Abstract
In this study, we examined the effect of bicarbonate transporters on ammonium/ammonia uptake in the medullary thick ascending limb cell line ST-1. Cells were treated with 1 mM ouabain and 0.2 mM bumetanide to minimize carrier-mediated NH4+ transport, and the intracellular accumulation of 14C-methylammonium/methylammonia (MA) was determined. In solution, cells at normal pH briefly accumulated 14C-MA over 7 min and reached a plateau. In solution, however, cells markedly accumulated 14C-MA over the experiment period of 30 min. This accumulation was reduced by the bicarbonate transporter blocker 4,4’-diisothiocyanatostilbene-2,2’-disulfonate (DIDS; 0.5 mM). Replacing Cl– with gluconate reduced the accumulation but the reduction was more substantial in the presence of DIDS. Incubating cells at pH 6.8 (adjusted with NaHCO3 in 5% CO2) for 24 h lowered the mean steady-state intracellular pH to 6.96, significantly lower than 7.28 for controls. DIDS reduced 14C-MA accumulation in controls but had no effect after acidic incubation. Immunoblot showed that NBCn1 was upregulated after acidic incubation and in NH4Cl-containing media. The Cl/HCO3 exchanger AE2 was present but its expression remained unaffected by acidic incubation. Expressed in Xenopus oocytes, NBCn1 increased carrier-mediated 14C-MA transport, which was abolished by replacing Na+. Two-electrode voltage clamp of oocytes exhibited negligible current after NH4Cl application. These results suggest that DIDS-sensitive extrusion normally governs NH4+/NH3 uptake in the MTAL cells. We propose that, under acidic conditions, DIDS-sensitive extrusion is inactivated while NBCn1 is upregulated to stimulate NH4+ transport.
Keywords: bicarbonate transporter, Xenopus oocytes, voltage-clamp, thick ascending limb
INTRODUCTION
One of the major tasks of the kidney is to maintain acid-base homeostasis in the body. The kidney regulates blood pH by reclaiming filtered acid or base equivalents or by releasing them into urine. The major acid-base component that the kidney regulates is . In the medullary thick ascending limb (MTAL), where 10-15% of filtered is reclaimed, absorption is initiated by H+ secretion into the lumen via the apical Na/H exchanger NHE3 (Good & Watts, III 1996), accompanied by exit to the medullary interstitium. The basolateral exit is probably mediated by the Cl/HCO3 exchanger AE2 (Brosius et al. 1995;Sun 1998). However, Na/HCO3 transporters (Kikeri et al. 1990;Vorum et al. 2000;Xu et al. 2003) and KCl cotransporter-mediated HCO3 exit (Bourgeois et al. 2002) have also been reported.
The MTAL also handles NH4+/NH3 that serve as major non-bicarbonate buffer components in the kidney (for review, see ref. (Weiner & Hamm 2007). NH4+, which is produced in the proximal tubules and secreted to the lumen, is absorbed via the apical Na/K/2Cl cotransporter NKCC2 (Kinne et al. 1986) and dissociates intracellularly into NH3 and H+. NH3 then moves to the medullary interstitium and re-associates with H+ to form NH4+. This process provides a concentration gradient of NH3 across the cells, which ultimately enables NH3 to diffuse into the collecting ducts for urinary excretion or to the renal vein for recycling. The blood type Rh glycoprotein family proteins are mammalian ammonium transporters (Marini et al. 2000;Liu et al. 2001;Liu et al. 2000) and are found in the proximal tubules and collecting ducts, but not in the MTAL (Verlander et al. 2003).
and NH4+ movements are associated with each other in many nephron segments including the MTAL (Good 1994;Wagner 2007;Kraut & Kurtz 2005). The physiological coupling between these two ion transport processes is particularly evident during cellular and systemic pH changes. The absorptive capacity for and NH4+ is increased in response to chronic metabolic acidosis, and this change typically involves up- or down-regulation of membrane proteins responsible for transporting acid/base equivalents (for review, see ref. (Good 1994;Wagner 2007). Among these proteins, NBCn1 is of particular interest. This transporter normally moves Na+ and into the cell, but it is localized to the basolateral membrane of the MTAL. In vivo animal studies show a nearly 10-fold increase in NBCn1 protein expression in chronic metabolic acidosis induced by NH4Cl load (Kwon et al. 2002;Odgaard et al. 2004). The upregulation of NBCn1 occurs probably to counter H+ overload caused by an increase in NH4+ uptake. Consistent with this compensatory function, NBCn1 in neurons is upregulated under acidic conditions (Park et al. 2010).
In this study, we examined the effect of transporters on NH4+/NH3 uptake in the MTAL cell line ST-1. This cell line is a non-polarized cell and exhibits many features characteristic for MTAL cells (Kone et al. 1995;Kone & Higham 1999). We measured intracellular accumulation of 14C-MA, and evaluated the effects of DIDS, as well as ion replacement, on 14C-MA accumulation. The effect of acidic pH on NH4+/NH3 uptake was evaluated by measuring 14C-MA accumulation after acidic incubation of the cells. NBCn1 was then expressed in Xenopus oocytes and examined for its ability to accumulate 14C-MA and affect pH changes mediated by NH4+ transport. Our data suggest that NBCn1 plays dynamic roles in regulating and NH4+ transport in the MTAL cells.
MATERIALS AND METHODS
ST-1 cells
The ST-1 cells (provided by Bruce Kone, University of Florida) were cultured in Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine serum, 50 U/ml penicillin and 50 μg/ml streptomycin in 5% CO2-equilibrated 37°C incubator (Kone & Higham 1999). For 14C-MA accumulation assay, cells were placed on 24-well plates at the density of 0.2–3.5 × 105 cells/well and incubated for 1–4 days. For acidification experiments, cells were placed on 6-well plates at the density of 2.2–8.8 × 104 cells/well until cells were > 90% confluent. The pH in the medium was adjusted by varying concentration according to the Henderson-Hasselbalch equation with the solubility coefficient of 0.03 mmol CO2/mmHg and PCO2 of 35.65 mmHg.
Measurements of pHi in ST-1 cells
Steady-state pHi was determined according to the protocol (Cooper et al. 2009) with a slight modification. Briefly, cells grown on a coverslip (>60% confluent) were loaded with 6.5 μM of 2',7'-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM) for 10 min and then mounted to a closed chamber RC-30 (Warner Instruments; Hamden, CT) affixed on the stage of a Zeiss Axiovert 135 inverted microscope. The microscope was equipped with a Lambda 10-2 filter wheel controller and a multi wavelength filter set (Sutter Instruments, Novato, CA). The dye was alternately excited with 490 nm and 440 nm light, and the emission light at 535 nm (i.e., I490 and I440) was captured. The ratio of I490/I440 was calculated after background subtraction. Dye calibration was done using nigericin. Steady-state pHi in was calculated by determining linear least square analysis over a minimum of 20 seconds. Data were acquired using Nikon NIS Elements AR 3.0 (Nikon; Melville, NY).
Immunoblot
Cells were scraped in ice-cold homogenization buffer containing 300 mM mannitol, 5 mM HEPES, pH 7.2, 0.1 mg/ml phenylmethanesulphonyl fluoride and 1 × protease inhibitor cocktail I (Calbiochem; San Diego, CA, USA). Cells were homogenized with a 26G needle and centrifuged at 810 × g for 10 min at 4°C, and supernatants were ultracentrifuged at 100,000 × g for 30 min at 4°C. Membrane pellets were collected and dissolved in phosphate buffered saline (PBS), and protein concentration was determined using the Bradford reagents (Sigma-Aldrich). The equal amounts of protein samples were separated on a 7.5% SDS polyacrylamide gel and blotted to a polyvinylidene fluoride membrane. The blot was incubated in PBS containing 0.05% Tween 20 and 5% nonfat dry milk for 1 h and then treated with antibodies to rat NBCn1 (Cooper et al. 2009) and AE2 (Frische et al. 2004). The blot was washed with PBS containing 0.05% Tween 20 and then incubated with a horseradish peroxidase-conjugated secondary antibody (1:2500) for 1 h (Millipore; Billerica, MA). The blot was washed and visualized by ECL chemiluminescence (GE Healthcare; Chicago, IL). For β-actin, the blot was striped with 62.6 mM Tris-HCl (pH 6.7), 2% SDS, and 0.7% β-mercaptoethanol at 50°C for 5 min, and reprobed with the mouse β-actin antibody (Millipore). For quantitation, mean pixel intensities of immunoreactive signals were measured by positioning boxes around protein bands using ImageJ image analysis software (NIH; Bethesda, MD, USA). The intensity values for NBCn1 and AE2 were normalized to the intensity value for β-actin after background subtraction.
14C-MA accumulation
Cells grown on a 24-well plate were treated with the assay solution (mM; 140 NaCl, 1 KCl, 2 CaCl2, 1 MgSO4, 2.5 NaH2PO4, 5.5 glucose, 10 HEPES, pH 7.4, 1 ouabain and 0.2 bumetanide; 2 ml/well) for 20–30 min, and then incubated in the fresh 14C-MA assay solution containing 0.5 mM CH3-NH3Cl and 1 μCi/ml 14CH3-NH3Cl (ICN; Costa Mesa, CA, USA). For solution, 33 mM NaHCO3 replaced the equimolar concentration of NaCl and the solution was equilibrated with 5% CO2 for 30 min. For ion replacement experiments, Na+ was substituted with N-methyl-D-glucamine (NMDG+) and Cl– with gluconate. To block transporters, 0.5 mM 4,4’-diisothiocyanato-2,2’-disulfonate stilbene DIDS (Sigma-Aldrich; St. Louis, MO) was included in the 14C-MA assay solution. Experiments were terminated at different time points by washing cells 4 times with ice-cold non-radioactive assay solution containing 1 mM MA. Cells were solubilized in 2% SDS/0.1 N NaOH, and the radioactivity was determined using a Packard Tricarb scintillation counter (PerkinElmer; Waltham, MA). Each time point was an independent measurement. The counts (count per minute; cpm) were divided by the mean specific activity of 14C-MA assay solution and presented as pmole per μg total protein. For 14C-MA accumulation experiments with oocytes, 3-5 oocytes were incubated with the 14C-MA assay solution in the presence/absence of 5% CO2, 25 mM (pH 7.4) without bumetanide and ouabain.
Expression of NBCn1 in Xenopus oocytes
Frogs were purchased from Xenopus Express (Brooksville, FL). A frog was anesthetized with fresh 0.1% 3-aminobenzoic acid ethyl ester (tricaine) in 5 mM HEPES (pH 7.5) for 20 min. The frog was then placed on ice and surgery was done to collect oocytes. After suture, the frog was placed in a recovery tank containing 0.1 M NaCl until the animal was fully recovered. Oocytes were washed with Ca2+-free solution containing (mM) 96 NaCl, 2 KCl, 1 MgCl2, 10 HEPES (pH 7.4) five times for 20 min each, and then agitated with 2 mg/ml type IA collagenase (Sigma-Aldrich) twice for 20 min each. Oocytes without follicles (stage V-VI) were manually sorted and stored in OR3 medium (Chang et al. 2008) at 18°C overnight. For cRNA injection, the plasmid containing rat NBCn1-E (Cooper et al. 2005) was linearized with Nhe I and in vitro transcribed using the mMessage/mMachine transcription kit (Ambion; Austin, TX). Injection was done with 46 nl of either NBCn1-E cRNA (25 ng) or sterile water. Oocytes were maintained at 18°C for 3 days. Experiments requiring the use of frogs were conducted under the NIH guideline for research on animals, and the protocols were approved by the Institutional Animal Care and Use Committee at Emory University and University of Alabama at Birmingham.
Electrophysiology
An oocyte was impaled with current and voltage electrodes filled with 3M KCl. The electrode resistance was < 1.5 MΩ for both. The oocyte was superfused with the recording solution (mM; 80 NaCl, 20 NMDG-Cl, 2 KCl, 1 MgCl2, 1.8 CaCl2, 5 HEPES, pH 7.5) and clamped at -60 mV using the oocyte clamp OC-725C (Warner). Step-voltage commands from -120 mV to +40 mV (100 msec, 20 mV increments) were applied before and ~1 min after the switch to the recording solution containing 20 mM NH4Cl. NMDG+ was replaced with NH4+. Current and voltage signals were collected using a Digidata 1322 interface (Molecular Devices; Sunnyvale, CA, USA) and data were acquired and analyzed using pClamp 8 (Molecular Devices). Experiments were done at room temperature.
Statistical analysis
Data were reported as mean ± SE. For level of significance, a one-way ANOVA with the Bonferroni post-test was used to compare the ratio of NBCn1/β-actin expression at different time points. A two-way ANOVA with the Bonferroni post-test was used to compare 14C-MA accumulation at different time points. An unpaired, 1-tailed Student t-test was used to analyze 1) 14C-MA accumulation and pHi after acidic incubation, 2) quantitation of immunoblot signals, and 3) conductance and zero-current voltage in oocytes. The p values less than 0.05 were considered significant. The n value referred to the number of independent experiments for 14C-MA accumulation assay and immunoblot, and the number of oocytes that were independently prepared from 4 experiments. Data were analyzed using Microsoft Excel and Origin 8.1 software (OriginLab Corporation; Northampton, MA).
RESULTS
14C-MA accumulation in ST-1 cells is stimulated by transporters
To examine whether transporters affect NH4+/NH3 uptake, we treated cells with the 14C-MA assay solution (pH 7.4) and measured the radioisotope accumulated in cells. Both CH3-NH2 diffusion and carrier-mediated CH3-NH3+ transport affect 14C-MA accumulation in the non-polarized ST-1 cells. This complicates our study examining the effect of transporters on NH4+/NH3 uptake in the cells because transporters change pHi, to which NH4+ transporters such as NKCC2 (Gamba & Friedman 2009) and Na,K-ATPase (Kaplan 2002) are sensitive. For this and subsequent experiments, 0.2 mM bumetanide and 1 mM ouabain (Kone & Higham 1999) were included in the assay solution to minimize carrier-mediated CH3-NH3+ transport. Thus, the 14C-MA accumulation value in our experiments primarily reflects the amount of CH3-NH3+ that is converted from CH3-NH2 and trapped in the cell.
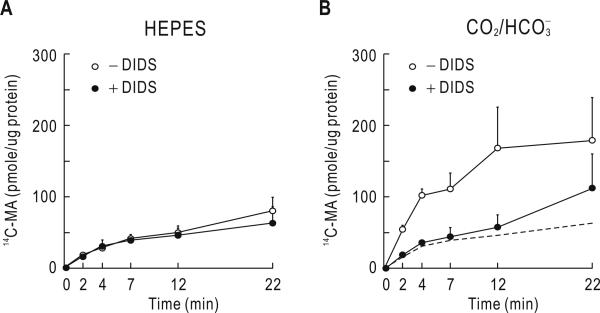
Fig. 1A shows the time course of mean 14C-MA accumulation in the absence of 5% CO2, 33 mM (pH 7.4). The accumulation increased over ~7 min and then reached a plateau, reflecting no net movement of CH3-NH3+/CH3-NH2 across the cell membrane. DIDS caused negligible effect under this condition (n = 3; p > 0.05; two-way ANOVA with Bonferroni post-test). In contrast, parallel experiments with sister plates showed that 14C-MA accumulation was greater in the presence of 5% CO2, 33 mM (Fig. 1B). The dotted line in the figure represents the time course of the accumulation measured in solution. The 14C-MA accumulation was markedly reduced by 0.5 mM DIDS (n = 3; p < 0.05; two-way ANOVA with Bonferroni post-test). The reduction was particularly substantial over 12 min and then less effective afterward. Thus, at 22 min, ~37% of the accumulation was inhibited by DIDS.
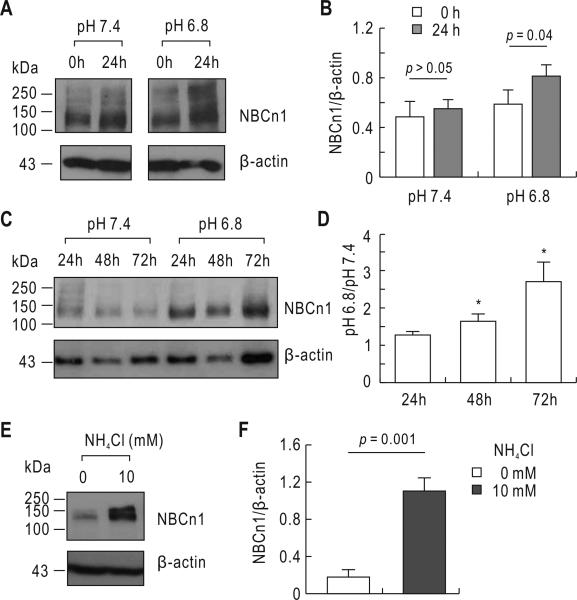
Fig. 1.
14C-MA accumulation in cells. A) 14C-MA accumulation in solution (pH 7.4). Cells grown on a 24-well plate were treated with 14C-MA assay solution. Cells were then rapidly rinsed with ice-cold wash solution containing unlabeled MA at different time points. The 14C-MA assay solution in this and subsequent experiments with ST-1 cells contained 0.2 mM bumetanide and 1 mM ouabain to minimize carrier-mediated CH3-NH3+ transport. Experiments were done in the presence or absence of 0.5 mM 4,4’-diisothiocyanatostilbene-2,2’-disulfonate (DIDS). The accumulation was presented as pmole/μg total protein. Data were averaged from 3 experiments. B) 14C-MA accumulation in solution. The experimental procedure was identical to that in A except that the assay solution contained 5% CO2, 33 mM (pH 7.4). The dotted line was derived from the line in A. Data were averaged from 3 experiments.
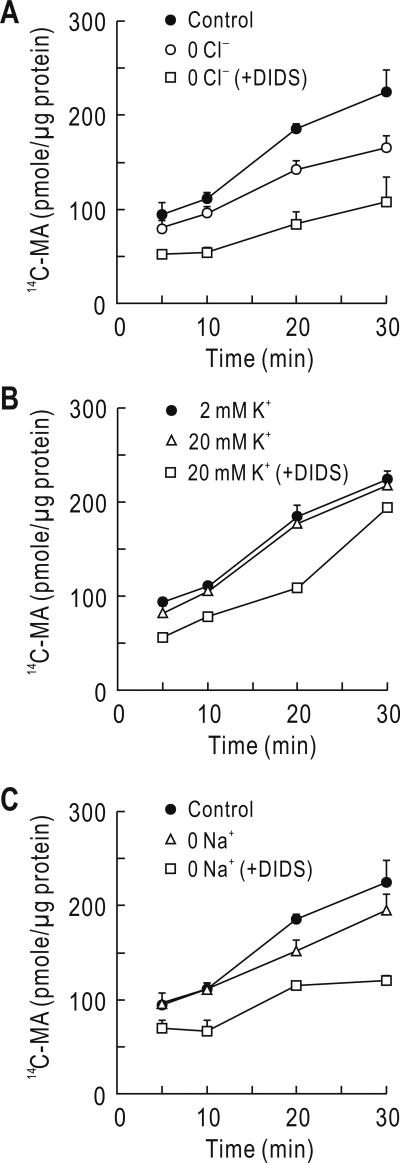
The above results indicate that DIDS-sensitive transporters assist in providing H+ and influence intracellular NH4+ trapping. Such a process is expected in an acid-loading transporter that removes from the cell. The DIDS-sensitive extrusion in the MTAL is governed by the Cl/HCO3 exchanger (Quentin et al. 2004). To test whether the Cl/HCO3 exchanger is involved in the process of NH4+ trapping in our experiments, we replaced Cl– in the assay solution with gluconate and measured 14C-MA accumulation over 10-30 min after treatment, during which the accumulation reached a plateau in solution. Replacing Cl– progressively reduced the accumulation over time compared to controls (Fig 2A), consistent with the idea that the absence of external Cl– decreases exit and the concomitant H+ needed to trap 14C-MA. Nonetheless, adding 0.5 mM DIDS to the Cl–-replaced assay solution caused a more profound decrease, comparable to the inhibition by DIDS in Cl–-containing assay solution (Fig. 1B). Thus, Cl– removal partially inhibited 14C-MA accumulation. In the MTAL, DIDS-sensitive exit can occur via the KCl cotransporter (Bourgeois et al. 2002) in addition to the Cl/HCO3 exchanger. We raised the K+ concentration by 10 fold (from 2 mM to 20 mM) to test whether the KCl transporter contributes to 14C-MA accumulation. Raising the K+ concentration produced negligible effect (p > 0.05, n = 4; Fig. 2B), implying that the KCl cotransporter is unlikely involved. In parallel experiments with sister plates, cells were treated with the assay solution that contained NMDG+ instead of Na+. Replacing Na+ caused a small reduction (Fig. 2C). Adding the NHE blocker dimethylamiloride (0.1 mM) had negligible effect on this reduction (data not shown).
Fig. 2.
Effects of Cl– & Na+ replacement and K+ concentration on 14C-MA accumulation. All assay solutions contained 5% CO2, 33 mM (pH 7.4). A) Effect of Cl– replacement. Cl– in the 14C-MA assay solution was replaced by gluconate in the absence or presence of 0.5 mM DIDS (n = 4 for each). B) Effect of raising K+ concentration. The K+ concentration in the assay solution was raised from 2 mM to 20 mM (n = 4). C) Effect of Na+ replacement. Na+ in the assay solution was replaced by NMDG+ (n = 4).
Acid-loading extrusion is found in ST-1 cells
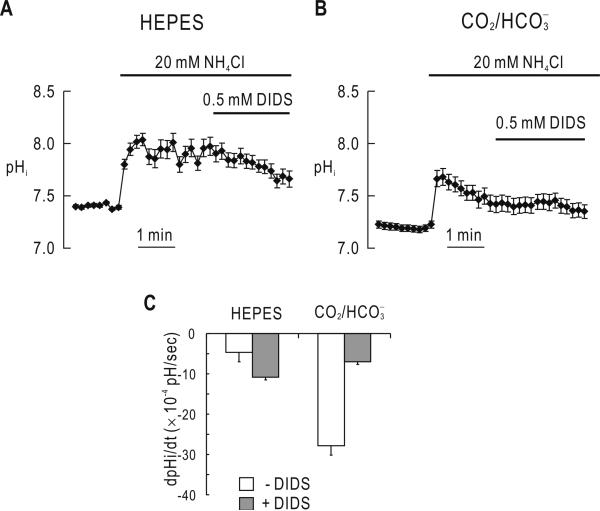
We monitored pHi changes from an alkali load using the pH-sensitive fluorescence dye BCECF. Upon the application of 20 mM NH4Cl in solution (Fig. 3A), pHi was rapidly increased and then nearly reached plateau (n = 5). Lack of intracellular acidification reflects inhibition of carrier-mediated CH3-NH3+ transport by bumetanide and ouabain in our experiments. The recovery was slightly enhanced by DIDS, the nature of which is unclear. Thus, DIDS had negligible effect on pHi alone. In the presence of 5% CO2, 33 mM (Fig. 3B), however, the pHi recovery toward the resting pH was significantly faster (dpHi/dt of -27.8 ± × 10-4 pH/sec; n =5), indicating acid-loading transport in the cells. This acid-loading process was substantially reduced by DIDS (-6.9 × 10-4; p < 0.05). Fig. 3C summarizes the pHi recovery rates from an alkali load in the absence and presence of . The dpHi/dt in the presence of was higher and inhibited by DIDS (75%).
Fig. 3.
stimulation of the pHi recovery from an alkali load. A) pHi recovery from an alkali load in the absence of . Cells were treated with 20 mM NH4Cl, and 0.5 mM DIDS was applied during the pHi recovery from an alkali load (n =5). B) pHi recovery from an alkali load in the presence of 5%CO2, 33 mM . Cells were subjected to the protocol as described in A except that the experiment was done in the presence of (n =5). C) Summary of the pHi recovery rate. Data were obtained from the experiments in A and B.
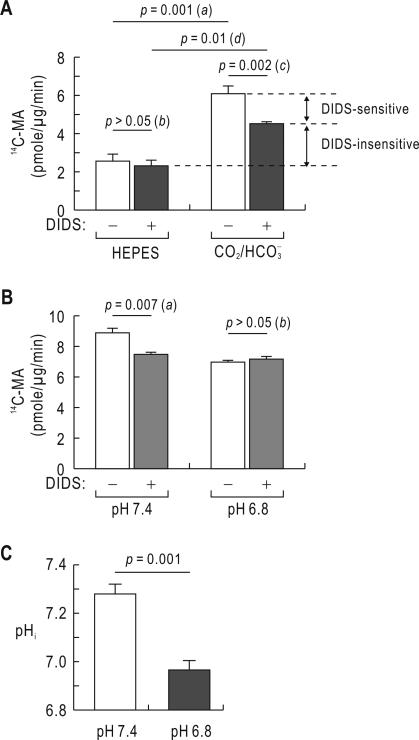
DIDS-sensitive 14C-MA accumulation is abolished at low pHi
Fig. 4A shows 14C-MA accumulation rates measured in the absence and presence of 5% CO2, 33 mM (pH 7.4). The measurements without DIDS (open boxes in the figure) revealed that the accumulation rate was higher in the presence of (2.5 ± 0.4 pmole/μg/min in HEPES versus 6.1 ± 0.4 pmole/μg/min in , n = 3 for each; the unit is pmole of 14C-MA per μg of total protein per min), corresponding to a 2.4-fold increase (p = 0.001; a in the figure). Adding 0.5 mM DIDS to the assay solution had negligible effect in the absence of , but reduced the accumulation (4.5 ± 0.1 pmole/μg/min, n = 3, p = 0.002; c in the figure). The remaining value after subtracting the value in HEPES represents the DIDS-insensitive accumulation (p = 0.01; d in the figure).
Fig. 4.
14C-MA accumulation in cells after acidic incubation. A) Effect of DIDS on 14C-MA accumulation. Cells were treated with 14C-MA assay solution in the absence and presence of 5% CO2, 33 mM (pH 7.4) for 30 min. The concentration of DIDS was 0.5 mM. The p values for comparison (a–d) are described in the text. Data were averaged from 3 experiments. B) Effect of acidic pHi on 14C-MA accumulation. Cells were incubated in the medium of pH 7.4 or 6.8 for 24 hours. Experiments were done by treating cells with 14C-MA assay solution (pH 7.4) for 20 min. Data were averaged from 5 experiments. C) Steady-state pHi. Cells were incubated in the medium of pH 7.4 or 6.8 for 24 h, and their pHi was measured in at the corresponding pH (n = 4 for each). The measurements were done using the ratiometric pH-sensitive dye BCECF-AM.
We then incubated cells at pH 7.4 and 6.8 (adjusted with NaHCO3 in 5% CO2) for 24 h and measured 14C-MA accumulation in the presence of 5% CO2, 33 mM (pH 7.4) (Fig. 4B). Acidic Incubation at pH 6.8-6.9 has been used for the in vitro study of metabolic acidosis in other systems (Tsuruoka et al. 2006;O'Hayre et al. 2006). Adding 0.5 mM DIDS to the assay solution reduced 14C-MA accumulation in control cells (p = 0.007; n = 5; a in the figure). However, DIDS showed negligible inhibition in cells incubated at pH 6.8. The 14C-MA accumulation was similar in the two groups of cells treated and untreated with DIDS (p > 0.05; b in the figure), indicating that DIDS-sensitive transport was inactivated after acidic incubation. This inactivation caused a substantial decrease in the accumulation.
Fig. 4C shows mean steady-state pHi in the presence of determined using the ratiometric pH-sensitive dye BCECF-AM. The steady-state pHi in cells incubated at pH 6.8 was 6.96 ± 0.03 (n = 4), significantly lower than 7.28 ± 0.04 (n = 4) for control cells (p = 0.001). Thus, acidic incubation lowered steady-state pHi. Incubating cells at pH 6.8 for 24 h caused 20% cell death compared to 8% for control, determined by Trypan Blue staining (data not shown). The unhealthy or dead cells are swollen, exhibit blebbing, and morphologically distinguishable from health cells. We used healthy cells for pH measurements.
NBCn1 in the MTAL cells is upregulated by acidic incubation
The above data show that the 14C-MA accumulation after acidic incubation is lower than that in control incubation, when compared in the absence of DIDS. This is probably due to inactivation of DIDS-insensitive extruders at low pHi, but it is also possible that acid-extruding loaders are stimulated under acidic conditions and reduce the conversion of CH3-NH3 to CH3-NH3+. To test whether NBCn1 protein expression is affected, we incubated cells at pH 6.8 for 24 h and performed immunoblot using the anti-NBCn1 antibody (Cooper et al. 2009). Control cells were incubated at pH 7.4 for 24 h.
Fig. 5A shows a representative immunoblot analysis of membrane preparations from cells at 0 and 24 h after acidic incubation. An immunoreactive band of 135 kDa was detected. A band of 250 kDa, which is a multimeric form of the protein as shown in neurons (Cooper et al. 2009), was additionally recognized. The detection of this higher molecular weight band varies depending upon experiments (Han Soo Yang, unpublished observation). NBCn1 expression was increased after acidic incubation. Analyzed by quantitative measurements of NBCn1 relative to β-actin (Fig. 5B), the increase was 48% (p = 0.04; n= 3). The upregulation was progressively larger at longer incubation periods (Fig. 5C and D) and thus, at 72 h, the NBCn1 expression level after acidic incubation was nearly 3 fold higher than controls. We also performed immunoblot of cells that were incubated in the medium containing 10 mM NH4Cl (pH 7.4) for 24 h and found a marked upregulation of NBCn1 after NH4Cl treatment (Fig. 5E and F). This result indicates that the pH in the media is not responsible for upregulating the transporter.
Fig. 5.
Effect of acidic incubation on NBCn1 expression. A) Immunoblot for NBCn1. Cells were incubated in the medium of pH 7.4 versus 6.8 for 0 or 24 h. Plasma membranes were isolated and subjected to immunoblot with the anti-NBCn1 antibody. The blots were striped and reprobed with the β-actin antibody. One of three experiments is shown. B) Quantitative measurements of NBCn1. The pixel intensity of NBCn1 was measured and normalized to β-actin (n = 3). The p value less than 0.05 was considered significant. C) Immunoblot for NBCn1 at normal and acidic incubation for 24, 48, and 72 hours. One of three experiments is shown. D) Quantitative measurements of NBCn1. The value of NBCn1/β-actin at pH 6.8 was presented relative to the value at pH 7.4 at each time point. The data were analyzed by one-way ANOVA with Bonferroni post-test. *p value of less than 0.05. E) NBCn1 expression in cells treated with NH4Cl. Cells were incubated in the medium (pH 7.4) containing 0 or 10 mM NH4Cl for 24 h. NH4Cl replaced NaCl. One of three experiments is shown. F) Quantitative measurements of NBCn1.
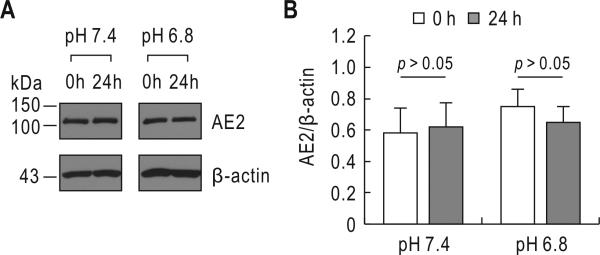
In other experiments, the blot was probed with the anti-AE2 antibody (Frische et al. 2004) to determine whether acidic incubation affects the Cl/HCO3 exchanger. An immunoreactive band of 110 kDa was recognized but remained unaffected after acidic incubation (Fig. 6A). The molecular weight of AE2 in ST-1 cells was smaller than ~150 kDa in the kidney (Frische et al. 2004). Quantitative measurements of AE2 relative to β-actin revealed negligible difference between control and acidic incubation (p > 0.05; n= 4) (Fig. 6B). We also performed immunoblot with antibodies to AE1 and AE3 but did not find immunoreactive bands (data not shown).
Fig. 6.
Effect of acidic incubation on AE2 expression. A) Immunoblot for AE2. The experimental procedure was similar to that in Fig. 5 except the blot was probed with the anti-AE2 antibody. One of four experiments is shown. B) Quantitative measurements of AE2. The pixel intensity of AE2 was measured and normalized to β-actin.
NBCn1 expressed in Xenopus oocytes increases 14C-MA accumulation
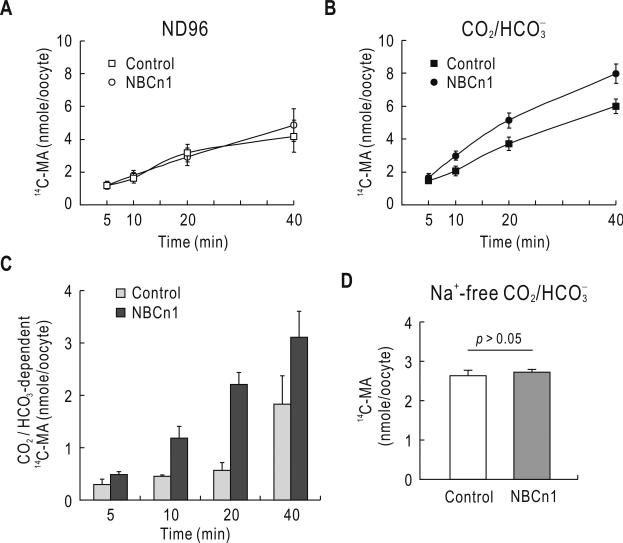
The above results led us to postulate that the loader NBCn1 might contribute to NH4+/NH3 uptake particularly under acidic conditions, in which the transporter is upregulated. To test this hypothesis, we expressed the rat renal NBCn1-E splice variant (Yang et al. 2009) in Xenopus oocytes and measured 14C-MA accumulation. Oocyte membranes are very impermeable to NH3 (Burckhardt & Frömter 1992;Nakhoul et al. 2001), indicating that oocytes can preferably trap NH3, opposite to our ST-1 cell system. For 14C-MA accumulation experiments with oocytes, therefore, bumetanide and ouabain were not added to the assay solution. In ND96 solution (Fig. 7A), oocytes expressing NBCn1-E and control oocytes similarly accumulated 14C-MA (p > 0.05, n = 16 oocytes for each). However, in a solution buffered with 5% CO2, 25 mM (Fig. 7B), oocytes expressing NBCn1-E showed a significant increase in 14C-MA accumulation (p < 0.05, n = 16 for each; two-way ANOVA with Bonferroni post-test). The difference between the two groups of oocytes was already substantial at 10 min after exposure to and continued afterward. Fig. 7C shows 14C-MA accumulation calculated by subtracting values in solution from values in solution. A profound increase was produced in oocytes expressing NBCn1-E. Na+ replacement abolished the difference in the accumulation (Fig. 7D).
Fig. 7.
Time course of 14C-MA accumulation in Xenopus oocytes expressing NBCn1. A & B) 14C-MA accumulation in oocytes injected with water or NBCn1-E cRNA. Oocytes were incubated with the 14C-MA assay solution (no bumetanide and ouabain) in the absence (A) or presence (B) of (pH 7.4), and then rapidly washed with ice-cold unlabeled MA solution at different time points. The accumulation was expressed as nmole/oocyte. Data were averaged from 4 independent experiments with total 16 oocytes per group. C) 14C-MA accumulation. The accumulation was calculated by subtracting values in ND96 solution from values in solution in A & B. D) Effect of Na+ replacement on 14C-MA accumulation. Na+ was replaced with NMDG+ (n = 3 for each).
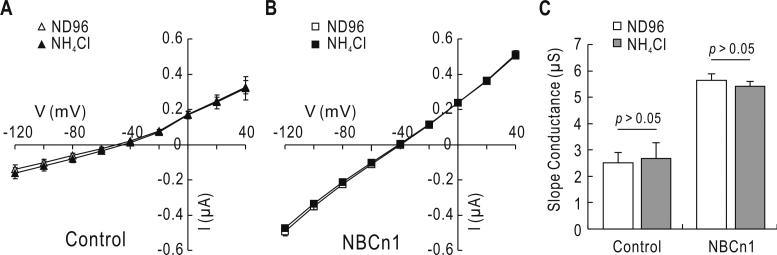
We then performed two-electrode voltage clamp to test whether NBCn1-E produces currents by NH4+. Determined before and 1 min after applying 20 mM NH4Cl, current-voltage relationships showed negligible change (Fig. 8A & B). The slope conductances were similar before and after NH4Cl application (p > 0.05 for both control and NBCn1-E; n = 6 for control and n = 8 for NBCn1-E) (Fig. 8C). Oocytes expressing NBCn1-E had more positive zero-current voltage and higher slope conductance due to the ionic conductance intrinsic to NBCn1 (Cooper et al. 2005).
Fig. 8.
Two-electrode voltage clamp of oocytes. A & B) Current-voltage relationships for currents before and after NH4Cl application. Oocytes were clamped at -60 mV and voltage commands from -120 to +40 mV were applied. Recordings were made in ND96 solution and 1 min after switching to a solution containing 20 mM NH4Cl. Data were averaged from 6 controls and 8 NBCn1-E-expressing oocytes. C) Slope conductance. The conductance was computed near the zero-current potential in A & B.
DISCUSSION
The major findings of the present study are the following: 1) enhances 14C-MA accumulation in the non-polarized MTAL cells and this effect is inhibited by DIDS; 2) 14C-MA accumulation is affected by replacing Cl–; 3) At low pHi, 14C-MA accumulation is not inhibited by DIDS; 4) NBCn1, but not AE2, in ST-1 cells is upregulated under acidic conditions; and 5) NBCn1 in Xenopus oocytes stimulates 14C-MA transport. These findings are important for understanding the effect of transporters on NH4+/NH3 uptake in the MTAL and provide evidence that cells regulate NH4+/NH3 uptake by altering the expression and activity of NBCn1 and AE2 in response to acid-base disturbance. A cell model for the effect of transporters on NH4+/NH3 uptake in ST-1 cells is described in the Supplementary Data.
We used radiolabeled MA to analyze the effect of transporters on NH4+/NH3 uptake. MA (pK of 10.3) has been extensively used as an NH4+/NH3 surrogate in many studies (Soupene et al. 2002;Verlander et al. 2003;Westhoff et al. 2002). When a cell is exposed to CH3-NH3+/CH3-NH2, membrane-permeable CH3-NH2 rapidly enters the cell and associates with H+ to form CH3-NH3+. The reaction then reaches a plateau as CH3-NH2 becomes equilibrated inside and outside the cell. Under this plateau phase, charged CH3-NH3+ is slowly transported into the cytosol via carrier proteins and then dissociates into CH3-NH2 and H+. If carrier-mediated CH3-NH3+ entry is inhibited, the total intracellular CH3-NH3+/CH3-NH2 (i.e., MA) is primarily determined by the amount of intracellular H+ supplied for the conversion of CH3-NH2 to CH3-NH3+. A high level of intracellular H+ supply increases the conversion and accumulates more 14C-MA, whereas a low level of intracellular H+ supply decreases it. Therefore, the magnitude of 14C-MA accumulation in our experiments reflects the activity of acid/base transporters in the cell.
In analyzing the effect of transporters on NH4+/NH3 uptake, we found that DIDS reduces 14C-MA accumulation (Fig. 1, Fig. 2, and Fig. 4). Additional experiments of Cl– replacement show the Cl/HCO3 exchanger influencing 14C-MA accumulation. In solution, CO2 enters the cell and produces H+ and . By moving out of the cell, the Cl/HCO3 exchanger concomitantly provides intracellular H+ available for the conversion of CH3-NH2 to CH3-NH3+. Nonetheless, our data suggest that the Cl/HCO3 exchanger would not be the major extruder affecting NH4+/NH3 uptake in ST-1 cells because Cl– replacement partially inhibits 14C-MA accumulation. DIDS in the Cl– replacement produces more severe inhibition than Cl– replacement alone (Fig. 2A), implying that there is an additional DIDS-sensitive process. This extrusion does not require K+ as raising the K+ concentration by 10 fold has no effect on 14C-MA accumulation (Fig. 2B and C). Similarly, it also dose not require Na+ because replacing Na+ has only a small effect. The molecular nature of this extrusion is unclear.
Under acidic conditions, the activity of Cl/HCO3 exchange appears to be minimal because DIDS has no significant effect on 14C-MA accumulation after acidic incubation (Fig. 3). Stewart et al. (Stewart et al. 2001) reported that AE2 is inactivated at low pHi. AE2-mediated 36Cl– uptake in Xenopus oocytes is almost abolished when the oocyte pHi is at 6.88. Furthermore, AE2 expression remains unaffected after acidic incubation (Fig. 6). This lack of AE2 upregulation is comparable to the previous report (Quentin et al. 2004) that AE2 in the MTAL of the kidney is not upregulated by chronic metabolic acidosis. Together with the lack of DIDS inhibition and the negligible change in AE2 expression, our data support the idea of Cl/HCO3 exchange being inactivated at low pHi.
In contrast to AE2, that NBCn1 in ST-1 cells is upregulated after acidic incubation (Fig. 5). This is in good agreement with the reports (Kwon et al. 2002;Odgaard et al. 2004) that NBCn1 expression and activity in rat MTAL are enhanced during chronic metabolic acidosis induced by NH4Cl feeding. It is also comparable to our recent report (Cooper et al. 2009) that NBCn1 in primary cultures of rat hippocampal neurons is upregulated after incubation at pH 6.2-6.5 for 1 hour. Others have reported that NBCn1 in rat MTAL is unaffected or down-regulated during chronic metabolic acidosis induced by ureter obstruction (Wang et al. 2008) and calcineurin inhibition (Mohebbi et al. 2009). The reason for the disparity among several reports remains unclear, but we think that the severity and duration of acidosis may determine the onset of NBCn1 upregulation. NBCn1 normally moves into the cell and extrudes acids, implying that its upregulation compensates excessive H+ load caused by acidification (Kwon et al. 2002;Odgaard et al. 2004). Consistent with this, we observe NBCn1 upregulation after incubating ST-1 cells with NH4Cl (Fig. 5E), further supporting the importance of pHi for NBC upregulation.
In Xenopus oocytes, NBCn1 increases 14C-MA accumulation (Fig. 7). Oocytes membranes are less permeable to NH3 than to NH4+ (Burckhardt & Frömter 1992;Nakhoul et al. 2001), similar to the apical membrane of the MTAL (Kikeri et al. 1989). Oocytes expressing NBCn1 show a relatively slow rise in pHi upon NH4Cl application compared to control oocytes (see Supplementary Data). Thus, the increased accumulation of 14C-MA in NBCn1-expressing oocytes reflects that carrier-mediated NH4+ transport is increased in these oocytes. It has been documented that NH4Cl induces a slow acidification with depolarization of oocyte membranes. However, we found no measurable change in Vm during NH4Cl application. The reason for this disparity is unclear although we note that NH4Cl-induced voltage changes in oocytes have been measured in solutions. It is also interesting to note that NH4+ transport in oocytes may be inhibited by acidic pHi (Boldt et al. 2003). Regardless, our data provide evidence that NBCn1 enhances NH4+ transport as determined by 14C-MA accumulation. This might be important for regulating NH4+ transport in the MTAL particularly during chronic metabolic acidosis.
In summary, our data provide in vitro evidence for NBCn1 upregulation in acidic pH environments. The upregulation may provide a constant supply of to the cell and enhance NH4+/NH3 uptake by buffering intracellular H+ load. It will be interesting to examine a possible NH4+/NH3 movement via NBCn1 in future experiments.
Supplementary Material
ACKNOWLEDGEMENT
The work was supported by the NIH R01-GM078502, National Kidney Foundation, and Emory URC grant (I.C). Dr. Soojung Lee was supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2007-357-C00121). Drs. Soojung Lee and Hye Jeong Lee equally contribute to the study and thus share the first authorship of the paper. The current address for Dr. Hye Jeong Lee is Department of Radiology and Radiological Sciences, Vanderbilt University, MCN CCC-1121, 1161 21st Avenue South, Nashville, TN 37232.
REFERENCES
- Boldt M, Burckhardt G, Burckhardt BC. NH4+ conductance in Xenopus laevis oocytes. III. Effect of NH(3). Pflügers Arch. 2003;446:652–657. doi: 10.1007/s00424-003-1122-z. [DOI] [PubMed] [Google Scholar]
- Bourgeois S, Masse S, Paillard M, Houillier P. Basolateral membrane Cl-, Na+, and K+coupled base transport mechanisms in rat MTALH. Am J Physiol Renal Physiol. 2002;282:F655–F668. doi: 10.1152/ajprenal.00220.2000. [DOI] [PubMed] [Google Scholar]
- Brosius FC, Nguyen K, Stuart-Tilley AK, Haller C, Briggs JP, Alper SL. Regional and segmental localization of AE2 anion exchanger mRNA and protein in rat kidney. Am J Physiol. 1995;269:F461–F468. doi: 10.1152/ajprenal.1995.269.4.F461. [DOI] [PubMed] [Google Scholar]
- Burckhardt BC, Frömter E. Pathways of NH3/NH4+ permeation across Xenopus laevis oocyte cell membrane. Pflügers Arch. 1992;420:83–86. doi: 10.1007/BF00378645. [DOI] [PubMed] [Google Scholar]
- Chang MH, Dipiero J, Sonnichsen FD, Romero MF. Entry to “Formula Tunnel” Revealed by SLC4A4 Human Mutation and Structural Model. J Biol Chem. 2008;283:18402–18410. doi: 10.1074/jbc.M709819200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature. 2000;405:571–575. doi: 10.1038/35014615. [DOI] [PubMed] [Google Scholar]
- Cooper DS, Saxena NC, Yang HS, Lee HJ, Moring AG, Lee A, Choi I. Molecular and functional characterization of the electroneutral Na/HCO3 cotransporter NBCn1 in rat hippocampal neurons. J Biol Chem. 2005;280:17823–17830. doi: 10.1074/jbc.M408646200. [DOI] [PubMed] [Google Scholar]
- Cooper DS, Yang HS, He P, Kim E, Rajbhandari I, Yun CC, Choi I. Sodium/bicarbonate cotransporter NBCn1/slc4a7 increases cytotoxicity in magnesium depletion in primary cultures of hippocampal neurons. Eur J Neurosci. 2009;29:437–446. doi: 10.1111/j.1460-9568.2008.06611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frische S, Zolotarev AS, Kim YH, Praetorius J, Alper S, Nielsen S, Wall SM. AE2 isoforms in rat kidney: immunohistochemical localization and regulation in response to chronic NH4Cl loading. Am J Physiol Renal Physiol. 2004;286:F1163–F1170. doi: 10.1152/ajprenal.00409.2003. [DOI] [PubMed] [Google Scholar]
- Gamba G, Friedman PA. Thick ascending limb: the Na+:K+:2Cl- co-transporter, NKCC2, and the calcium-sensing receptor, CaSR. Pflügers Arch. 2009;458:61–76. doi: 10.1007/s00424-008-0607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good DW. Ammonium transport by the thick ascending limb of Henle's loop. Annu Rev Physiol. 1994;56:623–647. doi: 10.1146/annurev.ph.56.030194.003203. [DOI] [PubMed] [Google Scholar]
- Good DW, Watts BA., III Functional roles of apical membrane Na+/H+ exchange in rat medullary thick ascending limb. Am J Physiol. 1996;270:F691–F699. doi: 10.1152/ajprenal.1996.270.4.F691. [DOI] [PubMed] [Google Scholar]
- Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- Kikeri D, Azar S, Sun A, Zeidel ML, Hebert SC. Na+-H+ antiporter and Na+-(HCO3-)n symporter regulate intracellular pH in mouse medullary thick limbs of Henle. Am J Physiol. 1990;258:F445–F456. doi: 10.1152/ajprenal.1990.258.3.F445. [DOI] [PubMed] [Google Scholar]
- Kikeri D, Sun A, Zeidel ML, Hebert SC. Cell membranes impermeable to NH3. Nature. 1989;339:478–480. doi: 10.1038/339478a0. [DOI] [PubMed] [Google Scholar]
- Kinne R, Kinne-Saffran E, Schutz H, Scholermann B. Ammonium transport in medullary thick ascending limb of rabbit kidney: involvement of the Na+, K+, Cl--cotransporter. J Membrane Biol. 1986;94:279–284. doi: 10.1007/BF01869723. [DOI] [PubMed] [Google Scholar]
- Kone BC, Higham S. Nitric oxide inhibits transcription of the Na+-K+-ATPase alpha1-subunit gene in an MTAL cell line. Am J Physiol. 1999;276:F614–F621. doi: 10.1152/ajprenal.1999.276.4.F614. [DOI] [PubMed] [Google Scholar]
- Kone BC, Schwobel J, Turner P, Mohaupt MG, Cangro CB. Role of NF-kappa B in the regulation of inducible nitric oxide synthase in an MTAL cell line. Am J Physiol. 1995;269:F718–F729. doi: 10.1152/ajprenal.1995.269.5.F718. [DOI] [PubMed] [Google Scholar]
- Kraut JA, Kurtz I. Metabolic acidosis of CKD: diagnosis, clinical characteristics, and treatment. Am J Kidney Dis. 2005;45:978–993. doi: 10.1053/j.ajkd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Kwon TH, Fulton C, Wang W, Kurtz I, Frokiaer J, Aalkjaer C, Nielsen S. Chronic metabolic acidosis upregulates rat kidney Na-HCO3 cotransporters NBCn1 and NBC3 but not NBC1. Am J Physiol Renal Physiol. 2002;282:F341–F351. doi: 10.1152/ajprenal.00104.2001. [DOI] [PubMed] [Google Scholar]
- Liu Z, Chen Y, Mo R, Hui C, Cheng JF, Mohandas N, Huang CH. Characterization of human RhCG and mouse Rhcg as novel nonerythroid Rh glycoprotein homologues predominantly expressed in kidney and testis. J Biol Chem. 2000;275:25641–25651. doi: 10.1074/jbc.M003353200. [DOI] [PubMed] [Google Scholar]
- Liu Z, Peng J, Mo R, Hui C, Huang CH. Rh type B glycoprotein is a new member of the Rh superfamily and a putative ammonia transporter in mammals. J Biol Chem. 2001;276:1424–1433. doi: 10.1074/jbc.M007528200. [DOI] [PubMed] [Google Scholar]
- Marini AM, Matassi G, Raynal V, Andre B, Cartron JP, Cherif-Zahar B. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat Genet. 2000;26:341–344. doi: 10.1038/81656. [DOI] [PubMed] [Google Scholar]
- Mohebbi N, Mihailova M, Wagner CA. The Calcineurin inhibitor FK506 (Tacrolimus) is associated with transient metabolic acidosis and altered expression of renal acid-base transport. Am J Physiol Renal Physiol. 2009 doi: 10.1152/ajprenal.90489.2008. [DOI] [PubMed] [Google Scholar]
- Nakhoul NL, Hering-Smith KS, Abdulnour-Nakhoul SM, Hamm LL. Ammonium interaction with the epithelial sodium channel. Am J Physiol Renal Physiol. 2001;281:F493–F502. doi: 10.1152/ajprenal.2001.281.3.F493. [DOI] [PubMed] [Google Scholar]
- O'Hayre M, Taylor L, Andratsch M, Feifel E, Gstraunthaler G, Curthoys NP. Effects of constitutively active and dominant negative MAPK kinase (MKK) 3 and MKK6 on the pH-responsive increase in phosphoenolpyruvate carboxykinase mRNA. J Biol Chem. 2006;281:2982–2988. doi: 10.1074/jbc.M510084200. [DOI] [PubMed] [Google Scholar]
- Odgaard E, Jakobsen JK, Frische S, Praetorius J, Nielsen S, Aalkjaer C, Leipziger J. Basolateral Na+-dependent HCO3- transporter NBCn1-mediated HCO3- influx in rat medullary thick ascending limb. J Physiol. 2004;555:205–218. doi: 10.1113/jphysiol.2003.046474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Rajbhandari I, Yang HS, Lee S, Cucoranu D, Cooper DS, Klein JD, Sands JM, Choi I. Neuronal Expression of Sodium/Bicarbonate Cotransporter NBCn1 (SLC4A7) and its Response to Chronic Metabolic Acidosis. Am J Physiol Cell Physiol. 2010 doi: 10.1152/ajpcell.00492.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quentin F, Eladari D, Frische S, Cambillau M, Nielsen S, Alper SL, Paillard M, Chambrey R. Regulation of the Cl-/HCO3- exchanger AE2 in rat thick ascending limb of Henle's loop in response to acid-base and sodium balance . J Am Soc Nephrol. 2004;15:2988–2997. doi: 10.1097/01.ASN.0000146426.93319.16. [DOI] [PubMed] [Google Scholar]
- Soupene E, Lee H, Kustu S. Ammonium/methylammonium transport (Amt) proteins facilitate diffusion of NH3 bidirectionally. Proc Natl Acad Sci U S A. 2002;99:3926–3931. doi: 10.1073/pnas.062043799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AK, Chernova MN, Kunes YZ, Alper SL. Regulation of AE2 anion exchanger by intracellular pH: critical regions of the NH(2)-terminal cytoplasmic domain. Am J Physiol Cell Physiol. 2001;281:C1344–C1354. doi: 10.1152/ajpcell.2001.281.4.C1344. [DOI] [PubMed] [Google Scholar]
- Sun AM. Expression of Cl-/HCO3- in the basolateral membrane of mouse medullary thick ascending limb. Am J Physiol. 1998;274:F358–F364. doi: 10.1152/ajprenal.1998.274.2.F358. [DOI] [PubMed] [Google Scholar]
- Tsuruoka S, Watanabe S, Purkerson JM, Fujimura A, Schwartz GJ. Endothelin and nitric oxide mediate adaptation of the cortical collecting duct to metabolic acidosis. Am J Physiol Renal Physiol. 2006;291:F866–F873. doi: 10.1152/ajprenal.00027.2006. [DOI] [PubMed] [Google Scholar]
- Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins RhBG and RhCG in mouse kidney. Am J Physiol Renal Physiol. 2003;284:F323–F337. doi: 10.1152/ajprenal.00050.2002. [DOI] [PubMed] [Google Scholar]
- Vorum H, Kwon TH, Fulton C, Simonsen B, Choi I, Boron W, Maunsbach AB, Nielsen S, Aalkjaer C. Immunolocalization of electroneutral Na-HCO3- cotransporter in rat kidney. Am J Physiol Renal Physiol. 2000;279:F901–F909. doi: 10.1152/ajprenal.2000.279.5.F901. [DOI] [PubMed] [Google Scholar]
- Wagner CA. Metabolic acidosis: new insights from mouse models. Curr Opin Nephrol Hypertens. 2007;16:471–476. doi: 10.1097/MNH.0b013e3282a4a69c. [DOI] [PubMed] [Google Scholar]
- Wang G, Li C, Kim SW, Ring T, Wen J, Djurhuus JC, Wang W, Nielsen S, Frokiaer J. Ureter obstruction alters expression of renal acid-base transport proteins in rat kidney. Am J Physiol Renal Physiol. 2008;295:F497–F506. doi: 10.1152/ajprenal.00425.2007. [DOI] [PubMed] [Google Scholar]
- Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol. 2007;69:317–340. doi: 10.1146/annurev.physiol.69.040705.142215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff CM, Ferreri-Jacobia M, Mak DO, Foskett JK. Identification of the erythrocyte Rh blood group glycoprotein as a mammalian ammonium transporter. J Biol Chem. 2002;277:12499–12502. doi: 10.1074/jbc.C200060200. [DOI] [PubMed] [Google Scholar]
- Xu J, Wang Z, Barone S, Petrovic M, Amlal H, Conforti L, Petrovic S, Soleimani M. Expression of the Na+-HCO3- cotransporter NBC4 in rat kidney and characterization of a novel NBC4 variant. Am J Physiol Renal Physiol. 2003;284:F41–F50. doi: 10.1152/ajprenal.00055.2002. [DOI] [PubMed] [Google Scholar]
- Yang HS, Cooper DS, Rajbhandari I, Park HJ, Lee S, Choi I. Inhibition of rat Na+-HCO3- cotransporter (NBCn1) function and expression by the alternative splice domain. Exp Physiol. 2009;94:1114–1123. doi: 10.1113/expphysiol.2009.048603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.