Abstract
Objective
Previous studies showed that sequential exposure to estrogen and progesterone or medroxyprogesterone acetate (MPA) stimulates vascularization and promotes the progression of BT-474 and T47-D human breast cancer cell xenografts in nude mice (Liang et al, Cancer Res 2007, 67:9929). In this follow-up study, the effects of progesterone, MPA, norgestrel (N-EL) and norethindrone (N-ONE) on BT-474 xenograft tumors were compared in the context of several different hormonal environments. N-EL and N-ONE were included in the study since synthetic progestins vary considerably in their biological effects and the effects of these two progestins on the growth of human tumor xenografts are not known.
Methods
Estradiol-supplemented intact and ovariectomized Immunodeficient mice were implanted with BT-474 cells. Progestin pellets were implanted either simultaneously with estradiol pellets 2-days prior to tumor cell injection (i.e. combined), or 5-days following tumor cell injections (i.e. sequentially).
Results
Progestins stimulated the growth of BT-474 xenograft tumors independent of exposure timing and protocol, MPA stimulated the growth of BT-474 xenograft tumors in ovariectomized mice and progestins stimulated VEGF elaboration and increased tumor vascularity. Progestins also increased lymph node metastasis of BT-474 cells. Therefore, progestins, including N-EL and N-ONE, induce the progression of breast cancer xenografts in nude mice and promote tumor metastasis.
Conclusions
These observations suggests that women who ingest progestins for HT or oral contraception could be more at risk for developing breast cancer as a result of proliferation of existing latent tumor cells. Such risks should be considered in the clinical setting.
Keywords: Hormone Therapy, Breast Cancer, Progestin, MPA, xenograft
BACKGROUND
Recent studies reported development of a mouse model for studying the effect of progestins on growth of breast cancer xenograft tumors in the context of different hormonal environments (1). In this model, nude mice are sequentially implanted with estradiol prior to inoculation with tumor cells followed by implantation with progestin pellets. Estradiol supplementation supports a short burst of tumor cell growth, followed by regression and tumor cell senescence and/or apoptosis. However, supplementation with progesterone or MPA rescues tumor growth, thus providing a good model in which to examine how the hormonal environment modulates tumor growth and progression. Here, this model is further developed to compare effects of clinically-relevant progestins that are common in clinical use.
Progestins, which are widely administered to post-menopausal women in the context of hormone therapy (HT), prevent estrogen-induced proliferation of uterine cells, which may play a role in endometrial abnormalities including endometrial cancer (2, 3). However, epidemiological studies suggest that progestin-containing HT may have the adverse effect of increasing breast cancer risk (4–6). Our experimental data, as described above, are consistent with this epidemiological finding; namely, that progesterone and medroxyprogesterone acetate (MPA), a synthetic progestin, induce progression of p53-deficient, but not p53-proficient human tumor xenografts (from MCF-7 cells) in nude mice (1).
The goal of this study was to compare the effects of commonly used progestins, which vary in their biological properties (7–10), on xenograft tumor growth in intact and ovarectomized nude mice. Two androgenic progestins, norgestrel (N-EL) and norethindrone (N-ONE), as well as MPA were used in this study. Prior to inoculation, hormone pellets were administered either sequentially or simultaneously. Animals were inoculated with estrogen and progesterone receptor positive, and p53 deficient, BT-474 breast cancer cells to establish xenografts and tumor size, expression of vascular endothelial growth factor (VEGF), and tumor vascularity were monitored over time. MPA was included in some experiments as a reference compound, because it is the most commonly used progestin in HT, and we have previously documented that MPA increases proliferation of BT-474 cells in vivo (1). Exposure of ovariectomized mice to MPA mimics the hormonal environment of HT-treated post-menopausal women.
MATERIALS AND METHODS
Cell lines and culture
BT-474 breast cancer cells, which contain both estrogen and progesterone receptors, were obtained from ATCC (Manassas, VA). Cells were maintained in phenol red-free DMEM: F12 medium (Invitrogen Corporation & Life Technologies, Grand Island, NY) supplemented with 10% FBS (JRH Biosciences Lenexa, KS) in 100 × 20 mm tissue culture dishes. Cells were harvested using 0.05% trypsin-EDTA (Invitrogen Corporation & Life Technologies, Grand Island, NY).
Hormone pellets
Sixty-day release pellets containing 17β-Estradiol (1.7mg), progesterone (10 mg), MPA (10mg), norgestrel (10 mg), norethindrone (10 mg) or placebo were from Innovative Research of America (Sarasota, FL). After implantation, steady-state blood levels of the injected compounds reaches 2–10 ng/ml (Innovative Research, USA).
Progestin-dependent growth of BT-474 human breast xenografts
Intact and ovariectomized female athymic nu/nu mice, 5–6 weeks old (18–22 g) were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, IN). Mice were housed in a laminar air-flow cabinet under specific pathogen-free conditions. All facilities were approved by the American Association for Accreditation of Laboratory Animal Care in accordance with the current federal regulations and standards and all procedures were approved by an institutional ACUC committee. Nude mice were inoculated with 17β-estradiol pellets 48 h before implantation of BT-474 cells. Cells were harvested by trypsinization and washed twice with DMEM/F12 medium. Cell pellets ((1× 107 cells) were re-suspended in 0.15 ml DMEM/F12 medium, and injected subcutaneously into left and right flanks of each mouse (0.15 ml/injection). Between 3–6 mice were used for each group resulting in 6–12 tumors in each treatment group as indicated by n numbers in figures. Tumor size was measured every three days using a digital caliper and tumor volume was calculated using the formula (L × W × H) × π/6 as previously described (1,11). Tumors began to regress after reaching 60–100 mm3 in size (approximately 6–10 days). When tumor volume had decreased by approximately 50%, mice were inoculated with placebo or progestin pellets. This is referred to as the sequential protocol. In some experiments estrogen and progestin or estrogen and placebo pellets were implanted at the same time, i.e., 48 h before tumor cell inoculation. This is referred to as the combined protocol. Throughout each study animal weight and behavior was monitored as an index of toxicity. At the end of the treatment period (between days 50–60 as indicated in figures), animals were sacrificed and tumors harvested and weighed. Fresh tumor tissue was immediately placed in 4% paraformaldehyde for immunohistochemical analysis (IHC).
Immunohistochemical Assays
Tumor tissue was fixed overnight in 4% paraformaldehyde, followed by paraffin infiltration and embedding. Five μm sections were mounted onto ProbeOn Plus microscope slides (Fisher Scientific Inc., Pittsburgh, PA), stained with hematoxylin-eosin and examined for cellularity by light microscopy. Sections were de-waxed in xylene, rehydrated through graded concentrations of ethanol, and rinsed in distilled water. Sections were subjected to heat-induced epitope retrieval in 10 mM citrate buffer (pH 6.0) for 30 min and then cooled to room temperature prior to treatment with 3% hydrogen peroxide in absolute methanol (to inactivate endogenousperoxidase activity). Sections were then washed 3X with PBS, incubated in blocking buffer with 5% bovine serum albumin for 20 min and probed for 60 minutes at room temperature with one of the following antibodies: VEGF (1:200 dilution of a rabbit anti-VEGF polyclonal antibody [sc-152], Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and CD34 (1:25 dilution of a rat monoclonal anti-CD34 [ab8158–100], Abcam, Inc., Cambridge, MA). Sections were then washed and sequentially incubated with a secondary antibody. VEGF-labeled sections were incubated for 30 minutes with EnVision+, a horseradish peroxidase–labeled polymer conjugated to anti-rabbit antibodies (DAKO). Sections probed with anti-CD34 were incubated for 30 minutes with a biotinylated rabbit anti-rat IgG [DAKO] and after a wash, with a streptavidin-linked horseradish peroxidase product (DAKO) for another 30 minutes at room temperature. Bound antibodies were visualized following incubation with 3, 3′-diaminobenzidine solution (0.05% with 0.015% H2O2 in PBS; DAKO) or NovaRED substrate (Vector Laboratories, Inc., Burlingame, CA) for 3–5 minutes. Sections were counterstained with Mayer’s hematoxylin, dehydrated, cleared, and cover-slipped for microscopic examination.
The distribution of immunolabeled cells in histologic sections of tumors was determined by use of a morphometric software (FoveaPro 3.0, ©2005 Reindeer Graphics) on images photographed at 20x magnification. Nine to 12 images from 3–5 tumors per treatment group were analyzed. VEGF distribution was determined on all cells in each tumor image. Results are expressed as area in square pixels.
For blood vessel enumeration, images of CD34-labeled sections from three to four tumors per treatment group were photographed at 20x magnification. Investigators performing this determination were blinded to treatment group assignments. Total number of vessels was counted in 3 to 4 fields from each tumor section (3–4 tumors; 15 sections in total). Vessel density was calculated using vessel number per field and plotted as mean ± SEM. Data was analyzed using ANOVA and p<0.05 was considered significant.
Lymph node Metastasis
Inguinal lymph nodes were collected from the nude mice at the end of experiment and analyzed by one of us (CBW) for the presence of tumor cells following H&E staining. Investigator performing this determination was blinded to treatment group assignments. Tumor cells were identified as cohesive pleomorphic polygonal to spindle-shaped cells with large vesicular nuclei and moderate to abundant cytoplasm. Immunohistochemical labeling of MHC-1 (anti-MHC-1, sc-25619, Santa Cruz Biotechnology, Inc, Santa Cruz, CA) on tumor cells was performed on a subset of tissues to verify identity of the cells (data not shown).
Statistical Analysis
Statistical significance was tested using one-way analysis of variance (ANOVA) using SigmaStat Software version 3.5 (Sigstat Software Inc., Richmond, CA, USA). For ANOVA, the assumption of analysis of variance was examined and non-parametric measure based on ranks was used, as needed. Values were reported as mean ± SE. When ANOVA indicated significant effect (F-ratio, p<0.05), the Student-Newman-Keuls multirange test was employed to compare the means of individual groups. When normality using Student-Newman-Keuls test failed, significance was determined by Kruskal Wallis test (one-way ANOVA by ranks) with Dunn’s test. Differences in the ability of progestins to increase lymph node metastasis was conducted using logistic analysis of variances performed using GENMOD procedure in the SAS program, Cary, NC.
RESULTS
Synthetic progestins rescue growth of BT-474 xenografts: sequential protocol
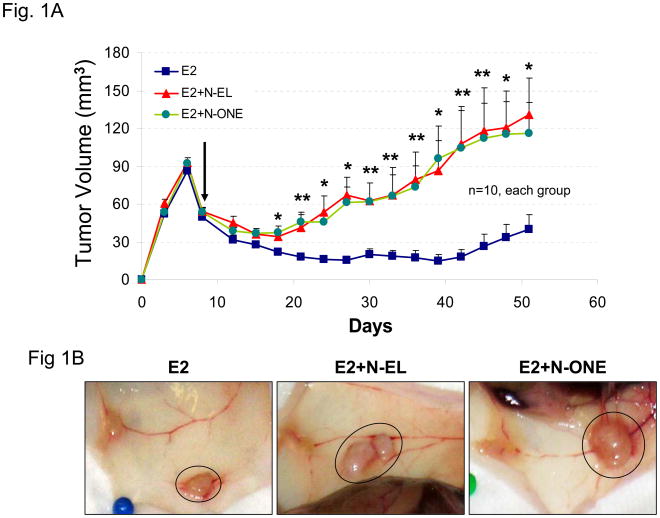
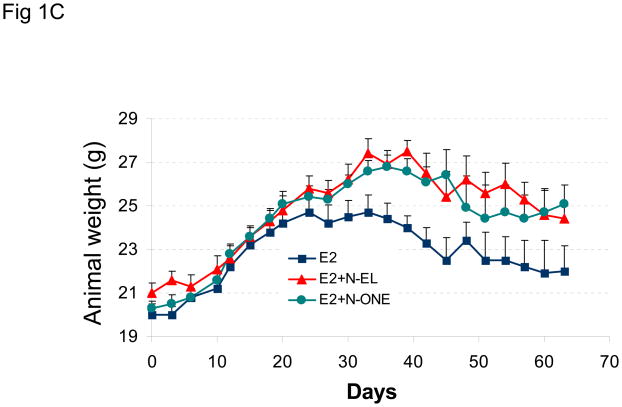
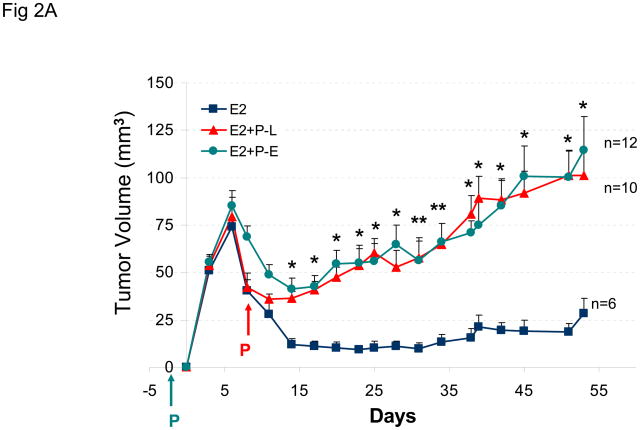
The goal of this study was to compare the effects of norgestrel (N-EL), norethindrone (N-ONE), and MPA on the growth of BT-474 tumor xenografts in nude mice. Figure 1A shows the change in tumor size over a 50 day treatment period in animals implanted sequentially with estrogen and N-EL or N-ONE hormone pellets. In animals implanted only with an estrogen pellet, tumors regressed from 6 to 10 days post-injection. In contrast, injection with N-EL or N-ONE pellets at day 8 rescued regressing tumors and promoted continued increase in the size of BT-474 xenograft tumors. Representative tumor size and morphology are shown in Figure 1B. After dosing with N-EL or N-ONE, tumor size and vascularization increased significantly. Average animal weights per treatment group over the course of the experiment are shown in Figure 1C. Progestin treatment did not reduce animal weight significantly due to toxicity. Thus treatment of BT-474 xenografts with N-EL and N-ONE led to progression of tumor growth as was observed with MPA in our previous study (1).
Figure 1. Synthetic progestins rescue growth of BT-474 xenograft tumors sequential protocol.
(A) Cells were injected into estradiol (E2)-supplemented intact nude mice (as described in Methods) followed by implantation of a norgestrel (N-EL) or norethindrone (N-ONE) pellet (arrow). Tumor size was monitored for 50 days, as indicated. (B) Representative tumors from each treatment group are shown. (C) Average body weight was calculated for each treatment group at the indicated time points. Five animals were used and n represents number of tumors in each group. *p <0.05, significantly different from E2 group (one-way ANOVA, Student-Newman-Keuls test), **p <0.05, significantly different from E2 group (one-way ANOVA, Kruskal-Wallis test). Significance values apply to both N-EL and N-ONE groups.
Synthetic progestins rescue growth of BT-474 xenografts: combined protocol
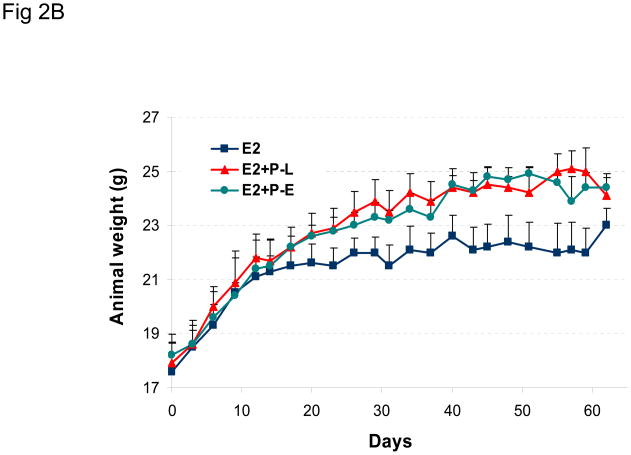
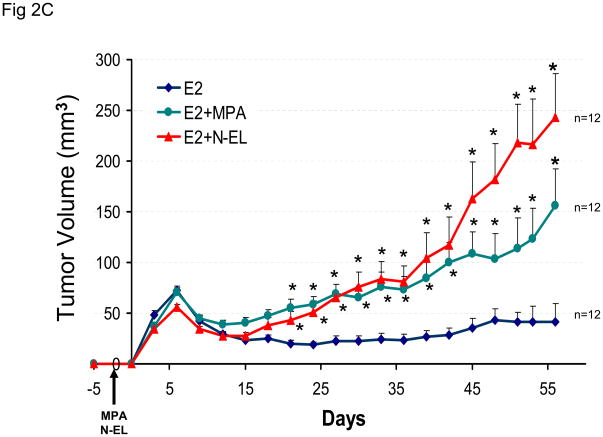
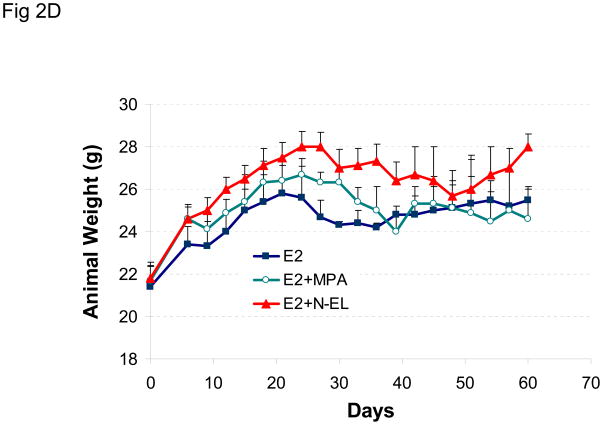
The effect of a different hormonal environment on tumor growth was tested by co-injecting mice with estradiol and progesterone prior to tumor cell inoculation. This combined treatment protocol was compared to the sequential treatment protocol, and the results are shown in Figure 2A. The results show that sequential or combined exposure to estrogen and progesterone promotes growth of BT-474 xenograft tumors to a similar extent. Similar results were obtained using the combined treatment protocol, when MPA or norgestrel was substituted for progesterone (Figure 2C). Animal weight did not vary significantly between any treatment groups (Figures 2B and 2D).
Figure 2. Synthetic progestins rescue growth of BT-474 xenograft tumors: combined protocol.
(A) BT-474 cells were injected into estrogen-supplemented intact nude mice as described in Methods and tumor growth monitored. A progesterone (P) pellet (arrow) was implanted on day 8 (red arrow; E2 +P-L) or co-implanted with estradiol (E2 +P-E) 48 h prior to injection with tumor cells (green arrow). *p <0.05, significantly different from E2 group (one-way ANOVA, Student-Newman-Keuls test), **p <0.05, significantly different from E2 group (one-way ANOVA, Kruskal-Wallis test). Significance values apply to both P-L and P-E groups. (B) Average body weight was calculated for each treatment group shown in (A) at the indicated time points. (C) MPA or norgestrel (N-EL) pellets (arrow) were co-implanted with estradiol 48 h prior to injection with tumor cells (arrow). *p <0.05, significantly different from E2 group (one-way ANOVA, Student-Newman-Keuls test) (D) Average body weight was calculated for each treatment group shown in (C) at the indicated time points.
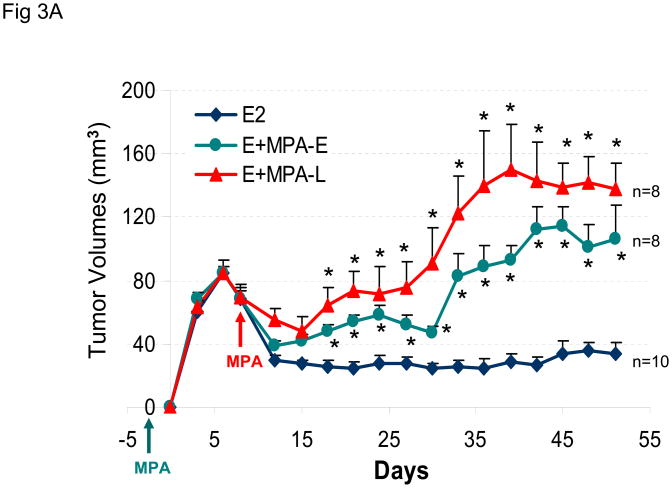
MPA enhances BT-474 tumor xenografts in ovariectomized nude mice
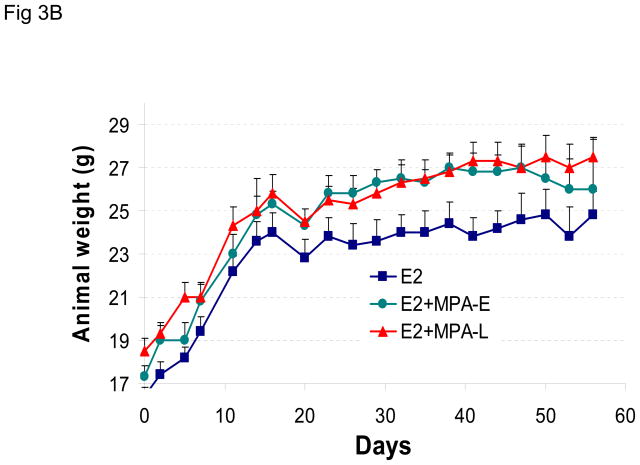
The effect of sequential or combined exposure to estrogen and MPA was also examined in ovariectomized nude mice. The results show that MPA promotes growth of BT-474 xenografts in ovariectomized nude mice, independent of the exposure protocol used in the experiment (Figure 3A). Animal weight was not adversely affected by hormone treatment during this experiment (Figure 3B).
Figure 3. MPA rescues growth of BT-474 xenograft tumors in ovariectomized mice.
(A) As in Figure 2A, except MPA pellets were implanted in ovariectomized mice 8 days after (MPA-L, red arrow) or 48 h before (MPA-E, green arrow) inoculation with tumor cells. Control animals (blue line) were co-injected with estrogen- and placebo-containing pellets. (B) Average body weight was calculated for each treatment group at the indicated time points. *p <0.05, significantly different from E2 only group ANOVA (Student-Newman-Keuls test).
Synthetic progestins increase VEGF and tumor blood vessel density
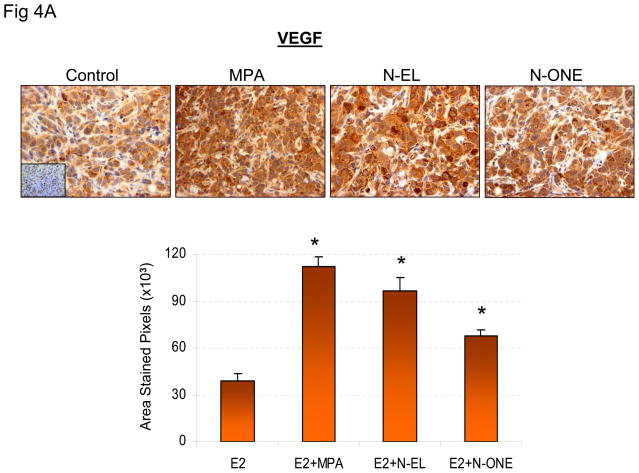
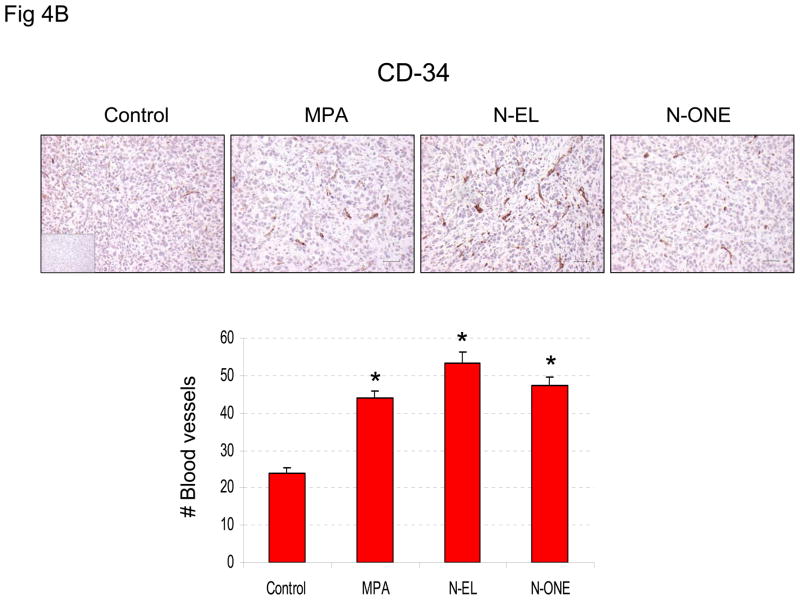
Previous in vitro and in vivo studies show that progestins induce VEGF and stimulate tumor vascularization (12–14). Therefore, the effect of N-EL and N-ONE on VEGF expression and vascularization was examined in the experimental system used here. The results (Fig 4A) showed that VEGF expression was 2- to 4-fold higher in tumors exposed to estrogen plus MPA, N-EL or N-ONE than in tumors exposed only to estrogen. Higher VEGF expression is expected to correlate with increased density of blood vessels and increased tumor vascularization. This expectation was verified by immunohistochemical quantification of CD-34, an indictor of blood vessel density (Fig 4B). The results show significantly higher expression of CD-34 in tumors exposed to estrogen plus MPA, N-EL or N-ONE than in tumors exposed only to estrogen.
Figure 4. Synthetic progestins stimulate VEGF expression (A) and CD-34 expression in BT-474 xenograft tumors.
(A) Animals were sacrificed and tumor samples collected at the end of the treatment period. Tissues were prepared and stained for VEGF (upper panel) as described in Methods. Quantification of VEGF immunoreactivity is shown in lower panel. *p<0.05, ANOVA; significantly different from E2 only group. (B) Animals were sacrificed and tumor samples collected at the end of the treatment period. Tissues were prepared and stained for CD-34 (upper panel) as described in Methods. Quantification of CD-34 immunoreactivity is shown in lower panel. *p <0.05, ANOVA (Student-Newman-Keuls test); significantly different from E2 only group. Insets, negative controls without primary antibody.
Synthetic progestins increase metastasis of tumor cells to lymph nodes
Since there have been suggestions of increased progestin-dependent metastasis in various models (15, 16), we isolated inguinal nodes from animals shown in Fig 2C and examined these for the presence of tumor cells. N-EL treatment significantly increased the frequency of tumor metastasis when compared to controls (Fig 5; p<0.01). While MPA increased lymph node infiltration of tumor cells this did not reach statistical significance compared with controls (Fig 5). However based on the differences of least squares means, the odds of MPA treatment increasing metastatic growth was 3.33 times greater than controls, and N-EL increased metastatic growth 8 times compared to MPA.
Figure 5. Incidence of lymph node metastasis in MPA- and norgestrel-stimulated BT-474 xenografts in nude mice.
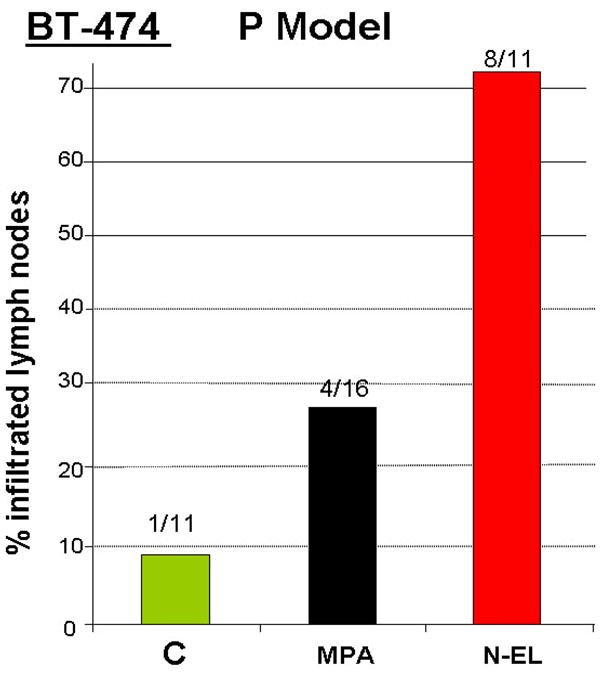
Nude mice were implanted with an E2 and progestin pellet and inoculated with BT-474 cells as described in the legend to Figure 2. Inguinal lymph glands were collected at the end of the experiment (Day 57) and the percentage of lymph nodes infiltrated with tumor cells quantified. Average tumor volume at day 57 was 50, 150, and 250 mm3 in control (C), MPA-, and norgestrel (N-EL)-treated animals, respectively. The incidence of lymph node infiltration in the norgestrel group was significantly different from control (p < 0.05, logistic analysis of variances performed using GENMOD procedure in the SAS program).
DISCUSSION
The role of progestins in the development of human breast cancer is controversial. Several recent clinical trials and clinical studies suggest that the risk of breast cancer is higher in post-menopausal women exposed to estrogen/progestin-containing HT than in those taking estrogen alone or placebo (4–6). These studies have led to a significant decrease in the use of HT, including those involving progestins, amongst postmenopausal women (17). While MPA is the most widely used progestin in HT and contraception, other progestins, including norgestrel and norethindrone are also used (18, 19). These progestins are thought to promote uterine cell differentiation, which antagonizes the proliferative effects of estrogens in the uterus. Although the exact role of progestins in the human breast is not known, a number of reports suggest they play a pro-proliferative, anti-apoptotic role in both human and rodent mammary gland (1,20–25).
Tumors appear rapidly in post-menopausal women exposed to estrogen/progestin-containing HT (4), suggesting that exposure to progestins promotes the proliferation of latent tumor cells within breast tissue (1, 6, 15). In cell culture and in vivo models, progestins induce VEGF expression in PR-positive/p53-deficient tumor cells (12, 13), which could further stimulate tumor cell growth via a paracrine mechanism (26). In a mouse model of human tumor cell-induced xenografts, the elaboration of progestin-induced VEGF was shown to be derived from the injected tumor cells and not stromal mouse cells, because the induced VEGF was targeted by an antibody to human (not murine) VEGF (1).
Due to the possibility of different synthetic progestins having varying effects on breast tumor growth (8–10), this study compared the effects of two commonly used progestins, norgestrel and norethindrone, on human breast cancer cell xenografts in nude mice. We used Her/2-neu p53-deficient BT-474 cells for this study since these cells grow aggressively and secrete VEGF in response to exposure to progestins (1). We have previously shown that RU-486, an anti-progestin, arrests progestin-dependent breast cancer cell progression, demonstrating the involvement of PR (1). Both norgestrel and norethindrone are as effective as MPA in promoting the growth of human breast cancer xenograft tumors in nude mice (Figures 1A and 1B). Not surprisingly, tumors exposed to treatment regimens containing a synthetic progestin and estrogen secrete higher levels of VEGF and express higher levels of CD34 than tumors exposed to estrogen alone, suggesting that all three progestins promote tumor vascularization (Figures 4 and 5).
In this study, the ability of progestins to rescue growth of regressing BT-474 xenograft tumors was determined, using two different hormone exposure protocols. In initial studies, progestin exposure was initiated after tumor regression began (i.e., approximately 6 days after tumor cell inoculation). However, a different hormonal environment, generated by co-implantation of estrogen and progestin pellets prior to inoculation with tumor cells, was equally effective in supporting tumor growth. Thus, in both of these hormonal environments, tumors are capable of recruiting components of extracellular matrix and stromal cells, forming blood vessels, and proliferating at a sufficient rate to support tumor growth. We believe that progestins play an active role in this process, at least in part by stimulating stromal cell functions in the vicinity of the tumor, as has been suggested by others (27). In addition, it has also been suggested that progestins may influence the epithelial-mesenchymal transition without having a direct effect on tumor cell proliferation (28). Both these possibilities will be examined further in future studies.
This study also shows that MPA promotes the growth of BT-474 xenografts in ovariectomized nude mice, independent of the exposure protocol used in the experiment. Thus, it is unlikely that intact and/or functional ovaries play a role in tumor progression in this experimental model. However, progestin-induced expression and secretion of VEGF by tumor epithelial cells is likely to be required for tumor growth and progression in this model.
Interestingly, our study demonstrated that progestins can increase the metastasis of breast cancer cells to lymph node. Although we did not detect significant differences in elevated VEGF levels and increased numbers of blood vessels in response to the different progestins (Fig 4), norgestrel exposure did lead to much bigger tumors (250 mm3) compared with MPA (150 mm3) or controls (50 mm3). Thus the increased infiltration of tumor cells observed with this particular progestin could arise as a consequence of a more aggressive growth of BT-474 tumors. It is known that progestins increase the invasiveness of tumor cells (7) and it may be that norgestrel is more potent than MPA in terms of its ability to induce such an effect, though this remains to be established. Further studies are required to determine the role played by PR in promoting metastasis. With this in mind we will use this model together with anti-progestins such as RU-486 to ascertain whether increased metastasis is PR dependent.
CONCLUSION
In conclusion, this study shows that several clinically-relevant progestins promote the growth of human breast cancer cells as xenograft tumors in nude mice. These synthetic progestins, which are administered widely as components of HT and oral contraception, could exert similar effects in human breast tissue that carry latent tumor cells or micro-tumors. Progestins also appear to increase the metastasis of breast cancer cells into lymph node. Women who are genetically-predisposed to developing breast cancer could be particularly at risk of adverse effects from progestin-containing HT. It is not yet known whether progestin-containing HT stimulates the growth of breast cancer cells at a certain pathological stage or within a critical kinetic “window” of exposure. Nevertheless, there is a need for selective progesterone receptor modulators that do not induce breast tumor cell proliferation but do inhibit estrogen-dependent proliferation of uterine cells. Recent studies have identified natural non-steroidal compounds of interest for such purposes, but their mechanism(s) of action and their ability to block estrogen-dependent proliferation of uterine cells are not yet known (29–31). Future studies should also examine the effect of progestins in the context of orthotopic cancer models in the mouse or other animal model systems.
Acknowledgments
This work was supported by NIH grant CA-86916, Komen for Cure grant BCTR 0600704, a COR award from the College of Veterinary Medicine and research funds from RADIL, University of Missouri.
We wish to thank Ms Vanessa Welbern and the RADIL Histology Laboratory (Ms Jill Hansen and Ms Jan Adair) for excellent technical assistance during this project. This work was supported by grants CA-86916, Komen for Cure grant BCTR 0600704, a COR award from the College of Veterinary Medicine and research funds from RADIL, University of Missouri. SMH is the Zalk Missouri Professor of Tumor Angiogenesis in the College of Veterinary Medicine, University of Missouri-Columbia.
Footnotes
Disclosure statement: The authors of this manuscript have nothing to disclose
References
- 1.Liang Y, Besch-Williford C, Brekken RA, Hyder SM. Progestin-dependent progression of human breast tumor xenografts: a novel model for evaluating anti-tumor therapeutics. Cancer Res. 2007;67:9929–9936. doi: 10.1158/0008-5472.CAN-07-1103. [DOI] [PubMed] [Google Scholar]
- 2.Feeley KM, Wells M. Hormone replacement therapy and the endometrium. J Clin Pathol. 2001;54:435–440. doi: 10.1136/jcp.54.6.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Gorp T, Neven P. Endometrial safety of hormone replacement therapy: review of literature. Maturitas. 2002;42:93–104. doi: 10.1016/s0378-5122(02)00031-2. [DOI] [PubMed] [Google Scholar]
- 4.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 5.Ross RK, Paganini-Hill A, Wan PC, Pike MC. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst. 2000;92:328–332. doi: 10.1093/jnci/92.4.328. [DOI] [PubMed] [Google Scholar]
- 6.Chen WY, Hankinson SE, Schnitt SJ, Rosner BA, Holmes MD, Colditz GA. Association of hormone replacement therapy to estrogen and progesterone receptor status in invasive breast carcinoma. Cancer. 2004;101:1490–1500. doi: 10.1002/cncr.20499. [DOI] [PubMed] [Google Scholar]
- 7.Fu XD, Giretti MS, Goglia L, et al. Comparative actions of progesterone, medroxyprogesterone acetate, drospirenone and nestorone on breast cancer cell migration and invasion. BMC Cancer. 2008;8:166. doi: 10.1186/1471-2407-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J, Brandt S, Hyder SM. Ligand- and cell-specific effects of signal transduction pathway inhibitors on progestin-induced vascular endothelial growth factor levels in human breast cancer cells. Mol Endocrinol. 2005;19:312–326. doi: 10.1210/me.2004-0252. [DOI] [PubMed] [Google Scholar]
- 9.Schindler AE. Differential effects of progestins on hemostasis. Maturitas. 2003;1:S31– 37. doi: 10.1016/j.maturitas.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Campagnoli C, Abba C, Ambroggio S, Lotano MR, Peris C. Differential effects of various progestogens on metabolic risk factors for breast cancer. Gynecol Endocrinol. 2007;1:22–31. doi: 10.1080/09513590701585037. [DOI] [PubMed] [Google Scholar]
- 11.El Etreby MF, Liang Y. Effect of antiprogestins and tamoxifen on growth inhibition of MCF-7 human breast cancer cells in nude mice. Breast Cancer Res Treat. 1998;49:109–117. doi: 10.1023/a:1006098910000. [DOI] [PubMed] [Google Scholar]
- 12.Hyder SM, Murthy L, Stancel GM. Progestin regulation of vascular endothelial growth factor in human breast cancer cells. Cancer Res. 1998;58:392–395. [PubMed] [Google Scholar]
- 13.Hyder SM, Chiappetta C, Stancel GM. Pharmacological and endogenous progestins induce vascular endothelial growth factor expression in human breast cancer cells. Int J Cancer. 2001;92:469–473. doi: 10.1002/ijc.1236. [DOI] [PubMed] [Google Scholar]
- 14.Mirkin S, Wong BC, Archer DF. Effects of 17beta-estradiol, progesterone, synthetic progestins, tibolone, and raloxifene on vascular endothelial growth factor and Thrombospondin-1 messenger RNA in breast cancer cells. Int J Gynecol Cancer. 2006;2:560–563. doi: 10.1111/j.1525-1438.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- 15.Carnevale RP, Proietti CJ, Salatino M, et al. Progestin effects on breast cancer cell proliferation, proteases activation, and in vivo development of metastatic phenotype all depend on progesterone receptor capacity to activate cytoplasmic signaling pathways. Mol Endocrinol. 2007;21:1335–1358. doi: 10.1210/me.2006-0304. [DOI] [PubMed] [Google Scholar]
- 16.Efeyan A, Fabris V, Merani S, Lanari C, Molinolo AA. Establishment of two hormone-responsive mouse mammary carcinoma cell lines derived from a metastatic mammary tumor. Breast Cancer Res Treat. 2004;83:233–244. doi: 10.1023/B:BREA.0000014044.02728.ac. [DOI] [PubMed] [Google Scholar]
- 17.Verkooijen HM, Bouchardy C, Vinh-Hung V, Rapiti E, Hartman M. The incidence of breast cancer and changes in the use of hormone replacement therapy: a review of the evidence. Maturitas. 2009;64:80–85. doi: 10.1016/j.maturitas.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Nath A, Sitruk-Ware R. Parenteral administration of progestins for hormonal replacement therapy. Eur J Contracept Reprod Health Care. 2009;14:88–96. doi: 10.1080/13625180902747425. [DOI] [PubMed] [Google Scholar]
- 19.Stahlberg C, Pedersen AT, Lynge E, Andersen ZJ, Keiding N, Hundrup YA, Obel EB, Ottesen B. Increased risk of breast cancer following different regimens of hormone replacement therapy frequently used in Europe. Int J Cancer. 2004;109:721–727. doi: 10.1002/ijc.20016. [DOI] [PubMed] [Google Scholar]
- 20.Soderqvist G, Isaksson E, von Schoultz B, Carlstrom K, Tani E, Skoog L. Proliferation of breast epithelial cells in healthy women during the menstrual cycle. Am J Obstet Gynecol. 1997;176:123–128. doi: 10.1016/s0002-9378(97)80024-5. [DOI] [PubMed] [Google Scholar]
- 21.De Lignieres B. Effects of progestogens on the postmenopausal breast. Climacteric. 2003;5:229–235. doi: 10.1080/713605271. [DOI] [PubMed] [Google Scholar]
- 22.Raafat AM, Li S, Bennett JM, Hofseth LJ, Haslam SZ. Estrogen and estrogen plus progestin act directly on the mammary gland to increase proliferation in a postmenopausal mouse model. J Cell Physiol. 2001;187:81–89. doi: 10.1002/1097-4652(2001)9999:9999<::AID-JCP1056>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Lydon JP, Ge G, Kittrell FS, Medina D, O’Malley BW. Murine mammary gland carcinogenesis is critically dependent on progesterone receptor function. Cancer Res. 1999;59:4276–4284. [PubMed] [Google Scholar]
- 24.Moore MR, Conover JL, Franks KM. Progestin effects on long-term growth, death, and Bcl-xL in breast cancer cells. Biochem Biophys Res Commun. 2000;277:650–654. doi: 10.1006/bbrc.2000.3728. [DOI] [PubMed] [Google Scholar]
- 25.Behera MA, Dai Q, Garde R, Saner C, Jungheim E, Price TM. Progesterone stimulates mitochondrial activity with subsequent inhibition of apoptosis in MCF-10A benign breast epithelial cells. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00209.2009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang Y, Hyder SM. Proliferation of endothelial and tumor epithelial cells by progestin-induced VEGF from human breast cancer cells: paracrine and autocrine effects. Endocrinology. 2005;146:3632–3641. doi: 10.1210/en.2005-0103. [DOI] [PubMed] [Google Scholar]
- 27.Giulianelli S, Cerliani JP, Lamb CA, et al. Carcinoma-associated fibroblasts activate progesterone receptors and induce hormone independent mammary tumor growth: A role for the FGF-2/FGFR-2 axis. Int J Cancer. 2008;123:2518–2531. doi: 10.1002/ijc.23802. [DOI] [PubMed] [Google Scholar]
- 28.Sartorius CA, Harvell DM, Shen T, Horwitz KB. Progestins initiate a luminal to myoepithelial switch in estrogen-dependent human breast tumors without altering growth. Cancer Res. 2005;65:9779–9788. doi: 10.1158/0008-5472.CAN-05-0505. [DOI] [PubMed] [Google Scholar]
- 29.Liang Y, Wu J, Stancel GM, Hyder SM. p53-dependent inhibition of progestin-induced VEGF expression in human breast cancer cells. J Steroid Biochem Mol Biol. 2005;93:173–1782. doi: 10.1016/j.jsbmb.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Carroll CE, Ellersieck MR, Hyder SM. Curcumin inhibits MPA-induced secretion of VEGF from T47-D human breast cancer cells. Menopause. 2008;15:570–574. doi: 10.1097/gme.0b013e31814fae5d. [DOI] [PubMed] [Google Scholar]
- 31.Carroll CE, Benakanakere I, Besch-Williford C, Ellersieck MR, Hyder SM. Curcumin delays development of medroxyprogesterone acetate-accelerated 7,12-dimethylbenz[a]anthracene-induced mammary tumors. Menopause. 2009 doi: 10.1097/gme.0b013e3181afcce5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]