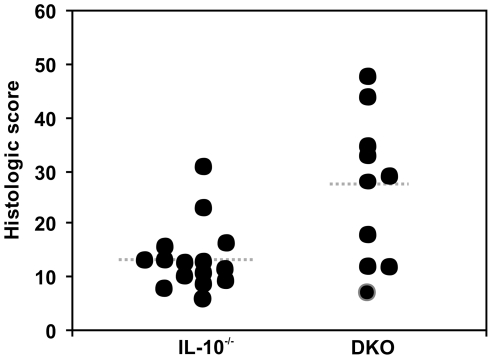
Since we did not see any contributory role for mast cells in actively induced inflammatory responses in gut of Il10 −/− mice, we investigated whether mast cells had a role in the spontaneous development of IBD in Il10 −/− vs. DKO mice. All mice were documented to remain free of helicobacter infection throughout the study. Whereas most mast cell-sufficient Il10 −/− mice did not develop colitis by 9 months of age (mean histologic score ± SEM = 13±2), most DKO mice developed at least moderate colitis (mean ± SEM histologic score = 27±4) (Figure 7). These DKO mice were significantly younger (31±2 wks; range = 25–38 wks; n = 10) than the Il10 −/− mice studied (36±1 wks; range 34–36 wks; n = 15; p = 0.02) due to more frequent occurrence of rectal prolapse (an indicator of severe IBD) in DKO mice that led to euthanasia for humane reasons prior to the planned study endpoint. Overall, these results demonstrate that mast cells protect Il10 −/− mice against the development of spontaneous colitis.
Figure 7. Severity of spontaneous IBD in specific pathogen-free Il10 −/− and DKO mice.
Il10 −/− mice on the C57BL/6 background rarely developed spontaneous colitis by 36 weeks of age when housed under clean conditions, free from specific pathogens including Helicobacter spp. (mean histologic scores ± SEM = 13±2; n = 15). In contrast, DKO mice that lacked mast cells frequently developed spontaneous colitis (mean histologic scores = 27±4; n = 10; p = 0.02), which was evident at an earlier age (31±2 wks vs. 36±1 wks; p = 0.02) compared with Il10 −/− mice.
Mast cells enhance epithelial barrier function
Il10 −/− mice have defects in immune regulation due to their genetic deficiency of IL-10 and their intestines contain potentially colitogenic antigens and adjuvants derived from their commensal intestinal microbiota. The three factor model presented earlier thus predicts that colitis will develop when the mucosal barrier is compromised, a prediction supported by the ability of NSAIDs and helicobacter infection to trigger colitis in these mice. Our finding that absence of mast cells predisposed to spontaneous development of IBD in Il10 −/− mice suggested that absence of mast cells might also decrease the effectiveness of the mucosal barrier.
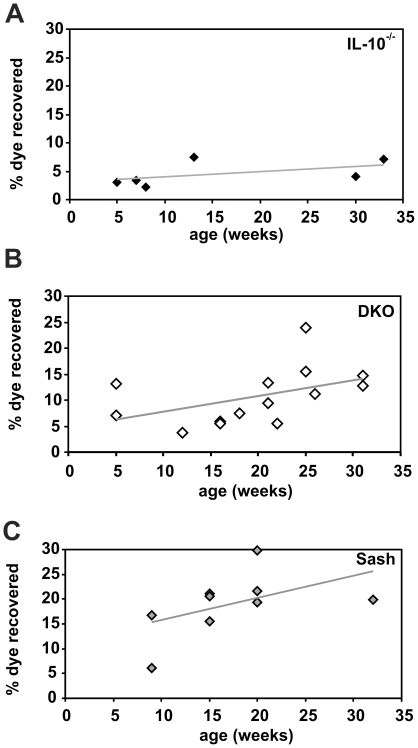
A quantitative assessment of barrier function was performed to determine the effect of mast cells on intestinal permeability. Phenol red dye was administered orally to Il10 −/− vs. DKO mice in vivo and the amount of dye recovered in urine was measured as an indicator of intestinal paracellular permeability. DKO mice exhibited higher permeability as indicated by increased dye recovery (Figure 8B) compared to Il10 −/− mice (Figure 8A; p = 0.02). Histologic scores indicated minimal to no inflammation in the animals tested (mean histologic score ± SEM = 7±1 (n = 33) for DKO and 15±3 (n = 18) for Il10 −/− mice). The increased intestinal permeability of DKO mice was due to the KitW-sh mutation that results in absence of mast cells, since it was also seen in sash mice that lacked mast cells, but were wild type with respect to IL-10 expression (Figure 8C). Taken together, these studies indicate that mice that lack mast cells have increased intestinal permeability. Furthermore, the data clearly show that the decreased barrier integrity observed in DKO relative to Il10 −/− mice occurs prior to, rather than in response to, the development of colon inflammation.
Figure 8. Permeability of the intestinal barrier in IL-10−/− and DKO mice.
Mice of the indicated ages and genotypes were gavaged with phenol red and the amount of dye absorbed was determined by spectrophotometric measurement of urine. Each point shown represents the average of 2–4 mice tested in a single experiment. Mast cell-deficient DKO and sash mice had significantly increased dye recovery compared with Il10 −/− mice (p = 0.02 for differences in data point elevation via linear regression analysis). The total numbers of mice tested were: Il10 −/− (n = 14), DKO (n = 18), and sash (n = 22).
Discussion
Although mast cells are best known for their pathogenic over-responses in immune-mediated diseases such as allergy and asthma, their protective role in innate immune responses is underscored by high susceptibility of mice deficient in mast cells to fatal infections with enteric bacteria [24], [25]. Mast cells aid the development of antigen-specific cellular and humoral adaptive immune responses by stimulating lymph node swelling and sequestration [26]. The data presented here suggest that mast cells also enhance intestinal mucosal barrier function in vivo and can protect against spontaneous development of IBD in susceptible mouse strains.
Intestinal epithelial barrier function is regulated by epithelial cells, innate and adaptive immunity, and the enteric nervous system and dysregulation of any of these alters the barrier [27]. Several mast cell-derived mediators, including histamine, serotonin, arachidonic acid, and mast cell proteinases have been shown to affect intestinal epithelial function, including ion channel conductance and activation/inhibition of secretion of electrolytes and mucus [8], [27], [28]. Our results show that mast cell-deficient sash and DKO mice had increased the baseline paracellular permeability of the intestine in vivo, independent of IL-10 status (Figure 8).
In addition to increased intestinal permeability, the data presented also show that mast cell-deficient DKO mice are at increased risk for spontaneous development of IBD compared to mast cell-sufficient Il10 −/− mice. These results parallel previous studies in humans demonstrating that patients with IBD and 10–25% of their first degree relatives have increased paracellular permeability relative to controls and that relapse of quiescent IBD was often preceded by increased intestinal permeability [29], [30], [31]. Interestingly, the Il10 −/− mice in our study had low gut permeability even at the relatively advanced ages of 30–35 wks. Others have shown that Il10 −/− mice develop increased gut permeability prior to development of colitis [32]. However, the lack of barrier dysfunction in the 30–35 wk old Il10 −/− mice reported here (Figure 8) correlates very well with the low incidence of spontaneous colitis (2 of 15 = 13%) that we observed in 36 wk old Il10 −/− mice (Figure 7).
A recent report by Groschwitz et al [8] showed that jejunal tissue from mast cell-deficient mice had decreased baseline permeability ex vivo compared to jejunal tissue from wild type mice. Our data differs since it represents an integration of the permeability of non-manipulated tissues throughout the gastrointestinal tract of live mice. The permeability characteristics of small intestine and colon are known to be different with respect to water as well as a variety of nutrients. Our in vivo studies also include potential contributions from different mast cell subsets that may be present in different parts of the intestinal tract, bacterial-mucosal interactions, leukocyte trafficking, and transient inflammation that can also affect net intestinal permeability in vivo. It is also possible that mast cells contribute differentially to homeostatic versus inflammation-induced barrier function. For example, during the normal non-inflamed state, mast cells may increase permeability (as reported by Groschwitz et al. [8]) to optimize small intestinal absorption of nutrients and antigens and to facilitate cellular interaction important for both digestive and immune system functions. However, mast cells may limit inflammation-induced barrier dysfunction by rapidly responding to pathogens that cross the mucosal barrier. The specific types of mast cells present and the quantity and type of mediators they release upon activation are likely to be critical in regulating their net effect [33].
We found that mast cell-sufficient Il10 −/− mice demonstrated decreased mucosal production of several pro-inflammatory cytokines (TNF, IFNγ, and IL12/23p40) compared with mice singly deficient in IL-10, mast cells, or TNF (Figure 4). Several previous studies using murine models have shown these pro-inflammatory cytokines can directly increase intestinal epithelial permeability by inducing disassembly of epithelial cell tight junctions (reviewed in [1]). Based on current understanding of tight junction function, the decrease in claudin-3 documented in intestinal tissues from mast cell-deficient sash mice [8] would be expected to increase the in vivo permeability of their intestinal tissues in vivo [11]. Perturbation of tight junctions and increased mucosal permeability and activation of the mucosal immune system have been shown to affect the severity of mucosal inflammation in humans [34], [35], [36]. However, since effect of mast cells on the mucosal barrier likely results from a balance of multiple effector and immunoregulatory functions [10], more studies will be required to precisely determine the mechanisms involved.
The KitW-sh allele that renders sash mice mast cell-deficient contains an inversion that abolishes Kit expression in mast cells and melanocytes but does not affect Kit expression in most other cell types [37]. Recent work has further identified that this inversion also inhibits the production of corin, a proteinase responsible for the activation of atrial natriuretic peptide [38]. It is also possible that the increased permeability may be due to this or to other factors in sash mice that render them susceptible to inflammation independent of their mast cell deficiency. However, we note that, despite their increased intestinal permeability, mice bearing the KitW-sh mutation alone do not have added susceptibility to IBD in the absence of concomitant IL-10 deficiency. Furthermore, reconstitution of Il10 −/− mice also homozygous for the KitW-sh mutation (DKO) mice with mast cells does not affect IBD severity (Figures 2 and 3).
Since mast cells protect Il10 −/− mice from spontaneous IBD, their failure to influence the severity of inflammation triggered by exposure of Il10 −/− mice to piroxicam or co-infection with H. rodentium and H. typhlonius may seem paradoxical at first glance. However, this result is predicted by the three factor model of IBD pathogenesis presented earlier [1]. Il10 −/− mice have bacteria within their intestine and their IL-10 deficiency results in defective immunoregulation. The model thus predicts that colitis will develop when the mucosal barrier is compromised. Exposure to piroxicam or infection with helicobacter already compromises mucosal barrier sufficiently to result in colitis. Thus further compromise of the barrier by mast cell deficiency would be predicted to have little to no additional effect, as was observed experimentally. Additional studies using mice with mast cell deficiency and/or alterations in intestinal permeability derived via other mechanisms will be useful for further confirming (or disproving) this hypothesized mechanism.
We also found that the presence or absence of mast cells had no effect on the incidence or progression of inflammation-associated colon cancers in Il10 −/− mice with colitis. There is currently controversy in the literature regarding the role of mast cells in tumor progression in the colon. Mice with a genetic susceptibility to intestinal neoplasia (ApcMin) had a greater frequency and size of adenomas in the absence of mast cells, suggesting a protective function for mast cells in colorectal carcinogenesis [23]. However, another report [22] showed recently that mast cells were required for the growth of adenomatous polyps in mice. Of note, both of these studies used different strains of mice genetically susceptible to polyp formation in models with minimal inflammation. Mast cells may thus have varying effects on the inflammatory milieu in these models based on any additional environmental triggers of intestinal inflammation that may be present.
In summary, our studies suggest that mast cells have primarily a protective role within the colonic microenvironment, serving to enhance barrier function and limit spontaneous inflammation. However, the presence of mast cells is insufficient to affect the severity of IBD triggered by massive disruption of the mucosal barrier and does not affect the development and progression of colorectal neoplasia associated with severe IBD.
Materials and Methods
Ethics Statement
All animal studies were approved by the Institutional Animal Care and Use Committee of Duke University, an institution accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International. The protocol numbers covering this work were A225-06-07 and A151-09-05.
Animal Studies
Breeding pairs of IL-10-deficient mice (strain name = B6.129P2-Il10tm1Cgn/J; stock # 002251), sash mice (strain name = B6.Cg-KitW-sh/HNihrJaeBsmJ; stock #005051), mice transgenic for global expression of green fluorescent protein (GFP) (strain name = C57BL/6-Tg(UBC-GFP)30Scha/J; stock # 004353), and mice deficient in TNF (strain name = B6.129S6-Tnftm1Gkl/J; stock # 005540) were obtained from Jackson Laboratories (Bar Harbor, ME). IL-10-deficient mice have been documented to be highly susceptible to developing IBD, either spontaneously or in response to triggers such as infection with intestinal helicobacter species or exposure to NSAIDs that damage their mucosal barrier [4], [5], [6], [39]. The KitW-sh allele contains an inversion located proximal to the Kit locus that abolishes Kit expression in mast cells and melanocytes but does not affect Kit expression in germ cells or erythrocytes [37]. Thus, although sash mice are white and lack mast cells, they can be propagated as homozygotes with normal litter sizes [40]. Il10 −/− and sash strains were crossed to generate DKO mice that were IBD-susceptible and lacked mast cells. All of these strains were on the C57BL/6 background.
Mice were housed in polycarbonate micro-isolator cages in individually ventilated racks under BSL-2 conditions, with access to food and water ad libitum. Mice were observed daily for clinical signs of distress and weight was monitored three times per week. Humane endpoints included >15% loss of body weight and development of rectal prolapse, a well-recognized complication of chronic inflammation in the colon.
Sentinel mice exposed repetitively to dirty bedding from the mice used in this study were negative for parasites by microscopic exam, negative for Citrobacter rodentium by fecal culture, and negative by serology for a panel of 22 murine protozoal, bacterial, and viral pathogens, including murine parvovirus, murine hepatitis virus, and murine norovirus. All mice not intentionally infected with Helicobacter spp. were confirmed to be Helicobacter-free by PCR of feces using a species-specific primer.
Models of Inflammatory Bowel Disease
For studies of spontaneous colitis, cohorts of mice were housed under specific pathogen-free conditions and euthanized for histologic scoring of colon inflammation if they experienced >15% loss of body weight, rectal prolapse, or reached pre-determined time points. Some cohorts of mice were exposed to 200 ppm piroxicam in powdered LabDiet 5001 chow (Purina, Framingham, MA) for 7 days to trigger the onset of colitis as previously described [6]. Based on measured food consumption, the dose of piroxicam averaged 40 mg/kg/day (range 21–62 mg/kg/day over 35 cage-days). The mice were then placed back on pelleted 5001 chow without piroxicam and observed for an additional 16 days before euthanasia for histologic scoring of colon inflammation. For studies of colitis triggered by helicobacter infection, 6–8 wk old mice were infected with H. typhlonius (clinical isolate DU-01) [41] and H. rodentium (MIT 95–1707 = ATCC type strain 700285) [42] by gavage of a single dose of 500 µl culture (generally 108 organisms) as previously described [6]. Infection was confirmed 7 days post-infection (PI) by quantitative real time PCR of feces (see below). Similar numbers of helicobacter organisms were present in feces on day 7 post-infection for Il10 −/− mice infected with 104, 106, or 108 organisms and all infected mice developed severe colitis (data not shown). Thus the severity of colonic inflammation was insensitive to inoculum size over the range of 104–108 helicobacter organisms due to their rapid in vivo multiplication. Mice were euthanized by CO2 asphyxiation in accordance with the American Veterinary Medical Association Recommendations on Euthanasia if they developed 15% body weight loss, rectal prolapse, or at 25–36 wks after infection. All mice in this study were evaluated pathologically for both colitis and neoplasia.
Tissue collection
After euthanasia, the digestive tract from stomach to anus was removed and divided into segments representing the stomach, cecum, and proximal, mid-, distal, and terminal colon/rectum. Portions of each gastrointestinal segment were rinsed briefly with PBS to remove non-adherent organisms. Tissues for molecular analysis were immediately frozen and stored at -20°C for subsequent quantitation of mRNAs or associated Helicobacter organisms by quantitative real-time PCR. The remaining tissues were fixed in Carnoy's solution for 2–4 hrs, then processed and embedded into paraffin.
Histological Scoring
The severity of colonic inflammation and incidence of colon neoplasia seen in hematoxylin and eosin-stained sections was scored by a board-certified pathologist blinded to experimental group. Histologic scores were calculated as described ([43]; modified from [44]), using a scale that takes into account mucosal changes in 5 different bowel segments, including hyperplasia and ulceration, degree of inflammation, and % of each bowel segment affected by these changes. Using this scale, the maximum score is 75 and a score >12 indicates the presence of colitis.
Neoplastic lesions were classified according to a consensus report for intestinal neoplasia in mouse models as gastrointestinal intraepithelial neoplasia or invasive carcinoma [45]. The category of gastrointestinal intraepithelial neoplasia is synonymous with atypical hyperplasia, atypia, microadenoma, carcinoma in situ, and dysplasia, which are non-invasive neoplastic lesions. A diagnosis of invasive carcinoma required the presence of a desmoplastic response to differentiate invasion from mucosal herniation or pseudoinvasion. Regions of neoplasia that were separated by regions of normal mucosa were scored as separate lesions.
Real-time PCR Assays
For detection of helicobacter organisms, DNA was extracted from 40 mg frozen tissue or 20 mg feces using the DNeasy Tissue kit (Qiagen, Valencia, CA). Real-time PCR was performed to quantify the relative concentrations of fecal and mucosa-associated H. rodentium and H. typhlonius organisms per milligram of feces or tissue based on comparison with a standard, as described previously [6].
For analysis of cytokine expression, total RNA was extracted from ∼30 mg cecal tissue using the RNA extraction kit (Ambion, Austin, TX). Complementary DNA (cDNA) was synthesized using 1 μg of RNA through a reverse transcription reaction (Applied Biosystems, Foster City, CA). Real-time PCR quantitative mRNA analyses were performed using SYBR green fluorescence (Stratagene, Cedar Creek, TX). The standard PCR conditions were 95°C for 10 min, 40 cycles for 1 min at 94°C, 56°C for 1 min and 72°C for 2 min, followed by the standard denaturation curve. The primer sequences used in this analysis were modified from Cardoso et al. [46] and were as follows: β-actin (fwd, 5′-AGT TGC GTT TTA CAC CCT TT-3′; rev, 5′-AAG CCA TGC CAA TGT TGT CT-3′), IL-4 (fwd, 5′-CTG ACG GCA CAG AGC TAT TGA-3′; rev, 5′-TAT GCG AAG CAC CTT GGA AGC-3′), TNF (fwd, 5′-TGT GCT CAG AGC TTT CAA CAA-3′; rev, 5′-CTT GAT GGT GGT GCA TGA GA-3′), IL-12p40 (fwd, 5′-AGC ACC AGC TTC TTC ATC AGG-3′; rev, 5′-GCG CTG GAT TCG AAC AAA G- 3′), IFN-γ (fwd,5′-GCA TCT TGG CTT TGC AGC T-3′ ; rev, 5′-CTT TTT TCG CCT TGC TGT TG-3′). SYBR Green PCR Master Mix (Stratagene, Cedar Creek, TX), specific primers, and cDNA template were used in each reaction. The relative mRNA amount of each sample was calculated based on its threshold cycle, Ct, in comparison to the Ct of housekeeping gene β-actin. The results for cytokine mRNAs were demonstrated as mRNA expression relative to non-infected mice of the same genotype. The purity of amplified product was determined as a single peak of dissociation curve. Negative controls without RNA were also performed.
Culture and In vitro Stimulation of Mast Cells
To obtain BMMC, mouse femurs were flushed with RPMI 1640 media using a 22G needle and 10 ml syringe. The bone marrow cells were cultured for 4 weeks in RPMI 1640 (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum, essential and non-essential amino acids, 100 U/ml penicillin, 100 µg/ml streptomycin, 10 mM HEPES, 5.5×10−5 M 2-mercaptoethanol, 10 ng/ml stem cell factor, and 5 ng/ml IL-3 (R&D Systems, Minneapolis, MN) to stimulate mast cell differentiation. Non-adherent cells were harvested to flasks containing fresh media once weekly. The percentage of mast cells was determined by performing a differential on a cytocentrifuge preparation stained with a modified Wright-Giemsa stain; % of mast cells averaged 95%. 107 BMMC in 100 µl PBS were injected intraperitoneally to reconstitute mast cell-deficient mice, which were used 12 wks after reconstitution.
The in vitro reactivity of BMMC derived from C57BL/6 wild type or Il10 −/− mice was compared by measuring degranulation, viability, and cytokine production post-degranulation. BMMC (1×106 cells/well) in Tyrode's buffer were sensitized by overnight incubation with 1 µg/ml IgE then stimulated by cross-linking with 1 µg/ml goat anti-mouse IgE (BD, San Jose, CA) or by incubation with a mixture of enteric bacterial antigens prepared as 100 mg cecal contents per ml of saline or Tyrode's buffer then filtered at 0.2 µm. Degranulation was assessed by β-hexoseaminidase activity using a colorimetric substrate assay as previously described [47]. Viability after degranulation was measured using a tetrazolium-based colorimetric assay (CellTiter96 Aqueous, Promega, Madison, WI). Media was harvested from some wells 4 hrs after stimulation for measurement of cytokine content using a Luminex bead-based multiplex fluorescent immunoassay (BioRad, Hercules, CA).
Intestinal permeability
The permeability of the mucosal layer was assessed using phenolsulfonphthalein (phenol red) [48]. Under normal conditions, phenol red is not absorbed in the intestine. However under conditions of increased intestinal permeability, the dye is absorbed then excreted in the urine. The urinary dye recovery was measured after administration of 2 µmol (∼700 µg) phenol red in 0.5 ml of saline by oral gavage. Groups of 2–4 animals were placed in metabolic caging that is optimized to separate urine from feces. Urine was collected for 18 hrs, alkalinized extracts were prepared, and the phenol red content of each sample was determined spectrophotometrically at 560 nm by comparison to standards of known concentration. The urinary recovery of phenol red was expressed as percent of the dose administered.
Statistical Analysis
Statistical comparison of groups was performed using Student's t-test. Survival rates were calculated using Kaplan-Meier test with p-values calculated using the log rank test. Linear regression was performed using GraphPad prism software. A value of p≤0.05 was considered to be significant.
Acknowledgments
The authors would like to thank Paula K. Greer and Chau T. Trinh for expert technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by the National Institutes of Health R21-DK075522 and 1R01-CA115480. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chichlowski M, Hale LP. Bacterial-mucosal interactions in inflammatory bowel disease—an alliance gone bad. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1139–1149. doi: 10.1152/ajpgi.90516.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sollid LM, Johansen F-E. Animal Models of Inflammatory Bowel Disease at the Dawn of the New Genetics Era. PLoS Med. 2008;5:e198. doi: 10.1371/journal.pmed.0050198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inaba Y, Ashida T, Ito T, Ishikawa C, Tanabe H, et al. Expression of the antimicrobial peptide alpha-defensin/cryptdins in intestinal crypts decreases at the initial phase of intestinal inflammation in a model of inflammatory bowel disease, IL-10-deficient mice. Inflammatory Bowel Diseases. 2010;9999:NA. doi: 10.1002/ibd.21253. [DOI] [PubMed] [Google Scholar]
- 4.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 5.Hale LP, Gottfried MR, Swidsinski A. Piroxicam treatment of IL-10-deficient mice enhances colonic epithelial apoptosis and mucosal exposure to intestinal bacteria. Inflamm Bowel Dis. 2005;11:1060–1069. doi: 10.1097/01.mib.0000187582.90423.bc. [DOI] [PubMed] [Google Scholar]
- 6.Chichlowski M, Sharp JM, Vanderford DA, Myles MH, Hale LP. Helicobacter typhlonius and Helicobacter rodentium differentially affect the severity of colon inflammation and inflammation-associated neoplasia in IL10-deficient mice. Comp Med. 2008;58(6):534–541. [PMC free article] [PubMed] [Google Scholar]
- 7.Hofmann AM, Abraham SN. New roles for mast cells in modulating allergic reactions and immunity against pathogens. Current Opinion in Immunology. 2009;21:679–686. doi: 10.1016/j.coi.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groschwitz KR, Ahrens R, Osterfeld H, Gurish MF, Han X, et al. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proceedings of the National Academy of Sciences. 2009;106:22381–22386. doi: 10.1073/pnas.0906372106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mekori YA, Metcalfe DD. Mast cell-T cell interactions. J Allergy Clin Immunol. 1999;104:517–523. doi: 10.1016/s0091-6749(99)70316-7. [DOI] [PubMed] [Google Scholar]
- 10.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thuijls G, Derikx JPM, Haan J-Jd, Grootjans J, Bruïne Ad, et al. Urine-based Detection of Intestinal Tight Junction Loss. Journal of Clinical Gastroenterology. 2010;44:e14–e19. doi: 10.1097/MCG.0b013e31819f5652. 10.1097/MCG.1090b1013e31819f35652. [DOI] [PubMed] [Google Scholar]
- 12.Santos J, Yates D, Guilarte M, Vicario M, Alonso C, et al. Stress neuropeptides evoke epithelial responses via mast cell activation in the rat colon. Psychoneuroendocrinology. 2008;33:1248–1256. doi: 10.1016/j.psyneuen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Fox CC, Lazenby AJ, Moore WC, Yardley JH, Bayless TM, et al. Enhancement of human intestinal mast cell mediator release in active ulcerative colitis. Gastroenterology. 1990;99:119–124. doi: 10.1016/0016-5085(90)91238-2. [DOI] [PubMed] [Google Scholar]
- 14.Raithel M, Matek M, Baenkler HW, Jorde W, Hahn EG. Mucosal histamine content and histamine secretion in Crohn's disease, ulcerative colitis and allergic enteropathy. Int Arch Allergy Immunol. 1995;108(2):127–133. doi: 10.1159/000237129. [DOI] [PubMed] [Google Scholar]
- 15.Heatley RV, Calcraft BJ, Rhodes J, Owen E, Evans BK. Disodium cromoglycate in the treatment of chronic proctitis. Gut. 1975;16:559–563. doi: 10.1136/gut.16.7.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nolte H, Spjeldnaes N, Kruse A, Windelborg B. Histamine release from gut mast cells from patients with inflammatory bowel diseases. Gut. 1990;31:791–794. doi: 10.1136/gut.31.7.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, et al. Mast Cells: A Cellular Link Between Autoantibodies and Inflammatory Arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 18.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamid Q, Tulic M. Immunobiology of Asthma. Annual Review of Physiology. 2009;71:489–507. doi: 10.1146/annurev.physiol.010908.163200. [DOI] [PubMed] [Google Scholar]
- 20.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48(4):526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, et al. Mast cells are an essential hematopoietic component for polyp development. Proceedings of the National Academy of Sciences. 2007;104:19977–19982. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinnamon MJ, Carter KJ, Sims LP, LaFleur B, Fingleton B, et al. A protective role of mast cells in intestinal tumorigenesis. Carcinogenesis. 2008;29:880–886. doi: 10.1093/carcin/bgn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Echtenacher B, Männel DN, Hültner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 25.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 26.McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, et al. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol. 2003;4:1199–1205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 27.Wood JD. Enteric neuroimmunophysiology and pathophysiology. Gastroenterology. 2004;127:635–657. doi: 10.1053/j.gastro.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Bischoff S. Physiological and pathophysiological functions of intestinal mast cells. Seminars in Immunopathology. 2009;31:185–205. doi: 10.1007/s00281-009-0165-4. [DOI] [PubMed] [Google Scholar]
- 29.Suenaert P, Bulteel V, Lemmens L, Noman M, Geypens B, et al. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn's disease. Am J Gastroenterol. 2002;97:2000–2004. doi: 10.1111/j.1572-0241.2002.05914.x. [DOI] [PubMed] [Google Scholar]
- 30.Peeters M, Geypens B, Claus D, Nevens H, Ghoos Y, et al. Clustering of increased small intestinal permeability in families with Crohn's disease. Gastroenterology. 1997;113:802–807. doi: 10.1016/s0016-5085(97)70174-4. [DOI] [PubMed] [Google Scholar]
- 31.Söderholm JD, Olaison G, Lindberg E, Hannestad U, Vindels A, et al. Different intestinal permeability patterns in relatives and spouses of patients with Crohn's disease: an inherited defect in mucosal defence? Gut. 1999;44:96–100. doi: 10.1136/gut.44.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, et al. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999;5(4):262–270. doi: 10.1097/00054725-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Weidner N, Austen KF. Ultrastructural and Immunohistochemical Characterization of Normal Mast Cells at Multiple Body Sites. J Investig Dermatol. 1991;96:26S–31S. [PubMed] [Google Scholar]
- 34.Gibson P, Rosella O, Nov R, Young G. Colonic epithelium is diffusely abnormal in ulcerative colitis and colorectal cancer. Gut. 1995;36:857–863. doi: 10.1136/gut.36.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, et al. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105(6):883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 36.Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, et al. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol. 2001;281:G216–228. doi: 10.1152/ajpgi.2001.281.1.G216. [DOI] [PubMed] [Google Scholar]
- 37.Berrozpe G, Timokhina I, Yukl S, Tajima Y, Ono M, et al. The Wsh, W57, and Ph Kit Expression Mutations Define Tissue-Specific Control Elements Located Between -23 and -154 kb Upstream of Kit. Blood. 1999;94:2658–2666. [PubMed] [Google Scholar]
- 38.Nigrovic PA, Gray DHD, Jones T, Hallgren J, Kuo FC, et al. Genetic Inversion in Mast Cell-Deficient Wsh Mice Interrupts Corin and Manifests as Hematopoietic and Cardiac Aberrancy. Am J Pathol. 2008;173:1693–1701. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berg DJ, Zhang J, Weinstock JV, Ismail HF, Earle KA, et al. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123:1527–1542. doi: 10.1053/gast.2002.1231527. [DOI] [PubMed] [Google Scholar]
- 40.Wolters PJ, Clair JM-S, Lewis CC, Villalta SA, Baluk P, et al. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient KitW-sh/KitW-sh sash mice. Clinical & Experimental Allergy. 2005;35:82–88. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hale LP, Perera D, Gottfried MR, Maggio-Price L, Srinivasan S, et al. Neonatal Co-Infection with Helicobacter Species Markedly Accelerates the Development of Inflammation-Associated Colonic Neoplasia in IL-10−/− Mice. Helicobacter. 2007;12:598–604. doi: 10.1111/j.1523-5378.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 42.Shen Z, Fox JG, Dewhirst FE, Paster BJ, Foltz CJ, et al. Helicobacter rodentium sp. nov., a urease-negative Helicobacter species isolated from laboratory mice. Int J Syst Bacteriol. 1997;47:627–634. doi: 10.1099/00207713-47-3-627. [DOI] [PubMed] [Google Scholar]
- 43.Hale LP, Greer PK, Trinh CT, Gottfried MR. Treatment with oral bromelain decreases colonic inflammation in the IL-10-deficient murine model of inflammatory bowel disease. Clinical Immunology. 2005;116:135–142. doi: 10.1016/j.clim.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Burich A, Hershberg R, Waggie K, Zeng W, Brabb T, et al. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2001;281:G764–778. doi: 10.1152/ajpgi.2001.281.3.G764. [DOI] [PubMed] [Google Scholar]
- 45.Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, et al. Pathology of mouse models of intestinal cancer: Consensus report and recommendations. Gastroenterology. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 46.Cardoso CR, Teixeira G, Provinciatto PR, Godoi DF, Ferreira BR, et al. Modulation of mucosal immunity in a murine model of food-induced intestinal inflammation. Clinical & Experimental Allergy. 2008;38:338–349. doi: 10.1111/j.1365-2222.2007.02866.x. [DOI] [PubMed] [Google Scholar]
- 47.Shin J-S, Shelburne CP, Jin C, LeFurgey EA, Abraham SN. Harboring of Particulate Allergens within Secretory Compartments by Mast Cells following IgE/Fc(epsilon}RI-Lipid Raft-Mediated Phagocytosis. J Immunol. 2006;177:5791–5800. doi: 10.4049/jimmunol.177.9.5791. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura J, Takada S, Ueda S, Hamaura T, Yamamoto A, et al. Assessment of pharmaceutical excipient-induced gastrointestinal mucosal damage in rats in vivo by measuring the permeation of phenolsulfonphthalein. Chem Pharm Bull. 1985;33(8):3527–3529. doi: 10.1248/cpb.33.3527. [DOI] [PubMed] [Google Scholar]