Abstract
Autism is a behaviorally defined neurodevelopmental disorder and among its symptoms are disturbances in face and emotional processing. Emerging evidence demonstrates abnormalities in the GABAergic (gamma-aminobutyric acid) system in autism, which likely contributes to these deficits. GABAB receptors play an important role in modulating synapses and maintaining the balance of excitation-inhibition in the brain. The density of GABAB receptors in subjects with autism and matched controls was quantified in the anterior and posterior cingulate cortex, important for socio-emotional and cognitive processing, and the fusiform gyrus, important for identification of faces and facial expressions. Significant reductions in GABAB receptor density were demonstrated in all three regions examined suggesting that alterations in this key inhibitory receptor subtype may contribute to the functional deficits in individuals with autism. Interestingly, the presence of seizure in a subset of autism cases did not have a significant effect on the density of GABAB receptors in any of the three regions.
Keywords: GABA, Anterior Cingulate, Posterior Cingulate, autistic, seizure
Introduction
Autism is a pervasive developmental disorder (PDD) that shares many clinical characteristics with other PDDs such as Asperger syndrome and Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS; Volkmar et al., 1996). The shared phenotypes of these disorders suggest some common neurobiological and genetic mechanisms. The core features of the disorder include restricted and repetitive behaviors, delayed language, and abnormal socio-emotional behaviors (APA, 1994). Although the etiology of autism is not known, there is growing consensus that the disorder, which ranges from mild to severe, results from a combination of genetic and environmental components (Fombonne, 1999). An important consideration when thinking about the neurobiology of the disorder is whether the multitude of symptoms is the result of a number of developmental “insults” to multiple regions of the brain, or if one “insult” results in a multitude of symptoms.
Neuropathology has been reported in the cerebellum, limbic system, and fusiform gyrus. Postmortem neuropathological studies have found reduced numbers of Purkinje cells (Bauman and Kemper, 1985; Ritvo et al., 1986; Bailey et al., 1998; Fatemi et al., 2002; Whitney et al., 2008), abnormal levels of glutamic acid decarboxylase (GAD) 65 and 67 (Fatemi et al., 2002) and GAD 65/67 mRNA levels (Yip et al., 2007, 2008, 2009), GABA receptors in the cerebellum (Fatemi et al., 2002), decreased neuron size and increased cell-packing density (Bauman and Kemper, 1985) in the hippocampus and anterior cingulate cortex (Simms et al., 2009), increased relative density of GABAergic interneurons in the hippocampus (Lawrence et al., 2010), and a reduced number of neurons in the lateral amygdala (Schumann and Amaral, 2006) and fusiform gyrus (Van Kooten et al., 2008). Bailey and colleagues (1998) and Simms et al. (2009) have also reported atypical laminar patterns in the frontal cortex and anterior cingulate gyrus, respectively. The abnormal cytoarchitecture observed in the autistic brain is indicative of an early developmental insult within these brain regions.
Functionally, the ACC has been associated with the pain system (Craig et al., 1996), regulation of attention (Botvinick et al., 1999), emotion (Davidson et al., 1999), vocalization (Jurgens and Ploog, 1970), cognition (MacDonald et al., 2000), and reward expectancy (Shidara and Richmond, 2002). The posterior cingulate cortex appears preferentially involved in visuospatial cognition (Olson et al., 1992; 1996), and is part of a network recruited when typically developing subjects see the faces or hear the voices of emotionally significant people in their lives (Maddock, 2001) and modulates emotion by responding to emotional scripts and faces (Mayberg et al., 1999). Individuals with autism are known to have difficulties in the perception of faces, direction of eye gaze, lack of eye contact and are impaired in face recognition abilities failing to use eye gaze and facial expression to regulate social interaction (Braverman et al., 1989; Davies et al., 1994; Joseph and Tanaka, 2003).
Functional brain imaging studies have described an extensive neural network implicated in face processing in humans. This network includes the fusiform gyrus, the superior temporal sulcus, anterior temporal pole, amygdala, orbitofrontal cortex, retrosplenial cortex, and the anterior and posterior cingulate cortices (Kanwisher et al., 1997; Shah et al., 2001). Several neuroimaging studies have found that individuals with autism display hypoactivation of the fusiform gyrus when compared to controls during a face recognition task (Schultz et al., 2000; Critchley et al., 2000; Pierce et al., 2001) but, there are also reports of normal activation of the fusiform gyrus during face processing tasks in autism (Pierce et al., 2004; Hadjikhani et al., 2004; Dalton et al., 2005).
Schultz et al. (2000) hypothesized that pathology in the fusiform gyrus may account for the hypoactivation during face processing. However, more recently Schultz’s group and others have suggested the hypoactivation of the FFG is a consequence of abnormalities of regions within the face-processing circuit (Grelotti et al., 2002, 2005). Therefore it is important to determine if neuropathology exists in the FFG and/or if areas conveying information about emotional salience (ACC, PCC) may contribute to the deficits observed in face-processing in autism. Evidence is mounting that the GABAergic system is affected in multiple brain regions in adults with autism (Blatt et al., 2001; Fatemi et al., 2002, 2009a, b; Guptill et al., 2007; Yip et al., 2007, 2008, 2009; Oblak et al., 2009a,b; Lawrence et al., 2010).
GABA is the main inhibitory neurotransmitter in the brain and is important for proper cortical and synapse formation during development. GABAA and GABAC receptors are ligand gated ion channels and GABAB receptors are metabotropic. Activation of presynaptic GABAB receptors, inhibits the release of neurotransmitters and neuropeptides via inhibition of Ca2+ channels. Postsynaptic GABAB receptors activate inwardly rectifying potassium channels and induce the slow, long-lasting component of inhibitory postsynaptic potentials, the fast component of which is mediated through GABAA receptors.
GABA dysregulation has been suggested to play a key role in the increased rate of seizures in autism and others have suggested an imbalance of GABA and glutamate in autism, (Rubenstein and Merzenich, 1998; Hussman, 2001). All reports of decreased GABA receptors in autism have targeted GABAA receptors and benzodiazepine binding sites (Blatt et al., 2001; Guptill et al., 2007; Oblak et al., 2009a, b). Fatemi et al. (2009a, b) has provided further molecular evidence by showing decreased protein levels of both GABAA and GABAB subunits in the cerebellum and cortex of individuals with autism. These results raise the question as to whether there are consistent and common alterations in the GABA system throughout affected areas in autism. The current study utilized on-the-slide ligand binding autoradiographic techniques to examine and quantify the density of the GABAB receptors in three areas involved in socio-emotional and face-processing behaviors, the anterior and posterior cingulate cortex, and fusiform gyrus.
Methods and Materials
Brain Tissue
Fresh frozen brain tissue from the anterior and posterior cingulate cortices and fusiform gyrus was obtained from The Autism Research Foundation, the Autism Tissue Program, Harvard Brain Tissue Resource Center, and the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD. A total of thirty-five blocks were obtained (15 autistic and 19 controls) and stored at −80°C. A summary of the case details is seen in Table 1. There was no significant difference in age or post-mortem interval between autism and control groups (student t-test).
Table 1.
Anterior Cingulate, Posterior Cingulate, and Fusiform Gyrus case information.
| CASE | DIAGNOSIS | AGE | PMI (HOURS) | CAUSE OF DEATH | GENDER | AREA | ||
|---|---|---|---|---|---|---|---|---|
| ACC | PCC | FFG | ||||||
| 1078*† | Autism | 22 | 14.3 | Drowning | Male | X | ||
| 1401 | Autism | 21 | 20.6 | Sepsis | Female | X | X | |
| 1484* | Autism | 19 | 15 | Burns | Male | X | X | |
| 1664 | Autism | 20 | 15 | Unknown | Male | X | ||
| 2825* ▲ | Autism | 19 | 9.5 | Heart attack | Male | X | X | |
| 3845*‡ | Autism | 30 | 28.4 | Cancer | Male | X | X | |
| 4099 | Autism | 19 | 3 | Congestive Heart Failure | Male | X | X | |
| 4899 | Autism | 14 | 9 | Drowning | Male | X | ||
| 5000 | Autism | 27 | 8.3 | Drowning | Male | X | ||
| 5027 | Autism | 37 | 26 | Bowel Obstruction | Male | X | ||
| 5144 | Autism | 20 | 23.7 | Auto Trauma | Male | X | ||
| 5173* ◇ | Autism | 30 | 20.3 | GI bleeding | Male | X | ||
| 5754 | Autism | 20 | 29.98 | Unknown | Male | X | X | |
| 6337* | Autism | 22 | 25 | Choked | Male | X | ||
| 6677* | Autism | 30 | 16 | Congestive heart failure | Male | X | ||
| 602 | Control | 27 | 15 | Accident | Male | X | ||
| 1026 | Control | 28 | 6 | Congenital Heart Disease | Male | X | ||
| 1365 | Control | 28 | 17 | Multiple Injuries | Male | X | ||
| 4103 | Control | 43 | 23 | Heart attack/disease | Male | X | X | |
| 4104 | Control | 24 | 5 | Gun shot | Male | X | X | |
| 4188 | Control | 16 | 13 | Gun Shot | Male | X | ||
| 4267 | Control | 26 | 20 | Accidental | Male | X | X | |
| 4268 | Control | 30 | 22 | Heart attack/disease | Male | X | X | |
| 4269 | Control | 28 | 24 | Heart disease | Male | X | X | |
| 4271 | Control | 19 | 21 | Epiglotitus | Male | X | X | |
| 4275 | Control | 20 | 16 | Accidental | Male | X | X | |
| 4364 | Control | 27 | 27 | Motor Vehicle Accident | Male | X | ||
| 4605 | Control | 29 | 18.3 | Renal Failure | Male | X | ||
| 4642 | Control | 28 | 13 | Cardiac Arrythmia | Male | X | ||
| 4916 | Control | 19 | 5 | Drowning | Male | X | ||
| 5873 | Control | 28 | 23.3 | Unknown | Male | X | ||
| 6004 | Control | 36 | 18 | Unknown | Female | X | ||
| 6207 | Control | 16 | 26.2 | Heart Attack | Male | X | ||
| 6221 | Control | 22 | 24.2 | Unknown | Male | X | ||
Note: Cases with an asterisk (*) had a history of at least one seizure.
The following symbols indicate medication history:
Dilantin, Tegretol, Theodur, Phenobarbital
Klonopin, Mysoline, Phenobarbitol, Thorazine
Dilantin, Mellaril, Phenobarbital
Cisapride, Clorazepate, Depakote, Dilantin, Mysoline, Phenobarbital
Case Data
The following is a list of cases used in the study (Table 1). Note that seven of sixteen cases from the autism group had a history of at least one seizure (1078, 1484, 2825, 3845, 5173, 6337, 6677). As indicated in Table 1, based on availability of tissue blocks, there were seven autistic cases and nine control cases in the ACC study; six autistic and nine control cases for PCC study; and nine autistic and ten control cases from the FFG. The cases used in the study are designated in the far right column of Table 1 with an “X.” All cases used in the study had an autism diagnosis of moderate to severe. Detailed information about the regions of interest can be found in Supporting Information section.
Single-Concentration Binding Assay
Tissue blocks were sectioned coronally at 20 μm using a Hacker/Brights motorized cryostat at −20°C. Sections were thaw mounted on 2x3 inch gelatin coated glass slides. “Total binding” was measured using two sections per case and non-specific binding was determined using one section from each case. The tritiated antagonist ([3H])CGP54626 (1.5 nM; specific activity 50.0 Ci/mmol; Perkin Elmer; Scheperjans et al., 2005) was used to label GABAB receptors. This antagonist selectively binds to the GABAB receptor subunits 1a and 1b (Kaupmann et al., 1998a,b). Non-specific binding was measured by adding a high concentration of a competitive displacer (CGP55845; 100μM; Scheperjans et al., 2005) to the tritiated ligand and buffer solution (50mM Tris-HCl, 2.5mM CaCl2, pH 7.2). The following steps were completed at 4°C: pre-incubation with buffer (15 minutes), incubation with ligand (plus blocker for non-specific binding; 1 hour), 3 washes in buffer (5 minutes each), and a dip in double deionized water. Variability in binding conditions was minimized by running all cases in parallel. Slides were dried overnight and loaded into X-ray cassettes with a set of 3H polymer autoradiographic brain tissue standards (Autoradiographic 3H Microscales, GE Healthcare) and apposed to tritium-sensitive film (3H-Hyperfilm, Kodak) for 10 weeks. The exposed films were processed as follows at room temperature: developed with Kodak D19 (4 minutes), fixed with Kodak Rapidfix (3 minutes), rinsed in water (10 minutes) and air dried. Slides were stained with thionin to determine cytoarchitecture and laminar distribution of the ACC, PCC, and FFG. In the ACC, superficial layers corresponded to layers I–III, and deep layers to cortical layers V–VI (Vogt et al., 1995). In the PCC and FFG, superficial layers correspond to layers I–IV and deep layers correspond to layers V–VI.
Data Analysis
The film autoradiograms were digitized using an Inquiry densitometry system (Loats Associates) to gather quantitative measurements of optical density. Samples were obtained from the superficial and deep layers of the anterior cingulate cortex, posterior cingulate cortex, and fusiform gyrus. For interpolation of measured optical densities, a standard curve was constructed by fitting the optical densities for the 3H tissue standards to the single-hit sensitometric equation (Zhu et al., 2003): optical density = B1*(1 − 10k1(specific activity))+B3, to generate a standard curve relating optical density to nCi per estimated tissue equivalent wet weight (Calon et al., 2001). Optimization of the adjustable parameters k1, B1, and B3 by nonlinear least squares regression using the Solver tool of Microsoft Excel (Microsoft Office 2007) yielded an excellent fit to the measured optical densities of the standards. The standard curve was then used to interpolate the measured optical densities for the tissue sections into nCi/mg. Binding in femtomoles per milligram (fmol/mg) was calculated based on the specific activity of the ligand.
Statistical Analyses
Student’s t-tests with unequal variances were performed to determine if there were significant differences in the binding density between autistic and control cases in the superficial and deep layers of the ACC, PCC, and FFG. Mann-Whitney U non-parametric tests were also performed to determine if there were differences between the autism subgroup with a history of seizure and the autism subgroup with no seizure history. Although the number of cases with a history of seizure is small in each region (ranging from 3–4), this meets the minimal requirements for a non-parametric test of statistical significance. Mann-Whitney U tests were also performed on the autism subgroups receiving anticonvulsant therapy and those not receiving anticonvulsants in the ACC study because the number of cases (n=3) met the criteria to perform this statistical test; however, the PCC and FFG did not meet the requirements and therefore statistical tests were not performed.
Results
Overall, significant decreases were found in GABAB receptor binding density in autistic cases throughout the cingulate cortex and fusiform gyrus. Specific binding densities can be found in the supporting information (Tables I and II). Figure 1a and 1b are pseudocolored digitized images from hyperfilm to demonstrate the division of the anterior cingulate cortex into superficial and deep layers.
Figure 1.
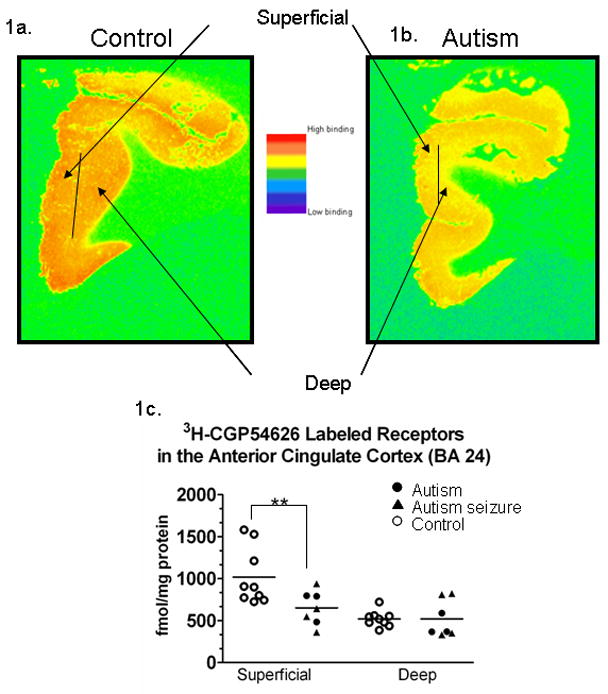
Pseudocolored image of a control case (1a.) and an autism case (1b.) from the ACC off [3H]-sensitive hyperfilm. The images demonstrate the superficial (I–III) and deep (V–VI) layers that were sampled (1a.) In 1c, a graph demonstrating [3H] labeled GABAB receptor binding density in the anterior cingulate cortex. Each symbol represents the GABAB receptor density from an individual case (see key). There was a significant (**) reductions (p=0.018) in the density of receptors in the autism cases in the superficial layers. Note these are sample sections from individual cases, and although it appears that there is a difference in the deep layers in these cases, statistically there was no significant difference in binding density between autism and control cases.
GABAB Receptor Binding in the Anterior Cingulate Cortex
In Figure 1a–c, in the anterior cingulate cortex, significant decreases in the binding density of GABAB receptors in the superficial layers of the autistic cases when compared to controls (p=0.018, ↓36.0%). In contrast there was no change in GABAB receptor density in the deep layers. Of the seven autistic cases, four had a history of seizure. There was also no difference between autistic seizure and autistic non-seizure subgroups or autistic antiepileptic therapy and autistic non-therapy, using a Mann-Whitney U non-parametric test (p>0.05).
GABAB Receptor Binding in the Posterior Cingulate Cortex
In Figure 2a–c, GABAB receptor binding studies in the posterior cingulate cortex showed a significant decrease in 3H-CGP54626 binding in the superficial layers (p=0.0076; ↓36.3%), but unlike the ACC, there was also a significant difference in GABAB receptor binding density in the deep layers of the PCC (p=0.050; ↓22.8%). Three of the six autistic cases had a history of seizure and when these cases were compared to the autistic cases without seizure (Mann-Whitney U), there was no significant difference between the groups in either the superficial or deep layers of the PCC.
Figure 2.
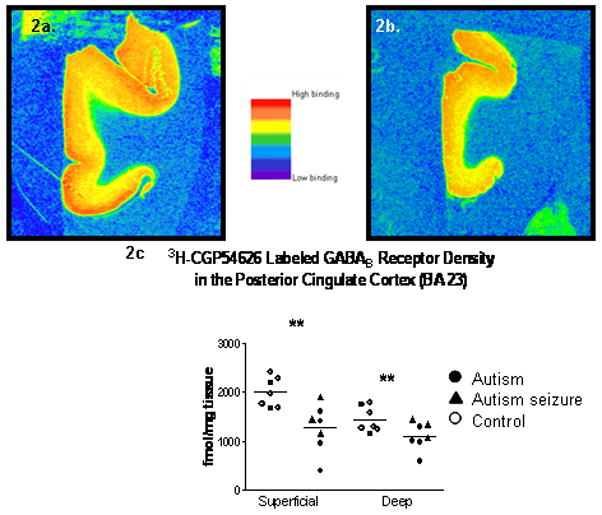
Pseudocolored images from a control (2a.) and autistic (2b.) case from the PCC. Graphs of the [3H]-CGP54626 labeled GABAB receptor binding density in the posterior cingulate cortex. Significant (**) decreases were found in the superficial (p=0.0076) and deep (p=0.050) layers of the autistic cases when compared to controls.
GABAB Receptor Binding in the Fusiform Gyrus
Similar to the PCC results, Figure 3a–c demonstrates that the binding of 3H-CGP54626 to GABAB receptors was significantly decreased in the superficial layers (p=0.019; ↓24.0 %) and in the deep layers (p=0.00095; ↓29.8 %). Four of the nine autistic cases had a history of seizure, but the comorbid seizure disorder did not have a significant effect on receptor binding in any layer. Note that there appears to be one outlier case with low binding in the autism group (1664) and one in the control group (1026).
Figure 3.
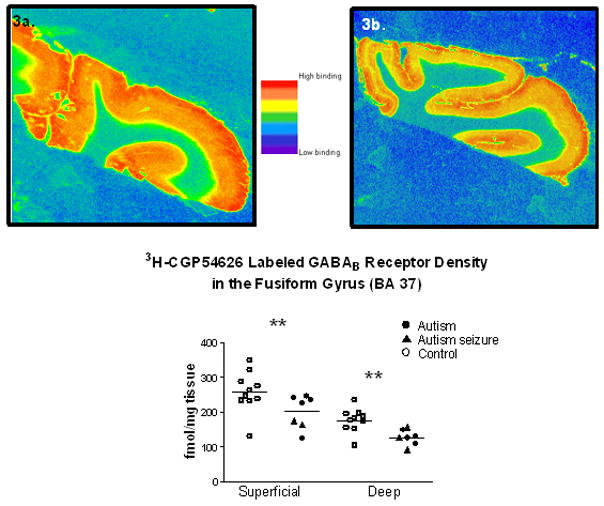
Graph demonstrating [3H]-CGP54626 labeled GABAB receptor binding density in the fusiform gyrus from a control (3a.) and an autistic case (3b.). Figure 3c is a scatter plot of all cases included in the study. Significant reductions (**) in the superficial (p=0.019) and deep (p=0.00095) layers were found in the autism cases.
Discussion
The anterior and posterior cingulate cortex and fusiform gyrus are among many cortical structures implicated in autism due to their correlative functions with core behaviors including social-emotional and face recognition/expression deficits. Abnormal cortical circuitry is a common theory in autism (e.g. Kleinhans et al., 2008; Weng et al., 2009) and mounting evidence directly implicates cellular and molecular components of the GABAergic system (e.g. Fatemi et al., 2009a; Lawrence et al., 2010), including the GABAB receptor and its subunits (Fatemi et al., 2009b)
The GABAB receptor
The GABAB receptor is composed of GABAB1 and GABAB2 subunits that must both be present for functional GABAB receptors to be expressed on the cell surface. Cell culture studies have shown that the GABAB1 subunit binds GABA; however, binding of GABA or any other endogenous ligand to the GABAB2 subunit has not been demonstrated (Kniazeff et al., 2002). The GABAB2 subunit, which exists as two isoforms, GABAB1a and GABAB1b, is necessary for trafficking of GABAB1 to the cell surface, and mediates G protein activation in response to binding of agonists to a binding site located on the GABAB1 subunit (Gi/Go; Kniazeff et al., 2002). Activation of GABAB receptors can influence long-term changes in synaptic strength and modulation of cortical circuits, and has been reported to restrict long-term potentiation (LTP) via hyperpolarization, whereas GABAB autoreceptors have been shown to promote the induction of LTP by disinhibiting the postsynaptic neuron in rat hippocampal slices (Davies et al., 1991; Olpe et al, 1993). Further evidence suggests that GABAB receptors have a critical role in developmental processes.
GABAB receptors during development
During pre- and postnatal brain development in the rat, GABAB receptors have a similar pre- and postsynaptic distribution as in adulthood in the neocortex, hippocampus, and dorsal cochlear nucleus (Lopez-Bendito et al., 2002 Lopez-Bendito et al., 2004; Lujan et al., 2004). Studies of the development of GABAB receptors in the cerebellum and olfactory cortex of rats suggest that GABAB receptors have a role in synapse and circuitry formation, and blockade of GABAB receptor signaling had a significant impact on the distribution of migratory neurons during corticogenesis (Lopez-Bendito et al., 2003; Panzanelli et al., 2004). Neuropathologic studies in the autistic brain have demonstrated abnormal neuronal migration and cytoarchitecture in the frontal cortex (Bailey et al., 1998) and anterior cingulate cortex (Simms et al., 2009). Casanova et al. (2002; 2003; 2006; 2009) reported an increase in the number of minicolumns and a decrease in neuropil in a number of cortical regions in autism. Furthermore, Van Kooten et al. (2008) found reduced volume and neuron number in the fusiform gyrus in autism cases; however, to our knowledge, neuropathology in the PCC has not yet been reported.
Insulin-like growth factor 1 (IGF-1) is important for normal development of the brain and promotes neuronal survival by rescuing neurons from apoptosis (Cheng et al., 2000; D’Mello et al., 1993). Reduced IGF-1 has been found in the cerebral spinal fluid (CSF) of children with autism (Vanhala et al., 2001; Riikonen et al., 2006). Tu and colleagues (2010) have recently found that GABAB receptors can protect neurons from apoptosis through a mechanism that involves transactivation of the IGF-1 receptor. IGF-1 triggers autophosphorylation of the IGF-1 receptor and activates the PI3 kinase/Akt signaling cascade which mediates the neuroprotective action of IGF-1 (Delcourt et al., 2007). Akt functions downstream of PI3 kinase and is critical for neuron survival (Bondy and Cheng, 2004). Therefore, reductions in GABAB receptors and/or IGF-1 in autism may result in the death of neurons, possibly contributing to reduced neuron numbers observed in the fusiform gyrus, amygdala, and anterior cingulate cortex (Schumann and Amaral, 2006; Van Kooten et al., 2008; Simms et al., 2009).
The current study demonstrates significant reductions in GABAB receptor density throughout the cingulate cortex and fusiform gyrus, and although the developmental origin of the GABAB receptor deficit is unknown, if this system is disturbed during the prenatal or early postnatal period, it could have profound implications towards the maturation of specific behaviors.
GABAB receptors and socio-emotional and face processing
Individuals with autism have severe deficits in processing faces (Klin et al., 1999; Grelotti et al., 2002; Joseph and Tanaka, 2003) that may be due to alterations in cortical network signaling because of deficits in the GABA system. The superficial layers of the cortex (layers I–IV) receive information from the thalamus and other cortical regions and project to inter- and intracortical areas while the deep layers also receive thalamic input and project to other cortical and subcortical regions. Based on the present findings, cortical information may be potentially disrupted because of reduced inhibitory receptors that could result in a failure to recruit cortical regions needed for processing emotional responses and facial recognition.
At the synaptic level, neuroligins are a family of postsynaptic cell adhesions molecules in the brain that interact with neurexins and are localized to the postsynaptic specializations of excitatory and inhibitory (including GABAergic) synapses (Ichtchenko et al., 1995, 1996). In vitro transfection studies have suggested a role for neuroligins in synapse formation (Dean et al., 2003); however, cell culture studies suggest that neuroligins are not required for synapse formation but for synapse specification and modulation (Varoqueaux et al., 2006). In autism, mutations in both neuroligins and neurexins have been reported (Chih et al., 2004; Feng et al., 2006; Yan et al., 2008). Therefore, reductions in inhibitory receptors in the present study may result from an alteration in neuroligins and/or neurexins resulting in reduced GABAergic synaptic protein recruitment and stabilization within the cingulate cortex and fusiform gyrus.
Seizures and GABAB receptors
A subgroup of approximately 25–33% of individuals with autism have a relatively high frequency of seizures (Olsson et al., 1988; Volkmar and Nelson, 1990). Knock-out mice lacking the GABAB1 or GABAB2 subunit exhibit epileptiform activity, enhanced prepulse inhibition, altered locomotor activity, and impaired memory processing (Prosser et al., 2001; Schuler et al., 2001; Vacher et al., 2006). These knock-out mice suggest that differences in the number of GABAB receptor subunits may lead to the development of seizures due to changes in neurotransmitter release or inhibition of local circuits. Similar deficiencies in GABAB receptors in autism may result in seizures and decreased effectiveness in controlling cortical circuits.
In the current study, the density of GABAB receptors in ACC, PCC or FFG did not differ significantly between the autism subgroup with a history of seizure and the subgroup with no history of seizure. However this result is met with caution since the number of cases (n=3) was small for seizures. Although several of the cases used in this study were receiving or had received anticonvulsant therapy during their life, none of the drugs used are known to target GABAB receptors (Table 1). We were unable to determine if the anticonvulsants had an effect on GABAB binding density in all three regions because of the small sample size (n=2 in PCC, n = 1 in FFG). However, in the ACC, there was no effect of pharmacotherapy on binding density, but again the number of cases was small and the results should be met with caution.
We are unable to determine if the significant decrease in the density of binding is due to a loss of GABAB receptors on GABAergic or glutamatergic neurons of if there is a reduction in one of the GABAB receptor subunits. Fatemi et al. (2009b) found significant reductions in the protein level of GABAB1 subunit in the parietal lobe, frontal lobe, and cerebellum of autistic individuals as well as reduced protein levels of the GABAB2 subunit in the cerebellum. Based on these results it is possible that the reductions in the GABAB binding density in the present study are due to decreased availability of either of the subunits required for proper GABAB receptor surface expression and functioning. A detailed molecular analysis of the subunits across brain areas is thus needed to determine whether the autistic group has fewer GABAB receptor subunit(s) than controls.
Closing Comments
The cingulate cortex, an area that modulates emotion, attention, and gaze fixation and activated by socio-emotional events, may be responsible for the proper function of the fusiform gyrus through mechanisms that modulate activity in this region. The alterations in GABAB receptor densities in the three regions further support the theory that deficits in the GABA system are widespread in autism (Blatt et al., 2010) and that this alteration is not restricted to GABAA receptors as previously reported in these regions (Oblak et al., 2009a, b). The changes in GABAB receptors thus suggest possible pharmacotherapies since GABAB receptors are not responsive to antiepileptic drugs that typically target GABAA receptors. GABAB agonists such as baclofen might be considered as possible agents for clinical trials.
Finally, although the full syndrome as expressed in later childhood and adolescence appears to involve insults to multiple brain regions, it is not clear if these multiple systemic brain disturbances reflect deficits in multiple independent control processes, or whether the initial insult might have been more restricted. It is possible that deficits initially confined to one system might negatively impact the development of other neural systems, such that a more pervasive set of impairments evolves. This would suggest that multiple brain areas within specific networks become involved and more widespread behavioral effects emerge.
Supplementary Material
Acknowledgments
This work was supported by a “Studies to Advance Autism Research and Treatment” grant from the National Institutes of Health (NIH U54 MH66398) and the Hussman Foundation. Human tissue was obtained from the Harvard Brain Tissue Resource Center, The Autism Tissue Program (ATP), The Autism Research Foundation (TARF), and the NICHD Brain and Tissue Bank for Developmental Disorders at The University of Maryland, Baltimore, Maryland. The authors have no conflicts of interest.
Contributor Information
Adrian L. Oblak, Email: aoblak@bu.edu, Boston University School of Medicine, Department of Anatomy and Neurobiology, Laboratory of Autism Neuroscience Research, 72 East Concord Street, L-1004, Boston, MA 02118, Phone: 617-638-5261, Fax: 617-638-4216
Terrell T. Gibbs, Boston University School of Medicine, Department of Pharmacology and Experimental Therapeutics, 72 East Concord Street, L-606, Boston, MA 02118, Phone: 617-638-5325, Fax: 617-638-4329
Gene J. Blatt, Boston University School of Medicine, Department of Anatomy and Neurobiology, Laboratory of Autism Neuroscience Research, 72 East Concord Street, L-1004, Boston, MA 02118, Phone: 617-638-5260, Fax: 617-638-4216
References
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Annals of the New York Academy of Science. 2001;935:107–117. [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A clinicopathological study of autism. Brain. 1998;121 ( Pt 5):889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35(6):866–874. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Soghomonian JJ, Yip J. Glutamic acid decarboxylase (GAD) as a biomarker of GABAergic activity in autism: impact on cerebellar circuitry and function. In: Blatt GJ, editor. The Neurochemical Basis of Autism: From Molecules to Minicolums. Springer; New York, Dordrecht, Heidelberg, London: 2010. [Google Scholar]
- Blatt GJ, Fitzgerald CM, Guptill JT, Booker AB, Kemper TL, Bauman ML. Density and distribution of hippocampal neurotransmitter receptors in autism: an autoradiographic study. J Autism Dev Disord. 2001;31(6):537–543. doi: 10.1023/a:1013238809666. [DOI] [PubMed] [Google Scholar]
- Bondy CA, Cheng CM. Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol. 2004;490:25–31. doi: 10.1016/j.ejphar.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402(6758):179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Braverman M, Fein D, Lucci D, Waterhouse L. Affect comprehension in children with pervasive developmental disorders. J Autism Dev Disord. 1989;19(2):301–316. doi: 10.1007/BF02211848. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Großhirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig; Barth JA: 1909. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Calon F, Lavertu N, Lemieux AM, Morissette M, Goulet M, Grondin R, Blanchet PJ, Bédard PJ, Di Paolo T. Effect of MPTP-induced denervation on basal ganglia GABA(B) receptors: correlation with dopamine concentrations and dopamine transporter. Synapse. 2001;40:225–34. doi: 10.1002/syn.1045. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58(3):428–32. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden D, Gomez J. Disruption in the inhibitory architecture of the cell minicolumn: implications for autisim. Neuroscientist. 2003;9(6):496–507. doi: 10.1177/1073858403253552. [DOI] [PubMed] [Google Scholar]
- Casanova MF, van Kooten IA, Switala AE, van Engeland H, Heinsen H, Steinbusch HW, Hof PR, Trippe J, Stone J, Schmitz C. Minicolumnar abnormalities in autism. Acta Neuropathol. 2006;112(3):287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- Casanova MF, El-Baz A, Vanbogaert E, Narahari P, Switala A. A topographic study of minicolumnar core width by lamina comparison between autistic subjects and controls: possible minicolumnar disruption due to an anatomical element in-common to muliple laminae. Brain Pathol. 2009 doi: 10.1111/j.1750-3639.2009.00319.x. (E–pub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17(1):77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Cheng CM, Reinhard RR, Lee WH, Joncas G, Patel SC, Bondy CA. Insulin-like growth factor 2 regulates developing brain glucose metabolism. Proc Natl Acad Sci USA. 2000;97:10236–10241. doi: 10.1073/pnas.170008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B, Afridi SK, Clark L, Scheiffele P. Disorder-associated mutations lead to functional inactivation of neuroligins. Human Molecular Genetics. 2004;13(14):1471–1477. doi: 10.1093/hmg/ddh158. [DOI] [PubMed] [Google Scholar]
- Craig AD, Reiman EM, Evans A, Bushnell MC. Functional imaging of an illusion of pain. Nature. 1996;384(6606):258–260. doi: 10.1038/384258a0. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, Williams SC, Van Amelsvoort T, Robertson DM, Rowe A, Phillips M, McAlonan G, Howlin P, Murphy DG. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123 ( Pt 11):2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8(4):519–26. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Abercrombie H, Nitschke JB, Putnam K. Regional brain function, emotion and disorders of emotion. Curr Opin Neurobiol. 1999;9(2):228–234. doi: 10.1016/s0959-4388(99)80032-4. [DOI] [PubMed] [Google Scholar]
- Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABA autoreceptors regulate the induction of LTP. Nature. 1991;349(6310):609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- Davies S, Bishop D, Manstead AS, Tantam D. Face perception in children with autism and Asperger's syndrome. J Child Psychol Psychiatry. 1994;35(6):1033–1057. doi: 10.1111/j.1469-7610.1994.tb01808.x. [DOI] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nature Neuroscience. 2003;6(7):708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118 ( Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- D’Mello SR, Galli C, Ciotti T, Calissano P. Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of death by insulin–like growth factor I and cAMP. Proc Natl Acad Sci US. 1993;90:10989–10993. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52(8):805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD. GABA(A) receptor downregulation in brains of subjects with autism. J Autism Dev Disord. 2009a;39(2):223–230. doi: 10.1007/s10803-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Thuras PD. Expression of GABA(B) receptors is altered in brains of subjects with autism. Cerebellum. 2009b;8(1):64–69. doi: 10.1007/s12311-008-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Schroer R, Yan J, Song W, Yang C, Bockholt A, Cook EH, et al. High frequency of neurexin 1beta signal peptide structural variants in patients with autism. Neuroscience Letters. 2006;409(1):10–13. doi: 10.1016/j.neulet.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Fombonne E. The epidemiology of autism: a review. Psychol Med. 1999;29(4):769–786. doi: 10.1017/s0033291799008508. [DOI] [PubMed] [Google Scholar]
- Grelotti DJ, Gauthier I, Schultz RT. Social interest and the development of cortical face specialization: what autism teaches us about face processing. Dev Psychobiol. 2002;40(3):213–225. doi: 10.1002/dev.10028. [DOI] [PubMed] [Google Scholar]
- Grelotti DJ, Klin AJ, Gauthier I, Skudlarski P, Cohen DJ, Gore JC, Volkmar FR, Schultz RT. fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. 2005;43(3):373–85. doi: 10.1016/j.neuropsychologia.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Guptill JT, Booker AB, Gibbs TT, Kemper TL, Bauman ML, Blatt GJ. [3H]-flunitrazepam-labeled benzodiazepine binding sites in the hippocampal formation in autism: a multiple concentration autoradiographic study. J Autism Dev Disord. 2007;37(5):911–920. doi: 10.1007/s10803-006-0226-7. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Chabris CF, Clark J, Steele S, McGrath L, Vangel M, Aharon I, Feczko E, Harris GJ, Tager-Flusberg H. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage. 2004;22(3):1141–1150. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Hussman JP. Suppressed GABAergic inhibition as a common factor in suspected etiologies of autism. J Autism Dev Disord. 2001;31(2):247–248. doi: 10.1023/a:1010715619091. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Südhof TC. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81(3):435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Nguyen T, Südhof TC. Structures, alternative splicing, and neurexin binding of multiple neuroligins. The Journal of Biological Chemistry. 1996;271(5):2676–2682. doi: 10.1074/jbc.271.5.2676. [DOI] [PubMed] [Google Scholar]
- Joseph RM, Tanaka J. Holistic and part-based face recognition in children with autism. J Child Psychol Psychiatry. 2003;44(4):529–542. doi: 10.1111/1469-7610.00142. [DOI] [PubMed] [Google Scholar]
- Jurgens U, Ploog D. Cerebral representation of vocalization in the squirrel monkey. Exp Brain Res. 1970;10(5):532–554. doi: 10.1007/BF00234269. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupmann K, Schuler V, Mosbacher J, Bischoff S, Bittiger H, Heid J, Froestl W, Leonhard S, Pfaff T, Karschin A, Bettler B. Human γ-aminobutyric acid type B receptors are differently expressed and regulate inwardly rectifying K channels. Proc Natl Acad Sci USA. 1998a;95:14991–14996. doi: 10.1073/pnas.95.25.14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B. GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998b;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A. 2006;103(21):8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Richards R, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131(4):1000–12. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Klin A, Sparrow SS, de Bildt A, Cicchetti DV, Cohen DJ, Volkmar FR. A normed study of face recognition in autism and related disorders. J Autism Dev Disord. 1999;29(6):499–508. doi: 10.1023/a:1022299920240. [DOI] [PubMed] [Google Scholar]
- Kniazeff J, Galvez T, Labesse G, Pin JP. No ligand binding in the GB2 subunit of the GABA(B) receptor is required for activation and allosteric interaction between the subunits. J Neurosci. 2002;22(17):7352–7361. doi: 10.1523/JNEUROSCI.22-17-07352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik A, Vida I, Fukazawa Y, Guetg N, Kasugai Y, Marker CL, Rigato F, Bettler B, Wickman K, Frotscher M, Shigemoto R. Compartment-dependent colocalization of Kir3.2-containing K+ channels and GABAB receptors in hippocampal pyramidal cells. J Neurosci. 2006;26(16):4289–4297. doi: 10.1523/JNEUROSCI.4178-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence YA, Kemper TL, Bauman ML, Blatt GJ. Parvalbumin-, calbindin-, and calretinin-immunoreactive hippocampal interneuron density in autism. Acta Neurologica Scandinavica. 2010;121(2):99–108. doi: 10.1111/j.1600-0404.2009.01234.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Shigemoto R, Kulik A, Paulsen O, Fairen A, Lujan R. Expression and distribution of metabotropic GABA receptor subtypes GABABR1 and GABABR2 during rat neocortical development. Eur J Neurosci. 2002;15(11):1766–1778. doi: 10.1046/j.1460-9568.2002.02032.x. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22(7):310–6. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104(3):667–676. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- McDonald B, Highley JR, Walker MA, Herron BM, Cooper SJ, Esiri MM, Crow TJ. Anomalous asymmetry of fusiform and parahippocampal gyrus gray matter in schizophrenia: A postmortem study. Am J Psychiatry. 2000;157(1):40–47. doi: 10.1176/ajp.157.1.40. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav Brain Res. 1982;6(1):57–77. doi: 10.1016/0166-4328(82)90081-x. [DOI] [PubMed] [Google Scholar]
- Oblak A, Gibbs TT, Blatt GJ. Decreased GABAA receptors and benzodiazepine binding sites in the anterior cingulate cortex in autism. Autism Res. 2009a;2(4):205–219. doi: 10.1002/aur.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblak A, Gibbs TT, Blatt GJ. GABAergic alterations in the cingulate cortex and fusiform gyrus in autism. Presented at the Society for Neuroscience Conference; October 19th, 2009; Chicago, IL. 2009b. p. 437.12. [Google Scholar]
- Olpe HR, Worner W, Ferrat T. Stimulation parameters determine role of GABAB receptors in long-term potentiation. Experientia. 1993;49(6–7):542–546. doi: 10.1007/BF01955159. [DOI] [PubMed] [Google Scholar]
- Olson CR, Musil SY. Posterior cingulate cortex: sensory and oculomotor properties of single neurons in behaving cat. Cereb Cortex. 1992;2(6):485–502. doi: 10.1093/cercor/2.6.485. [DOI] [PubMed] [Google Scholar]
- Olson CR, Musil SY, Goldberg ME. Single neurons in posterior cingulate cortex of behaving macaque: eye movement signals. J Neurophysiol. 1996;76(5):3285–3300. doi: 10.1152/jn.1996.76.5.3285. [DOI] [PubMed] [Google Scholar]
- Olsson I, Steffenburg S, Gillberg C. Epilepsy in autism and autistic like conditions. A population-based study. Archives of Neurology. 1988;45(6):666–668. doi: 10.1001/archneur.1988.00520300086024. [DOI] [PubMed] [Google Scholar]
- Panzanelli P, Lopez-Bendito G, Lujan R, Sassoe-Pognetto M. Localization and developmental expression of GABA(B) receptors in the rat olfactory bulb. J Neurocytol. 2004;33(1):87–99. doi: 10.1023/B:NEUR.0000029650.28943.b2. [DOI] [PubMed] [Google Scholar]
- Paus T, Koski L, Caramanos Z, Westbury C. Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: a review of 107 PET activation studies. Neuroreport. 1998;9(9):R37–47. doi: 10.1097/00001756-199806220-00001. [DOI] [PubMed] [Google Scholar]
- Pierce K, Redcay E. Fusiform function in children with an autism spectrum disorder is a matter of “who. Bio Psychiatry. 2008;64:552–560. doi: 10.1016/j.biopsych.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform 'face area' in autism: evidence from functional MRI. Brain. 2001;124(Pt 10):2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Prosser HM, Gill CH, Hirst WD, Grau E, Robbins M, Calver A, Soffin EM, Farmer CE, Lanneau C, Gray J, Schenck E, Warmerdam BS, Clapham C, Reavill C, Rogers DC, Stean T, Upton N, Humphreys K, Randall A, Geppert M, Davies CH, Pangalos MN. Epileptogenesis and enhanced prepulse inhibition in GABA(B1)-deficient mice. Mol Cell Neurosci. 2001;17(6):1059–1070. doi: 10.1006/mcne.2001.0995. [DOI] [PubMed] [Google Scholar]
- Riikonen R, Makkonen I, Vanhala R, Trupeinen U, Kuikka J, Kokki H. Cerebrospinal fluid insulin-like growth factors IGF-1 and IGF-2 in infantile autism. Dev Med Child Neuro. 2006;48:751–755. doi: 10.1017/S0012162206001605. [DOI] [PubMed] [Google Scholar]
- Ritvo ER, Freeman BJ, Scheibel AB, Duong T, Robinson H, Guthrie D, Ritvo A. Lower Purkinje cell counts in the cerebella of four autistic subjects: initial findings of the UCLA-NSAC autopsy research report. Am J Psychiatry. 1986;146:862–866. doi: 10.1176/ajp.143.7.862. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2(5):255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Grefkes C, Palomero-Gallagher N, Schleicher A, Zilles K. Subdivisions of human parietal area 5 revealed by quantitative receptor autoradiography: a parietal region between motor, somatosensory, and cingulate cortical areas. NeuroImage. 2005;25(3):975–992. doi: 10.1016/j.neuroimage.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Schuler V, Luscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid J, Gentry C, Urban L, Fox A, Spooren W, Jaton AL, Vigouret J, Pozza M, Kelly PH, Mosbacher J, Froestl W, Kaslin E, Korn R, Bischoff S, Kaupmann K, van der Putten H, Bettler B. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)) Neuron. 2001;31(1):47–58. doi: 10.1016/s0896-6273(01)00345-2. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, Skudlarski P, Lacadie C, Cohen DJ, Gore JC. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry. 2000;57(4):331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. J Neurosci. 2006;26(29):7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NJ, Marshall JC, Zafiris O, Schwab A, Zilles K, Markowitsch HJ. The neural correlates of person familiarity, A functional magnetic resonance study with clinical implications. Brain. 2001;124(4):804–815. doi: 10.1093/brain/124.4.804. [DOI] [PubMed] [Google Scholar]
- Shidara M, Richmond BJ. Anterior cingulate: single neuronal signals related to degree of reward expectancy. Science. 2002;296(5573):1709–1711. doi: 10.1126/science.1069504. [DOI] [PubMed] [Google Scholar]
- Simms ML, Kemper TL, Timbie CM, Bauman ML, Blatt GJ. The anterior cingulate cortex in autism: heterogeneity of qualitative and quantitative cytoarchitecttonic features suggests possible subgroups. Acta Neuropathol. 2009;118(5):673–84. doi: 10.1007/s00401-009-0568-2. [DOI] [PubMed] [Google Scholar]
- Takarae Y, Minshew NJ, Luna B, Sweeney JA. Atypical involvement of frontostriatal systems during sensorimotor control in autism. Psychiatry Res. 2007;156(2):117–127. doi: 10.1016/j.pscychresns.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, Barton JJ, Manoach DS. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131(Pt 9):2464–78. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Xu C, Zhang W, Liu Q, Rondard P, Pin JP, Liu J. GABAB receptor activation protects neurons from apoptosis via IGF-1 receptor transactivation. J Neurosci. 2010;30(2):749–759. doi: 10.1523/JNEUROSCI.2343-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchman R, Rapin I. Epilepsy in autism. Lancet Neurol. 2002;1(6):352–358. doi: 10.1016/s1474-4422(02)00160-6. [DOI] [PubMed] [Google Scholar]
- Uylings HB, van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Prog Brain Res. 1990;85:31–62. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- Vacher CM, Gassmann M, Desrayaud S, Challet E, Bradaia A, Hoyer D, Waldmeier P, Kaupmann K, Pevet P, Bettler B. Hyperdopaminergia and altered locomotor activity in GABAB1-deficient mice. J Neurochem. 2006;97(4):979–991. doi: 10.1111/j.1471-4159.2006.03806.x. [DOI] [PubMed] [Google Scholar]
- van Kooten IA, Palmen SJ, von Cappeln P, Steinbusch HW, Korr H, Heinsen H, Hof PR, van Engeland H, Schmitz C. Neurons in the fusiform gyrus are fewer and smaller in autism. Brain. 2008;131(Pt 4):987–999. doi: 10.1093/brain/awn033. [DOI] [PubMed] [Google Scholar]
- Vanhala R, Turpeinen U, Riikonen R. Low levels of insulin-like growth factor-I in cerebrospinal fluid in children with autism. Dev Med Child Neuro. 2001;43:614–616. doi: 10.1017/s0012162201001116. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, et al. Neuroligins determine synapse maturation and function. Neuron. 2006;51(6):741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol. 1995;359(3):490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L. Cytology of human dorsal midcingulate and supplementary motor cortices. J Chem Neuroanat. 2003;26(4):301–309. doi: 10.1016/j.jchemneu.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29(2):452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, Klin A, Schultz R, Bronen R, Marans WD, Sparrow S, Cohen DJ. Asperger's syndrome. J Am Acad Child Adolesc Psychiatry. 1996;35(1):118–123. doi: 10.1097/00004583-199601000-00020. [DOI] [PubMed] [Google Scholar]
- Weng SJ, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C, Monk CS. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2009 doi: 10.1016/j.brainres.2009.11.057. (E–pub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney ER, Kemper TL, Bauman ML, Rosene DL, Blatt GJ. Cerebellar Purkinje cells are reduced in a subppoulation of autistic brains: a stereological experiment using calbindin-D28k. Cerebellum. 2008;7(3):406–16. doi: 10.1007/s12311-008-0043-y. [DOI] [PubMed] [Google Scholar]
- Yan J, Feng J, Schroer R, Li W, Skinner C, Schwartz CE, Cook EH, et al. Analysis of the neuroligin 4Y gene in patients with autism. Psychiatric Genetics. 2008;18(4):204–207. doi: 10.1097/YPG.0b013e3282fb7fe6. [DOI] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD65 mRNA levels in select subpopulations of neurons in the cerebellar dentate nuclei in autism: an in situ hybridization study. Autism Res. 2009;2(1):50–59. doi: 10.1002/aur.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Increased GAD67 mRNA expression in cerebellar interneurons in autism: implications for Purkinje cell dysfunction. J Neurosci Res. 2008;86(3):525–530. doi: 10.1002/jnr.21520. [DOI] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathol. 2007;113(5):559–568. doi: 10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]
- Zhu XR, Yoo S, Jursinic PA, Grimm DF, Lopez F, Rownd JJ, Gillin MT. Characteristics of sensitometric curves of radiographic films. Med Phys. 2003;30:912–9. doi: 10.1118/1.1568979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


