Abstract
Brain-derived neurotrophic factor (BDNF) is a prototypic neurotrophin that regulates diverse developmental events from the selection of neural progenitors to the terminal dendritic differentiation and connectivity of neurons. We focus here on activity-dependent synaptic regulation by BDNF and its receptor, full length TrkB. BDNF-TrkB signaling is involved in transcription, translation, and trafficking of proteins during various phases of synaptic development and has been implicated in several forms of synaptic plasticity. These functions are carried out by a combination of the three signaling cascades triggered when BDNF binds TrkB: the mitogen-activated protein kinase (MAPK), the phospholipase Cγ (PLC PLCγ), and the phosphatidylinositol 3-kinase (PI3K) pathways. MAPK and PI3K play crucial roles in both translation and/or trafficking of proteins induced by synaptic activity while PLCγ regulates intracellular Ca2+ that can drive transcription via cyclic AMP and a Protein Kinase C. Conversely, the abnormal regulation of BDNF is implicated in various developmental and neurodegenerative diseases that perturb neural development and function. We will discuss the current state of understanding BDNF signaling in the context of synaptic development and plasticity with a focus on the post-synaptic cell and close with the evidence that basic mechanisms of BDNF function still need to be understood in order to effectively treat genetic disruptions of these pathways that cause devastating neurodevelopmental diseases.
Keywords: BDNF, TrkB, PSD-95, LTP, Synaptic Tag
Introduction
Brain-derived neurotrophic factor (BDNF) was the second neurotrophin, after nerve growth factor (NGF), to be isolated and sequenced. This feat was accomplished by Ives Barde and his colleagues (Barde et al., 1982). They isolated 2 µg of BDNF from 3 kg of pig brains to show that it supported the survival and fiber outgrowth of cultured embryonic chick sensory neurons and they cloned the molecule (Leibrock et al., 1989). With the sequence of both NGF and BDNF available, conserved sequences were identified and many laboratories began to probe DNA searching for other members of the neurotrophin family which now totals 4–5 depending on the species referred to. In 1986 and '87, two laboratories reported on the first neurotrophin receptor p75NTR which binds the neurotrophins with relatively low affinity (Johnson et al., 1986; Radeke et al., 1987) and in 1991 the orphan receptors, tropomyosin sensitive receptor kinases (Trks), were identified as the high affinity receptors for the neurotrophin family of growth factors (Kaplan et al., 1991a; Kaplan et al., 1991b; Klein et al., 1991a; Klein et al., 1991b; Soppet et al., 1991; Squinto et al., 1991). The Trks frequently work together with p75NTR to register neurotrophin signaling at nanogram levels. Since their discovery, Trk signaling, and in particular BDNF-TrkB signaling has been implicated in a plethora of brain functions: neuronal cell survival, neurite growth, cell migration, regulation of inhibitory-excitatory balance, and, more specifically, glutamate dependent spine and dendritic growth, synapse formation, stabilization and potentiation. This review discusses the synaptic effects of BDNF-TrkB signaling on, and within, post-synaptic neurons during glutamatergic synaptic development. It closes with a hypothesis or, more accurately a speculation, concerning how this particular locus of BDNF action may be involved in mechanisms critical to neuron-wide stabilization of synapses as a first step in establishing global brain circuitry in normal development and disease.
Regulation of BDNF / TrkB Signaling Pathway
The details of molecular properties of BDNF-TrkB signaling are beyond the scope of this article and are reviewed elsewhere (Huang and Reichardt, 2003; Reichardt, 2006). Here, we briefly summarize the findings. The Trk receptor tyrosine kinase family contains TrkA and TrkC, receptors for NGF and neurotrophic factor 3 (NT3) respectively, as well as TrkB which mediates the effect of BDNF and also neurotrophic factor 4/5 (NT 4/5).
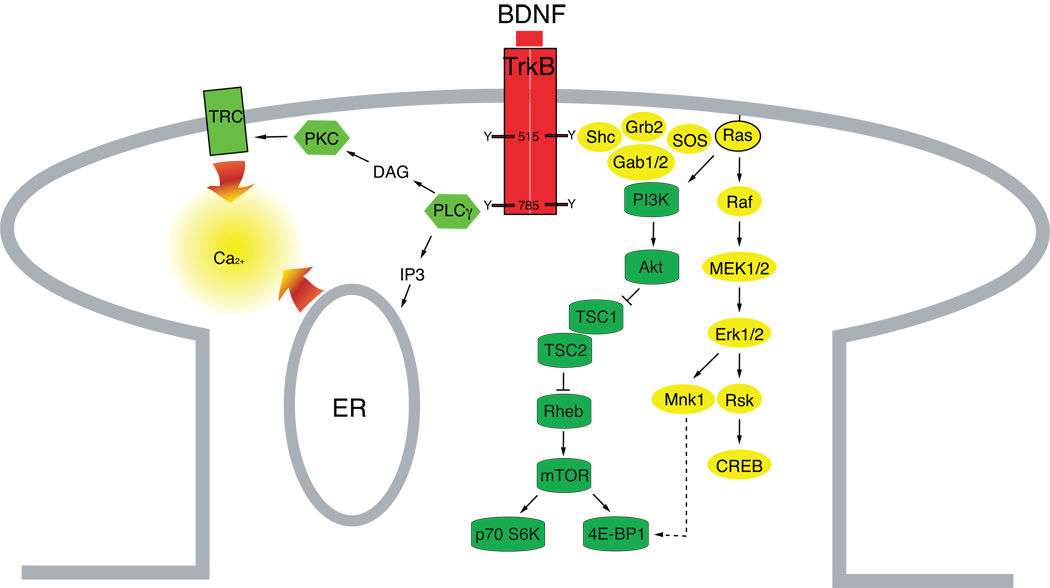
There are several TrkB isoforms in the mammalian CNS. The full-length TrkB isoform discussed here is a typical tyrosine kinase in that homodimerization during ligand binding causes cross tyrosine phosphorylations in the intracellular Trk domains thereby initiating transduction of the BDNF signal. These phosphorylation events then trigger MAPK, PI3K or PLCγ intracellular cascades (Fig 1). Synaptic activity drives these signaling pathways that regulate the assembling of TrkB with synaptic proteins (Fig. 2a), gene transcription (Fig. 2b), protein translation and trafficking (Fig. 2c). The three pathways can, at least theoretically, work in parallel (Fig. 2d). Thus for most TrkB investigations, one Trk signaling pathway is generally identified as necessary for an outcome, but seldom do these experiments eliminate possible contributions, to the particular effect, of the other Trk signaling pathways.
Figure 1.
Binding of BDNF activates PLCγ, PI3K and MAPK pathways.
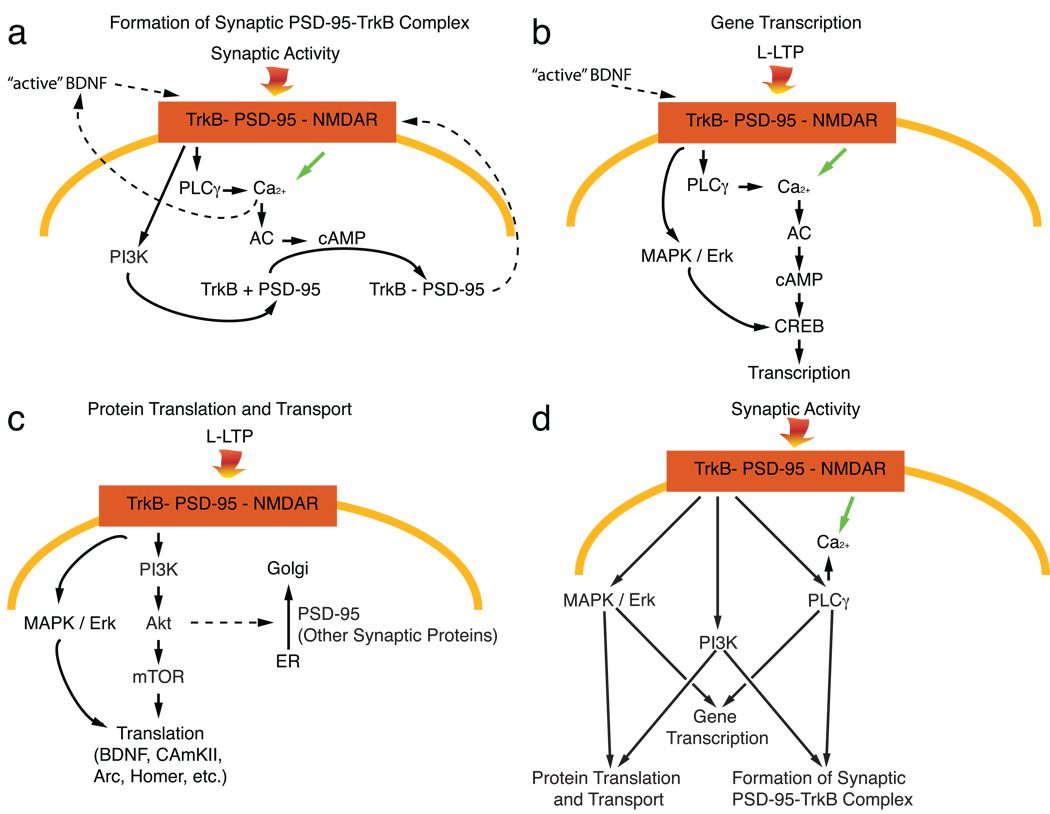
Figure 2. BDNF-TrkB signaling Regulates Multiple Events through PLCγ, PI3K and MAPK pathways.
(a) PI3K pathway regulates trafficking and PSD-95 to a synapse and cAMP regulates formation of synaptic PSD-95-TrkB complex.
(b) BDNF-TrkB signaling activates MAPK/Erk, increasese cAMP and activate CREB-regulated gene transcription.
(c) BDNF-TrkB signaling regulates protein translation through both MAPK/Erk and PI3K-Akt-mTOR pathways. PI3K-Akt also regulates protein trafficking in ER and Golgi.
(d) A diagram showing BDNF-TrkB signaling regulates multiple molecular events through three pathways in parallel.
Phospholipase Cγ (PLCγ)
There are two tyrosine phosphorylation residues outside the kinase activation domain of TrkB. Phospho-Tyr785 recruits and activates PLCγ which in turn hydrolyses phosphatidylinositol 4,5-bisphosphate, to produce diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) (Huang and Reichardt, 2003; Reichardt, 2006). DAG activates protein kinase C (PKC), and IP3 releases Ca2+ from intracellular stores. In developing hippocampal neurons, focal application of BDNF results in fast calcium transients at postsynaptic sites (Lang et al., 2007). The BDNF-induced release of intracellular Ca2+ together with DAG activate the plasma membrane transient receptor potential canonical subfamily channel 3/6 (TRPC3/6) that contribute to BDNF-induced Ca2+ elevations at growth cones and synapses (Li et al., 2005; Amaral and Pozzo-Miller, 2007).
Some studies link the PLCγ pathways underlying BDNF induced Ca2+ transients directly to synaptic plasticity. For example, in cultured cortical pyramidal neurons these transients translocate GluR1 subunit of α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate receptors (AMPAR), but not N-methyl-D-aspartic acid receptors (NMDAR) subunits to synapses. The transients were due to release from intracellular stores requiring active IP3 receptors and a contribution from TRPCs (Nakata and Nakamura, 2007). The Ca2+ elevation triggered by PLCγ also increases Ca2+-sensitive adenyl cylclase (AC) activity that is necessary for the formation of synaptic PSD-95-TrkB complexes (Ji et al., 2005) (Figure 2a) and that also is involved in cyclic AMP responsive element binding (CREB)-dependent transcription (Nguyen et al., 1994; Shaywitz and Greenberg, 1999) (Figure 2b).
Mitogen-Activated Protein Kinase (MAPK)
The other TrkB phosphorylation site on Tyr515 recruits Shc to TrkB and phosphorylates it. In turn, Shc interacts with an adaptor protein Grb2 that recruits and activates the guanine nucleotide exchange factor SOS. SOS promotes the removal of GDP from Ras that can then bind GTP and become active. Ras activates the downstream kinase B-raf, MEK and MAPK/Erk (Huang and Reichardt, 2003; Reichardt, 2006). MEK-MAPK/Erk signaling influences transcription events, such as the activation of the CREB transcription factor (Shaywitz and Greenberg, 1999) (Figure 2b). MAPK/Erk also regulates protein-synthesis dependent plasticity by increasing phosphorylation of eukaryotic initiation factor 4E (eIF4E), the 4E-binding protein 1 (4E-BP1) and ribosomal protein S6 (Kelleher et al., 2004; Klann and Dever, 2004) (Figure 2c). Thus, MAPK/Erk plays a critical role in protein-synthesis dependent plasticity.
Phosphatidylinositol 3-kinase (PI3K)
Recruitment of Shc to the Trk receptors also allows activation of the PI3K pathway by Ras, via Grb2 (Reichardt, 2006). Activation of PI3K changes the composition of inositol phospholipids in the inner leaflet of the plasma membrane. This results in the translocation of Akt/protein kinase B to the plasma membrane. Activated Akt is involved in a variety of functions such as cell survival, and protein translation. The TrkB-PI3K-AKT pathway activates translation via a cascade driving the mammalian target of rapamycin (mTOR), a major regulator of protein synthesis (Sarbassov et al., 2005). Akt accomplishes this by downregulating the tuberous sclerosis complex (TSC) protein, TSC2 that forms a complex with TSC1: a complex that otherwise inhibits Rheb a normal activator of mTOR. Active mTOR phosphorylates p70S6 kinase and 4E-BP1 permitting mRNA translation. The PI3K-Akt pathway also regulates the trafficking of synaptic proteins (Yoshii and Constantine-Paton, 2007). Thus, this pathway plays pivotal roles in a long-term maintenance of synaptic plasticity through translation and transport of synaptic proteins (Figure 2c).
BDNF-Dependent Structual Plasticity of Dendritic Spines
Spine Addition
There exists considerable evidence that BDNF-TrkB signaling can play a direct role in excitatory synaptic morphogenesis. TrkB receptors have been found in postsynaptic densities in rat cerebral cortex and hippocampus (Wu et al., 1996), and surface TrkB is enriched at glutamatergic synapses colocalizing with NMDARs in cultured dissociated cortical neurons (Gomes et al., 2006). In cerebellar cultures, BDNF increases the spine density of Purkinje cells without affecting dendritic branching complexity (Shimada et al., 1998). Chronic treatment of hippocampal slice cultures with BDNF increases synapse number and spine density in apical dendrites of CA1 pyramidal neurons acting predominately on short stubby spines without affecting dendritic length or branching. However, when BDNF is applied along with botulinum toxin C to block even miniature synaptic release of neurotransmitter, the proportion of long thin, possibly immature, spines is increased (Tyler and Pozzo-Miller, 2003). In the developing intact Xenopus tadpole, BDNF also increases synapse density of tectal neurons (Sanchez et al., 2006).
In forebrain-specific transgenic mice expressing a dominant negative TrkB tagged with an enhanced green fluorescent protein (EGFP), adult visual cortical neurons showed a reduction in mushroom, (believed to be mature), spine maintenance and synaptic efficacy, as well as an increase in long thin, spines and filopodia. However, in this same mouse, CA1 mushroom spine maintenance was unaffected suggesting that TrkB may play fundamentally different roles in structural plasticity in different brain areas (Chakravarthy et al., 2006). However, a recent study provides further evidence supporting a critical role of BDNF and protein synthesis in hippocampal neuron spine growth (Tanaka et al., 2008). In rat hippocampal slices, the repetitive pairing of postsynaptic spikes and two-photon uncaging of glutamate at single spines (a spike timing plasticity protocol) produced both immediate and gradual spine enlargement in CA1 pyramidal neurons. Protein synthesis inhibitors as well as BDNF scavengers suppressed the gradual phase of enlargement.
There are also several mechanisms through which BDNF appears to facilitate spine formation. An increase in spine density induced by BDNF in the hippocampus has been shown to be dependent on TRPC3 channels which generate sustained cationic currents and are activated by the TrkB-PLCγ pathway (Amaral and Pozzo-Miller, 2007). Also, through another branch of the TrKB-PLCγpathway, enhancement of cyclic-AMP, a product of a Ca2+-sensitive adenyl cylclase (AC) at synapses, or application of BDNF to cultured neurons, increased synaptic colocalization of TrkB with the dominant glutamate receptor scaffolding protein postsynaptic density protein-95 (PSD-95) and coimmunoprecipitation of PSD-95 and TrkB increased in an activity-dependent manner (Ji et al., 2005; Yoshii and Constantine-Paton, 2007) (Figure 2a). However, there is a second BDNF-dependent mechanism that rapidly transports PSD-95 from the soma to the dendritic regions of developing neurons in a PI3K-AKT- and microtubule- dependent, protein synthesis-independent, process (Yoshii and Constantine-Paton, 2007). Thus two different TrkB-driven pathways accomplish the trafficking of PSD-95 to dendrites and then to postsynaptic densities: cAMP regulates formation of PSD-95-TrkB complex (Ji et al., 2005; Yoshii and Constantine-Paton, 2007). PSD-95 binds many postsynaptic molecules that can regulate activity-dependent spine growth. For example, PSD-95 binds Kalirin, a neuronal Rho-GEF that facilitates actin polymerization and thereby contributes to spine growth (Penzes et al., 2001). Simple overexpression of PSD-95 will increase spine number, size and synaptic efficacy (El-Husseini et al., 2000), suggesting that TrkB signaling may play a central role in facilitating glutamate synapse stabilization/maturation through the PSD-95 scaffold and thereby exert its effects on glutamatergic synapse formation and function. MAPK / ERK1/2 activation is also necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons (Alonso et al., 2004). However, it is not clear if this response requires transcription or translation.
In short, despite some regional differences in observations on TrkB-induced spine development, it is likely that BDNF-TrkB signaling in the presence of synaptic activity can facilitate spine morphogenesis particularly because similar changes in spine morphology have been associated with long-term synaptic potentiation where, as will be discussed below, considerable evidence for the requirement of TrkB signaling exists. Increase in glutamatergic synapse activity stimulates the release of BDNF. BDNF, in turn, facilitates the growth of immature spines into mature spines by controlling the assembly of TrkB-PSD-95 complex and protein synthesis (Figure 2).
Spine Retraction
Spine retraction is another mechanism crucial to the regulation of dendritic spine density. p75NTR can negatively modulate dendrite morphology and dendritic spine number of hippocampal pyramidal neurons (Zagrebelsky et al., 2005). In wild-type animals, overexpression of full-length p75NTR in pyramidal neurons resulted in a marked reduction in dendritic length and complexity as well as spine density. Conversely, total spine density increased by 100% and dendritic complexity was much greater in EGFP-labeled pyramidal neurons in p75NTR−/− mice than in wild-type mice. As will be discussed further below, it is an intriguing possibility that p75NTR signaling induces spine retraction by facilitating long-term synaptic depression.
Physiological Synaptic Plasticity
Identifying any single mechanism for BDNF functional changes at synapses is difficult because BDNF-TrkB signaling, either directly or indirectly work together with synaptic activity to regulate synaptic strength of both excitatory and inhibitory connections. The latter effect could be due to a BDNF effect on inhibitory hippocampal neurons of reducing the reversal potential for GABAA currents thereby selectively decreasing the effect of inhibition on GABA neurons and effectively increasing excitation in the neuropil (Wardle and Poo, 2003). The BDNF-TrkB induced changes in excitatory neurons results from increases in internal Ca2+ (Balkowiec and Katz,2002;) and Ca2+ activation of adenyl cylase that can activate transcription (Fig. 2) or the formation of PSD-95-TrkB complexes (Ji et al., 2005) and NMDA-dependent BDNF activation can promote BDNF dependent synaptic delivery of PSD-95 to dendrites and synapses through PI3K-Akt pathway as described above (Yoshii and Constantine-Paton, 2007). BDNF also increases presynaptic, glutamate release. Thus in the brief overview below we outline major findings without an attempt to identify the progression of interacting events that produce functional synaptic change.
Changes in the strength of individual excitatory synapses are thought to enable developmental refinement and information storage within neuronal circuits, and to represent a cellular correlate of learning and memory. Long-term potentiation, also termed early LTP (E-LTP), and NMDAR-dependent long-term depression (LTD) of synaptic efficacy, elicited by a short period of synaptic stimulation, are partially mediated by local effects at the stimulated synapse, including the incorporation or removal of AMPARs and the regulation of dendritic spine turnover (Malinow and Malenka, 2002; Malenka and Bear, 2004; Shepherd and Huganir, 2007). Increases in AMPAR at synapses occur in the early phase of LTP (E-LTP) accompanied by rapid modification of synaptic proteins and the actin cytoskeleton. E-LTP results in increases of synaptic strength that are of relatively short duration (approximately 1 hour). A stronger synaptic stimulation induces late-LTP (L-LTP). L-LTP lasts longer than a few hours (Huang and Kandel,1994) and requires the synthesis of proteins that stably alter neuronal function (Nguyen et al, 1994). Accumulating evidence suggests that BDNF affects both E- and LLTP through both pre- and post-synaptic mechanisms and that both of these mechanisms are involved in learning and initiating activity-dependent tuning of developing circuits (Poo, 2001; Lu et al., 2005).
Hippocampal LTP
Of all the neurotrophins, BDNF is by far the best characterized for its role in regulating hippocampal LTP that occurs at the synapses from CA3 pyramidal neurons onto CA1 pyramidal neurons. LTP in the hippocampus is attenuated in a variety of experiments where BDNF signaling is decreased in BDNF −/− or TrkB mutant mice, or by application of TrkB ligand binding sites fused to the Fc region of immunoglobulin G (TrkB-Fc) that adsorb endogeneous BDNF (Korte et al., 1995; Patterson et al., 1996; Chen et al., 1999; Xu et al., 2000; Minichiello et al., 2002). Impaired LTP in BDNF −/− mice can be recovered by increasing BDNF either by direct application (Patterson et al., 1996; Pozzo-Miller et al., 1999) or virus mediated transfer of the BDNF gene (Korte et al., 1996). In addition, a weak synaptic activation, below the LTP threshold, induces LTP when BDNF is simultaneously applied (Figurov et al., 1996; Kovalchuk et al., 2002). It is thought that the potentiating effect of BDNF on E-LTP is achieved, at least in part, by enhancing synaptic responses to high frequency stimulation and by presynaptically facilitating synaptic vesicle docking (Pozzo-Miller et al., 1999; Jovanovic et al., 2000; Xu et al., 2000; Tyler and Pozzo-Miller, 2001). A brief dendritic BDNF application causes postsynaptic Ca2+ transients through Ca2+ channels and NMDARs (Kovalchuk et al., 2002). In addition, Man et al. applied a cocktail of NMDAR, antagonists of inhibitory receptors, and sodium channels, along with a short burst of the NMDAR co-agonist glycine to produce AMPA incorporation into the synapses of cultured hippocampal neurons (a chemical LTP) (Man et al., 2003). They found that antagonism of PI3K blocked this effect. We also used the chemical LTP regime and found that the activation of NMDAR triggers synaptic accumulation of PSD-95 (Yoshii and Constantine-Paton, 2007) that can scaffold TARP-AMPAR complexes at the synapse (Tomita et al., 2004). Anti-BDNF antibodies that scavenge BDNF blocked the effect of chemical LTP indicating that glutamatergic activity is upstream of the BDNF-TrkB signaling in this process (Yoshii and Constantine-Paton, 2007).
Ample studies indicate that BDNF is also involved in L-LTP. Acute application of BDNF to hippocampal slices induces synaptic potentiation in the hippocampal CA1 region (Kang and Schuman, 1995; Kang and Schuman, 1996; Kang et al., 1997). Also, in vivo infusion of BDNF in the hippocampal dentate gyrus acutely induces an increase in field EPSP for 2 to10 hours indicative of L-LTP (Messaoudi et al., 1998). Treatment of hippocampal slices with BDNF scavenging TrkB-Fc, or anti-BDNF can inhibit L-LTP (Kang et al., 1997). L-LTP is impaired in TrkB knockout mice (Minichiello et al., 1999). Furthermore, an impairment of L-LTP observed in BDNF−/− and +/− mice can be rescued by perfusion with exogenous BDNF (Korte et al., 1996; Pang et al., 2004). Nevertheless many investigators have been unable to reproduce LTP with BDNF alone. Thus the current evidence suggests that BDNF facilitates activity of glutamatergic synapses and is crucial for the maintenance of L-LTP but BDNF does not, by itself, induce L-LTP (Figurov et al., 1996; Patterson et al., 1996; Tanaka et al., 1997; Frerking et al., 1998; Gottschalk et al., 1998; Huber et al., 1998). In addition, BDNF is not involved in all forms of NMDAR-dependent LTP. Classic theta burst stimulation which activates significant Ca2+ influx through L-type Ca2+ channels of CA1 pyramidal cells required BDNF for L-LTP but a similar BDNF treatment applied with 100 Hz stimulation did not induce L-LTP (Kang et al., 1997; Chen et al., 1999; Patterson et al., 2001).
Assuming that BDNF is required for long-term maintenance of hippocampal LTP, one premise is that it facilitates the synthesis of proteins that sustain a prolonged increase in synaptic weight. Like BDNF-TrkB signaling, L-LTP is known to induce gene expression through PI3K-Akt-mTOR and MAPK/Erk pathways in parallel (Klann and Dever, 2004). Increases in cAMP induced by Forskolin can rapidly activate TrkB, (possibly by associating it with PSD-95 and glutamate receptors) (Ji et al., 2005) and induce BDNF-dependent long-lasting potentiation at the Schaffer collateral-CA1 synapse in hippocampus (Patterson et al., 2001). TrkB activation by cAMP induces nuclear translocation of the activated MAPK to the nucleus and CREB protein phosphorylation (Pizzorusso et al., 2000; Ying et al., 2002). Studies exploring mTOR involvement in LLTP demonstrate that rapamycin, an mTOR antagonist, blocks L-LTP and that proteins involved in mTOR-dependent protein elongation are close to postsynaptic sites (Tang et al., 2002). This is a potential positive feedback mechanisms that could produce L-LTP and a BDNF-induced synthesis of BDNF itself. Supporting such a mechanism, BDNF mRNA levels increase in the hippocampal CA1 region and dentate gyrus within 2–4 hours after the application of L-LTP-inducing stimulation (Patterson et al., 1992; Castren et al., 1993; Dragunow et al., 1993) and BDNF application to cultured neurons activates CREB which promotes transcription of BDNF (Shaywitz and Greenberg, 1999).
Despite the large literature on PI3K and MAPK signaling pathways in BDNFdependent plasticity, some dissenting evidence also exists. Both PI3K and the MAPK signaling pathways require Shc as an adaptor protein, therefore Minichiello et al. (2002) compared mice with targeted mutations in either the Shc- or the PLCγ-binding sites of TrkB (Minichiello et al., 2002). Only the mutation of the PLCγ-binding sites blocked the early and late phases of LTP in the CA1 region of the hippocampus whereas mutation of the Shc docking site on TrkB was without effect on both early and late LTP. Also, simultaneous pre- and post-synaptic expression of the PLC pleckstrin homology domain with viral vectors, (these constructs block PLCγ signaling and IP3 production), suppressed the effect of TrkB receptors on 80 minute LTP (Gartner et al., 2006). However, selective blockade of either pre- or post-synaptic PLCγ signaling alone did not result in a significant reduction of LTP, indicating that both sides of the synapse contribute to the effects of BDNF in LTP. This role of TrkB coupling to PLCγ in LTP induced by high-frequency stimulation correlates with a role of this signaling pathway in associative learning (Gruart et al., 2007). How this PLCγ mechanism coordinates with PI3K and MAPK pathway signaling requires further study.
Proposed Model -Step I
Based on the experiments outlined above we postulate a biphasic scenario. First, similar to E-LTP, NMDARs produce Ca2+ influx through both the NMDAR pore and L-type Ca2+ channels (Grover and Tyler, 1990) and BDNF-TrkB signaling also induces an increase in internal Ca2+ increase through the PLCγ pathway. Pre-synaptically this PLCγ pathway induces an increase in docked vesicles thereby releasing more glutamate. BDNF activation due to NMDAR activity also increases delivery of PSD-95 to synapses (Yoshii and Constantine-Paton, 2007). This initial phase sets the stage for the maintenance phase (L-LTP) which depends on protein synthesis through PI3K pathway and may produce additional synaptic proteins or mRNAs via MAPK and CREB activation. The outcome (described below) is the formation of synaptic tags (Frey and Morris, 1997) that will allow synapses throughout a neuron to be readily potentiated for a long period of time by even mild synaptic activation.
LTD
While the contribution of BDNF-TrkB signaling to LTP has been well established, it is unclear whether this pathway is involved in LTD. In visual cortical layer 2/3 neurons, an application of BDNF prevents LTD which is otherwise induced by low frequency stimulation of layer 4 neurons (Kinoshita et al., 1999). Conversely, recent studies indicate that activation of p75NTR alone is important for NMDAR-dependent LTD in CA1 hippocampal neurons (Rosch et al., 2005; Woo et al., 2005). NMDAR-dependent LTD is also absent in the hippocampal CA1 region of p75NTR-knockout mice without affecting NMDAR-dependent LTP or NMDAR-independent LTD (Rosch et al., 2005; Woo et al., 2005). Consistent with this functional data, other studies indicate that p75NTR−/− mice have increased hippocampal neuron spine numbers (Koshimizu et al., 2009); that overexpression of p75NTR in these neurons produces a decrease in spine number (Zagrebelsky et al., 2005); and that application of a cleavage-resistant proBDNF reduces the number of spines in cultured neurons (Koshimizu et al., 2009).
The p75NTR is known to bind pro-BDNF which can be cleaved by extracellular proteases such as plasmin and induces neuronal apoptosis (Lee et al., 2001) and few dispute the observation that p75NTR can function without Trk receptors. However, the issue is whether there is sufficient extracellular proBDNF neurotrophin in vivo to be the actual endogenous ligand. The effect of synaptic attenuation by the recombinant proBDNF lead to endeavors by two groups to determine how much pro-BDNF is secreted by neurons. Matsumoto et al. used a mouse line in which the Bdnf gene was substituted with Bdnf-Myc and observed secretion of Myc-tagged BDNF but not proBDNF, indicating efficient intracellular cleavage to mature BDNF (Matsumoto et al., 2008). Over expression of BDNF in COS cells however produced considerable more proBDNF in the extra-cellular medium. On the other hand, Yang et al. (2009) used knock-in mice in which the Bdnf gene was tagged with a hemagglutinin (HA) epitope (Yang et al., 2009). By immunoprecipitating the proteins from the extra-cellular media and immunoblotting with antibodies against HA, they showed that both BDNF and proBDNF were secreted by neurons but that proBDNF secretion is highest in young neurons. Consequently, the nature of the p75NTR receptor ligand remains contentious. Nevertheless, the correlation of extra-cellular proBDNF with highly expressed BDNF is intriguing particularly since the exon structure of BDNF is complex with different signaling pathways controlling each exon. As developmental neurobiologists it does not seem impossible to us that there is developmental regulation of this exon usage allowing high BDNF expression at some stages of development with consequently more secretion of proBDNF and more p75NTR activation. For example see (Pruunsild et al., 2007; Tian et al., 2009).
Visual Cortical Plasticity
The other region where BDNF involvement in synaptic plasticity has been studied extensively is in the vertebrate visual pathway where many studies have focused on the developmental plasticity that occurs during the critical period for ocular dominance competition which is an important model for the mechanisms underlying development of amblyopia in humans. The expression of BDNF increases during development and the onset of pattern vision associated with eye-opening, or exposure to light after dark-rearing causes a superimposed rapid increase in this neurotrophin (Castren et al., 1992). Also, because of the extensive literature on its mature function as well as development, the visual system is an excellent system to study BDNF/TrkB signaling among forebrain circuits. We concentrate here only on the work dealing with mammalian visual cortex since the Cohen-Corey article in this volume will be covering the exciting work on the amphibian retinal-tectal pathway.
In many highly binocular mammals, cortical ocular dominance is accompanied by segregation of the retinotopically organized thalamocortical axon terminals from left and right retinas into eye-specific stripes within the afferent layers of visual cortex. These are known as ocular dominance (OD) columns (Hubel et al., 1977). Visual deprivation is a classical paradigm to study the plasticity of visual cortical connections. Prolonged or later eyelid closure in young animals, known as monocular deprivation (MD), causes visual cortical neurons to become dominated by the open eye. This is the phenomenon known as OD plasticity and if the deprivation is prolonged through the "critical period", permanent disruption of pattern vision develops in the deprived eye. Infusion of NT-4/5 or BDNF into primary visual cortex of kittens blocks OD column formation around the infusion site (Cabelli et al., 1995) but infusion of TrkB-Fc also inhibits the formation of OD patches within layer 4 (Cabelli et al., 1997). These data suggest that the optimal level of BDNF is necessary for the selective remodeling of visual thalamic axons into eyespecific zones and that visual activity can regulate the BDNF-TrkB signaling in visual cortex. The latter notion is supported by the findings that phosphorylated Trk (activated) receptors are reduced in dark-reared, relative to light-reared animals (Viegi et al., 2002), and that BDNF is reduced in visual cortex receiving most of its input from a MD eye (Rossi et al., 1999). However, infusion of BDNF into 4–5 week old kittens who were monocularly deprived caused an enlargement of OD columns of the deprived eye contrary to a postulate that more active synapses are more likely to take advantage of available BDNF than inactive ones (Galuske et al., 2000). Furthermore, in contrast to the infusion experiments, BDNF overexpressing transgenic mice showed the same degree of susceptibility to MD as wild type mice, but only during a precocious critical period (Hanover et al., 1999; Huang et al., 1999).
In visual cortex, there is an age-dependent decline of cortical LTP that appears to be mediated by the development of cortical inhibition. BDNF overexpression resulted in this cortical inhibition occurring earlier. These overexpressing transgenic mice also showed a precocious development of visual acuity and an earlier termination of the critical period for MD induced OD plasticity, indicating that BDNF regulates many aspects of cortical development probably through direct (or indirect effects) on cortical inhibition (Huang et al., 1999). As discussed above, BDNF induces the formation of dendritic spines that are sites of excitatory synapses. Therefore results indicating that over-expression of BDNF causes earlier development of inhibitory circuitry in mouse visual cortex were somewhat surprising (Hanover et al., 1999; Huang et al., 1999). However, the effect on inhibition could be indirect, resulting from early increases in excitatory drive. This interpretation is consistent with the observation that overexpressing mice also have a precocious onset of the critical period (Hanover et al., 1999; Huang et al., 1999). The normal timing of critical period onset has been shown to be dependent on light stimulation that increases excitatory activity in the visual pathway.
A recent study on OD plasticity took advantage of knock-in mice with a mutation in the ATP binding site that causes TrkB kinase activity to be eliminated by the compound 1NM-PP1 (Chen et al., 2005). The acute blockade of TrkB kinase activity in MD mouse cortex by osmotic mini-pump infusion of 1NM-PP1 did not interrupt the shift of OD plasticity. However, in control animals, reopening of the deprived eye corrects the OD shift during the critical period. However, 1NM-PP1 inhibition of TrkB blocked this recovery. These findings suggest a trophic role for TrkB signaling in enhancement of newly active synapses or growth of new connections from the previously deprived eye (Kaneko et al., 2008). They could also reflect the absence of BDNF mediated PSD-95 increases in nascent synapses from the previously deprived eye. The lack of BDNF-triggered increase in PSD-95 could suppress any increase in the strength of the newly active synapses from a previously-deprived eye and/or eliminate any competition between inputs from a previously-deprived eye and from a non-deprived eye. Suppression of PSD-95 expression using siRNA has been shown to decrease developmental increases of AMPARs in cultured hippocampal slices (Ehrlich et al., 2007), to eliminate a rapid increase in PSD-95 upon controlled eye-opening (Yoshii et al., 2003), and also the rapid appearance of LTP in the superior colliculus at this time (Zhao et al., 2006; Zhao et al., 2007). These findings are tied to TrkB function because in ongoing studies in intact visual cortex (Yoshii et al., 2008) we are finding that TrkB inhibition with 1NM-PP1 suppresses an increase in PSD-95 at visual cortical synapses similar to that previously described in cultured visual cortical cells (Yoshii and Constantine-Paton, 2007).
Taken together, the work on BDNF in the hippocampus and visual pathway implicate BDNF in the maturation of excitatory synapse by participating in L-LTP and, at least partly by regulating trafficking of PSD-95 to glutamate synapses and also, either directly or indirectly, by selectively increasing excitation and decreasing inhibition, accelerates (Wardle and Poo, 2003) the integration of inhibitory neurons into the CNS circuitry.
Synaptic Tagging
NMDA receptor-dependent LTP and LTD are major synaptic mechanisms that either strengthen or weaken a neuron's connectivity with neighboring neurons and with long axon inputs from other regions of the brain using correlation or lack of correlation in the temporal patterning of the target cell's inputs. At the cellular level, the neuronal output is based on the integrated activity of individual synapses across the a neuron's dendritic tree. In learning and memory as well as initial development of circuitry, this integration must be ongoing over intervals longer than single synaptic responses. The latter requires some form of dendrite-wide or target-cell-population-wide long-term cellular memory. One of the most carefully documented examples of such a memory mechanism is "synaptic tagging" first described by Frey and Morris in 1997 (Frey and Morris, 1997). The tagging process is outlined here because it is likely to involve BDNF-Trk signaling at several stages.
Frey and Morris examined this integration mechanism using the hippocampal slice preparation and stimulated CA3 inputs onto the apical dendritic trees of CA1 pyramidal neurons (Frey and Morris, 1997). They activated one synaptic input with repetitive tetanization to produce protein synthesis-dependent L-LTP. If a second input onto the same apical dendritic tree was activated to produce E-LTP within 3 hours of the first induction, this stimulation would evoke L-LTP at the second input without the requirement of protein synthesis. They proposed that the initial L-LTP initiates the creation of short-lasting (less than 3 hours) 'tags' and plasticity related proteins (PRP) at the heavily potentiated synapses and these molecules diffuse through the dendrites. Other synapses on these dendrites become labeled with the tag and weakly potentiated synapses that develop within 3 hrs can use this tag to "capture" the PRPs. The capture of the PRPs by the weakly driven synapses as a result of their "tag" then allows these E-LTP expressing synapses to develop L-LTP independent of local proteins synthesis. A similar scenario occurs even between L-LTD and E-LTD. Specifically, an initial activation of a protein-synthesis-dependent, late, long-term depression in synaptic strength at one synapse allows a subsequent weaker stimulus on the same dendritic tree to develop a protein-synthesis-dependent, late, long-term depression in synaptic strength. Furthermore, a phenomenon of "cross-tagging" has also been described in which proteins induced by prior L-LTD can be captured by "tagged" E-LTP” synapse and transformed into L-LTP or vice versa (Sajikumar and Frey, 2004). This raises the intriguing possibility that the same PRPs are synthesized in response to either L-LTP- or L-LTD inducing stimuli and the type of stimulus delivered to a tagged synapse determines the direction of the synaptic change that is consolidated. Protein kinase Mζ (PKMζ), an isoform of PKC, is induced by L-LTP-inducing stimulation, and its activity is required for the maintenance of L-LTP (Sajikumar et al., 2005). PI3K-Akt-mTOR and MAPK/Erk pathways are required for L-LTP because of their role in local protein synthesis. However, an additional function of TrkB in the synaptic tagging process could be to activate PKMζ via PLCγ signaling.
The properties of the BDNF-TrkB signaling system that suggest its role in the synaptic tagging process are: 1) The requirement of BDNF-TrkB activation for dendritic translation of proteins that could be PRPs and the observation that exogenous BDNF is sufficient to maintain L-LTP in the presence of protein synthesis inhibitors suggesting that BDNF itself could be the most important PRP (Pang et al., 2004); 2) The fact that the BDNF-TrkB pathway interacts with the scaffolding and trafficking systems of glutamatergic synapses. For example, as mentioned previously, increases in neuronal activities after eye-opening, and associated increases in cyclic AMP, TrkB-PSD-95 association and rapid addition of PSD-95 at synapses (Ji et al., 2005; Yoshii and Constantine-Paton, 2007). Also PSD-95, the major scaffold for NMDA and AMPA receptors at glutamatergic synapses, when knocked down (KD) with siRNA in postsynaptic neurons, reduces the number of active synapses and decreases LTP in the KD neurons even when the LTP stimulus response in the cells is increased to the same levels as control cells (Zhao et al., 2007). Furthermore, when PSD-95 is over-expressed in particular neurons, it facilitates synaptic strengthening as evident by the fact that LTP is occluded (saturated) and LTD is increased (Beique and Andrade, 2003; Stein et al., 2003; Ehrlich and Malinow, 2004); 3) In addition in VP16-CREB mice which have an elevated BDNF level caused by constitutive activity of CREB, a weak tetanus can induce L-LTP, that is reversed by TrkB-Fc application (Barco et al., 2005). Furthermore, mice with the BDNF gene deleted only in CA1 neurons that would otherwise produce PRPs in response to strong LTP stimulation seem to lack synaptic "tags": that is, a weak second tetanus at a different synapse of the same cell can no produce L-LTP (Barco et al., 2005).
We became interested in the synaptic tagging hypothesis as a result of experiments showing that eye-opening significantly increased PSD-95 in developing visual synapses within 2–3 hours (Yoshii et al., 2003). In pursing the mechanism of this increase we discovered that full length TrkB co-immunopercipitated with PSD-95 but not with SAP 102, a glutamate receptor scaffold which appears earlier than PSD-95 (Yoshii and Constantine-Paton, 2007). Furthermore, the amount of TrkB associated with PSD-95 increased after eye-opening in visual cortex.
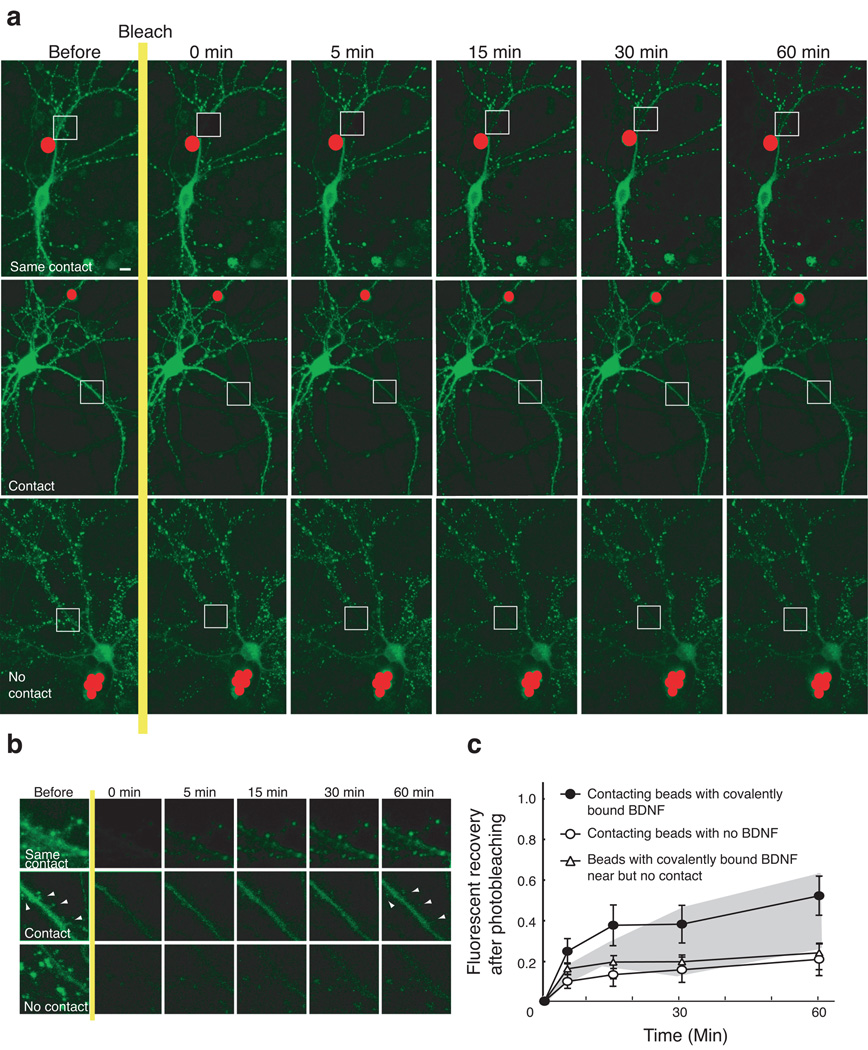
To determine if TrkB signaling was involved in the synaptic increase of PSD-95 throughout the dendrites of young visual cortical neurons in culture, we employed application of BDNF or the NMDA stimulation regime known to produce chemical LTP through PI3K (Man et al., 2003). These experiments used a fluorescent recovery after photobleaching (FRAP) assay in dendritic segments of visual cortical neurons transfected with GFP-tagged PSD-95. By measuring the rate and degree of PSD-95 FRAP we showed that BDNF as well as chemical LTP applied to the visual cortical neuronal cultures increased the rate of trafficking of GFP-tagged PSD-95 into these bleached dendritic segments and into synaptic puncta within 1hour. This acceleration of trafficking was not protein synthesis dependent but it was completely eliminated by antagonism of TrkB, PI3K and Akt regardless of whether the increase in trafficking was induced by chemical LTP or BDNF application. In addition, the BDNF signaling proved to be downstream of chemical LTP because addition of a BDNF-blocking antibody to the cultures inhibited the dendritic increases in PSD-95 induced by chemical LTP. To more closely mimic BDNF activity at a synapse, we placed single microspheres coated with BDNF against a dendrite of PSD-95-GFP expressing neurons with a bleached segment (Fig. 3). PSD-95 trafficking with this local BDNF stimulation was as effective as BDNF application to the entire culture. BDNF- coated bead clusters close to but not touching a dendrite had no effect on the FRAP of the cell as did contacting beads lacking BDNF. Unexpectedly, however, we observed a BDNF-induced facilitation of PSD-95 trafficking in a bleached segment even when a bead touched an entirely separate dendrite of the cell.
Figure 3. BDNF bead application to a short dendritic segment facilitates PSD-95-GFP FRAP throughout the dendritic tree of locally-stimulated neurons.
(a) (Top row) BDNF covalently linked to a bead (pseudo-colored red) was placed in contact with one dendritic branch and a dendritic segment nearby (white square) was bleached. (Middle row) A segment of a completely separate dendrite was bleached after a BDNF-coated bead was applied. (Bottom row) A group of BDNF-coated beads was placed near a dendrite without contact. Scale, 10 µm.
(b) Magnified images of dendrites inside the white squares in Fig. 6(a). The branches from a neuron contacting the BDNF-coated bead or a separate dendritic branch from a neuron with a BNDF-coated bead contacting another one of its dendrites, show a more pronounced FRAP of PSD-95-GFP than a branch from the neuron with near-by beads that have no dendritic contact. Each image corresponds to a 21 µm × 21 µm bleached frame. Filled arrowheads in the second row identify weak puncta and their recovery in a dendrite that was expressing relatively low intensity PSD-95 puncta.
(c) FRAP graph summarizing the results of the dendrite-wide BDNF-bead-mediated PSD-95-GFP FRAP. Application of BDNF-coated beads to one dendrite of transfected neurons showed significantly facilitated PSD-95-GFP FRAP on distant dendrites of the same cells. Beads coated only with BSA and touching a dendrite had no effect above baseline on PSD-95-GFP FRAP in the dendrites of the contacted cell. Dendrites of nearby cells that were not contacted by beads covalently coated with BDNF showed the same baseline FRAP response seen with the BSA-coated beads. This lack of effect indicates that the PSD-95-GFP FRAP shown by dendrites distant from the one contacted by the BDNF bead were not driven by trace amounts of BDNF escaping from the bead. FRAP with bead treatments are shown against shaded areas reflecting the difference between baseline FRAP and FRAP with BDNF in the perfusion fluid. P=0.02 Error bar, s.e.m. (Adopted from Yoshii and Constantine-Paton, 2007)
Furthermore, consistent with the pan-dendritic trafficking of PSD-95 as a result of local stimulation, we found that BDNF stimulation decreased endogenous PSD-95 in the somatic endoplasmic reticulum (ER) and increased the amount of PSD-95 in the somatic Golgi complex. Thus PSD-95, or perhaps some molecule transported to the dendrites along with PSD-95, or docked at synapses as a result of the synaptic presence of this multi-molecular scaffold, could be the synaptic tag that enables other synapses on young neurons to obtain L-LTP without new protein synthesis.
Proposed Model Step 2
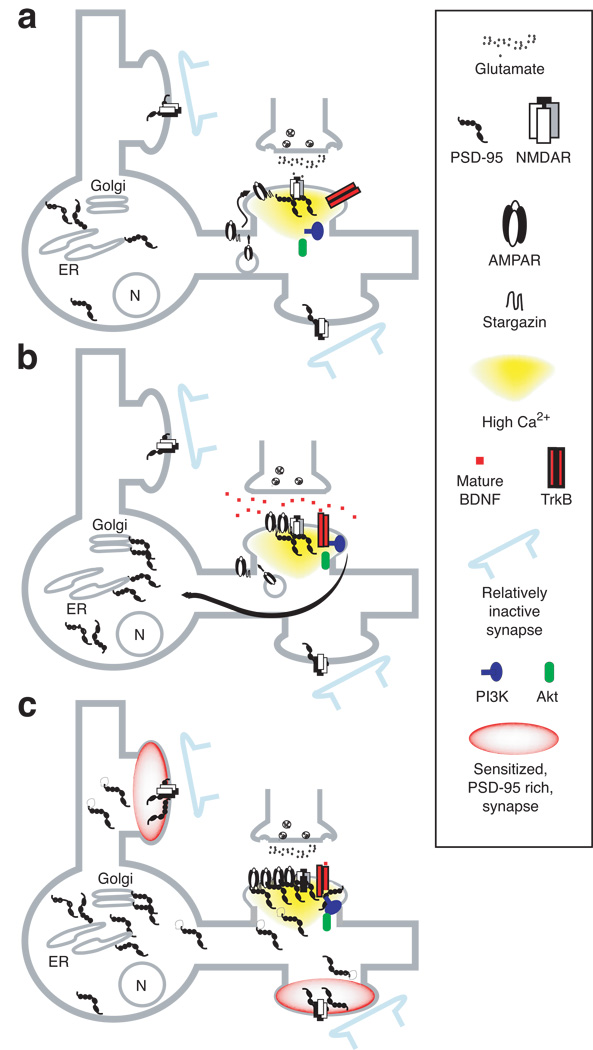
Consequently, we propose the following sequence of events to get young neurons primed to achieve L-LTP and stabilized synapses. 1) Strong glutamate activation at one or a small number of synapses (Fig. 4a); 2) subsequent activation of BDNF-TrkB-PI3K signaling; 3) facilitation of PSD-95 export to the Golgi (Fig. 4b); 4) tagging/priming of other synapses for facilitated L-LTP as a result of PSD-95 transport throughout the other dendrites of the same neuron (Fig. 4c). In this variation of the tagging model, at least within an hour or so following the induction of L-LTP, local protein synthesis of the "tag" (PSD-95) is not initially required because PI3K-Akt signaling can move PSD-95 from ER to Golgi and then to trans-Golgi vesicles for microtubular transport to dendrites. However for long-term changes such as the developmental stabilization of connections, protein synthesis would have to refill a local pool of the tag because, if PSD-95 is a tag, it is known to turn over rapidly at the synapse (Colledge et al., 2003). In this context it is interesting that PSD-95 mRNA has been found at dendrites in older neurons (Zalfa et al., 2007) suggesting that, to maintain PSD-95 at selected synapses into adulthood, local protein synthesis could provide a mechanism to reinforce only synapses receiving very active inputs.
Figure 4. A model for rapid, dendrite-wide, sensitization for synaptic potentiation conveyed by local NMDAR and BDNF activity-driven-PSD-95 trafficking to synapses throughout the neuron.
(a) Strong local NMDAR stimulation recruits TrkB to synapses, inserts AMPARs into extrasynaptic membrane, and initiates BDNF signaling.
(b) Mature BDNF near the synapse stimulates TrkB. TrkB activates PI3K and Akt resulting in facilitated PSD-95 localization to the Golgi and microtubule based transport along dendrites.
(c) An increased probability (sensitization) of LTP occurs at synapses throughout the dendritic tree as a result of dendrite-wide trafficking of PSD-95 from the Golgi. Synapses with a surplus of PSD-95 can readily bind AMPAR-stargazin complexes at the synapse and consequently strengthen that contact if a young synapse activates NMDARs sufficiently to drive AMPAR-stargazin complexes to the extrasynaptic membrane. (Adopted from Yoshii and Constantine-Paton, 2007)
Taken together, BDNF secretion following a local increase in synaptic activity regulates both pan-dendritic trafficking of synaptic proteins such as PSD-95 and local protein synthesis. PSD-95 serves as a platform to hold glutamate receptors as well as newly synthesized PRPs and facilitates coincidence detection by allowing a potent synaptic input to increase the probability that a subsequent, potentially weaker, different synapses of the same neuron.
Implications for Therapeutic Strategies to treat for Developmental Disorders
In closing, with this admittedly speculative and focused scenario for synapse stabilization as a result of activity and BDNF signaling, it is worthwhile reflecting on whether the interaction between BDNF and glutamate activity at synapses is, in fact, relevant to human health and development. The explosion of research on the bases of genetic disease suggests very strongly that this is the case. Diseases associated with some perturbation of BDNF signaling are diverse: epilepsy (He et al., 2004); mood disorders (Neves-Pereira et al., 2002; Sklar et al., 2002; Schumacher et al., 2005; Strauss et al., 2005), neurodegenerative diseases such Huntington's Disease (Zuccato et al., 2001; Zuccato et al., 2003; Lynch et al., 2007), Alzheimers Disease (Nagahara et al., 2009); and neurodevelopmental diseases including Autism Spectrum Disorders (ASD). Rett Syndrome and Tuberous sclerosis represent two of the best examples of diseases involving disrupted BDNF signaling and often presenting with ASD. We use them as examples of how BDNF pathways can be involved in producing complex and devastating childhood onset diseases.
Rett syndrome is an X-linked dominant disease, affecting girls almost exclusively because males die as fetuses (Moretti and Zoghbi, 2006). Symptoms of Rett syndrome start between 6–18 months and include microcephaly, hand stereotypies, loss of purposeful hand use, regression of language and motor milestones, autistic-like behavior and breathing irregularities. Rett syndrome is usually caused by a mutation in the gene encoding methyl CpG binding protein 2 (MeCP2) which is involved in chromatin remodeling and control of gene transcription (Amir et al., 1999). MeCP2 mutant mice show impaired LTP, reduced size of the PSD, deficits of memory and spatial learning (Moretti et al., 2006) and smaller, less complex pyramidal neurons in cortical layers II and III, (Kishi and Macklis, 2004). In cultured neurons, MeCP2 binds the promoter region of a Bdnf gene and suppresses its transcription (Chen et al., 2003; Martinowich et al., 2003). However, BDNF protein levels are decreased, not increased in brains of MeCP2 knockout mice (Chang et al., 2006). Recent studies show that the Bdnf gene can be regulated by MeCP2 and that MeCP2 associates with CREB1 at the promoter of an activated target, but not at the promoter of a repressed target (Chahrour et al., 2008). Overexpression of BDNF restores locomotor activity levels in MeCP2 mutant mice and relieves some symptoms of the mutant phenotype (Chang et al., 2006). Another rescue study used insulin-like growth factor (IGF-1) that permeates the blood–brain barrier more effectively, yet shares overlapping neurotrophic effects with BDNF. Systemic injection of IGF-1 partially restores spine density and synaptic amplitude, increases PSD-95, and stabilizes cortical plasticity to wild-type levels in MeCP2 mutant mice (Tropea et al., 2009). Nevertheless, little is known about whether cell type differences or age dependent changes in BDNF pathway responses to these treatments exist or, in the latter experiment, whether the overlapping effects that IGF shares with BDNF are responsible for the changes observed.
The Tuberous sclerosis complex (TSC) is another childhood onset genetic disease that involves multiple organs including brain, skin, lung, kidney, and heart (Crino et al., 2006). The neurological symptoms include epilepsy, developmental delays, autistic behavior and giant cell astrocytomas. TSC1 and TSC2 are the two causative genes. TSC-1 and −2 proteins form a complex that negatively regulates the PI3K-AKT-mTOR cascade initiated by BDNF and other growth factors (Crino et al., 2006). Loss of TSC1 or TSC2 results in enlargement of somas and dysmorphic dendritic spines and also increases spontaneous miniature AMPAR current (Tavazoie et al., 2005). Furthermore, synapsinpromoter driven conditional Tsc1 knock-out mice show spontaneous seizures along with dysplastic and ectopic neurons (Meikle et al., 2007). In cultured hippocampal neurons, the dendritic arbor growth stimulated by either BDNF or overexpression of constitutively active mutants of Ras, PI3K and Akt can be blocked by inhibition of mTOR (Kumar et al., 2005). Also mTOR inhibitors including rapamycin can correct somal and dendritic hypertrophies, and improve survival, seizure frequency and behavioral abnormalities in Tsc mutant mice (Ehninger et al., 2008; Meikle et al., 2008). These results are consistent with the up-regulation of mTOR pathway in TSC.
Collectively, Rett syndrome and TSC show that both up- and down-regulation of neurotrophin-triggered signaling result in abnormal morphological and behavioral phenotypes, indicating a requirement for the optimal level of BDNF-TrkB signaling in normal development. While the pilot studies using mouse models indicate that therapeutic intervention of neutorophin pathways is feasible in ameliorating some of the symptoms of these neurodevelopmental disorders, it is not yet clear how these effects can be transferred to afflicted patients. This is largely because, we still have a long way to fully understand how neurotrophic signaling in general, and BDNF signaling in the brain in particular, is differentially regulated. We also have little data on how the various pathways through which BDNF pathways can signal, interact with the necessary processes of selective synapse formation and maintenance, pruning of exuberant circuitry, and fine control of both protein synthesis in general and local protein synthesis at synapses. Much more mechanistic knowledge of the complexities of these pathways will be necessary before any interventions developed in engineered mice can be brought to the bedside.
Acknowledgement
AY is the PI on DoD grant TS080074 and is partially supported by the latter grant and R01EY006039. Our own BDNF work discussed here is being supported by NIH grant R01EY006039 to MCP.
References
- Alonso M, Medina JH, Pozzo-Miller L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn Mem. 2004;11:172–178. doi: 10.1101/lm.67804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral MD, Pozzo-Miller L. TRPC3 channels are necessary for brain-derived neurotrophic factor to activate a nonselective cationic current and to induce dendritic spine formation. J Neurosci. 2007;27:5179–5189. doi: 10.1523/JNEUROSCI.5499-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpGbinding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Barco A, Patterson S, Alarcon JM, Gromova P, Mata-Roig M, Morozov A, Kandel ER. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron. 2005;48:123–137. doi: 10.1016/j.neuron.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. Embo J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beique JC, Andrade R. PSD-95 regulates synaptic transmission and plasticity in rat cerebral cortex. J Physiol. 2003;546:859–867. doi: 10.1113/jphysiol.2002.031369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabelli RJ, Hohn A, Shatz CJ. Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science. 1995;267:1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- Cabelli RJ, Shelton DL, Segal RA, Shatz CJ. Blockade of endogenous ligands of trkB inhibits formation of ocular dominance columns. Neuron. 1997;19:63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- Castren E, Pitkanen M, Sirvio J, Parsadanian A, Lindholm D, Thoenen H, Riekkinen PJ. The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. Neuroreport. 1993;4:895–898. doi: 10.1097/00001756-199307000-00014. [DOI] [PubMed] [Google Scholar]
- Castren E, Zafra F, Thoenen H, Lindholm D. Light regulates expression of brainderived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci U S A. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy S, Saiepour MH, Bence M, Perry S, Hartman R, Couey JJ, Mansvelder HD, Levelt CN. Postsynaptic TrkB signaling has distinct roles in spine maintenance in adult visual cortex and hippocampus. Proc Natl Acad Sci U S A. 2006;103:1071–1076. doi: 10.1073/pnas.0506305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006;49:341–348. doi: 10.1016/j.neuron.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Chen G, Kolbeck R, Barde YA, Bonhoeffer T, Kossel A. Relative contribution of endogenous neurotrophins in hippocampal long-term potentiation. J Neurosci. 1999;19:7983–7990. doi: 10.1523/JNEUROSCI.19-18-07983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Colledge M, Snyder EM, Crozier RA, Soderling JA, Jin Y, Langeberg LK, Lu H, Bear MF, Scott JD. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron. 2003;40:595–607. doi: 10.1016/s0896-6273(03)00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Beilharz E, Mason B, Lawlor P, Abraham W, Gluckman P. Brainderived neurotrophic factor expression after long-term potentiation. Neurosci Lett. 1993;160:232–236. doi: 10.1016/0304-3940(93)90420-p. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Klein M, Rumpel S, Malinow R. PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci U S A. 2007;104:4176–4181. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA. Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J Neurophysiol. 1998;80:3383–3386. doi: 10.1152/jn.1998.80.6.3383. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Galuske RA, Kim DS, Castren E, Singer W. Differential effects of neurotrophins on ocular dominance plasticity in developing and adult cat visual cortex. Eur J Neurosci. 2000;12:3315–3330. doi: 10.1046/j.1460-9568.2000.00213.x. [DOI] [PubMed] [Google Scholar]
- Gartner A, Polnau DG, Staiger V, Sciarretta C, Minichiello L, Thoenen H, Bonhoeffer T, Korte M. Hippocampal long-term potentiation is supported by presynaptic and postsynaptic tyrosine receptor kinase B-mediated phospholipase Cgamma signaling. J Neurosci. 2006;26:3496–3504. doi: 10.1523/JNEUROSCI.3792-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes RA, Hampton C, El-Sabeawy F, Sabo SL, McAllister AK. The dynamic distribution of TrkB receptors before, during, and after synapse formation between cortical neurons. J Neurosci. 2006;26:11487–11500. doi: 10.1523/JNEUROSCI.2364-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk W, Pozzo-Miller LD, Figurov A, Lu B. Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the developing hippocampus. J Neurosci. 1998;18:6830–6839. doi: 10.1523/JNEUROSCI.18-17-06830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruart A, Sciarretta C, Valenzuela-Harrington M, Delgado-Garcia JM, Minichiello L. Mutation at the TrkB PLC{gamma}-docking site affects hippocampal LTP and associative learning in conscious mice. Learn Mem. 2007;14:54–62. doi: 10.1101/lm.428307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci. 1999;19:RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XP, Kotloski R, Nef S, Luikart BW, Parada LF, McNamara JO. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron. 2004;43:31–42. doi: 10.1016/j.neuron.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Huber KM, Sawtell NB, Bear MF. Brain-derived neurotrophic factor alters the synaptic modification threshold in visual cortex. Neuropharmacology. 1998;37:571–579. doi: 10.1016/s0028-3908(98)00050-1. [DOI] [PubMed] [Google Scholar]
- Ji Y, Pang PT, Feng L, Lu B. Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nat Neurosci. 2005;8:164–172. doi: 10.1038/nn1381. [DOI] [PubMed] [Google Scholar]
- Johnson D, Lanahan A, Buck CR, Sehgal A, Morgan C, Mercer E, Bothwell M, Chao M. Expression and structure of the human NGF receptor. Cell. 1986;47:545–554. doi: 10.1016/0092-8674(86)90619-7. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Hanover JL, England PM, Stryker MP. TrkB kinase is required for recovery, but not loss, of cortical responses following monocular deprivation. Nat Neurosci. 2008;11:497–504. doi: 10.1038/nn2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophininduced hippocampal synaptic plasticity. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- Kang H, Welcher AA, Shelton D, Schuman EM. Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Hempstead BL, Martin-Zanca D, Chao MV, Parada LF. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991a;252:554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Martin-Zanca D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991b;350:158–160. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Yasuda H, Taniguchi N, Katoh-Semba R, Hatanaka H, Tsumoto T. Brain-derived neurotrophic factor prevents low-frequency inputs from inducing long-term depression in the developing visual cortex. J Neurosci. 1999;19:2122–2130. doi: 10.1523/JNEUROSCI.19-06-02122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi N, Macklis JD. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol Cell Neurosci. 2004;27:306–321. doi: 10.1016/j.mcn.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Klann E, Dever TE. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci. 2004;5:931–942. doi: 10.1038/nrn1557. [DOI] [PubMed] [Google Scholar]
- Klein R, Jing SQ, Nanduri V, O'Rourke E, Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991a;65:189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- Klein R, Nanduri V, Jing SA, Lamballe F, Tapley P, Bryant S, Cordon-Cardo C, Jones KR, Reichardt LF, Barbacid M. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell. 1991b;66:395–403. doi: 10.1016/0092-8674(91)90628-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic factor mutant mice. Proc Natl Acad Sci U S A. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimizu H, Kiyosue K, Hara T, Hazama S, Suzuki S, Uegaki K, Nagappan G, Zaitsev E, Hirokawa T, Tatsu Y, Ogura A, Lu B, Kojima M. Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation and cell survival. Mol Brain. 2009;2:27. doi: 10.1186/1756-6606-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A. Postsynaptic Induction of BDNFMediated Long-Term Potentiation. Science. 2002;295:1729–1734. doi: 10.1126/science.1067766. [DOI] [PubMed] [Google Scholar]
- Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci. 2005;25:11288–11299. doi: 10.1523/JNEUROSCI.2284-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang SB, Stein V, Bonhoeffer T, Lohmann C. Endogenous brain-derived neurotrophic factor triggers fast calcium transients at synapses in developing dendrites. J Neurosci. 2007;27:1097–1105. doi: 10.1523/JNEUROSCI.3590-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, Thoenen H, Barde YA. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341:149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Li Y, Jia YC, Cui K, Li N, Zheng ZY, Wang YZ, Yuan XB. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434:894–898. doi: 10.1038/nature03477. [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Lynch G, Kramar EA, Rex CS, Jia Y, Chappas D, Gall CM, Simmons DA. Brainderived neurotrophic factor restores synaptic plasticity in a knock-in mouse model of Huntington's disease. J Neurosci. 2007;27:4424–4434. doi: 10.1523/JNEUROSCI.5113-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Man HY, Wang Q, Lu WY, Ju W, Ahmadian G, Liu L, D'Souza S, Wong TP, Taghibiglou C, Lu J, Becker LE, Pei L, Liu F, Wymann MP, MacDonald JF, Wang YT. Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron. 2003;38:611–624. doi: 10.1016/s0896-6273(03)00228-9. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Rauskolb S, Polack M, Klose J, Kolbeck R, Korte M, Barde YA. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci. 2008;11:131–133. doi: 10.1038/nn2038. [DOI] [PubMed] [Google Scholar]
- Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, Kwiatkowski DJ. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, Jensen FE, Kwiatkowski DJ. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27:5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Bardsen K, Srebro B, Bramham CR. Acute intrahippocampal infusion of BDNF induces lasting potentiation of synaptic transmission in the rat dentate gyrus. J Neurophysiol. 1998;79:496–499. doi: 10.1152/jn.1998.79.1.496. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Calella AM, Medina DL, Bonhoeffer T, Klein R, Korte M. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron. 2002;36:121–137. doi: 10.1016/s0896-6273(02)00942-x. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp HP, Bonhoeffer T, Klein R. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, Armstrong D, Arancio O, Sweatt JD, Zoghbi HY. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci. 2006;26:319–327. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P, Zoghbi HY. MeCP2 dysfunction in Rett syndrome and related disorders. Curr Opin Genet Dev. 2006;16:276–281. doi: 10.1016/j.gde.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Neuroprotective effects of brainderived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves-Pereira M, Mundo E, Muglia P, King N, Macciardi F, Kennedy JL. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. Am J Hum Genet. 2002;71:651–655. doi: 10.1086/342288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Pittenger C, Morozov A, Martin KC, Scanlin H, Drake C, Kandel ER. Some forms of cAMP-mediated long-lasting potentiation are associated with release of BDNF and nuclear translocation of phospho-MAP kinase. Neuron. 2001;32:123–140. doi: 10.1016/s0896-6273(01)00443-3. [DOI] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Sattler R, Zhang X, Huganir RL, Kambampati V, Mains RE, Eipper BA. The neuronal Rho-GEF Kalirin-7 interacts with PDZ domaincontaining proteins and regulates dendritic morphogenesis. Neuron. 2001;29:229–242. doi: 10.1016/s0896-6273(01)00193-3. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Ratto GM, Putignano E, Maffei L. Brain-derived neurotrophic factor causes cAMP response element-binding protein phosphorylation in absence of calcium increases in slices and cultured neurons from rat visual cortex. J Neurosci. 2000;20:2809–2816. doi: 10.1523/JNEUROSCI.20-08-02809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruunsild P, Kazantseva A, Aid T, Palm K, Timmusk T. Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics. 2007;90:397–406. doi: 10.1016/j.ygeno.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radeke MJ, Misko TP, Hsu C, Herzenberg LA, Shooter EM. Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature. 1987;325:593–597. doi: 10.1038/325593a0. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch H, Schweigreiter R, Bonhoeffer T, Barde YA, Korte M. The neurotrophin receptor p75NTR modulates long-term depression and regulates the expression of AMPA receptor subunits in the hippocampus. Proc Natl Acad Sci U S A. 2005;102:7362–7367. doi: 10.1073/pnas.0502460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi FM, Bozzi Y, Pizzorusso T, Maffei L. Monocular deprivation decreases brain-derived neurotrophic factor immunoreactivity in the rat visual cortex. Neuroscience. 1999;90:363–368. doi: 10.1016/s0306-4522(98)00463-1. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Frey JU. Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiol Learn Mem. 2004;82:12–25. doi: 10.1016/j.nlm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Navakkode S, Sacktor TC, Frey JU. Synaptic tagging and crosstagging: the role of protein kinase Mzeta in maintaining long-term potentiation but not long-term depression. J Neurosci. 2005;25:5750–5756. doi: 10.1523/JNEUROSCI.1104-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez AL, Matthews BJ, Meynard MM, Hu B, Javed S, Cohen Cory S. BDNF increases synapse density in dendrites of developing tectal neurons in vivo. Development. 2006;133:2477–2486. doi: 10.1242/dev.02409. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Schumacher J, Jamra RA, Becker T, Ohlraun S, Klopp N, Binder EB, Schulze TG, Deschner M, Schmal C, Hofels S, Zobel A, Illig T, Propping P, Holsboer F, Rietschel M, Nothen MM, Cichon S. Evidence for a relationship between genetic variants at the brain-derived neurotrophic factor (BDNF) locus and major depression. Biol Psychiatry. 2005;58:307–314. doi: 10.1016/j.biopsych.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Shimada A, Mason CA, Morrison ME. TrkB signaling modulates spine density and morphology independent of dendrite structure in cultured neonatal Purkinje cells. J Neurosci. 1998;18:8559–8570. doi: 10.1523/JNEUROSCI.18-21-08559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar P, Gabriel SB, McInnis MG, Bennett P, Lim YM, Tsan G, Schaffner S, Kirov G, Jones I, Owen M, Craddock N, DePaulo JR, Lander ES. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol Psychiatry. 2002;7:579–593. doi: 10.1038/sj.mp.4001058. [DOI] [PubMed] [Google Scholar]
- Soppet D, Escandon E, Maragos J, Middlemas DS, Reid SW, Blair J, Burton LE, Stanton BR, Kaplan DR, Hunter T, Nikolics K, Parade LF. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell. 1991;65:895–903. doi: 10.1016/0092-8674(91)90396-g. [DOI] [PubMed] [Google Scholar]
- Squinto SP, Stitt TN, Aldrich TH, Davis S, Bianco SM, Radziejewski C, Glass DJ, Masiakowski P, Furth ME, Valenzuela DM, et al. trkB encodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell. 1991;65:885–893. doi: 10.1016/0092-8674(91)90395-F. [DOI] [PubMed] [Google Scholar]
- Stein V, House DR, Bredt DS, Nicoll RA. Postsynaptic density-95 mimics and occludes hippocampal long-term potentiation and enhances long-term depression. J Neurosci. 2003;23:5503–5506. doi: 10.1523/JNEUROSCI.23-13-05503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J, Barr CL, George CJ, Devlin B, Vetro A, Kiss E, Baji I, King N, Shaikh S, Lanktree M, Kovacs M, Kennedy JL. Brain-derived neurotrophic factor variants are associated with childhood-onset mood disorder: confirmation in a Hungarian sample. Mol Psychiatry. 2005;10:861–867. doi: 10.1038/sj.mp.4001685. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Saito H, Matsuki N. Inhibition of GABAA synaptic responses by brainderived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycinsensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie SF, Alvarez VA, Ridenour DA, Kwiatkowski DJ, Sabatini BL. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat Neurosci. 2005;8:1727–1734. doi: 10.1038/nn1566. [DOI] [PubMed] [Google Scholar]
- Tian F, Marini AM, Lipsky RH. NMDA receptor activation induces differential epigenetic modification of Bdnf promoters in hippocampal neurons. Amino Acids. 2009 doi: 10.1007/s00726-009-0315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D, Giacometti E, Wilson NR, Beard C, McCurry C, Fu DD, Flannery R, Jaenisch R, Sur M. Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proc Natl Acad Sci U S A. 2009;106:2029–2034. doi: 10.1073/pnas.0812394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller L. Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J Physiol. 2003;553:497–509. doi: 10.1113/jphysiol.2003.052639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21:4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viegi A, Cotrufo T, Berardi N, Mascia L, Maffei L. Effects of dark rearing on phosphorylation of neurotrophin Trk receptors. Eur J Neurosci. 2002;16:1925–1930. doi: 10.1046/j.1460-9568.2002.02270.x. [DOI] [PubMed] [Google Scholar]
- Wardle RA, Poo MM. Brain-derived neurotrophic factor modulation of GABAergic synapses by postsynaptic regulation of chloride transport. J Neurosci. 2003;23:8722–8732. doi: 10.1523/JNEUROSCI.23-25-08722.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- Wu K, Xu JL, Suen PC, Levine E, Huang YY, Mount HT, Lin SY, Black IB. Functional trkB neurotrophin receptors are intrinsic components of the adult brain postsynaptic density. Brain Res Mol Brain Res. 1996;43:286–290. doi: 10.1016/s0169-328x(96)00211-2. [DOI] [PubMed] [Google Scholar]
- Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, Wang D, Nicoll RA, Lu B, Reichardt LF. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J Neurosci. 2000;20:6888–6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Siao CJ, Nagappan G, Marinic T, Jing D, McGrath K, Chen ZY, Mark W, Tessarollo L, Lee FS, Lu B, Hempstead BL. Neuronal release of proBDNF. Nat Neurosci. 2009;12:113–115. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat Neurosci. 2007;10:702–711. doi: 10.1038/nn1903. [DOI] [PubMed] [Google Scholar]
- Yoshii A, Sheng MH, Constantine-Paton M. Eye opening induces a rapid dendritic localization of PSD-95 in central visual neurons. Proc Natl Acad Sci U S A. 2003;100:1334–1339. doi: 10.1073/pnas.0335785100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii A, Tye LD, Shokat KM, Constantine-Paton M. Developmental increase of PSD-95 in the rodent visual cortex is suppressed by the blockade of TrkB using a chemical genetic approach. Washington D.C: Society for Neuroscience; 2008. [Google Scholar]
- Zagrebelsky M, Holz A, Dechant G, Barde YA, Bonhoeffer T, Korte M. The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J Neurosci. 2005;25:9989–9999. doi: 10.1523/JNEUROSCI.2492-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalfa F, Eleuteri B, Dickson KS, Mercaldo V, De Rubeis S, di Penta A, Tabolacci E, Chiurazzi P, Neri G, Grant SG, Bagni C. A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat Neurosci. 2007;10:578–587. doi: 10.1038/nn1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JP, Murata M, Constantine-Paton M. SiRNA against PSD-95 in intact superior colliculus blocks LTP. San Diego: Society for Neuroscience; 2007. [Google Scholar]
- Zhao JP, Phillips MA, Constantine-Paton M. Long-term potentiation in the juvenile superior colliculus requires simultaneous activation of NMDA receptors and Ltype Ca2+ channels and reflects addition of newly functional synapses. J Neurosci. 2006;26:12647–12655. doi: 10.1523/JNEUROSCI.3678-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, MacDonald ME, Friedlander RM, Silani V, Hayden MR, Timmusk T, Sipione S, Cattaneo E. Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease. Science. 2001;293:493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Tartari M, Crotti A, Goffredo D, Valenza M, Conti L, Cataudella T, Leavitt BR, Hayden MR, Timmusk T, Rigamonti D, Cattaneo E. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet. 2003;35:76–83. doi: 10.1038/ng1219. [DOI] [PubMed] [Google Scholar]