Abstract
Behavioral studies have suggested that the stabilization of motor memory varies depending on the practice schedule. The neural substrates underlying this schedule-dependent difference in memory stabilization are not known. Here, we evaluated the effects of 1-Hz repetitive transcranial magnetic stimulation (rTMS) applied to different cortical regions and sham after one session of training (Day 1) of sequential motor skills acquired through blocked (each sequence was completely trained before training the next)-practice schedules and random (random training of 3 sequences)-practice schedules. The recall of sequences learned on Day 1 by Day 2 was measured in different groups of healthy volunteers. The rTMS over the supplementary motor area (SMA) but not over control regions or over the primary motor cortex (M1) immediately after practice or over SMA 6 h later reduced recall relative to sham only in the blocked-practice group. In contrast, recall in the random-practice group was unaffected by rTMS. These results document a differential contribution of the SMA to the stabilization of motor memories acquired through different practice schedules. More generally, they indicate that the anatomical substrates underlying motor-memory stabilization (or their temporal operation) do differ depending on the practice schedule.
Keywords: magnetic stimulation, memory and learning, motor cortex, motor learning, training
Introduction
After practice, a newly learned skill undergoes a period of stabilization (a form of consolidation) before it becomes stable and resistant to disruption by subsequent interference (McGaugh 2000; Dudai 2004; Robertson et al. 2004; Krakauer and Shadmehr 2006). The time course of memory stabilization and its underlying neural circuitry differ depending on the sensory modalities where learning occurs and the type of task (e.g., Karni and Sagi 1993; Brashers-Krug et al. 1996; Muellbacher et al. 2002; Walker et al. 2003; Seitz et al. 2005).
One area of study that has elicited substantial interest in recent years has been the investigation of the influence of practice schedules on acquisition and retention of motor skills, an issue relevant to neurorehabilitation of the motor function (Schmidt 1975, 1991; Hanlon 1996; Krakauer, 2006; Lin et al. 2007; Reis et al. 2008). Previous work has proposed that practice schedule may influence the retention of motor memories (Shea and Morgan 1979; Schmidt 1988; Magill and Hall 1990; Hall and Magill 1995). Shea and Morgan (1979) reported that the retention of a learned sequence was better when practice of different motor sequences was random rather than when practice of each sequence was completed before the next was practiced. The authors found that randomizing the order of trained sequences from trial to trial produced a context that resulted in superior long-term retention, referred to as the contextual-interference effect. Consistent with these findings, recent behavioral studies have also indicated that practice schedule influences motor-memory stabilization after learning (Osu et al. 2004; Overduin et al. 2006). These findings suggest that simultaneous learning of multiple skills in randomized order may accelerate or facilitate posttraining memory stabilization and thus have advantageous effects in the long-term retention of a learned task (Robertson et al. 2004).
The learning of motor sequences engages an extensive network that includes the supplementary motor area (SMA), primary motor cortex (M1), parietal regions, cerebellum, and basal ganglia. The SMA, with its extensive interconnected network, plays a pivotal role in motor skill learning in general and in sequential motor behavior in particular (Tanji and Shima 1994; Tanji 1996; Gerloff et al. 1997; Honda et al. 1998; Nakamura et al. 1998, 1999; Doyon et al. 2002; Hikosaka et al. 2002; Doyon et al. 2003; Perez et al. 2007; Doyon et al. 2009). Previous work has provided evidence of the involvement of M1 in the stabilization of learning of simple ballistic movements as well as sequential movements (Muellbacher et al. 2002; Baraduc et al. 2004; Robertson et al. 2005). It is largely unknown, on the other hand, how the practice schedule influences the neural circuitry of posttraining motor-memory stabilization including the involvement of M1 and, the focus of this study, if SMA plays a contributory role. Previous behavioral and theoretical studies have proposed that multiple episodes of retrieval of a motor skill interspersed with the practice of another skill provide opportunities for better stabilization of memory traces (Shea and Morgan 1979; Lee and Magill 1983; Robertson et al. 2004). Therefore, it is possible that sequential motor memories encoded during random practice could have been more firmly stabilized in the SMA and be more resistant to physiological interference than during blocked practice. To address this question, we evaluated the “virtual lesion” effects of 1-Hz repetitive transcranial magnetic stimulation (rTMS) applied over the SMA and control cortical regions as well as sham stimulation immediately after the practice of 3 different motor sequences trained in 2 different practice schedules: blocked and random. We hypothesized that rTMS over the SMA would disrupt the recall of learned motor skills more after blocked practice than after random practice.
Materials and Methods
Subjects and Experimental Design
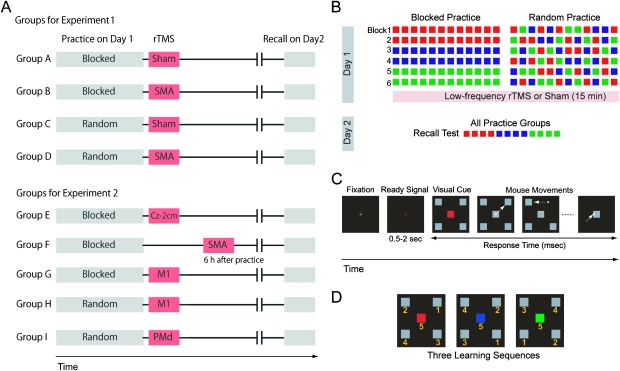
Sixty healthy volunteers (25 of them females, 27.7 ± 7.0 years) who were randomly assigned to 4 different groups (n = 15 each) participated in Experiment 1, which was geared to determine what effects the disruption of SMA activity with 1-Hz rTMS versus sham stimulation immediately after practice had on recall on Day 2 with the 2 different practice schedules (blocked and random). The 4 groups were as follows: (A) sham stimulation shortly after blocked practice, (B) 1-Hz rTMS over the SMA shortly after blocked practice, (C) sham stimulation shortly after random practice, or (D) 1-Hz rTMS over the SMA shortly after random practice (Fig. 1A). In Experiment 2, 63 additional subjects (20 of them females, 25.0 ± 4.6 years), who were divided into 5 groups, were later recruited to study what effects the disruption of SMA 6 h after practice had ended and the stimulation of other cortical regions also shortly after practice had ended had on recall on Day 2: (E) 1-Hz rTMS to a control position 2 cm posterior to Cz (international 10–20 system, n = 12), (G) to the left M1 (n = 13) shortly after blocked practice, (H) to the left M1 shortly after random practice (n = 13), (I) to the left dorsal premotor cortex (PMd) shortly after random practice (n = 13), and (F) 1-Hz rTMS to the SMA 6 h after blocked practice (n = 12) (Fig. 1A).
Figure 1.
Task and experimental design. (A) Healthy volunteers were divided into 9 groups. In the main experiment, 4 groups were evaluated in a 2 × 2 factorial design, defined by the type of practice (blocked/random) and rTMS (sham/SMA). Five more groups were studied in a set of additional experiments. (B) Experimental design. On Day 1, subjects practiced 6 training blocks (12 sequences/block). Different groups trained the 3 sequences in blocked (all sequences of each type were practiced before the next sequence was introduced, first red, then blue, and later green) or random (practice of the 3 sequences was intermixed) order. 1-Hz low-frequency rTMS or sham was applied over different brain regions shortly after or 6 h after the training ended. The recall test on Day 2 consisted of 12 trials of the previously learned sequences (4 trials per sequence), and the order of sequences was counterbalanced across the subjects. (C) Sequential visuomotor task, modified from Shea and Morgan (1979). Subjects were instructed to focus on a central fixation point and subsequently warned of the upcoming trial by the presentation of a ready (red cross) signal for 500–2000 ms. After the ready signal disappeared, a visual cue composed of a red, blue, or green central square surrounded by 4 gray squares was presented. Each color was associated with a particular order of 5 subsequent mouse movements that started and finished at the central square. The subjects’ instructions were to move a screen cursor with the mouse and click on each of the 4 targets one at a time. If it was the correct target, it disappeared from the screen and subjects were required to move and click the next target. If it was an incorrect target, it did not disappear and subjects were required to move the cursor toward the other peripheral targets (see Materials and Methods). The RT from the presentation of the visual cue to the end of each sequence represented the end point measure of a previous landmark study (Shea and Morgan 1979). (D) Practiced sequences. The numbers (not shown to the subjects in the actual experiment) indicate the order of the targets in each sequence.
The purpose of Experiment 1 (groups A–D) was to explore what differential effects a virtual lesion disruption of SMA (Pascual-Leone et al. 2000) immediately postpractice had on recall on Day 2 with the 2 practice schedules, blocked and random. Groups E–I (Experiment 2) were added to characterize the temporal and spatial specificity of the effects of SMA stimulation on stabilization with the 2 practice schedules. For random practice, the M1 (Muellbacher et al. 2002; Baraduc et al. 2004; Robertson et al. 2005; Lin et al. 2008, 2009) and PMd (Shadmehr and Holcomb 1997; Cross et al. 2007) were targeted because previous studies have suggested the possible involvement of these structures in the stabilization of motor skills. All subjects were right handed and had given written informed consent before the experiments. None of them had a history of psychiatric or neurological illness. The experiment was approved by the National Institute of Neurological Disorders and Stroke Ethics Committee and the local Ethics Committee of the National Institute for Neuroscience.
All subjects came to the laboratory on 2 subsequent days (Days 1 and 2). On Day 1, they practiced 3 motor sequences (6 training blocks of 12 sequences per block for a total of 72 sequences. Each sequence was practiced 24 times in a blocked- or a random-practice schedule (Fig. 1B). In the blocked-practice groups, they completed all practice trials of each sequence before proceeding to the next. In the random-practice groups, subjects practiced the 3 sequences intermixed in random order. The recall test on Day 2 consisted of 12 trials of the previously learned sequences (4 trials per sequence), and the order of sequences was counterbalanced across the subjects (Fig. 1B).
Sequential Visuomotor Task
We used a sequential-visuomotor task similar to that described by Shea and Morgan (1979) in which the contextual-interference effect was best described. Subjects sat in an armchair and visual stimuli were presented on a computer screen using a script based on Presentation software (Neurobehavioral Systems, Albany, CA). They were initially instructed to focus on a central fixation point (3–5 s) and subsequently warned of the upcoming trial by the presentation of a ready (red cross) signal for 0.5–2 s. After the ready signal disappeared, a visual cue composed of a red, blue, or green central square surrounded by 4 gray squares was presented. Each visual cue color was associated with a particular order of 5 subsequent mouse movements that started and finished at the central square (see Fig. 1C for an example of a sequence time course and Fig. 1D for the order of mouse movements in the 3 learning sequences). The subjects’ instructions were to move the screen's cursor with the mouse and click on each of the 4 targets one at a time until all targets were completed. Subjects were asked to be as fast and accurate as possible. The visual cue color was visible until subjects started to move the cursor and the center square changed color to gray at the onset of mouse movement. For each single mouse movement, if the chosen target was correct for that particular sequence, the target disappeared from the screen and subjects were required to move the cursor to the next target and click onto it. If it was the incorrect target, it did not disappear and they were required to move the cursor toward the other peripheral targets until they hit the correct one. For example, the correct sequence for the red visual cue was right-up, left-up, right-down, left-down, and central square (Fig. 1C,D). For all sequences, the last target was always the square at the center of the screen. The intertrial intervals ranged randomly between 3 and 5 s. The response time (RT), that is, the primary end point measure of the study, was defined as the time between the onset of the presentation of the visual cue and the mouse click onto the last target stimulus (central square). For each participant, the median RT of 12 sequences (4 for each of the 3 practiced types: red, blue, and green in Fig. 1B) was calculated for each practice block on Day 1 (e.g., the value representing RT in Block 1 in each individual was the median of the first 4 red, the first 4 blue, and the first 4 green practiced sequences; in Block 2, it was the median of the second 4 red, the second 4 blue, and the second 4 green sequences, etc.) and at recall time on Day 2. Group data were calculated as the mean ± standard error of the individual median values. This analysis was required to compare improvements in performance over the practice time across the 2 training types (random and blocked). We also evaluated performance in an untrained, nonsequential, simple visuomotor task as a control-motor task. The purpose was to determine if the hypothesized disruptive effects of rTMS were specific to the newly learned sequential skill or represented a less specific effect on motor function in general (see Supplementary material for details).
Transcranial Magnetic Stimulation
The rTMS was delivered from a Magstim Rapid Stimulator (Magstim Company, Whitland, UK) through 80-mm figure-eight coils that allowed delivery of real or sham stimuli. At the beginning of each experiment, we determined the resting motor threshold (rMT) for the right first dorsal interosseous (FDI) muscle over the left M1. The rMT was defined as the lowest intensity of transcranial magnetic stimulation (TMS) output required to elicit the motor-evoked potentials (MEPs) of at least a 50-μV peak-to-peak amplitude in at least 5 of the 10 consecutive trials (Rossini et al. 1994). The coil was placed tangential to the scalp with the junction region pointing backward and laterally at a 45° angle away from the midline (Di Lazzaro et al. 2004). In Experiment 1, we evaluated what effects 1-Hz rTMS over the SMA (15 min at 115% rMT intensity) or the sham applied shortly after practice had on recall on Day 2. The same intensity and duration of rTMS, which was started within 10 min after practice had ended in all groups, was used in all the experiments (115% rMT intensity for the FDI). The 1-Hz rTMS results in decreased excitability of the underlying cortical areas (Chen et al. 1997; Robertson et al. 2003) and can successfully downregulate activity in the SMA (Tanaka et al. 2005; Perez et al. 2007, 2008). When applied over the M1, this stimulation protocol decreases motor cortical excitability and influences the motor stabilization of tasks practiced in a blocked schedule (Muellbacher et al. 2002; Baraduc et al. 2004). The site of stimulation for each cortical area was determined using previously described procedures (Matsunaga et al. 2005; Perez et al. 2007, 2008; see Supplementary material for details).
Electromyographic Recordings
The electromyographic (EMG) activity was recorded from surface electrodes positioned on the skin overlying the FDI and tibialis anterior muscles in a bipolar montage (interelectrode distance, 2 cm). The EMG signals were amplified, filtered (band-pass, 25 Hz to 1 kHz), sampled at 2 kHz, and stored on a personal computer for off-line analysis.
Data Analysis
The median RT of 12 sequences (including the 3 practiced sequences) was calculated for each participant for each practice block on Day 1 and at the recall time on Day 2. Repeated measures analysis of variance (ANOVA) was implemented with “practice” (blocked vs. random) as a between-subjects factor and “time” (6 practice blocks) as a within-subject factor to evaluate Day 1’s practice. The effects that 1-Hz rTMS (real or sham) applied over the SMA immediately after practice on Day 1 had on recall on Day 2 with the 2 different practice schedules (random and blocked, groups A–D) were analyzed with a two-way factorial ANOVA design with between-subject factors of practice (blocked vs. random) and rTMS (SMA vs. sham): blocked/sham, blocked/SMA, random/sham, and random/SMA. To gain information on the temporal and spatial specificity of rTMS effects, we included the 5 additional groups described above (E–I). One-way ANOVA was performed for each practice schedule (blocked and random). The values were considered significant if P <0.05.
Results
Experiment 1
The male/female ratios, age, rMT, duration (h), and quality (a visual analog scale ranging from 1 to 10) of sleep time on the nights before recall testing, as well as experience (in years) and frequency of use of a computer mouse (days per week), were comparable across the 4 experimental groups (see Supplementary material). The mean RT and error response (the number of incorrect mouse clicks) on the initial block of training on Day 1 and the recall test on Day 2 had significant positive correlations (r = 0.59, P <0.001 for the initial block on training on Day 1; r = 0.45, P <0.001 for Day 2). No negative correlations were observed throughout the experiment, indicating there was no trade-off in speed accuracy.
Day 1
To evaluate the practice effects on training on Day 1 (preceding rTMS application), the RT data of the 4 groups (blocked/SMA, blocked/sham, random/SMA, and random/sham) were subjected to two-way ANOVA with factors practice (blocked vs. random) and time (6 practice blocks; Fig. 2).
Figure 2.
Effects of practice on RTs during learning on Day 1. As expected, RTs were initially longer in the random-practice groups (black circle and gray squares) than in the blocked (gray triangles and diamonds)-practice groups. It is evident that all 4 groups improved with training (although at different paces), reaching comparable RT at the end of the practice session (Block 6) on Day 1. Data are expressed as mean RT ± standard error. *P < 0.05.
There was a significant main effect of time (F5,290 = 57.69, P < 0.001, indicating that subjects improved RT as practice proceeded), practice (F1,58 = 14.67, P < 0.001, pointing to the fact that subjects in the blocked-practice groups responded faster overall than those in the random-practice groups), and practice × time interaction (F5,290 = 26.81, P < 0.001) on RT. RTs over the practice period were slower at the beginning of Day 1 in the random groups than in the blocked groups. However, the difference in RT became progressively shorter over the practice time. The results of one-way ANOVA in the last training block (number 6) across all 4 groups were not significant (F3,59 = 0.31, P = 0.82). Therefore, preceding the application of rTMS, subjects in the sham and real rTMS groups in both practice schedules had comparable performance, reaching similar RT.
Day 2
Despite the comparable performance at the end of the training on Day 1, we found a significant main effect of practice (F1,56 = 14.99, P < 0.001) and practice × rTMS interaction (F1,56 = 4.04, P < 0.05) but not rTMS (F1,56 = 2.43, P = 0.13) on RT at the recall time on Day 2 (Fig. 3A). These findings indicate that rTMS over the SMA shortly after the training period ended influenced recall differently on Day 2 depending on the practice schedule. Post hoc analysis revealed that RT on Day 2 for the blocked-practice group was significantly slower in the SMA-stimulation group (3256 ± 110 ms) relative to the sham group (2961 ± 69 ms, Bonferroni correction, P = 0.014), in the absence of differences between the random-practice groups, sham (2808 ± 64 ms), and SMA stimulation (2770 ± 81 ms, P = 0.75). By recall time on Day 2, subjects in the blocked-practice groups with rTMS over the SMA lost virtually all RT improvements acquired during learning on Day 1. rTMS did not significantly affect the error rate or reaction times (see Supplementary material for details).
Figure 3.
Effects of practice and SMA stimulation applied shortly after training on RTs and delta RT on Day 2. (A) RTs on Day 2 were shorter in the 2 random-practice groups than in the 2 blocked ones. With blocked practice, a virtual lesion of the SMA with 1-Hz rTMS shortly after practice on Day 1 resulted in longer RT on Day 2 relative to sham. By contrast, there were no significant differences in RT between rTMS and sham in the random-practice groups. The bars depict mean ± standard error of the mean. *P < 0.05. ***P < 0.001. (B) Delta RT, calculated by subtracting mean RTs in the last practice block of Day 1 minus RT in the testing block on Day 2 in each of the 4 groups, revealed an interaction comparable to that evidenced by the analysis of the raw RT data in (A).
Figure 3B shows the results for delta RT, defined as a between-session change in RT (mean RT in the last training block of Day 1 − RT in the recall testing on Day 2), which were comparable to those of raw RT: significant effects of practice (F1,56 = 30.46, P < 0.001) and practice × rTMS (F1,56 = 5.43, P < 0.05) interaction but not rTMS.
Control Task
The mean RTs of the 4 groups A–D before practice in Experiment 1 were comparable for the blocked and random schedules (two-way ANOVA, practice: F1,56 = 0.19, P > 0.66; rTMS: F1,56 = 0.01, P > 0.93). On Day 2 (Supplementary Fig. B), the main effect of practice on RT was significant (F1,56 = 8.71, P = 0.005), whereas there were no significant effects of rTMS (F1,56 = 1.01, P = 0.32) or practice × rTMS interaction (F1,56 = 0.97, P = 0.33). Post hoc comparisons between sham and rTMS conditions for each practice schedule also did not reveal significant differences (blocked practice, t28 = 1.24, P = 0.23; random practice, t28 = 0.02, P = 0.99). These results indicate that rTMS did not affect recall in the untrained control-motor task.
Experiment 2
The effects of 1-Hz rTMS over the SMA on the stabilization of RT after blocked practice had temporal and spatial specificity (Fig. 4). One-way ANOVA indicated a significant difference in recall on Day 2 for the 4 blocked-practice groups (F4,62 = 2.49, P = 0.05; Fig. 4A). Post hoc analysis revealed that relative to the sham group, the mean RT at recall time on Day 2 was significantly slower in the group that received 1-Hz rTMS over the SMA immediately after blocked practice (Dunnett t-test, P = 0.019) but not after 6 h of practice, after stimulation of the M1, or after stimulation of the control position, Cz-2. Thus, stabilization of the procedural skill memory acquired in a blocked schedule was SMA dependent immediately after but not 6 h following the practice. Recall testing on Day 2 after random practice was not affected by 1-Hz rTMS over any of the regions evaluated (SMA, left M1 or left PMd, one-way ANOVA, F3,52 = 0.15, P > 0.90; Fig. 4B).
Figure 4.
Effects of blocked (A) and random (B) practice and stimulation of different cortical sites on RTs on Day 2. With blocked practice (A), note that the group that received SMA stimulation shortly after practice on Day 1 had longer RT on Day 2 than the group that received sham. Stimulation of the control scalp position (2 cm posterior to Cz), stimulation of the left M1 shortly after practice, or SMA stimulation 6 h after practice resulted in RT values comparable to sham. (B) With random practice, there were no significant differences in RT on Day 2 between groups that received stimulation of the SMA, left M1, or left PMd.
Discussion
Two novel findings emerged from this study, in which subjects learned a procedural motor skill in different practice schedules: 1) rTMS over the SMA shortly after training disrupted the motor-memory stabilization of the learned sequential movements but not the control task, and 2) this effect was practice schedule specific, present with blocked but not with random practice.
The SMA plays an important role in the execution and learning of sequential movements (Tanji and Shima 1994; Tanji 1996; Nakamura et al. 1998, 1999; Hikosaka et al. 2002; Doyon et al. 2003; Perez et al. 2007; Doyon et al. 2009). Recent data, demonstrating the modulation of activation of SMA-based networks immediately after a sequential motor task was practiced (Peigneux et al. 2006) and during rapid eye movement sleep (Maquet et al. 2000), have been interpreted as suggestive of the involvement of SMA in motor-memory stabilization. We designed this study to evaluate what effects the disruption of SMA using a virtual lesion approach had on motor-memory stabilization.
The subjects in all 4 groups in Experiment 1 were matched for age and gender, had comparable initial performance levels in the control-motor task, experience with mouse use, sleep quality and time, and rMTs to TMS. At the onset of training, subjects in the random-practice groups started with slower RT in the sequential task than subjects in the blocked-practice groups, consistent with the need for frequent alternation of practice sequences (required for random practice) and in accordance with the contextual-interference effect (Shea and Morgan 1979; Magill and Hall 1990). Over the training period, the 4 groups (A–D) reached comparable RT levels by Block 6 (Fig. 2), indicating similar performance at the end of Day 1. rTMS was applied shortly after the training ended to all groups.
Stabilization of Motor Memory in Blocked-Practice Schedule
In the blocked-practice groups, 1-Hz rTMS over the SMA immediately after practice resulted in longer RT at recall time on Day 2 relative to sham. By contrast, there were no significant effects on RT in the group that received 1-Hz rTMS over the SMA after 6 h of practice, after stimulation of the M1, or after stimulation of the control position, Cz-2. This demonstrated spatial and temporal specificity of the consequences of SMA disruption. The timing at which rTMS over the SMA disrupted stabilization of this motor memory indicated that the stabilization process required more than 10 min following the end of training (when rTMS application started) but less than 6 h (when the application of rTMS did not affect subsequent recall). An alternative view could be that rTMS over SMA accelerated the memory decay seen partially in the sham group (Fig. 3B).
Such temporal specificity is consistent with the proposal for a migration of consolidated memories over time postpractice to other brain regions (Shadmehr and Holcomb 1997; Hikosaka et al. 2002; Doyon et al. 2003; Perez et al. 2007; Doyon et al. 2009). The short lasting duration of the effects of 1-Hz rTMS on cortical excitability (approximately 30 min) (Chen et al. 1997) as well as the specificity for stabilization of the practiced sequence but not the control task rule out a direct effect or rTMS applied on Day 1 on general motor performance tested on Day 2. One interesting feature of our investigation using motor sequences was that the stimulation of SMA disrupted subsequent stabilization while the stimulation of M1 did not. This result is consistent with a previous report in which subjects learned to compensate for dynamic force fields in a way that required precisely coordinated muscle activity (Baraduc et al. 2004) and this differs from another study that used a maximal-force generation task (Muellbacher et al. 2002). It is conceivable that the relative involvement of M1 and SMA in the stabilization of motor memories may depend on the type of task that has been learned or even on the learning strategy used to learn it.
Stabilization of Motor Memory in Random-Practice Schedule
Contrary to the findings with blocked practice, recall on Day 2 in the random-practice group was unaffected by rTMS. These results indicate that the anatomical structures underlying motor-memory consolidation differ depending on the training schedule. What are the possible mechanisms that could mediate this difference? One possibility is that motor memories encoded during random practice could have been more rapidly and firmly stored in the SMA than those stored through blocked practice, becoming more rapidly stabilized and resistant to rTMS interference in general or requiring higher parameters for SMA stimulation (Gerloff et al. 1997), which we did not use in this study. Alternatively, motor memories encoded during random practice may become more rapidly SMA independent before the rTMS is applied, migrating to other brain regions like the striatum (Miyachi et al. 1997; Maquet et al. 2000; Doyon et al. 2002; Miyachi et al. 2002; Lehericy et al. 2005; Peigneux et al. 2006) and/or parietal cortex (Shadmehr and Holcomb 1997; Huber et al. 2004; Fischer et al. 2005; Walker et al. 2005; Robertson and Cohen 2006). The rapid-stabilization hypothesis is consistent with a recent electrophysiological study in which rodent's spatial memory became more rapidly stabilized and hippocampus independent when each practiced task fits into a previously acquired framework of reference (Tse et al. 2007). The presence of such a preexistent framework may help to interpret and consolidate new incoming practiced sequences. With a random schedule, each time a subject is confronted with a changed sequence, memories of the previously practiced sequence may persist (possibly partially consolidated as a framework). The presence of the previous framework of reference may enable new incoming practice to consolidate the task more efficiently (Schmidt 1975, 1988, 1991; Lee and Magill 1983; Hanlon 1996; Robertson et al. 2004). With blocked practice, subjects train each sequence for the same amount of time as with random practice but may have less opportunity (in the absence of frequent changes in the order of practiced sequences) to relate each newly presented sequence with a previously partially consolidated framework of reference. A third interpretation is that the sequences practiced in the random schedule may not require stabilization.
Our empirical findings indicate that the anatomical structures underlying motor-memory consolidation differ depending on the training schedule. Previous studies have shown that the time course of memory stabilization and its underlying neural circuitry differ depending on the motor task (e.g., Brashers-Krug et al. 1996; Muellbacher et al. 2002; Walker et al. 2003; Baraduc et al. 2004; Robertson et al. 2004; Krakauer and Shadmehr 2006). Our present results demonstrate that the neural circuitry involved in consolidation differs even when subjects practice the same task with different schedules. This finding is consistent with molecular studies in which protein expression required for long-term memory formation depends on practice schedule (Yin et al. 1995; Genoux et al. 2002). Recent theoretical and behavioral work proposed multiple processes in motor-memory stabilization (Smith et al. 2006; Lee and Schweighofer 2009). Smith et al. (2006) postulated a two-state model, one supporting fast initial motor learning followed by rapid forgetting and a second, slower process, contributing to longer-lasting, more stable, motor-memory formation. In our experimental design, it is possible that random practice influences the latter by facilitating protein expression required for long-term potentiation-like mechanisms in the SMA, a region engaged in learning and memory of sequential multiple movements, similar to those used in our investigation (Tanji and Shima 1994; Shima et al. 1996; Tanji 1996; Shima and Tanji 1998, 2000).
A recent study indicated that performance in the last block of training in a sequential-movement task affects later stabilization and associated changes in the blood oxygen level-dependent signal (Albouy et al. 2008). Performance levels in the last block of training were comparable across blocked- and random-practice groups in our study, and therefore, the previous explanation could not account for across-group differences in memory stabilization (Fig. 2). It is conceivable, on the other hand, that the different rate of learning from first to last training blocks (faster in the blocked than in the random group) played a contributory role.
Finally, it should be kept in mind that the behavioral effects of applying rTMS over the SMA could represent the consequences of focal disruption of activity in this region, of disruption of its interconnected areas, and/or of disruption of the ability of the rest of the brain to compensate for the SMA disruption (Perez et al. 2007).
Conclusions
In summary, our results provided direct evidence, using a virtual lesion approach, of 1) the involvement of the SMA in stabilization of motor memories and 2) the differential contribution of the SMA to motor-memory stabilization depending on the practice schedule. One implication of these results is that the anatomical substrates underlying motor-memory stabilization (or their temporal operation) do differ depending on the practice schedule. It is important to understand these contributions since training strategies based on the contextual-interference effect have important clinical implications in neurorehabilitation.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
Intramural Research Program of the National Institute of Neurological Disorder and Stroke, National Institute of Health (NIH); Japan Society for the Promotion of Science Research Fellowship for Japanese Biomedical and Behavioral Researchers at NIH; Exploratory Research for Advanced Technology Shimojo Implicit Brain Function Project; KAKENHI (20019041, 20033030) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Supplementary Material
Acknowledgments
This work was partly presented at the Neural Control of Movement 18th Annual Meeting, Naples, FL, USA, May 2008. We would like to thank Dr John Krakauer, Ms Kazumi Kasahara, and Dr Sylvie Song for their helpful comments. Conflict of Interest: None declared.
References
- Albouy G, Sterpenich V, Balteau E, Vandewalle G, Desseilles M, Dang-Vu T, Darsaud A, Ruby P, Luppi PH, Degueldre C, et al. Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron. 2008;58:261–272. doi: 10.1016/j.neuron.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Baraduc P, Lang N, Rothwell JC, Wolpert DM. Consolidation of dynamic motor learning is not disrupted by rTMS of primary motor cortex. Curr Biol. 2004;14:252–256. doi: 10.1016/j.cub.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature. 1996;382:252–255. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Cross ES, Schmitt PJ, Grafton ST. Neural substrates of contextual interference during motor learning support a model of active preparation. J Cogn Neurosci. 2007;19:1854–1871. doi: 10.1162/jocn.2007.19.11.1854. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehericy S, Benali H. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res. 2009;199:61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41:252–262. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci USA. 2002;99:1017–1022. doi: 10.1073/pnas.022615199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Fischer S, Nitschke MF, Melchert UH, Erdmann C, Born J. Motor memory consolidation in sleep shapes more effective neuronal representations. J Neurosci. 2005;25:11248–11255. doi: 10.1523/JNEUROSCI.1743-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418:970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG. Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain. 1997;120:1587–1602. doi: 10.1093/brain/120.9.1587. [DOI] [PubMed] [Google Scholar]
- Hall KG, Magill RA. Variability of practice and contextual interference in motor skill learning. J Mot Behav. 1995;27:299–309. doi: 10.1080/00222895.1995.9941719. [DOI] [PubMed] [Google Scholar]
- Hanlon RE. Motor learning following unilateral stroke. Arch Phys Med Rehabil. 1996;77:811–815. doi: 10.1016/s0003-9993(96)90262-2. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Curr Opin Neurobiol. 2002;12:217–222. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- Honda M, Deiber MP, Ibanez V, Pascual-Leone A, Zhuang P, Hallett M. Dynamic cortical involvement in implicit and explicit motor sequence learning. A PET study. Brain. 1998;121:2159–2173. doi: 10.1093/brain/121.11.2159. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Karni A, Sagi D. The time course of learning a visual skill. Nature. 1993;365:250–252. doi: 10.1038/365250a0. [DOI] [PubMed] [Google Scholar]
- Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Shadmehr R. Consolidation of motor memory. Trends Neurosci. 2006;29:58–64. doi: 10.1016/j.tins.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Schweighofer N. Dual adaptation supports a parallel architecture of motor memory. J Neurosci. 2009;29:10396–10404. doi: 10.1523/JNEUROSCI.1294-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TD, Magill RA. The locus of contextual interference in motor-skill acquisition. J Exp Psychol Learn Mem Cog. 1983;9:730–746. [Google Scholar]
- Lehericy S, Benali H, Van de Moortele PF, Pelegrini-Issac M, Waechter T, Ugurbil K, Doyon J. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci USA. 2005;102:12566–12571. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Fisher BE, Winstein CJ, Wu AD, Gordon J. Contextual interference effect: elaborative processing or forgetting-reconstruction? A post hoc analysis of transcranial magnetic stimulation-induced effects on motor learning. J Mot Behav. 2008;40:578–586. doi: 10.3200/JMBR.40.6.578-586. [DOI] [PubMed] [Google Scholar]
- Lin CH, Fisher BE, Wu AD, Ko YA, Lee LY, Winstein CJ. Neural correlate of the contextual interference effect in motor learning: a kinematic analysis. J Mot Behav. 2009;41:232–242. doi: 10.3200/JMBR.41.3.232-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Sullivan KJ, Wu AD, Kantak S, Winstein CJ. Effect of task practice order on motor skill learning in adults with Parkinson disease: a pilot study. Phys Ther. 2007;87:1120–1131. doi: 10.2522/ptj.20060228. [DOI] [PubMed] [Google Scholar]
- Magill RA, Hall KG. A review of the contextual interference effect in motor skill acquisition. Hum Mov Sci. 1990;9:241–289. [Google Scholar]
- Maquet P, Laureys S, Peigneux P, Fuchs S, Petiau C, Phillips C, Aerts J, Del Fiore G, Degueldre C, Meulemans T, et al. Experience-dependent changes in cerebral activation during human REM sleep. Nat Neurosci. 2000;3:831–836. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- Matsunaga K, Maruyama A, Fujiwara T, Nakanishi R, Tsuji S, Rothwell JC. Increased corticospinal excitability after 5 Hz rTMS over the human supplementary motor area. J Physiol. 2005;562:295–306. doi: 10.1113/jphysiol.2004.070755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Memory—a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Lu X. Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Exp Brain Res. 2002;146:122–126. doi: 10.1007/s00221-002-1213-7. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Miyashita K, Karadi Z, Rand MK. Differential roles of monkey striatum in learning of sequential hand movement. Exp Brain Res. 1997;115:1–5. doi: 10.1007/pl00005669. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sakai K, Hikosaka O. Neuronal activity in medial frontal cortex during learning of sequential procedures. J Neurophysiol. 1998;80:2671–2687. doi: 10.1152/jn.1998.80.5.2671. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sakai K, Hikosaka O. Effects of local inactivation of monkey medial frontal cortex in learning of sequential procedures. J Neurophysiol. 1999;82:1063–1068. doi: 10.1152/jn.1999.82.2.1063. [DOI] [PubMed] [Google Scholar]
- Osu R, Hirai S, Yoshioka T, Kawato M. Random presentation enables subjects to adapt to two opposing forces on the hand. Nat Neurosci. 2004;7:111–112. doi: 10.1038/nn1184. [DOI] [PubMed] [Google Scholar]
- Overduin SA, Richardson AG, Lane CE, Bizzi E, Press DZ. Intermittent practice facilitates stable motor memories. J Neurosci. 2006;26:11888–11892. doi: 10.1523/JNEUROSCI.1320-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience—virtual lesion, chronometry, and functional connectivity. Curr Opin Neurobiol. 2000;10:232–237. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Orban P, Balteau E, Degueldre C, Luxen A, Laureys S, Maquet P. Offline persistence of memory-related cerebral activity during active wakefulness. PLoS Biol. 2006;4:e100. doi: 10.1371/journal.pbio.0040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Tanaka S, Wise SP, Sadato N, Tanabe HC, Willingham DT, Cohen LG. Neural substrates of intermanual transfer of a newly acquired motor skill. Curr Biol. 2007;17:1896–1902. doi: 10.1016/j.cub.2007.09.058. [DOI] [PubMed] [Google Scholar]
- Perez MA, Tanaka S, Wise SP, Willingham DT, Cohen LG. Time-specific contribution of the supplementary motor area to intermanual transfer of procedural knowledge. J Neurosci. 2008;28:9664–9669. doi: 10.1523/JNEUROSCI.3416-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, Perez MA, Ragert P, Rothwell JC, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. 2008;586:325–351. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM, Cohen DA. Understanding consolidation through the architecture of memories. Neuroscientist. 2006;12:261–271. doi: 10.1177/1073858406287935. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Pascual-Leone A, Miall RC. Current concepts in procedural consolidation. Nat Rev Neurosci. 2004;5:576–582. doi: 10.1038/nrn1426. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Press DZ, Pascual-Leone A. Off-line learning and the primary motor cortex. J Neurosci. 2005;25:6372–6378. doi: 10.1523/JNEUROSCI.1851-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM, Theoret H, Pascual-Leone A. Studies in cognition: the problems solved and created by transcranial magnetic stimulation. J Cogn Neurosci. 2003;15:948–960. doi: 10.1162/089892903770007344. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Schmidt RA. A schema theory of discrete motor skill learning. Psychol Rev. 1975;82:225–260. [Google Scholar]
- Schmidt RA. Motor control and learning. Champaign, IL: Human Kinetics Publisher; 1988. [Google Scholar]
- Schmidt RA. Motor learning principles for physical therapy. Alexandria, VA: Foundation for Physical Therapy; 1991. [Google Scholar]
- Seitz AR, Yamagishi N, Werner B, Goda N, Kawato M, Watanabe T. Task-specific disruption of perceptual learning. Proc Natl Acad Sci USA. 2005;102:14895–14900. doi: 10.1073/pnas.0505765102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science. 1997;277:821–825. doi: 10.1126/science.277.5327.821. [DOI] [PubMed] [Google Scholar]
- Shea JB, Morgan RL. Contextual interference effects on the acquisition, retention, and transfer of a motor skill. J Exp Psychol Hum Learn Mem. 1979;5:179–187. [Google Scholar]
- Shima K, Mushiake H, Saito N, Tanji J. Role for cells in the presupplementary motor area in updating motor plans. Proc Natl Acad Sci USA. 1996;93:8694–8698. doi: 10.1073/pnas.93.16.8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Tanji J. Both supplementary and presupplementary motor areas are crucial for the temporal organization of multiple movements. J Neurophysiol. 1998;80:3247–3260. doi: 10.1152/jn.1998.80.6.3247. [DOI] [PubMed] [Google Scholar]
- Shima K, Tanji J. Neuronal activity in the supplementary and presupplementary motor areas for temporal organization of multiple movements. J Neurophysiol. 2000;84:2148–2160. doi: 10.1152/jn.2000.84.4.2148. [DOI] [PubMed] [Google Scholar]
- Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol. 2006;4:e179. doi: 10.1371/journal.pbio.0040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Honda M, Sadato N. Modality-specific cognitive function of medial and lateral human Brodmann area 6. J Neurosci. 2005;25:496–501. doi: 10.1523/JNEUROSCI.4324-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J. New concepts of the supplementary motor area. Curr Opin Neurobiol. 1996;6:782–787. doi: 10.1016/s0959-4388(96)80028-6. [DOI] [PubMed] [Google Scholar]
- Tanji J, Shima K. Role for supplementary motor area cells in planning several movements ahead. Nature. 1994;371:413–416. doi: 10.1038/371413a0. [DOI] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RG. Schemas and memory consolidation. Science. 2007;316:76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R, Alsop D, Gaab N, Schlaug G. Sleep-dependent motor memory plasticity in the human brain. Neuroscience. 2005;133:911–917. doi: 10.1016/j.neuroscience.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Yin JC, Del Vecchio M, Zhou H, Tully T. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.