Abstract
Disruptions of glutamatergic and noradrenergic signaling have been postulated to occur in depressive disorders. Glutamate provides excitatory input to the noradrenergic locus coeruleus (LC). In this study, the location of immunoreactivity against neuronal nitric oxide synthase (nNOS), an intracellular mediator of glutamate receptor activation, was examined in the normal human LC, and potential changes in nNOS immunoreactivity that might occur in major depression were evaluated. Tissue containing LC, and a non-limbic, LC projection area (cerebellum) was obtained from 11 to 12 matched pairs of subjects with major depression and control subjects lacking major psychiatric diagnoses. In the LC region, nNOS immunoreactivity was found in large neuromelanin-containing neurons, small neurons lacking neuromelanin, and glial cells. Levels of nNOS immunoreactivity were significantly lower in the LC (− 44%, p < 0.05), but not in the cerebellum, when comparing depressed with control subjects. nNOS levels were positively correlated with brain pH values in depressed, but not control, subjects in both brain regions. Low levels of nNOS in the LC may reflect altered excitatory input to this nucleus in major depression. However, pH appears to effect preservation of nNOS immunoreactivity in subjects with depression. This factor may contribute, in part, to low levels of nNOS in depression.
Keywords: cerebellum, locus coeruleus, major depression, neuronal nitric oxide synthase
The locus coeruleus (LC) is the largest noradrenergic nucleus in the brain and projects to several cortical and subcortical structures. Previous observations reveal that depression is associated with altered concentrations of several noradrenergic proteins in the LC. For example elevated levels of tyrosine hydroxylase (TH) (Ordway et al. 1994a; Zhu et al. 1999), increased agonist binding to α2-adrenergic receptors (Ordway et al. 1994b; Ordway et al. 2003) and reduced levels of norepinephrine transporters (Klimek et al. 1997) were previously reported in major depressive subjects and in suicide victims. Based on these observations, it is tempting to hypothesize that monoaminergic abnormalities in depression might be induced by factors that drive LC activity, leading to compensatory changes in proteins.
LC neuronal activity is under the control of a number of excitatory inputs, including glutamate that originates predominately in the glutamatergic paragigantocellularis nucleus (Aston-Jones et al. 1991). Interestingly, several lines of evidence suggest a crucial involvement of the glutamate signaling pathway in the pathophysiology of depression and in the mechanism of action of antidepressant drugs. For example, NMDA receptor antagonists and nitric oxide synthase (NOS) inhibitors exhibit antidepressant-like properties in the animal-screening procedures (Moryl et al. 1993; Papp and Moryl 1994; Layer et al. 1995; Harkin et al. 1999; Skolnick 1999; Yildiz et al. 2000). Chronic treatment with a NOS inhibitor down-regulates β-adrenergic receptors in mice frontal cortex with a magnitude comparable to imipramine (Karolewicz et al. 1999). Moreover, ketamine, an NMDA receptor antagonist, exhibits antidepressant activity in humans (Berman et al. 2000).
Glutamate receptors that modulate LC activity include the NMDA receptor. Stimulation of NMDA receptors results in, at least in part, the activation of neuronal nitric oxide synthase (nNOS). The LC contains a high density of NMDA receptors (Shaw et al. 1992; Van Bockstaele and Colago 1996; Allgaier et al. 2001), NOS expressing cells (Xu et al. 1994; Cuellar et al. 2000), and a high density of soluble guanylyl cyclase (GC) (Xu et al. 1998; Vulliemoz et al. 1999), suggesting the existence of a glutamate/nitrergic transduction pathway. However, the exact cellular localization of these glutamate/nitrergic signaling proteins within the human LC is still unknown.
The aim of the present study was to examine the distribution of nNOS within the human LC, and to investigate potential changes in nNOS immunoreactive protein in the LC that might occur in subjects diagnosed with major depression. For the study of depression, subjects were matched closely for age, sex, and cigarette smoking history, and post mortem delay. Levels of nNOS were measured in the LC and cerebellum from 11 to 12 pairs of control and major depressive subjects. Additionally, the effect of confounding variables (such as age of subjects, post mortem delay, and brain pH) on protein levels in post mortem material was carefully evaluated. Brain tissue was collected from carefully screened subjects (post mortem) who were diagnosed retrospectively with major depression at the time of death and from subjects who lacked any major (axis I) psychiatric disorders (controls).
Materials and methods
Subjects
Tissues from 12 depressed subjects and 12 psychiatrically normal control subjects were obtained at autopsy at the Coroner's Office of Cuyahoga County, Cleveland, OH, USA. An ethical protocol approved by the Institutional Review Board of the University Hospitals of Cleveland was used and informed written consent was obtained from the next-of-kin for all subjects. Blood and urine samples from all subjects were examined by the coroner's office for psychotropic medications and substances of abuse.
Retrospective, informant-based psychiatric assessments were performed for all depressed and control subjects (see Tables 1 and 2 for information on all subjects). The Structured Clinical Interview for DSM-IV Psychiatric Disorders (SCID-IV) was administered to next-of-kin of the 10 of depressed subjects (First et al. 1996). A trained interviewer administered the Schedule for Affective Disorders and Schizophrenia: lifetime version (SADS-L) to knowledgeable next-of-kin of the two remaining depressed subjects. Axis I psychopathology was assessed and consensus diagnosis was reached in conference using information from the interview and medical records. The deaths of nine of the 12 depressed subjects were ruled a suicide by the coroner. No antidepressant drugs were detected in the post mortem toxicology screening of subjects in the present study (Table 1). The control subjects did not meet criteria for an axis I disorder at the time of their deaths. Blocks of brain tissue were dissected, frozen in dry-ice cooled isopentane and stored at − 80°C.
Table 1.
Vital data of subjects
| Subject | Age (years) | Brain pH | Sex | PMD (h) | Toxicology | Cause of death | Diagnosis |
|---|---|---|---|---|---|---|---|
| RRa,b | 37 | 6.47 | M | 17 | NDD | Acute hemorrhagic pancreatitis | None |
| VVa,b | 54 | 6.52 | M | 19 | Lidocaine | Hypertensive coronary sclerotic heart disease | None |
| FF1a,b | 27 | 6.88 | M | 17 | NDD | Gun shot, chest | None |
| HH1a,b | 54 | 6.87 | M | 17 | Brompheniramine | Heart disease | None |
| BB1a,b | 52 | 6.28 | M | 17 | NDD | Heart disease | None |
| KS6a,b | 43 | 6.58 | M | 22 | NDD | Crushing impacts to head, trunk and extremities | None |
| KS18a | 41 | 6.74 | F | 24 | Diazepam, chlorpheniramine, lidocaine | Heart disease | None |
| NDD | |||||||
| KS21a,b | 48 | 6.98 | M | 9 | NDD | Heart disease | None |
| KS23a,b | 58 | 6.78 | M | 21 | NDD | Heart disease | None |
| KS27a,b | 74 | 6.62 | M | 21 | NDD | Abdominal aortic aneurysm | None |
| IIa,b | 27 | 7.01 | F | 15 | NDD | Heart disease | None |
| KKa,b | 43 | 6.49 | M | 23 | Propoxyphene, norpropoxyphene, oxycodone | Pulmonary embolism | None |
| TTa,b | 38 | 6.52 | F | 24 | NDD | OD unspecified | MDD |
| WWa,b | 65 | 6.24 | M | 30 | Codeine | Gun shot | MDD |
| GG1a,b | 30 | 6.91 | M | 18 | NDD | Gun shot | MDD |
| JJ1a,b | 54 | 6.24 | M | 23 | CO, Phenobarbital Phenytoin | CO poisoning | MDD |
| DD1a,b | 52 | 6.48 | M | 18 | CO | CO poisoning | MDD |
| KS8a,b | 42 | 6.67 | M | 44 | NDD | Hanging | MDD |
| KS17a | 45 | 6.84 | F | 27 | NDD | Pulmonary embolism | MDD |
| KS12a,b | 41 | 6.24 | M | 19 | Chlorpheniramine | Heart disease | MDD |
| KS24a,b | 64 | 6.85 | M | 26 | NDD | Gun shot | MDD |
| KS28a,b | 81 | 6.78 | M | 33 | NDD | Drowning | MDD |
| JJa,b | 34 | 6.27 | F | 24 | CO, Alprazolam | CO poisoning | MDD |
| MMa,b | 36 | 6.96 | M | 20 | Diphenhydramine | Undetermined | MDD |
PMD, post mortem duration; MDD, major depressive disorder; NDD, no drugs detected; M, male; F, female. Subjects used in
locus coeruleus and
cerebellum study.
Table 2.
History of medication of subjects included in the study
| Subject | Drugs |
|---|---|
| RRa,b | Amlodipine, Ranitidine |
| VVa,b | Digoxin, dipyridamole |
| FF1a,b | None |
| HH1a,b | Clonazapam, Nicardipine, Dijoxin, Metaprolol, Prednisone, Simvastatin, Torsemide, Glipizide, Lisinopril, Ranitidine |
| BB1a,b | None |
| KS6a,b | None |
| KS18a | None |
| KS21a,b | None |
| KS23a,b | Digoxin |
| KS27a,b | Propranolol, Chlorthalidone |
| IIa,b | Captopril, Endurolyl, Enalapril maleate, Topral, Lopressor |
| KKa,b | Glyburide, Methylprednisolone, Propoxyphene |
| TTa,b | Elmiron, Lorazepam, Citalopram, Paroxetine |
| WWa,b | Unknown |
| GG1a,b | Unknown |
| JJ1a,b | Sertaline |
| DD1a,b | Sumatriptan, Loracarbef |
| KS8a,b | Paxil, Trazedone, Xanax, Accupro, Hydrochlorothiazide |
| KS17a | None |
| KS12a,b | None |
| KS24a,b | Lisinopril, Potassium, Spironolactone |
| KS28a,b | Sertaline, Prednisone |
| JJa,b | Alprazolam, Risperidone, Amoxicillin, Valproic Acid, Nitrofurintoin |
| MMa,b | Flomax, Etodolac, Ranitidine, Terazosin |
Subjects used in
locus coeruleus and
cerebellum study.
Dissection and anatomical positioning of measurement
Frozen tissue blocks were cut along the entire length of the LC, with histological sections taken at 1-mm intervals to evaluate anatomical position along the LC axis. The LC was punched from sections and punches were stored in microfuge tubes. The exact location of the rostral and caudal end of the LC was individualized for each subject based on Nissl staining and subsequent cell counting. The LC had its rostral border defined as a point with at least 25 ± 5 neuromelanin-containing cells identified. The caudal border was defined near the caudal end at a point where 25 ± 5 or less neuromelanin-containing cells were present. The whole LC was punched into 50-μm thick sections. Anatomical resolution was restricted to three positions along the LC axis: rostral, middle and caudal. For each anatomical level of LC, tissue was collected from 2-mm sections that were centered at points that represented 25, 50, and 75% of the total length of the LC. All results shown below were generated from the rostral, middle and caudal portion of the LC. For the study of cerebellum, several sections of the right cerebellar hemisphere weighing approximately 80 mg were collected into tubes and stored in − 80°C until assayed. From homogenized sections, cytoplasmic protein was extracted and used in the western blot assays for nNOS.
nNOS immunohistochemistry
Frozen tissue was sectioned (20 μm) and collected by thaw-mounting onto gelatin-coated glass slides. Sections containing LC were fixed in 4% paraformaldehyde in 0.05 m phosphate-buffered saline (PBS) for 1 h at room temperature, and preincubated in 5% normal horse serum in 0.05 m PBS (containing 0.1% Triton X-100 and 0.005% sodium azide) for 30 min. Sections were then incubated for 24 h at 4°C in the same solution containing mouse anti-nNOS monoclonal antibody (1 : 500; BD Transduction Laboratories/BD Biosciences, Lexington, KY, USA). The sections were subsequently washed in 0.05 m PBS, and incubated for 4 h at room temperature in biotinylated horse anti-mouse IgG (1 : 200) in 0.05 m PBS (Vector, Burlingame, CA, USA). The sections were then processed using the Vectastain ABC immunoperoxidase kit (Vector, Burlingame, CA, USA) for 24 h at 4°C. Antibody distribution was detected using 0.05% 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma, St. Louis, MO, USA) method. The sections were then dehydrated, cleared with xylene, and coverslipped.
nNOS immunoreactivity: western blots
LC and cerebellum samples were homogenized in ice-cold 10 mm HEPES buffer (pH 7.9) containing 10 mm KCl, 0.1 mm ethylenediaminetetraacetate (EDTA), 0.1 mm ethyleneglycol bis(aminoethylether)tetraacetate (EGTA), 1 mm dithiothreitol, 0.5 mm phenylmethylsulfonyl fluoride, and 0.5% nonylphenyl-polyethylene glycol (NP-40). Insoluble proteins were removed by centrifugation for 1 min at 12 000 × g. Protein concentration was determined using the bicinchoninic acid method (Pierce Biotechnology, Inc, Rockford, IL, USA). Samples were mixed with sample buffer containing: 0.125 m Tris base, 20% glycerol, 4% sodium dodecyl sulfate, 10% mercaptoethanol, 0.05% bromophenol blue, pH 6.8, and heated at 95°C for 6 min. Solubilized proteins (10 μg per lane) were separated by electrophoresis through a 7.5% sodium dodecyl sulfate–polyacrylamide gel and transferred to nitrocellulose membrane (Hybond ECL; Amersham Biosciences, Buckinghamshire, UK). Non-specific binding to the membranes was blocked with 5% non-fat dried milk/TBS (20 mm Tris base and 0.5 m NaCl, pH 7.5) for 2 h and then incubated overnight at 4°C with mouse anti-nNOS monoclonal antibody (diluted 1 : 1000; BD Transduction Laboratories/BD Biosciences). The anti-nNOS monoclonal antibody was raised against a carboxy end sequence (amino acids 1095–1289) of nNOS. As an additional verification of nNOS immunoreactivity, a polyclonal antibody (Chemicon, International Inc., Temecula, CA, USA) was used in randomly selected pairs of control/MDD subjects. Membranes were washed three times for 10 min in PBS buffer and incubated with secondary anti-mouse antibody (diluted 1 : 2000, Amersham Biosciences). After incubation, blots were washed three times for 15 min in TBS buffer and developed using enhanced chemiluminescence detection (ECL, Perkin-Elmer Life Sciences, Inc., Boston, MA, USA) and immediately exposed to film (Hyperfilm-ECL, Amersham Biosciences). As a control for transfer and loading, actin on each blot was detected using an anti-actin monoclonal antibody (Chemicon, International Inc., Temecula, CA, USA). Immunoreactivity of nNOS was investigated in 11–12 pairs of controls and matched depressive subjects. Each subject pair was immunoblotted twice, each time on a separate gel (gel duplicates).
Monoclonal and polyclonal antibodies used in the present study recognize a single band at approximately 155 kDa corresponding to nNOS (Bredt et al. 1991; Boissel et al. 1998; Wang et al. 1999). These antibodies have been used previously to identify nNOS in numerous regions of the human brain (Gerlach et al. 2001; Benavides-Piccione and DeFelipe 2003).
Tissue pH and effect of low pH on nNOS
The pH of cerebellar tissue from all subjects was determined as previously described by (Harrison et al. 1995). In order to examine the potential effect of low tissue pH on nNOS protein immunoreactivity, an in vitro experiment was performed. Lateral cerebellum tissue (1 g) from a control subject was homogenized in 10 volumes of distilled water at neutral pH, yielding two samples with a pH measured at 6.73. The pH of one of the samples was adjusted to 6.02 with lactic acid (0.01%, Sigma, St. Louis, MO, USA). Both samples of cerebellum (pH 6.73 and pH 6.02) were stored for 24 h at 4°C. After 24 h, cytoplasmic protein was extracted and nNOS immunoreactivity was measured using western blot method as described above.
Analyses of data
Band densities for nNOS and actin were analyzed using imaging software (MCID Elite 7.0; Imaging Research, St. Catherines, Ontario, Canada). Relative optical density values (G/Area – ROD × pixel) of experimental protein were normalized to values of control protein (actin) on the same gel. For the study of depression, normalized values from each depressive subject are expressed as percentages (averaged from two gels) of the normalized value from paired control subject. Results were analyzed statistically using the two–tail paired Student's t-test (GraphPad Prism 3.0, GraphPad Software Incorporated, San Diego, CA, USA). Summary statistics are reported as the mean ± SEM. A p-value < 0.05 was considered statistically significant.
The potential contribution of confounding factors (age, post mortem interval and brain pH) was evaluated in separate experiments. All control subjects (n = 11–12) were blotted together and all depressive subjects (n = 11–12) were run together on the same gel. Experiments were performed for LC (only middle level) and for cerebellum. Linear regression analysis (GraphPad Prism 3.0) was used to compute potential correlations between the amount of nNOS immunoreactivity and potentially confounding factors.
Results
Localization and distribution of nNOS immunoreactivity in the human LC
Immunohistochemical labeling of nNOS in the region of the LC of a psychiatrically normal subject revealed localization of nNOS to multiple cell types. nNOS immunoreactivity was colocalized with neuromelanin in large neurons (Fig. 1a). In addition, nNOS immunoreactivity was observed in smaller neurons void of neuromelanin (Fig. 1b) and in glial cells (Fig. 1c). A rostral-caudal gradient of nNOS immunoreactivity was observed based on western blots, with the highest density of nNOS immunoreactivity detected at the rostral level of the LC (see Dissection and anatomical positioning of measurement) (Fig. 2). In control subjects the average levels of nNOS immunoreactivity (expressed in relative optical density values) were 3.56 ± 1.64; 2.08 ± 0.57 and 1.18 ± 0.24 at the rostral, middle and caudal levels, respectively.
Fig. 1.
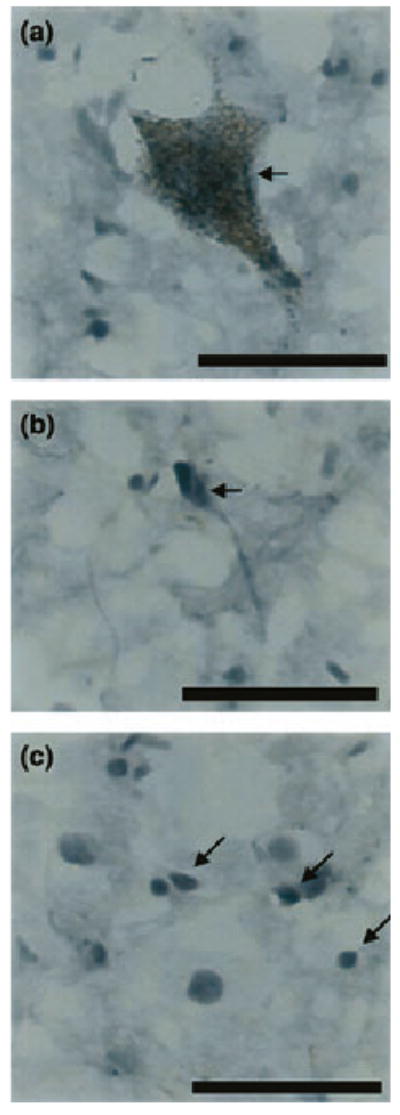
Photomicrographs showing nNOS – immunoreactive cells from the region of the human LC: (a) large neuron containing neuromelanin, (b) smaller neuron void of neuromelanin, (c) glial cells. Scale bars = 50 μm.
Fig. 2.
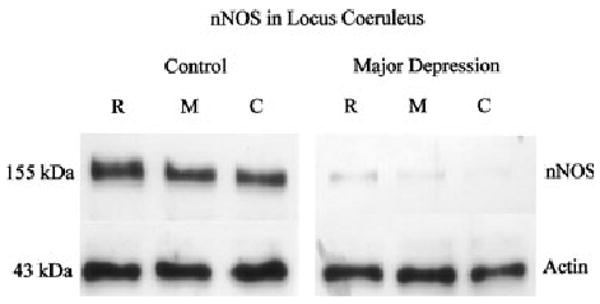
nNOS immunoreactivity in locus coeruleus (LC) from a single pair used in the analysis, representing three separate anatomical levels: rostral (R), middle (M) and caudal (C). Each well was loaded with 10 μg of total protein. The bottom panel (right and left) shows immunoreactive actin probed with anti-actin antibody on the same blot as a control for protein loading and transfer.
nNOS in depressive subjects vs. control subjects
Amounts of nNOS protein were analyzed in the LC from 12 pairs of depressive and individually matched control subjects. A single band with a molecular mass of 155 kDa was detected with the anti-nNOS monoclonal antibody. The highest amount of nNOS immunoreactivity was observed at rostral level of the human LC in all examined subjects. Representative immunoblots of nNOS and actin from a single experiment used in the analyses are shown in Fig. 2. Amounts of nNOS immunoreactivity in the LC were lower in major depressive subjects as compared with normal control subjects in 10 of the 12 matched pairs of subjects (Fig. 3). One matched pair exhibited no difference between the depressive and control subject and one pair demonstrated more nNOS in the depressive than in the control. The average relative amounts of nNOS were significantly lower in the LC (− 44%, p < 0.05), among depressive subjects compared with matched control subjects.
Fig. 3.
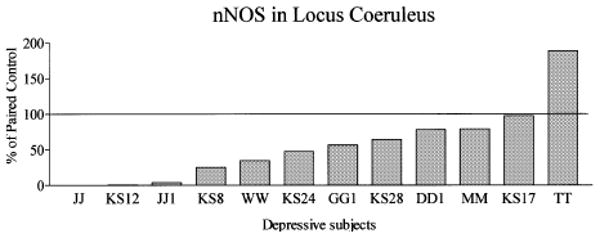
nNOS immunoreactivity in LC of depressive subjects expressed as percentages of values from paired control subjects. Each bar is an average of duplicate comparisons. The overall relative amount of nNOS was lower in the LC (− 44%, p < 0.05) among depressive subjects compared with individually matched control subjects.
To determine whether alterations of nNOS in the LC of major depressives would be observed anywhere in the brain, nNOS immunoreactivity were measured in cerebelli from 11 of the 12 major depressive/control pairs (cerebelli from one pair not available) used to study the LC (Figs 4 and 5). nNOS levels were lower in depressive compared with control subjects in eight of the 11 pairs, while nNOS levels were higher in three pairs (Fig. 5). The average relative amounts of nNOS were slightly lower (− 12%) in the cerebellum among depressive subjects compared with matched control subjects. However, this difference did not reach statistical significance.
Fig. 4.
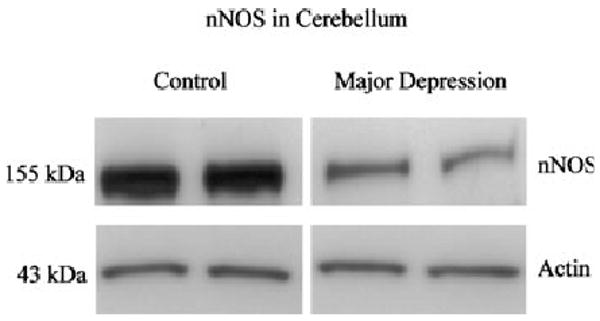
nNOS immunoreactivity in cerebellum from a single pair used in the analysis. Each well was loaded with 10 μg of total protein. The bottom panel (right and left) shows immunoreactive actin probed with anti-actin antibody on the same blot as a control for protein loading and transfer.
Fig. 5.
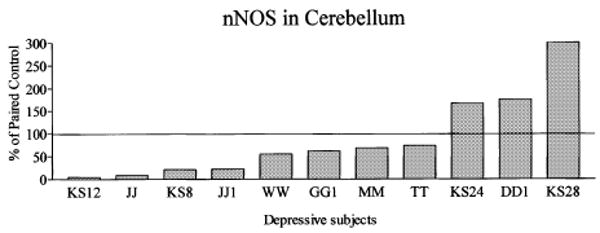
nNOS immunoreactivity in cerebelli of depressive subjects expressed as percentages of values from paired control subjects. Each bar is the average of duplicate comparisons. The overall relative amount of nNOS was modestly lower (− 12%) in the cerebellum among depressive subjects compared with individually matched control subjects, although this difference did not reach statistical significance.
In order to further verify the relative amount of nNOS immunoreactivity in control-depressive subject pairs, a polyclonal anti-human nNOS antibody was used in randomly selected pairs of control/MDD subjects. There was no difference between the amounts of nNOS detected by the polyclonal and monoclonal antibodies, i.e. the same differences between depressive and control subjects were observed (data not shown).
Age, post mortem delay and brain pH
The age of control subjects ranged from 27 to 74 years (46.5 ± 4 years) and depressed subjects ranged from 30 to 81 years (48.5 ± 4 years). The average ages of depressed and control subjects were not significantly different. The potential effect of age on nNOS levels was examined in separate experiments where all LC and cerebellar samples from control and major depressive subjects were run on the same gels. There was no significant correlation between age and the amount of nNOS immunoreactivity in the LC. As for cerebellum, a significant correlation between age and the amount of nNOS was observed only in control subjects (r2 = 0.46; p < 0.05). In this latter group, lower amounts of nNOS immunoreactivity were detected in older subjects.
Post mortem intervals of control and depressed subjects ranged from 9 to 24 h (18.5 ± 1.2 h) and from 18 to 44 h (25.5 ± 2.3 h), respectively. The average post mortem intervals of depressed and control subjects were significantly different (p < 0.01). Using data from the experiment to examine the effect of age on nNOS, the potential effect of post mortem interval on nNOS levels was evaluated. There was no significant correlation between post mortem intervals and amounts of nNOS in LC and cerebellum in controls and depressive subjects.
Brain pH of control subjects ranged from 6.28 to 7.01 (6.69 ± 0.07) and depressed subjects ranged from 6.24 to 6.96 (6.58 ± 0.09). Brain pH values of depressive and controls subjects were not significantly different. A significant correlation was observed between the amount of nNOS and brain pH in depressive subjects in LC (r2 = 0.55, p < 0.01, Fig. 6b), and in cerebellum (r2 = 0. 47, p < 0.05, Fig. 6d). These relationships did not reach statistical significance in the control group in LC and in cerebellum (Fig. 6a,c). Very low levels of nNOS were detected in subjects with the lowest pH values in both investigated areas. For example, in major depressive subjects JJ, KS12, and JJ1 where nNOS levels were barely detectable (see Figs 3 and 5), the brain pH values were 6.27, 6.24 and 6.24, respectively.
Fig. 6.
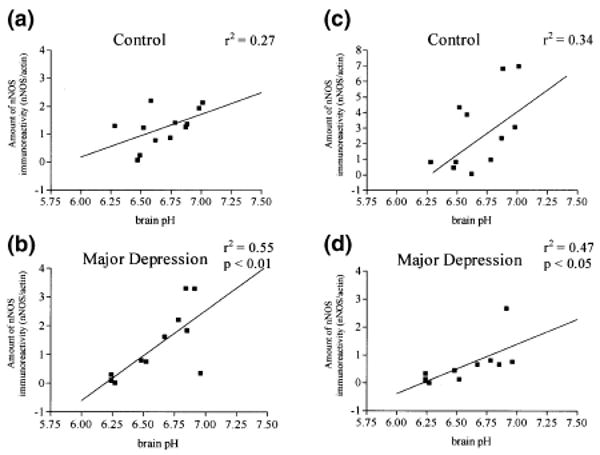
Relationships between the amount of nNOS and brain pH in control and major depressives subjects: (a and b) locus coeruleus (c and d) cerebellum.
The effect of low pH on nNOS protein immunoreactivity in cerebellum: in vitro study
To determine whether low tissue pH directly influences nNOS immunoreactivity, lactic acid was used to reduce the pH of a cerebellar homogenate from 6.72 to 6.02. After a 24 h (at 4°C) incubation in low pH, cytoplasmic protein was extracted, and western blot analysis of nNOS immunoreactivity was performed. As a result, the amount of nNOS immunoreactivity was found unchanged in the sample with reduced pH 6.02 as compared with nNOS immunoreactivity revealed in cerebellar sample with original pH 6.73 (data not shown).
Discussion
The present study is the first post mortem examination of nNOS in the human LC in major depression. This report demonstrates that nNOS is found in LC neurons and glial cells, and that overall amounts of nNOS are significantly lower in the LC, but not in the cerebellum, of major depressive subjects relative to carefully matched control subjects lacking a major psychiatric diagnosis. As nine out of the 12 major depressive subjects committed suicide, it is possible that behaviors related to suicide, but distinct from those of major depression, might contribute to altered nNOS in the LC. However, it is worth noting that of the three major depressive subjects that died of natural causes, two had amounts of nNOS lower than their respective control subjects. Hence, the present findings are suggestive of altered glutamate/nitrergic signaling in the LC in depression.
NOS catalyzes the conversion of l-arginine to l-citrulline with subsequent release of nitric oxide (NO). Of three identified isoforms, neuronal (nNOS), endothelial (eNOS) and inducible (iNOS), nNOS predominates in the central nervous system. Constitutive nNOS activity is dependent on intracellular [Ca2+] and is predominantly associated with the function of NMDA receptors. In the central nervous system, nitric oxide acts as a messenger and appears to be critically involved in various physiological and pathological processes, such as promotion of hippocampal long-term potentiation (Schuman and Madison 1991), glutamate-mediated neurotoxicity, and ischemic neuronal cell death (Dawson et al. 1991; Choi and Hartley 1993). Activation of nNOS is a part of the cascade of subcellular events linking activation of the NMDA receptor to stimulation of soluble guanylyl cyclase (GC) and production of intracellular cyclic guanosine monophosphate (cGMP) (Garthwaite et al. 1988, 1989; Bredt and Snyder 1989). nNOS is found in tyrosine hydroxylase-positive neurons (Simonian and Herbison 1996; Benavides-Piccione and DeFelipe 2003) or in neuromelanin-containing cells (present study). These catecholaminergic neurons are apparently capable of generating nitric oxide, and nitric oxide may directly regulate noradrenergic activity within the brain. Therefore, loss of nNOS immunoreactivity as seen in depressed subjects in the present study could be due to a loss of nNOS-containing neurons, their processes, or a compensatory reduction in nNOS protein within these neurons. No changes in the noradrenergic (neuromelanin-containing) cell numbers were found in depressed subjects as compared with controls, using the same brain specimens examined in this study (data not shown). In spite of this, the possibility of a selective loss of non-noradrenergic, neighboring neurons or efferents innervating the LC that express nNOS cannot be ruled out. Trends of lower NOS activity have been previously observed in the prefrontal cortex of unipolar and bipolar subjects (Xing et al. 2002). Moreover, a reduction in the number of NOS immunoreactive cells in the paraventricular nucleus and suprachiasmaticus nucleus in the hypothalamus of depressive subjects has been observed (Bernstein et al. 1998, 2002). However, it remains uncertain whether changes in NOS immunoreactivity represents a change in the production of NOS protein by cells or a change in the number of NOS-reactive cells.
Immunohistochemical evaluation of the LC region also revealed nNOS immunoreactivity in glial cells. nNOS appears to play a role in signaling in glial cells, particularly in astrocytes and Bergmann glia (Arbones et al. 1996; Kugler and Drenckhahn 1996). Furthermore, the NMDA-R1 receptor has been observed in astrocytes and presynaptic terminals in the rat LC (Van Bockstaele and Colago 1996). Nitrergic signaling via non-NMDA receptors, i.e. α-amino-3-hydroxy-5-methyl-4-isoxazole proprionate receptors, has been demonstrated in cultured rat cerebellar astroglial cells (Baltrons and Garcia 1997). The availability of the nNOS substrate, l-arginine, is regulated by glial cells, where l-arginine is synthesized and stored (Aoki et al. 1991; Grima et al. 1997; Do et al. 2002). Hence, glial cells utilize nitrergic signaling and may contribute to nitrergic signaling in neurons.
Given that glial cells express nNOS, an issue raised by the present study is whether lower levels of nNOS in depression occur in glia. It is interesting to note that a strong rostrocaudal gradient of nNOS immunoreactivity was observed in the LC, with the highest amount of nNOS immunoreactivity occurring at the rostral level and progressively lower levels of nNOS immunoreactivity at middle and caudal levels of the LC. The middle level of the LC has the highest number of neuromelanin-containing neurons (approximately twice the number in the rostral level). Hence, amounts of nNOS immunoreactivity do not appear to correlate with numbers of noradrenergic neurons of the LC, pointing to the possibility that changes in nNOS in depression are occurring in non-noradrenergic neurons or glial cells in the region of the LC. Recently, quantitative post mortem investigations of human cerebral cortex and amygdala have convincingly demonstrated reductions in glial cell number and/or density in subjects with major depression (Ongur et al. 1998; Rajkowska et al. 1999; Rajkowska 2000; Cotter et al. 2001, 2002; Bowley et al. 2002). Thus, it is tempting to speculate that abnormalities of glial cells may contribute to the pathology of nNOS proteins in major depression. Further study directed at potential glial cell pathology in the human LC is needed to elucidate the possible role of glia in LC neuropathology associated with depression.
Previous research has shown that interruption of nNOS signaling produces antidepressant effects. For example, NOS inhibitors exhibit antidepressant-like properties in animal-screening procedures (Harkin et al. 1999; Yildiz et al. 2000; Volke et al. 2003). Moreover, chronic treatment with a NOS inhibitor down-regulates β-adrenergic receptors in mouse frontal cortex with a magnitude comparable to imipramine (Karolewicz et al. 1999). These data are somewhat difficult to understand given demonstrations of reduced nNOS in depression. One would assume that if NOS inhibitors are antidepressant, then NOS activity or protein levels would be elevated in depression, rather than reduced as has been observed by Xing et al. (2002), Bernstein et al. (1998, 2002) and in the present study. However, this may be an over-simplistic explanation of the pathology of depression. If reduced NOS in the brain is a result of compensation for elevated glutamatergic activity, then further inhibition or complete blockade of nitrergic signaling by NOS inhibitors could conceivably produce therapeutic benefit. Together, preclinical and clinical findings suggest a role for NOS in the pathology of depression, as well as, antidepressant-sensitive behavior and neurochemical responses.
The possibility that treatment of depression could contribute to the adaptive changes in nNOS protein should be considered. No antidepressant drugs were detected in the post mortem toxicology screening of subjects in the present study (Table 1). However, four depressive subjects had a history of medication with antidepressants (serotonin reuptake inhibitors) at some point during their lifetime. Three of these four subjects had nNOS levels in LC near the average level of immunoreactivity of the depressive subjects lacking antidepressant drug exposure. However, one subject (TT) with a medication history of treatment with paroxetine and citalopram had higher nNOS immunoreactivity compared with the paired control subject. This is a difference opposite to the reduction in nNOS observed for the average of depressives as compared with control subjects. Hence, it is unlikely that past antidepressant drug treatment of depressed subjects contributed to the reduction of nNOS observed.
The present data indicate that the amount of nNOS immunoreactivity in human post mortem brain of depressive subjects may be affected by lower brain pH. Four major depressive subjects with the lowest brain pH (6.24–6.27) had low nNOS protein immunoreactivity in both the LC and cerebellum. Although there was no significant difference in average pH values between controls and depressives, a positive correlation between the amount of nNOS and brain pH was observed in the depressive subjects. This correlation suggests that pH may contribute to low levels of nNOS observed in depression. One possibility is that depressed subjects are more prone to pH instability and/or vulnerable to neurochemical alteration caused by lower pH. Exposure of brain tissue to low pH in vitro did not directly influence the nNOS immunoreactivity as measured by western blotting. However, pH is used as an index of hypoxia-related acidosis associated with prolonged time between major bodily insult and death (agonal state). Hypoxia-related acidosis can influence a number of neurochemical parameters in human brain, including mRNA quality and levels (Harrison et al. 1991; Kingsbury et al. 1995; Tomita et al. 2004), enzyme activities, and neuropeptide concentrations (Perry et al. 1982; Corder et al. 1990; Yates et al. 1990). Events that trigger low tissue pH may influence a wide spectrum of biological processes antemortem that could conceivably elicit changes in nNOS protein, if the length of time to death is prolonged and hypoxic. Three major depressive subjects with the lowest nNOS levels had their estimated average time between the insult and death as less than 1 h. Thus, agonal state does not convincingly contribute to the low levels of nNOS observed in these subjects. It is noteworthy that of three depressed subjects with lowest nNOS and low pH levels, two died of carbon monoxide poisoning and one died of cardiovascular collapse. Another depressed subject who had died of CO poisoning had a brain pH of 6.48 and an elevated amount of nNOS in the cerebellum as compared with paired control. Hence, there is no consistent association of CO poisoning and low levels of nNOS.
Considerable evidence suggests that depression is associated with dysregulation of LC activity. In fact, we have previously postulated that major depression is associated with noradrenergic overdrive, as well as norepinephrine depletion, based on biochemical alterations in the LC of major depressives (Ordway et al. 2002). Abnormalities of nNOS protein in major depression raise speculation that LC pathobiology may be secondary to altered glutamatergic input to the LC. Elevated levels of CSF glutamine (glutamate metabolite/precursor) (Levine et al. 2000), and high levels of serum glutamate have been observed in depressed subjects (Kim et al. 1982; Altamura et al. 1993; Mauri et al. 1998) with exception (Altamura et al. 1995). In addition, reduction in glutamate decarboxylase activity (GAD) has been demonstrated in neuropsychiatric disorders including depression. Interestingly, glutamatergic, NMDA receptor antagonists have antidepressant actions in animal models of depression (Moryl et al. 1993; Papp and Moryl 1994), as do NOS inhibitors (Harkin et al. 1999; Yildiz et al. 2000; Karolewicz et al. 2001; Volke et al. 2003). NMDA receptor antagonists have demonstrated antidepressant effects in humans (Berman et al. 2000). It seems reasonable to speculate that a part of the pathology of the LC in major depression could be triggered by altered excitatory input to the LC.
In summary, low levels of nNOS in subjects diagnosed with major depression in LC may reflect altered excitatory neurotransmission in major depression. However, it is noteworthy that brain pH may influence nNOS immunoreactivity in brains of depressive subjects. Further study of the glutamatergic/nitrergic signaling pathway in major depression will lead to a better understanding of the role of glutamate in the pathology of depression.
Acknowledgments
The authors wish to gratefully acknowledge Dr Jun Pan for technical assistance in the preparation of tissues for assays. The authors also gratefully acknowledge Dr Herbert Meltzer, Lesa Dieter and Ginny Dilley for assistance with family interviews and psychiatric diagnoses. This work was supported by MH63187, MH46692, MH/AG02031, and RR17701.
Abbreviations used
- GC
guanylyl cyclase
- LC
locus coeruleus
- nNOS
neuronal nitric oxide synthase
- PBS
phosphate-buffered saline
- TH
tyrosine hydroxylase
References
- Allgaier C, Durmaz M, Muller D, Franke H, Poelchen W, Wirkner K, Illes P. Single-cell RT-PCR analysis of N-methyl-d-aspartate receptor subunit expression in rat locus coeruleus neurones. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:120–123. doi: 10.1007/s002100000348. [DOI] [PubMed] [Google Scholar]
- Altamura CA, Mauri MC, Ferrara A, Moro AR, D'Andrea G, Zamberlan F. Plasma and platelet excitatory amino acids in psychiatric disorders. Am J Psychiatry. 1993;150:1731–1733. doi: 10.1176/ajp.150.11.1731. [DOI] [PubMed] [Google Scholar]
- Altamura C, Maes M, Dai J, Meltzer HY. Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. Eur Neuropsychopharmacol. 1995;5:71–75. doi: 10.1016/0924-977x(95)00033-l. [DOI] [PubMed] [Google Scholar]
- Aoki E, Semba R, Mikoshiba K, Kashiwamata S. Predominant localization in glial cells of free 1-arginine: immunocytochemical evidence. Brain Res. 1991;547:190–192. doi: 10.1016/0006-8993(91)90961-t. [DOI] [PubMed] [Google Scholar]
- Arbones ML, Ribera J, Agullo L, Baltrons MA, Casanovas A, Riveros-Moreno V, Garcia A. Characteristics of nitric oxide synthase type I of rat cerebellar astrocytes. Glia. 1996;18:224–232. doi: 10.1002/(SICI)1098-1136(199611)18:3<224::AID-GLIA6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Chouvet G, et al. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog Brain Res. 1991;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- Baltrons MA, Garcia A. AMPA receptors are coupled to the nitric oxide/cyclic GMP pathway in cerebellar astroglial cells. Eur J Neurosci. 1997;9:2497–2501. doi: 10.1111/j.1460-9568.1997.tb01667.x. [DOI] [PubMed] [Google Scholar]
- Benavides-Piccione R, DeFelipe J. Different populations of tyrosine-hydroxylase-immunoreactive neurons defined by differential expression of nitric oxide synthase in the human temporal cortex. Cereb Cortex. 2003;13:297–307. doi: 10.1093/cercor/13.3.297. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Stanarius A, Baumann B, Henning H, Krell D, Danos P, Falkai P, Bogerts B. Nitric oxide synthase-containing neurons in the human hypothalamus: reduced number of immunoreactive cells in the paraventricular nucleus of depressive patients and schizophrenics. Neuroscience. 1998;83:867–875. doi: 10.1016/s0306-4522(97)00461-2. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Heinemann A, Krell D, Mawrin C, Bielau H, Danos P, Diekmann S, Keilhoff G, Bogerts B, Baumann B. Further immunohistochemical evidence for impaired NO signaling in the hypothalamus of depressed patients. Ann N Y Acad Sci. 2002;973:91–93. doi: 10.1111/j.1749-6632.2002.tb04613.x. [DOI] [PubMed] [Google Scholar]
- Boissel JP, Schwarz PM, Forstermann U. Neuronal-type NO synthase: transcript diversity and expressional regulation. Nitric Oxide. 1998;2:337–349. doi: 10.1006/niox.1998.0189. [DOI] [PubMed] [Google Scholar]
- Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci USA. 1989;86:9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Glatt CE, Hwang PM, Fotuhi M, Dawson TM, Snyder SH. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991;7:615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- Choi DW, Hartley DM. Calcium and glutamate-induced cortical neuronal death. Res Pub Assoc Res Nerv Ment Dis. 1993;71:23–34. [PubMed] [Google Scholar]
- Corder R, Pralong FP, Muller AF, Gaillard RC. Regional distribution of neuropeptide Y-like immunoreactivity in human hypothalamus measured by immunoradiometric assay: possible influence of chronic respiratory failure on tissue levels. Neuroendocrinology. 1990;51:23–30. doi: 10.1159/000125311. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- Cuellar B, Fernandez AP, Lizasoain I, Moro MA, Lorenzo P, Bentura ML, Rodrigo J, Leza JC. Up-regulation of neuronal NO synthase immunoreactivity in opiate dependence and withdrawal. Psychopharmacology (Berl) 2000;148:66–73. doi: 10.1007/s002130050026. [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do KQ, Grima G, Benz B, Salt TE. Glial-neuronal transfer of arginine and S-nitrosothiols in nitric oxide transmission. Ann N Y Acad Sci. 2002;962:81–92. doi: 10.1111/j.1749-6632.2002.tb04058.x. [DOI] [PubMed] [Google Scholar]
- First MB, Donovan S, Frances A. Nosology of chronic mood disorders. Psychiatr Clin N Am. 1996;19:29–39. doi: 10.1016/s0193-953x(05)70271-9. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Charles SL, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Garthwaite G, Palmer RM, Moncada S. NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur J Pharmacol. 1989;172:413–416. doi: 10.1016/0922-4106(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Blum-Degen D, Ransmayr G, Leblhuber F, Pedersen V, Riederer P. Expression, but not activity, of neuronal nitric oxide synthase is regionally increased in the alcoholic brain. Alcohol Alcohol. 2001;36:65–69. doi: 10.1093/alcalc/36.1.65. [DOI] [PubMed] [Google Scholar]
- Grima G, Benz B, Do KQ. Glutamate-induced release of the nitric oxide precursor, arginine, from glial cells. Eur J Neurosci. 1997;9:2248–2258. doi: 10.1111/j.1460-9568.1997.tb01643.x. [DOI] [PubMed] [Google Scholar]
- Harkin AJ, Bruce KH, Craft B, Paul IA. Nitric oxide synthase inhibitors have antidepressant-like properties in mice. 1. Acute treatments are active in the forced swim test. Eur J Pharmacol. 1999;372:207–213. doi: 10.1016/s0014-2999(99)00191-0. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Procter AW, Barton AJ, Lowe SL, Najlerahim A, Bertolucci PH, Bowen DM, Pearson RC. Terminal coma affects messenger RNA detection in post mortem human temporal cortex. Brain Res Mol Brain Res. 1991;9:161–164. doi: 10.1016/0169-328x(91)90143-l. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Heath PR, Eastwood SL, Burnet PW, McDonald B, Pearson RC. The relative importance of premortem acidosis and post mortem interval for human brain gene expression studies: selective mRNA vulnerability and comparison with their encoded proteins. Neurosci Lett. 1995;200:151–154. doi: 10.1016/0304-3940(95)12102-a. [DOI] [PubMed] [Google Scholar]
- Karolewicz B, Bruce KH, Lee B, Paul IA. Nitric oxide synthase inhibitors have antidepressant-like properties in mice. 2. Chronic treatment results in downregulation of cortical beta-adrenoceptors. Eur J Pharmacol. 1999;372:215–220. doi: 10.1016/s0014-2999(99)00192-2. [DOI] [PubMed] [Google Scholar]
- Karolewicz B, Paul IA, Antkiewicz-Michaluk L. Effect of NOS inhibitor on forced swim test and neurotransmitters turnover in the mouse brain. Pol J Pharmacol. 2001;53:587–596. [PubMed] [Google Scholar]
- Kim JS, Schmid-Burgk W, Claus D, Kornhuber HH. Increased serum glutamate in depressed patients. Arch Psychiatr Nervenkr. 1982;232:299–304. doi: 10.1007/BF00345492. [DOI] [PubMed] [Google Scholar]
- Kingsbury AE, Foster OJ, Nisbet AP, Cairns N, Bray L, Eve DJ, Lees AJ, Marsden CD. Tissue pH as an indicator of mRNA preservation in human post-mortem brain. Brain Res Mol Brain Res. 1995;28:311–318. doi: 10.1016/0169-328x(94)00219-5. [DOI] [PubMed] [Google Scholar]
- Klimek V, Stockmeier C, Overholser J, Meltzer HY, Kalka S, Dilley G, Ordway GA. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci. 1997;17:8451–8458. doi: 10.1523/JNEUROSCI.17-21-08451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler P, Drenckhahn D. Astrocytes and Bergmann glia as an important site of nitric oxide synthase I. Glia. 1996;16:165–173. doi: 10.1002/(SICI)1098-1136(199602)16:2<165::AID-GLIA8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Layer RT, Popik P, Olds T, Skolnick P. Antidepressant-like actions of the polyamine site NMDA antagonist, eliprodil (SL-82.0715) Pharmacol Biochem Behav. 1995;52:621–627. doi: 10.1016/0091-3057(95)00155-p. [DOI] [PubMed] [Google Scholar]
- Levine J, Panchalingam K, Rapoport A, Gershon S, McClure RJ, Pettegrew JW. Increased cerebrospinal fluid glutamine levels in depressed patients. Biol Psychiatry. 2000;47:586–593. doi: 10.1016/s0006-3223(99)00284-x. [DOI] [PubMed] [Google Scholar]
- Mauri MC, Ferrara A, Boscati L, Bravin S, Zamberlan F, Alecci M, Invernizzi G. Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology. 1998;37:124–129. doi: 10.1159/000026491. [DOI] [PubMed] [Google Scholar]
- Moryl E, Danysz W, Quack G. Potential antidepressive properties of amantadine, memantine and bifemelane. Pharmacol Toxicol. 1993;72:394–397. doi: 10.1111/j.1600-0773.1993.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13, 290–13, 295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway GA, Smith KS, Haycock JW. Elevated tyrosine hydroxylase in the locus coeruleus of suicide victims. J Neurochem. 1994a;62:680–685. doi: 10.1046/j.1471-4159.1994.62020680.x. [DOI] [PubMed] [Google Scholar]
- Ordway GA, Widdowson PS, Smith KS, Halaris A. Agonist binding to alpha 2-adrenoceptors is elevated in the locus coeruleus from victims of suicide. J Neurochem. 1994b;63:617–624. doi: 10.1046/j.1471-4159.1994.63020617.x. [DOI] [PubMed] [Google Scholar]
- Ordway GA, Klimek V, Mann JJ. Neurocircuitry of mood disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: the Fith Generation of Progress. Lippincott Williams & Wilkins; Philadelphia: 2002. pp. 1051–1064. [Google Scholar]
- Ordway GA, Schenk J, Stockmeier CA, May W, Klimek V. Elevated agonist binding to alpha(2)-adrenoceptors in the locus coeruleus in major depression. Biol Psychiatry. 2003;53:315–323. doi: 10.1016/s0006-3223(02)01728-6. [DOI] [PubMed] [Google Scholar]
- Papp M, Moryl E. Antidepressant activity of non-competitive and competitive NMDA receptor antagonists in a chronic mild stress model of depression. Eur J Pharmacol. 1994;263:1–7. doi: 10.1016/0014-2999(94)90516-9. [DOI] [PubMed] [Google Scholar]
- Perry EK, Perry RH, Tomlinson BE. The influence of agonal status on some neurochemical activities of post mortem human brain tissue. Neurosci Lett. 1982;29:303–307. doi: 10.1016/0304-3940(82)90334-2. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Post mortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Schuman EM, Madison DV. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991;254:1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Ince PG, Johnson M, Perry EK, Candy JM. The quantitative autoradiographic distribution of [3H]MK-801 binding sites in the normal human brainstem in relation to motor neuron disease. Brain Res. 1992;572:276–280. doi: 10.1016/0006-8993(92)90484-q. [DOI] [PubMed] [Google Scholar]
- Simonian SX, Herbison AE. Localization of neuronal nitric oxide synthase-immunoreactivity within sub-populations of noradrenergic A1 and A2 neurons in the rat. Brain Res. 1996;732:247–252. doi: 10.1016/0006-8993(96)00687-7. [DOI] [PubMed] [Google Scholar]
- Skolnick P. Antidepressants for the new millennium. Eur J Pharmacol. 1999;375:31–40. doi: 10.1016/s0014-2999(99)00330-1. [DOI] [PubMed] [Google Scholar]
- Tomita H, Vawter MP, Walsh DM, et al. Effect of agonal and post mortem factors on gene expression profile: quality control in microarray analyses of post mortem human brain. Biol Psychiatry. 2004;55:346–352. doi: 10.1016/j.biopsych.2003.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE. Selective distribution of the NMDA-R1 glutamate receptor in astrocytes and presynaptic axon terminals in the nucleus locus coeruleus of the rat brain: an immunoelectron microscopic study. J Comp Neurol. 1996;369:483–496. doi: 10.1002/(SICI)1096-9861(19960610)369:4<483::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Volke V, Wegener G, Bourin M, Vasar E. Antidepressant- and anxiolytic-like effects of selective neuronal NOS inhibitor 1-(2-trifluoromethylphenyl)-imidazole in mice. Behav Brain Res. 2003;140:141–147. doi: 10.1016/s0166-4328(02)00312-1. [DOI] [PubMed] [Google Scholar]
- Vulliemoz Y, Whittington RA, Virag L. The nitric oxide-cGMP system of the locus coeruleus and the hypnotic action of alpha-2 adrenergic agonists. Brain Res. 1999;849:169–174. doi: 10.1016/s0006-8993(99)02147-2. [DOI] [PubMed] [Google Scholar]
- Wang Y, Newton DC, Marsden PA. Neuronal NOS: gene structure, mRNA diversity, and functional relevance. Crit Rev Neurobiol. 1999;13:21–43. doi: 10.1615/critrevneurobiol.v13.i1.20. [DOI] [PubMed] [Google Scholar]
- Xing G, Chavko M, Zhang LX, Yang S, Post RM. Decreased calcium-dependent constitutive nitric oxide synthase (cNOS) activity in prefrontal cortex in schizophrenia and depression. Schizophr Res. 2002;58:21–30. doi: 10.1016/s0920-9964(01)00388-7. [DOI] [PubMed] [Google Scholar]
- Xu ZQ, Pieribone VA, Zhang X, Grillner S, Hokfelt T. A functional role for nitric oxide in locus coeruleus: immunohistochemical and electrophysiological studies. Exp Brain Res. 1994;98:75–83. doi: 10.1007/BF00229111. [DOI] [PubMed] [Google Scholar]
- Xu ZQ, de Vente J, Steinbusch H, Grillner S, Hokfelt T. The NO-cGMP pathway in the rat locus coeruleus: electrophysiological, immunohistochemical and in situ hybridization studies. Eur J Neurosci. 1998;10:3508–3516. doi: 10.1046/j.1460-9568.1998.00359.x. [DOI] [PubMed] [Google Scholar]
- Yates CM, Butterworth J, Tennant MC, Gordon A. Enzyme activities in relation to pH and lactate in post mortem brain in Alzheimer-type and other dementias. J Neurochem. 1990;55:1624–1630. doi: 10.1111/j.1471-4159.1990.tb04948.x. [DOI] [PubMed] [Google Scholar]
- Yildiz F, Erden BF, Ulak G, Utkan T, Gacar N. Antidepressant-like effect of 7-nitroindazole in the forced swimming test in rats. Psychopharmacology (Berl) 2000;149:41–44. doi: 10.1007/s002139900316. [DOI] [PubMed] [Google Scholar]
- Zhu MY, Klimek V, Dilley GE, Haycock JW, Stockmeier C, Overholser JC, Meltzer HY, Ordway GA. Elevated levels of tyrosine hydroxylase in the locus coeruleus in major depression. Biol Psychiatry. 1999;46:1275–1286. doi: 10.1016/s0006-3223(99)00135-3. [DOI] [PubMed] [Google Scholar]


