Abstract
The temporal pole (TP) is the rostralmost portion of the human temporal lobe. Characteristically, it is only present in human and nonhuman primates. TP has been implicated in different cognitive functions such as emotion, attention, behavior, and memory, based on functional studies performed in healthy controls and patients with neurodegenerative diseases through its anatomical connections (amygdala, pulvinar, orbitofrontal cortex). TP was originally described as a single uniform area by Brodmann area 38, and von Economo (area TG of von Economo and Koskinas), and little information on its cytoarchitectonics is known in humans. We hypothesize that 1) TP is not a homogenous area and we aim first at fixating the precise extent and limits of temporopolar cortex (TPC) with adjacent fields and 2) its structure can be correlated with structural magnetic resonance images. We describe here the macroscopic characteristics and cytoarchitecture as two subfields, a medial and a lateral area, that constitute TPC also noticeable in 2D and 3D reconstructions. Our findings suggest that the human TP is a heterogeneous region formed exclusively by TPC for about 7 mm of the temporal tip, and that becomes progressively restricted to the medial and ventral sides of the TP. This cortical area presents topographical and structural features in common with nonhuman primates, which suggests an evolutionary development in human species.
Keywords: cytoarchitecture, humans, MRI, temporal pole, temporopolar cortex
Introduction
Historical Account
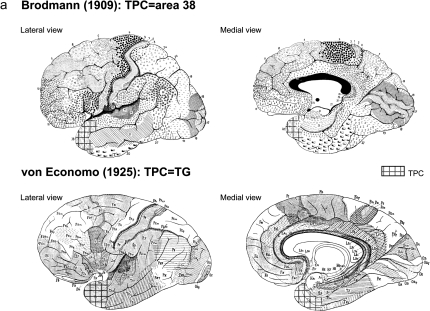
The rostralmost portion of the temporal lobe is known as the temporal pole (TP), a region completely formed by cortex that makes an extensive portion of cerebral cortex area encased in the anterior part of the middle cranial fossa. The temporal lobe is known to be implicated in many cognitive functions, including memory and emotional tagging of perceptive processes. The TP seems to play an important role in some of those functions, as revealed by the literature. Furthermore, from decades, both experimental and clinical evidence exist for the relation between lesions in the TP and different diseases such as Klüver–Bucy syndrome, frontal temporal dementia, Alzheimer's disease, epilepsy, head injury, as well as aging (Dupont 2002; Olson et al. 2007). However, the precise cytoarchitectonic organization, its location, and structure of this brain region still remain unclear. Originally described by Smith (1907) as temporopolar cortex (TPC), by Brodmann (1909) as area 38, and by von Economo and Koskinas (1925) as area TG of von Economo and Koskinas (TG) (Fig. 1a), the extent of the cortex lining the TP is often inaccurate in functional neuroimaging studies.
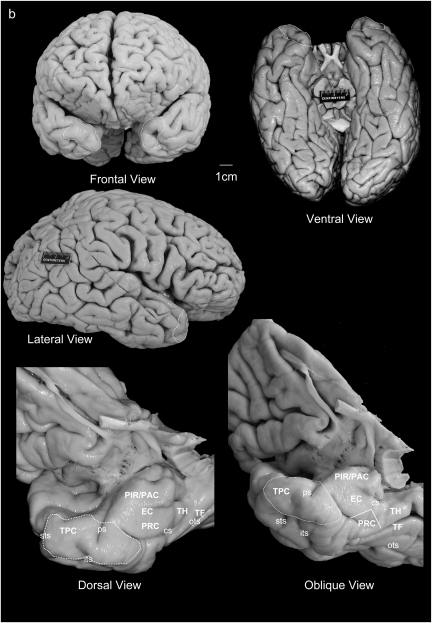
Figure 1.
(a) Location of the TPC according to Brodmann (1909) and von Economo and Koskinas (1925). (b) Frontal, ventral, and lateral aspects of the brain, with oblique views of the temporal lobe showing the whole parahippocampal gyrus including the TPC, piriform cortex, periamygdaloid, EC, PRC, and PPH cortex (areas TH and TF).
TPC, as a distinct cortical area, is macroscopically placed between the isocortex (laterally situated), proisocortex in a caudomedial continuation, and paleocortex located caudodorsal. The TPC, together with proisocortical areas of the perirhinal cortex (PRC) and posterior parahippocampal (PPH) cortex would form the parahippocampal region (Scharfman et al. 2000) that together account for about two-thirds of the cortical input to the entorhinal cortex (EC) in nonhuman primates (Insausti et al. 1987). In this report, we include under the commonly accepted term PRC, both Brodmann area (BA)35 and BA36, although only BA35 received the name of PRC originally, while BA36 received the term of ectorhinal cortex (Brodmann 1909). Likewise, under the term posterior parahippocampal gyrus, we include areas TF and TH of von Economo and Koskinas (1925). The parahippocampal region, plus other areas such as the EC, PRC, orbitofrontal cortex, cingulate cortex, and insular cortex, have been grouped under the term paralimbic structures (Mesulam and Mufson 1982).
Clinical Relevance of the TP
Klüver and Bucy Syndrome, Alzheimer's Disease, and Other Pathologies
In the past century, resection of large portions of the anterior temporal lobe in the nonhuman primate showed their involvement in several cognitive processes. Bilateral temporal lobe removals, performed by Klüver and Bucy, revealed strong impairments in emotional, social, and sexual behavior as well as in visual association referred to as “psychic blindness” or Klüver–Bucy syndrome (see for review Dupont 2002; Olson et al. 2007). Since, this syndrome has also been described in humans after ablation of the anterior temporal lobe in epileptic patients (Marlowe et al. 1975; Pestana and Gupta 2007). Similar symptoms (including “psychic blindness, prosopagnosia, oral exploration, hypermetamorphic impulse to action, lack of emotional responsiveness, aberrant sexual behavior, and insatiable appetite) were also observed in humans with herpetic meningoencephalitis, resulting in bilateral damage to the temporal lobes and especially to the TPs (Marlowe et al. 1975; Sanvito et al. 1982). Although these lesions surpassed the TP and involved other cortical areas, they nonetheless highlight the importance of this region in high-order cognitive processes (Patterson et al. 2007).
The TP is also largely involved in memory function and specifically in declarative memory either retrograde or anterograde as revealed by studies in epileptic patients (such as e.g., patient H.M., Scoville and Milner 1957; Corkin et al. 1997). The episodic memory impairments observed in those patients could be linked to the hypometabolism (Dupont et al. 2002) and atrophy (Ryvlin et al. 2002) observed in the TP.
Semantic memory is particularly affected in patients with lesion of the TP (Kapur et al. 1992), while procedural and working memory are generally preserved, stressing the specific involvement of the anterior part of the temporal lobe in memory processes. This is concordant with the declarative memory impairments observed in Alzheimer patients associated to an atrophy of the TP and a marked neuronal loss especially in layers III and V with an accumulation of neurofibrillary tangles (Arnold et al. 1991) as well as a decrease of glucose metabolism in the TP in addition to the hippocampus and amygdala (Ouchi et al. 1998; Zahn et al. 2004; Chételat et al. 2008).
Affection of the TP has also been reported in other neurodegenerative diseases such as in Pick's disease with an important neuronal loss, specifically in layer III (Arnold et al. 1994), and in schizophrenia with a loss of gray matter and atrophy (Wright et al. 1999), in frontal temporal dementia (see Olson et al. 2007 for review).
Functional Studies
The TP has been studied in several experiments using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) confirming both its involvement in different cognitive processes, and in declarative memory, especially in semantic memory (Schacter and Wagner 1999) as well as in face and name recognition (Olson et al. 2007). The TP is also involved in other cognitive processes such as emotion (Dolan et al. 2000; Pelletier et al. 2003). The TP also seems to play an important part in emotional saliency after presentation of stimuli from various modalities such as olfactory, visual and auditory inputs to subjects (Royet et al. 2000; Zald and Pardo 2002). The TP would act within a neural network including the orbitofrontal cortex and the superior frontal gyrus for the processing of the three sensory modalities taken together (Royet et al. 2000). Finally, the involvement of the TP has been demonstrated in other high-order cognitive processes such as satiation (Gautier et al. 2000), empathic behavior (Rankin et al. 2006), sadness (Eugène et al. 2003), anger (Blair et al. 1999; Kimbrell et al. 1999), anxiety and stress (Kimbrell et al. 1999; Tillfors et al. 2001), and language function (Deblaere et al. 2002). In the latter, the TP seems to be highly involved in linguistic integration, allowing semantic association of words, fundamental in the understanding of a story (Maguire and Mummery 1999), a feature that would also fit with purported implications in visual imagery of words, as described by Jackson and Schacter (2004).
Structural MRI Segmentation Studies
In order to assess the precise volume of cortical and subcortical brain areas, different structural MRI studies have been performed (Dade et al. 2004). Subregions have been described within the temporal lobe (TPC, EC, PRC, and PPH cortex, hippocampus, amygdala), and the determination of their volume/boundaries are used for diagnosis and indication of early treatment (Insausti, Juottonen, et al. 1998; Pruessner et al. 2002; Bonilha et al. 2004).
Segmentation of TP generally follows a rostrocaudal sequence, focusing on the collateral sulcus appearance and other external landmarks as the superior temporal and polar sulci taking into account the description of segmentation criteria reported by Insausti, Juottonen, et al. (1998).
Neuroanatomy in Nonhuman Primates
The neuroanatomy of the TP, and the whole parahippocampal gyrus in general, has been described in the macaque monkey brain since several decades (von Bonin and Bailey 1947; Van Hoesen and Pandya 1975; Insausti et al. 1987; Morán et al. 1987; Kondo et al. 2003; Suzuki and Amaral 2003) and in the baboon (Blaizot et al. 2004).
We adhere to the overall nomenclature of the temporal lobe described by Insausti et al. (1987) for the Macaca fascicularis temporal lobe. In our adaptation to the human brain, we have modified the nomenclature of the TPC. While it shares anatomic similarities with PRC (Insausti et al. 1987; Suzuki and Amaral 1994), and in some studies portions of it have been referred to as the polar part of the PRC (or area 36p, Suzuki and Amaral 1994; Saleem et al. 2007), in the monkey literature it is more often considered to be a separate region (Kondo et al. 2003; Saleem et al. 2007).
In the human brain (present study), TPC and PRC are considered as separate areas. Therefore, the TPC will be referred to as area TPC, although, in contrast to Brodmann (1909) and von Economo and Koskinas (1925) who present a much larger TPC, our results are in agreement with Ding et al. (2009), although we separate two specific subregions that remind the divisions already reported in macaque monkeys (Insausti et al. 1987) and baboons (Blaizot et al. 2004).
Aim of the Study
In this study, we hypothesize that the TP is not made up of one single cytoarchitectonic area as classically described. We aim at providing a more precise delimitation of the human TPC organization to determine with the highest possible accuracy its real extent in the human brain taking into consideration the variability in the sulcal pattern (Ono et al. 1990). The determination of its divisions and boundaries, based on its spatial relationship to anatomical landmarks within the temporal lobe, would greatly facilitate the correlation with MR and other neuroimaging techniques. We will also take into account the known connections of the TP in the human brain (by extrapolating data obtained in the Macaca fascicularis monkey brain). The data will also be compared with those observed in other nonhuman primate species (macaque and baboon) to explore the homology of the TPC among these species.
Subjects and Methods
Cases
Histological examinations were performed in 20 control cases, ages ranging from 12 to 96 years of both sexes. Postmortem time interval (PMTI, range = 1–12 h) was available for 10 patients (Table 1). In addition, 5 brains were encased in agar after immersion fixation in 10% formalin to obtain ex vivo MR images. Additional MRI examinations were performed, in vivo, in three healthy adults between 33 and 35 years old, who gave their consent to the study after detailed information. The study was done in line with the Declaration of Helsinki. Additional data were obtained from 24 control cases used in another clinical study (Abizanda P, Mansilla F, Insausti AM, Maicas L, López-Ramos B, Artacho-Pérula E, Insausti R, in preparation).
Table 1.
Descriptive data of the 20 studied cases
| Case | Age (y) | Sex | Hemisphere | Weight | PMTI | Fixation | Death |
| 1 | 12 | F | R | 1330 | 6 | I | Hypovolemic shock |
| 2 | 16 | M | R | 1300 | NC | I | Respiratory insufficiency |
| 3 | 20 | M | R | 1600 | NC | I | Head traumatism |
| 4 | 24 | M | R | 1600 | NC | I | Pulmonar embolism |
| 5 | 35 | F | R | 1750 | NC | I | Septicemia |
| 6 | 37 | M | R | 1600 | 12 | I | Myocardial infarction |
| 7 | 41 | F | R | 1400 | NC | I | Pulmonary edema |
| 8 | 47 | M | L | 1170 | NC | I | Peritoneal hemorrhage |
| 9 | 54 | M | R | 1400 | 6 | I | Hypovolemic shock |
| 10 | 64 | F | L | 1240 | 5 | I | Septicemia |
| 11 | 65 | M | L | 1400 | NC | I | Pulmonary carcinoma |
| 12 | 66 | M | L | NC | 2 | P | Liver failure |
| 13 | 70 | F | L | NC | 00:45 | P | Myocardial infarction |
| 14 | 77 | F | R | 1150 | 01:30 | P | Liver carcinoma |
| 15 | 78 | M | R | 1520 | NC | I | Cardiac insufficiency |
| 16 | 83 | F | R | 1150 | 2 | I | Pancreatic carcinoma |
| 17 | 84 | M | R | 1500 | 3 | I | Acute kidney insufficiency |
| 18 | 85 | F | R | NC | NC | I | Respiratory insufficiency |
| 19 | 96 | M | R | 1116 | 1 | P | Digestive hemorrhage |
| 20 | 58 | F | R | NC | 5 | I | Pulmonary carcinoma |
Note: F, female; R, Right; I, immersion; M, male; L, left; P, perfusion; NC, noncommunicated.
Fixation Procedures
Fixation was made either by immersion in 4% paraformaldehyde in 0.1 M phosphate buffer (4 °C, pH 7.4) for 4 days (PMTI between 3 and 6 h) or by 10% formalin in cases with a PMTI longer than 6 h. A few cases with a PMTI shorter than 3 h were perfused with 4% paraformaldehyde in 0.1 M phosphate buffer through the carotids as described (Insausti et al. 1995).
Sectioning and Staining
After fixation, the brain was bisected and both hemispheres separated and sectioned into 1-cm thick slabs. The plane of section was determined to be orthogonal to the anterior–posterior commissure (AC–PC) line. The first cut was performed at the level of the AC. One-centimeter–thick slabs were then obtained parallel to the first one (Insausti et al. 1995). Brains were photographed in toto as well as the anterior and posterior surfaces of each slab prior to dissecting the temporal lobe. Fifty-micrometer serial sections were obtained in a sliding microtome coupled to a freezing unit and mounted onto gelatin-coated slides at 500-μm intervals immediately after sectioning and stained with 0.25% thionin for cytoarchitectonic analysis. Sections were obtained in the axial (1 hemisphere) and parasagittal planes (2 hemispheres). Boundaries of the TPC with adjacent cortical areas were charted onto camera lucida drawings of the sections at ×7.5 magnification.
Immunohistochemistry
Series of sections every 500–1000 μm were immunostained with antibodies against calcium-binding proteins (calbindin, calretinin, and parvalbumin; 1:10 000; Swant, Bellinzona, Switzerland) and nonphosphorylated neurofilaments (SMI-32, Sternberger, working dilution 1:20 000). Sections were pretreated to eliminate endogenous peroxidase activity with a step of 10% hydrogen peroxide in phosphate-buffered saline (PBS) for 10 min. Nonspecific binding was blocked by a step in 1% normal goat serum for 2 h at room temperature. Free-floating sections were incubated overnight at 4 °C in a solution that contained 0.3% Triton-X-100, 1% normal horse serum, and the primary antibody in PBS, placed in the biotinylated secondary antibody (Vector, Burlingame, CA; working dilution 1:2000) and reacted with the avidin biotin method (BioStain; Biomeda, Foster City, CA) or the streptavidin method (1:2000) and 0.05% 3-3-′3′-diaminobenzidine and 0.04% hydrogen peroxide. Analysis was performed on an Olympus B-50 microscope coupled to an X–Y charting system (MDPLOT; AccuStage, Minneapolis, MN) after cytoarchitectonic evaluation following in general the criteria of Insausti, Juottonen, et al. (1998).
Two-Dimensional Reconstruction
We performed a 2D reconstruction (unfolded map) of the TP and the whole medial temporal lobe following the line of layer IV or the interval between layers III and V, using the fundus of the collateral sulcus, the location of the endorhinal sulcus (Gloor 1997), and the overall direction of the superior temporal sulcus as guide for orientation of the big expanse of the cortex at the TP (see Insausti et al. 1987, Fig. 1, for unfolded map of the monkey temporal lobe and Insausti R, Insausti AM, et al. 1998, Fig. 2, for unfolded map of the human medial temporal lobe).
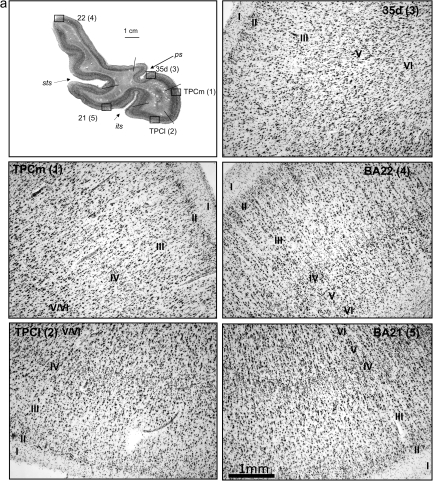
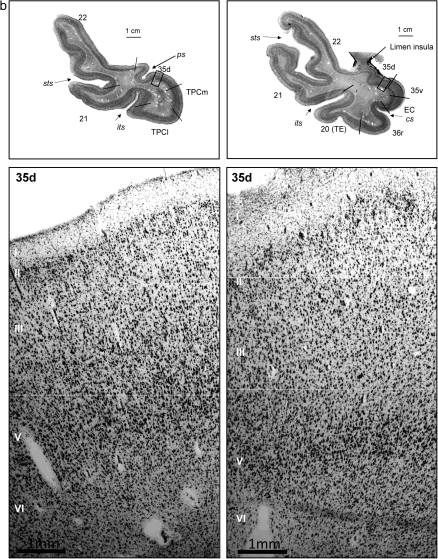
Figure 2.
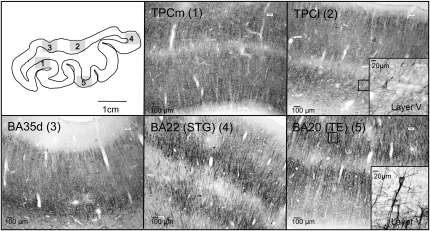
(a) Histological appearance and cytoarchitecture of the areas constituting the TP: TPC (TPCm and TPCl), areas BA35d, BA22 (or area TE of von Economo and Koskinas), and BA21. (b) Histological appearance and cytoarchitecture of area BA35d in two sections rostrocaudally distant from 6 mm. Scale bar in low-power photomicrograph is 1 cm and 1 mm in high-magnification photomicrographs (taken with a ×5 lens).
Structural MRI
In Vivo Studies
This protocol was carried out in the MR unit of the Hospital of Caen using a 1.5-T LX General Electric apparatus. MR acquisitions consisted in T1- and T2-weighted images and 3D T1 with inversion recovery. For the purpose of the study, only T1-weigthed images were employed. The 2D T1-weighted images were acquired with the following parameters: 1.5-mm thickness in the sagittal plane, fast spoiled gradient-echo, echo time (TE) = 4.2 ms, repetition time (TR) = 255 ms, number of excitation (NEX) = 1, field of view (FOV) = 26 × 26, and a matrix of 352 × 256; 1.5-mm thickness in the axial and coronal planes, TE = 9 ms, TR = 500 ms, NEX = 2, FOV = 24 × 18, and a matrix of 512 × 224.
Ex Vivo Studies and Additional Volumetric Data
Both hemispheres were taken during routine autopsies from subjects with no neurological or psychiatric alteration. Brains were fixed in 10% formalin and encased in 1.5% agar solution. Hemispheres were then studied using a MRI Siemens and a Philips Intera apparatus of 1.5 T, in a sequence with the following parameters: Flair 3, TR = 6000 ms, TE = 353 ms, inversion time (TI) = 2200 ms, 1-mm thickness with no interval, FOV = 26 × 26. Brains were oriented along the anterior–posterior intercommissural line, and series of images were obtained in the three planes. Brains were then removed from agar and cut in the coronal plane orthogonal to the AC–PC line, as detailed above. The additional cases (Abizanda P, Mansilla F, Insausti AM, Maicas L, López-Ramos B, Artacho-Pérula E, Insausti R, unpublished observations) were also obtained in the same MRI machine with the following parameters: T1 WIR, TR = 2863 ms, TE = 10 ms, TI = 400 ms, 40 sections, THK = 2.0/0.0 mm, FOV = 20 cm, Sc time 4.35.
Serial sections of 50-μm thickness were obtained throughout the temporal lobe in the same way as stated above, and a Nissl-stained series every 500 μm was prepared for analysis and correlation with MR images.
Results
Gross Anatomy
The temporal lobe is a brain region that extends into the TP with no obvious demarcation (Fig. 1). It is encased in the internal surface of the greater sphenoid wings at the middle cranial fossa. Its gross anatomic description and general boundaries offer little doubt, as it is self-contained rostrally while dorsally becomes defined by the course of the middle cerebral artery on its way toward the Sylvian fissure. However, this brain area is made up of several different cytoarchitectonic fields, which lack distinctive landmarks, making impossible to separate them under macroscopic examination through a simple visual analysis. Therefore, to determine more precisely the structural organization of the TP and to clarify whether or not the TP is made up of one single cytoarchitectonic field (i.e., BA38, TG) or is made up of several cytoarchitectonically distinct areas, we will start by the description of the major anatomic landmarks used to identify each of the cortical regions from rostral to caudal.
The ventral portion of the TP is continuous with the rostralmost continuation of the parahippocampal gyrus (Fig. 1b), a gross morphological term that describes the ventromedial area of the temporal lobe (Van Hoesen 1982; Gloor 1997). The parahippocampal gyrus also includes the EC, PRC, and PPH cortex. As the EC is included among the components of the hippocampal formation (Insausti and Amaral 2004), the remainder of the parahippocampal gyrus has been termed, and we adopted it, parahippocampal region (Scharfman et al. 2000). Dorsolaterally, the TP continues inconspicuously with the rostralmost extension of the cortex of the superior temporal gyrus (STG). Roughly speaking, the limen insulae or frontotemporal junction is the natural gross anatomic landmark that indicates the caudal limit of the TP. In contrast, the dorsal and lateral limit of the TP is again inconspicuous and lacks any gross anatomical landmark other than the rostralmost extension of the superior and inferior temporal sulci that give an approximation to this limit (Ono et al. 1990). Ventral and medial in the TP, a little further caudally, the collateral sulcus usually correlates with the caudal limit of the TP as a gross anatomical region.
Medially, the TP is bordered caudomedially by the gyrus semilunaris, a rounded prominence at the rostral–medial temporal lobe that is formed by piriform and periamygdaloid cortices (Stephan 1975; Insausti and Amaral 2004; Gonçalves-Pereira et al. 2005). The gyrus semilunaris (mostly made up of periamygdaloid cortex, Insausti and Amaral 2004) is always caudal to the limen insulae.
The dorsal aspect of the TP is smooth, and usually 1 or 2 polar sulci (Bailey and von Bonin 1951) run longitudinally from the rostral tip of the TP as far caudally as the limen insulae. Their number and length may vary (Bailey and von Bonin 1951). One or 2 of these polar sulci define 1 or 2 transverse gyri of Schwalbe (1881).
Cytoarchitectonic Description
The TPC has a laminar organization in 6 layers as in the isocortex, although ill defined (Table 2, Figs 2 and 3). For this reason, the term proisocortex is applied in human and nonhuman primate studies (i.e., Pandya and Yeterian 1984). Rather than being homogeneous, TPC presents a gradient toward an increasing isocortical organization that extends from rostral and medial to caudal and lateral portions in the TP. The structural organization of TPC is as follows and is presented in Figure 2.
Table 2.
Cytoarchitectonic organization of the TP areas
| TPCm | TPCl | 35d | 36r (PC) | 20(area TE of von Economo and Koskinas), 21(ITG), 22 (STG) | |
| I | Fibers | Fibers | Fibers | Fibers | Fibers |
| II | Granular layer, patchy appearance, with big and darkly stained neurons grouped in clumps, discontinuous band | Smaller and more uniform neurons, comparable to those of the neighboring cortical areas | Nonuniform with numerous darkly stained neurons | Better lamination compared with area TPC; occasional clumps of darker neurons | Dense and uniform with numerous small stellate neurons |
| III | External pyramidal layer with small to medium pyramidal cells and a size gradient from superficial to deep portions | External pyramidal layer, neuronal clumps poorly defined | Directly adjacent to layer V | Shorter than TPC, irregular border with layer IV, no gradient size | Columnar organization of pyramids |
| IV | Internal granular layer thinner than in TPCl | Internal granular layer, rather thin | Agranular | Bigger than TPC but rather thin compared with neocortical areas | Prominent with columnar organization |
| V | Internal pyramidal layer, bigger and darker than in TPCl | Internal pyramidal layer, big and darkly stained neurons | Discontinuous big and darkly stained neurons | Large, darkly stained pyramids | Layers V and VI well separated with clear columnar organization |
| VI | Neurons of various sizes and shapes, extending from layer V | Clearer transition between layers V and VI compared with TPCm | Fused with layer V, dark neurons extending from layer V | Intermediate neurons in size, radial organization | Dense, Smaller neurons compared with layer V, columnar organization |
Note: I to VI: cortical layers of the TP; TPCm, TPCl (TPC, TPCm, and TPCl), BA35d, BA36r (rostral PRC); BA20 (von Economo area TE of von Economo and Koskinas); BA21 (inferior temporal cortex, ITG); BA22 (superior temporal cortex, STG).
Figure 3.
Series of SMI-32-immunostained coronal sections, focused on layer V, of areas TPCm, TPCl, BA35d, BA22, and BA21 of the TP. Inset shows a pyramidal neuron almost completely immunostained. Scale bars are indicated.
Layer I
The layer is made up mostly of fibers, although a few Cajal–Retzius cells can be observed.
Layer II
It makes the external granular layer. According to the location in the TPC, layer II acquires two distinct types. The first one extends ventromedially and presents a patchy appearance in Nissl preparations, with big and darkly stained neurons, usually grouped in clumps. The extent and continuity of this layer is highly variable from individual to individual and is often present as a discontinuous band. Topographically, it is present mostly at the rostral and medial portions of the TPC. The second type, which is far more predominant, presents a layer II invested with smaller, more uniform neurons, comparable to those of the neighboring neocortical areas, that is, the superior and inferior temporal cortices (inferior temporal gyrus [ITG] and STG). Topographically, it is present in the lateral and ventral parts of TPC.
Layer III
This layer forms the external pyramidal layer and is made up of small or medium pyramidal cells that follow a size gradient: Pyramids from superficial to deep portions of the layer increase progressively in size. Laterally, a tendency to form little defined neuronal clumps can be observed. Layer III is not columnar, and this lack of columnar organization is useful to delimitate TPC from BA20, BA21, and BA22 (areas area TE of von Economo and Koskinas, ITG, and STG), where layer III is more clearly columnar.
Layer IV
This layer forms the internal granular layer and is very thin, in particular medially and even absent at certain locations. A segment of cortex situated dorsomedially in the TP is totally agranular, what is compatible with a dorsal division of area 35 of the PRC.
Layer V
This layer forms the internal pyramidal layer. As in other proisocortical areas, layer V has been described as the most outstanding layer in the whole parahippocampal gyrus (Van Hoesen and Pandya 1975; Insausti et al. 1987). Layer V in the TPC is thick, and the big size of its darkly stained neurons renders it highly visible. A less noticeable mediolateral gradient can also be observed in this layer as neurons are bigger and darker medially in the TPC relative to more lateral portions. As we move in a caudal direction, a progressively better defined isocortical organization takes place.
Layer VI
This layer forms the polymorphic layer. It contains neurons of various sizes and shapes; it extends from layer V to the white matter. In the medial part of the TPC, the transition between layers V and VI is usually blurred compared with the lateral part, where both layers are better separated.
Considering the cytoarchitectonic differences in layers II and V, 2 divisions are proposed for the TPC: a medial, smaller, area medial temporopolar cortex (TPCm) and a lateral, broader area lateral temporopolar cortex (TPCl).
Neighboring Cytoarchitectonic Fields
TPC borders a number of cortical fields: the rostral portion of BA36 ventromedially; BA35, dorsomedially; rostral parts of the ITG ventrolaterally; and finally, the STG dorsolaterally.
Rostral BA36, although sharing some commonalities with TPC such as a clear layer V, presents better lamination compared with area TPC. Layer II presents occasional clumps of darker neurons of small to medium size, much less conspicuous than TPCm. Layer III is populated with small to medium pyramidal cells and does not present a gradient in size. Layer III is much thinner compared with TPC and makes an irregular border with layer IV, which, at some points, is discontinuous. Layer IV is present, but rather thin as compared with the cortex at the ITG and STG, in which the granular layer is more prominent and a columnar organization predominates. Layer V is very outstanding, with the presence of large, darkly stained pyramids although the layer as a whole is thinner as compared with TPC. Layer V is prominent, although not very thick, and merges with layer VI. Layer VI presents a variety of neurons, small to medium in size, and is not as thick as layer VI of TPC.
Area 35
This is the rostralmost component of PRC and is characterized by a lack of layer IV (agranular) (Fig. 2B). Area 35 lies at the fundus of the rhinal sulcus as it comes around the medial aspect of the anterior part of the TP, not far from the limen insulae, which is occupied by a portion of PRC. Caudally, it is, in turn, replaced by a type of cortex compatible with the parainsular cortex in the monkey. Area 35 is characterized by a nonuniform layer II with numerous darkly stained neurons; the lack of a layer IV makes layers III and V to be directly apposed. Layer V is also recognizable by its big and darkly stained neurons.
area TE of von Economo and Koskinas (BA20), Inferior Temporal Gyrus (area TE of von Economo and Koskinas, BA21), and Superior Temporal Gyrus (TA, BA22)
These are isocortical six-layered areas that present a better lamination. Also, they have a typical columnar organization and much thicker layer IV compared with TPC and PRC. Granular layers (II and IV) appear as defined bands made up of small, stellate neurons. In contrast to area TPC, layers V and VI are well separated.
Finally, one of the main distinguishing features is the immunoreactivity of neurons in layers III and V in preparations stained with nonphosphorylated neurofilaments (SMI-32). With this antibody, large pyramids are clearly stained in layers III and V, often showing long apical dendrites (Fig. 3). Thus, this is a useful marker that helps in the delimitation typical neocortex and proisocortex. Indeed, TPC pyramidal neurons in layer V appear only lightly stained with this antibody. In contrast, pyramids are darkly stained in BA21 and BA22. BA35, on the dorsal aspect of the TP, and BA36, ventromedially, are between areas TPC and BA21 and BA22 in terms of number of neurons and density of the immunostaining, which also helps in their delimitation.
Rostrocaudal Sequence of Cytoarchitectonic Fields of the TP
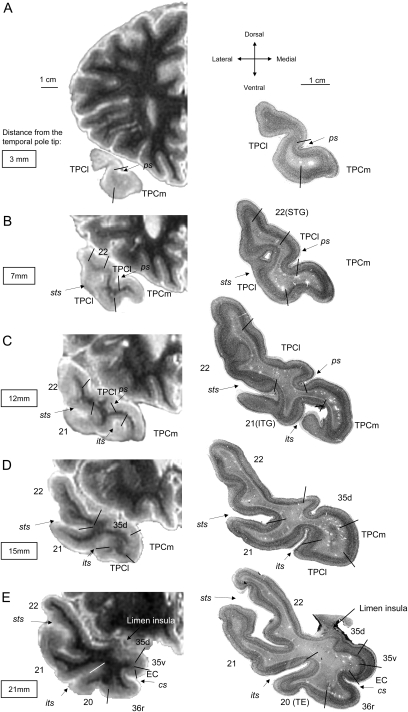
Given the cytoarchitectonic features described above, we can place the boundary between areas TPCl and TPCm at the fundus or within the lateral bank of the lateral polar sulcus, generally about 6 mm from the tip of TP (Fig. 4). The columnar organization of the cortex at the STG appears at about 7 mm from the TP tip, close to the TPCl. Both BA21 and BA22, start at about 12 mm from the rostral tip of the TP, along with the beginning of the superior temporal sulcus. Ventrally, TPC lies medial to BA20/area TE of von Economo and Koskinas at the medial edge of the inferior temporal sulcus, about 15 mm from the tip of TP. At this level, area 35d appears dorsally and replaces TPCl (Fig. 2A,B). When 2 polar sulci are present, 2 gyri of Schwalbe are formed, and the dorsolateral border of TPC (TPCl) extends up to the bottom of the fundus of the most lateral polar sulcus. In a few cases, no polar sulci could be identified, and the dorsal aspect of the TP had a smooth appearance, so that, and only as a compromise, we separate the TPC (TPCl) from the superior temporal cortex (BA22) at the midpoint of the dorsal aspect of the TP (Insausti, Juottonen, et al. 1998).
Figure 4.
T2-weighted MR images in the coronal plane of the left hemisphere of a brain embedded in agar from rostral (A) to caudal (E) levels of the TP (left). On the right-hand side of the illustration are the corresponding thionin-stained sections of the same subject (right), where cytoarchitectonically distinct fields have been depicted. Note the limited extent of TPC (labeled as TPCl or TPCm) and the heterogeneity (BA20, BA21, BA22, PRC) also present in the TP.
Correlation between Structural MRI and Cytoarchitectonics of the TP: Segmentation Criteria
Figure 4 shows the correlation between postmortem T2-weighted MR coronal images and corresponding Nissl-stained sections. Orientation of TP has been made taking into account the variability of the superior and inferior temporal sulci (Ono et al. 1990). As head tilting may occur at the acquisition period, differences in the specific shape of the cortex are very difficult to evaluate and are not of use in the segmentation of the rostral temporal lobe. For this reason, we have used ex vivo structural MRI of fixed, normal brains so that, after sectioning and staining, a cytoarchitectonic evaluation of the temporal lobe cortex could be performed. The resulting borders for different cytoarchitectonic areas of that evaluation have been transposed onto the coronal MR images of the same brain in sections and related to the sulci whose presence is more constant in the human brain.
With these considerations in mind, we defined a series of steps that allow a more detailed segmentation of the TPC. The basis for this segmentation was outlined in a previous report (Insausti, Juottonen, et al. 1998). We elaborate now in more detail the neuroanatomical basis for such a proposed segmentation.
The rostralmost portion of the TPC only contains cortex described above as TPC. While current MRI techniques do not allow the distinction between cytoarchitectonic features to set apart either of the 2 divisions, TPCm or TPCl, it can be said that by large, most of the cortex belongs to the TPCl type.
The series of coronal sections revealed that for the first 6 mm (range 5–10) only TPC is present. At this point, the cytoarchitectonic analysis reveals that features of area TPC are progressively replaced by the cortex of the STG (BA22, see above for cytoarchitectonic criteria), beginning at the lateral border of the TP. The microscopic analysis, in this and other cases, reveals that after the first 6 mm, area TPC no longer covers all the expanse of the TP, but it is limited to the lateral bank of the polar sulcus (or the most lateral polar sulcus if there is more than one present). The dorsocaudal limit of TPC is about 4 mm rostral to the limen insulae, where it becomes replaced by the dorsal portion of BA35 (see above for cytoarchitectonic criteria).
The ventromedial extent of BA38 extends a little more caudal relative to the dorsal part. The rostral tip of the inferior temporal sulcus marks the transition between the cortex of the ITG (BA20/area TE of von Economo and Koskinas). Area TPC extends ventromedially at this point as far as the fundus and medial bank of the inferior temporal sulcus. The rostral tip of the collateral sulcus sets the caudal limit of TPC, where it is replaced by BA36r. In this way, the MRI sequence of the sulci guides the segmentation of the TP approximately into the different cytoarchitectonic fields, what relates to functionally distinct cortical areas. The areal composition of the TP is represented in Figure 4.
Areal Extent and Volumetry of TPC
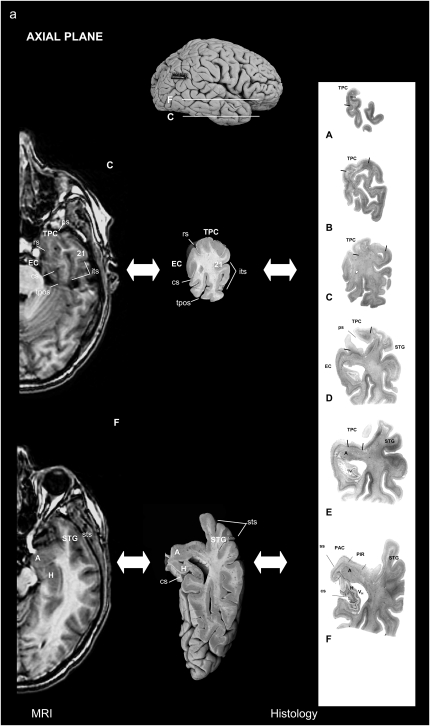
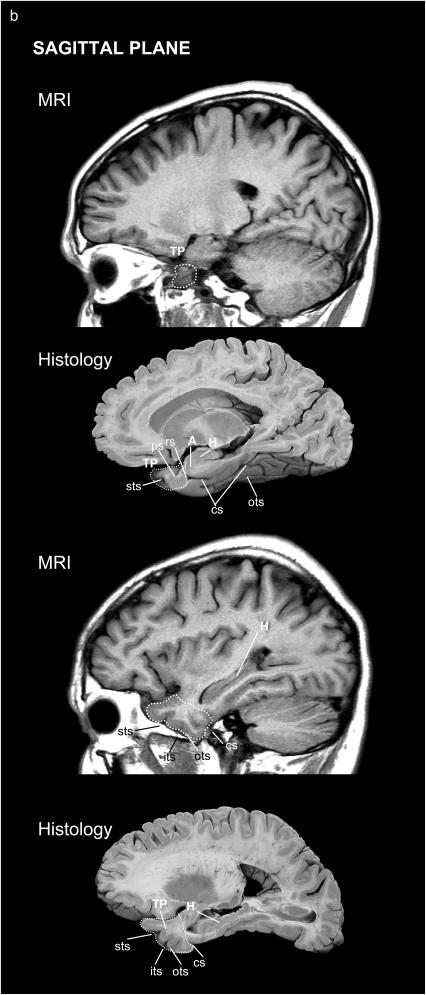
The volumetric segmentation of the TP was performed from the 3D T1-weighted MR sequence on a computer from coronal images with the corresponding location on the axial and sagittal MR images on the same screen (Fig. 5).
Figure 5.
Location of the TP and surrounding areas on both MRI and corresponding histological brain sections: in the axial (a) and sagittal (b) planes.
The main landmark used to delimitate the TP caudally was the limen insulae (Figs 4 and 5) rostral to the beginning of the amygdaloid complex. The appearance of the collateral sulcus generally confirmed the end of the TP, at the point where the EC and PRC first appear (Insausti, Juottonen, et al. 1998). Within the TP, we used the polar, inferior, and superior temporal sulci to delimitate the TPC from areas BA20, BA21, and BA22.
Volume of the TPC
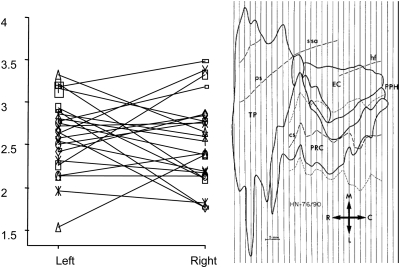
MRI measures performed in the 24 subjects revealed a 2.61 ± 0.42 and 2.54 ± 0.55 cm3. This represents 32% of the whole parahippocampal region including the rhinal cortex (BA28, BA35, and BA36) and the PPH cortex (areas TH and TF) (5.04 ± 0.96/15.78 ± 2.93 cm3). TPC represents as much as 35% (5.04 ± 0.96/14.11 ± 2.70 cm3) (excluding the PPH cortex). Our data indicate that the size of BA38 (TPC) is similar between both sides (P > 0.05) (Fig. 6).
Figure 6.
Left and right TPC volumes, in cubic centimeter, of the 24 subjects (left); 2D unfolded map of the parahippocampal region in one case (right).
Relative Surface of the TP
The 2D unfolded maps of the parahippocampal region (TP plus EC of the hippocampal formation, PRC and PPH cortex) was performed in 9 cases (5 males, 4 females), whose brain weight ranged from 1165 to 1750 g. The calculated area of those cases revealed a large contribution of TP of 8.98 ± 1.49 cm2, which represents 45%, (range = 40–55%) of the whole extent of the 2D reconstructions of the EC plus PRC (Brodman areas 28, 35, 36) and TP.
Variability
Figure 6 shows the MR volumes and their variability of the TPC of both hemispheres between subjects.
Discussion
Main Findings
The TPC described in the present study confirms the classical cytoarchitectonic descriptions of Brodmann (1909) and von Economo and Koskinas (1925) in which the thin layer II, the pyramids gradient size in layer III, thin layer IV, and big and dark pyramids in layer V, which fuse with a layer VI that spreads out in the subjacent white matter, are the main features of TPC. We describe for the first time in humans the presence of 2 divisions, TPCl, more extensive and placed mainly on the lateral aspect of the TP, and TPCm, more restricted to the medial and ventral aspects of the TP. This proposed division of TPC gains strength on considering that it provides some basis for homology with nonhuman primates. Indeed, two portions, TPCl and TPCm seem to exist also in macaques (Insausti et al. 1987; Morán et al. 1987; Kondo et al. 2003; Saleem et al. 2007) and in baboons (Blaizot et al. 2004). While the general location of BA38 and TG (Fig. 1a) is similar, another new finding is that our analysis reduces considerably the extent to which TPC extends in the TP, contrary to the accepted extent of this area by those authors. Moreover, our analysis of the human TP suggests a forward advance of the neocortex of the STG, middle temporal gyrus, and ITG in the human temporal lobe. As a consequence, some of the activations observed in neuroimaging studies could be ascribed to the rostral temporal neocortex of BA20, BA21, and BA22, which in nonhuman primates is polymodal or unimodal sensory association cortex (Desimone and Gross 1979). A recent report by Ding et al. (2009) also describes the characteristics of TPC. Our results are in agreement with that report in terms of the characteristics of TPC, as well as in the boundaries with adjoining cortical regions that Ding et al. (2009) further elaborate.
Our results indicate that TPC is organized in six layers as in the neocortex, but with less clear lamination than isocortex; thereby, it could be defined as proisocortex (Sanides and Sas 1970) or mesocortex (Klingler 1948; Gloor 1997). We found a gradient toward an increasing isocortical appearance. This gradient runs from medial to lateral aspects of the TP.
The TPC and the PRC seem to have increased in extent and volume in evolution, being noticeable in nonhuman primates and humans, as revealed by the 3D MR and the 2D unfolded maps measurements, in accordance to the literature (see Barbeau et al. 2004 for review). This evolutionary development is accompanied by an increase of variability in the shape and sulci pattern of the TP (Ono et al. 1990).
Comparative Anatomy
Although the overall anatomy and cytoarchitectonics of the parahippocampal region is comparable between human and nonhuman primates, we have found several differences in the TP. We first observed several macroscopic characteristics in humans such as the extent of the TP that is about 45% of the whole rhinal (entorhinal plus perirhinal) cortex compared with nonhuman primates (24% in the baboon and 17% in the macaque, Blaizot et al. 2004). Second, lamination is as little or less clear than in baboons (Blaizot et al. 2004) or macaques (Insausti et al. 1987; Morán et al. 1987; Kondo et al. 2003). Thus, this cytoarchitectonic analysis seems to reveal a gradient of complexity among primates (taking into account the gyrification degree, which is higher in baboons than in macaques, Zilles et al. 1989), especially at the level of the TP, which looks more developed in phylogenetically higher species, albeit keeping specific structural features. Among other putative functions, this region could represent an important site for 1) declarative memory by means of its connections with the amygdala (Stefanacci et al. 1996) and hippocampal formation and cortical association areas (Rosene and Van Hoesen 1977; Insausti et al. 1987; Muñoz and Insausti 2005) and 2) processing of emotional salience of stimuli elaborated by higher association cortices. Finally, gyrification within the TP seems to be higher in humans by the presence of 1 or 2 polar sulci (Bailey and von Bonin 1951) compared with nonhuman primates in which the dorsal aspect of the temporal is totally smooth. Thus, we previously showed in humans that 80% of cases had 2 temporopolar sulci, about 12% had a single temporopolar sulcus, and in 8% of the cases the dorsal aspect of the TP was almost completely smooth (Insausti R, Insausti AM, et al. 1998).
Variability of the TP in human subjects is high, not only in shape and aspect but in volume as well, as shown in Figure 6, while this variability is less pronounced in primates (Muñoz and Insausti 2005). Considering these data altogether, we could speculate whether the phylogenic development of the TP likely contributes to the specialization of declarative memory and other high-order cognitive processes such as emotion, language, satiation, learning, sexual and social behavior, and visual imaging (reviewed in Olson et al. 2007).
Interest for Segmentation Studies
The anatomical findings here presented are relevant for MRI measurements, as precise anatomical delimitation is required for medial temporal lobe volumes; however, detailed anatomical analyses are usually lacking in functional studies (Cabeza and Nyberg 2000) and in measurement in methods employing voxel based regression models, which have demonstrated morphological changes in neurodegenerative diseases (Baron et al. 2001). Several MRI segmentation studies separate regions within the temporal lobe (16, e.g., in the segmentation study of Kim et al. 2000), although delimitation criteria were unclear, especially at the level of the TP that was described simply as the rostral part of the temporal lobe. The present data support the outline of the segmentation procedure of Insausti, Juottonen, et al. (1998) also in agreement with Pruessner et al. (2002). However, the TPC in the study of Pruessner et al. (2002) was limited to the medial half of the TP contrasting to the present study and the study of Insausti, Juottonen, et al. (1998), in which the whole rostralmost portion of the TP (in coronal sections) is considered as area TPC. Finally, it should be taken into account that, besides the histological criteria, important factors such as MR acquisition, spatial normalization, or rostrocaudal axis used to segment images (AC–PC line compared with the line perpendicular to the long axis of the hippocampus) can account for differences in volume calculations.
Neuroanatomical Connections of the TP
The functionality of the TPC is heavily supported by its connections within the brain, not only with temporal lobe structures, such as the hippocampus, but also with the frontal lobe: The dorsal part (probably homologous to TPCl) is mainly interconnected with the medial frontal cortex (BAs 10m, 10o, 11m, 13a, 14c, 14r, 25, 32) (Kondo et al. 2003), while the ventral portion (probably homologous to TPCm) is interconnected to the orbitofrontal cortex (BAs 11l, 13b, 13l, 13m, Morecraft et al. 1992).
Contribution of experimental studies in primates, combined with anatomical and cytoarchitectonics data, is specially relevant to the understanding of the role played by the TPC in numerous cognitive functions. Thus, it has been reported in memory, and especially in object and visual recognition memory (Nakamura and Kubota 1995, 1996; Blaizot et al. 2000; Zola-Morgan et al. 1989; Delacour 1977; Murray and Mishkin 1986; Meunier et al. 1993), in visual discrimination (Gaffan 1994), memory at short delays (10 s), and learning (Horel et al. 1984; Voytko 1986). Other observations based on lesions, electrophysiology, or neuroimaging have demonstrated that the TPC is not only engaged in memory, emotion, and visual discrimination, but in several other functions as well, such as taste, olfaction, learning, and emotional relevance of stimuli.
Intrinsic connections of the TPC are also divided into two main pathways: The ventromedial part, more implicated in visual modalities, is mostly connected to visual areas of the inferior temporal cortex, and the dorsolateral portion, more implicated in auditory modalities, is mostly connected to the auditory areas of the superior temporal cortex. Ding et al. identify area TA in the TP, which can be assimilated to the rostral auditory field in macaques (Hackett et al. 1998). Indeed, while BA35 and BA36 of the PRC receive strong inputs from area TE of von Economo and Koskinas (Saleem and Tanaka 1996) and mainly project to the EC (Insausti et al. 1987; Lavenex and Amaral 2000), TPC projects to the EC (Insausti et al. 1987; Morán et al. 1987; Muñoz and Insausti 2005), PRC, (Suzuki and Amaral 1994; Lavenex and Amaral 2000; Muñoz and Insausti 2005), PPH (Lavenex and Amaral 2000; Muñoz and Insausti 2005), area TE of von Economo and Koskinas of the inferior temporal cortex (Van Hoesen and Pandya 1975; Morán et al. 1987; Muñoz and Insausti 2005), the hippocampus (Pandya and Kuypers 1969; Rosene and Van Hoesen 1977; Morán et al. 1987; Muñoz and Insausti 2005), and STG (from the dorsolateral part only, Muñoz and Insausti 2005).
Conclusion
The expansion of the TP surface in relation to the whole parahippocampal gyrus observed in humans, suggests that the TPC may participate in many cognitive functions, among them notably in encoding, consolidation, and retrieval of memory. Notably, the TPC, taking into account its cytoarchitecture, expanse, topography, and connections support its involvement in higher order cognitive functions, in declarative memory processing, as well as discrimination and/or recognition in polysensory modalities. Taken together, these data tend to confirm the hypothesis of Olson et al. 2007, indicating that the TP could modulate visceral emotional functions in response to emotionally evocative perceptual stimuli and are suggestive of an important role in the neuroanatomical substrate of personal semantic memory (Olson et al. 2007).
The correlation between cytoarchitecture of the TP either in the same or different subjects and MR images allows the delimitation of the subfields of the TP, which should be helpful in the interpretation of neurofunctional imaging such as fMRI or PET. These data provide thus a new tool to better understand the involvement of the different areas of the TP in cognitive functions and the organization of important neural networks in the human brain.
Funding
Ministry of Education (BFU 2003-09581, 2006-12964); Junta de Comunidades de Castilla-la Mancha (GC02-022).
Acknowledgments
The authors wish to thank Dr Jose Maria Gil Rubio, Head of the Department of Forensic Pathology of the Albacete, Cuenca and Guadalajara Legal Medicine Institute, Spain, for his help in the autopsies of several cases and Fabien Chaillot for technical help. Also, we would like to thank our technical staff at the Human Neuroanatomy Laboratory of the University of Castilla-La Mancha School of Medicine, Albacete. Conflict of Interest: None declared.
References
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Van Hoesen GW. Neuropathologic changes of the temporal pole in Alzheimer's disease and Pick's disease. Arch Neurol. 1994;51:145–150. doi: 10.1001/archneur.1994.00540140051014. [DOI] [PubMed] [Google Scholar]
- Bailey P, Bonin von G. The isocortex of man. Urbana, (IL): University of Illinois Press; 1951. [Google Scholar]
- Barbeau E, Sontheimer A, Joubert S, Didic M, Felician O, Tramoni E, Grimault S, Ceccaldi M, Poncet M. The human perirhinal cortex. Rev Neurol. 2004;160:401–411. doi: 10.1016/s0035-3787(04)70921-2. [DOI] [PubMed] [Google Scholar]
- Baron JC, Chételat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, Eustache F. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer's disease. NeuroImage. 2001;14:298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122(Pt 5):883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Blaizot X, Landeau B, Baron JC, Chavoix C. Mapping the visual recognition memory network with PET in the behaving baboon. J Cereb Blood Flow Metab. 2000;20:213–219. doi: 10.1097/00004647-200002000-00001. [DOI] [PubMed] [Google Scholar]
- Blaizot X, Martinez-Marcos A, Arroyo-Jimenez Md Mdel M, Marcos P, Artacho-Pérula E, Muñoz M, Chavoix C, Insausti R. The parahippocampal gyrus in the baboon: anatomical, cytoarchitectonic and magnetic resonance imaging (MRI) studies. Cereb Cortex. 2004;14:231–246. doi: 10.1093/cercor/bhg123. [DOI] [PubMed] [Google Scholar]
- Bonilha L, Kobayashi E, Cendes F, Min LL. Protocol for volumetric segmentation of medial temporal structures using high-resolution 3-D magnetic resonance imaging. Hum Brain Mapp. 2004;22:145–154. doi: 10.1002/hbm.20023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin G, Bailey P. The neocortex of Macaca mulatta. Urbana (IL): University of Illinois Press; 1947. [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehere der Grosshimrinde in ihren Principien dargestellt auf des Grund des Zellenbayes. Leipzig (Germany): Barth; 1909. [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Chételat G, Desgranges B, Landeau B, Mézenge F, Poline JB, de la Sayette V, Viader F, Eustache F, Baron JC. Direct voxel-based comparison between grey matter hypometabolism and atrophy in Alzheimer's disease. Brain. 2008;131:60–71. doi: 10.1093/brain/awm288. [DOI] [PubMed] [Google Scholar]
- Corkin S, Amaral DG, Gonzalez RG, Johnson KA, Hyman BT. H.M.’s medial temporal lobe lesion: findings from magnetic resonance imaging. J Neurosci. 1997;17:3964–3979. doi: 10.1523/JNEUROSCI.17-10-03964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dade LA, Gao FQ, Kovacevic N, Roy P, Rockel C, O'Toole CM, Lobaugh NJ, Feinstein A, Levine B, Black SE. Semiautomatic brain region extraction: a method of parcellating brain regions from structural magnetic resonance images. NeuroImage. 2004;22:1492–1502. doi: 10.1016/j.neuroimage.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Deblaere K, Backes WH, Hofman P, Vandemaele P, Boon PA, Vonck K, Boon P, Troost J, Vermeulen J, Wilmink J, et al. Developing a comprehensive presurgical functional MRI protocol for patients with intractable temporal lobe epilepsy: a pilot study. Neuroradiology. 2002;44:667–673. doi: 10.1007/s00234-002-0800-4. [DOI] [PubMed] [Google Scholar]
- Delacour J. Role of temporal lobe structures in visual short-term memory, using a new test. Neuropsychologia. 1977;15:681–683. doi: 10.1016/0028-3932(77)90072-0. [DOI] [PubMed] [Google Scholar]
- Desimone R, Gross CG. Visual areas in the temporal cortex of the macaque. Brain Res. 1979;178:363–380. doi: 10.1016/0006-8993(79)90699-1. [DOI] [PubMed] [Google Scholar]
- Ding SL, Van Hoesen GW, Cassell MD, Poremba A. Parcellation of human temporal polar cortex: a combined analysis of multiple cytoarchitectonic, chemoarchitectonic, and pathological markers. J Comp Neurol. 2009;514:595–623. doi: 10.1002/cne.22053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ, Lane R, Chua P, Fletcher P. Dissociable temporal lobe activations during emotional episodic memory retrieval. NeuroImage. 2000;11:203–209. doi: 10.1006/nimg.2000.0538. [DOI] [PubMed] [Google Scholar]
- Dupont S. Investigating temporal pole function by functional imaging. Epileptic Disord. 2002;4(Suppl 1):S17–S22. [PubMed] [Google Scholar]
- Dupont S, Ottaviani M, Thivard L, Semah F, Samson Y, Baulac M. Temporal pole hypometabolism may be linked to a reduction of grey matter in temporal lobe epilepsy. Neuroreport. 2002;13:2537–2541. doi: 10.1097/00001756-200212200-00031. [DOI] [PubMed] [Google Scholar]
- Eugène F, Lévesque J, Mensour B, Leroux JM, Beaudoin G, Bourgouin P, Beauregard M. The impact of individual differences on the neural circuitry underlying sadness. NeuroImage. 2003;19:354–364. doi: 10.1016/s1053-8119(03)00121-6. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Dissociated effects of perirhinal cortex ablation, fornix transection and amygdalectomy: evidence for multiple memory systems in the primate temporal lobe. Exp Brain Res. 1994;99:411–422. doi: 10.1007/BF00228977. [DOI] [PubMed] [Google Scholar]
- Gautier JF, Chen K, Salbe AD, Bandy D, Pratley RE, Heiman M, Ravussin E, Reiman EM, Tataranni PA. Differential brain responses to satiation in obese and lean men. Diabetes. 2000;49:838–846. doi: 10.2337/diabetes.49.5.838. [DOI] [PubMed] [Google Scholar]
- Gloor P. The temporal lobe and limbic system. New York: Oxford University Press; 1997. [Google Scholar]
- Goncalves Pereira PM, Insausti R, Artacho-Pérula E, Salmenperä T, Kälviäinen R, Pitkänen A. MR volumetric analysis of the piriform cortex and cortical amygdala in grug-refractory temporal lobe epilepsy. Amer J Neuroradiol. 2005;26:319–332. [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, Stepniewska I, Kaas JH. Subdivisions of auditory cortex and ipsilateral cortical connections of the parabelt auditory cortex in macaque monkeys. J Comp Neurol. 1998;394:475–495. doi: 10.1002/(sici)1096-9861(19980518)394:4<475::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Horel JA, Voytko ML, Salsbury KG. Visual learning suppressed by cooling the temporal pole. Behav Neurosci. 1984;98:310–324. doi: 10.1037//0735-7044.98.2.310. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG. Hippocampal Formation. In: Paxinos G, Mai JK, editors. The human nervous system: hippocampal formation. San Diego (CA): Academic Press; 2004. pp. 871–914. [Google Scholar]
- Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey: iII. Subcortical afferents. J Comp Neurol. 1987;264:396–408. doi: 10.1002/cne.902640307. [DOI] [PubMed] [Google Scholar]
- Insausti R, Insausti AM, Sobreviela MT, Salinas A, Martinez-Penuela JM. Human medial temporal lobe in aging: anatomical basis of memory preservation. Microsc Res Tech. 1998;43:8–15. doi: 10.1002/(SICI)1097-0029(19981001)43:1<8::AID-JEMT2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkänen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am J Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Tunon T, Sobreviela T, Insausti AM, Gonzalo LM. The human entorhinal cortex: a cytoarchitectonic analysis. J Comp Neurol. 1995;355:171–198. doi: 10.1002/cne.903550203. [DOI] [PubMed] [Google Scholar]
- Jackson O, 3rd, Schacter DL. Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. NeuroImage. 2004;21:456–462. doi: 10.1016/j.neuroimage.2003.09.050. [DOI] [PubMed] [Google Scholar]
- Kapur N, Ellison D, Smith MP, McLellan DL, Burrows EH. Focal retrograde amnesia following bilateral temporal lobe pathology. A neuropsychological and magnetic resonance study. Brain. 1992;115:73–85. doi: 10.1093/brain/115.1.73. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Crespo-Facorro B, Andreasen NC, O'Leary DS, Zhang B, Harris G, Magnotta VA. An MRI-based parcellation method for the temporal lobe. NeuroImage. 2000;11:271–288. doi: 10.1006/nimg.2000.0543. [DOI] [PubMed] [Google Scholar]
- Kimbrell TA, George MS, Parekh PI, Ketter TA, Podell DM, Danielson AL, Repella JD, Benson BE, Willis MW, Herscovitch P, et al. Regional brain activity during transient self-induced anxiety and anger in healthy adults. Biol Psychiatry. 1999;46:454–465. doi: 10.1016/s0006-3223(99)00103-1. [DOI] [PubMed] [Google Scholar]
- Klingler J. Die maksoskopische anatomie der Ammonsformation. Denkschr Schweiz Naturf Ges LXXVII. 1948:1–80. [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 2003;465:499–523. doi: 10.1002/cne.10842. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Amaral DG. Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ. Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus. 1999;9:54–61. doi: 10.1002/(SICI)1098-1063(1999)9:1<54::AID-HIPO6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Marlowe WB, Mancall EL, Thomas JJ. Complete Kluver-Bucy syndrome in man. Cortex. 1975;11:53–59. doi: 10.1016/s0010-9452(75)80020-7. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morán MA, Mufson EJ, Mesulam MM. Neural inputs into the temporopolar cortex of the rhesus monkey. J Comp Neurol. 1987;256:88–103. doi: 10.1002/cne.902560108. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol. 1992;323:341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- Muñoz M, Insausti R. Cortical efferents of the entorhinal cortex and the adjacent parahippocampal region in the monkey (Macaca fascicularis) Eur J Neurosci. 2005;22:1368–1388. doi: 10.1111/j.1460-9568.2005.04299.x. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Visual recognition in monkeys following rhinal cortical ablations combined with either amygdalectomy or hippocampectomy. J Neurosci. 1986;6:1991–2003. doi: 10.1523/JNEUROSCI.06-07-01991.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kubota K. Mnemonic firing of neurons in the monkey temporal pole during a visual recognition memory task. J Neurophysiol. 1995;74:162–178. doi: 10.1152/jn.1995.74.1.162. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kubota K. The primate temporal pole: its putative role in object recognition and memory. Behav Brain Res. 1996;77:53–77. doi: 10.1016/0166-4328(95)00227-8. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernathey CD. Atlas of the cerebral sulci. Stuttgart (NY): Georg Thieme verlag; 1990. [Google Scholar]
- Ouchi Y, Nobezawa S, Okada H, Yoshikawa E, Futatsubashi M, Kaneko M. Altered glucose metabolism in the hippocampal head in memory impairment. Neurology. 1998;51:136–142. doi: 10.1212/wnl.51.1.136. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Kuypers HG. Cortico-cortical connections in the rhesus monkey. Brain Res. 1969;13:13–36. doi: 10.1016/0006-8993(69)90141-3. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH. Proposed neural circuitry for spatial memory in the primate brain. Neuropsychologia. 1984 doi: 10.1016/0028-3932(84)90055-1. 1984;22:109–122. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Pelletier M, Bouthillier A, Lévesque J, Carrier S, Breault C, Paquette V, Mensour B, Leroux JM, Beaudoin G, Bourgouin P, et al. Separate neural circuits for primary emotions? Brain activity during self-induced sadness and happiness in professional actors. Neuroreport. 2003;14:1111–1116. doi: 10.1097/00001756-200306110-00003. [DOI] [PubMed] [Google Scholar]
- Pestana EM, Gupta A. Fluctuating Kluver-Bucy syndrome in a child with epilepsy due to bilateral anterior temporal congenital malformations. Epilepsy Behav. 2007;10:340–343. doi: 10.1016/j.yebeh.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Köhler S, Crane J, Pruessner M, Lord C, Byrne A, Kabani N, Collins DL, Evans AC. Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high-resolution MR images: considering the variability of the collateral sulcus. Cereb Cortex. 2002;12:1342–1353. doi: 10.1093/cercor/12.12.1342. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, Miller BL. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129:2945–2956. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198:315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- Royet JP, Zald D, Versace R, Costes N, Lavenne F, Koenig O, Gervais R. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. J Neurosci. 2000;20:7752–7759. doi: 10.1523/JNEUROSCI.20-20-07752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvlin P, Coste S, Hermier M, Mauguiere F. Temporal pole MRI abnormalities in temporal lobe epilepsy. Epileptic Disord. 2002;4(Suppl 1):S33–S39. [PubMed] [Google Scholar]
- Saleem KS, Price JL, Hashikawa T. Cytoarchitectonic and chemoarchitectonic subdivisions of the perirhinal and parahippocampal cortices in macaque monkeys. J Comp Neurol. 2007;500:973–1006. doi: 10.1002/cne.21141. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Tanaka K. Divergent projections from the anterior inferotemporal area TE to the perirhinal and entorhinal cortices in the macaque monkey. J Neurosci. 1996;16:4757–4775. doi: 10.1523/JNEUROSCI.16-15-04757.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanides F, Sas E. Persistence of horizontal cells of the Cajal foetal type and of the subpial granular layer in parts of the mammalian paleocortex. Z Mikrosk Anat Forsch. 1970;82:570–588. [PubMed] [Google Scholar]
- Sanvito WL, Tilbery CP, Ribeiro-Pinto L, Soares CA, Oliveira SV, Lancellotti CL. Kluver-Bucy syndrome caused by viral encephalitis. Report of a case. Arq Neuropsiquiatr. 1982;40:251–259. doi: 10.1590/s0004-282x1982000300006. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Witter M, Schwarcz R. The parahippocampal region. Implications for neurological and psychiatric diseases. Ann N Y Acad Sci. 2000;911:ix–xiii. [PubMed] [Google Scholar]
- Schwalbe B. Beitrag zur Enwicklungsgeschichte des Zwischenhirns. Sitzungsberichte der Jenaischen Gesellschaft für Medicin und Naturwissenschaft. 1881;4:2–7. [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GE. A new topgraphical Survey of the human cerebral cortex, being an account of the distribution of the anatomically distinct cortical areas and their relationship to the cerebral sulci. J Anat. 1907;41:237–254. [PMC free article] [PubMed] [Google Scholar]
- Stefanacci L, Suzuki WA, Amaral DG. Organization of connections between the amygdaloid complex and the perirhinal and parahippocampal cortices in Macaque monkeys. J Comp Neurol. 1996;375:552–582. doi: 10.1002/(SICI)1096-9861(19961125)375:4<552::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Stephan H. Handbuch der mikroskopischen Anatomie des Menschen. Vol IV/9. Heidelberg (Germany): Springer Verlag; 1975. Allocortex; p. 998. [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cytoarchitectonic and chemoarchitectonic organization. J Comp Neurol. 2003;463:67–91. doi: 10.1002/cne.10744. [DOI] [PubMed] [Google Scholar]
- Tillfors M, Furmark T, Marteinsdottir I, Fischer H, Pissiota A, Långström B, Fredrikson M. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. Am J Psychiatry. 2001;158:1220–1226. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW. The parahippocampal gyrus. New observations regarding its cortical connections in the monkey. Trends Neurosci. 1982;5:345–350. [Google Scholar]
- Van Hoesen GW, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. III. Efferent connections. Brain Res. 1975;95:39–59. doi: 10.1016/0006-8993(75)90206-1. [DOI] [PubMed] [Google Scholar]
- von Economo C, Koskinas GN. Die Cytoarchitectonic de Hirnrinde der erwachsenen Menschen. Vienna (Berlin): Springer Verlag; 1925. p. 810. [Google Scholar]
- Voytko ML. Visual learning and retention examined with reversible cold lesions of the anterior temporal lobe. Behav Brain Res. 1986;22:25–39. doi: 10.1016/0166-4328(86)90078-1. [DOI] [PubMed] [Google Scholar]
- Wright IC, Ellison ZR, Sharma T, Friston KJ, Murray RM, McGuire PK. Mapping of grey matter changes in schizophrenia. Schizophr Res. 1999;35:1–14. doi: 10.1016/s0920-9964(98)00094-2. [DOI] [PubMed] [Google Scholar]
- Zahn R, Juengling F, Bubrowski P, Jost E, Dykierek P, Talazko J, Huell M. Hemispheric asymmetries of hypometabolism associated with semantic memory impairment in Alzheimer's disease: a study using positron emission tomography with fluorodeoxyglucose-F18. Psychiatry Res. 2004;132:159–172. doi: 10.1016/j.pscychresns.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. The neural correlates of aversive auditory stimulation. NeuroImage. 2002;16:746–753. doi: 10.1006/nimg.2002.1115. [DOI] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Moser KH, Schleicher A, Stephan H. Gyrification in the cerebral cortex of primates. Brain Behav Evol. 1989;34:143–150. doi: 10.1159/000116500. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG, Suzuki WA. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J Neurosci. 1989;9:4355–4370. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]