Abstract
Objective
Increased adolescent obesity rates in the United States are a significant public health concern. Obesity or increased adiposity during puberty in girls, an important period of breast development and a window of exposure sensitivity, may influence breast development and cancer risk. The purpose of this study was to investigate the impact of a high fat diet on mammary gland development in obesity-susceptible C57BL/6 and obesity-resistant BALB/c mice.
Design
Pubertal or adult C57BL/6 and BALB/c mice were fed a high fat diet (HFD) or control diet (CD) from 3 to 7 weeks of age or from 10 to 14 wks of age, respectively. The effects of HFD diet on body weight, adiposity, mammary gland development, and mammary gland response to estrogen were evaluated.
Results
Pubertal C57BL/6 mice fed the HFD had a significant increase in body weight and adiposity, and this was accompanied by stunted mammary duct elongation and reduced mammary epithelial cell proliferation. Ovariectomy and estrogen (E) treatment of pubertal HFD-fed C57BL/6 mice showed decreased mammary gland stimulation by E. Amphiregulin, a downstream mediator of pubertal E action, was reduced in mammary glands of HFD-fed C57BL/6 mice. Weight loss and reduced adiposity initiated by switching C57BL/6 mice from HFD to CD restored ductal elongation. Pubertal BALB/c mice fed the HFD did not exhibit a significant increase in body weight or adiposity; HFD caused increased mammary epithelial cell proliferation and had no effect on response to E. HFD had no effect on body weight or the mammary glands of adult mice.
Conclusions
HFD during puberty had a profound strain-specific effect on murine mammary gland development. Obesity and increased adiposity were associated with reduced responsiveness to estrogen and stunted ductal growth. Importantly, the effect of diet and adiposity on the mammary gland was specific to the pubertal period of development.
Keywords: obesity, mammary gland, puberty, estrogen, amphiregulin
Introduction
In general, early onset of puberty and breast development in girls is associated with increased body mass index (BMI) (1). Increased BMI during childhood and at puberty is also associated with reduced risk of future breast cancer (2-7), whereas early age onset of menses increases risk (8, 9). The apparently contradictory associations between childhood BMI, age of menarche and breast cancer suggest the involvement of different biological pathways that determine the effect of childhood BMI on breast cancer risk. In recent years, the incidence of pediatric obesity has rapidly increased (10), yet little is known about how childhood obesity affects breast development, which may subsequently alter breast cancer risk.
Puberty is a period when the mammary duct epithelium rapidly proliferates and invades the mammary fat pad thereby establishing the ductal tree. In pubertal rodents, terminal end buds (TEBs) are the proliferative epithelial structures responsible for ductal morphogenesis (11). TEBs can also give rise to malignant mammary tumors after exposure to chemical carcinogens (12), strongly indicating that puberty is a unique window of susceptibility. It is well established that high levels of dietary fat increase the development of mammary tumors in rodents, although it is dependent upon period of exposure and types of dietary fat (reviewed in 13). Dietary fat may influence mammary tumorigenesis by altering development of the mammary gland. In support of this, pubertal offspring of rodents exposed during pregnancy to isocaloric diets high in n-6 polyunsaturated fatty acids (e.g. corn oil) have reduced mammary differentiation (e.g. increased TEB number and decreased alveolar-lobular density) (14-16) and increased carcinogen-induced mammary tumorigenesis (14, 15). Exposure of pubertal Balb/c mice to high fat diets only marginally affected mammary differentiation (17, 18), but increased duct epithelial cell proliferation in control and estradiol:progesterone-treated mice (17).
Although high fat diets can impact mammary gland development, most of the studies used feeding protocols that did not cause substantial body weight gain during puberty. Recently, it has been reported that female C57BL/6 mice fed an obesogenic diet from puberty to early adulthood (20 wks) had enlarged mammary gland fat pads, and less dense and branched ductal tree (19). The impact of this obesogenic diet on mammary gland development during puberty, however, was not examined. The purpose of the present studies was to investigate the effects of a high fat diet and diet-induced obesity during puberty on mammary gland development in the mouse model. Two mouse strains, BALB/c and C57BL/6, with differing susceptibility to diet-induced obesity were studied. High fat diet feeding of pubertal C57BL/6 mice resulted in marked adiposity, stunted pubertal mammary gland development and decreased proliferation of the mammary epithelium. In contrast, in BALB/c mice the high fat diet caused a significantly smaller increase in body weight and increased proliferation in the mammary epithelium.
Materials & Methods
Animals
Three-week old female BALB/c and C57BL/6 mice were obtained from Charles River Laboratories, Portage, MI. Mothers of these mice were maintained on the same diet (LabDiet 5L79, PMI Nutrition International LLC, St. Louis, MO) before and during pregnancy, and while nursing. Upon arrival, mice were randomly distributed into two diet groups – control diet (CD) or high fat diet (HFD). Animals were housed in polysulfone cages, and received food and water ad libitum. Housing facilities were maintained on a 12:12 h light-dark cycle, at 20-24°C with 40-50% relative humidity. The two purified diets, 58G7 (12% kcal fat – CD) and 58G9 (60% kcal fat – HFD), were purchased from TestDiet, PMI Nutrition International LLC, St. Louis, MO. For both diets, 11% kcal fat came from corn oil, with the remainder of the fat (1% or 49% kcal) coming from lard. Composition of the diets is further described in Supplemental Table 1. For time course studies, mice were maintained on CD or HFD for 2, 3 or 4 wks (i.e. 5, 6 or 7 wks of age). During this period, body weight, food consumption and vaginal opening were monitored twice weekly. For hormone-response studies, mice maintained on CD or HFD for 4 wks (i.e. 7 wks of age) were ovariectomized (OVX), maintained for three additional weeks on given diets, and then subcutaneously injected daily for 5 days with saline control (C) or 17-ß-estradiol (E, 1 μg). Adult female BALB/c and C57BL/6 mice were fed CD or HFD from 13 to 17 weeks of age. Two h prior to termination, mice were injected with 5-bromo-2′-deoxyuridine (BrdU) (70 μg/g body weight). At termination of all feeding studies, mammary tissues were procured for RNA isolation (described below), or fixed and processed as whole mounts (20) or paraffin-embedded for H&E staining and immunohistochemistry (21). Plasma insulin and leptin levels were determined by RIA (Linco, St. Louis, MO). Plasma estradiol levels were determined by ELISA (Perkin Elmer, Watham, MA). All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Michigan State University.
Mammary Gland Whole Mount Analysis
Whole mount preparations of inguinal glands were scored for growth and development status as previously described (22). Microphotographs were taken at 10× magnification using a Nikon SMZ-2T microscope with QImaging MicroPublisher 5.0 RTV Camera (Surrey BC, Canada) and longitudinal growth and numbers of terminal end buds (TEBs) were assessed. Longitudinal growth was determined by measuring duct length extending from the lymph node to the most distal terminal branches. TEBs were defined as enlarged multilayered ductal tips with a diameter greater than 100 μm that were surrounded by adipocytes and located in the periphery of the gland. Mean values for each treatment group were calculated and analyzed statistically for treatment-related differences.
Immunofluorescence
Detection of progesterone receptor A (PRA) and estrogen receptor α (ERα) using anti-PRA (1:50; hPRa7, Neomarkers, Fremont, CA) or anti-ERα (1:10; Novocastra, Newcastle, United Kingdom) antibodies was previously described (21). PCNA was detected using mouse monoclonal anti-PCNA (1:100, Calbiochem, Gibbstown, NJ). Amphiregulin was detected using a goat polyclonal anti-mouse amphiregulin (1:40, R&D Systems, Minneapolis, MN). Primary antibodies were detected by appropriate secondary antibody conjugated to Alexa 488 (Molecular Probes, Eugene, OR). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole, dilactate (DAPI) (1:10,000; Molecular Probes). Sections were visualized and images captured using a Nikon inverted epifluorescence microscope (Mager Scientific, Dexter, MI) with MetaMorph software (Molecular Devices Corp. Downingtown, PA).
PCNA, PRA, or ERα were quantified for number of positive luminal epithelial cells from captured images using MetaMorph software as previously described (23). Nuclei with a punctuate pattern of PCNA staining was quantitated as a measure of proliferation (24) and this was determined on mammary glands from mice in the estrus stage of the estrus cycle. Fluorescence intensity was determined by the average pixel intensity of all positively stained nuclei within the ductal epithelium. Image thresholds were set to exclude background fluorescence and gated to include intensity measurements only from positively staining epithelial cells. Background staining in epithelial cells was determined through setting thresholds to exclude positive staining, and the level of positive staining above background was calculated by subtracting background fluorescence intensity. When measures of fluorescence intensity were used as a semiquantitative measure of protein expression level, image capture conditions and settings were identical for all samples analyzed. A minimum of 3 mice per treatment group and a minimum of 1000 cells in three independent sections per mouse were analyzed.
Immunoperoxidase
Tissue sections were incubated in 3% H2O2 in methanol for 10 min, and subjected to antigen retrieval by heat and pressure in 10mM sodium citrate solution (pH 6.0) for 20 min (21). Sections were incubated with goat anti-mouse IgG Fab fragments (MP Biomedicals, Solon, OH) [1:100 in PBS containing 1% BSA) for 60 min, rinsed then blocked with normal goat serum (Vector Laboratories, Burlingame, CA) (1:1 in PBS) for 30 min. Tissue sections were incubated with mouse monoclonal anti-BrdU antibody with nuclease (anti-BrdU detection kit; Amersham, Little Chalfont, Buckinghamshire, UK) for 60 min at room temperature. This was followed by incubation with goat anti-mouse biotinylated antibody (Dako, Carpinteria, CA) (1:400 in PBS) for 30 min, then avidin-biotin-horseradish peroxidase complex (ABC reagent; Vector Laboratories). Immunoperoxidase localization of antibody staining was obtained using 3′-3′-diaminobenzidine (Thermo Scientific, Rockford, IL). Sections were counterstained with hematoxylin and visualized using a Nikon Eclipse 400 microscope.
Quantitative PCR arrays and quantitative RT-PCR analysis (qRT-PCR)
Total RNA was extracted from mammary glands using TRIzol® reagent (Invitrogen, Carlsbad, CA). First strand cDNA was synthesized using RT2 First Strand kit (SABiosciences). cDNAs were then used to quantify mRNA levels using mouse growth factor PCR arrays (SABiosciences, Cat #: PAMM-041) and qRT-PCR analysis. Primers pairs for qRT-PCR analysis of the following genes were purchased from Integrated DNA Technologies (Coralville, IA): leptin 5′-ACAGTTTCGTGCTCAGCTCTGTCT-3′, 5′-TGTCACCCTCAGCTCAGGTTCTTT-3′; aromatase (CYP19A1) 5′-GCCTTTGGAGAACAATTCGCCCTT-3′, 5′-AGGTGTCCAGCATGATGTGTCTCA-3′; amphiregulin 5′-TCTGCCATCATCCTCGCAGCTATT-3′, 5′-CGGTGTGGCTTGGCAATGATTCAA-3′; estrogen receptor α 5′-TGAGGGCTGTAGGGTGTGTTGATT-3′, 5′-AAGGCTAGCATCCTGTGCTGAAGA-3′. The q-PCR was performed with an ABI7500Fast (Applied Biosystems Inc., Foster City, CA).
Quantitation and statistical analyses
Results are expressed as mean ± SEM, and differences are considered significant at p < 0.05 by using Student's t test or ANOVA followed by a Tukey's multiple comparison test as appropriate.
Results
Strain-specific effect of high fat diet on pubertal mammary gland development
To investigate the impact of a high fat diet during puberty on the mammary gland development, C57BL/6 and BALB/c mice were fed a control diet (CD) or high fat diet (HFD) from weaning (3 wks of age) to 7 weeks of age. The HFD consisted of 60% kcal fat, 21% kcal carbohydrate and 19% kcal protein, whereas the CD was 12% kcal fat, 69% kcal carbohydrate and 19% kcal protein (Supplemental Table 1). Daily food consumption (g) was not significantly different between mouse strains irrespective of diets. For example, three weeks into the feeding study C57BL/6 mice consumed 2.46 ± 0.05 or 2.38 ± 0.16 g/day of CD or HFD, respectively. While Balb/c mice consumed 2.42 ± 0.03 or 2.45 ± 0.62 g/day of CD or HFD, respectively. C57BL/6 mice fed HFD for 4 wks weighed 1.2 ± 0.39 g (p < 0.05) more than CD fed C57BL/6 mice (Table 1). In contrast, BALB/c mice fed HFD did not exhibit a statistically significant gain in body weight.
Table 1. Impact of HFD on pubertal C57BL/6 and BALB/c mice.
| Mouse strain/Diet | Body Weight (g) | Fasting blood glucose (mg/dl) | Fasting plasma insulin (ng/ml) | Leptin (ng/ml) | Mammary gland 4 weight (mg) | Mammary gland 2 & 3 weight (mg) | Parametrial fat pad weight (mg) | Liver weight (mg) |
|---|---|---|---|---|---|---|---|---|
| C57BL/6 CD | 18.1 ± 0.2 | 139 ± 5.6 | 0.48 ± 0.06 | 2.79 ± 0.58 | 113.6 ± 5.2 | 147.9 ± 6.2 | 89.3 ± 11.9 | 798.4 ± 14.8 |
| C57BL/6 HFD | 19.3 ± 0.4a,b | 120.9 ± 4.5b | 0.47 ± 0.05 | 8.74 ± 1.99a,b | 206.4 ± 18.6a,b | 252.9 ± 2.1a,b | 233.6 ± 30.6a,b | 680.5 ± 18.0a |
| BALB/c CD | 17.9 ± 0.2 | 102.9 ± 4.7 | 0.42 ± 0.06 | 1.96 ± 0.20 | 92.5 ± 5.0 | 124.2 ± 6.2 | 100.0 ± 11.3 | 736.5 ± 17.3 |
| BALB/c HFD | 18.5 ± 0.3 | 118.3 ± 4.9d | 0.60 ± 0.04d | 2.39 ± 0.16c | 111.7 ± 7.6c | 170.0 ± 8.6c,d | 156.7 ± 22.1d | 712.3 ± 12.3 |
p < 0.001 when compared to CD fed C57BL/6 mice (n = 7 to 8, ANOVA).
p < 0.02 when compared to CD fed C57BL/6 mice (n = 7 to 8, Student's t-test).
p < 0.001 when compared to HFD fed C57BL/6 mice (n = 6 to 7, ANOVA).
p < 0.05 when compared to CD fed BALB/c mice (n = 6, Student's t-test).
A time course study showed that after 2 weeks on CD or HFD, C57BL/6 mammary glands had similar mammary gland development as evidenced by similar degree of ductal elongation and numbers of terminal end buds (TEBs) (data not shown). After 3 and 4 weeks, however, HFD-fed C57BL/6 mice had reduced duct length and numbers of TEB compared to CD-fed C57BL/6 mice (Fig. 1A). Mammary glands of CD-fed C57BL/6 mice contained well-arborized ducts, whereas HFD-fed mice had a sparse mammary ductal tree (Fig. 1A). Quantitation showed that duct length and numbers of TEBs in C57BL/6 mice fed HFD for 4 weeks were significantly reduced (Fig. 1B). Analysis of epithelial cell proliferation showed a significant decrease in HFD fed C57BL/6 mice (Fig. 1C).
Figure 1. Mammary duct epithelium of peripubertal C57BL/6 mice fed HFD has reduced outgrowth and terminal end bud formation.
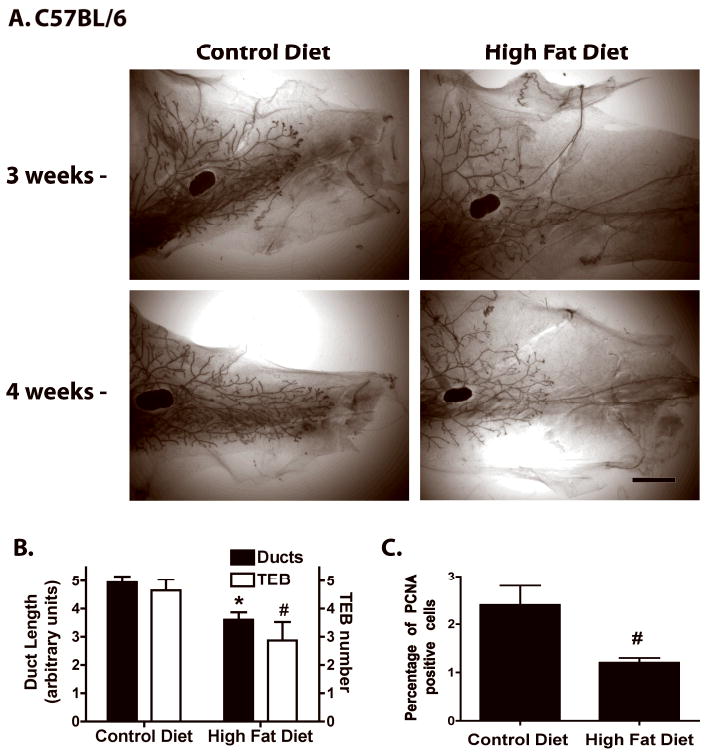
A. Whole mounts of representative mammary glands from peripubertal C57BL/6 mice fed CD or HFD for 3 or 4 weeks. Scale bar, 300 μm. B. Mammary duct length and TEB number in C57BL/6 mice fed CD or HFD for 4 weeks (n = 15). *, p < 0.001 or #, p < 0.05 when compared to CD fed C57BL/6 mice. C. Percentage of PCNA-positive epithelial cells in ducts, duct ends and TEB of C57BL/6 mice during estrus and fed CD or HFD (n = 3). #, p < 0.05 when compared to CD fed C57BL/6 mice.
The mammary glands of BALB/c mice fed CD or HFD for 4 weeks exhibited similar ductal morphologies, extent of ductal elongation and numbers of TEBs (Fig 2A,B). Analysis of epithelial cell proliferation showed a significant increase in HFD-fed mice (Fig. 2C).
Figure 2. Effect of HFD on mammary duct epithelium development in pubertal BALB/c mice.
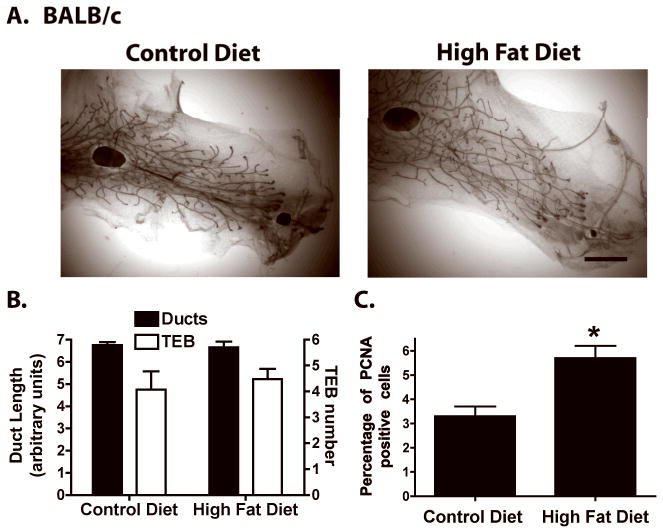
A. Representative whole mounts of mammary glands from peripubertal BALB/c mice fed CD or HFD for 4 weeks (n = 7). Whole mounts from BALB/c mice fed CD or HFD for 2 or 3 weeks are not shown because no distinct differences were observed. Scale bar, 300 μm. B. Mammary duct length and TEB number in BALB/c mice fed CD or HFD for 4 weeks (n = 14). C. Percentage of PCNA-positive epithelial cells in ducts, duct ends and TEB of BALB/c mice during estrus and fed CD or HFD (n = 3). *, p < 0.05 when compared to CD fed BALB/c mice.
Effect of HFD on adult mammary gland
The effect of diet on mammary gland development described above was observed during pubertal development. This raised the question as to whether 4 weeks of HFD feeding would affect mammary gland morphology in sexually mature mice. BALB/c and C57BL/6 mice were fed CD or HFD from 13 to 17 weeks of age. During this feeding period, HFD-fed C57BL/6 mice did not exhibit significant body or mammary gland weight gain compared to CD-fed mice (Fig. 3B and data not shown). BALB/c mice fed HFD showed a trend toward increased body weight and this was reflected in a significant increase in mammary gland weight (Fig. 3B). Analysis of mammary gland whole mounts showed no consistent effect of HFD on mammary gland morphology in either strain (Fig. 3A). Analysis of proliferation as assessed by percentage of cells labeled with BrdU revealed no significant differences between CD and HFD in either mouse strain (1.97 ± 1.82 vs 1.33 ± 0.55 for C57BL/6; 1.47 ± 0.84 vs 1.10 ± 0.17 for Balb/c; n=3).
Figure 3. Effect of HFD on mammary gland morphology in adult BALB/c and C57BL/6 mice.
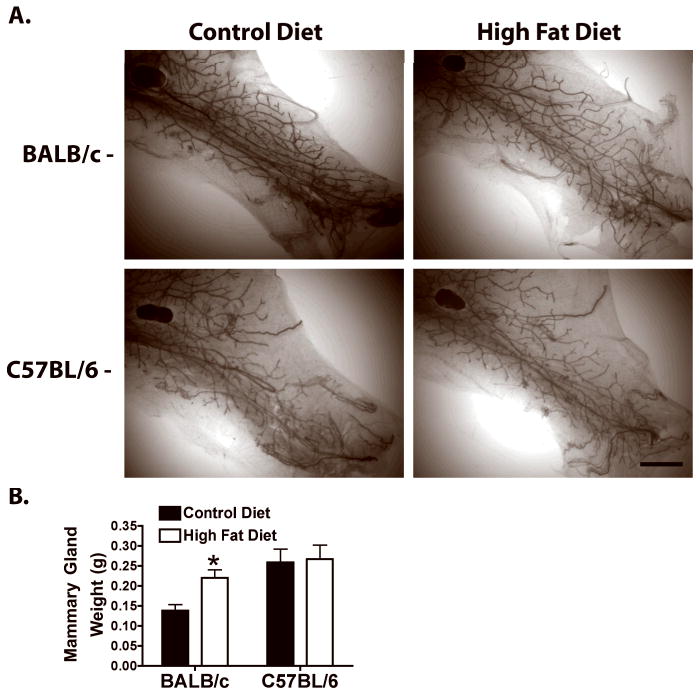
Adult BALB/c and C57BL/6 mice were fed CD or HFD from 13 to 17 weeks of age. A. Mammary gland whole mounts. Scale bar, 300 μm. B. Mammary gland weights. *, p < 0.05 HFD fed compared to CD fed BALB/c mice.
Systemic effects of HFD in pubertal mice
In order to determine if the effects HFD on mammary gland development observed in C57BL/6 and BALB/c mouse strains were mediated systemically we investigated the effects on tissue adiposity, glucose metabolism, and liver histology (Table 1). C57BL/6 mice fed HFD for 4 wks weighed significantly more than CD fed C57BL/6 mice. This increase in body weight was associated with a 1.8- and 2.6-fold increase in mammary gland and parametrial fat pad weights, respectively. Livers from C57BL/6 mice fed HFD weighed significantly less than livers of CD-fed C57BL/6 mice. Consistent with increased adiposity, plasma leptin levels were significantly elevated in C57BL/6 mice fed HFD. Leptin mRNA levels were also increased 5.9-fold in mammary glands in C57BL/6 mice fed HFD (Fig. 4). Fasting blood glucose levels were slightly decreased in C57BL/6 mice fed HFD, however, there were no significant differences in fasting plasma insulin levels.
Figure 4. Effect of HFD on leptin, ERα and amphiregulin mRNA levels in peripubertal C57BL/6 and BALB/c mice.
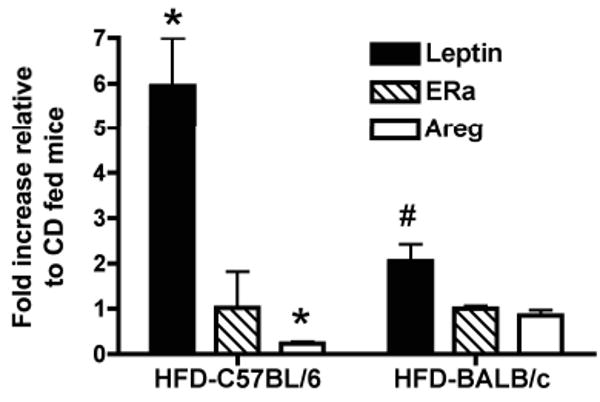
Peripubertal C57BL/6 and BALB/c mice were fed CD or HFD for 4 weeks, after which total RNA was isolated from mammary glands. Leptin, ERα and amphiregulin mRNA levels were determined as described in the Materials & Methods. Data are expressed as fold change in HFD-fed mice relative to mice fed CD. Values are mean ± SEM (n = 3). *, p < 0.005 when compared to CD-fed C57BL/6 mice. #, p < 0.05 when compared to CD-fed BALB/c mice.
BALB/c mice fed HFD did not exhibit a statistically significant gain in body weight (Table 1). HFD feeding, however, led to a 1.2- and 1.6-fold increase in mammary gland and parametrial fat pad weights, respectively. There were no significant changes in liver weights between diet groups. Plasma leptin levels were not significantly elevated in BALB/c mice fed HFD. Leptin mRNA levels, however, were increased 2-fold in mammary glands in BALB/c mice fed HFD (Fig. 4). Fasting blood glucose levels were slightly elevated in BALB/c mice fed HFD and this occurred with a small but significant increase in plasma insulin levels. Overall these findings demonstrate that C57BL/6 mice are more susceptible to diet-induced obesity (DIO) than BALB/c mice and this was reflected by marked increases in body, mammary gland and parametrial fat pad weights.
To determine if alterations in mammary gland development were due to differences in onset of puberty, the age at vaginal opening was examined. There was no significant difference in mean age at vaginal opening for CD- or HFD-fed mice in either mouse strain (data not shown). Analysis of plasma estradiol levels after 4 weeks on the diets revealed no significant differences between CD and HFD in either mouse strain (27.6 ± 4.2 vs 31.4 ± 3.7 pg/ml for C57BL/6, n=7-8; 36.3 ± 4.8 vs 36.3 ± 3.4 pg/ml for BALB/c, n=8-9). There were also no discernible differences in ovarian histology between CD and HFD in either mouse strain (data not shown).
Mammary gland specific effects of HFD in pubertal mice
The most notable effect of HFD was inhibition of mammary gland development in the C57BL/6 mouse strain. Since none of the systemic parameters measured above provided a plausible explanation for the observed inhibitory effects, we investigated potential mammary gland-specific effects of HFD. Combined actions of estrogen and progesterone regulate the development of the mammary duct epithelium, with estrogen playing a crucial role in ductal elongation through stimulation of proliferation in TEBs. The inhibition of ductal elongation and TEB formation in C57BL/6 mice fed HFD suggested that increased adiposity might alter ERα expression. There were no significant differences in ERα mRNA levels (Fig. 4) or intensity of ERα immunofluorescent staining in luminal epithelial cells between CD and HFD fed C57BL/6 mice (data not shown). Progesterone receptor isoform A (PRA), the major PR isoform expressed prior to pregnancy, was also investigated as a downstream transcriptional target of E. There were also no notable changes in intensity of PRA immunofluorescent staining between C57BL/6 mice fed CD or HFD (data not shown). The percent of PRA positive duct luminal epithelial cells in HFD fed C57BL/6 mice was not significantly different from mice fed CD (50.10 ± 1.48% (CD) versus 51.85 ± 1.60% (HFD)).
There were also no notable differences in PRA or ERα immunofluorescent staining (data not shown), or ERα mRNA levels between BALB/c mice fed CD or HFD (Fig. 4). The percent of PRA positive cells was 51.6 ± 1.3% and 52.1 ± 1.2% for BALB/c mice fed CD and HFD, respectively.
Strain-specific effect of HFD on mammary gland responses to exogenous estrogen
To assess the impact of DIO on responsiveness of mammary epithelium to estrogen (E), C57BL/6 and BALB/c mice fed CD or HFD for 4 wks were ovariectomized (OVX), maintained for 3 wks on their respective diets to allow pubertal mammary duct and TEB regression (25), and then treated with E for 5 days. As expected, ovariectomy led to regression of the mammary gland in CD and HFD fed C57BL/6 and BALB/c mice (Fig. 5A and B). Treatment of CD fed OVX-C57BL/6 with E caused stimulation of duct ends (Fig. 5A). In contrast, HFD fed OVX-C57BL/6 mice exhibited a reduced stimulatory response to E. OVX-BALB/c mice fed either CD or HFD exhibited similar duct end stimulation in response to E (Fig 5B). Treatment with E increased uterine weights in both OVX-BALB/c and OVX-C57BL/6 mice irrespective of diet (data not shown) indicating that the HFD-induced reduced response to E was specific to the mammary gland.
Figure 5. HFD modifies mammary gland response to E in ovariectomized C57BL/6 mice treated with estrogen (E).
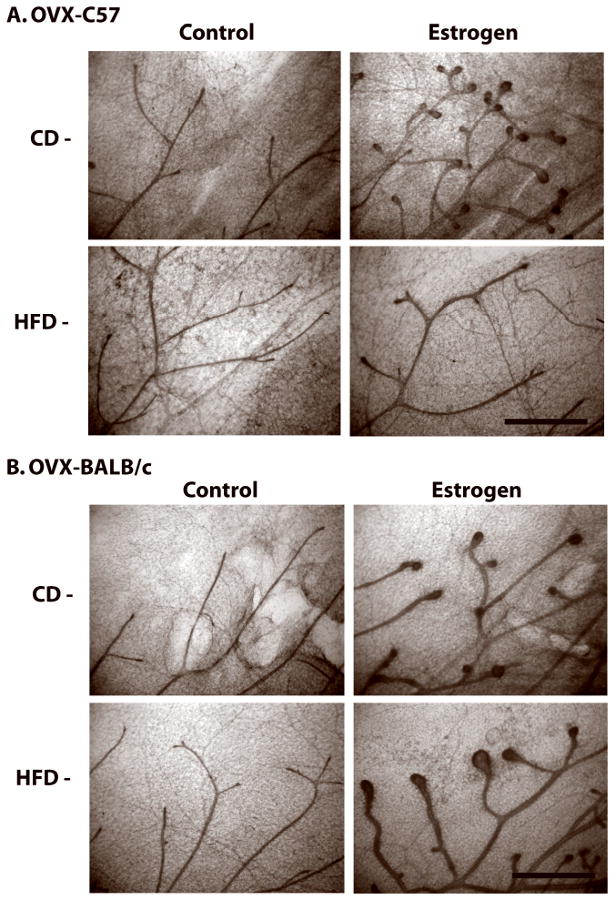
Peripubertal C57BL/6 or BALB/c mice were fed CD or HFD for 4 weeks (7 weeks of age), after which they were ovariectomized and maintained on respective diets for 3 additional weeks to allow for TEB regression. The mice were then treated with saline (control) or E for 5 days as described in Materials & Methods. Representative mammary gland whole mounts from (A) C57BL/6 mice or (B) BALB/c mice. Scale bar, 100 μm.
Effect of HFD on amphiregulin expression in pubertal C57BL/6 mice
Estrogenic stimulation of pubertal mammary gland development involves a paracrine mechanism via E regulation of amphiregulin (Areg) (26). Since HFD fed C57BL/6 mice had a reduced response to E, we analyzed the effect of HFD on Areg expression. Areg mRNA levels were decreased by 77% in HFD-fed C57BL/6 mice (Fig. 4). Areg immunofluorescent staining was also markedly reduced in HFD fed C57BL/6 mice (Fig. 6).
Figure 6. HFD-fed C57BL/6 mice have reduced amphiregulin protein expression.
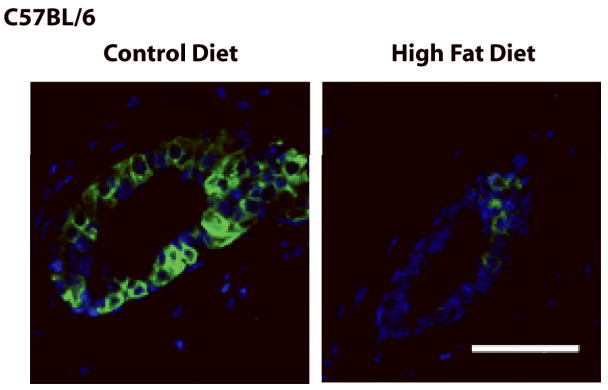
Representative merged images of mammary gland Areg (green) immunofluorescent staining in mammary glands of peripubertal C57BL/6 mice fed CD or HFD for 4 weeks. Nuclei were counterstained with DAPI. Scale bar, 25 μm.
Effect of weight loss in C57BL/6 mice fed HFD on mammary gland development
The increase in mammary gland weight in HFD-fed C57BL/6 mice (Table 1) corresponded to an enlargement in mammary adipocyte size (Fig. 7A). HFD also increased adipocyte size in BALB/c mice, but to a lesser extent compared to C57BL/6 mice. These findings suggest that increased adiposity of the mammary fat pad in C57BL/6 mice might be associated with delayed mammary gland development. To examine this possibility, C57BL/6 mice fed HFD for 4 weeks were switched to CD for a subsequent 6 weeks. Switching from HFD to CD led to a 16% decrease in body weight (Table 2). The decrease in body weight was reflected in a 47% decrease in mammary gland weight and a 64% decrease in parametrial fat pad weight. The decrease in mammary gland weight was also accompanied by a reduction in mammary adipocyte size (Fig. 7B). Importantly, the decrease in adiposity in C57BL/6 mice switched from HFD to CD corresponded with restoration of TEBs and ductal elongation. Increased adipocyte size was also maintained in mammary glands of HFD-fed OVX C57BL/6 mice treated with E (Fig. 7C).
Figure 7. Effect of switching C57BL/6 mice fed HFD to CD on adipocyte size and mammary gland development.
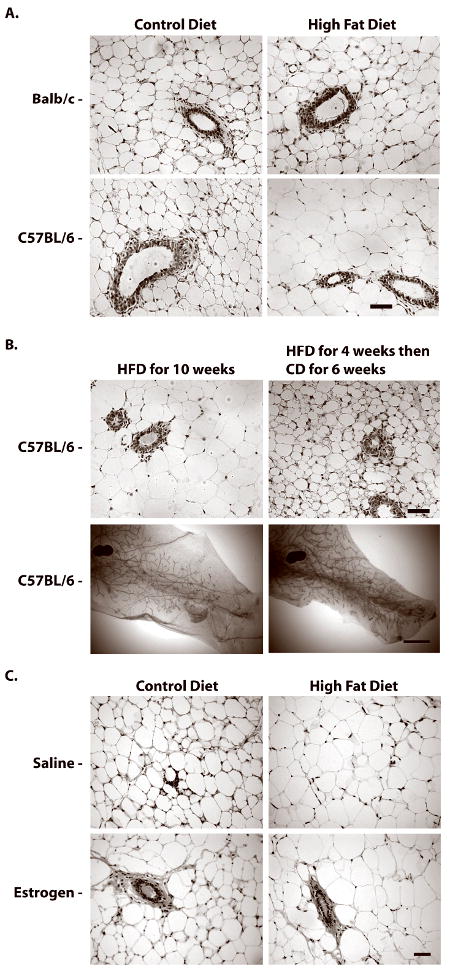
A. Representative H&E staining of mammary glands from BALB/c and C57BL/6 mice fed CD or HFD for 4 weeks; note significant increase in adipocyte size in C57BL/7 glands in HFD group. Scale bar, 60 μm. B. Representative H&E staining and mammary gland whole mounts from peripubertal C57BL/6 mice fed HFD for 4 weeks, then switched to CD or maintained on HFD for an additional 6 weeks; note decrease in adipocyte size and increase in duct length in C57BL/6 glands in group switched to CD. C. Representative H&E staining of mammary glands from CD- or HFD-fed OVX-C57BL/6 mice treated with saline (control) or estrogen. Note that E treatment for 5 days had no discernible effect on adipocyte size. H&E images scale bar, 60 μm. Whole mount images scale bar, 300 μm.
Table 2. Switching pubertal C57BL/6 mice fed HFD for 4 weeks to CD for an additional 6 weeks reduces body weight and adiposity.
| Diet and Time | Body weight (g) | Mammary gland #4 weight (mg) | Mammary gland #2 and 3 weight (mg) | Parametrial fat pad weight (mg) | Uterus weight (mg) |
|---|---|---|---|---|---|
| 10 wks HFD | 24.99 ± 0.52 | 326.7 ± 32.7 | 358.3 ± 25.2 | 413.3 ± 38.3 | 65.0 ± 8.5 |
| 4 wks HFD and 6 wks CD | 20.98 ± 0.21a | 163.3 ± 6.6a | 200.0 ± 6.8a | 148.3 ± 12.8a | 65.0 ± 5.6 |
p < 0.005 when compared to mice fed HFD for 10 weeks (Student's t-test).
Discussion
Strain-specific inhibition of pubertal mammary gland development by HFD
When fed a standard rodent chow (Teklad 8640, 16% kcal fat, major source soybean oil) pubertal (6 wk old) C57BL/6 and BALB/c mice have similar ductal development including the degree of ductal branching and TEB number (27). As shown herein these developmental parameters are sensitive to the effects of a HFD, with the most pronounced impact occurring in the C57BL/6 mouse strain. Pubertal C57BL/6 mice fed a HFD had reduced mammary epithelial cell proliferation, TEB number, and ductal elongation. Strikingly, delayed mammary ductal elongation and reduced numbers of TEBs was evident after 3 wks (at 6 wks of age) on HFD and this coincided with a significant increase in mammary gland weight. The HFD did not affect TEB number or elongation in BALB/c mice but caused an increase in mammary epithelial cell proliferation.
C57BL/6 mice are susceptible to HFD-induced obesity, hyperglycemia and hyperinsulinemia relative to A/J mice (28). BALB/c mice, which are genetically related to A/J mice (29), are much less susceptible to HFD-induced obesity than C57BL/6 mice (30). These strain differences suggest that the delay in mammary development in HFD fed C57BL/6 mice may result from increased adiposity and associated changes. Consistent with this, pubertal C57BL/6 mice fed HFD have significantly heavier mammary and parametrial fat pads than HFD-fed BALB/c mice. Moreover, weight loss initiated by switching C57BL/6 mice from HFD to CD restored TEB formation and ductal elongation. It has been previously reported that C57BL/6 mice fed a HFD from 4 to 20 wks of age had a ∼4-fold increase in mammary gland weights and less densely distributed ducts and diminished branching frequency (19). In our studies both mouse strains consumed similar amounts of HFD, which suggests that the calories and type of fat consumed were not the underlying cause of the strain differences in mammary gland development. Feeding HFD to adult mice from 13-17 weeks of age did not significantly increase body weight but increased mammary gland weights in only BALB/c mice. The HFD, however, had no significant effect on mammary gland morphology. These results demonstrate that the major effect of HFD on weight gain and mammary gland development occurs during pubertal development.
Effect of HFD on hormonal regulation of mammary gland development
Estrogen has been shown to stimulate ductal morphogenesis during puberty (25, 31). Moreover, ERα-knockout mice do not form TEB and have only rudimentary mammary glands that fail to grow (32). These reports suggested that changes in estrogen levels or estrogen signaling might account for the stunted mammary gland development in C57BL/6 mice fed HFD. HFD, however, did not alter the mean age of vaginal opening, an index of pubertal onset that occurs within a week prior to first estrus in rodents (33). Estradiol levels and ovarian morphology were also not significantly changed in either strain of mice fed HFD for 4 wks. These findings suggest that HFD delayed mammary gland development independent of changes in sexual maturation, ovarian function, or estradiol levels. HFD also did not alter the percentage of mammary luminal epithelial cells that expressed ERα in either mouse strain. Consistent with this observation, the percentage of mammary luminal epithelial cells that expressed PRA, a transcriptional target for ERα that is extensively colocalized with ERα in normal mouse mammary epithelium (23), was also not affected by HFD in either mouse strain. The lack of an affect of HFD on ERα levels in pubertal Balb/c mice conflicts with Hilakivi-Clarke et al. who report that mammary glands of Balb/c mice fed a high corn oil diet have increased ER binding activities (16).
To determine if HFD caused altered responsiveness to E, the effect of exogenous E was assessed in OVX-C57BL/6 or OVX-BALB/c mice fed CD and HFD. Treatment of OVX-C57BL/6 mice fed CD with E stimulated duct ends, whereas HFD fed OVX-C57BL/6 mice had a dramatic reduced stimulatory response to E. Because of the prominent role of E in mammary duct development, loss of E responsiveness in HFD fed C57BL/6 mice might account for the stunted duct elongation and reduced numbers of TEBs. Estrogen is not considered to be a mitogen that directly acts on pubertal mouse mammary epithelial cells. Instead, it is thought to act through a paracrine mechanism via the induction of Areg, which acts on stromal cells to induce growth factors such as fibroblast growth factors, hepatic growth factor and/or insulin-like growth factor that then act on the mammary epithelium to stimulate proliferation (reviewed in 34, 35). Interestingly, HFD significantly reduced Areg mRNA levels and immunofluorescent staining in C57BL/6 mice, thus providing a plausible explanation for reduced growth of mammary ducts in HFD-fed C57BL/6 mice. In contrast, treatment of OVX-BALB/c mice fed CD or HFD with E stimulated duct ends. The difference between C57BL/6 and BALB/c mice raises the possibility that it is the level of adiposity induced by the HFD that dictates the sensitivity to E.
Reversal of DIO and adiposity on mammary gland development
The differential effect of HFD on mammary gland development in DIO-susceptible (C57BL/6) versus DIO-non-susceptible (BALB/c) mice, and the restoration of development upon weight loss in C57BL/6 mice, suggests that increased adiposity and related changes are associated with delayed mammary gland development. Leptin, a hormone produced by adipocytes that regulates energy balance, is secreted proportional to the level of adiposity. Leptin has been shown to be a mitogen for normal and malignant breast epithelial cells in vitro (36). Because leptin-signaling deficient mice have atrophic mammary glands it has been proposed that leptin also serves as a mitogen for mammary epithelial cells in vivo. The effect of leptin-signaling deficiency on mammary gland development, however, is likely due to global changes in the reproductive endocrine system because lack of leptin signaling also results in atrophic ovaries and uteri, and infertility (37, 38). In contrast to leptin serving as a mitogen in vivo, intra-mammary infusion of leptin has been shown to diminish proliferation of mammary duct epithelial cells in prepubertal heifers (39). Treatment of cultured bovine mammary epithelial cells with leptin also reduced cell proliferation (19). These reports suggest that delayed mammary gland development in HFD fed C57BL/6 mice might be related to increased systemic or local production of leptin. In support of this possibility, HFD fed C57BL/6 mice had increased parametrial fat pad and mammary gland weights, which corresponded with increased expression of leptin mRNA in the mammary gland and increased circulating leptin levels. The changes in leptin expression and secretion occurred prior to any notable change in fasting insulin or glucose levels, indicating that the length or severity of the increased adiposity was in our study insufficient to cause insulin resistance. In a previous study, C57BL/6 mice fed a HFD from 4 to 20 wks of age have increased expression of leptin mRNA in the mammary gland and increased plasma leptin levels, which are associated with elevated mammary gland weight and disrupted ductal development (19). That feeding study analyzed mammary glands of older mice that were insulin resistant as indicated by elevated plasma glucose and insulin levels, therefore it was not possible to separate the effects of increased adiposity and leptin from the effects of insulin resistance on mammary gland development. In our study pubertal BALB/c mice fed HFD also had increased parametrial fat pad and mammary gland weights compared to control mice fed CD. This increase in adiposity, however, was insufficient to increase circulating leptin levels and resulted in increased mammary gland proliferation. Its interesting to note that HFD-fed BALB/c mice had elevated insulin levels, a known mitogen of mammary epithelial cells (reviewed in 40), and it is possible that elevated insulin levels this was associated with increased ductal epithelial cell proliferation.
Strain-specific differences in duct elongation and sidebranching have been shown to be dependent upon signals from the mammary stroma (41, 42). The most obvious difference between the mammary stroma of HFD-fed C57BL/6 and BALB/c mice was the extent to which adipocytes were engorged with lipid. Lipid-ladened adipocytes in HFD-fed C57BL/6 mice might influence duct epithelium growth by altered release of adipokines (e.g. leptin, adiponectin or resistin), and inflammatory cytokines and chemokines (e.g. TNFα, IL6 or MCP-1) (43). Increased cytokines and chemokines can recruit and activate inflammatory macrophages, which may disrupt the normal balance of eosinophils and macrophages that are necessary for TEB formation and ductal outgrowth into the fat pad (44). Larger adipocytes also have greater basal lypolytic rates than small adipocytes (45), which may increase local concentrations FFA that potentially influence stromal cell function. Enlarged adipocytes may also establish physical and/or functional barriers that prevent elongation of duct epithelium into the fat pad. Placement of plastic barriers into the mammary fat pads cause ductal bifurcation and sidebranching (46), however, this pattern of duct growth was not observed in HFD-fed C57BL/6 mice. Obese C57BL/6 mice have reduced blood flow and increased hypoxia in white adipose tissue (47), which could establish a functional barrier that limits mammary duct elongation. Consistent with a hypoxic environment, others and we observed “crown-like structures” – necrotic adipocytes surrounded by macrophages – in mammary glands of HFD-fed C57BL/6 mice (19 and data not shown). Adipocytes exposed to hypoxia have increased cytokine expression (48), which may directly or indirectly through the recruitment of inflammatory macrophages alter mammary gland development.
Our results are consistent with the hypothesis that increased adiposity, specifically during puberty, delays mammary gland development. The mechanisms involved are uncertain, but likely involve an alteration in stromal-epithelial cell interactions and/or local hormonal environment. It has been proposed that dietary components that increase or decrease TEB formation and ductal proliferation during puberty also increase or decrease carcinogen-induced mammary tumorigenesis, respectively (49). Based on this, increased adiposity during puberty would be expected reduce mammary tumorigenesis. This hypothesis remains to be tested in diet-induced obese mice, but is consistent with epidemiologic studies indicating that body fatness at childhood and puberty lowers breast cancer risk (2-7). Conversely, HFD feeding without substantial weight gain would be expected to increase mammary tumorigenesis as shown in many rodent studies (13). How this is related to breast cancer risk in humans is uncertain, however, increased ductal proliferation throughout mammary gland development might increase mammographic density, as defined as the relative amounts of radiodense epithelium and connective tissue to radiolucent adipose, a marker for breast cancer risk (50). Additional mechanistic studies on how adiposity and diet alters mammary gland development are warranted.
Supplementary Material
Supplemental Table 1. Dietary Formulations
Acknowledgments
The authors sincerely thank Sarah Woiderski, Sara Hawes and Christopher D. Green for their excellent technical support.
Grant support: This work was supported by the Breast Cancer and the Environment Research Centers Grant U01 ES/CA 012800 from the National Institute of Environment Health Science (NIEHS) and the National Cancer Institute (NCI), National Institutes of Health (NIH), Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS or NCI, NIH.
The abbreviations used are
- BMI
body mass index
- CD
control diet
- HFD
high fat diet
- TEBs
terminal end buds
- ERα
estrogen receptor α
- PRA
progesterone receptor A
- E
17-ß-estradiol
- P
progesterone
- OVX
ovariectomized
- Areg
amphiregulin
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Rosenfield RL, Lipton RB, Drum ML. Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics. 2009;123:84–88. doi: 10.1542/peds.2008-0146. [DOI] [PubMed] [Google Scholar]
- 2.Marchand L, Kolonel LN, Earle ME, Mi MP. Body size at different periods of life and breast cancer risk. Am J Epidemiol. 1988;128:137–152. doi: 10.1093/oxfordjournals.aje.a114936. [DOI] [PubMed] [Google Scholar]
- 3.Magnusson C, Baron J, Persson I, Wolk A, Bergstrom R, Trichopoulos D, et al. Body size in different periods of life and breast cancer risk in post-menopausal women. Int J Cancer. 1998;76:29–34. doi: 10.1002/(sici)1097-0215(19980330)76:1<29::aid-ijc6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Berkey CS, Frazier AL, Gardner JD, Colditz GA. Adolescence and breast carcinoma risk. Cancer. 1999;85:2400–2409. doi: 10.1002/(sici)1097-0142(19990601)85:11<2400::aid-cncr15>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Hilakivi-Clarke L, Forsen T, Eriksson JG, Luoto R, Tuomilehto J, Osmond C, et al. Tallness and overweight during childhood have opposing effects on breast cancer risk. Br J Cancer. 2001;85:1680–1684. doi: 10.1054/bjoc.2001.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahlgren M, Melbye M, Wohlfahrt J, Sorensen TI. Growth patterns and the risk of breast cancer in women. The New England journal of medicine. 2004;351:1619–1626. doi: 10.1056/NEJMoa040576. [DOI] [PubMed] [Google Scholar]
- 7.Baer HJ, Colditz GA, Rosner B, Michels KB, Rich-Edwards JW, Hunter DJ, et al. Body fatness during childhood and adolescence and incidence of breast cancer in premenopausal women: a prospective cohort study. Breast Cancer Res. 2005;7:R314–325. doi: 10.1186/bcr998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garland M, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Spiegelman D, et al. Menstrual cycle characteristics and history of ovulatory infertility in relation to breast cancer risk in a large cohort of US women. American journal of epidemiology. 1998;147:636–643. doi: 10.1093/oxfordjournals.aje.a009504. [DOI] [PubMed] [Google Scholar]
- 9.Titus-Ernstoff L, Longnecker MP, Newcomb PA, Dain B, Greenberg ER, Mittendorf R, et al. Menstrual factors in relation to breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7:783–789. [PubMed] [Google Scholar]
- 10.Hannon TS, Rao G, Arslanian SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics. 2005;116:473–480. doi: 10.1542/peds.2004-2536. [DOI] [PubMed] [Google Scholar]
- 11.Watson CJ, Khaled WT. Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development (Cambridge, England) 2008;135:995–1003. doi: 10.1242/dev.005439. [DOI] [PubMed] [Google Scholar]
- 12.Russo J, Russo IH. Biological and molecular bases of mammary carcinogenesis. Lab Invest. 1987;57:112–137. [PubMed] [Google Scholar]
- 13.Welsch CW. Relationship between dietary fat and experimental mammary tumorigenesis: a review and critique. Cancer research. 1992;52:2040s–2048s. [PubMed] [Google Scholar]
- 14.Hilakivi-Clarke L, Clarke R, Onojafe I, Raygada M, Cho E, Lippman M. A maternal diet high in n - 6 polyunsaturated fats alters mammary gland development, puberty onset, and breast cancer risk among female rat offspring. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:9372–9377. doi: 10.1073/pnas.94.17.9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilakivi-Clarke L, Cho E, Cabanes A, DeAssis S, Olivo S, Helferich W, et al. Dietary modulation of pregnancy estrogen levels and breast cancer risk among female rat offspring. Clin Cancer Res. 2002;8:3601–3610. [PubMed] [Google Scholar]
- 16.Hilakivi-Clarke L, Stoica A, Raygada M, Martin MB. Consumption of a high-fat diet alters estrogen receptor content, protein kinase C activity, and mammary gland morphology in virgin and pregnant mice and female offspring. Cancer research. 1998;58:654–660. [PubMed] [Google Scholar]
- 17.Welsch CW, DeHoog JV, O'Connor DH, Sheffield LG. Influence of dietary fat levels on development and hormone responsiveness of the mouse mammary gland. Cancer research. 1985;45:6147–6154. [PubMed] [Google Scholar]
- 18.Welsch CW, O'Connor DH. Influence of the type of dietary fat on developmental growth of the mammary gland in immature and mature female BALB/c mice. Cancer research. 1989;49:5999–6007. [PubMed] [Google Scholar]
- 19.Kamikawa A, Ichii O, Yamaji D, Imao T, Suzuki C, Okamatsu-Ogura Y, et al. Diet-induced obesity disrupts ductal development in the mammary glands of nonpregnant mice. Dev Dyn. 2009;238:1092–1099. doi: 10.1002/dvdy.21947. [DOI] [PubMed] [Google Scholar]
- 20.Banerjee M, Wood B, Lin F, Crump L. Organ culture of whole mammary gland of the mouse. Tissue Culture Association Manual. 1976;2:457–462. [Google Scholar]
- 21.Aupperlee MD, Smith KT, Kariagina A, Haslam SZ. Progesterone receptor isoforms A and B: temporal and spatial differences in expression during murine mammary gland development. Endocrinology. 2005;146:3577–3588. doi: 10.1210/en.2005-0346. [DOI] [PubMed] [Google Scholar]
- 22.Yang C, Tan YS, Harkema JR, Haslam SZ. Differential effects of peripubertal exposure to perfluorooctanoic acid on mammary gland development in C57Bl/6 and Balb/c mouse strains. Reproductive toxicology (Elmsford, NY. 2009;27:299–306. doi: 10.1016/j.reprotox.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aupperlee MD, Haslam SZ. Differential hormonal regulation and function of progesterone receptor isoforms in normal adult mouse mammary gland. Endocrinology. 2007;148:2290–2300. doi: 10.1210/en.2006-1721. [DOI] [PubMed] [Google Scholar]
- 24.van Dierendonck JH, Wijsman JH, Keijzer R, van de Velde CJ, Cornelisse CJ. Cell-cycle-related staining patterns of anti-proliferating cell nuclear antigen monoclonal antibodies. Comparison with BrdUrd labeling and Ki-67 staining. The American journal of pathology. 1991;138:1165–1172. [PMC free article] [PubMed] [Google Scholar]
- 25.Daniel CW, Silberstein GB, Strickland P. Direct action of 17 beta-estradiol on mouse mammary ducts analyzed by sustained release implants and steroid autoradiography. Cancer research. 1987;47:6052–6057. [PubMed] [Google Scholar]
- 26.Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5455–5460. doi: 10.1073/pnas.0611647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aupperlee MD, Drolet AA, Durairaj S, Wang W, Schwartz RC, Haslam SZ. Strain-specific differences in the mechanisms of progesterone regulation of murine mammary gland development. Endocrinology. 2009;150:1485–1494. doi: 10.1210/en.2008-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, et al. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism: clinical and experimental. 1995;44:645–651. doi: 10.1016/0026-0495(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 29.Clee SM, Attie AD. The genetic landscape of type 2 diabetes in mice. Endocrine reviews. 2007;28:48–83. doi: 10.1210/er.2006-0035. [DOI] [PubMed] [Google Scholar]
- 30.Haramizu S, Nagasawa A, Ota N, Hase T, Tokimitsu I, Murase T. Different contribution of muscle and liver lipid metabolism to endurance capacity and obesity susceptibility of mice. J Appl Physiol. 2009;106:871–879. doi: 10.1152/japplphysiol.90804.2008. [DOI] [PubMed] [Google Scholar]
- 31.Silberstein GB, Van Horn K, Shyamala G, Daniel CW. Essential role of endogenous estrogen in directly stimulating mammary growth demonstrated by implants containing pure antiestrogens. Endocrinology. 1994;134:84–90. doi: 10.1210/endo.134.1.8275973. [DOI] [PubMed] [Google Scholar]
- 32.Korach KS, Couse JF, Curtis SW, Washburn TF, Lindzey J, Kimbro KS, et al. Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes. Recent progress in hormone research. 1996;51:159–186. discussion 186-158. [PubMed] [Google Scholar]
- 33.Eckstein B, Golan R, Shani J. Onset of puberty in the immature female rat induced by 5 - androstane-3,17 -diol. Endocrinology. 1973;92:941–945. doi: 10.1210/endo-92-3-941. [DOI] [PubMed] [Google Scholar]
- 34.Sternlicht MD, Sunnarborg SW. The ADAM17-amphiregulin-EGFR axis in mammary development and cancer. Journal of mammary gland biology and neoplasia. 2008;13:181–194. doi: 10.1007/s10911-008-9084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McBryan J, Howlin J, Napoletano S, Martin F. Amphiregulin: role in mammary gland development and breast cancer. Journal of mammary gland biology and neoplasia. 2008;13:159–169. doi: 10.1007/s10911-008-9075-7. [DOI] [PubMed] [Google Scholar]
- 36.Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. Journal of the National Cancer Institute. 2002;94:1704–1711. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- 37.Coleman DL, Hummel KP. Studies with the mutation, diabetes, in the mouse. Diabetologia. 1967;3:238–248. doi: 10.1007/BF01222201. [DOI] [PubMed] [Google Scholar]
- 38.Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, et al. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144–3147. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- 39.Silva LF, Etchebarne BE, Nielsen MS, Liesman JS, Kiupel M, VandeHaar MJ. Intramammary infusion of leptin decreases proliferation of mammary epithelial cells in prepubertal heifers. Journal of dairy science. 2008;91:3034–3044. doi: 10.3168/jds.2007-0761. [DOI] [PubMed] [Google Scholar]
- 40.Hadsell DL, Bonnette SG. IGF and insulin action in the mammary gland: lessons from transgenic and knockout models. Journal of mammary gland biology and neoplasia. 2000;5:19–30. doi: 10.1023/a:1009559014703. [DOI] [PubMed] [Google Scholar]
- 41.Naylor MJ, Ormandy CJ. Mouse strain-specific patterns of mammary epithelial ductal side branching are elicited by stromal factors. Dev Dyn. 2002;225:100–105. doi: 10.1002/dvdy.10133. [DOI] [PubMed] [Google Scholar]
- 42.Yant Y, Gusterson B, Kamalati T. Induction of strain-specific mouse mammary gland ductal architecture. The Breast. 1998;7:269–272. [Google Scholar]
- 43.Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. The Journal of clinical endocrinology and metabolism. 2008;93:S64–73. doi: 10.1210/jc.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development (Cambridge, England) 2000;127:2269–2282. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 45.Wueest S, Rapold RA, Rytka JM, Schoenle EJ, Konrad D. Basal lipolysis, not the degree of insulin resistance, differentiates large from small isolated adipocytes in high-fat fed mice. Diabetologia. 2009;52:541–546. doi: 10.1007/s00125-008-1223-5. [DOI] [PubMed] [Google Scholar]
- 46.Hinck L, Silberstein GB. Key stages in mammary gland development: the mammary end bud as a motile organ. Breast Cancer Res. 2005;7:245–251. doi: 10.1186/bcr1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. International journal of obesity (2005) 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 48.Trayhurn P, Wang B, Wood IS. Hypoxia and the endocrine and signalling role of white adipose tissue. Archives of physiology and biochemistry. 2008;114:267–276. doi: 10.1080/13813450802306602. [DOI] [PubMed] [Google Scholar]
- 49.Hilakivi-Clarke L. Nutritional modulation of terminal end buds: its relevance to breast cancer prevention. Curr Cancer Drug Targets. 2007;7:465–474. doi: 10.2174/156800907781386641. [DOI] [PubMed] [Google Scholar]
- 50.Oza AM, Boyd NF. Mammographic parenchymal patterns: a marker of breast cancer risk. Epidemiol Rev. 1993;15:196–208. doi: 10.1093/oxfordjournals.epirev.a036105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Dietary Formulations


