Abstract
Recent advances in neuroimaging have permitted testing of hypotheses regarding the neural bases of individual differences, but this burgeoning literature has been characterized by inconsistent results. To test the hypothesis that differences in task demands could contribute to between-study variability in brain-behavior relationships, we had participants perform 2 tasks that varied in the extent of cognitive involvement. We examined connectivity between brain regions during a low-demand vigilance task and a higher-demand digit–symbol visual search task using Granger causality analysis (GCA). Our results showed 1) Significant differences in numbers of frontoparietal connections between low- and high-demand tasks 2) that GCA can detect activity changes that correspond with task-demand changes, and 3) faster participants showed more vigilance-related activity than slower participants, but less visual-search activity. These results suggest that relatively low-demand cognitive performance depends on spontaneous bidirectionally fluctuating network activity, whereas high-demand performance depends on a limited, unidirectional network. The nature of brain-behavior relationships may vary depending on the extent of cognitive demand. High-demand network activity may reflect the extent to which individuals require top-down executive guidance of behavior for successful task performance. Low-demand network activity may reflect task- and performance monitoring that minimizes executive requirements for guidance of behavior.
Keywords: connectivity, functional imaging, individual differences, prefrontal cortex, processing speed
Introduction
One aim of cognitive neuroscience has been to identify those aspects of neurophysiology that underlie the consistent individual differences in performance that have long been observed in experimental psychology. Spearman's (1904) observation that some individuals consistently perform better than others across a broad range of tasks has spawned generations of research investigating the hypothesis that a limited set of resources govern cognitive performance (Spearman 1904; Kahneman 1973; Norman and Bobrow 1975; Vernon 1983; Baddeley 1986; Just and Carpenter 1992).
One such resource, processing speed, may emerge from individual differences in the efficiency with which cognitive operations can be performed. Cognitive efficiency theories suggest that when these operations can be performed quickly, resource allocation can be minimized and performance maximized. Efficiency theorists have hypothesized that oft-observed correlations between reaction time (RT) and intelligence measures reflect individual differences in “neural efficiency” which, they argue, permit some individuals to overcome cognitive capacity limits more than others (e.g., Jensen 1982, 1998; Vernon 1983). The advent of modern neuroimaging techniques has made it possible to test such hypotheses by permitting more direct observation of brain-behavior relationships than was possible in the past.
Neuroimaging studies in healthy adults support efficiency explanations of individual differences. Results from electroencephalography (EEG) studies have shown differences in amplitude and coherence measures between individuals that correspond to their performance differences (e.g., Gevins and Smith 2000; Grabner et al. 2003; Reiterer et al. 2005). In one study, for instance, Gevins and Smith (2000) required high-ability and low-ability (as measured by WAIS-R performance) participants to perform an n-back working memory (WM) task during EEG recording. The important result was that high-ability participants showed less prefrontal cortex (PFC) and more parietal activity than their low-ability counterparts.
In other EEG studies, reduced “event-related desynchronizations” (ERDs) in alpha frequencies (8–12 Hz), coupled with reduced “event-related synchronizations” (ERSs) in theta frequencies (4–8 Hz), in higher as compared to lower performing individuals have been observed (e.g., Grabner et al. 2003; Babiloni et al. 2009; Del Percio et al. 2009). An ERD is said to occur when the power of some frequency or band of frequencies decreases in response to a stimulus event, whereas an ERS is said to occur when the power of a frequency band increases. ERD in the alpha band, coupled with ERS in the theta band have been interpreted as an index of mental effort (Nunez et al. 2001) . Thus, these results suggest reduced mental effort in higher performers compared to lower performers.
Finally, EEG results suggesting reduced neural activity in experts and professional athletes, compared with novices and amateur athletes, suggest support for efficiency explanations of individual differences. In one study, for instance, Babiloni et al. (2009) observed reduced ERDs in the scalp potentials of gymnasts, compared with nongymnasts, while they viewed films of gymnastic performances and judged the artistic and athletic level of the performer. Similar results have been observed when expert performers were compared with nonexperts during actual athletic performance (Del Percio et al. 2009).
Results from positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) studies also show reduced activation in faster than in slower individuals (e.g., Haier et al. 1988, 1992; Larson et al. 1995; Kosslyn et al. 1996; Rypma and D'Esposito 1999; Rypma et al. 2002, 2005). In one study for instance, Haier et al. (1992) had 8 participants perform a spatial reasoning task, Raven progressive matrices (RPM). Next, they recorded participants’ glucose metabolic rate (GMR; measured by PET) during performance of a complex visual manipulation task (“tetris”) both before and after extensive practice. In addition to observing GMR reduction after learning, they observed that higher RPM scores were associated with greater GMR reduction demonstrating that greater visuospatial capacity was associated with less task-related neural activity. Similar neural activity reductions have been observed for faster, compared with slower, participants on mental imagery tasks (Kosslyn et al. 1996) and WM tasks (e.g., Rypma et al. 1999, 2002, 2006). These results suggest a specific model of neural efficiency in which the integrity of structural connections between task–critical brain regions is reflected in PET and fMRI activation. Specifically, they suggest that more direct connections between task–critical brain regions may correspond to decreases in task-related neural activity and improvements in performance (cf. Vernon 1983; Cerella 1991; Rypma and D'Esposito 1999, 2000; Rypma et al. 2006; see also Neubauer and Fink 2009).
Despite the explanatory power of the neural efficiency hypothesis and suggestive data, neuroimaging findings have not been replicated consistently across studies. Specifically, some studies have shown between-subject performance differences in which greater task-dependent activation was observed in higher than in lower performing individuals (e.g., Larson et al. 1995; Gray et al. 2003; Newman et al. 2003) and suggest that neural activity increases with task-related cognitive capacity. Mixed results in ERD measurements have been observed as well. For instance, unlike the Grabner et al. (2003) results reviewed above, Klimesch (1997, 1999) has observed greater ERD for higher than lower performing participants (see Jausovec N and Jausovec K 2005, for a review).
Similar discrepant results have been reported in PET and fMRI studies. Gray et al. (2003), for instance, performed a study similar to Haier et al.’s (1992) (see above) in which, prior to fMRI scanning, participants performed the RPM task. During scanning, participants performed a complex WM task in which they viewed single letters that appeared sequentially. They were required to respond each time they observed the appearance of a letter that had also occurred 3 trials earlier. The difficulty of the task was varied by the occasional occurrence of “lure” trials in which a letter presented on a current trial had also appeared 2, 4, or 5 trials previously. Unlike the results of Haier et al. (1992) described above, they observed, on the lure trials, greater neural activity within PFC, in participants with higher, compared with those with lower RPM performance (see also Brand and Deary 1982; Callicott et al. 2000 see Toffanin et al. 2007, for further review), suggesting that greater visuospatial WM capacity was associated with greater task-related PFC neural activity.
Divergent patterns of activation–performance relations across neuroimaging studies may occur for a number of reasons. In the studies reviewed above, different tasks were employed in the different studies. One possibility suggested by the discrepant results in the Gray et al. (2003) and Haier et al. (1992) studies is that the nature of activation–performance relations may be task dependent. It may be that the n-back task used by Gray et al. (2003) and the tetris task used by Haier et al. (1992) emphasize different cognitive mechanisms. Other studies have also shown divergent results (e.g., Tower of London; Newman et al. 2003; Sternberg-type WM; Rypma et al. 1999; backward digit span; Larson et al. 1995). Indeed, even subtle variations in task parameters have been shown to influence activation–performance relations in both EEG and fMRI studies (Johnson et al. 1997; Rypma, 2006).
The between-study variation in brain-behavior relationships that have been observed in prior studies suggests that these relationships could vary on the basis of task demand. Looking across a broad range of studies (Bressler 1995; Corbetta et al. 1995; Larson et al. 1995; Petersen et al. 1998; Poldrack et al. 1998; Smith and Jonides 1999; Rypma et al. 2002; Newman et al. 2003; Maccotta and Buckner 2004; Landau et al. 2007; Bressler et al. 2008), 3 observations can be made about the variation in brain-behavior relationships. The first observation is that these studies have been consistent in identifying a frontoparietal network in which neural activity varies on the basis of individual participants’ performance, repeated performance of a single task, or repeated stimulus exposure. These results are important because they suggest that performance might vary between individuals on the basis of interactive functions of relatively distant brain regions whose communication depends on the integrity of large-scale networks. Bressler (1995, 1996) and Bressler et al. (2008), for example, have pioneered the concept of large scale–distributed processing in functionally localized brain regions. Using local field potentials from up to 15 cortical sites in 1 hemisphere of functioning adult rhesus monkeys, Bressler et al. (1993) found task-related multiregional synchronization over the entire frequency range examined. In subsequent studies, using local field potential data from extracellular recordings, they demonstrated synchrony on physically distant but functionally related regions during task performance. Thus, it may be that the efficiency of coordinating such complex systems, involving the integration of multiple distributed areas differs between individuals, and is reflected in interactions between frontal and parietal regions (Bressler 1995; Brovelli et al. 2004, 2008).
The second observation is that results have been inconsistent with respect to the direction of brain-behavior relationships. That is to say, some studies have shown activation increases with increases in performance, whereas others have shown activation decreases with increases in performance. Resolving these discrepancies would have important implications for, for instance, how we ascribe cognitive functions to brain regions and whether optimal performance depends on the amount of activation that accompanies task performance (e.g., Klimesch 1997, 1999; Gray et al. 2003) or the speed and efficiency of activation and communication between brain regions (e.g., Vernon 1983; Haier et al. 1992; Grabner et al. 2003; Neubauer et al. 2004; Rypma et al. 2006). The third observation about brain-behavior studies is that different tasks ranging from simple digit span to RPM, with differing cognitive requirements eliciting different levels of performance, have been employed across these studies. Therefore, it has been difficult to ascertain the contribution of task demands to the variance observed across studies.
In the present study, we sought to examine relationships between neural activity and performance in this frontoparietal network, in a single group of participants, using tasks that varied in the extent of cognitive demand. We employed GCA to develop a network-based model and to investigate effective connectivity relationships between brain regions, how they varied with task demand, and how they varied with individual differences in participants’ performance.
Previous studies of functional cerebral connectivity have relied on methods that identify sets of brain regions with correlated signal-change patterns (e.g., ICA and PCA; McKeown et al. 1998; Biswal and Ulmer 1999; Allen et al. 2005). These studies have yielded robust delineation of functional connectivity between brain regions by locating discrete temporal structures (e.g., Biswal et al. 1995; Hyde and Biswal 1998; Gusnard et al. 2001; Greicius et al. 2003; Fox et al. 2005). GCA adds important information to that derived from these methods by assessing time-lagged relationships between functionally connected regions, permitting inferences about the directional influences of effective connections. In the present study, we used GCA to gain clues regarding the role of effective connectivity in performance differences between individuals. Brain-behavior relationships have been investigated in the context of fMRI activity (e.g., Rypma and D'Esposito 1999; Rypma et al. 2002, 2006; Grabner et al. 2003; Gray et al. 2003; Beschoner et al. 2008; Perfetti et al. 2009; Rypma and Prabhakaran 2009). How relationships between neural connectivity and behavior vary based on task demands, however, has not yet been systematically investigated.
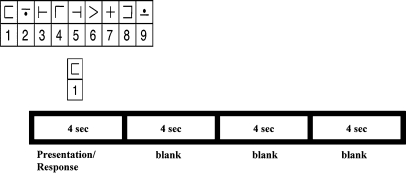
In this study, a single group of participants performed 2 kinds of tasks during fMRI scanning. The first task was a vigilance task in which participants passively viewed a fixation point and were periodically signaled to press a button. The second task was a digit–symbol substitution task (DSST) in which, on each trial, participants viewed a code table containing digit–symbol pairs, and a single digit–symbol probe that appeared simultaneously below the key (Fig. 1). If the probe pair matched one of those in the table, participants pressed a right-thumb button; otherwise they pressed a left-thumb button. A generation of research on DSST performance indicates that it is maximally sensitive to individual performance differences, that it requires a circumscribed set of cognitive mechanisms (including visual search, pattern matching, and response selection), and that it is minimally sensitive to individual strategy differences (Erber 1976; Grant et al 1978; Beres and Baron 1981; Wechsler 1981; Joy et al. 2000). Thus, the vigilance task was used to evoke neural activity on the basis of minimal cognitive demand, requiring vigilant attention to stimulus presentation and a simple response. The DSST was used to evoke neural activity that was more cognitively demanding, involving not only attention and simple button-press requirements, but also visual-search and choice-response requirements. Directional influences that were evoked during task performance were assessed using GCA performed on time series data from PFC and parietal regions where neural activity is known to vary between individuals and where DSST-related neural activity has been observed before (Rypma et al. 2006).
Figure 1.
Trial sequence of the modified DSST. On each trial, a code table appeared in the middle of the screen while a probe digit–symbol pair appeared below it. These stimuli stayed on the screen for 3.5 s followed by variable intertrial intervals (0.5, 4.5, 8.5, or 12.5 s).
Materials and Methods
Participants
Twelve participants (ages 18–27, 7 males and 5 females) were recruited from the Rutgers University and New Jersey Medical School campuses. Participants were excluded if they had any medical, neurological, or psychiatric illness, or if they were taking any type of prescription medication. Participants were screened for depression using the Beck Depression Inventory, which is a 21-item screener for depressive symptoms (BDI; Beck and Steer 1987). Individuals scoring above 14 (i.e., mild depression) were excluded because of the potential for depression to influence brain activity. The study was approved by the University of Medicine and Dentistry of New Jersey and Rutgers University Institutional Review Boards.
Behavioral Tasks
Participants were brought into the behavioral laboratory, signed consent and given a standard battery of tests and questionnaires, and were trained on the computerized DSST by one of the authors (D.A.E.). Participants were then brought to the neuroimaging laboratory. Prior to scanning, they were given brief practice with each of 2 tasks they were to perform during scanning.
Vigilance Task
Each subject performed a task in which he or she stared at a central white fixation cross for 18-s intervals after which the cross changed briefly (500 ms) to a circular checkerboard that flickered at 8 Hz for 500 ms, cueing participants to make a bilateral button press. Twenty such events occurred during the 320-s scan (160 images). All scanning parameters were identical to those used for the DSST.
Digit–Symbol Substitution Test
Following performance of the vigilance task, participants performed a task modeled after the DSST from the Wechsler Adult Intelligence Scale (1981). On each fMRI scanning trial, a code table containing digit–symbol pairs and a single digit–symbol probe appeared simultaneously (Fig. 1) for 3.5 s. If the probe pair matched one of those in the table, participants pressed a right-thumb button; otherwise, they pressed a left-thumb button. There were a total of 260 trials in 5 scanning runs (ca., 52 trials per run); the trials for each run were randomly intermixed (jittered) with 23 4-s rest periods. On half the trials, the probe pair matched one of the digit–symbol pairs in the code table, on the other half, the probe pair did not match one of the pairs in the code table. RT was measured as the time from the onset of the stimulus (i.e., code table and probe-pair presentation) to the time that the subject made a response. Participants were required to respond within the 3.5 s that the stimuli appeared on the screen. To discourage WM-based strategies, the digit–symbol pairings in the code table changed randomly from trial to trial. We used an event-related design that allowed us to examine blood-oxygenation-level-dependent (BOLD) signal changes separately during each trial event.
MRI Technique
Imaging was performed on a 3-T head-only Allegra scanner (Siemens Medical Systems, Ehrlangen, Germany) equipped with a fast gradient system for echoplanar imaging. A standard radiofrequency head coil was used with foam padding to comfortably restrict head motion. High-resolution T1-weighted sagittal images were collected. A gradient echo, echoplanar sequence (repetition time [TR] = 2000 ms, echo time [TE] = 30 ms, DSST = 150 vol, Vigilance = 180 vol) was used to acquire data sensitive to the BOLD signal. Resolution was 3.5 × 3.5 mm in-plane and 4 mm between planes (thus 32 axial slices were acquired). Eighteen seconds of gradient and radiofrequency pulses preceded the actual data acquisition to allow tissue to reach steady state magnetization.
Image Analysis
FMRI data were analyzed using AFNI software (Cox 1996). Participant-level task-related effects were identified using conventional linear deconvolution. A regressor was constructed by convolving a hemodynamic response model (a gamma-variate function; Cohen 1997, parameters b = 8.6, c = 0.547) with each trial onset in a task-reference function. The t-value matrix for each subject was resampled to a 2-mm isovoxel resolution and then spatially normalized to Talairach space (Talairach and Tornoux 1988). Each participant's 3D structural image (coregistered to the functional data) was transformed, via a 12-parameter affine transformation, to fit it to a Talairach template (i.e., the Colin-brain template), and then the t-value matrix was transformed to Talairach space based on structural image transformation parameters. Regressors for motion correction estimates and linear, quadratic, and cubic trends for each run were included in the baseline regression model. Any subjects with greater than 3 mm of motion were not considered for further analysis. No subjects met this criterion for exclusion (see Supplementary Table 1). For each participant, the preprocessed BOLD data per voxel were then regressed on the resulting model to obtain model scaling parameter estimates (i.e., task-related percent signal-change estimates) and corresponding t-values.
To plot mapwise activation at the group level, the data for individual participants were corrected for slice-timing offset, motion corrected, and then spatially filtered with a Gaussian kernel (full-width at half-maximum = 8 mm). For each run, data then were scaled by the mean for that run (i.e., 100 × yt/My) in each voxel so that the deconvolution parameter estimates would be expressed in terms of percent signal change.
GCA was performed using code written in MATLAB. Data analyses were performed on the time series from 12 regions of interest (ROIs). These ROIs were drawn on individual subjects’ anatomical images to include Brodmann's areas (BAs) where DSST activation was observed. ROIs were drawn on each subject's T1 axial slices by one of the authors (D.A.E.) using software from the VoxBo statistical package. These regions included middle and superior frontal gyri, corresponding to BAs 9 and 46, ventral PFC ROIs including inferior frontal gyri corresponding to BAs 44, 45, and 47, and superior parietal gyrus corresponding to BAs 39 and 40, in each hemisphere, according to the Talairach and Tournoux (1988) and Duvernoy (1999) atlases. For individual participants, time series from all voxels within an ROI were averaged to create a single time series for each ROI.
Each time series was detrended to remove any systematic variation in the data sets that could result from machine system noise leading to linear, cubic, or quadratic drift. To minimize the effect of physiological noise sources like respiration rate and heart rate (so called nuisance covariates), a low-pass filter (with a cutoff frequency of 0.10 Hz) was used. Granger causality between 2 regions can be defined as the extent to which the data from 1 region at 1 point in time significantly improves the prediction of another region's data at a later point in time (Goebel et al. 2003).
Bivariate Granger analyses were performed using F-statistics to test whether lagged data from a time series (variable) y improved the prediction of a later value in a time series (variable) x to a degree that was statistically significant over that provided by lagged x alone. If not, then “y did not influence x.” The model assumed a model order (i.e., lag length) p = 5 TRs, and estimated a residual for the following unrestricted equation by ordinary least squares (OLS):
| (1) |
where x(t) and y(t) are the 2 time series being evaluated for influence, t is the current time point, a(i) and b(i) are the linear prediction coefficients for x and y, u is the residual error of the fit, and p is the lag length.
The residual variance from this full model (eq. 1) was compared with the residual variance from the following reduced autoregressive model:
| (2) |
where x(t) is the time series being evaluated for influence, t is the current time point, g(i) is the linear prediction coefficient for x, e is the residual error in fit, and p is the lag length.
If the F-test comparing the residual variance from the full model to the residual variance from the reduced model
| (3) |
(where T is the total number of time points, p is the model order) was greater than tabular significance values, then the null hypothesis (y does not influence x) was rejected (Greene 2008).
Thus, a time series from each ROI was fit using a full autoregressive model. Briefly, in an autoregressive process the time-series data sets assume that the current time point is functionally related to its N previous time points. For this study a fifth order autoregressive process was used for each of the 8 ROIs.
Five time points (10 s) in the y time series were sequentially assessed for their effect in the prediction of a point immediately preceding them in the x time series as the difference in error terms between the full and reduced models. Thus, for each such assessment, the time point in the x time series served as the dependent variable whereas the time points in the y time series served as independent variables.
Ten-second model orders were adopted to account for delay that may arise due to differences between time series in the hemodynamic response. Although 10 s is orders of magnitude larger than neuronal delays, the fMRI signal represents a convolution of the neuronal signal with the vascular response function. A number of methods have been proposed to estimate the appropriate model order, including Bayesian and Akaike information criterion methods that permit selection between models on the basis of the extent to which variance can be explained with the fewest parameters. Application of these methods to determine a model-order parameter for GCA of fMRI data depends on the assumption that a single model fits the data across all voxels involved in the analysis. FMRI signal, however, is comprised of several noise components that arise from respiration, cardiac pulsatility, and machine noise, differentially affect different voxels, and impose influences upon the signal arising from neural activity. Therefore different model orders may be obtained for different voxels, as calculated by information criterion methods. Based on these considerations, we sought to determine an optimal model order based on known properties of the hemodynamic response function.
We determined an optimal autoregressive order based on prior estimates of onset-delay and phase-delay variances of the vascular response function, observed to be around 10–12 s by a number of different investigators (Lee et al. 1995; Boynton et al. 1996; Saad et al. 2003). Thus, our 5-TR model order was relatively conservative in the context of the hemodynamic filter.
Submodel fits were then carried out for each time series data set compared with the other time series data sets. The significance level for each of them was tabulated for group analysis. For each bivariate model, the F value for each individual subject was calculated, resulting in an interregional matrix consisting of M*(M − 1)/2 values (where M is the number of matrix elements) that were then z-transformed. An average z-score map was obtained for each task and converted to significance values. We used a false discovery rate procedure to correct for multiple comparisons at a q value of 0.05 (Benjamini and Yekutieli 2001).
This bivariate procedure was performed on all possible ROI pairs such that Granger influences were computed in both directions for all ROI pairs (Brovelli et al. 2004). Unidirectional influences between each ROI pair were calculated for 2 different significance levels. Influences were considered significant for Ps < 0.05, and they were considered trends when 0.05 < P < 0.10. Influences were considered “unidirectional” if the influence of 1 region of the pair was significant. They were considered “bidirectional” when the influences of both pairs were significant.
Results
Behavioral Performance
One participant's data were lost due to equipment failure. Behavioral analyses (using the SAS statistical package, SAS Institute, Cary, NC) of the vigilance data indicated uniformly high accuracy with minimal interindividual variability (M = 98.8%, standard deviation [SD] = 0.001). RTs were fast, also with minimal interindividual variability (M = 507.5 ms, SD = 41.0). Analysis of DSST data also indicated uniformly high accuracy with minimal interindividual variability (M = 95.8%, SD = 0.01) that was not significantly different from vigilance task accuracy. DSST RTs were significantly slower than button-press RT (M = 1641.5 ms, SD = 238.8), t(10) = 16.45, P < 0.005, and showed greater variability.
DSST Activation
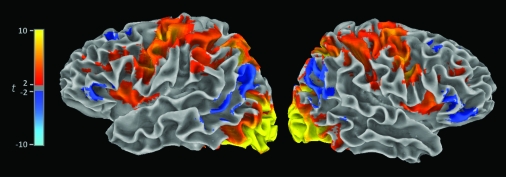
Figure 2 shows the average t-values (thresholded at t ≥ 2.00, P < 0.05 uncorrected). The results of this analysis indicated task-related signal-change in the previously identified target regions, including dorsolateral PFC (BA 9 and BA 46), ventrolateral (BA 44, BA 45, and BA 47) PFC, and inferior parietal cortex (BA 39 and BA 40).
Figure 2.
Average t-values (thresholded from cyan to blue and red to yellow, respectively, at −2.00 ≥ t ≥ 2.00, P < 0.05 uncorrected), spatially normalized to Talairach space via affine transformation to the Colin-brain template. The mean t-values are shown on surface models created from the Colin template. These results show task-related signal change in the previously identified target regions, including dorsolateral PFC (BA 9 and BA 46), ventrolateral (BA 44, BA 45, and BA 47) PFC, and inferior parietal cortex (BA 39 and BA 40).
FMRI Connectivity: between-Task Differences
Analysis of DSST fMRI data (filtered for temporal drift and high-frequency noise to minimize nuisance-covariate effects) using a modified (to account for serially correlated error terms; Worsley and Friston 1995) general linear model indicated activity in several cortical regions including the 12 ROIs across the 2 hemispheres, dorsal PFC (DPFC; Brodmann Area 9; BA 9), a relatively more posterior and inferior regions of PFC (BA 46), ventral PFC (BAs 44, 45, and 47), and parietal cortex (BA 40). Anatomical ROIs were drawn on these regions and GCA methods were used to assess influences between them. GC between 2 regions can be defined as the extent to which the data from one region at one point in time improves the prediction of another region's data at a later point in time (Goebel et al. 2003). GCA was used to evaluate causal influences between ROIs by measuring the extent to which activation changes in one region affected (i.e., reliably preceded) those in other regions at later points in time. Thus, it permitted characterization of the strength and direction of influence between discrete brain regions (Goebel et al. 2003).
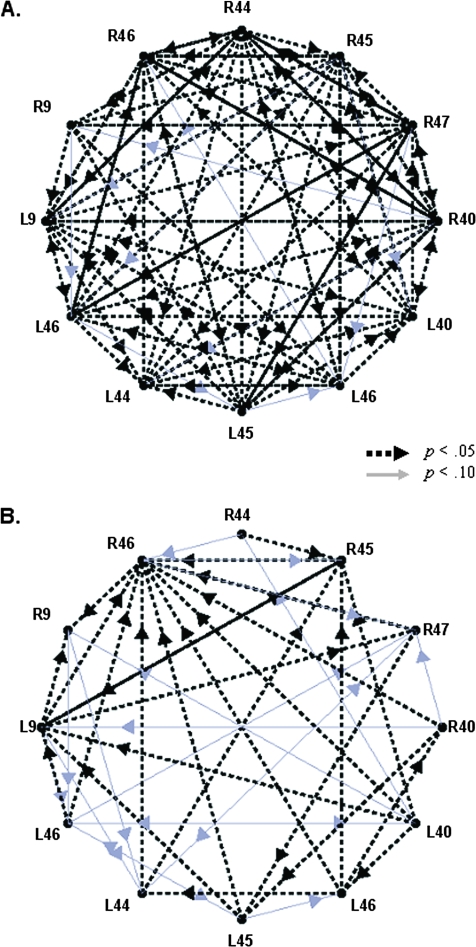
Figure 3 shows the GCA results for the vigilance task (A) and for the DSST (B). For the results of both tasks, the results were arranged in a circular fashion with rostral information represented on the left side of each circle, relatively ventral and posterior regions are illustrated in the middle portions so that the caudal-most ROIs are on the right side of the circle. Arrows indicate significant influences; black dashed-line arrows represent influences with Ps < 0.05; thinner gray arrows represent influences with Ps < 0.10.
Figure 3.
Results of the GCAs for the vigilance task (A) and for the DSST task (B). For both tasks, the results are arranged in a circular fashion with rostral information represented on the left side of each circle, relatively ventral and posterior regions are illustrated in the middle portions so that the caudal-most ROIs are on the right side of the circle. Arrows indicate significant influences; thicker black and dashed arrows represent influences with P < 0.05; thinner gray arrows represent influences with 0.05 < P < 0.10.
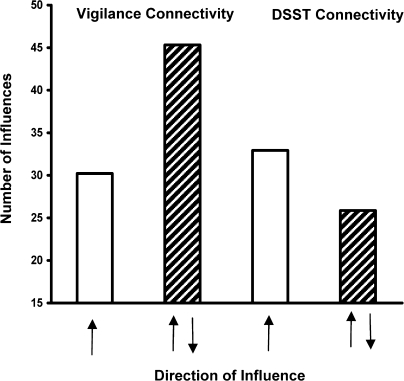
The results illustrated in Figure 3 show 2 differences between the vigilance task and the DSST. First it can be observed that, overall, there were more significant influences between ROIs for the vigilance task than for the DSST. This difference was significant t(10) = 14.96, P < 0.0005. Second, it can be observed that there were more bidirectional influences between ROIs for the vigilance task than for the DSST. This difference is illustrated in Figure 4 which shows mean numbers of unidirectional and bidirectional influences for both task types. It can be seen in Figure 4 that unidirectional influences are equivalent between the 2 tasks but that there are more bidirectional influences for the vigilance task than for the DSST. The interaction of Task-type (vigilance vs. DSST) and Influence type (unidirectional vs. bidirectional) was significant, F(1, 10) = 11.4, P < 0.007. Posthoc t-tests indicated significant differences between tasks for bidirectional but not for unidirectional influences.
Figure 4.
Mean numbers of unidirectional and bidirectional influences for both task types across ROIs. Unidirectional influences are illustrated in solid open bars and labeled with single arrows. Bidirectional influences are illustrated as filled bars and labeled with 2 arrows.
FMRI Connectivity: between-Subject Differences
To test relationships between the nature of directional influences in each ROI and performance, we made 2 calculations for each subject and each task within each ROI. First, we calculated the number of regions that exerted influences upon each ROI (i.e., the number of “input influences”) and second, we calculated the number of regions that each ROI had influences upon (i.e., the number of “output influences”; Brovelli et al. 2004). To examine how brain-behavior relationships during DSST activity differ from vigilance activity, we first calculated, for each ROI in each subject, differences in the numbers of influences for the vigilance task and the DSST. These calculations were performed separately for input and output influences. It is worth noting that, in correlation-based analyses, these types of connections are considered to be identical. GCA however permits separate assessment of both input influences from other regions and output influences to other regions for each ROI. Miniscule intersubject variability in vigilance RT performance obviated meaningful analysis with these behavioral data. Thus, we performed a series of linear regressions, with Bonferroni correction (Holm 1979), on individual participants’ DSST RT, and differences between tasks in the numbers of input and output influences. This approach is similar to that performed by Brovelli et al. (2004).
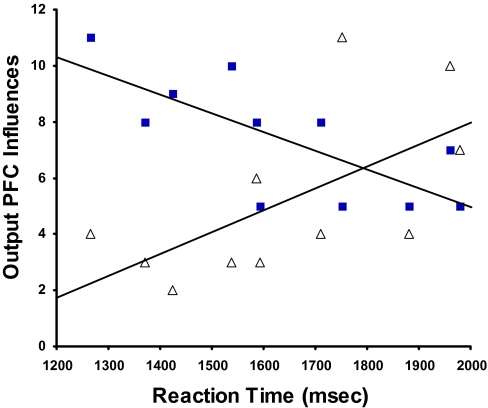
There were no input influences to any ROIs that were significantly different between the 2 tasks after Bonferroni correction. The linear regression of DSST RT and task differences in numbers of output influences from BA 9 to inferior PFC and parietal regions in the right hemisphere was significant (slope = −0.78; r2 = 0.61; t = −3.8; P < 0.004) and accounted for fully 61% of the variance (see Supplementary Table 2). Thus, inter-task differences in numbers of output influences depended on individual participants’ RTs. Faster participants showed greater numbers of output influences during vigilance-task performance than during DSST performance whereas slower participants showed the opposite pattern Specifically, numbers of BA 9 output influences were negatively correlated with individual participants’ DSST RT (slope = −0.78; r2 = 0.49; t = −3.3; P < 0.01) in the vigilance task, but positively correlated with their DSST RT (slope = 0.62; r2 = 0.31; t = 2.4; P < 0.04; Fig. 5) in the DSST.
Figure 5.
Scatterplot showing relationships between DSST RT and unidirectional output PFC influences (determined by GCA) from BA 9 for the vigilance (filled squares) and DSST (open triangles) tasks.
Discussion
In this study, we tested the hypothesis that the nature of activation–performance relations varies with the extent of cognitive involvement required by the task. We compared effective connectivity differences between brain regions where neural activity was elicited in 2 simple tasks that varied in the extent of cognitive demand. We used an analysis method that permitted unambiguous assignment of the direction of connectivity, GCA. Participants were faster when they were cued periodically to press a button than when they were required to determine the presence or absence of a probe digit–symbol pair among a string of such pairs. There was greater effective connectivity in the vigilance task compared with the DSST. Analyses of directional connectivity indicated that there were significantly more bidirectional influences in the vigilance task than in the DSST. Unidirectional influences were, however, equivalent between the 2 tasks. Finally, analysis of individual differences in Granger causal influences indicated that individuals with faster DSST RTs showed reduced dorsal PFC influence extending to ventral PFC and posterior parietal regions than slower individuals during DSST performance. These same faster individuals, however, showed increased dorsal PFC influence compared with slower individuals during vigilance task performance.
Vigilance-Related Activity
The present results clearly suggest an association between activation–performance relations and task demand. When task demand was low, requiring participants only to maintain vigilance for a signal to press a button, processing-speed ability (as measured by DSST) was negatively associated with neural connectivity. This increased vigilance-related connectivity mainly resulted from increases in bidirectional connectivity between brain regions. Such activity may reflect in part vigilance operations of participants awaiting the response signal. Passive vigilance has been known to elicit increased activity in previous studies, compared with that elicited during task performance (e.g., Fransson 2006). Increased bidirectional connectivity might suggest an increased equilibrium in signal transmission between brain regions during vigilance compared with DSST performance. Increased bidirectional fluctuation during sustained vigilance has important implications for speculation regarding the mechanisms that could give rise to interregional connectivity when cognitive mechanisms are relatively minimally engaged. To support such activity, brief neural pulses may fluctuate in synchrony between functionally related regions. Such spontaneous fluctuation has been observed in low-frequency ranges during other tasks that minimally involve cognition (e.g., Cordes et al. 2001; Goldman et al. 2002).
A number of mechanisms have been hypothesized that could give rise to such phenomena. For instance, Zonta et al. (2003) have suggested that glutamate-mediated Ca2+ fluctuation in astrocytes could mediate arteriole dilation. Ca2+ transient pulses in astrocyte end feet (part of the astrocyte that makes contact with the arteriole) also cause cerebrovascular contraction (Mulligan and McVicar 2004). Thus, the balance between these opposing cellular signaling mechanisms may govern vigilant rest-related activity. Ca2+ channel inhibition led to disruption of this signaling. A shift in the balance between cell signaling mechanisms could mediate a modal shift from vigilant rest to active task performance. Indeed, other similar mechanisms, including nitric oxide synthase have also been shown to disrupt low-frequency fluctuations (Biswal and Hudetz 1996).
DSST-Related Activity
In contrast to vigilance-related connectivity, when task demand was relatively high, requiring participants to not only maintain vigilance but also to search an array for the presence of a digit–symbol target, connectivity was 1) reduced relative to the lower demand vigilance task and 2) dominated by more unidirectional than bidirectional activity, as measured by GCA. The relative reduction in bidirectional connections during DSST suggests that task-related activity reflects disengagement of equilibrium mechanisms that dominate low-demand task activity and engagement of mechanisms that involve more directed activity between brain regions. This directed activity may reflect the goal-oriented executive control that dorsal PFC regions exert upon more ventral and more posterior brain regions that are involved in the execution of the visual search, target-detection, and response-selection processes required by the DSST. The relationship we observed between connections that extended from dorsal PFC to other brain regions replicates earlier results from our laboratory (Rypma et al. 2006; Motes MA, Rypma B, unpublished data) and suggests support for the hypothesis that individuals vary in the extent to which cognitive processes can be implemented automatically. It may be that some individuals implement the cognitive operations necessary for successful task performance (mediated by ventral PFC and parietal brain regions) automatically, with minimal reliance on PFC regions involved in executive operations (e.g., dorsolateral PFC; Jung and Haier 2007; Prabhakaran and Rypma 2007). Other individuals may implement these operations in a more controlled fashion. In these individuals, PFC mediation may serve to guide more ventral and posterior brain regions in the service of successful task performance.
Task-Dependent Variation in Brain-Behavior Relationships
Relationships between neural activity and behavioral state have been observed in studies comparing waking, sleeping, and anesthesis in animals and humans. When humans are minimally engaged in cognitive activity (i.e., during “resting state”), neuroimaging signal exhibits higher amplitude and interregional correlation depending upon whether individuals are anesthetized or not (Kiviniemi et al. 2005), whether they are asleep or awake (Fukunaga et al. 2006; Horovitz et al. 2008;), or whether they have their eyes open or closed (e.g., Yang et al. 2007; Bianciardi et al. 2009). Although the origin of these state-related signal changes are not yet completely understood (e.g., Birn et al. 2006), these findings have important implications for a complete theory of functional neural circuitry at rest, during task-related activity, and the interaction of resting and active functional circuitry. For instance, some studies indicate that distinct networks of activity during spontaneous and evoked activity interact such that increases in spontaneous network activity result in reduced activity evoked by sensory stimulation (Sachdev et al. 2004; Hasenstaub et al. 2007). Such results suggest a dynamic balancing mechanism in which resting neural activity levels mediate the responsiveness of networks to stimulation. The differences observed in DSST-related Granger connectivity, compared with vigilance-related connectivity, suggest that such balancing mechanisms play an important role in determining levels of activation observed during task performance.
Participant-Dependent Variation in Functional Connectivity
In the present results, task-dependent connectivity changes differed between individuals. Specifically, within dorsal PFC, faster participants (as measured by DSST) showed more vigilance-related connectivity than slower participants, but faster participants showed less DSST-related connectivity than slower participants. The results suggest that the balancing mechanisms that mediate differences in low- versus high-demand PFC connectivity are intimately related to individual differences in cognitive efficiency (as measured by DSST).
The finding of participant-dependent connectivity differences has implications for both cognitive and neural explanations of individual differences. At a cognitive level, one possibility is that dorsal PFC regions subserve the simultaneous monitoring of task-demand information and performance accuracy in the service of task learning. This region has been implicated in performance monitoring (e.g., Sharp et al. 2004). Such task- and performance-monitoring may aid in the development of more efficient, or “automatic” task-performance. Those individuals without such extra-task processing capability may rely on less efficient “controlled” processing (cf. Schneider and Shiffrin 1977), mediated by right-hemisphere regions of dorsal PFC. Consistent with our findings, frontal activity declines have been observed with skill improvements that reflect development of task automaticity (Petersen et al. 1998).
At a neural level, participant-dependent connectivity changes may reflect individual differences in the integrity of large-scale networks composed of computational nodes comprised of physically distant but functionally related brain regions that must coordinate and integrate functions (e.g., Bressler and Kelso 2001). Findings of activation synchrony across relatively distal brain regions support this notion. It may be that white-matter integrity affects neural transmission efficiency between these brain regions which in turn, affects performance (e.g., Jensen 1982; Vernon 1983; Rypma and D'Esposito 1999; Grabner et al. 2003; Rypma and Prabhakaran 2009). Precise explication of the mechanisms that govern differences in white-matter integrity has only begun to emerge as measures that distinguish these mechanisms in pathological and aging populations have been developed (e.g., Song et al. 2003; Nair et al. 2005; Bennett et al. 2009). Studies utilizing these measurement methods indicate that such mechanisms could take several forms including changes in axon number, size, and myelination extent. More research is certainly required before any precise inferences could be made about the nature of the white-matter differences that distinguish between relatively good and poor performers on cognitive tasks.
Granger Causality and Neural Connectivity
Other multivariate statistical methods including ICA and PCA have been used to decompose fMRI data into independent components on the basis of distinct sets of linear parameters. The “images,” or time-series data sets, derived from these analyses represent functional connectivity maps that have been important for understanding connectivity relations between regions (e.g., McKeown et al. 1998; Biswal and Ulmer 1999).
GCA improves upon these methods because Granger regression explicitly accounts for interregional temporal variability that has been demonstrated in previous reports (Lee et al. 1995; Buckner et al. 1996; Miezin et al. 2000), whereas ICA and PCA assume that the exact sequence of information flow cannot be obtained from the data. Thus, Granger correlation adds to information obtained from ICA and PCA about functionally connected brain regions by explicitly accounting for interregional temporal variability.
The use of temporal variability in BOLD signal to make inferences about regional connectivity could be considered hazardous if it were known that interregional differences in hemodynamic delays varied systematically between regions. On one hand, explicit tests of such systematicity in humans have yielded null results (e.g., Miezin et al. 2000). These results suggest that the Granger correlations we observed here reflect systematic functional relationships between brain regions that emerge on the basis of task-related neural activity. On the other hand, some studies with rodents suggest that further development of connectivity analysis methods are required to minimize the influence of region-specific hemodynamic activity differences on estimates of neural connectivity (e.g., David et al., 2008). It is clear that more research is required to completely understand how relationships between interregional BOLD signal differences affect estimates of cortical influences, but current evidence suggests the bona fides of the Granger relationships that we have observed here (see also Kayser et al. 2009). The results of the present study, task- and subject-related differences in the extent to which PFC activity drives activity in other PFC and posterior brain regions, provides important clues regarding individual differences in cortical function and how they influence performance.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
NIH grants 1R01AG029523 (BR) and 5R01NS049176 (BB).
Supplementary Material
Acknowledgments
The authors wish to thank Elizabeth Bukowski, Jacqueline Witham, Eric Zaccone, and Mary Jo Maciejewski for assistance in data collection and analysis. Conflict of Interest: None declared.
References
- Allen G, McColl R, Barnard H, Ringe WK, Fleckenstein J, Cullum CM. Magnetic resonance imaging of cerebellar-prefrontal and cerebellar-parietal functional connectivity. Neuroimage. 2005;28:39–48. doi: 10.1016/j.neuroimage.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Del Percio C, Rossini PM, Marzano N, Iacoboni M, Infarinato F, Lizio R, Piazza M, Pirritano M, Berlutti G, et al. Judgment of actions in experts: a high-resolution EEG study in elite athletes. Neuroimage. 2009;45:512–521. doi: 10.1016/j.neuroimage.2008.11.035. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working Memory. NY: Oxford University Press; 1986. [Google Scholar]
- Beck A, Steer R. Manual for the Beck Depression Inventory. San Antonio (TX): Psychological Corporation. 1987 [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29:1165–1188. [Google Scholar]
- Bennett IJ, Madden DJ, Howard DV, Howard JH Forthcoming 2009. Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Mapp. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beres CA, Baron A. Improved digit symbol substitution by older women as a result of extended practice. J. Gerontol. 1981;36:591–597. doi: 10.1093/geronj/36.5.591. [DOI] [PubMed] [Google Scholar]
- Beschoner P, Richter S, Lo H, Sim EJ, Baron K, Osterfeld N, Horn AB, Viviani R. Baseline brain perfusion and working memory capacity: a neuroimaging study. Neuroreport. 2008;19:1803–1807. doi: 10.1097/WNR.0b013e32831997f1. [DOI] [PubMed] [Google Scholar]
- Bianciardi M, Fukunaga M, van Gelderen P, Horovitz SG, de Zwart JA, Duyn JH. Modulation of spontaneous fMRI activity in human visual cortex by behavioral state. Neuroimage. 2009;45:160–168. doi: 10.1016/j.neuroimage.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Hudetz AG. Synchronous oscillations in cerebrocortical capillary red blood cell velocity after nitric oxide synthase inhibition. Microvasc Res. 1996;52:1–12. doi: 10.1006/mvre.1996.0039. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Ulmer JL. Blind source separation of multiple signal sources of fMRI data sets using independent component analysis (neuroradiology) J Comput Assist Tomog. 1999;23:265–271. doi: 10.1097/00004728-199903000-00016. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Mag Res Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand CR, Deary IJ. Intelligence and “inspection time”. In: Eysenck hJ., editor. A model for intelligence. New York: Springer-Verlag; 1982. pp. 133–148. [Google Scholar]
- Bressler SL. Large-scale cortical networks and cognition. Brain Res Brain Res Rev. 1995;20:288–304. doi: 10.1016/0165-0173(94)00016-i. [DOI] [PubMed] [Google Scholar]
- Bressler SL. Interareal synchronization in the visual cortex. Behav Brain Res. 1996;76:37–49. doi: 10.1016/0166-4328(95)00187-5. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Coppola R, Nakamura R. Episodic multiregional cortical coherence at multiple frequencies during visual task performance. Nature. 1993;366:153–156. doi: 10.1038/366153a0. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Kelso JAS. Cortical coordination dynamics and cognition. Trends Cog Sci. 2001;5:26–36. doi: 10.1016/s1364-6613(00)01564-3. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Tang W, Sylvester CM, Shulman GL, Corbetta M. Top-down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. J Neurosci. 2008;28:10056–10061. doi: 10.1523/JNEUROSCI.1776-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler SL. Beta oscillations in a large-scale sensorimotor cortical network: Directional influences revealed by Granger causality. Proc Nat Acad Sci U S A. 2004;101:9849–9854. doi: 10.1073/pnas.0308538101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Bandettini PA, O'Craven KM, Savoy RL, Petersen SE, Raichle ME, Rosen BR. Detection of cortical activation during averaged single trials of a cognitive task using functional magnetic resonance imaging. Proc Nat Acad Sci USA. 1996;93:14878–14883. doi: 10.1073/pnas.93.25.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJP, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Cerella J. Age effects may be global, not local: comment on Fisk and Rogers (1991) J Exp Psychol Gen. 1991;2:215–223. doi: 10.1037/0096-3445.120.2.215. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Shulman GL. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, Miezin FM, Petersen SE. Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science. 1995;270:802–805. doi: 10.1126/science.270.5237.802. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA. Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting state” data. Am J Neurorad. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comp Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- David O, Guillemain I, Saillet S, Reyt S, Deransart C, Segebarth C, Depaulis A. Identifying neural drivers with functional MRI: an electrophysiological validation. PLoS Biol. 2008;6:2683–2697. doi: 10.1371/journal.pbio.0060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Percio C, Babiloni C, Bertollo M, Rossini P, Marzano N, Iacobini M, Infarinato F, Lizio R, Stocchi M, Robazza C, Cibelli G, Comani S, Eusebi F. Visuo-attentional and sensorimotor alpha rhythms are related to visuo-motor performance in athletes. Hum Brain Mapp. 2009;30:3527–3540. doi: 10.1002/hbm.20776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The human brain: surface, blood supply, and three-dimensional sectional anatomy. 2nd ed. New York, Wien: Springer-Verlag; 1999. [Google Scholar]
- Erber JT. Age differences in learning and memory on a digit–symbol substitution task. Exp Aging Res. 1976;2:45–53. doi: 10.1080/03610737608257976. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Nat Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function?: Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Horovitz SG, van Gelderen P, de Zwart JA, Jansma JM, Ikonomidou VN, Chu R, Deckers RH, Leopold DA, Duyn JH. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Mag Res Imag. 2006;24:979–992. doi: 10.1016/j.mri.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME. Neurophysiological measures of working memory and individual differences in cognitive ability and cognitive style. Cereb Cortex. 2000;10:829–839. doi: 10.1093/cercor/10.9.829. [DOI] [PubMed] [Google Scholar]
- Goebel R, Roebroeck A, Kim DS, Formisano E. Investigating directed cortical interactions in time-resolved fMRI data using vector autoregressive modeling and Granger causality mapping. Mag Res Imag. 2003;21:1251–1261. doi: 10.1016/j.mri.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Goldman RI, Stern JM, Engel J, Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002;13:2487–2492. doi: 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner RH, Stern E, Neubauer AC. When intelligence loses its impact: neural efficiency during reasoning in a familiar area. Int J of Neurophys. 2003;49:89–98. doi: 10.1016/s0167-8760(03)00095-3. [DOI] [PubMed] [Google Scholar]
- Grant EA, Storandt M, Botwinick J. Incentive and practice in the psychomotor performance of the elderly. J Gerontol. 1978;33:413–415. doi: 10.1093/geronj/33.3.413. [DOI] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nat Neurosci. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Greene WH. Econometric analysis. Upper Saddle River (NJ): Prentice Hall; 2008. [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Nat Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle M. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Nat Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier RJ, Siegel BV, Nuechterlein KH, Hazlett E, Wu JC, Paek J, Browning HL, Buchsbaum MS. Cortical glucose metabolic rate correlates of abstract reasoning and attention studied with positron emission tomography. Intelligence. 1988;12:199–217. [Google Scholar]
- Haier RJ, Siegel BV, Tang C, Abel L, Buchsbaum MS. Intelligence and changes in regional cerebral glucose metabolic rate following learning. Intelligence. 1992;16:415–426. [Google Scholar]
- Hasenstaub A, Sachdev RN, McCormick DA. State changes rapidly modulate cortical neuronal responsiveness. J Neurosci. 2007;27:9607–9622. doi: 10.1523/JNEUROSCI.2184-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Smith DV, Platt ML. Electrophysiological correlates of default-mode processing in macaque posterior cingulated cortex. Proc Nat Acad Sci. 2009;106:5948–5953. doi: 10.1073/pnas.0812035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- Horovitz SG, Fukunaga M, de Zwart JA, van Gelderen P, Fulton SC, Balkin TJ, Duyn JH. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG–fMRI study. Hum Brain Mapp. 2008;29:671–682. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JS, Biswal B. Functionally related correlation in noise. In: Moonen CT, Bandettini PA, editors. Functional MRI. Berlin (Germany): Springer-Verlag; 1998. pp. 263–275. [Google Scholar]
- Jausovec N, Jausovec K. Differences in induced gamma and upper alpha oscillations in the human brain related to verbal/performance and emotional intelligence. Int J Psychophysiol. 2005;56:223–235. doi: 10.1016/j.ijpsycho.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Jensen AR. Reaction time and psychometric g. In: Eysenck HJ, editor. A model for intelligence. Berlin and New York: Springer-Verlag; 1982. pp. 93–132. [Google Scholar]
- Jensen AR. The g factor: the science of mental ability. Westport (CT): Praeger; 1998. [Google Scholar]
- Johnson M, Nolde S, Mather M, Kounios J, Schacter DL, Curran T. The similarity of brain activity associated with true and false recognition memory depends on test format. Psychol Sci. 1997;8:250–257. [Google Scholar]
- Joy S, Fein D, Kaplan E, Freedman M. Speed and memory in WAIS-R-NI digit symbol performance among healthy older adults. J Int Neuropsychol Soc. 2000;6:770–780. doi: 10.1017/s1355617700677044. [DOI] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. The parieto-frontal integration theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci. 2007;30:135–189. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA. A capacity theory of comprehension: individual differences in working memory. Psychol Rev. 1992;99:122. doi: 10.1037/0033-295x.99.1.122. [DOI] [PubMed] [Google Scholar]
- Kahneman D. Attention and effort. Englewood Cliffs (NJ): Prentice-Hall; 1973. [Google Scholar]
- Kayser AS, Sun FT, D'Esposito M. A comparison of Granger causality and coherency in fMRI-based analysis of the motor system. Hum Brain Mapp. 2009;30:3475–3494. doi: 10.1002/hbm.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL, Kim IJ, Rauch SL, Alpert NM. Individual differences in cerebral blood flow in Area 17 predicts the time to evaluate visualized letters. J Cog Neurosci. 1996;8:78–82. doi: 10.1162/jocn.1996.8.1.78. [DOI] [PubMed] [Google Scholar]
- Kiviniemi VJ, Haanpää H, Kantola JH, Jauhiainen J, Vainionpää V, Alahuhta S, Tervonen O. Midazolam sedation increases fluctuation and synchrony of the resting brain BOLD signal. Mag Res Imag. 2005;23:531–537. doi: 10.1016/j.mri.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG-alpha rhythms and memory processes. Int J Psychophysiol. 1997;26:319–340. doi: 10.1016/s0167-8760(97)00773-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Landau SM, Garavan H, Schumacher EH, D'Esposito M. Regional specificity and practice: dynamic changes in object and spatial working memory. Brain Res. 2007;1180:78–89. doi: 10.1016/j.brainres.2007.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson GE, Haier RJ, LaCasse L, Hazen K. Evaluation of a “mental effort” hypothesis for correlations between cortical metabolism and intelligence. Intelligence. 1995;21:267–278. [Google Scholar]
- Lee AT, Glover GH, Meyer CH. Discrimination of large venous vessels i time-course spiral blood-oxygen-level-dependent magnetic resonance imaging. Mag Res Med. 1995;33:745–754. doi: 10.1002/mrm.1910330602. [DOI] [PubMed] [Google Scholar]
- Maccotta L, Buckner RL. Evidence for neural effects of repetition that directly correlate with behavioral priming. J Cog Neurosci. 2004;16:1625–1632. doi: 10.1162/0898929042568451. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Jung TP, Makeig S, Brown G, Kindermann SS, Lee TW, Sejnowski TJ. Spatially independent activity patterns in functional MRI data during the stroop color-naming task. Proc Natl Acad Sci USA. 1998;95:803–810. doi: 10.1073/pnas.95.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Mulligan SJ, McVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Nair G, Tanahashi Y, Low HP, Billings-Gagliardi S, Schwartz WJ, Duong TQ. Myelination and long diffusion times alter diffusion-tensor-imaging contrast in myelin-deficient shiverer mice. Neuroimage. 2005;28:165–174. doi: 10.1016/j.neuroimage.2005.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer AC, Fink A. Intelligence and neural efficiency. Neurosci Biobehav Rev. 2009;33:1004–1023. doi: 10.1016/j.neubiorev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Neubauer AC, Grabner RH, Freudenthaler HH, Beckmann JF, Guthke J. Intelligence and individual differences in becoming neurally efficient. Acta Psychol. 2004;116:55–74. doi: 10.1016/j.actpsy.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Newman SD, Carpenter PA, Varma S, Just M. Frontal and parietal participation in problem solving in the Tower of London. Neuropsychologia. 2003;41:1668. doi: 10.1016/s0028-3932(03)00091-5. [DOI] [PubMed] [Google Scholar]
- Norman DA, Bobrow DG. On data-limited and resource limited processes. Cog Psychol. 1975;7:44–64. [Google Scholar]
- Nunez PL, Wingeier BM, Silberstein RB. Spatial-temporal structures of human alpha rhythms: theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum Brain Mapp. 2001;13:125–164. doi: 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti B, Saggino A, Ferretti A, Caulo M, Romani GL, Onofrj M. Differential patterns of cortical activation as a function of fluid reasoning complexity. Hum Brain Mapp. 2009;30:497–510. doi: 10.1002/hbm.20519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. Proc Nat Acad Sci USA. 1998;95:853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Desmond JE, Glover GH, Gabrieli JDE. The neural basis of visual skill learning: an fMRI study of mirror reading. Cereb Cortex. 1998;8:1–10. doi: 10.1093/cercor/8.1.1. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Rypma B. P-fit and the neuroscience of intelligence: how well does P fit? Behav Brain Sci. 2007;30:166. [Google Scholar]
- Reiterer S, Berger ML, Hemmelmann C, Rappelsberger P. Decreased EEG coherence between prefrontal electrodes: a correlate of high language proficiency? Exp Brain Res. 2005;163:109–113. doi: 10.1007/s00221-005-2215-z. [DOI] [PubMed] [Google Scholar]
- Rypma B. Factors controlling neural activity during delayed-response task performance: testing a memory organization hypothesis of prefrontal function. Neuroscience. 2006;139:223–235. doi: 10.1016/j.neuroscience.2005.07.062. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, D'Esposito M. The influence of working memory demand and subject performance on prefrontal cortical activity. J Cog Neurosci. 2002;14:721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Genova HM, Rebbechi D, D'Esposito M. Dissociating age-related changes in cognitive strategy and neural efficiency using event-related fMRI. Cortex. 2005;41:582–594. doi: 10.1016/s0010-9452(08)70198-9. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Prabhakaran V, Bly BM, Kimberg DY, Biswal BH, D'Esposito M. Neural correlates of cognitive efficiency. Neuroimage. 2006;33:145–156. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proc Nat Acad Sci. 1999;96:6558–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V. When less is more and when more is more: the mediating roles of capacity and speed in brain-behavior efficiency. Intelligence. 2009;37:207–222. doi: 10.1016/j.intell.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JDE. Load-dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage. 1999;37:216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- Saad Z, DeYoe EA, Ropella KM. Estimation of fMRI response delays. Neuroimage. 2003;18:494–504. doi: 10.1016/s1053-8119(02)00024-1. [DOI] [PubMed] [Google Scholar]
- Sachdev RN, Ebner FF, Wilson CJ. Effect of subthreshold up and down states on the whisker-evoked response in somatosensory cortex. J Neurophys. 2004;92:3511–3521. doi: 10.1152/jn.00347.2004. [DOI] [PubMed] [Google Scholar]
- Schneider W, Shiffrin R. Controlled and automatic human information processing: 1. Detection, search, and attention. Psychol Rev. 1977;84:1–66. [Google Scholar]
- Sharp DJ, Scott SK, Wise RJ. Monitoring and the controlled processing of meaning: distinct prefrontal systems. Cereb Cortex. 2004;14:1–10. doi: 10.1093/cercor/bhg086. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Spearman C. General intelligence, objectively determined and measured. Am J Psychol. 1904;15:201. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Toffanin P, Johnson A, de Jong R, Martens S. Rethinking neural efficiency: effects of controlling for strategy use. Behav Neurosci. 2007;121:854–870. doi: 10.1037/0735-7044.121.5.854. [DOI] [PubMed] [Google Scholar]
- Vernon PA. Speed of information processing and general intelligence. Intelligence. 1983;7:53–70. [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale—revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited—again. Neuroimage. 1995;2:173–182. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Yang H, Long XY, Yang Y, Yan H, Zhu CZ, Zhou XP, Zang YF, Gong QY. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage. 2007;36:144–152. doi: 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.