Abstract
Background
The Centers for Medicare and Medicaid Services (CMS) considered whether to reimburse computed tomographic colonography (CTC) for colorectal cancer screening of Medicare enrollees. To help inform its decision, we evaluated the reimbursement rate at which CTC screening could be cost-effective compared with the colorectal cancer screening tests that are currently reimbursed by CMS and are included in most colorectal cancer screening guidelines, namely annual fecal occult blood test (FOBT), flexible sigmoidoscopy every 5 years, flexible sigmoidoscopy every 5 years in conjunction with annual FOBT, and colonoscopy every 10 years.
Methods
We used three independently developed microsimulation models to assess the health outcomes and costs associated with CTC screening and with currently reimbursed colorectal cancer screening tests among the average-risk Medicare population. We assumed that CTC was performed every 5 years (using test characteristics from either a Department of Defense CTC study or the National CTC Trial) and that individuals with findings of 6 mm or larger were referred to colonoscopy. We computed incremental cost-effectiveness ratios for the currently reimbursed screening tests and calculated the maximum cost per scan (ie, the threshold cost) for the CTC strategy to lie on the efficient frontier. Sensitivity analyses were performed on key parameters and assumptions.
Results
Assuming perfect adherence with all tests, the undiscounted number life-years gained from CTC screening ranged from 143 to 178 per 1000 65-year-olds, which was slightly less than the number of life-years gained from 10-yearly colonoscopy (152–185 per 1000 65-year-olds) and comparable to that from 5-yearly sigmoidoscopy with annual FOBT (149–177 per 1000 65-year-olds). If CTC screening was reimbursed at $488 per scan (slightly less than the reimbursement for a colonoscopy without polypectomy), it would be the most costly strategy. CTC screening could be cost-effective at $108–$205 per scan, depending on the microsimulation model used. Sensitivity analyses showed that if relative adherence to CTC screening was 25% higher than adherence to other tests, it could be cost-effective if reimbursed at $488 per scan.
Conclusions
CTC could be a cost-effective option for colorectal cancer screening among Medicare enrollees if the reimbursement rate per scan is substantially less than that for colonoscopy or if a large proportion of otherwise unscreened persons were to undergo screening by CTC.
CONTEXT AND CAVEATS
Prior knowledge
Although computed tomographic colonography (CTC) and colonoscopy have similar sensitivities for detection of large (≥10 mm) adenomas, the former is less invasive and thus may be more acceptable to patients. To inform a national coverage determination by Medicare, a cost-effectiveness analysis of CTC screening was conducted among the average-risk Medicare population to identify the reimbursement rate at which CTC would be cost-effective compared with currently reimbursed colorectal cancer screening tests, including 10-yearly colonoscopy.
Study design
Three independently developed microsimulation models of colorectal cancer were used to assess the health outcomes and costs associated with 15 screening strategies, including no screening, CTC screening every 5 years, annual fecal occult blood test (FOBT), flexible sigmoidoscopy every 5 years, flexible sigmoidoscopy every 5 years in conjunction with annual FOBT, and colonoscopy every 10 years, among a previously unscreened cohort of 65-year-old average-risk Medicare beneficiaries.
Contribution
Assuming perfect adherence to all tests, the number of life-years gained from 5-yearly CTC was similar to the number gained from 10-yearly colonoscopy. If CTC was reimbursed at roughly the same rate as a colonoscopy without polypectomy, it would be the most costly of all the strategies and the cost, relative to the benefit derived and to the availability and costs of other colorectal cancer screening tests, would be too high for it to be a cost-effective screening strategy.
Implications
CTC could be a cost-effective option for colorectal cancer screening among Medicare enrollees if the test cost was substantially less than that of colonoscopy or if a large proportion of otherwise unscreened persons were to undergo screening by CTC.
Limitations
Excess risks and costs associated with radiation exposure or with the detection of extracolonic findings on CTC were not considered. Estimates of the numbers of life-years were not quality adjusted. Conditional independence of repeat screenings was assumed. Patient time costs were based on assumptions.
From the Editors
Computed tomographic colonography (CTC) is a promising technique for colorectal cancer screening. With CTC, two- and three-dimensional images are reconstructed to allow the visualization of protrusions in the bowel walls, such as those produced by polyps and cancer. Recent studies (1–7) have demonstrated that CTC and colonoscopy have similar sensitivities for detection of adenomas 10 mm or larger. Because CTC is less invasive than colonoscopy, it may be more acceptable to patients (8,9). However, patients undergoing CTC must undergo extensive bowel preparation similar to that required for colonoscopy, and suspicious lesions identified on CTC require the patient to undergo a second procedure (colonoscopy) for biopsy or removal.
In May 2008, the Coverage and Analysis Group at the Centers for Medicare and Medicaid Services (CMS) requested an analysis of CTC screening for colorectal cancer among Medicare enrollees from the Technology Assessment Program at the Agency for Healthcare Research and Quality (AHRQ) to inform a national coverage determination (http://www.cms.gov/mcd/viewdecisionmemo.asp?id=220; tracking number CAG-00396N). AHRQ commissioned the three colorectal cancer modeling groups from the National Cancer Institute’s Cancer Intervention and Surveillance Modeling Network (CISNET) to perform a cost-effectiveness analysis of CTC screening among the average-risk Medicare population. The objective of this analysis was to identify the reimbursement rate at which CTC would be cost-effective compared with the colorectal cancer screening tests that are currently reimbursed by CMS. The findings were presented to the Medicare Evidence Development and Coverage Advisory Committee in November 2008 and are described here.
Methods
Models
We evaluated the cost-effectiveness of CTC screening by using three microsimulation models of colorectal cancer that were developed independently within the CISNET consortium: the Microsimulation Screening Analysis (MISCAN) model from Erasmus University Medical Center (Rotterdam, the Netherlands) and Memorial Sloan-Kettering Cancer Center (New York, NY), the Simulation Model of Colorectal Cancer (SimCRC) from the University of Minnesota (Minneapolis, MN) and Massachusetts General Hospital (Boston, MA), and the Colorectal Cancer Simulated Population model for Incidence and Natural History (CRC-SPIN) from Group Health Research Institute (Seattle, WA). Standardized profiles of the structure and assumptions of each model are available at http://cisnet.cancer.gov/profiles/ and are summarized in the Supplementary Material (available online). Each model simulates the life histories of a large population of individuals from birth to death and has a natural history component that tracks the progression of colorectal disease in the absence of screening (Figure 1). As a simulated individual ages, one or more adenomas may develop, and the risk of developing an adenoma depends on the individual's age, sex, and individual risk; sessile-serrated adenomas are not simulated in any of the models. Adenomas may grow in size, and some may become malignant. A preclinical (ie, undetected) cancer has a chance of progressing from stage I to stage IV and may be detected by symptoms at any stage.
Figure 1.
The natural history of colorectal cancer as simulated by the Microsimulation Screening Analysis (MISCAN), Simulation Model of Colorectal Cancer (SimCRC), and Colorectal Cancer Simulated Population model for Incidence and Natural History (CRC-SPIN) models. The opportunity to intervene in the natural history through screening is indicated by the dotted lines. Screening can remove a precancerous lesion (ie, adenoma), thus preventing it from progressing to cancer, or it can detect a preclinical cancer at a potentially earlier stage of disease when it is more amenable to treatment.
These natural history models were calibrated to data from autopsy studies (10–19) and to Surveillance, Epidemiology, and End Results (SEER) Program data for 1975–1979, which was before the introduction of colorectal cancer screening (20). The models use all-cause mortality estimates from US life tables and stage-specific colorectal cancer survival data from SEER (1996–1999) (20). The outcomes predicted by the natural history models for individuals at age 65 years are compared in Appendix Table 1.
Each model also has a screening component that simulates the ability of a screening test to detect adenomas or preclinical cancer. During a screening round, a simulated individual with an underlying adenoma or preclinical cancer has a chance of having the lesion detected depending on the sensitivity of the test for that lesion and, for testing by colonoscopy or sigmoidoscopy, whether the lesion is within the reach of the endoscope. For an individual without an underlying adenoma or cancer, we applied the test's false-positive rate (ie, 1 minus specificity) to determine whether or not that individual will undergo an unnecessary follow-up colonoscopy. Although nonadenomatous polyps are not modeled explicitly, they are reflected in the false-positive rate of the test. Thus, individuals may be referred for a follow-up colonoscopy and incur polypectomy costs as a result of the detection of nonadenomatous polyps. The models incorporate the risk of fatal complications that are associated with perforation during colonoscopy and, to a lesser extent, sigmoidoscopy and CTC.
To ensure that differences in predictions among the three models were due solely to differences among the natural history models themselves, we standardized all model inputs, including test characteristics, costs, and screening and follow-up assumptions, as well as all-cause mortality rates and survival following cancer diagnosis.
Screening Strategies
We evaluated the health effects and costs associated with CTC screening every 5 years (21) and compared them with the health effects and costs associated with the screening tests that are currently covered by Medicare (22) and are included in most colorectal cancer screening guidelines (21,23–25): annual fecal occult blood test (FOBT), flexible sigmoidoscopy every 5 years, flexible sigmoidoscopy every 5 years in conjunction with annual FOBT, and colonoscopy every 10 years. We considered three commonly used FOBTs (Hemoccult II, Hemoccult SENSA, and immunochemical FOBT) and two strategies for sigmoidoscopy (with and without biopsy). Although Medicare covers screening with barium enema, we did not include this procedure in our analysis because it is rarely used for routine screening (26,27). In our primary or base-case analysis, we assumed that all individuals begin colorectal cancer screening at age 65 years and stop at age 80 years.
Follow-up, Surveillance, and Adherence Assumptions
We assumed that individuals with a positive FOBT or sigmoidoscopy or with a CTC finding of 6 mm or larger were referred for diagnostic follow-up with colonoscopy (21). We assumed that if no adenomas or cancer were detected at follow-up, the individual underwent subsequent screening with colonoscopy every 10 years (as long as the subsequent colonoscopies were negative); no other switching between screening tests was allowed. Individuals with adenomas that were detected and removed by colonoscopy (screening or diagnostic) were assumed to undergo colonoscopy surveillance per guidelines (ie, every 3 years for individuals with an adenoma 10 mm or larger or with three or more adenomas of any size detected at the last colonoscopy, or every 5 years otherwise) (28,29). We assumed that surveillance continued until a diagnosis of colorectal cancer or death. For the base-case analysis, we assumed that individuals were 100% adherent with the screening test of interest and with the recommended follow-up and surveillance; alternative adherence assumptions were explored in a sensitivity analysis.
Test Characteristics
Table 1 presents the screening test characteristics used in the analyses. We considered two sets of CTC performance characteristics: those from a Department of Defense study of colorectal cancer screening by CTC in asymptomatic adults (3) and those from the National CTC Trial (5), which assessed the accuracy of CTC in detecting histologically confirmed, large (≥10 mm in diameter) colorectal adenomas and cancers. We considered the test performance characteristics in these studies separately rather than pooling them because doing so allowed us to explore the impact of somewhat divergent estimates of CTC accuracy on our predictions (6).
Table 1.
Screening test characteristics and costs used in the analyses*
| Test characteristics |
Test costs by perspective, $ | ||||||||
| Sensitivity† for adenomas by size and for CRC, % | Specificity, % | Source | |||||||
| Analysis/screening test | 1–5 mm | 6–9 mm | ≥10 mm | CRC | CMS‡ | Modified societal§ | Source | ||
| Base-case analysis | |||||||||
| Hemoccult II | 2 | 5 | 12 | 40 | 98 | (30) | 5 | 22 | (30) |
| Hemoccult SENSA | 7 | 12 | 24 | 70 | 93 | (30) | 5 | 22 | (30) |
| Immunochemical FOBT | 5 | 10 | 22 | 70 | 95 | (30) | 22 | 39 | (30) |
| Sigmoidoscopy without biopsy║ | 75 | 85 | 95 | 95 | 92¶ | Assumption | 161 | 270 | (30) |
| Sigmoidoscopy with biopsy║ | 75 | 85 | 95 | 95 | 100 | Assumption | 348 | 497 | (30) |
| Colonoscopy without polypectomy | 75 | 85 | 95 | 95 | 90¶ | (31) | 498# | 795# | (30) |
| Colonoscopy with polypectomy | 75 | 85 | 95 | 95 | 90¶ | (31) | 649# | 979# | (30) |
| CT colonography DoD | — | 84** | 92 | 92†† | 80‡‡ | (3) | 488 | 644 | Assumption |
| CT colonography NCTC | — | 57** | 84 | 84†† | 88‡‡ | (5) | 488 | 644 | Assumption |
| Sensitivity analysis | |||||||||
| CT colonography DoD | — | — | 92 | 92†† | 96‡‡ | (3) | 488 | 644 | Assumption |
| CT colonography NCTC | — | — | 84 | 84†† | 86‡‡ | (5) | 488 | 644 | Assumption |
— = Sensitivity is not provided because adenoma size is smaller than the referral threshold for a colonoscopy of 6 mm (base-case analysis) or 10 mm (sensitivity analysis); CMS = Centers for Medicare and Medicaid Services; CRC = colorectal cancer; CT = computed tomographic; DoD = Department of Defense study; FOBT = fecal occult blood test; NCTC = National CT Colonography Trial.
Sensitivity is provided per individual for FOBTs and per lesion for endoscopy and CT colonography.
Costs reflect 2007 CMS payment rates and do not include beneficiary copayments or patient time costs.
Costs reflect 2007 CMS payment rates, beneficiary copayments, and patient time costs.
Test characteristics for sigmoidoscopy apply only to lesions in the distal colon and rectum.
The lack of specificity with endoscopic tests reflects the detection of nonadenomatous lesions. With sigmoidoscopy, the presence of nonadenomatous lesions induces biopsy costs (in the case of sigmoidoscopy with biopsy) or results in referral for colonoscopy (in the case of sigmoidoscopy without biopsy). With colonoscopy, nonadenomatous lesions are removed and therefore induce polypectomy and biopsy costs.
The cost of colonoscopy includes the cost of sedation, assuming that it is not delivered by an anesthesiologist. Higher colonoscopy cost estimates were explored in a sensitivity analysis.
Sensitivity for CT colonography for adenomas 6–9 mm in size was calculated from published tables (3,5).
Sensitivity for colorectal cancer was assumed to be the same as for adenomas ≥10 mm because of the small number of colorectal cancers detected in the DoD and NCTC studies.
The lack of specificity with CT colonography reflects the detection of nonadenomatous polyps, artifacts, and adenomas smaller than the colonoscopy referral threshold (ie, adenomas <6 mm for the base-case analysis and adenomas <10 mm for the sensitivity analysis).
The sensitivity and specificity of each of the FOBTs were based on values reported in a literature review (30). Sensitivities for colonoscopy were from a systematic review and a meta-analysis (31); we assumed that the sensitivities for sigmoidoscopy were the same as those for colonoscopy for adenomas and cancers located within the reach of the sigmoidoscope and were 0 for lesions located beyond the reach of the scope. We assumed that 5% of individuals had more than one colonoscopy to visualize the entire colon and that the cecum was ultimately reached in 98% of individuals screened. For sigmoidoscopy, we assumed that 80% of examinations reached the junction of the sigmoid and descending colon and that 40% reached the beginning of the splenic flexure (32,33).
Costs
The base-case cost-effectiveness analysis was conducted from the payer's (ie, CMS) perspective; accordingly, beneficiary copayments, patient time costs, and the costs of lost productivity because of death or disability were not included. Screening costs were based on the national average (ie, unadjusted for geographic location) Medicare payments in 2007 for procedures and tests associated with colorectal cancer screening and complications of screening (30). These payments reflect approximately 80% of the allowable charges, including the facility charges (as applicable) and physician services charges. The estimates for the cost of colonoscopy with and without polypectomy are weighted averages of the costs across each point of service (ie, ambulatory surgery centers, hospital outpatient settings, and office settings) and include the cost of sedation, assuming that it was not delivered by an anesthesiology professional.
Currently, there is no national CMS payment rate for a screening CTC. Therefore, for the base-case analysis, we considered the reimbursement cost of CTC to be the sum of the national average CMS payments for abdominal and pelvic computed tomography without contrast plus the national average CMS payments for image processing on an independent workstation. For consistency with our other cost estimates (ie, those of other screening tests, complications, and treatment), we converted the 2008 CMS payment rates for the computed tomography procedures to 2007 dollars by using a decrease of 3.5% in medical care costs, yielding a base-case unit cost for CTC of $488. This cost estimate is only slightly lower than the cost of a colonoscopy without polypectomy ($498) (Table 1).
We also considered the rates and associated costs of the major complications of screening (Table 2). There are no complications associated with FOBT. Patients undergoing a colonoscopy and, to a lesser extent, sigmoidoscopy or CTC are at risk of a colon perforation; we assumed that 5.2% of perforations resulted in death (35). Colonoscopy is also associated with serosal burns and with bleeding that may or may not, require transfusion (36–41). We assumed that an individual who has a perforation or bleeding that requires transfusion would be hospitalized. The cost of perforation was based on the 2007 payments for Diagnosis-Related Group code 442 (other operating room procedures for injuries with colon cancer), and the cost of bleeding with transfusion was based on the 2007 payment for Diagnosis-Related Group code 452 (complications with treatment of colon cancer) (42). We assumed that a serosal burn would cost the same as bleeding that requires transfusion. For bleeding that does not require transfusion, we assumed that the individual would be treated in an emergency room visit and that the cost of this visit would be based on the 2007 physician and facility payments for Physicians’ Current Procedural Terminology code 99284 (emergency department visit for a level 4 patient) (43).
Table 2.
Model inputs for complication rates and costs and colorectal cancer (CRC) treatment costs*
| Complication rates and complication costs by perspective, $ |
Annual cost of CRC treatment by stage at diagnosis, phase of care§, and perspective, $ |
|||||||
| Test complications | Rate per 1000 | Perspective | AJCC stage (34) | Phase of care§ | ||||
| CMS† | Modified societal‡ | Initial | Continuing | Terminal, death from CRC | Terminal, death from other cause | |||
| CT colonography | CMS perspective† | |||||||
| Perforation | 0.0456 | 12 446 | 12 712 | I | 25 487 | 2028 | 45 689 | 11 257 |
| Sigmoidoscopy | II | 35 173 | 1890 | 45 560 | 9846 | |||
| Perforation | 0.02 | 12 446 | 12 712 | III | 42 885 | 2702 | 48 006 | 13 026 |
| Colonoscopy | IV | 56 000 | 8375 | 64 428 | 34 975 | |||
| Perforation | 0.7 | 12 446 | 12 712 | Modified societal perspective‡ | ||||
| Serosal burn | 0.3 | 5208 | 5474 | I | 32 720 | 2719 | 56 640 | 17 408 |
| Bleed with transfusion | 0.4 | 5208 | 5474 | II | 43 752 | 2561 | 56 417 | 15 740 |
| Bleed without transfusion | 1.1 | 320 | 586 | III | 53 003 | 3573 | 59 481 | 19 413 |
| IV | 68 853 | 10 743 | 78 227 | 44 384 | ||||
AJCC = American Joint Committee on Cancer; CMS = Centers for Medicare and Medicaid Services; CT = computed tomographic.
Costs reflect 2007 CMS payment rates and do not include beneficiary copayments or patient time costs.
Costs reflect 2007 CMS payment rates, beneficiary copayments, and patient time costs.
The initial phase of care is the first 12 months following diagnosis, the terminal phase is the final 12 months of life, and the continuing phase is all the months between the initial and terminal phases.
Net costs of colorectal cancer–related care by stage and phase of care (Table 2) were obtained from an analysis of 1998–2003 SEER–Medicare linked data that compared the Medicare claims of colorectal cancer patients with those of control subjects without colorectal cancer who were matched by age, sex, and SEER area (R. Yabroff and M. Brown, personal communication, National Cancer Institute) and used the same methodology reported by Yabroff et al. (44), with stage reclassified using the American Joint Committee on Cancer staging algorithm (as opposed to SEER historic stage) and costs in the last year of life stratified according to whether the individual died from colorectal cancer or from other causes. The costs were converted to 2007 dollars using the Consumer Price Index for all items (45).
Cost-Effectiveness Analysis
We used the simulation models to calculate the lifetime costs, life expectancy, and total number of colonoscopies (ie, those for screening, follow-up of individuals with a positive result on another screening test, surveillance of individuals with a history of an adenoma, and diagnosis of individuals with symptomatic colorectal cancer) for a previously unscreened cohort of 65-year-old Medicare beneficiaries under 15 screening strategies, including no screening. We tallied costs from the perspective of CMS and discounted future costs and total life-years by 3% annually (46). We assessed the relative performance of each economically efficient (ie, nondominated) strategy using the incremental cost-effectiveness ratio, which is the additional cost of a strategy divided by its additional clinical benefit compared with the next less expensive nondominated strategy. Dominated strategies included those that were more costly and less effective than a competing option (ie, strongly dominated strategies) and those that had a higher incremental cost per life-year gained compared to a more costly strategy (ie, weakly dominated strategies). All nondominated strategies represent the set of potentially cost-effective (depending on the willingness to pay for a life-year gained) or “efficient” options. When the discounted total costs and the discounted life-years gained associated with each strategy are plotted on a graph, the line connecting the subset of efficient strategies is called the efficient frontier (47). The incremental cost-effectiveness ratios were calculated using each set of CTC test characteristics in turn because the CTC strategies using the parameters from the Department of Defense study and from the National CTC Trial are not competing options.
Threshold Analyses
We performed two types of threshold analyses. In the first threshold analysis, if CTC was found to be dominated by any of the currently reimbursed screening options, we calculated the maximum cost per scan (ie, the threshold cost) for the CTC strategy to lie on the efficient frontier (ie, to be included among the set of efficient strategies). If the CTC strategy was found to be the most effective of the strategies considered, we identified the threshold cost that would yield an incremental cost-effectiveness ratio compared with the next least effective strategy of $50 000 per life-year gained. In the second threshold analysis, because the availability of CTC might entice previously unscreened individuals to undergo screening, we also identified threshold costs for CTC for scenarios in which we allowed adherence to CTC screening to be greater than that of all other screening modalities. For that analysis, we assumed an overall adherence-to-screening rate of 57% for each test [based on the percentage of Medicare-eligible individuals in the 2005 National Health Interview Survey (48) who were adherent with colorectal cancer screening recommendations], and we assumed that this 57% of the population was completely adherent to screening and the rest of the population was completely nonadherent. Modeling adherence in this fashion allowed us to evaluate the impact of increased screening participation among a previously unscreened segment of the population. To do so, we then allowed better adherence to CTC screening than to other tests, increasing overall adherence rates for CTC by 10% (to an overall rate of 62.7%) and by 25% (to an overall rate of 71.3%).
Sensitivity Analyses
We performed a number of sensitivity analyses to evaluate how threshold costs for CTC were influenced by our assumptions. First, we evaluated a scenario in which we restricted diagnostic follow-up to individuals with CTC findings of 10 mm or larger (see Table 1 for test characteristics). Second, we evaluated a scenario in which the screening interval for CTC was 10 years (with diagnostic follow-up for individuals with lesions ≥6 mm). Third, because the specificity of colonoscopy is a function of the extent to which nonadenomatous polyps are removed by the endoscopist, we assessed whether the threshold costs of CTC changed if the specificity of colonoscopy was reduced from 90% to 80%, which reflects an approximate 20% prevalence of nonadenomatous as the most advanced finding on colonoscopy (36). Fourth, we explored how the threshold costs of CTC change when the cost of a colonoscopy increases. Next, to explore the implications of our assumption of a population of unscreened 65-year-olds, we repeated our analysis for a cohort of 50-year-olds with screening beginning at age 50 years as recommended in screening guidelines (23–25) and life-years and lifetime costs tallied from age 50 years.
Finally, we repeated the analysis of a cohort of previously unscreened 65-year-olds by using a modified societal perspective that included direct costs borne by beneficiaries and patient time costs. We labeled this a modified societal perspective because we did not incorporate productivity costs nor did we quality adjust the life-years associated with each strategy. Cost inputs for the modified societal perspective analysis are shown in Table 1 for the test costs and in Table 2 for the costs of complications from screening and of cancer treatment. We assumed that the (nonsleeping) patient times associated with screening were 8 hours for colonoscopy, 4 hours for sigmoidoscopy, 2 hours for CTC, and 1 hour for FOBT and that the average (nonsleeping) time associated with a complication was 16 hours. Annual patient time associated with colorectal cancer care was estimated by R. Yabroff and M. Brown (personal communication) using previously described methodology (49). The value of an hour of patient time was assigned the 2007 median wage rate from the Bureau of Labor Statistics of $17. We identified the threshold CTC costs from the modified societal perspective and then subtracted beneficiary copayments and patient time costs from these threshold costs to yield the corresponding CMS payment rates.
Results
Projected Undiscounted Outcomes
In the absence of screening, the models projected that 53–60 per 1000 individuals alive and free of colorectal cancer at age 65 years would be diagnosed with the disease during their lifetime (Table 3). With screening, many of these cases could be prevented. Assuming 100% adherence to screening, the reduction in colorectal cancer incidence projected by the three models ranged from 32% to 46% with annual Hemoccult II screening to 53%–85% with 10-yearly colonoscopy screening. In the SimCRC and CRC-SPIN models, compared with no screening, 10-yearly colonoscopy was the most effective strategy in terms of life-years gained (171 and 185 life-years gained, respectively, per 1000 65-year-olds). In the MISCAN model, the combination of 5-yearly flexible sigmoidoscopy with an annual highly sensitive FOBT (ie, Hemoccult SENSA or immunochemical FOBT) was the most effective strategy compared with no screening, saving 154 life-years per 1000 65-year-olds; with 10-yearly colonoscopy, 152 life-years were saved per 1000 65-year-olds screened. In all three models, 10-yearly colonoscopy screening was associated with the largest number of colonoscopies (range = 2674–2794 per 1000 65-year-olds). For the MISCAN model, annual screening with Hemoccult II was associated with the fewest number of colonoscopies (1217 per 1000 65-year-olds), followed by 5-yearly sigmoidoscopy with biopsy (1430 per 1000 65-year-olds). For the CRC-SPIN and SimCRC models, 5-yearly sigmoidoscopy screening with biopsy was associated with the fewest number of colonoscopies (791 and 901 per 1000 65-year-olds, respectively), followed by annual screening with Hemoccult II (823 and 955 per 1000 65-year-olds, respectively).
Table 3.
Total costs, number of life-years gained, and number of cases of colorectal cancer (CRC) per 1000 65-year-olds by screening strategy*
| Strategy | MISCAN | SimCRC | CRC-SPIN | |||||||||
| Total costs, million $ | LYG | No. of CRC cases | Reduction in cases, % | Total costs, million $ | LYG | No. of CRC cases | Reduction in cases, % | Total costs, million $ | LYG | No. of CRC cases | Reduction in cases, % | |
| No screening | 4.03 | 0 | 57 | — | 3.54 | 0 | 60 | — | 3.00 | 0 | 53 | — |
| HII yearly | 3.70 | 116.5 | 39 | 32 | 2.80 | 113.9 | 36 | 41 | 2.17 | 114.5 | 29 | 46 |
| HS yearly | 3.75 | 142.8 | 32 | 44 | 2.65 | 150.7 | 26 | 58 | 1.99 | 155.1 | 18 | 67 |
| IFOBT yearly | 3.82 | 141.0 | 33 | 42 | 2.75 | 148.3 | 27 | 55 | 2.11 | 150.4 | 20 | 63 |
| SIGB 5 yearly | 3.81 | 132.2 | 30 | 47 | 2.85 | 120.6 | 29 | 52 | 2.29 | 133.7 | 21 | 60 |
| SIG 5 yearly | 3.80 | 135.4 | 30 | 48 | 2.81 | 128.0 | 27 | 56 | 2.16 | 142.2 | 18 | 65 |
| HII yearly + SIGB 5 yearly | 3.67 | 149.1 | 29 | 50 | 2.64 | 157.7 | 23 | 63 | 2.15 | 163.7 | 15 | 72 |
| HII yearly + SIG 5 yearly | 3.86 | 149.9 | 28 | 51 | 2.62 | 160.1 | 22 | 64 | 2.06 | 166.7 | 14 | 74 |
| HS yearly + SIGB 5 yearly | 3.75 | 154.1 | 27 | 52 | 2.69 | 169.3 | 19 | 68 | 2.12 | 175.9 | 11 | 78 |
| HS yearly + SIG 5 yearly | 3.93 | 154.1 | 27 | 53 | 2.69 | 170.2 | 19 | 69 | 2.05 | 176.8 | 11 | 79 |
| IFOBT yearly + SIGB 5 yearly | 4.03 | 154.3 | 27 | 52 | 2.81 | 168.9 | 20 | 67 | 2.24 | 174.4 | 12 | 77 |
| IFOBT yearly + SIG 5 yearly | 4.00 | 154.3 | 27 | 52 | 2.80 | 169.9 | 19 | 68 | 2.16 | 175.8 | 11 | 78 |
| COL 10 yearly | 3.84 | 151.6 | 27 | 53 | 2.68 | 171.3 | 17 | 72 | 1.98 | 184.9 | 8 | 85 |
| CT colonography DoD 5 yearly† | 4.54 | 149.5 | 28 | 51 | 3.28 | 168.2 | 18 | 71 | 2.65 | 177.7 | 10 | 81 |
| CT colonography NCTC 5 yearly† | 4.59 | 142.7 | 30 | 48 | 3.35 | 160.2 | 20 | 67 | 2.70 | 172.2 | 11 | 78 |
— = not applicable; COL = colonoscopy; CRC-SPIN = Colorectal Cancer Simulated Population model for Incidence and Natural History; CT = computed tomographic; DoD = Department of Defense study parameters; HII = Hemoccult II; HS = Hemoccult SENSA; IFOBT = immunochemical fecal occult blood test; LYG = life-years gained compared with no screening; MISCAN = Microsimulation Screening Analysis model; NCTC = National CT Colonography Trial parameters; SIG = sigmoidoscopy without biopsy; SIGB = sigmoidoscopy with biopsy; SimCRC = Simulation Model of Colorectal Cancer.
† Six-millimeter threshold for colonoscopy referral.
When CTC test characteristics from the Department of Defense study (3) were used in the three simulation models, 5-yearly CTC screening with a 6-mm threshold for colonoscopy referral resulted in 2–7 fewer life-years gained and 672–1003 fewer colonoscopies per 1000 individuals compared with 10-yearly colonoscopy screening. Assuming that a CTC scan was reimbursed at $488 (only slightly less than the reimbursement for a colonoscopy without polypectomy), 5-yearly CTC screening was more costly than 10-yearly colonoscopy by approximately $600 000–$700 000 per 1000 65-year-olds. When CTC test characteristics from the National CTC Trial (5) were used in the three simulation models, 5-yearly CTC screening resulted in 9–13 fewer life-years gained and 992–1291 fewer colonoscopies per 1000 65-year-olds compared with 10-yearly colonoscopy screening, with an increase in costs similar to that obtained using the CTC test characteristics from the Department of Defense study.
Cost-Effectiveness Analysis
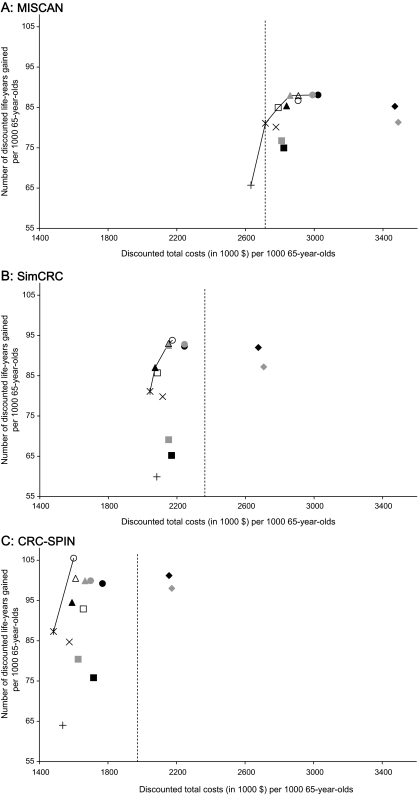
Figure 2 shows the numbers of discounted life-years gained compared with no screening, the discounted lifetime costs (from the CMS perspective), and the cost-efficient frontier showing the set of efficient (nondominated) strategies for the three simulation models. CTC screening using either set of test characteristics provided fewer discounted life-years gained than colonoscopy screening for all three models. Assuming a cost to CMS of $488 per CTC scan, all three models showed that CTC would be the most costly strategy. Although CTC screening at that test cost was dominated by at least one of the currently recommended screening options, the incremental cost per discounted life-year gained of CTC compared with no screening was less than $10 000 in all three models and for each set of CTC test characteristics and ranged from $1800 to $9500 (Appendix Table 2).
Figure 2.
Discounted costs and discounted life-years gained per 1000 65-year-olds screened for 14 colorectal cancer screening strategies and the efficient frontier connecting the efficient strategies. The two computed tomographic colonography (CTC) strategies are not competing options; they represent a range of CTC test characteristics. They are shown together for comparison purposes only. A) Microsimulation Screening Analysis (MISCAN). B) Simulation Model of Colorectal Cancer (SimCRC). C) Colorectal Cancer Simulated Population model for Incidence and Natural History (CRC-SPIN). COL = colonoscopy; DoD = Department of Defense study parameters; HII = Hemoccult II; HS = Hemoccult SENSA; IFOBT = immunochemical fecal occult blood test; NCTC = National CT Colonography Trial parameters; SIG = sigmoidoscopy without biopsy; SIGB = sigmoidoscopy with biopsy; y = years.
Threshold and Sensitivity Analyses
Threshold analyses indicated that for the base-case 5-yearly CTC strategies to be on the efficient frontier (ie, to be included among the set of efficient strategies), a CTC scan would have to cost $122–$199 using the test characteristics from the Department of Defense study and $108–$205 using the test characteristics from the National CTC Trial, depending on the simulation model used (Table 4). In sensitivity analyses that considered the CTC screening interval and the lesion size that triggers diagnostic follow-up, the ranges of threshold costs for CTC were wider than those using the two sets of test characteristics and the base-case assumptions of 5-yearly CTC with a 6-mm referral threshold for colonoscopy, but the threshold costs for CTC were still lower than the base-case cost estimate of $488 for all three models and never exceeded $371 (Table 4). Lengthening the screening interval to 10 years yielded higher threshold costs for CTC in the SimCRC model ($266 and $241 using test characteristics from the Department of Defense study and the National CTC Trial, respectively) and CRC-SPIN model ($352 and $371 for the two sets of test characteristics, respectively) and slightly lower threshold costs in the MISCAN model (to $103 and $108, respectively). In all three models, restricting follow-up colonoscopy to individuals with CTC findings of 10 mm or larger lowered threshold costs for CTC compared with the threshold costs from the base-case analysis. Decreasing the specificity of colonoscopy from 90% to 80% by assuming that one or more nonadenomatous polyp was removed for biopsy in 20% of colonoscopies modestly increased the threshold costs for CTC (data not shown).
Table 4.
Computed tomographic colonography (CTC) unit cost thresholds ($) at which CTC strategies are efficient screening options compared with other Medicare-reimbursed colorectal cancer screening strategies*
| Analysis† | DoD study test characteristics | NCTC trial test characteristics | ||||
| MISCAN | SimCRC | CRC-SPIN | MISCAN | SimCRC | CRC-SPIN | |
| Base-case analysis | ||||||
| 5-yearly CTC with 6-mm threshold for colonoscopy referral; equal adherence for all tests | 122 | 199 | 196 | 108 | 183 | 205 |
| Sensitivity analyses | ||||||
| 5-yearly CTC with 10-mm threshold for colonoscopy referral; equal adherence for all tests | 98 | 192‡ | 132‡ | 49 | 135‡ | 90‡ |
| 10-yearly CTC with 6-mm threshold for colonoscopy referral; equal adherence for all tests | 103 | 266 | 352 | 108 | 241‡ | 371 |
| 5-yearly CTC with 6-mm threshold for colonoscopy referral; relative adherence with CTC 10% higher than that with other tests | 293§ | 408§ | 360§ | 204§ | 286§ | 290§ |
| 5-yearly CTC with 6-mm threshold for colonoscopy referral; relative adherence with CTC 25% higher than that with other tests | 547§ | 694§ | 668§ | 433§ | 544§ | 571§ |
CRC-SPIN = Colorectal Cancer Simulated Population model for Incidence and Natural History; DoD = Department of Defense study; MISCAN = Microsimulation Screening Analysis model; NCTC = National CT Colonography Trial; SimCRC = Simulation Model of Colorectal Cancer.
See Table 1 for the test characteristics used in these analyses.
CTC strategy is on the efficient frontier as the least effective and least costly nondominated strategy if the cost is, at most, this amount.
CTC strategy is the most effective strategy evaluated. The threshold cost shown here is the cost at which the CTC strategy is on the efficient frontier with an incremental cost-effectiveness ratio of $50 000 per life-year gained.
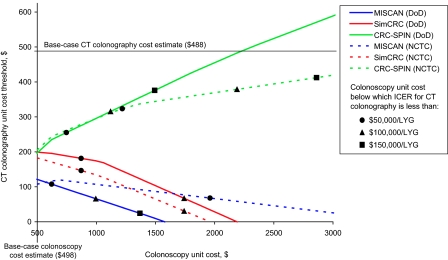
Results of a sensitivity analysis on the unit cost of a colonoscopy are shown in Figure 3. In the MISCAN and SimCRC models, threshold costs for CTC decreased as the cost of a colonoscopy increased and never exceeded the base-case estimate of $488. In the CRC-SPIN model, threshold costs for CTC increased as the cost of a colonoscopy increased and exceeded the base-case estimate of $488 per scan only when the unit cost of a colonoscopy was nearly 4.5 times higher than the base-case colonoscopy cost estimate of $498; at these higher colonoscopy costs, the incremental cost-effectiveness ratio for CTC screening exceeded $150 000 per discounted life-year gained.
Figure 3.
Computed tomographic (CT) colonography unit cost thresholds at which the base-case CT colonography strategies are efficient screening options compared with other reimbursed colorectal cancer screening strategies for different values for the unit cost of a colonoscopy without polypectomy. The CT colonography cost thresholds for unit colonoscopy costs as high as $4000 were calculated and used to fit the lines. The data points shown illustrate the colonoscopy cost below which the incremental cost-effectiveness ratio is less than reference values ($50 000, $100 000, and $150 000). CRC-SPIN = Colorectal Cancer Simulated Population model for Incidence and Natural History; DoD = Department of Defense study parameters; ICER = incremental cost-effectiveness ratio; LYG = discounted life-year gained; MISCAN = Microsimulation Screening Analysis; NCTC = National CT Colonography Trial parameters; SimCRC = Simulation Model of Colorectal Cancer.
These differences in estimated threshold costs for CTC across models are because of differences in the screening strategies that are efficient at each colonoscopy cost level and, thus, against which the CTC strategy was compared. The MISCAN and SimCRC models determined threshold costs for CTC relative to FOBT and sigmoidoscopy strategies. The CRC-SPIN model determined threshold costs for CTC relative to 10-yearly colonoscopy, the strategy that was associated with the largest number of colonoscopies and is therefore the strategy that is most sensitive to changes in the cost of a colonoscopy. We observed a pattern of increasing threshold costs for CTC similar to that observed with the CRC-SPIN model when we used the MISCAN and SimCRC models to estimate threshold costs for CTC relative to 10-yearly colonoscopy screening (data not shown).
When we considered the costs and health effects of colorectal cancer screening among a previously unscreened 50-year-old cohort, we found cost thresholds for CTC that ranged from $72 to $179 per scan (data not shown). This range was lower than the range found for a cohort of previously unscreened 65-year-olds. The cost thresholds did not differ between the two sets of CTC test characteristics.
Cost thresholds for CTC were lower when the analysis was performed from the modified societal perspective rather than from the CMS perspective. After subtracting patient copayments and time costs from the threshold costs from the modified societal perspective, the CMS payment rates ranged from $26 to $177 with the CTC test parameters from the Department of Defense study and less than $0 (ie, not cost-effective at any payment) to $181 with the test characteristics from the National CTC Trial.
If individuals who would not be screened otherwise would agree to be screened with CTC, the threshold costs would increase compared with the base-case estimates. With 10% of otherwise unscreened individuals adopting CTC screening (ie, an increase in overall adherence from 57% to 62.7%), CTC screening became the most effective strategy and threshold costs ranged from $204 to $408 (Table 4). With 25% of otherwise unscreened persons adopting CTC screening (ie, an increase in overall adherence from 57% to 71.3%), the threshold costs ranged from $433 to $694.
Discussion
We used three independently developed microsimulation models to evaluate the cost-effectiveness of CTC screening for colorectal cancer and found that the number of life-years gained from 5-yearly CTC (with referral of individuals with findings of ≥6 mm for a diagnostic colonoscopy) were similar to the number gained from 10-yearly colonoscopy screening, assuming perfect adherence to all tests. However, if CTC was reimbursed at $488 per test, slightly less than the reimbursement for a colonoscopy without polypectomy, the overall costs of the CTC strategies were greater than the costs of all of the other screening strategies considered, and CTC was therefore dominated by other screening strategies in the cost-effectiveness analysis.
At first, it may seem surprising that CTC was not cost-effective when compared with the other colorectal cancer screening tests, considering that the sensitivity of CTC for large adenomas and colorectal cancer was almost comparable to that of colonoscopy and that the base-case estimate of the cost to CMS per CTC scan was slightly less than the CMS reimbursement rate for colonoscopy. However, the recommended screening interval is 5 years with CTC vs 10 years with colonoscopy (21), and individuals with CTC findings measuring 6 mm or larger require a follow-up colonoscopy. Thus, even for people who never have an abnormality detected, the costs of CTC are incurred twice as frequently as the cost of colonoscopy, whereas those who have a positive finding on CTC accrue the cost of a diagnostic colonoscopy in addition to the cost of the screening CTC. We found that at a cost of $108–$205 per scan, CTC would be a cost-effective strategy for colorectal cancer screening, assuming that the CTC performance characteristics in the Department of Defense study and the National CTC Trial are achievable in routine clinical practice.
CTC is a rapidly evolving technology. After we presented these findings to CMS, the Munich Colorectal Cancer Prevention Trial (7), a prospective trial that compared the performance characteristics of CTC, colonoscopy, flexible sigmoidoscopy, immunochemical FOBT, and FOBT for the detection of advanced colonic neoplasia among 307 asymptomatic adults, reported a CTC specificity of 93% using a 5-mm threshold for referral for a follow-up colonoscopy, which is higher than the CTC specificity of 80% in the Department of Defense study and of 88% in the National CTC Trial (both with 6-mm thresholds) and CTC sensitivity estimates that were as high or higher than those in the Department of Defense study and the National CTC Trial. If these more favorable test characteristics are replicated in larger studies, then the threshold cost of a CT scan would increase. However, the increase is likely to be small because, as noted above, individuals with positive findings on CTC require a second procedure, the follow-up colonoscopy. Therefore, research on techniques to improve detection rates with CTC may be less important in informing future coverage decisions than studies that assess whether or not individuals who have yet to be screened for colorectal cancer deem CTC more acceptable than other colorectal cancer screening options. Our analysis showed that if the availability of CTC enticed 25% of otherwise unscreened individuals to be screened, CTC would be cost-effective at the base-case cost estimate of $488. If only 10% of unscreened persons adopted CTC screening, CTC would become cost-effective at $204–$408 per scan. To our knowledge, no clinical study has evaluated whether the addition of CTC to the menu of colorectal cancer screening options increases adherence among those who were previously unwilling to be screened.
Because colonoscopy reimbursement rates might be higher among non-Medicare providers, we evaluated how threshold costs for CTC changed as the cost of a colonoscopy increased. In two of the models, threshold costs for CTC decreased as the cost of a colonoscopy increased. In one model, threshold costs exceeded the base-case estimate of $488 per scan when the cost of a colonoscopy was 4.5 times higher than the base-case estimate, although at this high colonoscopy cost the incremental cost-effectiveness ratio for CTC screening was in excess of $150 000 per life-year gained.
There are several limitations to this analysis. First, we did not consider excess risks and costs that are associated with radiation exposure or with the detection of extracolonic findings on CTC. The magnitude of these risks and costs are unclear, but their inclusion would likely yield lower threshold costs for CTC. With regard to extracolonic findings on CTC, there has been considerable discussion about whether they represent an asset or liability (50–52). Longitudinal studies are needed to determine the long-term clinical outcomes and the potential benefits and harms associated with the spectrum of extracolonic diseases and conditions that become evident with CTC. At present, such data are not available. In the Department of Defense study (3), 56 (4.5%) of the 1233 subjects had extracolonic findings that were deemed highly important, and a higher proportion of subjects had findings that were deemed moderately important (total number with findings of moderate importance was not reported). In the National CTC Trial (5), 16% of subjects had a finding that was deemed to require follow-up or urgent care. Estimates of the costs associated with extracolonic findings vary considerably, ranging from $28 (53) to $248 (54) per person screened. Although there is uncertainty regarding the magnitude of the costs of extracolonic findings, their inclusion in analyses such as this one would likely result in lower threshold costs for CTC, even if treatment of the extracolonic findings led to a small gain in life expectancy.
Second, we did not quality adjust our estimates of the numbers of life-years. Although data on the quality of life among individuals with cancer have been reported (55), there is a lack of data on the quality-of-life implications of undergoing screening, follow-up, and surveillance procedures and, in particular, of waiting for test results.
Third, because there are no nationally representative longitudinal data to our knowledge concerning colorectal cancer screening patterns among individuals younger than 64 years, we assumed that all 65-year-olds were previously unscreened. Many individuals who are entering the Medicare program will have had prior colorectal cancer screening, whereas others will have had none; only those with no prior screening and those with no adenomas or cancer detected at a prior screening are eligible for average-risk screening. The first group may be at higher-than-average risk of harboring colorectal neoplasia, whereas the latter group would likely be at lower risk. The risk of colorectal cancer for a previously unscreened population may therefore not be very different from the true risk. If the risk of colorectal cancer for the Medicare-eligible population differs from that for a previously unscreened cohort, our estimates of the life-years gained from screening may be overstated or understated depending on the true risk. However, because the threshold costs of CTC are assessed by comparing the relative costs and life-years gained between screening strategies, the threshold CTC costs may not be greatly affected by the baseline colorectal cancer risk. Indeed, in the sensitivity analysis on a population (ie, 50-year-olds), the threshold CTC costs did not change substantially ($108–$205 for previously unscreened 65-year-olds vs $72–$179 for 50-year-olds).
Fourth, in the sensitivity analysis of screening adherence, we assumed that individuals either were fully adherent with a screening strategy or were never screened. This assumption is an oversimplification of what occurs in practice but is closer to reality than assuming that all individuals show up randomly to their scheduled screens. Furthermore, because of a lack of data on test-specific adherence patterns with repeated screening, we could not account for the fact that adherence may differ between stool-based and endoscopic tests. It is unclear which screening methods would have greater adherence, those that are less invasive but that require more frequent testing (eg, stool-based tests) or those that are more invasive but require less frequent testing (eg, endoscopic and radiological tests).
Fifth, we assumed conditional independence of repeat screenings (ie, no systematic false-negative or false-positive results on repeat screens). This is a reasonable assumption for the FOBTs because bleeding of a lesion is thought to be a random event and for CTC because it can detect lesions that may be difficult to find with endoscopy, such as those located on folds (56). It may be a less reasonable assumption for endoscopy, in which case our estimates of the effectiveness of endoscopic strategies could be overstated. However, because colonoscopy is used for follow-up of positive results on all other screening tests and for surveillance, the effectiveness of all strategies would be similarly overstated. Accordingly, the overall effect of the assumption of conditional independence on the threshold cost of CTC is unclear.
Sixth, patient time costs were based on assumptions because estimates were not available for most screening modalities. To our knowledge, patient time costs have only been assessed for one screening modality, that is, colonoscopy. Jonas et al. (57) reported a median time of 37 hours from initiation of colonic preparation to return to routine activities among 110 patients undergoing screening colonoscopy. This estimate is considerably higher than our estimate of 8 hours because it also includes the patients’ sleep time. Our estimates of the time spent with complications (16 hours) may also be underestimated. Although the costs of colonoscopy screening would be most affected by our assumptions about patient time, the costs of all strategies ultimately would be affected because colonoscopy is used for follow-up of positive findings on other tests and for surveillance. Accordingly, we do not expect that increasing our estimates of the amount of patient time involved with screening and complications would have a large impact on the threshold costs of CTC from the modified societal perspective.
Finally, although we performed sensitivity analyses on key parameters, we did not specify distributions around the uncertain parameters and sample from those distributions in a probabilistic sensitivity analysis. Instead, to address uncertainties among model parameters that relate to the natural history of colorectal cancer, we used multiple models that were developed independently before we performed the analyses presented here and as such, they provide a sensitivity analysis on the structural assumptions of the models. The models differ in their estimates of dwell time, that is, the total amount of time a clinically detected colorectal cancer spends in the adenoma and preclinical cancer phases. The dwell time in the MISCAN model is, on average, shorter than the dwell times in the SimCRC and CRC-SPIN models. On the basis of this difference, the MISCAN model estimates fewer life-years saved as a result of removing adenomas through screening compared with the SimCRC and CRC-SPIN models, and it estimates a greater benefit for shorter screening intervals for tests with relatively high sensitivities for detecting adenomas, such as colonoscopy and CTC than do the other models. Nevertheless, all three models came to similar conclusions about the cost-effectiveness and threshold costs of CTC screening, demonstrating the robustness of these results to uncertainties about the duration of the adenoma–carcinoma sequence.
Several other studies on the cost-effectiveness of CTC screening in the United States have been published (52,58–63), and their findings are summarized in Table 5. Because these studies used different unit cost estimates, we compared the findings by calculating the threshold cost of CTC as a percentage of the study-specific estimate of the cost of a colonoscopy. We refer to this as the threshold cost percentage. In all of these studies, the threshold cost percentages for CTC were higher than the 22%–41% found in this study. However, direct comparison of threshold costs percentages across studies is difficult for three reasons. First, the studies differ with respect to the strategies against which CTC screening was compared. We compared CTC screening with all other currently reimbursed and widely used test strategies, whereas most of the other studies compared CTC with colonoscopy or with colonoscopy, sigmoidoscopy, and Hemoccult II. In only one of the three models used in this analysis (CRC-SPIN), were the costs and health effects of the colonoscopy strategy relevant in assessing the threshold cost of a CTC scan. The recommended approach for conducting a cost-effectiveness analysis is to consider all relevant comparators (46,64). Second, the studies used different estimates of CTC test characteristics, different assumptions about which findings at CTC would trigger referral for a follow-up colonoscopy, and different screening intervals. Finally, the previous studies used different approaches for identifying the threshold cost of a CTC scan. In one study (61), threshold costs were identified so that the incremental cost per life-year gained for CTC compared with no screening was equal to the incremental cost per life-year gained for colonoscopy compared with no screening. In other studies, threshold costs were identified such that the incremental cost-effectiveness ratio of CTC screening compared with the next less effective strategy was less than a specified amount (60,63) or equal to the incremental cost-effectiveness ratio for colonoscopy screening (62), whereas in another study (59), threshold costs were assessed to yield a prohibitively high incremental cost-effectiveness ratio of colonoscopy compared with CTC. In yet another study (58), the threshold cost of CTC was calculated such that the total cost of the CTC strategy per life-year gained vs no screening equaled the total cost of the colonoscopy strategy per life-year gained vs no screening.
Table 5.
Overview of studies that estimated the cost-effectiveness of computed tomographic colonography (CTC) screening in the US population*
| First author, year of publication (reference) | Tests evaluated and screening intervals considered, y | Starting age of cohort, y | CTC sensitivity for adenomas, % | CTC specificity, % (referral threshold) | Screening strategy yielding greatest number of life-years | Method of assessing threshold cost per CTC scan† | CTC threshold cost, % of study-specific COL cost | |||||
| CTC | COL | HII | HS | IFOBT | SIG | |||||||
| Sonnenberg, 1999 (58) | 10 | 10 | — | — | — | — | 50 | Small: 80, medium: 80, large: 80 | 95 (0 mm) | COL 10 yearly | Cost that yields the same total cost/LYG‡ as COL | 46 |
| Ladabaum, 2004 (59) | 10 | 10 | — | — | — | — | 50 | Small: 87, medium: 87, large: 94 | 80 (0 mm) | COL 10 yearly | Cost below which ICER of COL vs CTC would exceed $230 000/LYG | 60 |
| Vijan, 2007 (60) | 5,10 | 10 | 1§ | — | — | 5 | 50 | Small: 46, medium: 83, large: 91 | 91 (0 mm) | CTC 5 yearly | Cost at which 5-yearly CTC is on efficient frontier with LCER of $50 000/LYG | 69 |
| Pickhardt, 2007 (61) | 10 | 10 | — | — | — | 10 | 50 | Small: 48, medium: 70, large: 85 | 86 (0 mm) | COL 10 yearly | Cost at which cost/LYG of CTC vs no screening is equal to the cost/LYG of COL vs no screening | 87 |
| Hassan, 2008║(52) | 10 | 10 | — | — | — | — | 50 | Small: NA, medium: 70, large: 85 | 86 (6 mm) | COL 10 yearly | Cost at which ICER of COL vs CTC is $51 000/LYG | 76 |
| Lansdorp-Vogelaar, 2009 (62) | 5,10,15,20 | 5,10,15,20 | — | — | — | — | 50 | Small: NA, medium: 66, large: 87 | 84 (6 mm) | COL 5 yearly | Cost at which 5-yearly CTC has the same ICER as 10-yearly COL | 43 |
| Pickhardt, 2009║ (63) | 5,10 | 10 | — | — | — | — | 65 | Small: NA, medium: 70, large: 90 | 86 (6 mm) | CTC 5 yearly | Cost below which ICER of 5-yearly CTC vs COL less than $50 000/LYG | 72 |
| Knudsen, this study (DoD parameters) | 5,10 | 10 | 1§ | 1§ | 1§ | 5 | 65 | Small: NA, medium: 84, large: 92 | 80 (6 mm) | HS yearly + SIGB 5 yearly (MISCAN); COL 10 yearly (SimCRC, CRC-SPIN) | Cost below which 5-yearly CTC is on the efficient frontier | 25–40 |
| Knudsen, this study (NCTC parameters) | 5,10 | 10 | 1§ | 1§ | 1§ | 5 | 65 | Small: NA, medium: 57, large: 84 | 88 (6 mm) | HS yearly + SIGB 5 yearly (MISCAN); COL 10 yearly (SimCRC, CRC-SPIN) | Cost below which 5-yearly CTC is on the efficient frontier | 22–41 |
— = Not evaluated; COL = colonoscopy; CTC = computed tomographic colonography; CRC-SPIN = Colorectal Cancer Simulated Population model for Incidence and Natural History; DoD = Department of Defense study; HII = Hemoccult II; HS = Hemoccult SENSA; ICER = incremental cost-effectiveness ratio; IFOBT = immunochemical fecal occult blood test; LYG = life-years gained; MISCAN = Microsimulation Screening Analysis model; NA = not applicable because lesion size is smaller than colonoscopy referral threshold of 6 mm; NCTC = National CT Colonography Trial; SIG = sigmoidoscopy; SIGB = sigmoidoscopy with biopsy; SimCRC = Simulation Model of Colorectal Cancer.
Not all studies reported threshold costs. In such cases, we calculated threshold costs using the data reported in the studies.
As opposed to incremental cost.
Evaluated alone and in conjunction with 5-yearly sigmoidoscopy screening.
The authors reported the costs and health effects associated with extracolonic findings on CTC. For comparison with the other studies shown here, these costs and health effects were not included.
In conclusion, with the ongoing discussions about modifying the health-care system in the United States, much emphasis is being placed on comparative effectiveness research. Some worry that comparative effectiveness research will lead to the rationing of care (65). This analysis highlights that comparative effectiveness research, and cost-effectiveness analyses in particular, can also be used to inform reimbursement levels. Our results suggest that CTC screening every 5 years provides a benefit in terms of life-years gained compared with no screening and provides only slightly fewer life-years gained than colonoscopy screening every 10 years. If CTC screening is reimbursed at roughly the same rate as colonoscopy, the cost, relative to the benefit derived and to the availability and costs of other colorectal cancer screening tests, is too high for it to be a cost-effective screening strategy. At the current test characteristics, CTC could be a cost-effective option for colorectal cancer screening among Medicare enrollees if the test cost was substantially less than that of colonoscopy or if its availability would entice a large proportion of otherwise unscreened persons to be screened.
Funding
Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (HHSP233200800231A [Memorial Sloan-Kettering Cancer Center], HHSP233200800323A [Erasmus MC], HHSP233200800270A [University of Minnesota], and HHSP233200800234A [Group Health Cooperative]). The models used in the analysis were supported by awards from the National Cancer Institute (NCI) (U01-CA-088204, U01-CA-097426, U01-CA-097427, and U01-CA-115953).
Supplementary Material
Appendix
Appendix Table 1.
Comparison of the Microsimulation Screening Analysis (MISCAN), Simulation Model of Colorectal Cancer (SimCRC), and Colorectal Cancer Simulated Population model for Incidence and Natural History (CRC-SPIN) models on natural history outcomes at age 65 years
| Outcome | MISCAN | SimCRC | CRC-SPIN |
| Adenoma prevalence, age 65 y, % | 39.8 | 37.2 | 30.7 |
| Number of adenomas per 1000 by site and size at age 65 y | |||
| Proximal colon | |||
| 1–5 mm | 121.2 | 171.7 | 190.2 |
| 6–9 mm | 69.9 | 186.2 | 67.8 |
| 1–10 mm | 61.8 | 23.9 | 40.8 |
| Distal colon | |||
| 1–5 mm | 134.4 | 124.2 | 124.5 |
| 6–9 mm | 77.4 | 18.2 | 44.4 |
| ≥10 mm | 68.4 | 41.6 | 26.7 |
| Rectum | |||
| 1–5 mm | 133.5 | 8.7 | 14.1 |
| 6–9 mm | 76.8 | 16.0 | 9.1 |
| ≥10 mm | 68.1 | 15.8 | 20.2 |
| Distribution of adenomas by site and size at age 65 y, % | |||
| Proximal colon | |||
| 1–5 mm | 15 | 28 | 35 |
| 6–9 mm | 9 | 31 | 13 |
| ≥10 mm | 8 | 4 | 8 |
| Total | 31 | 63 | 56 |
| Distal colon | |||
| 1–5 mm | 17 | 20 | 23 |
| 6–9 mm | 10 | 3 | 8 |
| ≥10 mm | 8 | 7 | 5 |
| Total | 35 | 30 | 36 |
| Rectum | |||
| 1–5 mm | 16 | 1 | 3 |
| 6–9 mm | 9 | 3 | 2 |
| ≥10 mm | 8 | 3 | 4 |
| Total | 34 | 7 | 8 |
| Cumulative incidence of colorectal cancer among those cancer free at age 65 y, % | |||
| 10 y | |||
| Stage I | 0.4 | 0.4 | 0.3 |
| Stage II | 0.7 | 0.7 | 0.7 |
| Stage III | 0.5 | 0.5 | 0.5 |
| Stage IV | 0.5 | 0.5 | 0.3 |
| Total | 2.1 | 2.2 | 1.8 |
| 20 y | |||
| Stage I | 0.8 | 0.8 | 0.7 |
| Stage II | 1.6 | 1.5 | 1.4 |
| Stage III | 1.0 | 1.0 | 1.0 |
| Stage IV | 1.0 | 1.2 | 0.7 |
| Total | 4.4 | 4.6 | 3.9 |
| Lifetime | |||
| Stage I | 1.0 | 1.0 | 0.9 |
| Stage II | 2.1 | 2.0 | 1.9 |
| Stage III | 1.3 | 1.4 | 1.4 |
| Stage IV | 1.3 | 1.6 | 1.0 |
| Total | 5.7 | 6.0 | 5.3 |
Appendix Table 2.
Discounted costs and discounted life-years gained per 1000 65-year-olds without colorectal cancer screening and with 14 colorectal cancer screening strategies and associated incremental and average cost-effectiveness ratios*
| Strategy | MISCAN | SimCRC | CRC-SPIN | |||||||||
| Discounted costs, million $ | Discounted LYG | ICER, $ | Cost/LYG vs no screening, $ | Discounted costs, million $ | Discounted LYG | ICER, $ | Cost/LYG vs no screening, $ | Discounted costs, million $ | Discounted LYG | ICER, $ | Cost/LYG vs no screening, $ | |
| No screening | 2.715 | 0 | d | NA | 2.368 | 0 | d | NA | 1.977 | 0 | d | NA |
| HII yearly | 2.632 | 65.7 | — | CS | 2.083 | 59.9 | d | CS | 1.536 | 64.0 | d | CS |
| HS yearly | 2.716 | 81.1 | 5500 | 10 | 2.043 | 81.1 | — | CS | 1.482 | 87.3 | — | CS |
| IFOBT yearly | 2.777 | 80.1 | d | 800 | 2.117 | 79.8 | d | CS | 1.575 | 84.7 | d | CS |
| SIGB 5 yearly | 2.823 | 75.0 | d | 1400 | 2.169 | 65.2 | d | CS | 1.716 | 75.8 | d | CS |
| SIG 5 yearly | 2.810 | 76.7 | d | 1200 | 2.152 | 69.1 | d | CS | 1.626 | 80.4 | d | CS |
| HII yearly + SIGB 5 yearly | 2.791 | 84.9 | 19 400 | 900 | 2.086 | 85.7 | d | CS | 1.656 | 92.9 | d | CS |
| HII yearly + SIG 5 yearly | 2.839 | 85.4 | d | 1500 | 2.073 | 87.0 | 5100 | CS | 1.590 | 94.5 | d | CS |
| HS yearly + SIGB 5 yearly | 2.860 | 88.0 | 22 900 | 1700 | 2.152 | 92.5 | d | CS | 1.667 | 99.9 | d | CS |
| HS yearly + SIG 5 yearly | 2.907 | 87.9 | d | 2200 | 2.151 | 93.0 | 12 900 | CS | 1.611 | 100.5 | d | CS |
| IFOBT yearly + SIGB 5 yearly | 3.022 | 88.1 | d | 3500 | 2.244 | 92.3 | d | CS | 1.769 | 99.2 | d | CS |
| IFOBT yearly + SIG 5 yearly | 2.991 | 88.1 | 988 700 | 3100 | 2.245 | 92.8 | d | CS | 1.699 | 99.9 | d | CS |
| COL 10 yearly | 2.906 | 86.7 | d | 2200 | 2.174 | 93.8 | 27 700 | CS | 1.600 | 105.5 | 6500 | CS |
| CTC DoD 5 yearly† | 3.470 | 85.3 | d | 8900 | 2.675 | 92.0 | d | 3300 | 2.157 | 101.2 | d | 1800 |
| CTC NCTC 5 yearly† | 3.489 | 81.3 | d | 9500 | 2.706 | 87.2 | d | 3900 | 2.173 | 98.0 | d | 2000 |
— = default strategy (ie, the least costly and least effective nondominated strategy); COL = colonoscopy; CS = cost saving; CTC = computed tomographic colonography; CRC-SPIN = Colorectal Cancer Simulated Population model for Incidence and Natural History; d = dominated; DoD = Department of Defense study parameters; HII = Hemoccult II; HS = Hemoccult SENSA; ICER = incremental cost-effectiveness ratio; IFOBT = immunochemical fecal occult blood test; LYG = life-years gained compared with no screening; MISCAN = Microsimulation Screening Analysis; NA = not applicable; NCTC = National CT Colonography Trial parameters; SIG = sigmoidoscopy without biopsy; SIGB = sigmoidoscopy with biopsy; SimCRC = Simulation Model of Colorectal Cancer.
The two CTC strategies are not competing options; they represent a range of estimates of CTC test characteristics. They are shown here together for comparison purposes only. The ICERs are assessed separately using each CTC strategy in turn.
Footnotes
Neither AHRQ nor the NCI had any role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The findings and conclusions are those of the authors. No statement in this article should be construed as an official position of AHRQ, NCI, the National Institutes of Health, or the US Department of Health and Human Services.
We acknowledge Martin Brown, PhD, and Robin Yabroff, PhD, of the NCI for their assistance with obtaining cancer treatment costs using SEER–Medicare data; Joan Warren, PhD, and Carrie Klabunde, PhD, of the NCI for sharing their preliminary analysis of SEER–Medicare data on colonoscopy-related complications; John Allen, MD, of Minnesota Gastroenterology and Joel Brill, MD, of Predictive Health for their assistance in deriving coding for screening and complications; Beth McFarland, MD, of the Center of Diagnostic Imaging and Pamela Kassing, MS, of the American College of Radiology for assistance in coding and costs for CTC; William Lawrence, MD, and Kim Wittenberg, MA, of AHRQ for contextual and administrative assistance, respectively; William Larson, MA, of CMS, and Chuck Shih, MHS, of the Johns Hopkins School of Public Health for providing Medicare cost data; and Eric (Rocky) Feuer, PhD, of the NCI for continued support of the work and infrastructure of the CISNET consortium. We thank the following individuals for helpful comments and review of earlier versions of this article: Robert Fletcher, MD, MSc, of Harvard Medical School; G. Scott Gazelle, MD, MPH, PhD, of the MGH Institute for Technology Assessment; Uri Ladabaum, MD, and Judy Yee, MD, of the University of California, San Francisco; Michael Pignone, MD, MPH, and David Ransohoff, MD, of the University of North Carolina; and Paul Pinsky, PhD, of the NCI.
References
- 1.Sosna J, Morrin MM, Kruskal JB, et al. CT colonography of colorectal polyps: a metaanalysis. AJR Am J Roentgenol. 2003;181(6):1593–1598. doi: 10.2214/ajr.181.6.1811593. [DOI] [PubMed] [Google Scholar]
- 2.van Dam J, Cotton P, Johnson CD, et al. AGA future trends report: CT colonography. Gastroenterology. 2004;127(3):970–984. doi: 10.1053/j.gastro.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349(23):2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 4.Rosman AS, Korsten MA. Meta-analysis comparing CT colonography, air contrast barium enema, and colonoscopy. Am J Med. 2007;120(3):203–210. doi: 10.1016/j.amjmed.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 5.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359(12):1207–1217. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitlock EP, Lin JS, Liles E, et al. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):638–658. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 7.Graser A, Stieber P, Nagel D, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009;58(2):241–248. doi: 10.1136/gut.2008.156448. [DOI] [PubMed] [Google Scholar]
- 8.Taylor SA, Halligan S, Saunders BP, et al. Acceptance by patients of multidetector CT colonography compared with barium enema examinations, flexible sigmoidoscopy, and colonoscopy. AJR Am J Roentgenol. 2003;181(4):913–921. doi: 10.2214/ajr.181.4.1810913. [DOI] [PubMed] [Google Scholar]
- 9.Thomeer M, Bielen D, Vanbeckevoort D, et al. Patient acceptance for CT colonography: what is the real issue? Eur Radiol. 2002;12(6):1410–1415. doi: 10.1007/s003300101082. [DOI] [PubMed] [Google Scholar]
- 10.Clark JC, Collan Y, Eide TJ, et al. Prevalence of polyps in an autopsy series from areas with varying incidence of large-bowel cancer. Int J Cancer. 1985;36(2):179–186. doi: 10.1002/ijc.2910360209. [DOI] [PubMed] [Google Scholar]
- 11.Blatt L. Polyps of the colon and rectum: incidence and distribution. Dis Colon Rectum. 1961;4(4):277–282. [Google Scholar]
- 12.Arminski TC, McLean DW. Incidence and distribution of adenomatous polyps of the colon and rectum based on 1,000 autopsy examinations. Dis Colon Rectum. 1964;7(4):249–261. doi: 10.1007/BF02630528. [DOI] [PubMed] [Google Scholar]
- 13.Vatn MH, Stalsberg H. The prevalence of polyps of the large intestine in Oslo: an autopsy study. Cancer. 1982;49(4):819–825. doi: 10.1002/1097-0142(19820215)49:4<819::aid-cncr2820490435>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 14.Jass JR, Young PJ, Robinson EM. Predictors of presence, multiplicity, size and dysplasia of colorectal adenomas. A necropsy study in New Zealand. Gut. 1992;33(11):1508–1514. doi: 10.1136/gut.33.11.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johannsen LG, Momsen O, Jacobsen NO. Polyps of the large intestine in Aarhus, Denmark. An autopsy study. Scand J Gastroenterol. 1989;24(7):799–806. doi: 10.3109/00365528909089217. [DOI] [PubMed] [Google Scholar]
- 16.Bombi JA. Polyps of the colon in Barcelona, Spain. An autopsy study. Cancer. 1988;61(7):1472–1476. doi: 10.1002/1097-0142(19880401)61:7<1472::aid-cncr2820610734>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut. 1982;23(10):835–842. doi: 10.1136/gut.23.10.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rickert RR, Auerbach O, Garfinkel L, et al. Adenomatous lesions of the large bowel: an autopsy survey. Cancer. 1979;43(5):1847–1857. doi: 10.1002/1097-0142(197905)43:5<1847::aid-cncr2820430538>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 19.Chapman I. Adenomatous polypi of large intestine: incidence and distribution. Ann Surg. 1963;157(2):223–226. doi: 10.1097/00000658-196302000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Surveillance Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 9 Regs Public-Use, November 2003 Sub (1973-2001). www.seer.cancer.gov. Accessed March 1, 2005. [Google Scholar]
- 21.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 22. Colorectal cancer screening tests: conditions for and limitations on coverage. In: 62 Federal Register 59100; October 31, 1997, as amended at 66 FR 55329, November 1, 2001; 67 FR 80040, December 31, 2002. [Google Scholar]
- 23.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology. 2003;124(2):544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 24.Smith RA, Cokkinides V, Eyre HJ. American Cancer Society guidelines for the early detection of cancer, 2006. CA Cancer J Clin. 56(1):11–25. doi: 10.3322/canjclin.56.1.11. quiz 49–50. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 26.Robertson RH, Burkhardt JH, Powell MP, et al. Trends in colon cancer screening procedures in the US Medicare and Tricare populations: 1999-2001. Prev Med. 2006;42(6):460–462. doi: 10.1016/j.ypmed.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 27.El-Serag HB, Petersen L, Hampel H, et al. The use of screening colonoscopy for patients cared for by the Department of Veterans Affairs. Arch Intern Med. 2006;166(20):2202–2208. doi: 10.1001/archinte.166.20.2202. [DOI] [PubMed] [Google Scholar]
- 28.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130(6):1872–1885. doi: 10.1053/j.gastro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin. 2006;56(3):143–159. doi: 10.3322/canjclin.56.3.143. quiz 184–185. [DOI] [PubMed] [Google Scholar]
- 30.Zauber AG, Lansdorp-Vogelaar I, Wilschut J, et al. 2007. Cost-Effectiveness of DNA Stool Testing to Screen for Colorectal Cancer: Report to AHRQ and CMS from the Cancer Intervention and Surveillance Modeling Network (CISNET) for MISCAN and SimCRC Models. https://www.cms.hhs.gov/mcd/viewtechassess.asp?from2=viewtechassess.asp&id=212& Accessed July 1, 2009. [PubMed] [Google Scholar]
- 31.van Rijn JC, Reitsma JB, Stoker J, et al. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101(2):343–350. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 32.Doria-Rose VP, Levin TR, Selby JV, et al. The incidence of colorectal cancer following a negative screening sigmoidoscopy: implications for screening interval. Gastroenterology. 2004;127(3):714–722. doi: 10.1053/j.gastro.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 33.Adam AS. How accurate is the endoscopist's assessment of visualization of the left colon seen at flexible sigmoidoscopy? Colorectal Dis. 2000;2(1):41–44. doi: 10.1046/j.1463-1318.2000.00091.x. [DOI] [PubMed] [Google Scholar]
- 34.American Joint Committee on Cancer. Manual for Staging of Cancer. 3rd ed. Philadelphia, PA: JB Lippincott Co; 1988. [Google Scholar]
- 35.Gatto NM, Frucht H, Sundararajan V, et al. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;95(3):230–236. doi: 10.1093/jnci/95.3.230. [DOI] [PubMed] [Google Scholar]
- 36.Lieberman DA, Weiss DG, Bond JH, et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 37.Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355(18):1863–1872. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 38.Pox C, Schmiegel W, Classen M. Current status of screening colonoscopy in Europe and in the United States. Endoscopy. 2007;39(2):168–173. doi: 10.1055/s-2007-966182. [DOI] [PubMed] [Google Scholar]
- 39.Levin TR, Conell C, Shapiro JA, et al. Complications of screening flexible sigmoidoscopy. Gastroenterology. 2002;123(6):1786–1792. doi: 10.1053/gast.2002.37064. [DOI] [PubMed] [Google Scholar]
- 40.Levin TR, Zhao W, Conell C, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145(12):880–886. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]
- 41.Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150(12):849–857. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
- 42. Federal Register Vol. 71. No. 196, Wednesday, October 11, 2006, p 59886-60042. Medicare Program; Changes to the Hospital Inpatient Prospective Payment Systems and Fiscal Year 2007 Rates; Final Fiscal Year 2007 Wage Indices and Payment Rates After Application of Revised Occupational Mix Adjustment to the Wage Index; Corrections. [Google Scholar]
- 43. Federal Register Vol. 71. No. 226, Friday, November 24, 2006, p 67960-68401. Medicare Program: Revisions to Hospital Outpatient Prospective Payment System and Calendar Year 2007 Payment Rates; Final Rule. [PubMed] [Google Scholar]
- 44.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100(9):630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 45.U.S. Census Bureau. Statistical Abstract of the United States: 2009. 128th ed. Section 14: Prices, Table 703. Consumer Price Indexes (CPI-U) by Major Groups: 1990 to 2007. Washington, DC: US Government Printing Office; 2008. http://www.census.gov/compendia/statab/2009/2009edition.html. Accessed June 24, 2010. [Google Scholar]
- 46.Gold MR, Siegel JE, Russell LB, et al. Cost-effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 47.Mark DH. Visualizing cost-effectiveness analysis. JAMA. 2002;287(18):2428–2429. doi: 10.1001/jama.287.18.2428. [DOI] [PubMed] [Google Scholar]
- 48.Shapiro JA, Seeff LC, Thompson TD, et al. Colorectal cancer test use from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17(7):1623–1630. doi: 10.1158/1055-9965.EPI-07-2838. [DOI] [PubMed] [Google Scholar]
- 49.Yabroff KR, Warren JL, Knopf K, et al. Estimating patient time costs associated with colorectal cancer care. Med Care. 2005;43(7):640–648. doi: 10.1097/01.mlr.0000167177.45020.4a. [DOI] [PubMed] [Google Scholar]
- 50.Pickhardt PJ, Hanson ME, Vanness DJ, et al. Unsuspected extracolonic findings at screening CT colonography: clinical and economic impact. Radiology. 2008;249(1):151–159. doi: 10.1148/radiol.2491072148. [DOI] [PubMed] [Google Scholar]
- 51.Fletcher RH, Pignone M. Extracolonic findings with computed tomographic colonography: asset or liability? Arch Intern Med. 2008;168(7):685–686. doi: 10.1001/archinte.168.7.685. [DOI] [PubMed] [Google Scholar]
- 52.Hassan C, Pickhardt PJ, Laghi A, et al. Computed tomographic colonography to screen for colorectal cancer, extracolonic cancer, and aortic aneurysm: model simulation with cost-effectiveness analysis. Arch Intern Med. 2008;168(7):696–705. doi: 10.1001/archinte.168.7.696. [DOI] [PubMed] [Google Scholar]
- 53.Yee J, Kumar NN, Godara S, et al. Extracolonic abnormalities discovered incidentally at CT colonography in a male population. Radiology. 2005;236(2):519–526. doi: 10.1148/radiol.2362040166. [DOI] [PubMed] [Google Scholar]
- 54.Kimberly JR, Phillips KC, Santago P, et al. Extracolonic findings at virtual colonoscopy: an important consideration in asymptomatic colorectal cancer screening. J Gen Intern Med. 2009;24(1):69–73. doi: 10.1007/s11606-008-0835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramsey SD, Andersen MR, Etzioni R, et al. Quality of life in survivors of colorectal carcinoma. Cancer. 2000;88(6):1294–1303. [PubMed] [Google Scholar]
- 56.Pickhardt PJ, Nugent PA, Mysliwiec PA, et al. Location of adenomas missed by optical colonoscopy. Ann Intern Med. 2004;141(5):352–359. doi: 10.7326/0003-4819-141-5-200409070-00009. [DOI] [PubMed] [Google Scholar]
- 57.Jonas DE, Russell LB, Sandler RS, et al. Patient time requirements for screening colonoscopy. Am J Gastroenterol. 2007;102(11):2401–2410. doi: 10.1111/j.1572-0241.2007.01387.x. [DOI] [PubMed] [Google Scholar]
- 58.Sonnenberg A, Delco F, Bauerfeind P. Is virtual colonoscopy a cost-effective option to screen for colorectal cancer? Am J Gastroenterol. 1999;94(8):2268–2274. doi: 10.1111/j.1572-0241.1999.01304.x. [DOI] [PubMed] [Google Scholar]
- 59.Ladabaum U, Song K, Fendrick AM. Colorectal neoplasia screening with virtual colonoscopy: when, at what cost, and with what national impact? Clin Gastroenterol Hepatol. 2004;2(7):554–563. doi: 10.1016/s1542-3565(04)00247-2. [DOI] [PubMed] [Google Scholar]
- 60.Vijan S, Hwang I, Inadomi J, et al. The cost-effectiveness of CT colonography in screening for colorectal neoplasia. Am J Gastroenterol. 2007;102(2):380–390. doi: 10.1111/j.1572-0241.2006.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pickhardt PJ, Hassan C, Laghi A, et al. Cost-effectiveness of colorectal cancer screening with computed tomography colonography: the impact of not reporting diminutive lesions. Cancer. 2007;109(11):2213–2221. doi: 10.1002/cncr.22668. [DOI] [PubMed] [Google Scholar]
- 62.Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, et al. At what costs will screening with CT colonography be competitive? A cost-effectiveness approach. Int J Cancer. 2009;124(5):1161–1168. doi: 10.1002/ijc.24025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pickhardt PJ, Hassan C, Laghi A, et al. CT colonography to screen for colorectal cancer and aortic aneurysm in the Medicare population: cost-effectiveness analysis. AJR Am J Roentgenol. 2009;192(5):1332–1340. doi: 10.2214/AJR.09.2646. [DOI] [PubMed] [Google Scholar]
- 64.Hunink M, Glasziou P, Siegel J, et al. Decision Making in Health and Medicine: Integrating Evidence and Values. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- 65.Avorn J. Debate about funding comparative-effectiveness research. N Engl J Med. 2009;360(19):1927–1929. doi: 10.1056/NEJMp0902427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.