Abstract
Breast density, as assessed by mammography, reflects breast tissue composition. Breast epithelium and stroma attenuate x-rays more than fat and thus appear light on mammograms while fat appears dark. In this review, we provide an overview of selected areas of current knowledge about the relationship between breast density and susceptibility to breast cancer. We review the evidence that breast density is a risk factor for breast cancer, the histological and other risk factors that are associated with variations in breast density, and the biological plausibility of the associations with risk of breast cancer. We also discuss the potential for improved risk prediction that might be achieved by using alternative breast imaging methods, such as magnetic resonance or ultrasound. After adjustment for other risk factors, breast density is consistently associated with breast cancer risk, more strongly than most other risk factors for this disease, and extensive breast density may account for a substantial fraction of breast cancer. Breast density is associated with risk of all of the proliferative lesions that are thought to be precursors of breast cancer. Studies of twins have shown that breast density is a highly heritable quantitative trait. Associations between breast density and variations in breast histology, risk of proliferative breast lesions, and risk of breast cancer may be the result of exposures of breast tissue to both mitogens and mutagens. Characterization of breast density by mammography has several limitations, and the uses of breast density in risk prediction and breast cancer prevention may be improved by other methods of imaging, such as magnetic resonance or ultrasound tomography.
The ability to predict the future occurrence of disease in individuals allows for improvements in the design and application of preventive strategies and intervention trials as well as improved clinical decision making (1). Cardiovascular medicine provides a paradigm for an approach to disease prevention based on risk prediction. Modification of risk factors has been estimated to account for approximately half of the 40% reduction in age-specific mortality from cardiovascular disease observed over the past three decades; the remainder of the risk reduction has been attributed to improvements in treatment (2).
Predicting the risk of developing breast cancer is less well developed than predicting the risk of cardiovascular disease (1). Currently, the most widely used method of predicting risk of breast cancer in individuals is the Gail model (3), which takes into account a woman’s age, age at menarche, age at first live birth, number of previous benign breast biopsies, and number of first-degree relatives with breast cancer.
Breast density reflects variations in breast tissue composition and is more strongly associated with breast cancer risk than the other variables included in the Gail model (4). Breast density is assessed by mammography and expressed as the percentage of the breast that is occupied by radiologically dense tissue (ie, percent mammographic density). The addition of breast density to the Gail model increased predictive accuracy, as shown by the concordance statistic, from 0.607 to 0.642 (5), whereas adding seven single-nucleotide polymorphisms that are associated with breast cancer to the Gail model increased the concordance statistic to 0.632 (6). Unlike most other risk factors for breast cancer that are included in the Gail model, breast density can be changed by hormone interventions that include a gonadotrophin-releasing hormone agonist (7), combined hormone therapy (8), or tamoxifen (9), suggesting that it may be a target for preventive interventions.
In this review, we provide an overview of selected areas of current knowledge about the relationship between breast density and the susceptibility to breast cancer. We review briefly the evidence that mammographic density is a risk factor for breast cancer, the histological and other factors that are associated with variations in breast density, and the biological plausibility of the association between breast density and the risk of breast cancer. We discuss the limitations of mammography as a method of characterizing breast cancer risk and describe some alternative methods that may improve risk prediction.
Mammographic Density
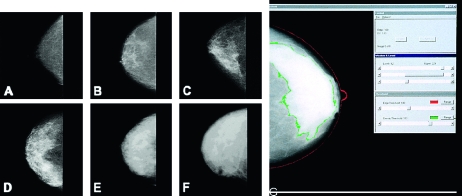
The radiographic appearance of the breast on mammography varies among women and reflects the variations in breast tissue composition and the different x-ray attenuation characteristics of these tissues (10). Fat is radiologically lucent and appears dark on a mammogram, whereas connective and epithelial tissues are radiologically dense and appear light. The proportion of the breast that comprises connective and epithelial tissues is usually expressed as a percentage of the breast area (ie, as the percent mammographic density). Examples of variations in percent mammographic density are shown in Figure 1.
Figure 1.
Mammographic density. Left, examples of variation in mammographic density: (A) 0%, (B) <10%, (C) <25%, (D) <50%, (E) <75%, (F) >75%. Right, (G) illustration of a computer-assisted measure. The outer (red) line shows the edge of the breast, the inner (green) line shows the edge of dense tissue. Percent density is calculated by dividing the dense area by the total area and multiplying by 100.
The late Dr John Wolfe was the first to describe differences in risk of breast cancer associated with variations in the mammographic appearance of the breast (11,12). Since then, other qualitative and quantitative methods of measuring percent mammographic density (13) have been applied to the assessment of percent mammographic density in relation to risk of breast cancer.
Methods of Classifying Mammographic Density
To date, five principal methods have been used to assess mammographic density. Wolfe (11,12) described four categories of breast density: N1 (predominately fat), P1 and P2 (ductal prominence in “less than one-fourth or more than one-fourth,” respectively, of the breast), and DY (extensive “dysplasia”). The American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) also has four categories of mammographic breast density: 1 (predominately fat), 2 (scattered densities), 3 (heterogeneously dense), and 4 (extremely dense) (14). Other methods include visual estimation of the proportion of the breast occupied by radiologically dense breast tissue (15), planimetry (16), and computer-assisted methods of measurement that are based on interactive thresholding (ie, Cumulus software and other similar programs) (13). An example of data generated by Cumulus is shown in Figure 1 (panel G). An observer places thresholds at the edge of the breast (the red line) and at the edge of density (the green line), and the areas so defined are recorded by the computer. Percent mammographic density is calculated by dividing the dense area by the total area and multiplying by 100 and can be treated as either a continuous or categorical variable in subsequent analysis.
These methods differ in their ease of application and in their reliability. The Wolfe categories have largely been replaced in the literature by quantitative methods of classification or by the BI-RADS score, the latter of which is included routinely for a large proportion of mammograms in the United States. Quantitative assessment of mammograms using Cumulus or other similar methods of measurement has been used mostly in research studies because it requires either a trained observer and digitized film images or processed images from digital mammography. Reliability between readers of mammograms is modest for BI-RADS (κ statistic = 0.56) (17) and good for estimation by radiologists (intraclass correlation coefficient [ICC] = .7) (15) and by Cumulus (ICC ≥ .9) (18).
Mammographic Density and Breast Cancer Risk
McCormack and dos Santos Silva (4) conducted a systematic meta-analysis of the association between percent mammographic density and risk of breast cancer using data for more than 14 000 women with breast cancer and 226 000 women without breast cancer from 42 studies. They found that percent extensive mammographic density was consistently associated with an increased risk of breast cancer. However, associations were stronger in studies that were conducted in the general population than for those conducted in symptomatic women, stronger for percent mammographic density than for Wolfe categories, and stronger in studies of incident vs prevalent cancer. The breast cancer risk associated with percent mammographic density did not differ by age, menopausal status, or ethnicity and could not be explained by the “masking” of cancers by dense tissue (18).
Table 1 summarizes selected features of the 10 studies reported to date, all carried out as case–control studies nested within cohorts, that used quantitative methods to classify percent mammographic density in the baseline mammogram taken at entry to the cohort (16,18–25). The studies were carried out in the United States, Canada, and Europe. The maximum interval between the baseline mammogram and the date of diagnosis of breast cancer ranged among studies from 5–10 years (16,18–25). The methods of categorizing percent mammographic density for statistical analysis varied among these studies; however, all studied showed a substantial and statistically significant increase in breast cancer risk across the categories of percent mammographic density examined. After adjustment for other risk factors, most odds ratios (ORs) were between 4 and 5. The three Canadian cohorts (18) showed similar odds ratios for radiologists’ estimation of percent mammographic density and for computer-assisted measurements.
Table 1.
Selected characteristics of cohort studies with quantitative classification of percent mammographic density*
| First author/study (reference), region | Subject age, y | Sample size† | Measurement‡ | Partition§ | OR (95% CI) | Follow-up, y | Adjustments║ |
| Kato (19), USA | 35–65 | 197/521 | Planimetry | Upper vs lower tertile | 3.6 (1.4 to 9.1) | 5.5 | BMI, parity, menopause |
| Saftlas (20), USA | 35–74 | 266/301 | Planimetry | <5% vs ≥65% | 4.3 (2.1 to 8.8) | 5 | Age, weight, parity |
| Byrne (16), USA | 35–74 | 1880/2152 | Planimetry | 0% vs ≥75% | 4.3 (3.1 to 6.1) | 10 | Weight, age at first birth, family history, years of education, alcohol use, previous benign biopsies, reproductive years. |
| Torres-Mejia (21), EUR | 40–80 | 111/3100 | Computer assisted | 0.5% vs >46% | 3.5 (1.4 to 5.2) | 14 | Age, education, parity, height, BMI |
| van Gils (22), EUR | >45 | 129/517 | Automated | <5% vs >25% | 2.9 (1.6 to 5.6) | 10 | Age, parity |
| Thomas (23), USA | <50 | 547/472 | Estimation | Upper vs lower quartiles | 4.4 (3.0 to 6.7) | >6 | Age, study |
| Maskarinec (24), USA | 60¶ | 607/667 | Computer assisted | <10% vs >50% | 3.6 (2.3 to 5.6) | 7 | Ethnicity, age, BMI, age at fist birth, number of births, age at menarche, age at menopause, HRT, family history of breast cancer |
| Boyd-NBSS (25), CAN | 40–59 | 330 | Estimation | 0% vs ≥75% | 6.0 (2.8 to 13.0) | 7 | Age, parity, age at first birth, weight, height, number of births, age at menarche, family history |
| Computer assisted | 4.0 (2.1 to 7.7) | ||||||
| Boyd-SMPBC (18), CAN | 40–70 | 398 | Estimation | <10% vs ≥75% | 4.5 (1.9 to 11.0) | 6 | Age, parity, age at first birth, weight, height, number of births, age at menarche, family history |
| Computer assisted | 4.4 (2.1 to 5.0) | ||||||
| Boyd-OBSP (18), CAN | 50–69 | 386 | Estimation | <10% vs ≥75% | 3.4 (1.1 to 10.3) | 8 | Age, parity, age at first birth, weight, height, number of births, age at menarche, family history |
| Computer assisted | 4.1 (2.0 to 8.6) | ||||||
| Boyd-Combined (18), CAN | 40–70 | 1114 | Estimation | <10% vs ≥75% | 4.7 (3.0 to 7.4) | 6–8 | Age, parity, age at first birth, weight, height, number of births, age at menarche, family history |
| Computer assisted | 4.4 (2.9 to 6.7) |
BMI = body mass index; CAN = Canada; CI = confidence interval; EUR = Europe; HRT = hormone replacement therapy; NBSS = National Breast Screening Study; OBSP = Ontario Breast Screening Program; OR = odds ratio; SMPBC = Screening Mammography Program of British Columbia.
Reported as the number of case subjects/number of control subjects or as the number of pairs of case and control subjects.
Estimation means visual estimation by an observer (radiologist).
The most and least extensive categories of density from which odds ratios were calculated.
Factors included in the analysis of risk associated with mammographic density. Factors controlled for by matching are also included.
Average age.
Three of these studies also showed that the association between percent mammographic density and risk of breast cancer could not be explained by the “masking” of cancer by dense breast tissue in the baseline mammogram (16,18,20). Masking is expected to distort estimates of risk only in the short term (26). However, the Saftlas cohort (20) was limited to women who developed breast cancer 5 years after the baseline mammogram, the Byrne cohort (16) showed persistence of risk associated with percent mammographic density in the baseline mammogram for up to 10 years, and the Canadian cohorts (18) showed both persistence of risk for up to 6–8 years after baseline and an increased risk of breast cancer associated with percent mammographic density for cancers detected at screening and between screening examinations (18,27). Furthermore, extensive percent mammographic density is common in the population, and estimates of attributable risk suggest that a percent mammographic density of greater than 50% may account for approximately one-third of breast cancers (9,16).
Factors Associated With Variations in Mammographic Density
Age, Mammographic Density, and the Incidence of Breast Cancer.
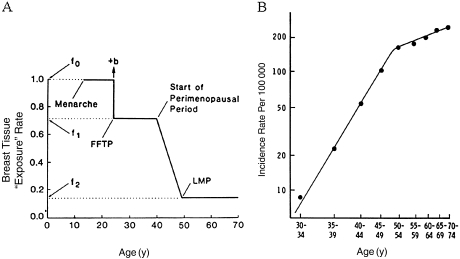
The average percent mammographic density in the population decreases with increasing age (16,28–30), which seems paradoxical given that breast cancer incidence increases with age. This apparent paradox may be explained by a model of breast cancer incidence proposed by Pike et al. (31). The Pike model is based on the concept that “breast tissue exposure,” rather than chronological age, is the relevant measure for describing the incidence of breast cancer. Breast tissue exposure refers to exposure of breast tissue to hormones and growth factors, and to the effects that menarche, pregnancy, and menopause have on these exposures and on the susceptibility of breast tissue to carcinogens. Breast tissue exposure is highest at the time of menarche, decreases at the time of pregnancy, is further reduced during the perimenopausal period, and is lowest after the menopause (Figure 2, A). Pike et al. (31) showed that cumulative breast tissue exposure, which corresponds to the area under the exposure–age curve (Figure 2, A), describes the age–incidence curve for breast cancer (Figure 2, B).
Figure 2.
The Pike model. A) Pike model of mammary carcinogenesis. b = short-term increase in risk after FFTP; FFTP = first full-term pregnancy; LMP = last menstrual period; f0, f1, f2 are parameters of the model. B) Age-specific incidence of breast cancer observed and predicted by the Pike model. Reproduced with permission from Pike et al. (31).
The variables that influence breast tissue exposure in the Pike model also influence percent mammographic density, and average percent mammographic density in the population has several features in common with the Pike concept of breast tissue exposure. Both percent mammographic density and breast tissue age are greatest at younger ages and decline with increasing age, and both are reduced by pregnancy and the menopause. Increasing age is also associated with a reduction in average amounts of stromal and epithelial tissues in the breast that are reflected in the percent mammographic density (32).
Percent mammographic density may thus reflect the cumulative exposure of breast stroma and epithelium to hormones and growth factors that stimulate cell division, and changes in percent mammographic density with age may reflect changes in breast histology that are commonly referred to as involution (33). The extent of involution has now been shown to vary inversely with percent mammographic density (34). Furthermore, a study of breast biopsy specimens showed that the extent of lobular involution increases with increasing age and is associated with risk of breast cancer; compared with age-matched women in the general population, the relative risk of breast cancer for women with no involution was 1.88 (95% confidence interval [CI] = 1.59 to 2.21), for those with partial involution was 1.47 (95% CI = 1.33 to 1.61), and for those in whom involution was complete was 0.91 (95% CI = 0.75 to 1.10) (30).
The prediction of the Pike model, that susceptibility of the breast to carcinogens will be greatest before the age of 20 years, is supported by data from female Japanese atomic bomb survivors (35). Risk of breast cancer in the survivors of those explosions was greatest in those who were younger than 20 years at the time of exposure. Breast cancer risk is also increased in women who were exposed as children to ionizing radiation during treatment for tuberculosis, Hodgkin disease, or an “enlarged” thymus (36–38). The possibility that the greater susceptibility of the breast to carcinogens at younger ages may be related to breast tissue composition in young women is considered in the following section.
Breast Tissue Composition in Young Women.
Exposure to radiation precludes the use of mammography to characterize breast tissue composition in healthy young women. However, the relative water content of the breast determined by magnetic resonance, which uses no radiation and like mammographic density reflects the fibroglandular tissue of the breast and, in adult women, is strongly correlated (r = .8) with percent mammographic density (39), can be used to characterize breast tissue composition in young women.
In a cross-sectional study of 400 young women aged 15–30 years, magnetic resonance imaging was used to measure the water and fat content of the breast during the follicular phase of the woman's menstrual cycle (40). Mothers of the young women provided mammograms (n = 365), and a random sample of the mothers (n = 100) also had breast magnetic resonance imaging. Percent breast water was highest at early ages, when susceptibility to breast carcinogens is also greatest, and, in young women, was inversely associated with weight and positively associated with height and the breast tissue characteristics of their mothers, all of which are risk factors for breast cancer. The mother–daughter correlation for percent breast water (Spearman r = .28), together with results from two studies in twins (41,42) that are described below, provide strong evidence that percent mammographic density is a heritable quantitative trait.
Figure 3 shows three simplified hypothetical models of ways in which percent mammographic density might begin and change throughout a woman's life. The models illustrate, by using the interquartile range (the 75th and 25th percentiles of the hypothetical distributions of percent mammographic density), how the distribution of percent mammographic density might change and vary in early, middle, and late life. In model A, all women have a high level of percent mammographic density in early life; differences in percent mammographic density during midlife are the result of differences in the rate of decrease of percent mammographic density with increasing age. A slower rate of decrease is associated with greater percent mammographic density in later life and a more rapid decrease with lower percent mammographic density. In model B, differences in percent mammographic density in early life decreases at the same rate with increasing age and persist throughout life. In model C, some women in early life have high levels of percent mammographic density, and others have low levels. In this model, percent mammographic density decreases with age in those with high levels at early ages and remains low in those with low levels at early ages.
Figure 3.
Potential models of change in mammographic density with age. A–C) Three hypothetical models of ways in which mammographic density might change over the life span. All models show a reduction in the values for percent density (on the y-axis) with increasing age (on the x-axis) but are distinguished by differences in the change of the interquartile range (IQR) with age. This is shown by the 25th and 75th percentiles of the distribution of percent density. D) Observed distributions of percent breast water in mothers and daughters. The y-axis shows the percent of subjects, and the x-axis the value for percent breast water. Vertical arrows indicate median percent breast water values for each group. Reproduced with permission from Boyd et al. (40). IQR = interquartile range.
In all models, the median level of percent mammographic density decreases with increasing age, but the interquartile range in percent mammographic density increases with increasing age in model A, does not change with age in model B, and is greatest at early ages and decreases with increasing age in model C. The observed distributions of percent water content in the breast tissue of daughters, who were stratified by the median age of 19 years, and the sample of mothers who also had magnetic resonance are shown in Figure 3, D. Daughters had a higher median percent water and interquartile range compared with mothers, and daughters aged 15 to less than 19 years had a higher median percent water and interquartile range compared with daughters aged 19–30 years. These data are consistent with model C but not with model A or B.
This hypothetical model (model C) suggests that extensive percent mammographic density in midlife, when, as discussed above, it is a strong risk factor for breast cancer (4), may arise from the subset of the female population with the most extensive fibroglandular tissue in early life. The observed associations between percent breast water and height and weight in young women and between percent breast water in young women and their mother's breast density suggest that genetic influences and growth and development in early life may determine breast tissue composition. The observation that median percent water was greater in women aged 15–19 years than in women aged 20–30 years or in the mothers also suggests that the greater quantity of fibroglandular tissue in early life may be a potential mechanism for the increased susceptibility to carcinogens at early ages. Interventions directed at the prevention of breast cancer may therefore be more effective if started in early life rather than in adult life.
Heritability.
Age, parity, and menopausal status account for only 20%–30% of the variation in percent mammographic density observed in the US population (43). However, results of small studies of mother–daughter sets (44) and twins (45) and of two segregation analyses (46) suggest that genetic factors may explain a proportion of the remaining variation (ie, the heritability) in percent mammographic density.
To our knowledge, only two sufficiently large studies of twins (41,47) have been published that estimate the proportion of the variance in percent mammographic density that could be explained by genetic factors. In one study (41), 951 pairs of twins aged 40–70 years were recruited in Australia and North America; mammograms for these women were obtained, and information was collected on factors known to be associated with variations in percent mammographic density. After adjustment for age and other covariates, the correlation coefficients for percent mammographic density were .61 and .67 for monozygotic pairs in Australia and North America, respectively, and .25 and .27 for dizygotic pairs in Australia and North America, respectively. The estimated proportion of the residual variation in percent mammographic density accounted for by additive genetic factors (heritability) was 60% (95% CI = 54% to 66%) for the Australian twins, 67% (95% CI = 59% to 75%) for the North American twins, and 63% (95% CI = 59% to 67%) for the two groups of subjects combined (41).
In a second study (47) of 553 twin pairs, before adjustment for age and other covariates, the correlation coefficients for percent mammographic density were .74 (95% CI = 0.68 to 0.79) for monozygotic twin pairs and .38 (95% CI = 0.28 to 0.47) for dizygotic twin pairs. The estimated proportion of the residual variation in percent mammographic density accounted for by additive genetic factors (ie, heritability) was 53% (47). Studies of the genetic variants associated with variations in percent mammographic density are in progress (48).
Breast Cancer Risk Factors.
As noted above, in addition to its decrease with increasing age (29), percent mammographic density is also less extensive in women who have given birth and in those with a larger number of live births (49,50) and declines at menopause (28,43,51). After adjustment for age and other potential risk factors, a family history of breast cancer has been found to be associated with more extensive percent mammographic density (52,53). Percent mammographic density has consistently been found to be inversely associated with body weight (54,55). Greater birth weight and greater adult height have been shown to be positively associated with percent mammographic density (54,56) and with an increased risk of breast cancer (57,58). Physical activity has not been shown consistently to be associated with mammographic density (56,59–61). It is not yet known to what extent the risk of breast cancer associated with risk factors such as height, parity, and menopause is mediated through their associations with percent mammographic density.
Factors That Change Mammographic Density.
Estrogen plus progestin therapy but not estrogen therapy alone is associated with a small increase in risk of breast cancer (62), and the combined therapy is associated with an increase in percent mammographic density (63–65), whereas estrogen alone is not (63). Tamoxifen treatment in postmenopausal women (9) and treatment with a gonadotrophin-releasing hormone agonist in premenopausal women (7) are associated with a reduction in percent mammographic density. Cuzick et al. (66) recently reported that women who used tamoxifen had a reduction in percent mammographic density and a reduction in breast cancer incidence. It remains to be determined whether the observed changes in mammographic density mediate the effect of tamoxifen in reducing breast cancer risk.
Biological Plausibility of the Association Between Mammographic Density and Risk of Breast Cancer
We summarize below, and describe in detail elsewhere (50), the epidemiological evidence that the risk of breast cancer associated with extensive percent mammographic density may arise from the combined effects of cell proliferation in response to mitogens and genetic damage caused by mutagens. There is also some evidence that molecules in the extracellular matrix may contribute to the susceptibility to breast cancer that is associated with percent mammographic density.
Breast Tissue Composition and Mammographic Density
Studies of surgical biopsy samples or mastectomy specimens [described in detail in reference (49)] have shown that greater amounts of epithelium and/or stroma are associated with more extensive percent mammographic density. Li et al. (32) used the forensic autopsy series of Bartow et al. (32,67,68), and quantitative microscopy to examine histological features of randomly selected tissue blocks from breast tissue obtained at forensic autopsy. The methods and results of this study are summarized in Figure 4. Random biopsy samples were taken from breast tissue slices (Figure 4, A) and used to prepare histological sections. The area of the tissue on the slide was outlined, and randomly selected areas within each section were selected (Figure 4, B), stained with hematoxylin–eosin to detect nuclei (Figure 4, C) and with trichrome to detect collagen (Figure 4, F), and staining was assessed using thresholding software to determine the total areas of nuclei (Figure 4, D) and collagen (Figure 4, G) within each section.
Figure 4.
Breast tissue composition and mammographic density. Random biopsy samples were taken from breast tissue slices (A), and histological sections were prepared. The area of the tissue on the slide was outlined, and the randomly selected areas within each section were selected (B). Tissue sections stained with hematoxylin and eosin (C) and trichrome (F) were prepared, and assessed using thresholding software to determine the total areas of nuclei (D) and collagen (G) within each section. Associations between these measurements and percent mammographic density are shown in box plots: nuclear area (E) and collagen (H); both were statistically significant (P < .001). Modified and reproduced with permission from Li et al. (32).
Greater percent mammographic density in the breast tissue from which the biopsy sample was taken was associated with a statistically significantly greater total nuclear area (P < .001) (Figure 4, E) and a statistically significantly greater proportion of collagen (P < .001) (Figure 4, H) and with a greater nuclear area of both epithelial and nonepithelial cells and a greater area of glandular structures. Of the tissue components that were measured by Li et al. (32), collagen was present in the greatest quantity, was most strongly associated with percent mammographic density, and explained 29% of the variance in percent mammographic density. Nuclear area accounted for 4% of the variance in percent mammographic density.
Greater body weight, parity and a greater number of births, and postmenopausal status—all factors that are associated with both variations in percent mammographic density (32,43,49) and risk of breast cancer (69)—were also associated with quantitative differences in one or more of the tissue features measured in the autopsy samples described above [see reference (32) for details].
Mitogens and Mammographic Density
To date, most studies that have examined percent mammographic density and circulating levels of ovarian hormones in premenopausal or postmenopausal women have found either no association or an inverse association (70–76). Two studies in postmenopausal women found a positive association between plasma estrogen level and percent mammographic density (76,77). In a cross-sectional study of 494 premenopausal women that accounted for cyclic variations in estrogen levels, Walker et al. (78) measured urinary levels of the estrogen metabolite estrone glucuronide in the periovulatory and luteal phases of the menstrual cycle. Mean ovulatory estrone glucuronide level and daily estrone glucuronide load were positively associated with percent mammographic density before adjustment for body mass index; however, these associations were attenuated after adjustment for body mass index. In postmenopausal women, serum levels of estradiol and testosterone and percent mammographic density appear to be independent risk factors for breast cancer (79).
We have found positive associations between serum levels of growth hormone and breast water (a surrogate for percent mammographic density) in young women aged 15–30 years (n = 280; P = .003) (40), in adult premenopausal women (n = 193; P = .03), and in postmenopausal women (n = 170; P = .003) (74). The association in young women remained statistically significant after adjustment for covariates, whereas those in older women lost statistical significance after adjustment for body size (74). However, because growth hormone influences body size, adjusting for body size may be overadjustment (80). Percent mammographic density has been found to be positively associated with serum levels of insulinlike growth factor I in premenopausal women in four (74,78,81,82) of the six studies (83,84) and in one study in postmenopausal women (85). The extent of immunostaining for insulinlike growth factor I in breast biopsy samples is also positively associated with percent mammographic density in the breast from which the biopsy sample was taken (86).
Mutagens and Mammographic Density
An excess of reactive oxygen species in relation to antioxidant defenses can cause oxidative damage to DNA, protein, and lipid molecules and may be associated with an increased risk of cancer (87). A validation study in rats showed that urinary malondialdehyde, a known mutagen (88,89), was among the best indicators of in vivo oxidative stress (90). Three independent studies in adult women found that 24-hour urinary malondialdehyde excretion was positively and statistically significantly associated with percent mammographic density and was 23%–30% higher in the highest quintile of percent mammographic density compared with the lowest quintile after adjustment for age and body mass index or waist circumference (91–93). The mechanisms that underlie the association between urinary malondialdehyde and percent mammographic density are unknown; however, the growth hormone–mediated release of free fatty acids from adipocytes, and an increase in the lipid substrate available for oxidative damage, might be involved (80). Other markers of oxidative stress have not yet to our knowledge been examined in relation to percent mammographic density.
Potential Biological Mechanisms
Epithelial and stromal cells, collagen, and fat—the tissue components that contribute to variations in mammographic density—are related to each other in several ways. Epithelial and stromal cells communicate with each other by means of paracrine growth factors (94). Collagen is a product of stromal fibroblasts, and adipocytes develop from the differentiation of stromal preadipocytes (95). Factors that affect one of these components may therefore affect the others, either directly or indirectly, and each component has properties that may influence the risk and progression of breast cancer.
Breast cancer arises from epithelial cells, and, thus, the number and proliferative state of these cells may influence both the radiological density of the breast and the probability of genetic damage that can give rise to cancer. In addition, collagen and the stromal matrix are products of stromal cells, which may, through their mechanical and other properties, facilitate tumor invasion (96–98). Interactions between stroma and epithelium are known to influence breast development and the changes in breast structure that take place during pregnancy, lactation, and involution and during tumorigenesis (99–101). The extracellular matrix, which comprises collagens, fibronectin, laminins, polysaccharides, and proteoglycans, plays a key role in these processes, and there is a large and rapidly growing literature on the molecules that mediate how the extracellular matrix influences the epithelium [see (99–102) for reviews].
To date, there has been limited application of these basic science findings to understanding the association between mammographic density and risk of breast cancer. In addition to having greater amounts of collagen, cells, and larger areas that are immunohistochemically positive for insulinlike growth factor I, radiologically dense breast tissue also has greater amounts of the stromal matrix regulatory protein tissue inhibitor metalloproteinase 3 (86). Metalloproteinases that regulate stromal matrix can also regulate the activation of growth factors and influence susceptibility to breast cancer (103,104). Expression of the proteoglycans lumican and decorin has been found to be increased in stromal tissue associated with breast cancer and in women with extensive mammographic density who do not have breast cancer (105). Proteoglycans bind growth factors, contribute to the mechanical integrity of tissues, may reflect the stiffness of breast tissue, and can modify tissue behavior (102).
Mammographic Density and Histological Precursors to Breast Cancer
Breast lesions including ductal carcinoma in situ, atypical hyperplasia, hyperplasia without atypia, and columnar cell lesions are, to different degrees, associated with an increased risk of breast cancer, and extensive percent mammographic density is associated with an increased risk of each of these lesions. Ductal carcinoma in situ is thought to be a nonobligatory precursor of invasive breast cancer. In the Multiethnic Cohort, compared with women with less than 10% percent mammographic density, those with more than 50% percent mammographic density had increased risks of both invasive breast cancer (OR = 3.58, 95% CI = 2.3 to 5.7) and ductal carcinoma in situ (OR = 2.9, 95% CI = 1.4 to 5.9) (106). Ursin et al. (107) found that ductal carcinoma in situ occurred in regions of the breast that were mammographically dense. A case–control study in the Canadian National Breast Screening Study (108) showed that compared with women with no mammographic density, women with greater than 75% mammographic density had increased risks of in situ breast cancer and atypical hyperplasia combined (OR = 9.7, 95% CI = 1.7 to 53.9) and of hyperplasia without atypia (OR = 12.2, 95% CI = 2.9 to 50.1).
Columnar cell lesions are thought to be the earliest recognizable histological features that are nonobligate precursors to breast cancer (109–111). In a study of tissue slides from the forensic autopsy series described above (32), 17% of women were found to have columnar cell lesions, and these lesions were more frequent in biopsy samples from breasts with greater than the median density of 30% compared with biopsy samples from breasts with less than the median density (OR = 2.2, 95% CI = 1.03 to 4.8). The presence of columnar cell lesions was also positively associated with the percentage of the biopsy sample that was occupied by collagen (P < .001) and the percentage that was glandular area (P < .001) (112).
Summary
Variations in percent mammographic density reflect variations in the amounts of collagen and the numbers of epithelial and nonepithelial cells in the breast. Extensive percent mammographic density is associated with increased risks of invasive breast cancer and all of the proliferative lesions that are thought to be precursors of breast cancer. Associations between percent mammographic density and variations in breast histology, risk of proliferative breast lesions, and risk of invasive breast cancer may all be the result of exposures to breast mitogens and mutagens. Metalloproteinases and proteoglycans in the extracellular matrix may be among the factors that contribute to the susceptibility to breast cancer that is associated with percent mammographic density.
Limitations of Using Mammography to Measure Breast Density
All of the methods currently used to assess breast density by mammography, including Wolfe categories, BI-RADS, visual estimation, planimetry, and computer-assisted thresholding, have limitations. None takes into account the thickness of the breast, and thus all are based on the projected area, rather than the volume, of breast tissue. Current computer-assisted methods of measuring breast density require the placement of a dichotomous threshold between dense and nondense tissue and do not allow for the gradual transition from dense to nondense tissue (and vice versa) that is likely to exist in reality. No allowance is made for variations in the current or voltage used in generating the image or for variations in film development or breast compression. All current methods require a trained observer and, thus, the measurements are subjective.
These potential sources of error in measurement are likely to attenuate the observed associations between percent mammographic density and other risk factors for breast cancer and risk of the disease itself. The risk prediction of all models that include percent mammographic density, such as the revised Gail model described above, will thus also be underestimated (5). Furthermore, the exposure of subjects examined by mammography to radiation limits the use of repeated measurements and, in the absence of a clinical indication, precludes the use of mammography to measure breast density in young women.
Potential Improvements in Characterizing Breast Tissue Composition
Mammography
To date, two studies have examined the association between percent mammographic density and risk of breast cancer by measuring breast tissue volumes. Ding et al. (113) carried out a large case–control study using standard mammography form (SMF) software to assess the association between volumetric breast density and risk of breast cancer; they compared the SMF measurements with those obtained by other methods of assessing density, including the computer-assisted threshold method described above. SMF uses information about the nonfat tissue in the breast, in conjunction with the thickness of the compressed breast and the breast imaging variables of tube voltage and exposure time, to generate estimates of breast tissue volumes (113). The volume-based measures of percent density generated by SMF were associated with breast cancer risk, albeit less strongly than the area-based measures of percent density. However, after adjustment for the area measure of percent density, the SMF-derived measures were no longer statistically significantly associated with breast cancer risk (113).
In an alternative approach, we acquired mammographic images prospectively under controlled conditions in a matched case–control study (114). Women with newly diagnosed breast cancer (case subjects) identified in selected mammography centers were compared with women without breast cancer (control subjects) who underwent mammography at the same center as the case subject. The mammography machines in these centers had been calibrated to allow examination of the relationship between the image signal in each pixel (ie, optical density or blackness of the processed film value), the exposure factors (ie, kilovoltage, mAs, tube target, and beam filter), and the amount of radiation transmitted by the breast (115). Corrected breast thickness at each pixel was calculated by using a mathematical formula (116), and the volumes of dense tissue and of the total breast were calculated [see (114)].
Analyses of percent density according to volume and area measures, considered separately and then together, showed that the volume measure alone was statistically significantly associated with risk of breast cancer after adjustment for other risk factors (P = .003). The area measure alone was also statistically significantly associated with risk of breast cancer (P < .001). When the volume and area measures of percent density were both included in the predictive model as continuous variables, the area measure remained statistically significantly associated with risk of breast cancer (P = .002), whereas the volume measure did not (P = .29). Thus, although the volume measure provided risk information similar to that of the area measure, it did not improve on the risk predictions made by the area measure. Knowledge of the thickness of the compressed breast was essential for the calculation of tissue volumes, and the volumes calculated were very sensitive to small variations in measured thickness (117). The thickness of the compressed breast varies between chest wall and nipple and from the midline to the edge of the breast. The corrections made for the thickness measurements from multiple machines may have failed to capture fully these variations and may have attenuated the breast cancer risk associated with the volume measures.
Other methods of measuring volumetric breast density from mammography have been described (118–121); however, to our knowledge, there are as yet no published data on their ability to predict risk of breast cancer. We next describe briefly two potential alternatives to mammography that have been used to measure breast density.
Magnetic Resonance Measures of Breast Tissue Composition
Breast stromal and epithelial tissues, whose x-ray attenuation properties are responsible for the radio-dense tissue that contributes to percent mammographic density, are also responsible for the water content measured by magnetic resonance (122). Furthermore, magnetic resonance assesses quantitatively the volume of breast tissue and is largely automated. It is therefore likely to have less measurement error than the subjective measurement of the two-dimensional representation of breast tissue in mammograms shown in Figure 1. Quantitative direct image-based measurements of water and fat (123) are now commercially available on many magnetic resonance imaging systems (124) and have been extensively calibrated (125).
The application of magnetic resonance to the characterization of breast tissue in the cross-sectional study of breast tissue composition in young women and their mothers is described above (35); in that study, measurements of the breast were made using a 1.5T Signa CVi MR system (GE, Waukesha, WI) with a breast coil that surrounded both breasts of a prone subject. Images were acquired in the sagittal plane. The sequence was calibrated for both the volumes and the percentages of fat and water, and a quality-control program verified the accuracy and stability of these measurements within 2% and the water and oil phantom content accuracy within 3%.
The output of the magnetic resonance examination (illustrated in Figure 5, A and B) is a series of “slices” at 7-mm intervals through both breasts. On each slice, an observer distinguished the breast from the surrounding tissues (as shown by the solid line), and the water and fat content within each slice was calculated pixel by pixel and summed over all slices using the three-point Dixon method (126,127), which acquires the water and fat signals with phase shifts of 0, pi, and 2pi.
Figure 5.
Magnetic resonance of the breast. A and B) Examples of magnetic resonance tissue slices with green lines showing definition of breast outlines. Images show a breast with little water (A) and a breast with extensive water (B). C) Scatter plot of percent breast water by magnetic resonance imaging vs percent mammographic density (n = 100 subjects). Spearman correlation coefficient = .85 (P < .001). Reproduced with permission from Boyd et al. (40).
Figure 5, C shows the correlation between the measurements made by magnetic resonance and mammography in 100 adult women who were examined by both methods (40). Percent mammographic density in the mammogram and percent water by magnetic resonance were strongly correlated (Spearman r = .85; P < .001), similar to previous findings (39).
Ultrasound Tomography
The average speed of sound (s) through human tissue is related to tissue density and elasticity in the following manner: s = (c/ρ)−1/2, where c is the elastic constant and ρ the material density of the tissue through which sound waves travel. In human tissue, the elastic constant is proportional to density cubed, suggesting that sound speed correlates linearly with density (128–130). Thus, breast density can be measured without exposing the patient to ionizing radiation by measuring sound speed (118,119,131,132).
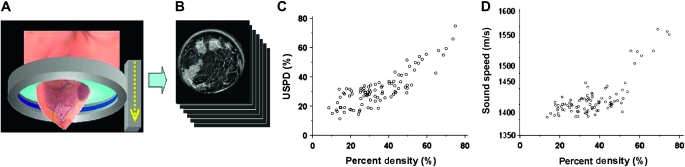
The application of ultrasound tomography to the measurement of breast density has been examined using a clinical prototype that was constructed at the Karmanos Cancer Institute (Detroit, MI). The patient lies prone on a table made of flexible sailcloth, with the breast suspended in an imaging tank filled with warm water below the table (Figure 6, A). The ultrasound sensor, in the shape of a ring, surrounds the breast and moves from the chest wall to the nipple on a motorized gantry, gathering data from 45 to 75 tissue slices, each 5 mm thick. A typical whole-breast exam takes approximately 1 minute to perform. A sound speed image is generated for each position of the transducer, yielding an image stack (Figure 6, B). The images are produced from algorithms that use “bent-ray” tomographic techniques that provide greater computational efficiency while accounting for refractive effects (131,132).
Figure 6.
Ultrasound tomography of the breast. A) The ultrasound ring array surrounds the breast as it moves on a vertical trajectory from the chest wall to the nipple, acquiring data at discrete steps along the way. B) Each acquired dataset yields images of sound speed. C) Scatter plot of percent density by ultrasound tomography (USPD) vs percent mammographic density (n = 90 subjects). Spearman correlation coefficient = .75 (P < .001). D) Scatter plot of volumetrically averaged breast sound speed vs percent mammographic density (n = 92 subjects). Spearman correlation coefficient = .59 (P < .001). Reproduced with permission from Schuchmann et al. (125).
Two methods have been used to assess breast density by ultrasound tomography: ultrasound percent density and volume-averaged sound speed. Ultrasound percent density is determined by segmenting high areas with sound speed from each sound speed tomogram by using a k-means clustering routine (133), integrating the segmented areas over the entire volume of the breast, and dividing by the volume of the whole breast. In the second method, the volume-averaged sound speed of the whole breast is determined from the pixel values of the entire image stack. This method avoids the use of thresholding and takes into account gradations of density in the breast.
Breast density measurements by both methods were positively correlated with percent mammographic density (Spearman correlation coefficient for ultrasound percent density =.75 [Figure 6, C] and for volume-averaged sound speed = .59 [Figure 6, D]) (N. Duric and N. F. Boyd, unpublished observations). To determine the stability of breast density measurements by ultrasound tomography in the same individuals over repeated examinations, we examined eight premenopausal women four times during their menstrual cycles; the ICC for the repeated measurements of average sound speed was .99 (N. Duric and N. F. Boyd, unpublished data).
Potential Impact on Risk Prediction of Improved Measures of Breast Density.
To our knowledge, no study has directly assessed the association between the risk of breast cancer and breast density measured by either magnetic resonance or ultrasound tomography. To estimate the magnitude of the gradient in risk expected with breast density measurements by magnetic resonance or ultrasound tomography, we assumed that measurements of breast density by these methods would be more accurate measures of breast density than mammography. We used the methods of Rosner et al. (134) to correct estimates of relative risk from logistic regression for the assumed measurement error associated with percent mammographic density. In a previous study of mammographic density and risk of breast cancer (18), the interquartile odds ratio for the association between percent mammographic density risk of breast cancer was 3.08 (beta coefficient = 0.2005; square root–transformed interquartile range = 5.5). The Spearman correlation coefficient for percent mammographic density and ultrasound tomography by ultrasound percent density was .75, and the ICC was .56. The corresponding values for percent mammographic density and percent water by magnetic resonance were .85 and .72, respectively. To estimate the beta coefficient for the measure of breast density by magnetic resonance or ultrasound tomography, the beta coefficient is divided by the ICC, resulting in beta coefficients of 0.3589 (0.2005/0.56) for ultrasound tomography and 0.2673 (0.2005/0.72) for magnetic resonance. The expected interquartile odds ratios are given by e(beta)(5.5) and are 7.19 for ultrasound tomography and 4.63 for magnetic resonance, both of which are substantially larger than the odds ratio of 3.08 for percent mammographic density by mammography. Substantial improvement in risk prediction may therefore be achieved by the use of measures of breast density that are more accurate than mammography.
Conclusions
There is now a substantial body of evidence showing that the variations in breast tissue composition that are reflected by mammographic density have the characteristics of a highly heritable quantitative trait and are associated with differences in risk of breast cancer. Current methods of measuring percent density by mammography are susceptible to several potential sources of error as a result of the subjectivity of the measurement, the omission of breast thickness, and the omission of potential sources of variation in the exposure and processing of the images, which are likely to attenuate the association between percent mammographic density and risk of breast cancer; these sources of measurement error may be reduced by using alternative methods for assessing breast density, such as magnetic resonance or ultrasound tomography. These alternatives to mammography can provide quantitative, objective, and volumetric measures of breast density that are immune to the potential sources of variations in image acquisition and processing that are associated with mammography and can be compared with external physical standards of measurement, such as the known fat and water composition in the case of magnetic resonance and the speed of sound for ultrasound. We expect that the reduction of measurement error will create larger gradients in breast cancer risk compared with those associated with percent mammographic density. The use of breast density in breast cancer risk prediction, clinical decision making, and breast cancer prevention is likely to be improved substantially through the elimination of error in the measurement of breast density by mammography. Improved accuracy in the measurement of breast density is also likely to strengthen etiological associations with breast density, such as those involving genetic variants and blood levels of hormones. Furthermore, methods for assessing breast composition that avoid exposure to radiation will allow the characterization of breast density in young women. The Pike model suggests, and empirical data have shown, that the breast is most susceptible to carcinogens at early ages, and thus, it may be that cancer prevention in early life will be most effective (135).
Funding
The work has been supported in part by grants from the National Cancer Institute of Canada, the Canadian Breast Cancer Research Alliance, the Komen Foundation, and the National Institutes of Health (RO1CA082826-01). This research was also funded in part by the Ontario Ministry of Health and Long Term Care. N.F.B. was supported by the Lau Chair in Breast Cancer Research, and M.J.Y. was supported by the Tory Family Chair in Cancer Research. N.D. acknowledges the support of the Komen Foundation and the Michigan Economic Development Corporation.
Footnotes
The funding bodies had no involvement in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
N. Duric is a coinventor of, and has intellectual property interests in, the ultrasound technology discussed in this review.
References
- 1.Freedman AN, Seminara D, Gail MH, et al. Cancer risk prediction models: a workshop on development, evaluation, and application. J Natl Cancer Inst. 2005;97(10):715–723. doi: 10.1093/jnci/dji128. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356(23):2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 3.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 4.McCormack VA. dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Pee D, Ayyagari R, et al. Projecting absolute invasive breast cancer risk in white women with a model that includes mammographic density. J Natl Cancer Inst. 2006;98(17):1215–1226. doi: 10.1093/jnci/djj332. [DOI] [PubMed] [Google Scholar]
- 6.Gail MH. Discriminatory accuracy from single-nucleotide polymorphisms in models to predict breast cancer risk. J Natl Cancer Inst. 2008;100(14):1037–1041. doi: 10.1093/jnci/djn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spicer DV, Ursin G, Parisky YR, et al. Changes in mammographic densities induced by a hormonal contraceptive designed to reduce breast cancer risk. J Natl Cancer Inst. 1994;86(6):431–436. doi: 10.1093/jnci/86.6.431. [DOI] [PubMed] [Google Scholar]
- 8.Greendale GA, Reboussin BA, Sie A, et al. Effects of estrogen and estrogen-progestin on mammographic parenchymal density. Ann Intern Med. 1999;130(4 pt 1):262–269. doi: 10.7326/0003-4819-130-4_part_1-199902160-00003. [DOI] [PubMed] [Google Scholar]
- 9.Cuzick J, Warwick J, Pinney E, Warren RML, Duffy SW. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96(8):621–628. doi: 10.1093/jnci/djh106. [DOI] [PubMed] [Google Scholar]
- 10.Johns PC, Yaffe MJ. X-ray characterisation of normal and neoplastic breast tissues. Phys Med Biol. 1987;32(6):675–695. doi: 10.1088/0031-9155/32/6/002. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer. 1976;37(5):2486–2492. doi: 10.1002/1097-0142(197605)37:5<2486::aid-cncr2820370542>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe JN. Breast patterns as an index of risk for developing breast cancer. Am J Roentgenol. 1976;126(6):1130–1137. doi: 10.2214/ajr.126.6.1130. [DOI] [PubMed] [Google Scholar]
- 13.Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ. The quantitative analysis of mammographic densities. Phys Med Biol. 1994;39(10):1629–1638. doi: 10.1088/0031-9155/39/10/008. [DOI] [PubMed] [Google Scholar]
- 14.American College of Radiology (ACR) Breast Imaging Reporting and Data System (BI-RADS) 3rd ed. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 15.Jong R, Fishell E, Little L, Lockwood G, Boyd NF. Mammographic signs of potential relevance to breast cancer risk: the agreement of radiologists’ classification. Eur J Cancer Prev. 1996;5(4):281–286. doi: 10.1097/00008469-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Byrne C, Schairer C, Wolfe J, et al. Mammographic features and breast cancer risk: effects with time, age, and menopause status. J Natl Cancer Inst. 1995;87(21):1622–1629. doi: 10.1093/jnci/87.21.1622. [DOI] [PubMed] [Google Scholar]
- 17.Kerlikowske K, Grady D, Barclay J, et al. Variability and accuracy in mammographic interpretation using the American college of radiology breast imaging reporting and data system. J Natl Cancer Inst. 1998;90(23):1801–1809. doi: 10.1093/jnci/90.23.1801. [DOI] [PubMed] [Google Scholar]
- 18.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 19.Kato I, Beinart C, Bleich A, Su S, Kim M, Toniolo PG. A nested case-control study of mammographic patterns, breast volume, and breast cancer (New York City, NY, United States) Cancer Causes Control. 1995;6(5):431–438. doi: 10.1007/BF00052183. [DOI] [PubMed] [Google Scholar]
- 20.Saftlas AF, Hoover RN, Brinton LA, et al. Mammographic densities and risk of breast cancer. Cancer. 1991;67(11):2833–2838. doi: 10.1002/1097-0142(19910601)67:11<2833::aid-cncr2820671121>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Torres-Mejia G, De Stavola B, Allen D, et al. Mammographic features and subsequent risk of breast cancer: a comparison of qualitative and quantitative evaluations in the Guernsey prospective studies. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1052–1059. doi: 10.1158/1055-9965.EPI-04-0717. [DOI] [PubMed] [Google Scholar]
- 22.van Gils CH, Hendriks JH, Otten JD, Holland R, Verbeek AL. Parity and mammographic breast density in relation to breast cancer risk: indication of interaction. Eur J Cancer Prev. 2000;9(2):105–111. doi: 10.1097/00008469-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Thomas DB, Carter RA, Bush WH, Jr, et al. Risk of subsequent breast cancer in relation to characteristics of screening mammograms from women less than 50 years of age. Cancer Epidemiol Biomarkers Prev. 2002;11(6):565–571. [PubMed] [Google Scholar]
- 24.Maskarinec G, Pagano I, Lurie G, Kolonel LN. A longitudinal investigation of mammographic density: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2006;15(4):732–739. doi: 10.1158/1055-9965.EPI-05-0798. [DOI] [PubMed] [Google Scholar]
- 25.Boyd NF, Byng JW, Jong RA, et al. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87(9):670–675. doi: 10.1093/jnci/87.9.670. [DOI] [PubMed] [Google Scholar]
- 26.Whitehead J, Carlile T, Kopecky KJ, et al. Wolfe mammographic parenchymal patterns. A study of the masking hypothesis of Egan and Mosteller. Cancer. 1985;56(6):1280–1286. doi: 10.1002/1097-0142(19850915)56:6<1280::aid-cncr2820560610>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Sala E, Warren R, McCann J, Duffy S, Day N, Luben R. Mammographic parenchymal patterns and mode of detection: implications for the breast screening programme. J Med Screen. 1998;5(4):207–212. doi: 10.1136/jms.5.4.207. [DOI] [PubMed] [Google Scholar]
- 28.Grove JS, Goodman MJ, Gilbert FI, Clyde D. Factors associated with breast structures in breast cancer patients. Cancer. 1979;43(5):1895–1899. doi: 10.1002/1097-0142(197905)43:5<1895::aid-cncr2820430546>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 29.Wolfe JN. Breast parenchymal patterns and their changes with age. Radiology. 1976;121(3 pt 1):545–552. doi: 10.1148/121.3.545. [DOI] [PubMed] [Google Scholar]
- 30.Milanese TR, Hartmann LC, Sellers TA, et al. Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst. 2006;98(22):1600–1607. doi: 10.1093/jnci/djj439. [DOI] [PubMed] [Google Scholar]
- 31.Pike MC, Krailo MD, Henderson BE, Casagrande JT, Hoel DG. “Hormonal” risk factors, “breast tissue age” and the age-incidence of breast cancer. Nature. 1983;303(5920):767–770. doi: 10.1038/303767a0. [DOI] [PubMed] [Google Scholar]
- 32.Li T, Sun L, Miller N, et al. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(2):343–349. doi: 10.1158/1055-9965.EPI-04-0490. [DOI] [PubMed] [Google Scholar]
- 33.Ginsburg OM, Martin LJ, Boyd NF. Mammographic density, lobular involution, and risk of breast cancer. Br J Cancer. 2008;99(9):1369–1374. doi: 10.1038/sj.bjc.6604635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh K, Hartmann LC, Reynolds C, et al. Association between mammographic density and age-related lobular involution of the breast. J Clin Oncol. 2010;28(13):2207–2212. doi: 10.1200/JCO.2009.23.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tokunaga M, Land CE, Yamamoto T, et al. Breast cancer among atomic bomb survivors. In: Boice JD Jr, Fraumeni JF Jr, editors. Radiation Carcinogenesis: Epidemiology and Biological Significance. 2nd ed. Bethesda, MA: Raven Press; 1984. pp. 45–56. [Google Scholar]
- 36.Hancock SL, Tucker MA, Hoppe RT. Breast cancer after treatment of Hodgkin's disease. J Natl Cancer Inst. 1993;85(1):25–31. doi: 10.1093/jnci/85.1.25. [DOI] [PubMed] [Google Scholar]
- 37.Hildreth NG, Shore RE, Dvoretsky PM. The risk of breast cancer after irradiation of the thymus in infancy. N Engl J Med. 1989;321(19):1281–1284. doi: 10.1056/NEJM198911093211901. [DOI] [PubMed] [Google Scholar]
- 38.Miller AB, Howe GR, Sherman GJ, et al. Mortality from breast cancer after irradiation during fluoroscopic examinations in patients being treated for tuberculosis. N Engl J Med. 1989;321(19):1285–1289. doi: 10.1056/NEJM198911093211902. [DOI] [PubMed] [Google Scholar]
- 39.Graham SJ, Bronskill MJ, Byng JW, Yaffe MJ, Boyd NF. Quantitative correlation of breast tissue parameters using magnetic resonance and X-ray mammography. Br J Cancer. 1996;73(2):162–168. doi: 10.1038/bjc.1996.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyd NF, Martin LJ, Chavez S, et al. Breast-tissue composition and other risk factors for breast cancer in young women: a cross-sectional study. Lancet Oncol. 2009;10(6):569–580. doi: 10.1016/S1470-2045(09)70078-6. [DOI] [PubMed] [Google Scholar]
- 41.Boyd NF, Dite GS, Stone J, et al. Heritability of mammographic density, a risk factor for breast cancer. N Engl J Med. 2002;347(12):886–894. doi: 10.1056/NEJMoa013390. [DOI] [PubMed] [Google Scholar]
- 42.Stone J, Dite GS, Gunasekara A, et al. The heritability of mammographically dense and nondense breast tissue. Cancer Epidemiol Biomarkers Prev. 2006;15(4):612–617. doi: 10.1158/1055-9965.EPI-05-0127. [DOI] [PubMed] [Google Scholar]
- 43.Vachon CM, Kuni CC, Anderson K. Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States) Cancer Causes Control. 2000;11(7):653–662. doi: 10.1023/a:1008926607428. [DOI] [PubMed] [Google Scholar]
- 44.Wolfe JN, Albert S, Belle S, Salane M. Familial influences on breast parenchymal patterns. Cancer. 1980;46(11):2433–2437. doi: 10.1002/1097-0142(19801201)46:11<2433::aid-cncr2820461123>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 45.Kaprio J, Alanko A, Kivisaari L. Mammographic patterns in twin pairs discordant for breast cancer. Br J Radiol. 1987;60(713):459–462. doi: 10.1259/0007-1285-60-713-459. [DOI] [PubMed] [Google Scholar]
- 46.Pankow JS, Vachon CM, Kuni CC, et al. Genetic analysis of mammographic breast density in adult women: evidence of a gene effect. J Natl Cancer Inst. 1997;89(8):549–556. doi: 10.1093/jnci/89.8.549. [DOI] [PubMed] [Google Scholar]
- 47.Ursin G, Lillie EO, Lee E, et al. The relative importance of genetics and environment on mammographic density. Cancer Epidemiol Biomarkers Prev. 2009;18(1):102–112. doi: 10.1158/1055-9965.EPI-07-2857. [DOI] [PubMed] [Google Scholar]
- 48.Kelemen LE, Sellers TA, Vachon CM. Can genes for mammographic density inform cancer aetiology? Nat Rev Cancer. 2008;8(10):812–823. doi: 10.1038/nrc2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyd NF, Lockwood GA, Byng J, Tritchler DL, Yaffe M. Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7(12):1133–1144. [PubMed] [Google Scholar]
- 50.Martin LJ, Boyd N. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008;10(1):1–14. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boyd N, Martin L, Stone J, Little L, Minkin S, Yaffe M. A longitudinal study of the effects of menopause on mammographic features. Cancer Epidemiol Biomarkers Prev. 2002;11(10 pt 1):1048–1053. [PubMed] [Google Scholar]
- 52.Ziv E, Shepherd J, Smith-Bindman R, Kerlikowske K. Mammographic breast density and family history of breast cancer. J Natl Cancer Inst. 2005;95(7):556–558. doi: 10.1093/jnci/95.7.556. [DOI] [PubMed] [Google Scholar]
- 53.Martin LJ, Melnichouk O, Guo H, et al. Family history, mammographic density, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(12):456–463. doi: 10.1158/1055-9965.EPI-09-0881. [DOI] [PubMed] [Google Scholar]
- 54.Brisson J, Morrison AS, Kopans DB. Height and weight, mammographic features of breast tissue, and breast cancer risk. Am J Epidemiol. 1984;119(3):371–381. doi: 10.1093/oxfordjournals.aje.a113755. [DOI] [PubMed] [Google Scholar]
- 55.Grove JS, Goodman MJ, Gilbert F, Mi MP. Factors associated with mammographic pattern. Br J Radiol. 1985;58(685):21–25. doi: 10.1259/0007-1285-58-685-21. [DOI] [PubMed] [Google Scholar]
- 56.Sellers TA, Vachon CM, Pankratz VS, et al. Association of childhood and adolescent anthropometric factors, physical activity, and diet with adult mammographic breast density. Am J Epidemiol. 2007;166(4):456–464. doi: 10.1093/aje/kwm112. [DOI] [PubMed] [Google Scholar]
- 57.Lawlor DA, Okasha M, Gunnell D, Smith GD, Ebrahim S. Associations of adult measures of childhood growth with breast cancer: findings from the British Women's Heart and Health Study. Br J Cancer. 2003;89(1):81–87. doi: 10.1038/sj.bjc.6600972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hunter DJ, Willett WC. Diet, body size, and breast cancer. Epidemiol Rev. 1993;15(3):110–132. doi: 10.1093/oxfordjournals.epirev.a036096. [DOI] [PubMed] [Google Scholar]
- 59.Suijkerbuijk KP, Van Duijnhoven FJ, van Gils CH, et al. Physical activity in relation to mammographic density in the Dutch prospect-European prospective investigation into cancer and nutrition cohort. Cancer Epidemiol Biomarkers Prev. 2006;15(3):456–460. doi: 10.1158/1055-9965.EPI-05-0569. [DOI] [PubMed] [Google Scholar]
- 60.Siozon CC, Ma H, Hilsen M, Bernstein L, Ursin G. The association between recreational physical activity and mammographic density. Int J Cancer. 2006;119(7):1695–1701. doi: 10.1002/ijc.22020. [DOI] [PubMed] [Google Scholar]
- 61.Woolcott CG, Courneya KS, Boyd NF, et al. Mammographic density change with 1 year of aerobic exercise among postmenopausal women: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2010;19(4):1112–1121. doi: 10.1158/1055-9965.EPI-09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chlebowski RT, Hendrix SL, Langer R, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women. The Women's Health Initiative Randomized Trial. JAMA. 2003;289(24):3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 63.Greendale GA, Reboussin BA, Slone S, Wasilauskas C, Pike MC, Ursin G. Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst. 2003;95(1):30–37. doi: 10.1093/jnci/95.1.30. [DOI] [PubMed] [Google Scholar]
- 64.Lundstrom E, Wilczek B, von Palffy Z, Soderqvist G, von Schoultz B. Mammographic breast density during hormone replacement therapy: differences according to treatment. Am J Obstet Gynecol. 1999;181(2):348–352. doi: 10.1016/s0002-9378(99)70560-0. [DOI] [PubMed] [Google Scholar]
- 65.Rutter CM, Mandelson MT, Laya MB, Seger DJ, Taplin S. Changes in breast density associated with initiation, discontinuation, and continuing use of hormone replacement therapy. JAMA. 2001;285(2):171–176. doi: 10.1001/jama.285.2.171. [DOI] [PubMed] [Google Scholar]
- 66.Cuzick J, Warwick J, Pinney L, et al. Change in breast density as a biomarker of breast cancer risk reduction: Results from IBIS-1. Cancer Res. 2009;69(suppl 2):61. [Google Scholar]
- 67.Hart BL, Steinbock RT, Mettler FA, Jr, Pathak DR, Bartow SA. Age and race related changes in mammographic parenchymal patterns. Cancer. 1989;63(12):2537–2539. doi: 10.1002/1097-0142(19890615)63:12<2537::aid-cncr2820631230>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 68.Bartow SA, Mettler FA, Jr, Black WC., III Correlations between radiographic patterns and morphology of the female breast. Rad Patterns Morph. 1997;13:263–275. [Google Scholar]
- 69.Kelsey JL, Gammon MD, John ES. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15(1):36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 70.Noh JJ, Maskarinec G, Pagano I, Cheung LW, Stanczyk FZ. Mammographic densities and circulating hormones: a cross-sectional study in premenopausal women. Breast. 2006;15(1):20–28. doi: 10.1016/j.breast.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 71.Tamimi RM, Hankinson SE, Colditz GA, Byrne C. Endogenous sex hormone levels and mammographic density among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14(11 pt 1):2641–2647. doi: 10.1158/1055-9965.EPI-05-0558. [DOI] [PubMed] [Google Scholar]
- 72.Aiello EJ, Tworoger SS, Yasui Y, et al. Associations among circulating sex hormones, insulin-like growth factor, lipids, and mammographic density in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1411–1417. doi: 10.1158/1055-9965.EPI-04-0920. [DOI] [PubMed] [Google Scholar]
- 73.Warren R, Skinner J, Sala E, et al. Associations among mammographic density, circulating sex hormones, and polymorphisms in sex hormone metabolism genes in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1502–1508. doi: 10.1158/1055-9965.EPI-05-0828. [DOI] [PubMed] [Google Scholar]
- 74.Boyd NF, Stone J, Martin LJ, et al. The association of breast mitogens with mammographic densities. Br J Cancer. 2002;87(8):876–882. doi: 10.1038/sj.bjc.6600537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verheus M, Peeters PHM, van Noord PAH, van der Schouw YT, Grobbee DE, van Gils CH. No relationship between circulating levels of sex steroids and mammographic breast density: the Prospect-EPIC cohort. Breast Cancer Res. 2007;9(4):R53. doi: 10.1186/bcr1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bremnes Y, Ursin G, Bjurstam N, Rinaldi S, Kaaks R, Gram IT. Endogenous sex hormones, prolactin and mammographic density in postmenopausal Norwegian women. Int J Cancer. 2007;121(1):2506–2511. doi: 10.1002/ijc.22971. [DOI] [PubMed] [Google Scholar]
- 77.Greendale GA, Palla SL, Ursin G, et al. The association of endogenous sex steroids and sex steroid binding proteins with mammographic density: results from the Postmenopausal Estrogen/Progestin Interventions Mammographic Density Study. Am J Epidemiol. 2005;162(9):826–834. doi: 10.1093/aje/kwi286. [DOI] [PubMed] [Google Scholar]
- 78.Walker K, Fletcher O, Johnson N, et al. Premenopausal mammographic density in relation to cyclic variations in endogenous sex hormone levels, prolactin, and insulin-like growth factors. Cancer Res. 2009;69(16):6490–6499. doi: 10.1158/0008-5472.CAN-09-0280. [DOI] [PubMed] [Google Scholar]
- 79.Tamimi RM, Byrne C, Colditz GA, Hankinson SE. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2007;99(15):1178–1187. doi: 10.1093/jnci/djm062. [DOI] [PubMed] [Google Scholar]
- 80.Nam SY, Lobie PE. The mechanism of effect of growth hormone on preadipocyte and adipocyte function. Obes Rev. 2000;1(2):73–86. doi: 10.1046/j.1467-789x.2000.00015.x. [DOI] [PubMed] [Google Scholar]
- 81.Battistutta D, Palmer J, Walters M, Walker G, Nancarrow D, Hayward N. Incidence of familial melanoma and MLM2 gene. Lancet. 1994;344(8937):1607–1608. doi: 10.1016/s0140-6736(94)90408-1. [DOI] [PubMed] [Google Scholar]
- 82.Byrne C, Colditz GA, Pollak M, Willet WC, Speizer FE, Hankinson SE. Plasma insulin-like growth factor-I, insulin-like growth factor binding protein-3 and mammographic density. Cancer Res. 2000;60(14):3744–3748. [PubMed] [Google Scholar]
- 83.Maskarinec G, Williams AE, Kaaks R. A cross-sectional investigation of breast density and insulin-like growth factor I. Int J Cancer. 2003;107(6):991–996. doi: 10.1002/ijc.11505. [DOI] [PubMed] [Google Scholar]
- 84.dos Santos Silva I, Johnson N, De Stavola B, et al. The insulin-like growth factor system and mammographic features in premenopausal and postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15(3):449–455. doi: 10.1158/1055-9965.EPI-05-0555. [DOI] [PubMed] [Google Scholar]
- 85.Bremnes Y, Ursin G, Bjurstam N, Rinaldi S, Kaaks R, Gram IT. Insulin-like growth factor and mammographic density in postmenopausal Norwegian women. Cancer Epidemiol Biomarkers Prev. 2007;16(1):57–62. doi: 10.1158/1055-9965.EPI-06-0788. [DOI] [PubMed] [Google Scholar]
- 86.Guo YP, Martin LJ, Hanna W, et al. Growth factors and stromal matrix proteins associated with mammographic densities. Cancer Epidemiol Biomarkers Prev. 2001;10(3):243–248. [PubMed] [Google Scholar]
- 87.Valko M, Izakovic M, Mazur M, Rhodes CJ, Talser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266(1-2):37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 88.Mukai FH, Goldstein BD. Mutagenicity of malondialdehyde, a decomposition product of peroxidized polyunsaturated fatty acids. Science. 1976;191(4229):868–869. doi: 10.1126/science.766187. [DOI] [PubMed] [Google Scholar]
- 89.Basu AK, Marnett LJ. Unequivocal demonstration that malondialdehyde is a mutagen. Carcinogenesis. 1983;4(3):331–333. doi: 10.1093/carcin/4.3.331. [DOI] [PubMed] [Google Scholar]
- 90.Kadiiska MB, Gladen BC, Baird DD, et al. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning. Free Radic Biol Med. 2005;38(6 ):698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 91.Boyd NF, McGuire V. Evidence of lipid peroxidation in premenopausal women with mammographic dysplasia. Cancer Lett. 1990;50(1):31–37. doi: 10.1016/0304-3835(90)90175-w. [DOI] [PubMed] [Google Scholar]
- 92.Boyd NF, Connelly P, Byng J, et al. Plasma lipids, lipoproteins, and mammographic densities. Cancer Epidemiol Biomarkers Prev. 1995;4(7):727–733. [PubMed] [Google Scholar]
- 93.Hong CC, Tang BK, Rao V, et al. Cytochrome P450 1A2 (CYP1A2) activity, mammographic density, and oxidative stress: a cross-sectional study. Breast Cancer Res. 2004;6(4):R338–R351. doi: 10.1186/bcr797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hagios C, Lochter A, Bissell M. Tissue architecture: the ultimate regulator of epithelial function? Philos Trans R Soc Lond B Biol Sci. 1998;353(1370):857–870. doi: 10.1098/rstb.1998.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zangani D, Darcy KM, Masso-Welch PA, Bellamy ES, Desole MS, Ip MM. Multiple differentiation pathways of rat mammary stromal cells in vitro: acquisition of a fibroblast, adipocyte or endothelial phenotype is dependent on hormonal and extracellular matrix stimulation. Differentiation. 1999;64(2):91–101. doi: 10.1046/j.1432-0436.1999.6420091.x. [DOI] [PubMed] [Google Scholar]
- 96.Provenzano PO, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4(1):38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. J Mammary Gland Biol Neoplasia. 2004;9(4):325–342. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- 98.Provenzano PP, Inman DR, Eliceiri KW, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ghajar CM, Bissell MJ. Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: insights from imaging. Histochem Cell Biol. 2008;130(6):1105–1118. doi: 10.1007/s00418-008-0537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kass L, Erler JT, Dembo M, Weaver VM. Mammary epithelial cell: Influence of extracellular matrix composition and organization dring development and tumorigenesis. Int J Biochem Cell Biol. 2007;39(11):1987–1994. doi: 10.1016/j.biocel.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9(2):108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432(7015):332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296(5570):1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alowami S, Troup S, Al-Haddad S, Kirkpatrick I, Watson PH. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res. 2003;5(5):R129–R135. doi: 10.1186/bcr622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gill JK, Maskarinec G, Pagano I, Kolonel LN. The association of mammographic density with ductal carcinoma in situ of the breast: the Multiethnic Cohort. Breast Cancer Res. 2006;8(3):R30. doi: 10.1186/bcr1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ursin G, Hovanessian-Larsen L, Parisky YR, Pike MC, Wu AH. Greatly increased occurrence of breast cancers in areas of mammographically dense tissue. Breast Cancer Res. 2005;7(5):R605–R608. doi: 10.1186/bcr1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boyd NF, Jensen H, Cooke G, Lee Han HW. Relationship between mammographic and histological risk factors for breast cancer. J Natl Cancer Inst. 1992;84(15):1170–1179. doi: 10.1093/jnci/84.15.1170. [DOI] [PubMed] [Google Scholar]
- 109.Simpson PT, Gale T, Reis-Filho JS, et al. Columnar cell lesions of the breast: the missing link in breast cancer progression? A morphological and molecular analysis. Am J Surg Pathol. 2005;29(6):734–746. doi: 10.1097/01.pas.0000157295.93914.3b. [DOI] [PubMed] [Google Scholar]
- 110.Feeley L, Quinn CM. Columnar cell lesions of the breast. Histopathology. 2008;52(1):11–19. doi: 10.1111/j.1365-2559.2007.02890.x. [DOI] [PubMed] [Google Scholar]
- 111.Pinder SE, Reis-Filho JS. Non-operative breast pathology: columnar cell lesions. J Clin Pathol. 2007;60(12):1307–1312. doi: 10.1136/jcp.2006.040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Turashvili G, McKinney S, Martin LJ, et al. Columnar cell lesions, mammographic density and breast cancer risk. Breast Cancer Res Treat. 2009;115(3):561–571. doi: 10.1007/s10549-008-0099-x. [DOI] [PubMed] [Google Scholar]
- 113.Ding J, Warren R, Warsi I, et al. Evaluating the effectiveness of using standard mammogram form to predict breast cancer risk: case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1074–1081. doi: 10.1158/1055-9965.EPI-07-2634. [DOI] [PubMed] [Google Scholar]
- 114.Boyd NF, Martin LJ, Gunasekara A, et al. Mammographic density and breast cancer risk: evaluation of a novel method of measuring breast tissue volumes. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1754–1762. doi: 10.1158/1055-9965.EPI-09-0107. [DOI] [PubMed] [Google Scholar]
- 115.Pawluczyk O, Augustine BJ, Yaffe MJ, et al. A volumetric method for estimation of breast density on digitized screen-film mammograms. Med Phys. 2003;30(3):352–364. doi: 10.1118/1.1539038. [DOI] [PubMed] [Google Scholar]
- 116.Mawdsley GE, Tyson AH, Peressotti CL, Jong RA, Yaffe MJ. Accurate estimation of compressed breast thickness in mammography. Med Phys. 2009;36(2):577–586. doi: 10.1118/1.3065068. [DOI] [PubMed] [Google Scholar]
- 117.Yaffe MJ, Boone JM, Packard N, et al. The myth of the 50-50 breast. Med Phys. 2009;36(12):5437–5443. doi: 10.1118/1.3250863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Glide C, Duric N, Littrup P. Novel approach to evaluating breast density utilizing ultrasound tomography. Med Phys. 2007;34(2):744–753. doi: 10.1118/1.2428408. [DOI] [PubMed] [Google Scholar]
- 119.Glide-Hurst CK, Duric N, Littrup P. Volumetric breast density evaluation from ultrasound tomography images. Med Phys. 2008;35(9):3988–3997. doi: 10.1118/1.2964092. [DOI] [PubMed] [Google Scholar]
- 120.Shepherd JA, Herve L, Landau J, Fan B, Kerlikowske K, Cummings SR. Clinical comparison of a novel breast DXA technique to mammographic density. Med Phys. 2006;33(5):1490–1498. doi: 10.1118/1.2193691. [DOI] [PubMed] [Google Scholar]
- 121.Wei J, Chan HP, Helvie MA, et al. Correlation between mammographic density and volumetric fibroglandular tissue estimated on breast MR images. Med Phys. 2004;31(4):933–942. doi: 10.1118/1.1668512. [DOI] [PubMed] [Google Scholar]
- 122.Graham SJ, Ness S, Hamilton BS, Bronskill MJ. Magnetic resonance properties of ex vivo breast tissue at 1.5 T. Magn Reson Med. 1997;38(4):669–677. doi: 10.1002/mrm.1910380422. [DOI] [PubMed] [Google Scholar]
- 123.Ma J. Dixon techniques for water and fat imaging. J Magn Reson Imaging. 2008;28(3):543–558. doi: 10.1002/jmri.21492. [DOI] [PubMed] [Google Scholar]
- 124.Costa DN, Pedrosa I, McKenzie C, Reeder SB, Rofsky NM. Body MRI using IDEAL. Am J Roentgenol. 2008;190(4):1076–1084. doi: 10.2214/AJR.07.3182. [DOI] [PubMed] [Google Scholar]
- 125.Schuchmann S, Weigel C, Albrecht L, et al. Non-invasive quantification of hepatic fat fraction by fast 1.0, 1.5 and 3.0 T MR imaging. Eur J Radiol. 2007;62(3):416–422. doi: 10.1016/j.ejrad.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 126.Dixon W. Simple proton spectroscopic imaging. Radiology. 1984;153(1):189–194. doi: 10.1148/radiology.153.1.6089263. [DOI] [PubMed] [Google Scholar]
- 127.Glover GH. Multipoint Dixon technique for water and fat proton and susceptibility imaging. J Magn Reson Imaging. 1991;1(5):521–530. doi: 10.1002/jmri.1880010504. [DOI] [PubMed] [Google Scholar]
- 128.Masugata H, Mizushige K, Senda S, et al. Relationship between myocardial tissue density measured by microgravimetry and sound speed measured by acoustic microscopy. Ultrasound Med Biol. 1999;25(9):1459–1463. doi: 10.1016/s0301-5629(99)00091-5. [DOI] [PubMed] [Google Scholar]
- 129.Mast TD. Empirical relationships between acoustic parameters in human soft tissues. Acoust Res Lett Online. 2000;1(2):37–42. [Google Scholar]
- 130.Weiwad W, Heinig A, Goetz L, et al. Direct measurement of sound velocity in various specimens of breast tissue. Invest Radiol. 2000;35(12):721–726. doi: 10.1097/00004424-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 131.Li C, Duric N, Huang L. Clinical breast imaging using sound-speed reconstructions of ultrasound tomography data. Proc SPIE. 2008;6920:6920–6929. [Google Scholar]
- 132.Li C, Huang L, Duric N, Zhang H, Rowe C. An improved automatic time-of-flight picker for medical ultrasound tomography. Ultrasonics. 2009;49(1):61–72. doi: 10.1016/j.ultras.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Glide-Hurst CK, Duric N, Littrup P. A new method for quantitative analysis of mammographic density. Med Phys. 2007;34(11):4491–4498. doi: 10.1118/1.2789407. [DOI] [PubMed] [Google Scholar]
- 134.Rosner B, Willett WC, Spiegelman D. Correction of logistic regression relative risk estimates and confidence intervals for systematic within-person measurement error. Stat Med. 1989;8(9):1051–1069. doi: 10.1002/sim.4780080905. [DOI] [PubMed] [Google Scholar]
- 135.Colditz GA, Frazier LA. Models of breast cancer show that risk is set by events of early life: prevention efforts much shift focus (review) Cancer Epidemiol Biomarkers Prev. 1995;4(5):567–571. [PubMed] [Google Scholar]