Abstract
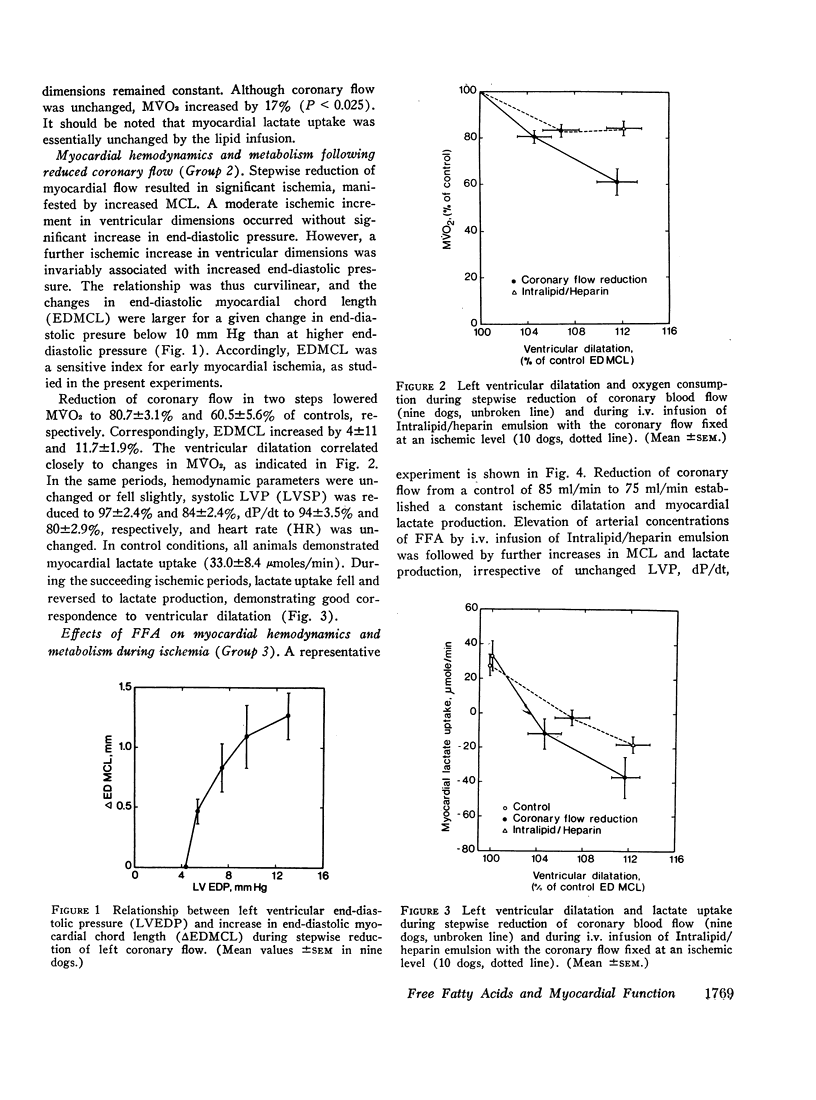
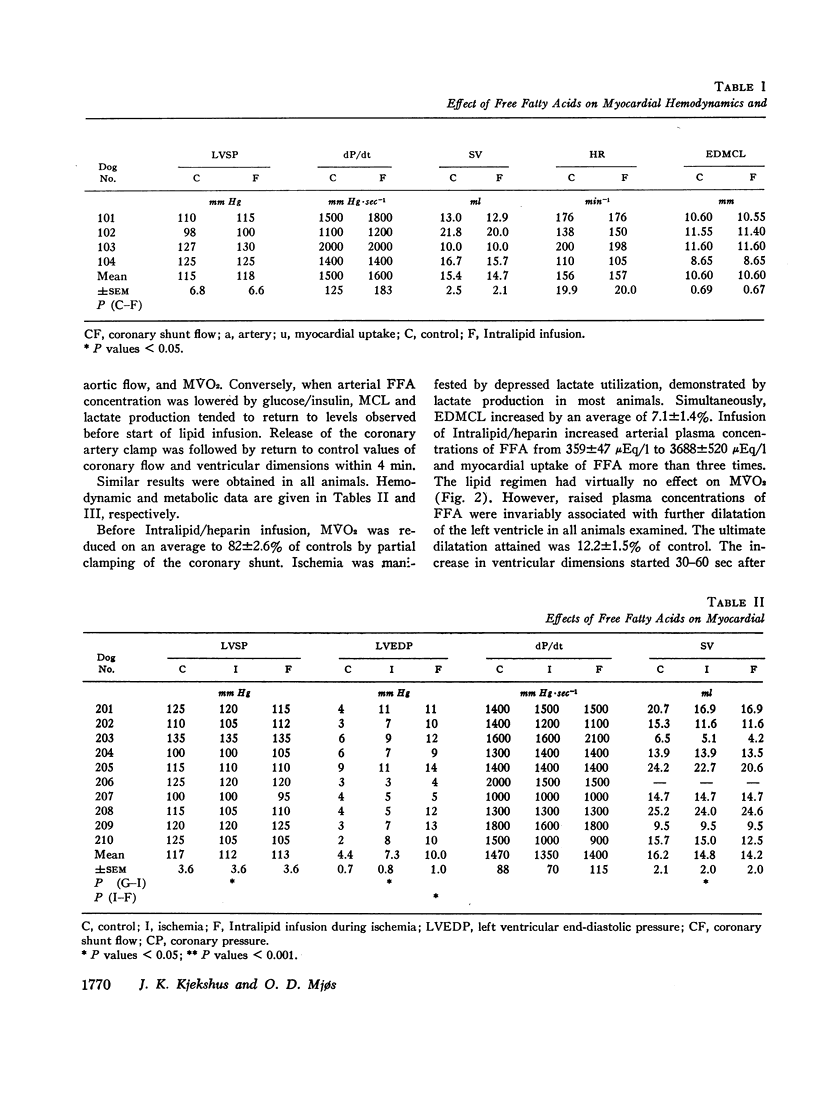
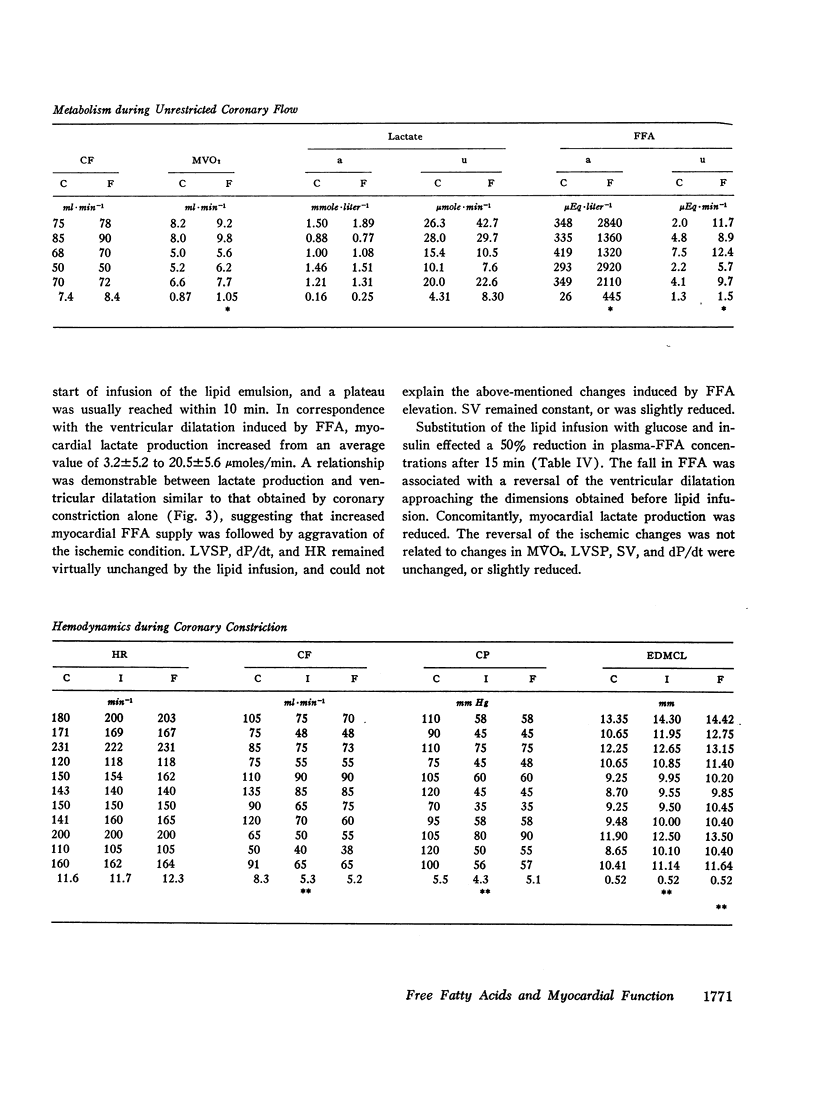
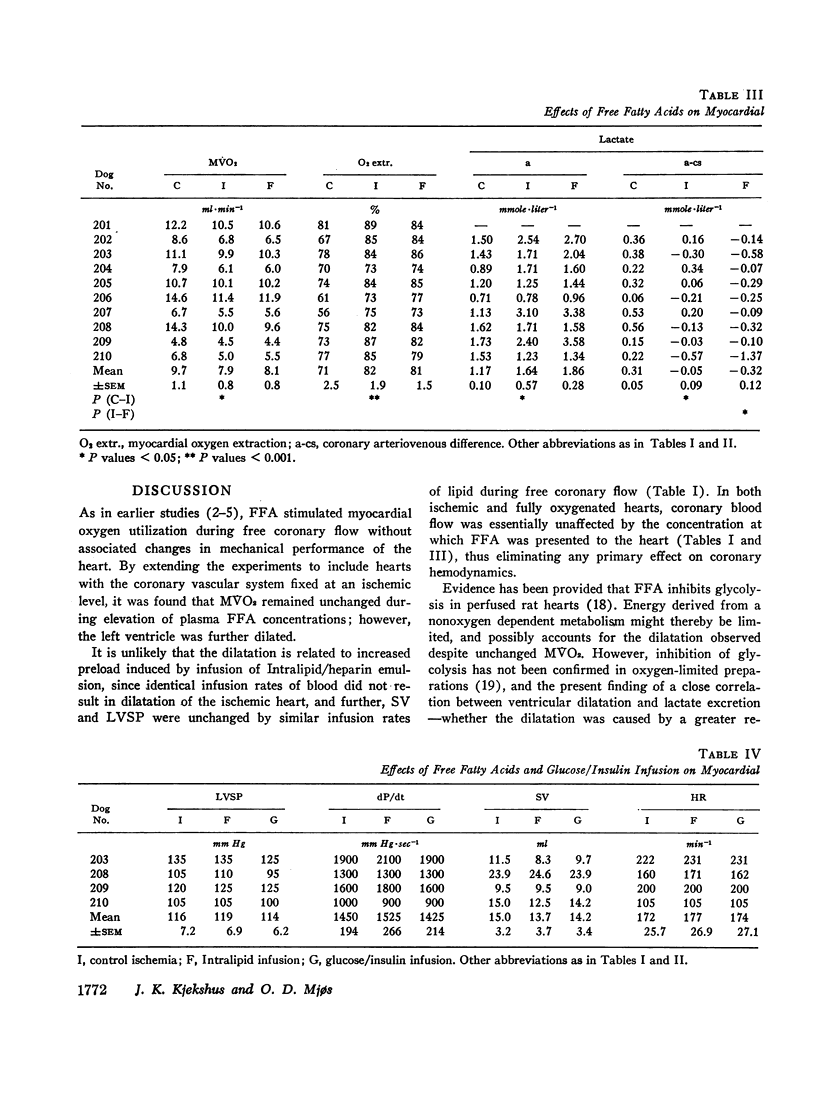
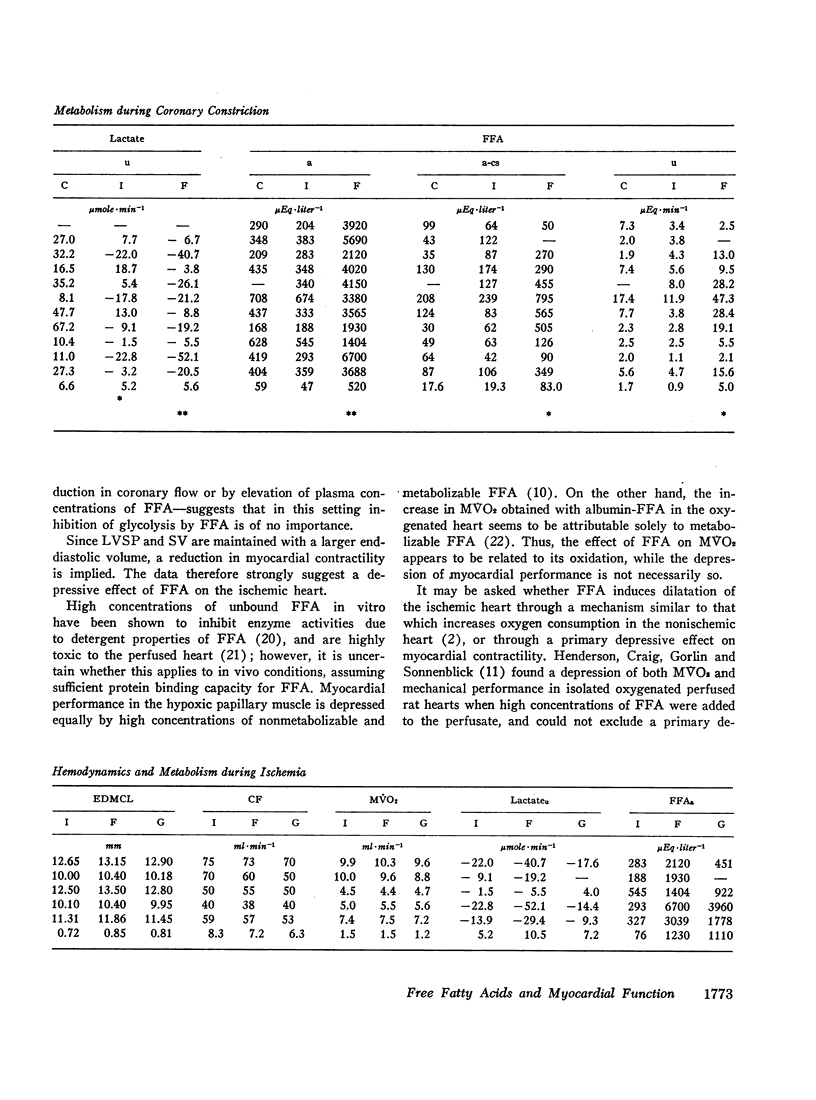
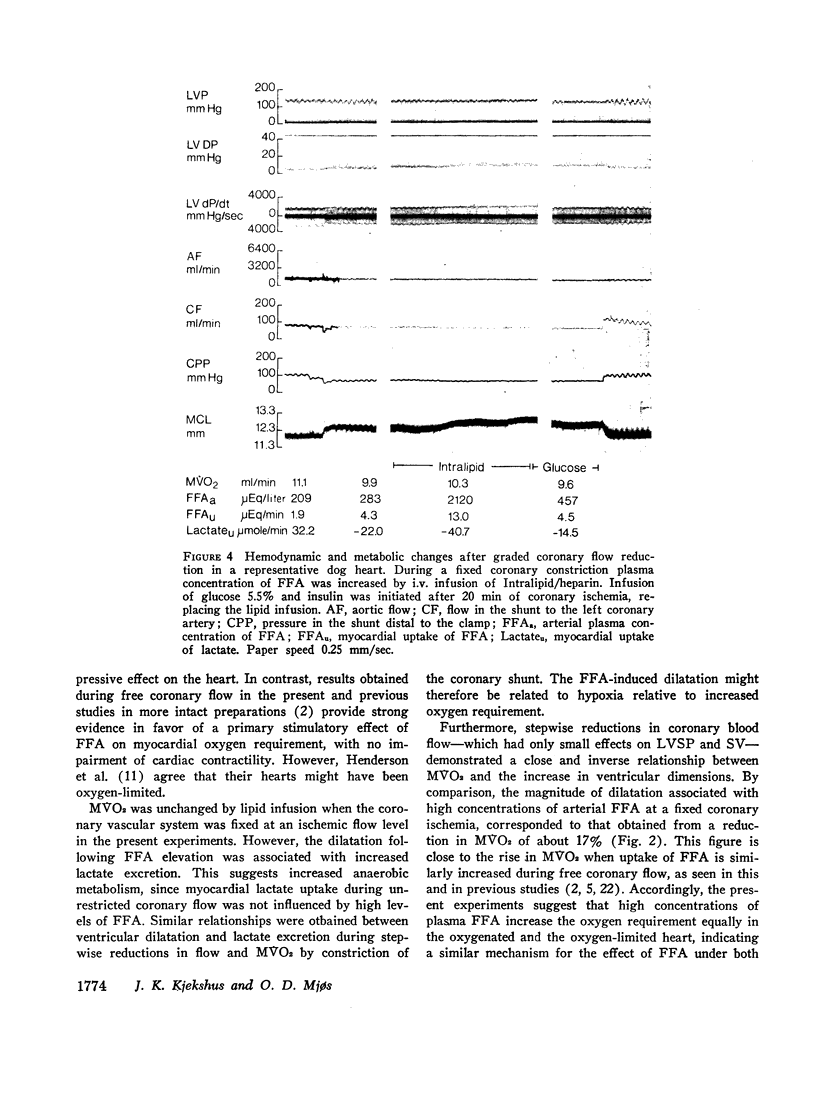
Since elevation of plasma concentrations of free fatty acids (FFA) increases myocardial oxygen consumption without influencing mechanical performance in normal hearts, it was the purpose of this study to determine whether FFA would modify mechanical performance at limited oxygen supply. Left coronary blood flow was reduced by gradual clamping of a shunt from the left carotid artery until moderate ventricular dilatation supervened. Left ventricular systolic pressure (LVSP), its maximal rate of rise (dP/dt) and stroke volume (SV) were unchanged or slightly reduced. The ischemia resulted in a decrease in myocardial oxygen consumption (MVO2) from 9.7±1.1 ml/min to 7.9±0.8 ml/min, and myocardial lactate uptake was reduced or reversed to excretion. Increasing the plasma concentrations of FFA from 359±47 μEq/1 to 3688±520 μEq/1 by intravenous infusion of a triglyceride emulsion and heparin resulted in further ventricular dilatation, accompanied by increased excretion of lactate. The ventricular decompensation and enhancement of anaerobic myocardial metabolism associated with increased uptake of FFA was not related to changes in coronary flow, MVO2, or LVSP. dP/dt and SV were virtually unchanged. Intravenous infusion of glucose/insulin, which lowered plasma concentrations of FFA, reversed ventricular dilatation and lactate excretion.
The data support the hypothesis that high concentrations of FFA play a significant role in increasing myocardial oxygen requirement and thereby promote depression of contractility of the hypoxic heart in experimental animals.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUKLAND K. Spectrophotometric determination of hemoglobin oxygen saturation in small blood samples. Scand J Clin Lab Invest. 1962;14:533–536. doi: 10.3109/00365516209051275. [DOI] [PubMed] [Google Scholar]
- BERNE R. M. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963 Feb;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- BORST P., LOOS J. A., CHRIST E. J., SLATER E. C. Uncoupling activity of long-chain fatty acids. Biochim Biophys Acta. 1962 Aug 27;62:509–518. doi: 10.1016/0006-3002(62)90232-9. [DOI] [PubMed] [Google Scholar]
- Ball E. G. Some energy relationships in adipose tissue. Ann N Y Acad Sci. 1965 Oct 8;131(1):225–234. doi: 10.1111/j.1749-6632.1965.tb34791.x. [DOI] [PubMed] [Google Scholar]
- Bugge-Asperheim B., Leraand S., Kiil F. Local dimensional changes of the myocardium measured by ultrasonic technique. Scand J Clin Lab Invest. 1969 Dec;24(4):361–371. doi: 10.3109/00365516909080172. [DOI] [PubMed] [Google Scholar]
- Carlson L. A. Inhibition of the mobilization of free fatty acids from adipose tissue. Physiological aspects on the mechanisms for the inhibition of mobilization of FFA from adipose tissue. Ann N Y Acad Sci. 1965 Oct 8;131(1):119–142. doi: 10.1111/j.1749-6632.1965.tb34784.x. [DOI] [PubMed] [Google Scholar]
- Challoner D. R. Evidence for uncoupled respiration in thyrotoxic and epinephrine-stimulated myocardium. Am J Physiol. 1968 Feb;214(2):365–369. doi: 10.1152/ajplegacy.1968.214.2.365. [DOI] [PubMed] [Google Scholar]
- Challoner D. R., Steinberg D. Effect of free fatty acid on the oxygen consumption of perfused rat heart. Am J Physiol. 1966 Feb;210(2):280–286. doi: 10.1152/ajplegacy.1966.210.2.280. [DOI] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson A. H., Craig R. J., Gorlin R., Sonnenblick E. H. Free fatty acids and myocardial function in perfused rat hearts. Cardiovasc Res. 1970 Oct;4(4):466–472. doi: 10.1093/cvr/4.4.466. [DOI] [PubMed] [Google Scholar]
- Henderson A. H., Most A. S., Parmley W. W., Gorlin R., Sonnenblick E. H. Depression of myocardial contractility in rats by free fatty acids during hypoxia. Circ Res. 1970 Apr;26(4):439–449. doi: 10.1161/01.res.26.4.439. [DOI] [PubMed] [Google Scholar]
- Kurien V. A., Oliver M. F. Serum-free-fatty-acids after acute myocardial infarction and cerebral vascular occlusion. Lancet. 1966 Jul 16;2(7455):122–127. doi: 10.1016/s0140-6736(66)92420-2. [DOI] [PubMed] [Google Scholar]
- Kurien V. A., Yates P. A., Oliver M. F. The role of free fatty acids in the production of ventricular arrhythmias after acute coronary artery occlusion. Eur J Clin Invest. 1971 Jan;1(4):225–241. doi: 10.1111/eci.1971.1.4.225. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., PRESSMAN B. C. Effect of surface active agents on the latent ATPase of mitochondria. Biochim Biophys Acta. 1956 Sep;21(3):458–466. doi: 10.1016/0006-3002(56)90182-2. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Kjekshus J. K., Sobel B. E., Watanabe T., Covell J. W., Ross J., Jr, Braunwald E. Factors influencing infarct size following experimental coronary artery occlusions. Circulation. 1971 Jan;43(1):67–82. doi: 10.1161/01.cir.43.1.67. [DOI] [PubMed] [Google Scholar]
- Mittra B. Potassium, glucose, and insulin in treatment of myocardial infarction. Lancet. 1965 Sep 25;2(7413):607–609. doi: 10.1016/s0140-6736(65)90516-7. [DOI] [PubMed] [Google Scholar]
- Mjos O. D. Effect of free fatty acids on myocardial function and oxygen consumption in intact dogs. J Clin Invest. 1971 Jul;50(7):1386–1389. doi: 10.1172/JCI106621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjos O. D. Effect of inhibition of lipolysis on myocardial oxygen consumption in the presence of isoproterenol. J Clin Invest. 1971 Sep;50(9):1869–1873. doi: 10.1172/JCI106679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjös O. D. Effect of isoproterenol, glucagon and calcium on myocardial oxygen consumption in intact dogs. A comparative study. Scand J Clin Lab Invest. 1971 Oct;28(2):127–132. doi: 10.3109/00365517109086893. [DOI] [PubMed] [Google Scholar]
- Mjös O. D. Free fatty acids and oxygen consumption in dogs. Scand J Clin Lab Invest. 1971 Oct;28(2):121–125. doi: 10.3109/00365517109086892. [DOI] [PubMed] [Google Scholar]
- Mjös O. D., Kjekshus J. Increased local metabolic rate by free fatty acids in the intact dog heart. Scand J Clin Lab Invest. 1971 Dec;28(4):389–393. doi: 10.3109/00365517109095714. [DOI] [PubMed] [Google Scholar]
- Oliver M. F., Kurien V. A., Greenwood T. W. Relation between serum-free-fatty acids and arrhythmias and death after acute myocardial infarction. Lancet. 1968 Apr 6;1(7545):710–714. doi: 10.1016/s0140-6736(68)92163-6. [DOI] [PubMed] [Google Scholar]
- Owen P., Thomas M., Opie L. Relative changes in free-fatty-acid and glucose utilisation by ischaemic myocardium after coronary-artery occlusion. Lancet. 1969 Jun 14;1(7607):1187–1190. doi: 10.1016/s0140-6736(69)92168-0. [DOI] [PubMed] [Google Scholar]
- Pande S. V., Mead J. F. Inhibition of enzyme activities by free fatty acids. J Biol Chem. 1968 Dec 10;243(23):6180–6185. [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- ROSSI C. R., GIBSON D. M. ACTIVATION OF FATTY ACIDS BY A GUANOSINE TRIPHOSPHATE-SPECIFIC THIOKINASE FROM LIVER MITOCHONDRIA. J Biol Chem. 1964 Jun;239:1694–1699. [PubMed] [Google Scholar]
- Regan T. J., Harman M. A., Lehan P. H., Burke W. M., Oldewurtel H. A. Ventricular arrhythmias and K+ transfer during myocardial ischemia and intervention with procaine amide, insulin, or glucose solution. J Clin Invest. 1967 Oct;46(10):1657–1668. doi: 10.1172/JCI105657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SODI-PALLARES D., BISTENI A., MEDRANO G. A., TESTELLI M. R., DE MICHELI A. The polarizing treatment of acute myocardial infarction. Possibility of its use in other cardiovascular conditions. Dis Chest. 1963 Apr;43:424–432. doi: 10.1378/chest.43.4.424. [DOI] [PubMed] [Google Scholar]
- Scheuer J., Brachfeld N. Myocardial uptake and fractional distribution of palmitate-1 C14 by the ischemic dog heart. Metabolism. 1966 Oct;15(10):945–954. doi: 10.1016/0026-0495(66)90165-x. [DOI] [PubMed] [Google Scholar]
- Scott J. C., Gold M., Bechtel A. A., Spitzer J. J. Influence of 2,4-dinitrophenol on myocardial metabolism and hemodynamics. Metabolism. 1968 Apr;17(4):370–376. doi: 10.1016/0026-0495(68)90107-8. [DOI] [PubMed] [Google Scholar]
- Sodi-Pallares D., Ponce de León J., Bisteni A., Medrano G. A. Potassium, glucose, and insulin in myocardial infarction. Lancet. 1969 Jun 28;1(7609):1315–1316. doi: 10.1016/s0140-6736(69)92251-x. [DOI] [PubMed] [Google Scholar]
- TROUT D. L., ESTES E. H., Jr, FRIEDBERG S. J. Titration of free fatty acids of plasma: a study of current methods and a new modification. J Lipid Res. 1960 Apr;1:199–202. [PubMed] [Google Scholar]
- WINBURY M. M. Influence of glucose on contractile activity of papillary muscle during and after anoxia. Am J Physiol. 1956 Sep;187(1):135–138. doi: 10.1152/ajplegacy.1956.187.1.135. [DOI] [PubMed] [Google Scholar]
- Weissler A. M., Kruger F. A., Baba N., Scarpelli D. G., Leighton R. F., Gallimore J. K. Role of anaerobic metabolism in the preservation of functional capacity and structure of anoxic myocardium. J Clin Invest. 1968 Feb;47(2):403–416. doi: 10.1172/JCI105737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG W. C. ANAEROBIC FUNCTIONAL ACTIVITY OF ISOLATED RABBIT ATRIA. Am J Physiol. 1963 Oct;205:781–784. doi: 10.1152/ajplegacy.1963.205.4.781. [DOI] [PubMed] [Google Scholar]