Abstract
Importance of the field
Due to growing concerns over toxic or active metabolites, significant efforts have been focused on qualitative identification of potential in vivo metabolites from in vitro data. However, limited tools are available to quantitatively predict their human exposures.
Areas covered in this review
Theory of clearance predictions and metabolite kinetics is reviewed together with supporting experimental data. In vitro and in vivo data of known circulating metabolites and their parent drugs was collected and the predictions of in vivo exposures of the metabolites were evaluated.
What the reader will gain
The theory and data reviewed will be useful in early identification of human metabolites that will circulate at significant levels in vivo and help in designing in vivo studies that focus on characterization of metabolites. It will also assist in rationalization of metabolite-to-parent ratios used as markers of specific enzyme activity.
Take home message
The relative importance of a metabolite in comparison to the parent compound as well as other metabolites in vivo can only be predicted using the metabolites in vitro formation and elimination clearances, and the in vivo disposition of a metabolite can only be rationalized when the elimination pathways of that metabolite are known.
Keywords: metabolite pharmacokinetics, in vitro-to-in vivo prediction, prediction of circulating metabolites, metabolic clearance
1. Introduction
A metabolite can be formed from any enzymatic transformation of a parent drug after the parent is administered in vivo or is incubated as substrate in vitro. Often, these metabolites prove to have in vivo pharmacologic activity. Classic examples of metabolites that have pharmacologic activity are metabolites of tricyclic antidepressants and benzodiazepine anxiolytics, where many of the metabolites are also marketed drugs [1, 2]. Metabolites can also possess toxicological activity. Examples of in vivo toxic metabolites have been well established for many parent drugs, such as carbamazepine, valproic acid and nefazodone [3–5]. Additionally, it has been demonstrated that some in vivo inhibitors have inhibitory metabolites of similar potencies, such as fluoxetine, itraconazole and atomoxetine [6–8]. Due to the realization that metabolites can oftentimes have in vivo activity, it is important to understand the disposition of a metabolite after the administration of a parent drug.
A recent FDA guidance on metabolites in safety testing (MIST) has drawn more attention to identifying and predicting human metabolites [9]. This guidance states that a metabolite found to circulate at equivalent or greater concentrations in at least one pre-clinical animal species when compared to in human has been adequately evaluated for safety and no further non-clinical testing is warranted. If this cannot be demonstrated, any metabolite with exposure > 10% of the parent at steady-state in humans warrants separate non-clinical toxicological and pharmacokinetic studies. In contrast to the MIST guidance, the European guidance states that separate studies are only warranted when a metabolite exposure is > 10 % of the total drug-related material exposure [10]. These guidance pose two important dilemmas in new drug development: 1) how to identify and reliably predict potentially important circulating metabolites sufficiently early in new drug development to allow timely synthesis of reference material, development of validated assays and toxicological evaluation, and 2) how to determine the steady-state area under the plasma concentration versus time curve for the metabolite (AUCm) relative to the parent (AUCp), or total drug related material, for relevant metabolites without performing elaborate multiple dose studies with radiolabeled drug.
In vitro metabolism and pre-clinical animal data as well as single dose pharmacokinetic data are often used to predict the in vivo steady-state disposition of new drug candidates, as well as the in vivo metabolite profile of a candidate drug. However, attempts to predict important circulating metabolites in humans from pre-clinical data are qualitative and have met with variable success [11]. Direct translation of the metabolite profile from animal species to humans may be confounded by species differences in enzyme activity and expression, whereas in vitro HLM and hepatocyte studies qualitatively identify the primary metabolites that are likely to be formed in vivo but detection of secondary metabolites remains challenging. In this review, established in vivo metabolite kinetic theory will be discussed and a method for predicting in vivo metabolite disposition from in vitro data will be presented and evaluated for its usefulness in preclinical prediction of metabolite exposure as well as in rationalization of in vivo metabolite exposures.
2. Metabolite Kinetic Theory: In vivo Aspects
During the late 1960’s through to the early 1980’s, much interest was paid to the development of pharmacokinetic theory that describes the in vivo disposition of a metabolite formed after administration of a parent drug. The metabolite plasma concentration (Cm) versus time (t) curve for a metabolite formed after intravenous (IV) administration will exhibit biphasic kinetics and depend on the dose of parent (D), the fraction of parent that is converted to metabolite (fm), the metabolite volume of distribution (Vd,m) and the formation (kf) and elimination (km) rate constants for the metabolite [12]:
| (1) |
This expression dictates that the slope of the linear terminal portion of a metabolite concentration versus time profile will either be equal to that of the parent, i.e. formation rate limited (FRL, kf < km) kinetics, or less than that of the parent, i.e. elimination rate limited (ERL, kf < km) kinetics [13]. After oral (PO) administration, the metabolite concentration versus time profile will further depend on the fraction of drug absorbed into the body (Fa) and will be either biphasic or triphasic, depending on the efficiency of metabolite formation during first pass metabolism [14].
The AUCm after either IV or PO administration of the parent was demonstrated to be determined by D, Fa (only after PO administration), fm and the clearance of the metabolite (Clm) [15, 16]. Additionally, the in vivo metabolite-to-parent AUC ratio (AUCm/AUCp) was determined to be dependent only on the in vivo formation and elimination clearances for the metabolite [17, 18]. Patel et al [18], demonstrated that after IV administration of a drug the ratio of AUCm to the AUCp is:
| (2) |
where Clp and Clm are the total in vivo clearances of the parent and the metabolite, respectively, and Clf is the formation clearance of the metabolite. The assumptions that are made in this model are that 1) the kinetics of the parent and metabolite are linear, 2) only one metabolite is formed from the parent (i.e. fm equals the fraction of drug excreted unchanged subtracted from unity) and 3) all metabolite formed is available to the systemic circulation. It should be noted, that the above expression can be adapted to PO administration if metabolism occurs only in the liver and Clp is the apparent oral clearance of parent, i.e. the quotient of the true clearance of parent and the fraction of parent that escapes first pass in the liver (Fh):
| (3) |
The AUCm/AUCp is a primary measure utilized in the MIST guidance and hence, this theory was reexamined to address the first dilemma posed by the guidance. Although the in vivo expression for AUCm/AUCp has been available for 25 years, it has only been tested in the norclobazam/clobazam metabolite/parent (M/P) pair [17]. In order to further validate the above kinetic theory, literature data for three M/P pairs, morphine/codeine, morphine-6-glucuronide/morphine and theophylline/caffeine, were reviewed with Equations 2 and 3 to test the existing metabolite kinetic theory in an in vivo-to-in vivo extrapolation of the AUCm/AUCp. For these three M/P pairs, the clearance values for both the parents and metabolites were available following IV administration. The literature clearance values utilized for codeine, morphine, morphine-6-glucuronide, caffeine and theophylline were 63, 120, 7.5, 6.7 and 0.59 L/hr, respectively [19–22]. The Fh values utilized for the parents codeine, morphine and caffeine were 0.5, 0.2 and 1, respectively [23, 24]. The fm values utilized for the formation of the metabolites morphine, morphine-6-glucuronide and theophylline were 0.1, 0.1 and 0.03, respectively [25–27]. Since codeine, morphine and caffeine are highly water soluble and readily absorbed from the gastrointestinal tract, Fa is expected to be unity for these drugs [24, 26, 27]; hence, the bioavailability values utilized for the predictions are considered to exclusively represent the fraction of drug eliminated during first pass hepatic metabolism (Fh). Table 1 summarizes the observed and predicted AUCm/AUCp values for these M/P pairs. Using the known in vivo clearance values, the predictions were within a 3-fold error in comparison to the average observed AUCm/AUCp for all 3 M/P pairs after both IV and PO administration.
Table 1.
The AUCm/AUCp observed in vivo and predicted using observed Clp, Clm, fm, and bioavailability (F) values for three M/P pairs for which in vivo clearance data of the metabolite were available.
| Parent | Metabolite | Route of Administration |
Observed AUCm/ AUCp (Range) |
Predicted AUCm/AUCp |
Predicted/ Observed (Range) |
References |
|---|---|---|---|---|---|---|
| Codeine | Morphine | IV | 0.068a | 0.053 | 0.71 | 1, 2, 3 |
| PO | 0.071 (0.019 – 0.17) | 0.11 | 1.5 (0.64 – 5.8) | |||
| Morphine | Morphine-6- glucuronide |
IV | 1.4a | 1.6 | 1.1 | 4, 5, 6 |
| PO | 2.8 (2.8 – 2.8) | 7.9 | 2.8 (2.8 – 2.8) | |||
| Caffeine | Theophylline | PO | 0.12 (0.11 – 0.14) | 0.34 | 2.8 (2.4 – 3.1) | 7, 8 |
Notes: Where multiple data sets exist, the ranges of AUCm/AUCp values are provided in parentheses. All observed AUCm/AUCp values were determined after single dose administration.
Superscript ‘a’ indicates that only one in vivo study was available. Reference numbers coincide with the below provided references and not with the bibliography references.
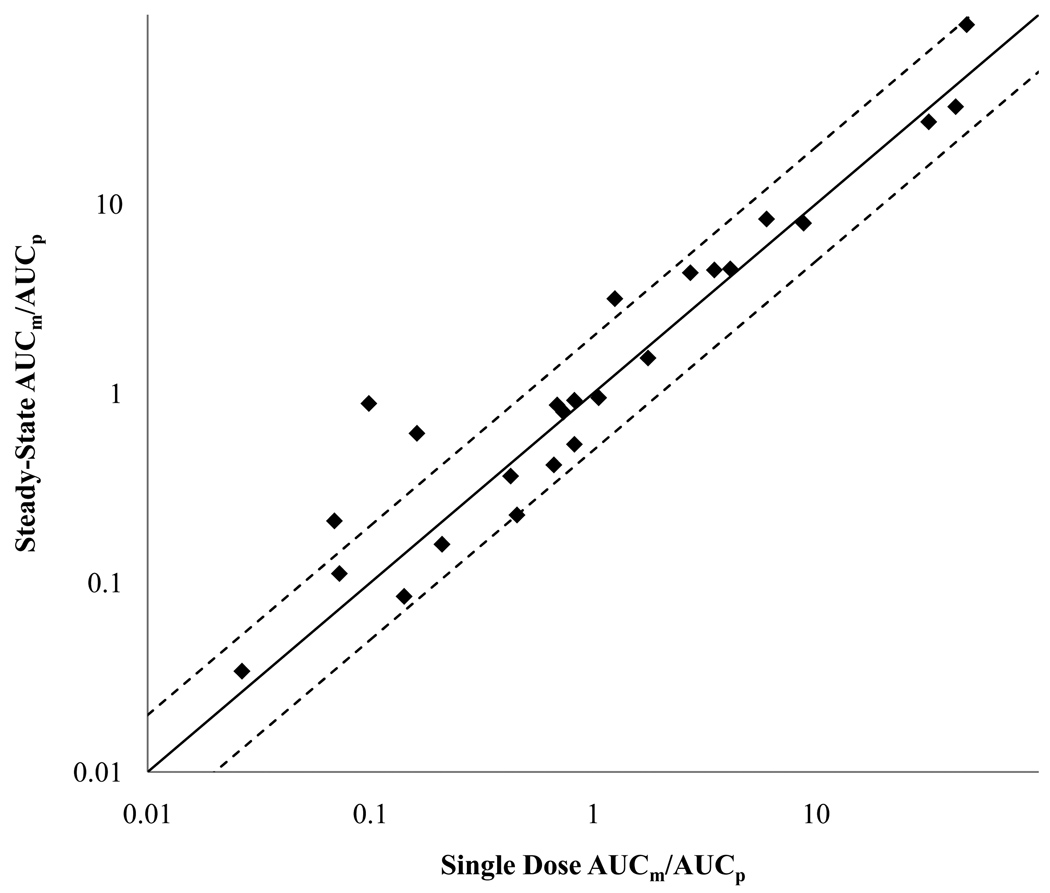
The second dilemma posed by the MIST guidance is how to determine the steady-state AUCm/AUCp for relevant, potentially yet unidentified, metabolites without performing elaborate multiple dose studies with radiolabeled drug. Based on established pharmacokinetic theory [28] single dose data could be used as a measure of steady-state AUCm/AUCp as long as clearance is constant (linear kinetics). A classic study demonstrated in nonhuman primates that the AUC0-∞ ratios of carbamazepine epoxide/carbamazepine and 3-bromocinnamamide/cinromide P/M pairs after single dose are identical to the AUC0-τ ratios at steady-state [29]. To further examine this relationship, the AUCm/AUCp for all M/P pairs with both single dose and steady-state in vivo data were collected. In total, 25 M/P pairs were examined (Figure 1 and Table 2). There was no statistically significant difference between single dose and steady-state AUCm/AUCp (Wilcoxon Signed Rank p-value > 0.47) and 84% of the M/P pairs included in the analysis had a single dose AUCm/AUCp within 2-fold of the steady-state AUCm/AUCp. 56% of the M/P pairs analyzed contained a metabolite that displayed ERL kinetics and hence the metabolite accumulation to steady-state is determined by its elimination half-life rather than the half-life of the parent (Table 2). Therefore parent drug will reach steady state before the metabolite. This presents a fundamental dilemma in study design since it is impossible to know whether a metabolite has reached steady-state, before relevant in vivo metabolites are identified and characterized. The ambiguity of when metabolite steady-state is reached is emphasized by the fact that over half of the metabolites examined between single dose and steady-state, displayed elimination rate limited kinetics. This analysis suggests that single dose AUCm/AUCp data can be used as a surrogate for steady-state AUCm/AUCp and may be more reliable. However, it is critical to confirm that after single dose administration, samples are collected for 4 to 6 parent or metabolite half-lives, whichever is longer, to capture AUC0-∞.
Figure 1. Relationship between the single dose and steady-state in vivo AUCm/AUCp for 25 M/P pairs.
The solid line indicates no difference and the dashed line indicates a 2-fold difference between single dose and steady-state dosage regimens.
Table 2.
The 25 M/P pairs utilized to examine the difference between single dose and steady-state AUCm/AUCp. Included is the AUCm/AUCp after a single dose and at steady-state and whether or not the metabolite is elimination rate limited.
| Parent | Metabolite | Elimination Rate Limited? |
Single Dose AUCm/AUCp |
Steady-State AUCm/AUCp |
References |
|---|---|---|---|---|---|
| Allopurinol | Oxypurinol | Yes | 42 | 33 | 1, 2 |
| Alprazolam | 4-hydroxyalprazolam | No | 0.073 | 0.11 | 3, 4, 5 |
| 1-hydroxyalprazolam | Yes | 0.027 | 0.034 | ||
| Atorvastatin | 2-hydroxyatorvastatin | Yes | 0.69 | 0.87 | 6, 7 |
| 4-hydroxyatorvastatin | Yes | 0.098 | 0.88 | ||
| Clarithromycin | 14-hydroxyclarithromycin | Yes | 0.67 | 0.42 | 8, 9 |
| Ezetimibe | Ezetimibe-O-glucuronide | No | 8.8 | 7.9 | 10, 11 |
| Hydralazine | Hydralazine pyruvic acid hydrazone |
Yes | 47 | 89 | 12 |
| Imatinib | N-desmethylimatinib | Yes | 0.21 | 0.16 | 13, 14 |
| Lansoprazole | 5-hydroxylansoprazole | No | 0.14 | 0.085 | 15, 16 |
| Lansoprazole sulfone | No | 0.16 | 0.61 | ||
| Modafinil | Modafinil acid | No | 0.43 | 0.37 | 17, 18 |
| Nelfinavir | Nelfinavir hydroxy-t-butylamide | No | 0.45 | 0.23 | 19 |
| Nortriptyline | 10-hydroxynortriptyline | No | 1.3 | 3.2 | 20, 21 |
| Omeprazole | 5-hydroxyomeprazole | No | 0.82 | 0.92 | 22, 23 |
| Omeprazole sulfone | Yes | 0.82 | 0.54 | ||
| Oxcarbazepine | 10-hydroxycarbazepine | Yes | 32 | 27 | 24, 25 |
| Pentoxifylline | 1-(3-carboxypropyl)-3,7- dimethylxanthine |
Yes | 6.0 | 8.3 | 26, 27 |
| 1-(4-carboxybutyl)-3,7- dimethylxanthine |
Yes | 0.74 | 0.80 | ||
| 1-(5-hydroxyhexyl)-3,7- dimethylxanthine |
Yes | 4.1 | 4.5 | ||
| Risperidone | Paliperidone | No | 3.5 | 4.5 | 28, 29 |
| Sertraline | N-desmethylsertraline | Yes | 1.8 | 1.5 | 30, 31 |
| Sulfinpyrazone | Sulfinpyrazone sulfone | No | 0.069 | 0.21 | 32, 33 |
| Terbinafine | N-desmethylterbinafine | No | 1.1 | 0.95 | 34, 35 |
| Venlafaxine | Desvenlafaxine | Yes | 2.7 | 4.3 | 36, 37 |
Notes: The AUC for both parent and metabolite after single dose was assured to represent the AUC0-∞, and the AUC0-τ for both parent and metabolite at steady-state were assured to be truly under steady-state conditions. In the studies of hydralazine and nelfinavir, the AUCm/AUCp was determined after pairing single dose and multiple doses in the same subjects. Reference numbers coincide with the below provided references and not with the bibliography references.
References: 1Ayalasomayajula et al, 2008; 2Guerra et al, 2001; 3Buch et al, 1993; 4Greene et al, 1995; 5Wennerholm et al, 2005; 6Lau et al, 2007; 7Tekturna New Drug Application, 2007; 8Cheng et al, 1998; 9Hassan-Alin et al, 2006; 10Bergman et al, 2006; 11Reyderman, 2005; 12Shepherd et al, 1980; 13Egorin et al, 2009; 14Van Erp et al, 2007; 15Kim et al, 2002; 16Ieiri et al, 2001; 17Wong et al, 1998; 18Wong et al, 1999; 19Damle et al, 2009; 20Laine et al, 2001; 21Yue et al, 1998; 22He et al, 2003; 23Shirai et al, 2001; 24Keranen et al, 1992; 25Theis et al, 2005; 26Mauro et al, 1992; 27Smith et al, 1986; 28Bialer et al, 2004; 29Bondolfi et al, 2002; 30Allard et al, 1999; 31Hamelin et al, 1996; 32Bradbrook et al, 1982; 33Rosenkranz et al, 1983; 34Kovarik et al, 1992; 35Robbins et al, 1996; 36Lindh et al, 2003 and 37Troy et al, 1995.
3. Metabolite Kinetic Theory: In vitro-to-In vivo Extrapolations
3.1 Qualitative Predictions of In vivo Metabolite Exposure
There has been a considerable amount of discussion on how to identify important circulating metabolites during pre-clinical phases of development of a new drug candidate [11, 30]. Advances in and increased access to analytical technologies have made metabolite identification a routine part of new drug development. Most techniques focus on metabolic incubations of either a radiolabeled (if available) or nonradiolabeled new drug candidate to generate potential metabolites. The products of these incubations are then subjected to ultra performance liquid chromatography, to separate closely chemically related species, and coupled to either accurate mass spectrometry or NMR spectroscopy for structural determination [31–33]. This procedure results in the identification and quantification of potential metabolites formed from the new drug candidate, but the relative abundance of each metabolite formed in vitro often does not agree with its relative abundance in vivo [11].
Intuitively, one would expect that a major metabolite in HLMs or hepatocytes would also be a major metabolite in plasma. However, the clearances of primary metabolites vary, even within closely chemically related species such as two primary metabolites of the same parent. The relative exposure to different metabolites formed from the same parent drug will depend on the rank order of the ratio of formation clearance to elimination clearance for each metabolite (Equation 2). Hence, a major metabolite observed in HLMs or hepatocytes will not be dominant in vivo unless it has sufficiently low elimination clearance in comparison to other metabolites formed and in comparison to the parent drug. Whether this is clinically important was tested using published literature data of all M/P pairs for which both in vivo AUCm/AUCp and in vitro metabolite intrinsic formation clearance (Cli,f) data for parent drug with at least two metabolites was available. In total, 31 M/P pairs from 14 parent drugs were examined. Table 3 summarizes the in vitro Cli,f and the observed in vivo AUCm/AUCp ratios for each M/P pair examined for a given parent drug. AUCm/AUCp ratios for each M/P pair, for a given parent, were considered to rank correctly with respect to in vitro Cli,f if the M/P pair with a > 15% higher Cli,f also had a > 15% AUCm/AUCp. Only 7 parent drugs (50%) had AUCm/AUCp ratios for their respective metabolites that rank ordered correctly. One of the parent drugs examined in the rank order analysis was clomipramine. Clomipramine has three important metabolites: 8-hydroxyclomipramine, N-desmethylclomipramine and 2-hydroxyclomipramine. Based solely on in vitro Cli,f, N-desmethylclomipramine would be predicted to be the major metabolite, followed by equal exposures to 8-hydroxy and 2-hydroxyclomipramine. However, 8-hydroxyclomipramine can be detected in vivo in plasma at 40% of the parent whereas 2-hydroxyclomipramine is undetectable in plasma. This can be explained by the greater intrinsic elimination clearance of 2-hydroxyclomipramine when compared to 8-hydroxyclomipramine, 6.5 versus 1.5 µL/min/mg microsomal protein [34]. Incorrect rank ordering of metabolites for a given parent drug for half of the cases examined demonstrates that the consideration of only in vitro metabolite formation clearance is not sufficient for predicting the relative importance of a given metabolite in vivo.
Table 3.
Rank order analysis of the in vitro Cli,f and the in vivo AUCm/AUCp for M/P pairs of a given parent drug.
| Parent | Metabolite |
In vitro Cli,f (µL/min/mg) |
In vivo AUCm/AUCp |
Rank Order Correctly? |
References |
|---|---|---|---|---|---|
| Alprazolam | 1-hydroxyalprazolam | 0.51 | 0.11 | No | 1, 2, 3 |
| 4-hydroxyalprazolam | 4.1 | 0.034 | |||
| Atorvastatin | 2-hydroxyatorvastatin | 20 | 0.87 | Nob | 4, 5 |
| 4-hydroxyatorvastatin | 9.1 | 0.88 | |||
| Caffeine* | Theophylline | 0.072 | 0.14 | Yes | 6, 7 |
| Paraxanthine | 1.4 | 0.48 | |||
| Theobromine | 0.019 | 0.080 | |||
| Clomipramine | 2-hydroxyclomipramine | 0.98 | NDa | Nob | 8, 9 |
| 8-hydroxyclomipramine | 0.94 | 0.36 | |||
| N-desmethylclomipramine | 16 | 2.2 | |||
| Codeine* | Morphine | 0.23 | 0.029 | No | 10, 11, 12 |
| Norcodeine | 1.1 | 0.25 | |||
| Codeine-6-glucuronide | 0.45 | 9.2 | |||
| Imipramine* | Desipramine | 8.3 | 0.54 | Yes | 13, 14 |
| 2-hydroxyimipramine | 2.8 | 0.37 | |||
| Lansoprazole | 5-hydroxylansoprazole | 19 | 0.085 | No | 15, 16 |
| Lansoprazole sulfone | 10 | 0.61 | |||
| Morphine* | Morphine-3-glucuronide | 8.4 | 23 | Yes | 17, 18 |
| Morphine-6-glucuronide | 1.8 | 2.8 | |||
| Nefazodone | Hydroxynefazodone | 239 | 0.36 | Yes | 2, 19 |
| Meta-chlorophenylpiperazine | 190 | 0.090 | |||
| Omeprazole | 5-hydroxyomeprazole | 24 | 0.92 | Yes | 20, 21 |
| Omeprazole sulfone | 20 | 0.54 | |||
| Propafenone | 5-hydroxypropafenone | 530 | 0.50 | Yes | 22, 23 |
| N-desalkylpropafenone | 3.5 | 0.064 | |||
| Quinidine | 3-hydroxyquinidine | 15 | 0.24 | No | 24, 25 |
| Quinidine N-oxide | 2.9 | 0.37 | |||
| Tamoxifen | 4-hydroxytamoxifen | 2.3 | 0.034 | Yes | 26, 27 |
| N-desmethyltamoxifen | 31 | 2.1 | |||
| Theophylline* | 3-methylxanthine | 0.012 | 0.035 | Nob | 28, 29 |
| 1,3-dimethyluric acid | 0.16 | 0.032 | |||
Notes: The in vitro Cli,f and in vivo AUCm/AUCp values are provided. Additionally, whether or not the metabolites of a given parent rank ordered correctly with respect to these two values is provided. All in vivo AUCm/AUCp values were determined under steady-state conditions except for those parent drugs marked with an asterisk, which were determined after single dose administration.
Superscript ‘a’ indicates a value not determined because the observed in vivo metabolite concentrations were not detectable.
Superscript ‘b’ indicates a set of M/P pairs for a given parent drug that were considered to not rank order correctly because the in vitro Cli,f values exhibited a > 15% difference but the AUCm/AUCp values did not or the AUCm/AUCp values exhibited a > 15% difference but the in vitro Cli,f values did not. Reference numbers coincide with the below provided references and not with the bibliography references.
References: 1Buch et al, 1993; 2Greene et al, 1995; 3Hirota et al, 2001; 4Park et al, 2008; 5Tekturna New Drug Application, 2007; 6Labedzki et al, 2002; 7Akinyinka et al, 2000; 8Kramer-Nielsen et al, 1996; 9Linnoila et al, 1982; 10Soars et al, 2001; 11Yue et al, 1997; 12Yue et al, 1991b; 13Skelbo et al, 1992; 14Koyama et al, 1994; 15Ieira et al, 2001; 16Kim et al, 2003; 17Morrish et al, 2006; 18Eliot et al, 2002; 19VonMoltke et al, 1999; 20Shirai et al, 2001; 21Shu et al, 2000; 22Dilger et al, 1999; 23Hemeryck et al, 2000; 24Nielsen et al, 1999; 25Schellens et al, 1991; 26Desta et al, 2004; 27Lien et al, 1990; 28Tjia et al, 1996 and 29Rodopoulos et al, 1996.
3.2 Quantitative Predictions of In vivo Metabolite Exposure
The in vivo metabolite kinetic theory developed by Pang et al, Houston et al and others laid the foundation of in vivo metabolite pharmacokinetics but these theories have not yet been applied to in vitro-to-in vivo extrapolation, a useful tool in anticipating the in vivo pharmacokinetics of a parent drug during new drug development. The prediction of in vivo clearance of drugs based on in vitro metabolism data is well established, although predictions have varying degrees of accuracy [35, 36]. In an extensive analysis of scaling in vitro HLM clearance values to in vivo clearance using multiple hepatic clearance models and plasma protein binding considerations, 29 drugs with varying physicochemical properties were predicted with a 2.14 to 4.39 average fold error [37]. Another study of scaling in vitro Cli values from human hepatocyte data for 50 drugs, obtained a 2.5 average fold error to the observed in vivo clearance, with outliers having up to 15-fold error [38]. Although quantitative in vitro-to-in vivo clearance prediction for a parent drug is now commonplace, little attention has been paid to the prediction of the in vivo disposition of a metabolite from in vitro metabolism data.
The MIST guidance requires the evaluation of absolute steady-state AUCm between pre-clinical animal species and human for major metabolites. If similar exposure is not obtained in animals, additional safety testing of the metabolite may be required. This requirement generates a need to predict, prior to clinical studies, what metabolites will be quantitatively important in humans. Predicting absolute AUCm values in humans poses a significant challenge because the result will depend on the dose of the parent, the fraction of the dose absorbed after PO administration, and the overall clearance of the parent drug. In addition, the AUCm will depend on the metabolite specific parameters, such as the fraction of the dose converted to the metabolite of interest and the metabolite clearance. Within the MIST guidance, the secondary qualification of the relative exposure to the metabolite in human, i.e. AUCm/AUCp > 0.1, appears more conducive to prediction. The AUCm/AUCp is independent of the parent dose and fraction absorbed after PO administration and the predicted AUCm/AUCp can be utilized as a proportionality constant for anticipating the absolute levels of the metabolite of interest when a desired AUC or steady-state concentration of parent is ascertained. This can be illustrated via a review of the existing data on desipramine as a metabolite of imipramine, which demonstrates that within a 4-fold range of in vivo doses of imipramine, the AUCm/AUCp remains constant (Table 4) in cytochrome P450 (CYP) 2D6 extensive metabolizers (EMs). The absolute steady-state concentration of desipramine could be predicted by use of the steady-state concentration of imipramine and the AUCm/AUCp ratio. Normalizing the predicted metabolite exposure to that of the parent also provides valuable insight into whether the metabolite will be quantitatively important in vivo, regardless of the parent dose.
Table 4.
The effect of dose and CYP2D6 phenotype on the imipramine to desipramine AUCm/AUCp ratio after PO administration of imipramine.
The prediction of in vivo AUCm/AUCp from in vitro parameters relies on methods for clearance predictions of both parent and metabolite. This is because an important principle of metabolite kinetics is that the in vivo disposition of a metabolite is dependent not only on its formation clearance, but also its elimination. Based on this principle, predicting relative exposure to human metabolites can only be done if the formation and elimination clearances for the metabolite are predicted.
To adapt Equation 2 to in vitro-to-in vivo extrapolation of AUCm/AUCp after intravenous (IV) or oral (PO) administration, four assumptions were made: 1) the kinetics of both parent and metabolite are linear, 2) all metabolite formed is available to the systemic circulation, 3) parent and metabolite elimination is via metabolism only and 4) metabolism occurs only in the liver which can be represented by the well-stirred model [39]:
| (4) |
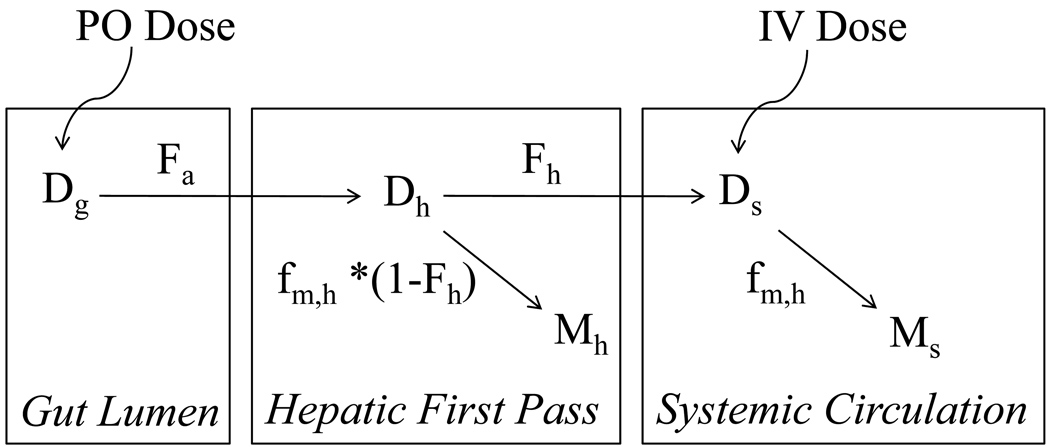
where CLh is the hepatic clearance, Q is the hepatic blood flow, fu, is the plasma fraction unbound and Cli is the hepatic intrinsic metabolic clearance. This clearance model can be applied to either the metabolite or the parent drug. In addition to this well-stirred model of the liver, two other prominent hepatic clearance models, the parallel tube and dispersion model, could be used for predictions [40]. At present the potential advantages of the alternative hepatic clearance models for predicting metabolite formation and elimination clearances are unknown and require further study. The well-stirred model was chosen for this analysis because it is the most commonly utilized hepatic clearance model and easiest to adapt for predictions. Furthermore, a general metabolic scheme based on the scheme of Houston and Taylor [14] can be considered for both IV and PO administration (Figure 2). In this scheme, Dg, Dh and Ds are the amounts of drug in the gut lumen, liver during first pass and systemic circulation, respectively. Fa and Fh are the fraction of drug absorbed from the gut lumen into the enterocytes and fraction of drug that escapes first pass elimination in the liver, respectively, and Mh and Ms refer to the amount of metabolite formed from first pass in the liver and from systemic elimination, respectively. The in vivo fraction of parent converted to the metabolite of interest, when the parent is cleared only through hepatic metabolism was previously defined as the fraction of hepatic parent drug clearance that results in the metabolite of interest [41]. This definition was adapted to in vitro parameters and defined as fm,h:
| (5) |
where Cli,f and Cli,f are the intrinsic formation clearance of the metabolite in vitro and intrinsic elimination clearance of the parent in vitro, respectively. When the parent is cleared entirely via hepatic metabolism, the in vivo fm for the metabolite of interest is equal to the in vitro fm,h. Utilizing this metabolic scheme, the AUCm after PO administration can be defined as:
| (6) |
and the AUCp after PO administration can be defined as:
| (7) |
Figure 2. Schematic representation of the metabolic fate of a parent drug after PO or IV administration.
In this scheme, Dg, Dh and Ds are the amounts of drug in the gut lumen, liver during first pass and systemic circulation, respectively. Fa and Fh are the fractions of drug that are absorbed into the enterocytes and that escape first pass elimination in the liver, respectively. The fm,h term is the fraction of hepatic metabolism that results in the metabolite of interest and Mh and Ms refer to the amount of metabolite of interest, formed from first pass in the liver and systemic elimination, respectively.
By definition [42], the hepatic bioavailability (Fh) is a function of the extraction ratio of the parent (ERp):
| (8) |
Substituting for Fh and the well-stirred model for Clp and Clm (as defined by Equations 9 and 5, respectively) into the quotient of Equations 7 and 8 yields:
| (9) |
After intravenous administration, by substituting fm,h for fm (Equation 6) and the well-stirred model for Clm and Clp (Equation 5), Equation 2 can be defined as:
| (10) |
Utilizing the common technique of evaluating the limits of pharmacokinetic models with respect to high ER (Q ≪ fu*Cli) or low ER (Q ≫ fu*Cli), the above two models presented result in three pharmacokinetic outcomes: 1) the relative exposure to the metabolite (AUCm/AUCp) will be different after IV and PO administration when the parent drug has a high ER, but the AUCm/AUCp is independent of route of administration when ERp is low, 2) changes in the intrinsic clearance of a metabolite with a low ER (ERm) will alter the exposure to the metabolite resulting in changes in the AUCm/AUCp and 3) the relative exposure to the metabolite depends on the ratio between its formation and elimination clearances, not on the absolute value of either of these two terms. To determine whether the relative exposure to a metabolite is dependent on route of administration when the parent drug has a high ER, the exposure to morphine after PO or IV administration of codeine and the exposure to morphine-6-glucuronide after PO or IV administration of morphine was revisited (Table 1). Based on literature in vivo clearance values, formation of morphine from codeine is classified as high ERp, high ERm, while formation of morphine-6-glucuronide from morphine is classified as high ERp, low ERm. The observed AUCm/AUCp values are shown in Table 1. Indeed, as the model suggests, the relative exposure to the metabolite was dependent on route of administration for morphine-6-glucuronide, where it was always greater after PO administration than IV administration. The relative exposure to morphine after IV administration of codeine ranged from 3.5-fold less to 2.4-fold greater than after PO administration. Given the variability in AUCm/AUCp for the morphine/codeine pair after PO administration, it was not possible to define a clear relationship between route of administration and AUCm/AUCp.
It has been theoretically demonstrated that changes in the elimination clearance of a low ER metabolite will alter the relative exposure to that metabolite [13]. This can be illustrated by considering the exposure to desipramine as a metabolite of imipramine. If the clearance pathway of the metabolite is subject to genetic polymorphisms, the AUCm/AUCp will depend on the individual’s genotype for that elimination pathway. The formation of desipramine from imipramine is mediated primarily by CYP2C19, whereas desipramine elimination is mediated by CYP2D6. The desipramine/imipramine AUCm/AUCp was 5.4-fold higher in CYP2D6 poor metabolizers (PMs) in comparison to EMs making desipramine the major circulating species in CYP2D6 PMs (Table 4). This increase in AUCm/AUCp was due to a 1.7- and 9.1-fold increase in the AUC of imipramine and desipramine, respectively.
To test the developed in vitro-to-in vivo extrapolation model (Equations 10 and 11 for PO and IV administration, respectively), the AUCm/AUCp of seven M/P pairs: morphine/codeine, theophylline/caffeine, desipramine/imipramine, nortriptyline/amitriptyline, N-desmethylclomipramine/clomipramine, 8-hydroxyclomipramine/clomipramine and 2-hydroxyclomipramine/clomipramine were predicted. The predictions were accomplished using literature in vitro metabolite Cli,f and Cli,m obtained in human liver microsomes (HLM), literature plasma fraction unbound values for both parent and metabolite and common methods of clearance scaling. The Cli values (in µL/min/mg microsomal protein) were first scaled to grams of liver, using the value of 54.7 mg microsomal protein per g liver, and then to kg body weight, using the value of 21.43 g liver per kg body weight [43, 44]. An average body weight of 70 kg was considered. Summary of the obtained predicted and observed AUCm/AUCp values are shown in Table 5. Four of the seven M/P pairs were accurately predicted (< 2-fold error in comparison to the average observed AUCm/AUCp) and two of the seven were predicted within < 5-fold error (Table 5). 2-hydroxyclomipramine had undetectable metabolite levels after clomipramine administration and hence the prediction of very low AUCm/AUCp for this pair is considered in agreement with the in vivo finding.
Table 5.
The in vivo observed and in vitro predicted AUCm/AUCp values for seven M/P pairs.
| Parent | Metabolite | Predicted AUCm/AUCp |
Observed AUCm/ AUCp (Range) |
Predicted/ Observed (Range) |
References |
|---|---|---|---|---|---|
| Codeine | Morphine | 0.051a | 0.068a,b | 0.75 | 1, 2, 3 |
| 0.042 | 0.071 (0.019 – 0.17) | 0.60 (0.26 – 2.2) | |||
| Caffeine | Theophylline | 0.50 | 0.12 (0.11 – 0.14) | 4.0 (3.6 – 4.6) | 4, 5 |
| Imipramine | Desipramine | 1.43 | 0.89 (0.50 – 1.6) | 1.6 (0.89 – 2.9) |
6, 7, 8, 9, 10, 11, 12 |
| Clomipramine | N-desmethylclomipramine | 7.49 | 1.7 (1.2 – 2.2) | 4.3 (3.4 – 6.1) | 13, 14 |
| 8-hydroxyclomipramine | 0.22 | 0.40 (0.36 – 0.44) | 0.55 (0.49 – 0.61) | ||
| 2-hydroxyclomipramine | 0.05 | NDc | ND | ||
| Amitriptyline | Nortriptyline | 0.45 | 0.47 ( 0.43 – 0.53) | 0.91 (0.81 – 1.0) | 15, 16, 17 |
Notes: All values are for after PO administration unless otherwise noted. All observed AUCm/AUCp ratios were determined after single dose administration, save for the two in vivo studies of clomipramine. ND indicates a value not determined.
Superscript ‘a’ indicates a predicted or observed AUCm/AUCp after IV administration.
Superscript ‘b’ indicates that only one in vivo study was available.
Superscript ‘c’ indicates a value not determined because the metabolite was not detected in vivo. Reference numbers coincide with the below provided references and not with the bibliography references.
References: 1Guay et al, 1988; 2Yue et al, 1991a; 3Yue et al, 1991b; 4Akinyinka et al, 2000; 5Rodopoulos et al, 1996; 6Abernethy et al, 1984; 7Albers et al, 1995; 8Albers et al, 2000; 9Bergstrom et al, 1992; 10Koyama et al, 1994; 11Kurtz et al, 1997; 12Wells et al, 1986; 13Linnoila et al, 1982; 14Shimoda et al, 1995; 15Jiang et al, 2002; 16Liedholm et al, 1998 and 17Venkatakrishnan et al, 2001.
It is unlikely that the major inaccuracies in the above AUCm/AUCp predictions are a result of in vitro to in vivo scaling, since the use of in vivo parameters did not yield more accurate predictions (Tables 1 and 5). The morphine/codeine AUCm/AUCp could be predicted accurately from both in vitro and in vivo parameters (< 2-fold error in comparison to the average observed AUCm/AUCp) after both IV and PO administration and the theophylline/caffeine M/P pair yielded similar prediction accuracies when using either in vitro values or in vivo (4-fold versus 3-fold error). Additionally, by utilizing a ratio as the primary predicted measure, any systematic error made in the scaling of in vitro-to-in vivo clearances is negated although random error in the prediction will propagate in the AUC ratio.
The model is designed to predict the nonparametric outcome of AUCm/AUCp. Oftentimes, the pharmacologic or toxicologic effect is a function of the maximum concentration (Cmax) and not the total body exposure. Predictions of metabolite Cmax would require additional parametric information about the input and disposition rates of both the parent and the metabolite only obtainable after in vivo administration of the compounds.
One obvious limitation to this model is that it can only address the disposition of primary metabolites formed after parent administration. Theoretically, the same necessity of predicting both formation and elimination are relevant for subsequent downstream metabolites making quantitative predictions of downstream metabolites very complicated. However, if primary metabolites are used in in vitro incubation experiments, the likelihood of qualitatively identifying downstream metabolites is greatly increased.
Renal clearance as well as biliary excretion and gut metabolism are often important elimination pathways for xenobiotics. These pathways were not considered for either the parent or metabolite in this review and it is likely that to be fully applicable in new drug development, prediction of the total clearance of the metabolite (sum of predicted hepatic and renal clearances) will be necessary. Unfortunately in vitro-to-in vivo extrapolation models for prediction of renal clearance, transport and gut metabolism are currently not as well established as hepatic clearance predictions. Nonetheless, the preliminary success of the predictions indicates that in vitro-to-in vivo extrapolation of AUCm/AUCp, after further development and validation, could prove to be a useful tool in addressing metabolite-related concerns in new drug development.
4. Conclusions
This review of the available data on metabolite disposition shows that as predicted by original metabolite kinetic theory, for any quantitative or semi-quantitative prediction of metabolite abundance or relative importance in vivo, the in vivo elimination clearance of the metabolite has to be predicted or rationalized in addition to basic metabolite profiling in vitro. This is shown by the fact that the in vivo abundance of metabolites of a given drug are no more likely to rank order correctly based on in vitro formation clearances than when left to random probability. An interesting outcome of the literature review is that in comparison to parent clearance predictions, the AUCm/AUCp predicts with similar accuracy suggesting that important human metabolites can be quantitatively predicted using in vitro data. This accuracy was achieved despite the fact that in vitro Cli,f and Cli,m were usually not determined in the same study. Finally, the fact that AUCm/AUCp ratios measured after single dose administration were not significantly different from multiple dose AUCm/AUCp ratios, but ERL kinetics of metabolites were common suggests that early single dose studies for metabolite identification may be justified.
5. Expert Opinion
Based on available pharmacokinetic theory and literature data, the AUCm/AUCp is most appropriate value to be used as the relevant outcome measure of metabolite exposure in in vitro-to-in vivo extrapolation. Since this value does not depend on dose or the Fa of the parent drug, it is a more robust and generally applicable measure of metabolite exposure than the dose-dependent measure of absolute AUCm. Predicting absolute AUCm would require knowledge of the clinical dose of the parent drug and prediction of its Fa. Additionally, the predicted AUCm/AUCp, when multiplied by the expected clinically effective average steady state concentration, can be used to determine prior to human studies how likely the need for additional safety evaluation is.
The results of this review clearly show that the formation clearance of a metabolite is not sufficient for understanding and predicting its in vivo relative exposure (AUCm/AUCp) or importance in comparison to other metabolites. Although the equally important role of metabolite elimination and metabolite formation in metabolite disposition in vivo is generally known, this concept has yet to be applied to in vitro-to-in vivo extrapolation in preclinical new drug development. Inclusion of metabolite formation and elimination clearances in predictions allows prediction of in vivo AUCm/AUCp or, if AUCp is known, the absolute AUCm from in vitro data. It is interesting that after development of the early metabolite kinetic theory it has not been thoroughly reexamined in light of modern experimental approaches. It is likely that better understanding of metabolite disposition can be obtained by further testing in vitro-to-in vivo extrapolation models applied to metabolites even by using a relatively simplistic model detailed in this review. It would also be beneficial if such models would be further developed and validated. This is important not only to address the MIST guidance, but to improve our current understanding of the in vivo pharmacokinetics of probe metabolic ratios that are used in drug-drug interaction studies as well as in pharmacogenetic studies.
The developed model and future models addressing the same primary outcome may be useful in lead compound selection and toxicology stages of new drug development, allowing early attention on potential quantitatively important metabolites. Since chemical synthesis of metabolites can be time consuming, expensive and difficult, it is likely that predictions of metabolite clearance need to be obtained from minimal amounts of primary metabolites generated in in vitro systems and isolated using chromatographic techniques to justify investment of resources to synthesis of reference materials. However, the obtained predictions can be used to guide prioritization of synthetic efforts of metabolite standards. It is noteworthy, that intrinsic metabolic elimination clearance of a metabolite can be predicted from a substrate depletion experiment conducted below Km (Michaelis-Menten affinity constant) concentrations of the metabolite [45, 46]. Additionally, pre-clinical animal studies can be utilized not only to determine the absolute abundance of a metabolite after parent administration, but also to examine the overall pharmacokinetics of the metabolite after administration of the metabolite or the parent. This data can be leveraged for in vivo human metabolite kinetic prediction and may provide further confidence in the in vivo predictions of metabolite clearance and AUCm/AUCp from in vitro data.
The in vivo AUCm/AUCp of only seven M/P pairs were predicted from in vitro data in this review. The unfortunate limitation was the dearth of literature on in vitro metabolite Cli values, and this review suggests that there is a great need to generate more metabolite relevant in vitro kinetic data. Even M/P pairs that are commonly utilized as in vivo CYP probes possess metabolite elimination pathways that are not kinetically characterized. For example, the urinary or plasma ratio of dextrorphan and dextromethorphan is a common probe for phenotyping CYP2D6, yet the major elimination pathway of dextrorphan is via glucuronidation, a pathway that has never been kinetically characterized in vitro [47, 48]. Additionally, the plasma ratios of 5-hydroxyomeprazole and omeprazole or omeprazole sulfone and omeprazole are common probes for CYP2C19 and CYP3A4 drug interactions, respectively, yet neither the kinetics of 5-hydroxyomeprazole nor omeprazole sulfone metabolism are characterized in vitro [49, 50]. This raises some concerns of the validity of these ratios, as genetic factors or drug-drug interactions affecting the unknown elimination pathways of the metabolite could result in skewed data.
Increasingly, M/P ratios are being utilized as specific in vivo CYP markers [51]. When a metabolite is considered pharmacokinetically relevant, the enzymes responsible for the elimination of that metabolite should be identified for proper interpretation of drug-drug interaction and genetic polymorphism studies. When a metabolite is considered pharmacologically relevant, again, these secondary metabolic pathways should be identified in order to understand the therapeutic impact of said drug-drug interaction or genetic polymorphism.
ARTICLE HIGHLIGHTS
There is an increased interest in qualitative and quantitative prediction of in vivo circulating metabolites and in rationalization of metabolite exposures.
The relative importance of metabolites in vivo is measured as the ratio between the metabolite’s and parent drug’s area under the plasma concentration versus time curve (AUCm/AUCp). This ratio can be predicted using in vitro-to-in vivo scaling of formation and elimination clearances of the metabolite of interest.
Single dose AUCm/AUCp data can be utilized as a surrogate for steady-state AUCm/AUCp when the compounds have linear kinetics. 56% of reviewed metabolites underwent elimination rate limited kinetics and hence time to reach steady-state for the metabolites is governed by the half-life of the metabolite, not the half-life of the parent drug.
When multiple metabolites are formed from the same parent drug, the rank order of importance observed in in vitro systems correctly predicts the rank order importance in vivo for only half of the drugs. This discrepancy is most likely due to great variability in the clearances of the metabolites. Prediction of the identity of major metabolites in contrast to minor metabolites in circulation is likely to require determination of the clearance of the metabolites as well. There is no significant correlation between the formation clearance of a given metabolite in vitro and the relative abundance (AUCm/AUCp) of that metabolite in vivo.
If a metabolite is used as a probe of specific enzyme activity, the elimination pathways of that metabolite should be characterized.
Acknowledgements
The authors wish to thank Dr. Thomas A. Baillie for helpful discussions during the preparation of this manuscript.
Declaration of Interest:
This work was supported in part by the National Institute of Health [Grant P01 GM32165]
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Riss J, Cloyd J, Gates J, et al. Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008;118(2):69–86. doi: 10.1111/j.1600-0404.2008.01004.x. [DOI] [PubMed] [Google Scholar]
- 2.Akiyoshi J, Isogawa K, Yamada K, et al. Effects of antidepressants on intracellular Ca2+ mobilization in CHO cells transfected with the human 5-HT2C receptors. Biol Psychiatry. 1996;39(12):1000–1008. doi: 10.1016/0006-3223(95)00309-6. [DOI] [PubMed] [Google Scholar]
- 3.Rambeck B, Sälke-Treumann A, May T, et al. Valproic acid-induced carbamazepine-10,11-epoxide toxicity in children and adolescents. Eur Neurol. 1990;30(2):79–83. doi: 10.1159/000117315. [DOI] [PubMed] [Google Scholar]
- 4.Ho PC, Abbott FS, Zanger UM, et al. Influence of CYP2C9 genotypes on the formation of a hepatotoxic metabolite of valproic acid in human liver microsomes. Pharmacogen J. 2003;3:335–342. doi: 10.1038/sj.tpj.6500210. [DOI] [PubMed] [Google Scholar]
- 5.Bauman JN, Frederick KS, Sawant A, et al. comparison of the bioactivation potential of the antidepressant and hepatotoxin nefazodone with aripiprazole, a structural analog and marketed drug. Drug Metab Dispos. 2008;36(6):1016–1029. doi: 10.1124/dmd.108.020545. [DOI] [PubMed] [Google Scholar]
- 6.Stevens JC, Wrighton SA. Interaction of the enantiomers of fluoxetine and norfluoxetine with human liver cytochromes P450. J Pharmacol Exper Ther. 1993;266(2):964–971. [PubMed] [Google Scholar]
- 7.Sauer JM, Long AJ, Ring B, et al. Atomoxetine hydrochloride: clinical drug-drug interaction prediction and outcome. J Pharmacol Exper Ther. 2004;308(2):410–418. doi: 10.1124/jpet.103.058727. [DOI] [PubMed] [Google Scholar]
- 8.Templeton IE, Thummel KE, Kharasch ED, et al. Contribution of itraconazole metabolites to inhibition of CYP3A4 in vivo. Clin Pharmacol Ther. 2008;83(1):77–85. doi: 10.1038/sj.clpt.6100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FDA. Guidance for industry: safety testing of drug metabolites. Rockville, MD: Food and Drug Administration; 2008. [Google Scholar]
- 10.EMA. Non-clinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals. London, UK: European Medicines Agency; 2008. [Google Scholar]
- 11. Anderson S, Luffer-Atlas D, Knadler MP. Predicting human circulating metabolites: how good are we? Chem Res Tox. 2009;22(2):243–256. doi: 10.1021/tx8004086. •• An interesting discussion on metabolite identification and correlation toin vivo.
- 12.Cummings AJ, Martin BK, Pang KS. Kinetic considerations relating to the accrual and elimination of drug metabolites. Br J Pharmac Chemother. 1967;29:136–149. doi: 10.1111/j.1476-5381.1967.tb01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Houston JB. Drug metabolite kinetics. Pharmacol Ther. 1981;15(3):521–552. doi: 10.1016/0163-7258(81)90056-5. •• A useful review on in vivo metabolite kinetics.
- 14. Houston JB, Taylor G. Drug metabolite concentration-time profiles: influence of route of drug administration. Br J Clin Pharmacol. 1984;17:385–394. doi: 10.1111/j.1365-2125.1984.tb02362.x. •• A theoretical discussion of possible metabolite concentration versus time profiles after PO administration.
- 15.Pang KS, Gillette JR. Theoretical relationship between area under the curve and route of administration of drugs and their precursors for evaluating sites and pathways of metabolism. J Pharm Sci. 1978;67(5):703–704. doi: 10.1002/jps.2600670536. [DOI] [PubMed] [Google Scholar]
- 16.Pang KS. Metabolite pharmacokinetics: the area under the curve for the metabolite and the fractional rate of metabolism of a drug after different routes of administration for renally and hepatically cleared drugs and metabolites. J Pharmacokinet Biopharm. 1981;9:477–487. doi: 10.1007/BF01060890. [DOI] [PubMed] [Google Scholar]
- 17. Levy RH, Lane EA, Guyot M, et al. Analysis of parent drug-metabolite relationship in the presence of an inducer. Application to the carbamazepine-clobazam interaction in normal man. Drug Metab Dispos. 1983 July 1;11(4):286–292. 1983. • In vivo validation of the discussed AUCm/AUCp theory.
- 18. Patel IH, Levy RH, Trager WF. Pharmacokinetics of carbamazepine-10,11-epoxide before and after autoinduction in rhesus monkeys. J Pharmacol Exper Ther. 1978;206(3):607–613. •• Initial development of the in vivo AUCm/AUCp theory and analysis of possible AUCm/AUCp outcomes.
- 19.Skarke C, Langer M, Jarrar M, et al. Probenecid interacts with the pharmacokinetics of morphine-6-glucuronide in humans. Anesthesiology. 2004;101:1394–1399. doi: 10.1097/00000542-200412000-00020. [DOI] [PubMed] [Google Scholar]
- 20.du Preez MJ, Botha JH, McFadyen ML, et al. The pharmacokinetics of theophylline in premature neonates during the first few days after birth. Ther Drug Monit. 1999 Dec;21(6):598–603. doi: 10.1097/00007691-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Guay DR, Awni WM, Findlay JW, et al. Pharmacokinetics and pharmacodynamics of codeine in end-stage renal disease. Clin Pharmacol Ther. 1988;43(1):63–71. doi: 10.1038/clpt.1988.12. [DOI] [PubMed] [Google Scholar]
- 22.Osborne R, Joel S, Trew D, et al. Morphine and metabolite behavior after different routes of morphine administration: demonstration of the importance of the active metabolite morphine-6-glucuronide. Clin Pharmacol Ther. 1990;47:12–19. doi: 10.1038/clpt.1990.2. [DOI] [PubMed] [Google Scholar]
- 23.Tegeder I, Lotsch J, Geisslinger G. Pharmacokinetics of opiods in liver disease. Clin Pharmacokinet. 1999;37(1):17–40. doi: 10.2165/00003088-199937010-00002. [DOI] [PubMed] [Google Scholar]
- 24.Roxane. CafCit Package Insert. Columbus, OH: 2000. [Google Scholar]
- 25.Rodopoulos N, Norman A. Assessment of dimethylxanthine formation from caffeine in healthy adults: comparison between plasma and saliva concentrations and urinary excretion of metabolites. Scand J Clin Lab Invest. 1996;56:259–268. doi: 10.3109/00365519609088615. [DOI] [PubMed] [Google Scholar]
- 26.Hospira. Codeine Phosphate Package Insert. Lafe Forest, IL: 2004. [Google Scholar]
- 27.AlPharma. Kadian Package Insert. Piscataway, NJ: 2007. [Google Scholar]
- 28.Rowland M, Tozer TN. Clinical pharmacokinetics: concepts and applications. 3rd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 1995. [Google Scholar]
- 29. Lane EA, Levy RH. Prediction of steady-state behavior of metabolite from dosing of parent drug. J Pharm Sci. 1980;69(5):610–612. doi: 10.1002/jps.2600690541. • Validation of equivalence of AUC0-∞ after single dose to AUC0-τ at steady state.
- 30.Smith DA, Obach RS. Metabolites in safety testing (MIST): considerations of mechanism of toxicity with dose, abundance and duration of treatment. Chem Res Tox. 2009;22(2):267–279. doi: 10.1021/tx800415j. [DOI] [PubMed] [Google Scholar]
- 31.Espina R, yu L, Wang J, et al. Nuclear magnetic resonance spectroscopy as a quantitative tool to determine the concentrations of biologically produced metabolites: implications in metabolites in safety testing. Chem Res Tox. 2009;22:299–310. doi: 10.1021/tx800251p. [DOI] [PubMed] [Google Scholar]
- 32.Vishwanathan K, Babalola K, Wang J, et al. Obtaining exposures of metabolites in preclinical species through plasma pooling and quantitative NMR: addressing metabolites in safety testing (MIST) guidance without using radiolabeled compounds and chemically synthesized metabolite standards. Chem Res Tox. 2009;22:311–322. doi: 10.1021/tx8003328. [DOI] [PubMed] [Google Scholar]
- 33.Leclercq L, Cuyckens F, Mannens GSJ, et al. Which human metabolites have we MIST? Retrospective analysis, practical aspects, and perspectives for metabolite identification and quantification in pharmaceutical development. Chem Res Tox. 2009;22:280–293. doi: 10.1021/tx800432c. [DOI] [PubMed] [Google Scholar]
- 34.Kramer-Nielsen K, Flinois JP, Beaune P, et al. The biotransformation of clomipramine in vitro, identification of the cytochrome P450s responsible for the separate metabolic pathways. J Pharmacol Exper Ther. 1996 June 1;277(3):1659–1664. 1996. [PubMed] [Google Scholar]
- 35.Obach RS, Baxter JG, Liston TE, et al. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J Pharmacol Exper Ther. 1997 October 1;283(1):46–58. 1997. [PubMed] [Google Scholar]
- 36.Carlile DJ, Hakooz N, Bayliss MK, et al. Microsomal prediction of in vivo clearance of CYP2C9 substrates in humans. Br J Clin Pharmacol. 1999;47:625–635. doi: 10.1046/j.1365-2125.1999.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obach RS. Prediction of human clearance of twenty-nine drugs from hepatic microsomal clearance data: and examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab Dispos. 1999;27(11):1350–1359. [PubMed] [Google Scholar]
- 38.McGinnity DF, Soars MG, Urbanowicz RA, et al. Evaluation of fresh and cryopreserved hepatocytes as in vitro drug metabolism tools for the prediction of metabolic clearance. Drug Metab Dispos. 2004 November;32(11):1247–1253. doi: 10.1124/dmd.104.000026. 2004. [DOI] [PubMed] [Google Scholar]
- 39. Wilkinson GR, Shand DG. A physiologic approach to hepatic drug clearance. Clin Pharmacol Ther. 1975;18:377–390. doi: 10.1002/cpt1975184377. • Development of the well-stirred model of hepatic clearance.
- 40.Ito K, Houston JB. Comparison of the use of liver models for predicting drug clearance using in vitro kinetic data from hepatic microsomes and isolated hepatocytes. Pharmaceutical Research. 2004;21(5):785–792. doi: 10.1023/b:pham.0000026429.12114.7d. [DOI] [PubMed] [Google Scholar]
- 41. Pang KS, Kwan KC. A commentary: methods and assumptions in the kinetic estimation of metabolite formation. Drug Metab Dispos. 1983 March;11(2):79–84. 1983. • An interesting discussion on the fraction metabolized to a metabolite of interest.
- 42.Rane A, Wilkinson GR, Shand DG. Prediction of hepatic extraction ratio from in vitro measurement of intrinsic clearance. J Pharmacol Exper Ther. 1977;200(2):420–424. [PubMed] [Google Scholar]
- 43. Iwatsubo T, Hirota N, Oois T, et al. Prediction of in vivo drug disposition from in vitro data based on physiological pharmacokinetics. Biopharm Drug Dispos. 1996;17:273–310. doi: 10.1002/(SICI)1099-081X(199605)17:4<273::AID-BDD961>3.0.CO;2-R. • A useful review of in vitro to in vivo extrapolation methodologies
- 44.Thummel KE, Shen DD, Podoll TD, et al. Use of midazolam as a human cytochrome P450 3A probe: I In vitro-in vivo correlations in liver transplant patients. J Pharmacol Exper Ther. 1994 October 1;271(1):549–556. 1994. [PubMed] [Google Scholar]
- 45.Mohutsky MA, Chien JY, Ring BJ, et al. Predictions of the in vivo clearance of drugs from rate of loss using human liver microsomes for phase I and phase II biotransformations. Pharm Res. 2006;23(4):654–662. doi: 10.1007/s11095-006-9663-4. [DOI] [PubMed] [Google Scholar]
- 46.Nath A, Atkins WM. A theoretical validation of the substrate depletion approach to determining kinetic parameters. Drug Metab Dispos. 2006 September;34(9):1433–1435. doi: 10.1124/dmd.106.010777. 2006. [DOI] [PubMed] [Google Scholar]
- 47.Chládek J, Zimová G, Beránek M, et al. In-vivo indices of CYP2D6 activity: comparison of dextromethorphan metabolic ratios in 4-h urine and 3-h plasma. Eur J Clin Pharmacol. 2000;56(9–10):651–657. doi: 10.1007/s002280000218. [DOI] [PubMed] [Google Scholar]
- 48.Ryu JY, Song IS, Sunwoo YE, et al. Development of the "Inje Cocktail" for high-throughput evaluation of five human cytochrome P450 isoforms in vivo. Clin Pharmacol Ther. 2007;82(5):531–540. doi: 10.1038/sj.clpt.6100187. [DOI] [PubMed] [Google Scholar]
- 49.Yang L-J, Fan L, Liu Z-Q, et al. Effects of allicin on CYP2C19 and CYP3A4 activity in healthy volunteers with different CYP2C19 genotypes. Eur J Clin Pharmacol. 2009;65(6):601–608. doi: 10.1007/s00228-008-0608-1. [DOI] [PubMed] [Google Scholar]
- 50.Abelo A, Andersson TB, Antonsson M, et al. Stereoselective metabolism of omeprazole by human cytochrome P450 enzymes. Drug Metab Dispos. 2000;28(8):966–972. [PubMed] [Google Scholar]
- 51.Streetman DS, Bertino JS, Nafziger AN. Phenotyping of drug-metabolizing enzymes in adults: a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics. 2000;10(3):187–216. doi: 10.1097/00008571-200004000-00001. [DOI] [PubMed] [Google Scholar]