Abstract
Adolescence is a unique period of physical and cognitive development that includes concurrent pubertal changes and sex-based vulnerabilities. While diffusion tensor imaging (DTI) studies show white matter maturation throughout the lifespan, the state of white matter integrity specific to adolescence is not well understood as are the contributions of puberty and sex. We performed whole-brain DTI studies of 114 children, adolescents, and adults to identify age-related changes in white matter integrity that characterize adolescence. A distinct set of regions across the brain were found to have decreasing radial diffusivity across age groups. Region of interest analyses revealed that maturation was attained by adolescence in broadly distributed association and projection fibers, including those supporting cortical and brain stem integration that may underlie known enhancements in reaction time during this period. Maturation after adolescence included association and projection tracts, including prefrontal–striatal connections, known to support top-down executive control of behavior and interhemispheric connectivity. Maturation proceeded in parallel with pubertal changes to the postpubertal stage, suggesting hormonal influences on white matter development. Females showed earlier maturation of white matter integrity compared with males. Together, these findings suggest that white matter connectivity supporting executive control of behavior is still immature in adolescence.
Keywords: diffusion tensor imaging, gender, myelination, puberty
Introduction
Adolescence is a unique period of development characterized by immature brain processes and limitations in decision making. While the gross morphology of the brain is in place by this time as are core cognitive abilities, there are significant refinements to brain processes (Yakovlev and Lecours 1967; Huttenlocher 1990) that continue into adulthood as cognitive control continues to improve (Demetriou et al. 2002; Luna et al. 2004; Luciana et al. 2005). This period in development is recognized for brain-based vulnerabilities that affect behavior, including the emergence of psychopathology (Everling and Fischer 1998; Chau et al. 2004; Sweeney et al. 2004; Paus et al. 2008) and increases in overall mortality rate due to risk-taking behavior (Spear 2000). Adolescence is also characterized by important pubertal changes that can influence behavior and brain processing. Puberty is a period of development intrinsically related to the timing and exposure to gonadal hormones on the brain (McEwen 2001) and through these mechanisms exerts unique effects on brain and behavior processes during adolescence (Spear 2000; Sisk and Zehr 2005). Finally, during this period there are important sex-based differences that emerge, including the increased incidence of mood disorders in females (Giaconia et al. 1994; Nolen-Hoeksema and Girgus 1994), higher mortality in males due to risk-taking behavior and conduct disorders (Arnett 1992; Zahn-Waxler et al. 2008), and sex-based disparities in visuospatial versus verbal abilities (Delgado and Prieto 1996; Collins and Kimura 1997). Understanding structural brain changes that are unique to adolescence can better inform us regarding the inherent vulnerabilities of this period of development.
Developmental vulnerabilities in adolescence occur in the context of immature ability to voluntarily control our behavior in a planned fashion compared with adults. Overall improvements in cognitive control and reaction time show a nonlinear developmental trajectory with a sharp increase in performance from childhood to adolescence supporting increased speed of information processing that may be supported by enhanced white matter connectivity. This pattern changes from adolescence to adulthood as developmental progressions are less steep, and performance then attains a relative plateau (Kail 1993; Luna et al. 2004). Functional neuroimaging studies of voluntary control also demonstrate that differences during childhood may be qualitatively different from those that occur during adolescence. Functional magnetic resonance imaging (MRI) studies show differential recruitment of prefrontal regions in childhood, adolescence, and adulthood during tasks requiring cognitive control (Luna et al. 2000, 2001; Tamm et al. 2002; Crone et al. 2006; Velanova et al. 2008; Geier et al. 2009). Functional connectivity analyses, which may be independent from structural connectivity, also show distinct patterns in childhood and adolescence indicating protracted integration of long range connectivity supporting cognitive control (Fair et al. 2007, 2009). Whether the supporting white matter circuitry also demonstrates stage-like developmental patterns has not yet been well understood.
Myelination, which speeds neuronal transmission by the elaboration of a concentric phospholipid layer of insulation around axons by oligodendrocytes, continues to occur through adolescence (Yakovlev and Lecours 1967; Huttenlocher 1990), particularly in association areas into adulthood (Yakovlev and Lecours 1967; Benes 1989). Histologically based findings are supported by structural MRI studies (Pfefferbaum et al. 1994; Giedd, Blumenthal, Jeffries, Castellanos, et al. 1999; Sowell et al. 2003), which also show decreases in cortical gray matter in the context of white matter development (Giedd, Blumenthal, Jeffries, Castellanos, et al. 1999; Sowell et al. 2001; Gogtay et al. 2004; Giorgio et al. 2009). The concurrent changes in gray and white matter may not represent a simple reciprocal relationship and may be influenced by other neurobiological processes and tissue properties related to MRI (Paus et al. 2008; Tamnes et al. 2009). While histological studies of myelination emphasize a posterior to anterior gradient (Yakovlev and Lecours 1967), these findings have been extended to include prolonged development in other regions such as the hippocampus (Benes et al. 1994) and temporal regions (Giedd, Blumenthal, Jeffries, Castellanos, et al. 1999; Paus et al. 1999). Taken together, these findings support an ongoing refinement of white matter integrity consisting of greater functional connectivity that may contribute to the establishment of widely distributed brain function believed to underlie complex voluntary control of behavior (Goldman-Rakic 1988; Luna 2009).
Diffusion tensor imaging (DTI) offers the ability to indirectly study the microstructural components of white matter, including myelination and axonal organization, in a quantitative manner that complements existing structural MRI studies of white matter volume. By visualizing water diffusion, the microstructural components of white matter can be represented through the diffusion of water that is directionally predisposed along the parallel axis of the axons making it anisotropic. The obtained diffusion-weighted images are used to generate a diffusion tensor matrix with 3 voxel-specific scalar values (eigenvalues λ1, λ2, and λ3), which can be used to calculate mean overall diffusion in the parallel or perpendicular directions with respect to axonal fibers.
Different DTI analyses approaches have consistently found protracted development of white matter integrity. Region of interest analyses, where brain areas are considered in a predetermined manner, allows for hypothesis-driven studies but is limited in the ability to assess whole-brain changes. Region of interest (ROI) studies confirm that commissural and projection fibers in the internal capsule (IC), basal ganglia, and thalamus show maturation from infancy and childhood (Mukherjee et al. 2001; Schneider et al. 2004) that continue to progress through adolescence (Bonekamp et al. 2007; Schneiderman et al. 2007). Tractography analyses consider changes to the extent of a whole tract but are not sensitive to detecting regions within a tract that may have a different maturational time line. Similar to ROI driven analyses, tractography studies also confirm maturation of white matter integrity in the IC, corpus callosum (CC), corticospinal tract (CST), and the uncinate fasciculus (UF) that continue through childhood and adolescence (Eluvathingal et al. 2007; Giorgio et al. 2008; Lebel et al. 2008). Voxel-wise analyses characterize maturational changes at the voxel level and allow precise identification of white matter regions, which may inform already known maturation of adjacent gray matter regions. These studies also support continued maturation of association and projection tracts through adolescence (Schmithorst et al. 2002; Barnea-Goraly et al. 2004; Ashtari et al. 2007; Giorgio et al. 2008).
While these studies do not focus on the specific aspects of white matter integrity that may characterize adolescence, they provide suggestive evidence that this stage of development may have a unique status of white matter maturity. These studies agree that the relationship between white matter integrity and age is nonlinear (Mukherjee et al. 2001; Hermoye et al. 2006; Lebel et al. 2008) with a steep increase in fractional anisotropy (FA) from childhood to adolescence and a less steep progression from adolescence to adulthood (Lebel et al. 2008), suggesting that there may be unique qualitative changes associated with different stages of development. Moreover, varying maturational schedules for different brain regions (Tamnes et al. 2009) also support the hypotheses that adolescence may involve a unique state of white matter maturation that contributes to the unique aspects of behavior and vulnerabilities characteristic of this stage of development. For example, age-related changes in radial diffusivity (RD) values are associated with developmental improvements in cognitive control (Liston et al. 2006), and maturation of fronto-parietal networks is related to increased working memory capacity (Nagy et al. 2004). Taken together, these studies suggest that there are continued immaturities in adolescence in white matter maturation. However, precisely what is immature and what matures during adolescence remains unclear undermining our ability to understand this period of development.
The main aim of this study was to identify age-related changes in white matter that are distinct in childhood from those that characterize adolescence. Given the protracted development of executive function (Luna 2009) and initial DTI findings, we hypothesized that tracts that support complex behavior, including association tracts, and projection fibers supporting integration of frontal regions with the rest of the brain would demonstrate prolonged development through adolescence.
Adolescence is not strictly defined by age and varies in great part due to the influence of puberty and sex-based differences (Spear 2000; Dahl 2004). Puberty involves the programmed release of gonadal hormones that are known to have effects on brain mechanisms (McEwen 2001; Sisk and Foster 2004). While the effects of puberty have begun to be explored (Peper et al. 2008; Perrin et al. 2008), the contribution of pubertal status with regards to white matter is relatively underexplored in typical developing populations using DTI. Pubertal status, while inevitably linked to age, may have independent contributions to white matter maturation that may help illustrate brain development transitions during adolescence. The second aim of this study was to investigate the relationship between pubertal status and white matter integrity that may differ from associations with age. Given hormonal influences on brain development, we hypothesized that the association between white matter maturation and puberty would be distinct from that of age more closely tied to pubertal transitions.
Finally, the relationship of brain maturation and sex has been well established with MRI studies of brain dimorphism that have shown greater brain total white matter volumes in males compared with females (Filipek et al. 1994; De Bellis et al. 2001; Lenroot et al. 2007). Developmental MRI studies have documented both steeper slopes of white matter growth curves in males compared with females and males having an overall more protracted period of development, suggesting brain dimorphism not only in overall growth but also in growth trajectories (De Bellis et al. 2001; Lenroot et al. 2007). The third aim of this study was to assess sex-based differences in the maturation of white matter integrity. Based on volumetric white matter MRI studies, we predicted that males would demonstrate a more protracted course of white matter maturation.
Materials and Methods
Participants
The group consisted of 114 typically developing individuals between ages 8 and 28 years (M = 15.5; SD = 4.49) with no self-reported history of neurological or psychiatric disorders in themselves or their first-degree relatives. To assess different stages of development, we defined three age groups: 36 children (8–12 years of age), 45 adolescents (13–17 years of age), and 33 adults (18–28 years of age). These age ranges were based on our earlier findings of significant stage-like shifts in the development of cognitive control (Luna et al. 2004), and a broad age range allowed us to investigate developmental processes occurring during the transitions from childhood to adolescence and into adulthood. IQ was obtained using the Wechsler Abbreviated Scale of Intelligence. Full-scale IQ for the entire cohort was 109.66 (SD 10.27) and the mean IQs for all 3 age groups were all over 100.
Pubertal staging was obtained using the Tanner Maturation Scale (TMS) (Marshall and Tanner 1969, 1970) self-report questionnaire, which provides a subjective assessment of pubertal stage and has been shown to demonstrate high agreement ratings with physician assessments (Duke et al. 1980; Morris and Udry 1985). The TMS is a clinical tool used to evaluate the phenotypic pubertal changes in pubic hair, breast and genital development. Subjects were given pictures representing Tanner stages of the development of secondary sex characteristics in a private office setting. Females were shown sets of drawings depicting 5 stages of breast and pubic hair development, while males were shown drawings of 5 stages of genital and pubic hair development. Subjects were then instructed to choose the level that most closely reflected their stage of physiologic development and to then place their responses in a sealed envelope to ensure privacy. Composite scores reflecting breast development and pubic hair development (females) and genital and pubic hair development (males) were calculated. Pubertal stages were grouped by Tanner staging scores reflecting the following: pre/early pubertal (maturational scores 1, 2) (N = 28, 13 females), midpubertal (maturational scores 3, 4) (N = 49, 28 females), and adult maturational/postpubertal (maturational score 5) (N = 35, 22 females). There were no differences in the distribution of pubertal groupings between males and females. All experimental procedures complied with the Code of Ethics of the World Medical Association (1996 Declaration of Helsinki) and were approved by the Institutional Review Board of University of Pittsburgh.
MRI Scanning Protocol
Images were acquired on a 3T MR Siemens MAGNETOM Allegra scanner (Erlangen, Germany) with a standard circularity-polarized head coil. Head movement was minimized by having subjects spend time in a simulator scan with head movement auditory feedback, and pillows were used to stabilize the head in the head coil during scanning.
A whole-brain spin-echo echo-planar imaging sequence was used to obtain DTI images, with a scanning protocol of 29 4 mm-thick contiguous axial slices with an in-plane resolution of 1.5625 × 1.5625 mm providing full brain coverage. As in other developmental DTI studies (Schmithorst et al. 2002; Barnea-Goraly et al. 2005), diffusion gradients were applied in 6 noncollinear directions averaged over 14 repetitions with b = 800 s/mm2 increasing the signal-to-noise ratio allowing our clusters to reach a corrected P value of 0.001. To ensure consistency of image acquisition across subjects, the axial plane was aligned with the anterior and posterior commissures. The Siemens’ stock gradient table for 6 gradient directions, or MDDW6, was rotated to account for the angle difference between the AC–PC axial plane and the z-axis of the scanner. To optimize coregistration and diffusion tensor calculation, we removed the skull and scalp from the b0 image and this was applied as a mask to remove these components from the diffusion-weighted images.
MRI DTI Analyses
The FMRIB Software Library (FSL; University of Oxford) linear image registration tool (“flirt”) was used for preprocessing, including eddy correction for the diffusion-weighted images, correction for spatial distortions, including head motion, and for transformation of each diffusion-encoded image accordingly. We used the eddy-corrected images to calculate the diffusion tensor components using “MedINRIA” (Asclepios Research Project; INRIA Sophia Antipolis, Cedex, France), which creates an initial estimate of DTI parameters using the method described by Basser and Pierpaoli (1998). MedINRIA was used to calculate 3 eigenvalues (λ1, λ2, and λ3) and FA. We used the λ2 and λ3 images to calculate RD by averaging the 2.
While both RD and FA values were analyzed for this study, RD results are presented. Given that animal studies have shown RD to be better representative of histologic changes in demyelination (Song 2005) and dysmyelination (Song 2002; Nair 2005; Harsan et al. 2006) models. The correlation of RD related to histology in human studies has been less exclusive to myelination (Pierpaoli et al. 2001; Cader et al. 2007). However, it is interesting to note that given the lack of differences that we found between these measures in showing developmental differences utilizing voxel-based measurements undermines the specificity of RD with respect to white matter development.
We used Tract-Based Spatial Statistics (TBSS) (Smith et al. 2006), an FSL software suite, to do the group analysis. Parameters for registration, skeletonizing, and group map averaging were first obtained with the FA data since TBSS is designed primarily for use with FA values. The registration parameters calculated using the FA images were then applied to the RD images. All FA maps were nonlinearly registered to an FA template brain in Montreal Neurological Institute (MNI) space using FSL. After transformation into MNI space, the individual FA images were averaged to create a group-wise mean FA image. A skeletonization procedure was utilized to derive a group-wise skeleton of the white matter tracts. Each subject's FA image was skeletonized; the individual skeletons were then projected onto the group-wise skeleton as a second localized coregistration. The RD images were then transformed using the same registration parameters as the FA data and projected in the same way onto the derived group white matter skeleton. Voxel-wise statistical analyses were then performed on only the skeletonized images, thus making analyses less vulnerable to partial volume effects. The MNI 152 structural brain was used as an underlay to visualize DTI data.
To determine a main effect of age on RD, we obtained a t statistic for each voxel using permutation methods in “randomize” in FSL. The program randomize also performed cluster-based thresholding to obtain a P value for each cluster, corrected for multiple comparisons, which allowed us to identify regions showing age-related changes considering age as a continuous variable. Significant RD clusters (P < 0.001, corrected) were identified and defined anatomically using the probabilistic John Hopkins University White Matter Atlas (Hua et al. 2008) provided by “fslview” as well as other neuroanatomic references (Catani et al. 2003; Mori et al. 2005; Schmahmann and Pandya 2006). The reported MNI coordinates reflect the cluster's center of gravity (Giorgio et al. 2008), which provided the most accurate depiction of the localization and identification of the tract contained within the cluster when directly compared with the voxel coordinate of maximum intensity (Barnea-Goraly et al. 2005) or the arithmetic mean of the two. The identified clusters were used for all subsequent ROI-based analyses and group comparisons.
From the randomize procedure, we analyzed the obtained 19 RD clusters by performing a repeated measures analysis to determine the interactions between region of interest (within-subjects variable) and age group, sex, and pubertal status (between-subjects variable). Based on these results, we conducted a 1-way analysis of variance (ANOVA) with planned post hoc corrected comparisons to determine points of developmental transition for each derived cluster by age group (child, adolescent, and adult). Specifically, between-group comparisons were performed on mean RD values on a ROI clusters between children and adolescent groups and between adolescent and adult groups in order to identify differences between these subgroups. Significant differences in the mean RD of a given cluster between children and adolescents but not between adolescents and adults were interpreted as maturation being attained by adolescence, whereas significant differences in the mean RD of a given cluster between adolescents and adults was interpreted as persistent immaturity during adolescence showing protracted maturation.
To characterize sex-based differences in white matter integrity, we identified regions of interest that demonstrated a sex by age interaction. We then did a ROI analyses on males and females separately to determine developmental effects. By using the skeletonization procedure, the need to correct for sex-based differences for intracranial volume was eliminated.
Similarly to investigate the effect of pubertal development, given an ROI by sex by puberty interaction, we then did a region of interest analyses where we ran planned corrected comparisons using a 1-way ANOVA between puberty groupings (pre/early pubertal, midpubertal, and adult maturation/postpubertal) for males and females separately to determine developmental patterns of each of the derived RD clusters based on puberty stage. All these analyses were also conducted by correcting for multiple comparisons using Bonferroni corrections.
Results
Voxel-wise Regions Of Interest
Effects of Age as a Continuous Variable
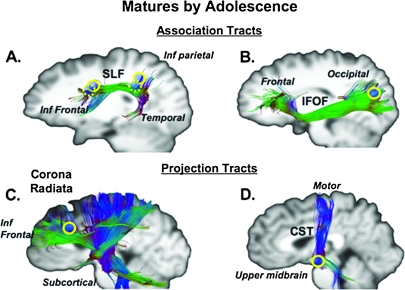
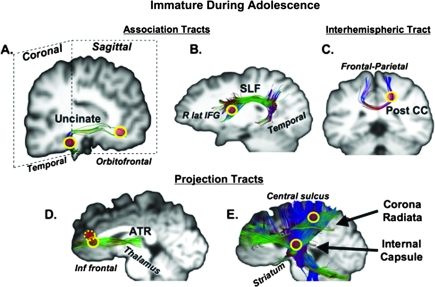
Using a cluster-based corrected P value <0.001 (t = 4), we identified 19 separate clusters that showed significant association with age as a continuous variable (see Figs 1 and 2 and Table 1). We identified 7 unique regions or tracts in the frontal, parietal, temporal, and occipital lobes, in the striatal region, CC, and the brain stem demonstrating significant effects of age using RD. These clusters were located in 1) association tracts: inferior fronto-occipital fasciculus/inferior longitudinal fasciculus (IFOF/ILF), the superior longitudinal fasciculus (SLF), and UF; 2) projection tracts: the CST, IC and contiguous corona radiata, and anterior thalamic radiations (ATRs); and 3) interhemispheric tract: CC. The cluster relating to the lateral occipital region was identified in a region where both the IFOF and the ILF are located and were thus considered as a single region of interest.
Figure 1.
Clusters showing significant effects of age maturing during adolescence are depicted on the MNI brain and on representative tracts to illustrate its approximate location. MNI coordinates for each ROI are detailed on Table 1. The clusters are depicted by yellow circles (dotted circles for closely neighboring ROIs). (A) SLF. (B) IFOF/ILF. (C) Corona Radiata. (D) CST.
Figure 2.
Clusters showing significant effects of age maturing after adolescence are depicted on the MNI brain and on representative tracts to illustrate its approximate location. MNI coordinates for each ROI are detailed on Table 1. The clusters are depicted by yellow circles (dotted circles for closely neighboring ROIs). (A) UF, (B) SLF, (C) Post CC = posterior corpus callosum, (D) ATR, and (E) corona radiata and IC.
Table 1.
Age-related changes in RD
| Developmental pattern | Tract type | Tract name | Associated gray matter connecting regions | Cluster location | MNI coordinates (x, y, z) |
| Maturation by Adolescence | Association | IFOF/ILF left (right) | Fronto-occipital/temporal–occipital | Lateral occipital | −35, −73, 26 (27, −69, 25) |
| SLF left frontal (2 clusters) and parietal portion right (2 clusters) | Fronto-temporal | Medial inferior frontal gyrus/precentral gyrus (lateral inferior frontal gyrus/precentral gyrus); medial (lateral) superior parietal | −37, −6, 28 (−51, 2, 25); 24, −37, 29 (36, −33, 33) | ||
| Projection | CST | Fronto-subcortical | Upper brain stem | −18, −21, −6 | |
| CR | Fronto-subcortical | Inferior/middle frontal gyrus | −31, 17, 34 | ||
| Still Immature in Adolescence | Association | SLF | Fronto-temporal | Inferior frontal gyrus | 47, 10, 16 |
| UF frontal portion, left (right) and temporal portion left (right) | Fronto-temporal | Medial orbitofrontal; middle temporal gyrus | −20, 24, −12 (14, 29, −13); −44, −14, −19 (45, −20, −12) | ||
| Projection | ATR right (2 clusters) | Corticothalamic | Inferior/middle frontal gyrus (superior/middle frontal gyrus) | 28, 17, 26 (20, 46, 13) | |
| IC left (right) | Fronto-subcortical | Genu region | −15, 5, 5 (13, 0, 0) | ||
| CR | Parietal–subcortical | Central sulcus | −34, −31, 53 | ||
| Interhemispheric | CC, posterior | Fronto-parietal (interhemispheric) | Central sulcus | −16, −13, 34 |
Note: RD clusters, tract localization, and maturation patterns (effect with age as a continuous variable, t = 4, P < 0.001, corrected). SLF = superior longitudinal fasciculus; UF = uncinate fasciculus; CST = corticospinal tract; CR = corona radiata; IC = internal capsule; ATR = anterior thalamic radiations; CC = corpus callosum.
Effect of Age Group and Developmental Transitions
ROIs determined by the clusters that showed a main effect of age as a continuous variable were used to investigate effects of developmental stage, puberty, and sex. All significant effects were corrected for multiple comparisons unless noted. We found main effects for age group (F2,98 = 3.19, P < 0.05) and pubertal status (F2,98 = 3.10, P < 0.05) but no age group by pubertal status interaction. A sex by puberty group interaction was found that approached significance (F2,98 = 3.00, P = 0.054). While there was no significant interaction of ROI and age group, there was a significant interaction between ROI, age group, sex, and puberty group (F18,98 = 1.90, P < 0.05, corrected) indicating that these regions varied based on stages of both age-based and puberty development by sex. Based on the significance of these interactions and the already known effect of chronological age on DTI measures, we performed corrected separate group comparisons based on age group, puberty, and sex for each of the 19 RD clusters. For the age group comparisons, these analyses revealed 4 different tracts showing significant maturation by adolescence, including the occipital portion of the IFOF/ILF, frontal and parietal portions of the SLF, upper brain stem regions of the CST, and frontal portions of the corona radiata (see Table 1 and Fig. 1). There were 6 tracts showing continued immaturity during adolescence, including the frontal and temporal portions of the UF, a frontal portion of the SLF, frontal portions of the ATRs, the genu of the IC, a frontal–parietal portion of the corona radiata, and the posterior portion of the CC (see Table 1 and Fig. 2).
Planned and corrected between-group comparisons of mean RD of the original 19 clusters between pre/early pubertal, midpubertal, and adult maturational/postpubertal groups indicated that all but one cluster continued to show immaturity during the midpubertal stage. The single cluster that showed maturity during the midpubertal stage was the occipital portion of the left IFOF/ILF (F2,111 = 10.27, P < 0.01).
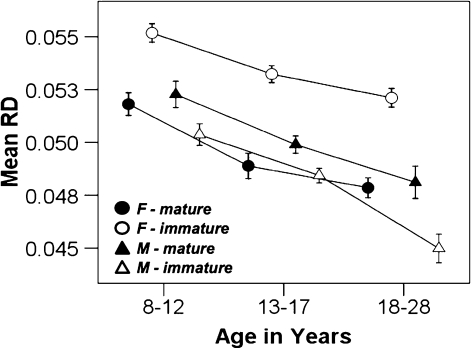
Between-subject comparisons demonstrated a significant age group × sex interaction in the left IC (F2,106 = 7.23, P < 0.01) and the right ATR (F2,106 = 3.28, P < 0.05). There were 2 other clusters that approached significance, which included the right genu of the IC (F2,106 = 2.62, P = 0.08) and the frontal portion of the corona radiata (F2,106 = 2.87, P = 0.06). In addition, we also performed planned comparisons in all ROIs given our interest in characterizing how development occurred in males and females across development. Results of planned and corrected between-group comparisons of mean RD of the original 19 clusters between groups of male and female children, adolescents, and adults showed an earlier maturational pattern in females compared with males (see Supplementary Table 1 and Fig. 3). All clusters in the female group showed maturation attained by adolescence except for one cluster located in the right frontal portion of the SLF that showed ongoing maturation into young adulthood. In the male group, one cluster was mature in adolescence that was located in the right parietal portion of the SLF. The clusters in the left and right IFOF/ILF and one cluster in the superior parietal region in the SLF did not show any age effects between age groups in the males. The rest of the clusters showed ongoing maturation into young adulthood.
Figure 3.
Graph demonstrating age × sex effects. Filled shapes depict the mean RD of the regions that showed RD values that were mature by adolescence. There were 4 regions that were mature in the male group and 18 regions in the female group by adolescence. The open shapes depict the mean RD of the regions that continued to show immature mean RD values in adolescence compared with adults. The one mature region showing late maturity in the females was the SLF. Specific mean RD values for each region are listed on Supplementary Table 1.
Discussion
Characterizing white matter microstructure through stages of development leading to adulthood can inform our understanding of the mechanisms that rely on complex brain connectivity such as cognitive control during adolescence and to gain insight into the neural basis of development and vulnerabilities that emerge during this period. We used DTI in a large community-based sample as an indirect measure of myelination to identify patterns of white matter development that characterized childhood and adolescence, as well as the contributions of puberty and sex.
In agreement with other studies, we found evidence for continuing maturation of white matter throughout distributed brain regions from childhood into adulthood (Mukherjee et al. 2001; Barnea-Goraly et al. 2005; Hermoye et al. 2006; Lebel et al. 2008). Using these results to define regions of interest we found different schedules of development corresponding to childhood and adolescence.
Stages of Development: Regions of Maturation Attained By Adolescence
We found that the CST in the brain stem region and in frontal portions of the corona radiata, which contain fibers connecting cortical and brain stem regions, matured by adolescence. We also found maturation by adolescence in the several key long association tracts, including the IFOF/ILF and the SLF, indicating relatively earlier establishment of a broadly distributed cortical network. The findings of the IFOF and SLF are similar to the results of Lebel et al. (2008) who found that these 2 tracts attain 90% of their maximum FA value between 13 and 20 years of age using a tractography-based approach. Similarly, another tractography-based study (Eluvathingal et al. 2007) found similar progression of RD in both the CST and the ILF and IFOF with age. Maturation in the SLF may relate to longitudinal structural MRI studies demonstrating gray matter volume changes in the posterior portion of the superior temporal gyrus region (Paus et al. 1999; Gogtay et al. 2004). This widespread development of white matter connectivity may support increases in the speed of information processing that underlie the known improvements in reaction time in cognitive control tasks that have been shown to reach adult levels by adolescence (Hale 1990; Luna et al. 2004).
The areas that continued to mature through adolescence were more localized in association and projection fibers related to frontal pathways. Regions within association tracts included the UF (frontal and temporal portions) and in a right frontal portion of the SLF. The primary projection fiber regions supporting frontal connections included the genu of the IC, a frontal portion of the corona radiata, and the frontal projection fibers of the ATR. The genu of the IC carries fibers from the prefrontal cortex as well as the more caudal orbital prefrontal regions while the ATR connects the dorsomedial and anterior thalamic nuclei with the prefrontal cortex (Schmahmann and Pandya 2006). Both these projection tracts thus carry important connections between the prefrontal and orbitofrontal cortices to subcortical regions. Similarly, the protracted maturation of the uncinate (Lebel et al. 2008) and inferior temporal regions (Schneiderman et al. 2007) in our studies and others may have functional contributions to the orbitofrontal cortex, a key region in reward processing (O'Doherty et al. 2001), which may bear significant implications in terms of development and reward modulation of cognitive control (Geier and Luna 2009). Finally, late developments in commissural connections in the posterior portion of the CC concur with MRI morphometric (Giedd, Rumsey, et al. 1996) and histological studies showing ongoing maturation, including high–fiber diameter density in the posterior callosal regions (Aboitiz et al. 1992). This portion of the CC is thought to carry interhemispheric connections for the superior temporal cortices and may thus facilitate refinements in higher language skills and other associative functions (Giedd, Blumenthal, Jeffries, Rajapakse, et al. 1999).
In summary, age group comparisons showed adolescence as being characterized by earlier maturation in tracts pertaining to intrahemispheric connections (i.e. IFOF and SLF) and projection fibers, including the CST and the corona radiata. The establishment of longer range connections in particular may contribute to the establishment of a whole brain network that matures with age into adulthood. Development through adolescence included projection fibers connecting striatum and thalamus to the prefrontal regions, other association fiber tracts, such as the UF, and more posterior mid portions of the CC. The finding of protracted development in projection fibers between the prefrontal cortex and subcortical regions (IC) may underlie improvements in top-down modulation of behavior that support complex behavior such as cognitive control that continues to improve through adolescence (Zald and Iacono 1998; Anderson et al. 2001; Demetriou et al. 2002; Luna et al. 2004, 2009) and structural MRI developmental studies documenting late maturation of these white matter tracts (Paus et al. 1999). Developmentally based increases in the integrity of frontostriatal tracts have been found to be associated with age-related increases in performance in a response inhibition task providing support for effects of enhanced white matter connectivity on cognitive control (Liston et al. 2006).
Developmental Differences Based on Pubertal Development
Although puberty and chronological age are tightly coupled, puberty is a complex biological process that includes physical and sexual growth and maturation, which may have independent effects from age. By Tanner staging, the cohort was divided into pre/early pubertal, midpubertal, and adult maturation/postpubertal subgroups to determine developmental pattern. Unlike results based on chronological age, which showed significant development during childhood, all the tracts except one cluster (localized in the left IFOF) demonstrated continued immaturities in midpuberty and only became adultlike by the postpubertal stage. This finding suggests that pubertal changes may be more tightly coupled to white matter maturation than had been previously considered. Specifically, it suggests that once adult maturational levels are attained, detected white matter changes decrease. These possible influences of pubertal processes on white matter maturation may contribute to the well-characterized vulnerabilities during adolescence and early adult years to psychiatric disorders.
Sex Differences in White Matter Maturation
Our sex comparisons suggest that by adolescence, females have developed earlier than males in the majority of the identified tracts. Males had a more protracted course with the majority of tracts continuing to develop into adulthood. Exceptions were the right SLF, in the parietal region, and the occipital portions of the IFOFs, which did not show any age effects for males indicating early maturity. Our results complement other studies showing that females generally complete maturation earlier in association regions, including the right occipito-parietal white matter and right arcuate fasciculus reflected in mean diffusivity values (Schmithorst et al. 2008), with overall lower RD measurements in some association areas such as the ILF and IFOF (Eluvathingal et al. 2007).
The earlier white matter maturation in females compared with males was surprising, however, given morphometric MRI studies indicating more protracted but steeper slopes depicting total white matter volume in males during adolescence across the frontal, temporal, parietal, and occipital lobes (Lenroot et al. 2007). However, the trend for females to undergo earlier white matter maturation has been suggested to be related to microstructural processes rather than increases in total white matter volume (De Bellis et al. 2001). Sex-based schedules of development may underlie the bias for females being more vulnerable to disorders such as depression and anorexia (Ge et al. 2001; Stice et al. 2001) in adolescence and higher occurrence of attention deficit hyperactivity disorder and autism in males. Given known differences in brain development in hormone-based and/or sex chromosomal disorders (Giedd et al. 2006), future studies in typical developing populations of pubertal timing by sex may further elucidate the contribution of sex and puberty to white matter development.
Significance of Association and Projection Tracts: Refinement of a Network Supporting Cognitive Control
A number of structural brain MRI studies have demonstrated a dynamic reciprocal relationship between gray and white matter volumes in childhood and into young adulthood. Regions showing protracted changes in gray matter thinning include the prefrontal region, the superior temporal gyrus, and posterior parietal regions (Paus et al. 1999; Giedd, Blumenthal, Jeffries, Castellanos, et al. 1999; Sowell et al. 2001; Gogtay et al. 2004; Toga et al. 2006) as well as key connecting regions such as the basal ganglia (Sowell et al. 1999). Gray matter changes occur in the context of white matter volumetric growth in the IC, the left arcuate fasciculus, and specific regions of the CC such as the posterior portion (Pujol et al. 1993; Giedd, Snell, et al. 1996; Paus et al. 1999; Lebel et al. 2008). Structural changes in gray and white matter concur with a large number of developmental studies that detail great strides in cognitive control (Levin et al. 1991; Dempster et al. 1993; Kail 1993; Case 1996; Fry and Hale 1996; Luciana and Nelson 1998; Demetriou et al. 2002; Luna et al. 2004; Asato et al. 2006). The ability to carry out voluntary and goal-directed activities while suppressing task inappropriate or irrelevant information is essential for mature control of behavior. However, mature performance is accompanied by differences in brain function underlying performance at younger ages (Thomas et al. 1999; Rubia et al. 2000, 2005, 2007; Luna et al. 2001; Bunge et al. 2002). The discrepancy between apparent mature skills and immature brain function may be accounted for in part by ongoing maturation of white matter and in particular tracts that involve the frontal lobe. It has traditionally been proposed that the exclusive late emergence of frontal systems supports behavioral improvements during this period of development (Hudspeth and Pribram 1990; Stuss 1992; Diamond and Taylor 1996; Luciana and Nelson 1998). Frontostriatal connectivity supports core cognitive skills such as response inhibition and spatial working memory, which is also known to have late development through adolescence (Zald and Iacono 1998; Demetriou et al. 2002; Chambers et al. 2003; Bjork et al. 2004; Luna et al. 2004; Ernst et al. 2006; Scherf et al. 2006). Additionally, given the role of the frontostriatal pathways in reward processing (Bjork et al. 2004; Ernst et al. 2005) and the importance of prefrontal function, demonstration of the protracted development of fronto-subcortical and cortico-cortical connections into adolescence may reflect a period of particular vulnerability to both the peak in risk-taking behavior during adolescence and the emergence and exacerbation of psychopathology, which is associated with abnormalities in rewards processing and cognitive control (Dahl 2001; Luna and Sweeney 2001; Chambers et al. 2003; Ernst et al. 2006).
While our study provided the sensitivity to identify unique immaturities related to different stages of development, a more extensive characterization of developmental transitions can be further investigated with new and more extensive methods. Similarly, cross-sectional samples can offer important insights into developmental processes in a timely fashion; however, longitudinal approaches allow for the assessment of within-subject change over time that can enhance our ability to identify different profiles of development. Additionally, our studies used a limited number of gradients (6), which have been successfully used in the literature (Schmithorst et al., 2002; Barnea-Goraly et al., 2005). However, recent DTI methods afford a higher number of gradient directions which paired with multiple scans can provide more precise DTI measurements that can increase sensitivity to characterize white matter immaturities (Ashtari et al. 2007; Tamnes et al. 2009).
In summary, our results provide evidence for a qualitatively unique state of white matter maturation in adolescence that is primarily characterized by enhanced connectivity between cortical and subcortical regions known to support top-down executive control of behavior. These developmental changes persisted past pubertal development suggesting possible hormonal associations with white matter structure maturation. Finally, females appeared to reach mature levels earlier than males suggesting different stages of vulnerability that may contribute to sex discrepancies in the emergence of psychopathology.
Supplementary Material
Supplementary material can be found at http://www.cercor.oxfordjournals.org/.
Funding
National Institutes of Health (K23 NS052234 to M.R.A. and R01 MH 067924, R01 MH 080243 to B.L.).
Supplementary Material
Acknowledgments
We would like to acknowledge the help of our Laboratory Coordinator, Melanie Wilds, for her help in recruiting and administrative aspects of this study. Conflict of Interest: None declared.
References
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Anderson P, Anderson V, Gartner AF. Assessment and development of organizational ability: the Rey Complex Figure Organizational Strategy Score (RCF-OSS) Clin Neuropsychol. 2001;15:81–94. doi: 10.1076/clin.15.1.81.1905. [DOI] [PubMed] [Google Scholar]
- Arnett J. Reckless behavior in adolescence: a developmental perspective. Dev Rev. 1992;12:339–373. [Google Scholar]
- Asato MR, Sweeney JA, Luna B. Cognitive processes in the development of TOL performance. Neuropsychologia. 2006;44:2259–2269. doi: 10.1016/j.neuropsychologia.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cervellione KL, Hasan KM, Wu J, McIlree C, Kester H, Ardekani BA, Roofeh D, Szeszko PR, Kumra S. White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. Neuroimage. 2007;35:501–510. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med. 1998;39:928–934. doi: 10.1002/mrm.1910390610. [DOI] [PubMed] [Google Scholar]
- Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophr Bull. 1989;15:585–593. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonekamp D, Nagae LM, Degaonkar M, Matson M, Abdalla WM, Barker PB, Mori S, Horska A. Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. Neuroimage. 2007;34:733–742. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cader S, Johansen-Berg H, Wylezinska M, Palace J, Behrens TE, Smith S, Matthews PM. Discordant white matter N-acetylasparate and diffusion MRI measures suggest that chronic metabolic dysfunction contributes to axonal pathology in multiple sclerosis. Neuroimage. 2007;36:19–27. doi: 10.1016/j.neuroimage.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Case R. Modeling the process of conceptual change in a continuously evolving hierarchical system. Monogr Soc Res Child Dev. 1996;61:283–295. [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Petenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau DT, Roth RM, Green AI. The neural circuitry of reward and its relevance to psychiatric disorders. Curr Psychiatry Rep. 2004;6:391–399. doi: 10.1007/s11920-004-0026-8. [DOI] [PubMed] [Google Scholar]
- Collins DW, Kimura D. A large sex difference on a two-dimensional mental rotation task. Behav Neurosci. 1997;111:845–849. doi: 10.1037//0735-7044.111.4.845. [DOI] [PubMed] [Google Scholar]
- Crone EA, Bunge SA, van der Molen MW, Ridderinkhof KR. Switching between tasks and responses: a developmental study. Dev Sci. 2006;9:278–287. doi: 10.1111/j.1467-7687.2006.00490.x. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Affect regulation, brain development, and behavioral/emotional health in adolescence. CNS Spectr. 2001;6:60–72. doi: 10.1017/s1092852900022884. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Delgado AR, Prieto G. Sex differences in visuospatial ability: do performance factors play such an important role? Mem Cognit. 1996;24:504–510. doi: 10.3758/bf03200938. [DOI] [PubMed] [Google Scholar]
- Demetriou A, Christou C, Spanoudis G, Platsidou M. The development of mental processing: efficiency, working memory, and thinking. Monogr Soc Res Child Dev. 2002;67:1–155. discussion 156. [PubMed] [Google Scholar]
- Dempster FN. Resistance to interference: developmental changes in a basic processing mechanism. In: Howe ML, et al., editors. Emerging themes in cognitive development, volume I: foundations. New York: Springer-Verlag; 1993. pp. 3–27. [Google Scholar]
- Diamond A, Taylor C. Development of an aspect of executive control: development of the abilities to remember what I said and to “do as I say, not as I do”. Dev Psychobiol. 1996;29:315–334. doi: 10.1002/(SICI)1098-2302(199605)29:4<315::AID-DEV2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Duke PM, Litt IF, Gross RT. Adolescents’ self-assessment of sexual maturation. Pediatrics. 1980;66:918–920. [PubMed] [Google Scholar]
- Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex. 2007;17:2760–2768. doi: 10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Fischer B. The antisaccade: a review of basic research and clinical studies. Neuropsychologia. 1998;36:885–899. doi: 10.1016/s0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS. The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex. 1994;267:344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Fry AF, Hale S. Processing speed, working memory, and fluid intelligence: evidence for a developmental cascade. Psychol Sci. 1996;7:237–241. [Google Scholar]
- Ge X, Conger RD, Elder GH., Jr Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Dev Psychol. 2001;37:404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Geier C, Luna B. The maturation of incentive processing and cognitive control. Pharmacol Biochem Behav. 2009;93:212–221. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Garver K, Terwilliger R, Luna B. Development of working memory maintenance. J Neurophysiol. 2009;101:84–99. doi: 10.1152/jn.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaconia RM, Reinherz HZ, Silverman AB, Pakiz B, Frost AK, Cohen E. Ages of onset of psychiatric disorders in a community population of older adolescents. J Am Acad Child Adolesc Psychiatry. 1994;33:706–717. doi: 10.1097/00004583-199406000-00012. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog Neuro-Psychopharmacol Biol Psychiatry. 1999;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, Molloy EA, Blumenthal JD, Tossell JW, Stayer C, et al. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006;254-255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rumsey JM, Castellanos FX, Rajapakse JC, Kaysen D, Vaituzis AC, Vauss YC, Hamburger SD, Rapoport JL. A quantitative MRI study of the corpus callosum in children and adolescents. Brain Res Dev Brian Res. 1996;91:274–280. doi: 10.1016/0165-3806(95)00193-x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, et al. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Chadwick M, James S, Winmill L, Douaud G, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H, et al. Longitudinal changes in grey and white matter during adolescence. Neuroimage. 2009;49:94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, Matthew PM, Smith SM, Johansen-Berg H. Changes in white matter microstructure during adolescence. Neuroimage. 2008;39:52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Ann Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Hale S. A global developmental trend in cognitive processing speed. Child Dev. 1990;61:653–663. [PubMed] [Google Scholar]
- Harsan LA, Poulet P, Guignard B, Steibel J, Parizel N, de Sousa PL, Boehm N, Grucker D, Ghandour MS. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res. 2006;83:392–402. doi: 10.1002/jnr.20742. [DOI] [PubMed] [Google Scholar]
- Hermoye L, Saint-Martin C, Cosnard G, Lee SK, Nassogne MC, Menten R, Clapuyt P, Donohue PK, Hua K, Wakana S, et al. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29:493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth WJ, Pribram KH. Stages of brain and cognitive maturation. J Educ Psychol. 1990;82:881–884. [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Kail R. Processing time decreases globally at an exponential rate during childhood and adolescence. J Exp Child Psychol. 1993;56:254–265. doi: 10.1006/jecp.1993.1034. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;140:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Culhane KA, Hartmann J, Evankovich K, Mattson AJ. Developmental changes in performance on tests of purported frontal lobe functioning. Dev Neuropsychol. 1991;7:377–395. [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex. 2006;16:553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Dev. 2005;76:697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Luciana M, Nelson C. The functional emergence of prefrontally-guided working memory systems in four- to eight-year-old children. Neuropsychologia. 1998;36:273–293. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Luna B. The maturation of cognitive control and the adolescent brain. In: Aboitiz F, et al., editors. From attention to goal-directed behavior: neurodynamical, methodological and clinical trends. Heidelberg (Germany): Springer-Verlag; 2009. pp. 249–274. [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O'Hearn K Forthcoming. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2009 doi: 10.1016/j.bandc.2009.08.005. Available online September 2009, doi: Luna 2009-10.1016/j.bandc.2009.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. Studies of brain and cognitive maturation through childhood and adolescence: a strategy for testing neurodevelopmental hypotheses. Schizophr Bull. 2001;27:443–455. doi: 10.1093/oxfordjournals.schbul.a006886. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Invited review: estrogens effects on the brain: multiple sties and molecular mechanisms. J Appl Psychol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM, VanZijl PCM. MRI atlas of human white matter. Amsterdam: Elsevier; 2005. [Google Scholar]
- Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BCP, Almli CR, McKinstry RC. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221:349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- Nair G, Tanahashi Y, Low HP, Billings-Gagliardi S, Schwartz WJ, Duong TQ. Myelination and long diffusion times alter diffusion-tensor-imaging contrast in myelin-deficient shiverer mice. Neuroimage. 2005;28:165–174. doi: 10.1016/j.neuroimage.2005.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychol Bull. 1994;115:424–443. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM, Delemarre-Van de Waal HA, Janke AL, Collins DL, Evans AC, et al. Cerebral white matter in early puberty is associated with luteinizing hormone concentrations. Psychoneuroendocrinology. 2008;33:909–915. doi: 10.1016/j.psyneuen.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Herve PY, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci. 2008;28:9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13:1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Junqué C, Martí-Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann Neurol. 1993;34:71–75. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Sweeney JA, Luna B. Brain basis of developmental change in visuospatial working memory. J Cog Neurosci. 2006;18:1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. New York: Oxford University Press; 2006. [Google Scholar]
- Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls. Hum Brain Mapp. 2008;29:696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JF, Il'yasov KA, Hennig J, Martin E. Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology. 2004;46:258–266. doi: 10.1007/s00234-003-1154-2. [DOI] [PubMed] [Google Scholar]
- Schneiderman JS, Buchsbaum MS, Haznedar MM, Hazlett EA, Brickman AM, Shihabuddin L, Brand JG, Torosjan Y, Newmark RE, Tang C, et al. Diffusion tensor anisotropy in adolescents and adults. Neuropsychobiology. 2007;55:96–111. doi: 10.1159/000104277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stice E, Presnell K, Bearman SK. Relation of early menarche to depression, eating disorders, substance abuse, and comorbid psychopathology among adolescent girls. Dev Psychol. 2001;37:608–619. doi: 10.1037//0012-1649.37.5.608. [DOI] [PubMed] [Google Scholar]
- Stuss DT. Biological and psychological development of executive functions. Brain Cogn. 1992;20:8–23. doi: 10.1016/0278-2626(92)90059-u. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Takarae Y, Macmillan C, Luna B, Minshew NJ. Eye movements in neurodevelopmental disorders. Curr Opin Neurol. 2004;17:37–42. doi: 10.1097/00019052-200402000-00007. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp118. Advance Access published June 11, doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, Birmaher V, Casey BJ. A developmental functional MRI study of spatial working memory. Neuroimage. 1999;10:327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Oxford: Blackwell Scientific; 1967. pp. 3–70. [Google Scholar]
- Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: gender and psychopathology. Annu Rev Clin Psychol. 2008;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]
- Zald DH, Iacono WG. The development of spatial working memory abilities. Dev Neuropsychol. 1998;14:563–578. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.