Abstract
The effects of conantokin (con)-G, con-R[1-17], and con-T on ion flow through N-methyl-D-aspartate receptor (NMDAR) ion channels were determined in cultured primary rat hippocampal neurons. The potency of con-G diminished, whereas inhibition by con-R[1-17] and con-T did not change, as the neurons matured. Con-G, con-R[1-17], and con-T effectively diminished NMDA-induced Ca2+ influx into the cells. A similar age-dependent decrease in con-G-mediated inhibition of the amplitude of spontaneous excitatory postsynaptic currents (sEPSCs) was observed, compared to con-R[1-17] and con-T. The effects of the conantokins on NMDA-induced cAMP response element-binding protein (CREB) phosphorylation in immature (DIV 9) and mature (DIV 16) neurons showed that, at DIV 9, con-G, con-R[1-17], and con-T inhibited NMDA-mediated P-CREB levels, whereas in DIV 16 neurons the conantokins did not inhibit overall levels of NMDA-induced P-CREB. In contrast, P-CREB levels were enhanced through inhibition of the protein phosphatases, PP1 and PP2B (calcineurin). This ability of conantokins to sustain CREB phosphorylation can thus enhance neuronal survival and plasticity.
Keywords: NMDA receptor, ion channels, calcium imaging, neuron signaling, neuron development, electrophysiology, conantokins
1. Introduction
The glutamate/glycine activated N-methyl-D-aspartate receptor (NMDAR) is a dynamic combination of NR subunits located in the central nervous system. In the presence of these coagonists, ion channels of the NMDAR open and allow Ca2+ to enter cells, thereby resulting in cellular responses. The levels and frequencies of Ca2+ influx into neurons have profound effects on normal processes, e.g., synaptic plasticity (Malenka and Nicoll, 1993), leading to long-term potentiation (LTP) and long-term depression (LTD), respectively, and also on neuropathies associated with stroke, brain injury, and pain, among others (McBain and Mayer, 1994). The functional NMDAR is composed of 2 heterodimers (Furukawa et al., 2005), with each of the dimers containing one of eight splice variants (NR1a-NR1h) of the glycine-binding NR1 subunits (Chatterton et al., 2002), in association with a glutamate binding NR2 subunit, consisting of 4 different gene products, NR2A-NR2D (Laube et al., 1997). Heterotrimeric complexes are also present in brain, such as NR1/NR2A/NR2B (Sheng et al., 1994), and are functional receptors. NR1 is ubiquitously present in cells of the CNS, whereas the specific NR2 component is regulated developmentally and regionally, and the exact nature of this subunit is responsible for many of the pharmacological responses of the NMDAR. The fact that distinct NMDAR subunit combinations are spatially and temporally regulated in brain (Tovar and Westbrook, 1999; Tovar and Westbrook, 2002), allows the possibility for development of subunit-specific pharmacological agents that target pathologies dependent on the locations of distinct combinations of subunits. In this regard, NR2B has been a subject of such efforts (Borza and Domány, 2006; Chazot, 2004; Nikam and Meltzer, 2002) because of its importance to drug responses in animal neuropathic models, e.g., stroke, pain, opiate dependence, and epilepsy (Wei et al., 2001; Wei et al., 2006; Williams et al., 2002a; Xiao et al., 2008).
The conantokins are small γ-carboxyglutamate (Gla)-containing gene products present in snails of the genus, Conus (Gowd et al., 2008; Haack et al., 1990; Jimenez et al., 2002; McIntosh et al., 1984; Teichert et al., 2007; White et al., 2000). These peptides inhibit opening of NMDAR ion channels via competitive inhibition of glutamate agonism (Donevan and McCabe, 2000). One member of this family, conantokin (con)-G, a 17-residue peptide, has been widely studied due to its high selectivity for inhibition of NR2B-containing NMDAR ion channels, whereas other members of this peptide family, viz., con-R and con-T, display broader NR2 activity, e.g., with NR2A and NR2B. Con-G has shown efficacy in animal models of pain (Malmberg et al., 2003; Xiao et al., 2008), in protection against ischemic brain injury (Williams et al., 2002b), and as a anticonvulsant (Hovinga, 2002). The differential NMDAR selectivity of conantokins thus provides opportunities to investigate the roles of NMDARs composed of different NR2 subunits in temporal- and regio-specific manners. In the current study, we have applied this strategy to evaluate the effects of conantokins on NMDA-evoked currents in developing cultured primary rat neurons. Furthermore, the antagonist effect of conantokins on intracellular Ca2+ (iCa2+) mobilization, which, in-turn, is coupled to downstream signaling events was also examined.
2. Results
2.1. Developmental decrease in NMDA-evoked currents by con-G
Primary rat hippocampal neurons at various developmental ages were used to study ion current flow through synaptic and extrasynaptic channels of NMDARs, in linkage with Ca2+-mediated intracellular signaling events, and the effect of NMDAR-subunit selective conantokins on these processes. Previous studies showed that extrasynaptic NMDARs predominate in neurons at early developmental stages, e.g., DIV 7 (Tovar and Westbrook, 1999), and, at later stages of development, e.g., DIV 16, synaptic NMDARs outnumber extrasynaptic NMDARs by at least 4:1 (Rosenmund et al., 1995). We also found lower steady state currents in immature neurons, where, at 20 αM NMDA/10 αM glycine, approximately 35% of the peak current was observed in DIV 7 neurons as compared to DIV 16 neurons. Nonetheless, NMDA concentration response curves of steady state currents showed similar EC50 values of NMDA, viz., 17.2 ± 1.7 μM, n = 6, in DIV 7 neurons, and 20.9 ± 1.3 μM, n = 8, in DIV 16 neurons, respectively. This suggests that a lower NMDAR density on the DIV 7 neurons was responsible for the reduced steady state currents in the immature neurons, not a difference in the response of developing neurons to NMDA.
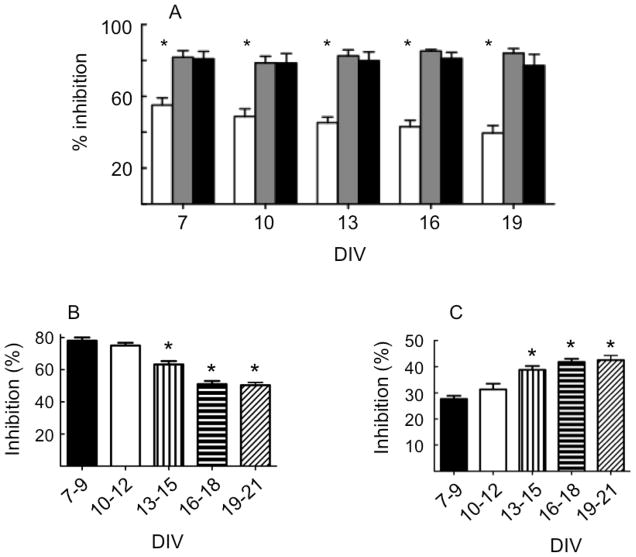
It has previously been shown that con-G and con-T inhibited NMDA-evoked currents in hippocampal neurons in a voltage-dependent manner (Klein et al., 1999). Since NMDA-evoked currents increased with neuronal age, we first determined the extent of inhibition of con-G, con-T, along with another conantokin, con-R[1-17], with respect to neuron maturity (Figure 1A). Exogenous application of 20 αM NMDA/10 αM glycine was utilized to elicit current in neurons at different levels of maturity, ranging from DIV 7-22. At DIV 7, con-R[1-17] and con-T showed greater inhibition than con-G, as was also the case with DIV 19 neurons (Fig. 1A). The differences between con-G, with con-R[1-17] and con-T, were larger as neurons matured, whereas con-T and con-R[1-17] functioned similarly to each other. Examination of these primary hippocampal neurons at a variety of ages in culture showed progressively increased differences between con-G with con-R[1-17] and con-T, primarily due to the age dependent-decrease in con-G inhibition, combined with the relative lack of age-dependency on con-R[1-17] and con-T inhibitions (Fig. 1A).
Fig. 1.
The inhibition by 20 μM con-G (white bars), 20 μM con-R[1-17] (black bars), and 20 μM con-T (grey bars), of NMDA-evoked currents as a function of developmental age of the neurons (*p<0.05 between con-G and either con-R[1-17] or con-T; n = 6-9 at each time). B, C. Age-dependency of ifenprodil and NVP-AAM077-inhibition of NMDA evoked whole cell currents in developing neurons. The changes in the relative inhibition by 3 μM ifenprodil (B) and 100 nM NVP (C) as a function of neuronal age in culture (*p<0.05 compared to DIV 7-9, n = 7-11 at each age).
One likely reason for the pharmacological differences between con-G as compared to con-R[1-17] and con-T lies in the subunit compositions of the NMDARs and their changes during development. Con-R[1-17] and con-T are less specific in this regard than con-G, the latter showing strong preference toward NR2B-containing NMDARs, especially NR1/NR2B receptors, as compared to receptor populations containing NR2A. As is known, mature hippocampal neurons in culture contain large numbers of NR1/NR2B and NR1/NR2A/NR2B receptors, with a more sparse population of NR1/NR2A receptors (Waxman and Lynch, 2005). Further, we have previously shown that con-G is fully active at NR1/NR2B receptors, inactive at NR1/NR2A receptors, and partially active at NR1/NR2A/NR2B receptors, while both con-R and con-T completely inhibit the currents from ion channels of all diheteromeric and triheteromeric receptors (Klein et al., 2001). Thus, taking advantage of the specific pharmacological inhibition of NR2B-containing NMDARs by ifenprodil (Williams, 1993) and of NR2A-containing NMDARs by NVP-AAM077 (Auberson et al., 2002; Wu et al., 2007), we determined the relative proportions of these subunits in developing neurons (Fig. 1B and 1C, respectively). From these data, it is seen that ifenprodil inhibition of whole cell NMDAR currents diminishes with neuronal development (Fig. 1B), indicating a relative decrease of NR2B-containing subpopulations in the whole cell NMDAR population. On the other hand, the increasing sensitivity of developing neurons to inhibition by NVP-AAM077 (Fig. 1C) demonstrates that the NR2A-containing subpopulation of NMDARs increase during development. Thus, since con-R[1-17] and con-T inhibition do not show NR2 subunit dependencies, at least with regard to NR2A and NR2B, it is not surprising that con-R[1-17] and con-T inhibitions are age-independent, whereas con-G inhibition, with a NR2B inhibitory specificity, decreases with age, because of the decreased relative proportion of NR2B containing subunits as compared to those containing NR2A.
Additionally, these conantokins did not inhibit voltage-dependent K+, Na+, and Ca2+ currents in neurons (not shown), assuring that our approaches were specifically targeted to NMDAR currents evoked by NMDA/glycine coagonism.
2.2 NMDAR-mediated sEPSC inhibition by con-G is dependent on neuron maturity
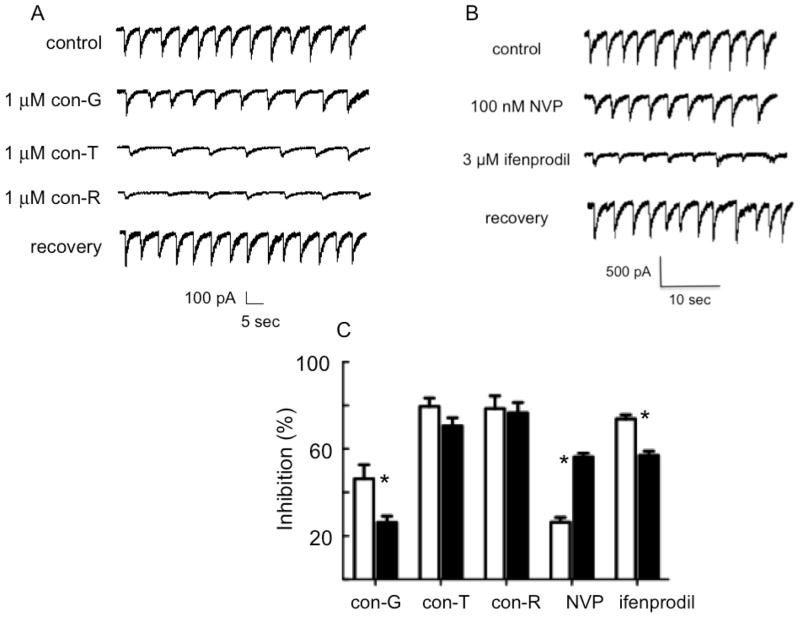
Inhibition of whole cell currents by conantokins reflects both extrasynaptic and synaptic NMDARs. Thus, we assessed the inhibitory properties of these peptides toward mature synaptic NMDARs in DIV 14 and DIV 19 neurons, after pharmacological isolation of NMDARs that result in the absence of exogenous glutamate. Bicuculine was added to eliminate inhibitory outward Cl− currents from GABAA receptors (Schneggenburger et al., 1992) and strychnine was added to inhibit glycinergic receptors (Smith et al., 2000). CNQX (6-cyano-7-nitroquinoxaline-2,3-dione) was added to eliminate the fast (early) sEPSCs from synaptically-expressed glutamate acting at AMPA/kainate channels (Muramatsu et al., 1998), thus leading to the slow sEPSCs that originate from NMDARs (Fig. 2A and 2B, controls). An example of the inhibition by con-G, con-R[1-17], and con-T (Fig. 2A) and by NVP-AAM077 and ifenprodil (Fig. 2B) of the synaptic sEPSCs from NMDARs is shown for DIV 14 neurons. In all cases, this inhibition is recovered by wash-out of the inhibitors. Quantitative results of the inhibition by all inhibitors of the amplitudes of the EPSCs are illustrated for DIV 14 and DIV 19 neurons in Figure 2C. Con-R[1-17] and con-T were more effective inhibitors than con-G at both developmental ages, consistent with the findings that synaptic NMDARs contain both NR2A and NR2B subunits, and reflect relative proportions these subunits seen in whole neurons (Fig. 1) (Tovar and Westbrook, 1999). Further, the NVP-AAM077 data show more directly that the relative proportion of NR2A increases in synapses as a function of neuronal developmental age, whereas the ifenprodil data demonstrate that the relative proportion of NR2B relatively diminishes in more mature synapses, consistent with similar observations in whole neurons (Fig. 1).
Fig. 2.
Inhibition of the sEPSCs of developing neurons by conantokins. An example of NMDAR-derived sEPSCs recorded from hippocampal neurons at DIV 14. The control buffer was 10 μM glycine, 20 μM bicuculline, 1 μM strychnine, and 20 μM CNQX. A. Conantokins inhibited the peak amplitudes of the sEPSCs, and this inhibition was reversible after wash-out of these inhibitors. B. Similar data are shown for inhibition of NMDA-elicited sEPSCs by 100 nM NVP-AAM077 (NVP) and 3 μM ifenprodil. C. Quantitation of the inhibition of the peak amplitudes of the sEPSCs of DIV 14 (white bars) and DIV 19 (black bars) neurons by various inhibitors (*p<0.05 for pairwise comparisons, n =7-11).
2.3 The effects of conantokins on iCa2+ transients in developing neurons
It has been established that robust electrical activity in excitatory neurons induces Ca2+ entry, which in-turn modulates a repertoire of transcription factors, key among them being the CREB protein (Bito and Takemoto-Kimura, 2003). Physiological involvement of the Ca2+/CREB signaling axis, resulting in phosphorylation (P) of CREB at Ser133, is important for maintaining long-term synaptic activity and neuronal activity. It has been demonstrated that NMDAR stimulation by NMDA, glutamate, LTP or LTD, or KCl depolarization, led to induction of dephosphorylation of P-CREB (Bito et al., 1996; Hardingham et al., 2002; Kopnisky et al., 2003; Sala et al., 2000). One study showed that NMDA-induced CREB phosphorylation was dependent on the maturity of the neurons. In immature neurons (DIV 7), NMDA induced a strong P-CREB response that was sustained for 2 hr, compared to mature neurons (DIV 14), where appearance of P-CREB was transient, peaking at 2 min and then quickly declining to basal levels. This dephosphorylation event in immature neurons was linked to developmentally regulated functioning of phosphatase PP1 (Sala, 2000;Kopnisky, 2003 #3527}.
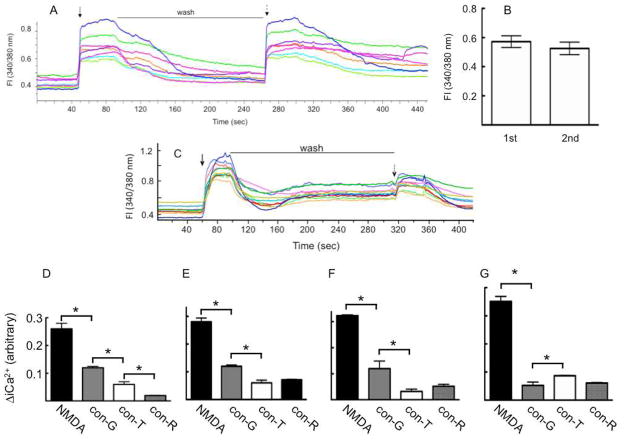
In the present study we determined the functional consequences of current flow through NMDARs in neurons, with emphasis on Ca2+-dependent cell signaling. Toward this end, we first performed whole cell intracellular Ca2+ (iCa2+) measurements upon NMDA stimulation of neurons in the absence and presence of con-G, con-R[1-17], or con-T (Fig. 3). An example of the imaging of iCa2+ transients after stimulation of DIV 14 neurons with 50 αM NMDA/10 αM glycine, as monitored by ratiometric fluorescence measurements (Fl), is shown in Figure 3A. Here, large increases in [iCa2+] were observed upon initial treatment with NMDA/glycine. The data show that Ca2+ flows into the cells via NMDARs, since other Ca2+ channels are not active under the conditions of the experiment. After wash-out of 50 αM NMDA/10 αM glycine, [iCa2+] returned to near basal levels and a second application of 50 αM NMDA/10 αM glycine increased the [iCa2+] to a level similar to the first application of these coagonists (Fig. 3A,B). This control result showed that the cells are capable of fully responding to a second round of coagonist application and that the neurons maintained their viability after coagonist washout. On the other hand, when the neurons were exposed to 50 αM NMDA/10 αM glycine/40 αM con-G, at 320 sec after washout of the first application of 50 αM NMDA/10 αM glycine, the increase in [iCa2+] was greatly diminished as compared to the response from the same solution that lacked con-G (Fig. 3C). In addition, further control experiments showed that addition of 40 αM con-G, at a time of 30 sec prior to addition of 50 αM NMDA/10 αM glycine, did not enhance the level of inhibition by con-G. Lastly, addition of CNQX to the initial buffer did not affect the levels of Ca2+ migrating into neurons, that were found in the absence of CNQX, upon treatment with 50 αM NMDA/10 αM glycine. Similar experiments were conducted with 40 μM con-G, con-R[1-17], or con-T using DIV 7 (Fig. 3D), DIV 13 (Fig. 3E), DIV 16 (Fig. 3F), and DIV 19 (Fig. 3G) neurons. The reduced current flow observed with con-G, con-R[1-17], or con-T is reflected in reduced flow of Ca2+ through the NMDAR ion channels, and con-R[1-17] and con-T are more effective inhibitors than con-G in all but DIV 19 neurons, where there are no significant differences in iCa2+ levels between the three conantokins.
Fig. 3.
Single cell iCa2+ measurements upon NMDA stimulation in the absence or presence of conantokins. (A). An example of the imaging of iCa2+ transients after stimulation (solid arrow) of DIV 13 neurons with 50 αM NMDA/10 αM glycine as monitored by the fluorescence (Fl) ratio at 340/380 nm. After wash-out (wash) of 50 αM NMDA/10 αM glycine, the neurons were reexposed to the same stimulation (dashed arrow) to assess the viability of the neurons to a second application of coagonists. (B). The maximum Fl 340/380 ratio as a result of the 1st and 2nd stimulation by 50 αM NMDA/10 αM glycine, showing that the neurons maintained the same level of viability. (C). Inhibition of the iCa2+ transients of DIV 13 neurons as a result of application of con-G. After washout of 50 αM NMDA/10 αM glycine from the first stimulation (solid arrow), the neurons were restimulated with 50 αM NMDA/10 αM glycine/40 αM con-G. A decrease in the maximum Fl (340/380) is seen, quantitatively demonstrating the effectiveness of con-G. (D-G). The effect of con-G, con-R[1-17] (con-R), and con-T on the increase in [iCa2+] at DIV 7 (D), DIV 13 (E), DIV 16 (F), and DIV 19 (G). The data are expressed as the mean ± S.E.M. of 3 independent experiments with 10–13 neurons per experiment. Statistical analysis * P < 0.05 compares iCa2+ changes between NMDA alone and NMDA/con-G, NMDA alone and NMDA/con-R[1-17], and NMDA alone and NMDA/con-T.
2.4 Effects of conantokins on CREB phosphorylation
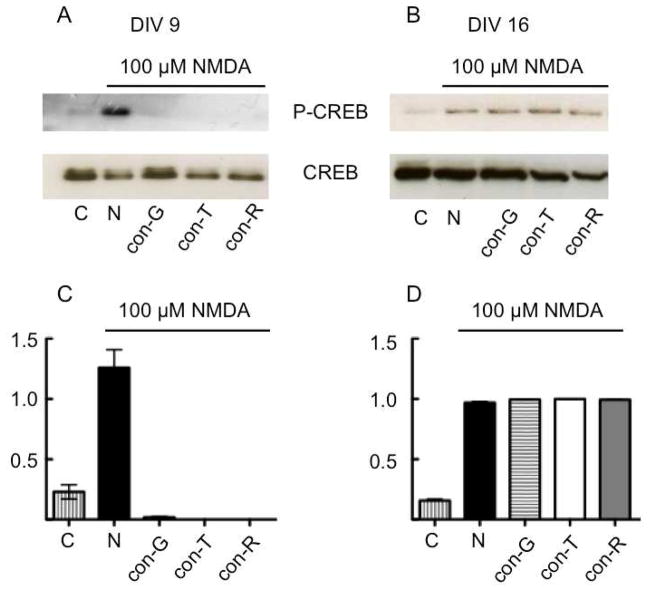
The effects of inhibition of Ca2+ movement through NMDAR ion channels on intracellular signaling was assessed by examination of the effects of con-G, con-T, and con-R[1-17] on CREB phosphorylation. Immature (DIV 9) and mature (DIV 16) neurons responded differently to conantokins in the presence of NMDA with regard to P-CREB levels. In both immature (DIV 9, Fig. 4A) and mature (DIV 16, Fig. 4B) neurons, NMDA elevated P-CREB levels (see lanes N vs C in Fig. 4A and Fig 4. B), although more robustly in DIV 9 immature neurons compared to DIV 16 mature neurons. The quantitative data of Figure 4C demonstrate that the inhibition of inward Ca2+ flow by conantokins in DIV 9 immature neurons substantially reduced P-CREB levels, without affecting total CREB levels, thus potentially reducing downstream Ca2+-induced signaling events. On the other hand, in mature neurons, neither con-G, con-R[1-17], nor con-T inhibited NMDA-stimulated P-CREB levels. The P-CREB levels remained constant in the presence of these antagonists in DIV 16 neurons (Fig. 4D). Similar effects of NMDA-mediated CREB phosphorylation was observed in immature and mature neurons in the presence of MK-801 (not shown), a pharmacological NMDAR antagonist, thus indicating that CREB activation events are initiated by Ca2+ influx through the NMDAR.
Fig. 4.
Inhibition of CREB phosphorylation by con-G, con-R[1-17] (con-R), and con-T. Cultured hippocampal neurons at DIV 9 (A) and DIV 16 (B) were stimulated with 100 αM NMDA or 100 αM NMDA/40 αM conantokins for 20 min. Total cell lysates were then obtained and immunoblotted for P-CREB, CREB, and the loading control, tubulin. C, D. Densitometric analysis is provided of the blots representing the ratio of P-CREB/CREB for treatments performed on neurons at (A) DIV 9 and (B) DIV 16.
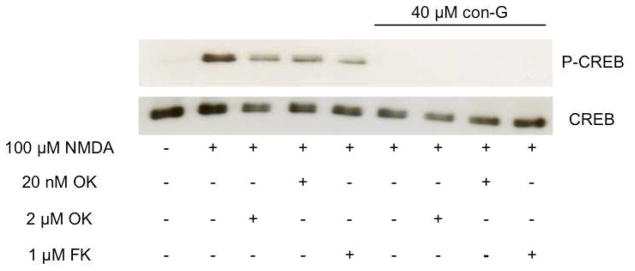
These data indicate that NMDA-stimulation of mature neurons in the presence of NMDAR antagonists allows for sustained phosphorylation of CREB, which is not observed in the younger neurons. To attempt to further explain mechanistically this difference in phosphorylation of CREB in a developmentally-dependent manner, levels of phosphatases, PP2BA-α and PP2BA-β, as well as kinases, CaMKII and CaMKIV, were evaluated. No differences in the levels of these phosphatases or kinases were observed in the DIV 9 or DIV 16 neurons treated with NMDA, NMDA/conantokins, or NMDA/MK-801 (not shown). Similarly, no differences in the levels of activated (P-Thr286)CaMKII and (P-Thr196)CaMKIV were observed in treated DIV 9 or DIV 16 neurons (data not shown). However, since NMDA-induced phosphorylation of CREB is not observed in DIV 9 neurons in the presence of NMDAR antagonists, we sought to determine whether inhibition of phosphatases would allow for CREB phosphorylation. Phosphatases PP1, PP2A, and PP2B were pharmacologically inhibited, and the resulting neurons assessed for CREB activation. It is known that 2 αM OA inhibits PP1 and PP2A and, at lower (20 nM) concentrations (Bialojan and Takai, 1988), selectively inhibits PP2A, whereas FK506 is a specific inhibitor of PP2B (Parsons et al., 1994). It was observed that both doses of OA (2 αM and 20 nM), as well as 1 αM FK506 failed to reverse the effects of con-G on CREB deactivation in DIV 9 neurons (Figure 5). Similar results were observed when the phosphatase inhibition experiments were performed with con-R[1-17], con-T, and MK-801 (data not shown). These data suggest that in DIV 9 neurons, NMDAR antagonists did not affect CREB dephosphorylation, at least via the major phosphatases tested.
Fig. 5.
Effect of protein phosphatase inhibitors on levels of P-CREB. Cultured hippocampal neurons at DIV 9 were treated with 100 αM NMDA in the presence or absence of 40 αM con-G for 5 min and then treated with the indicated phosphatase inhibitors for 20 min. Since nearly identical data were obtained with con-R[1-17], con-T, and MK-801 for DIV 9 neurons, only the immunoblot with con-G is shown as an example. Total cell lysates were immunoblotted for P-CREB and CREB.
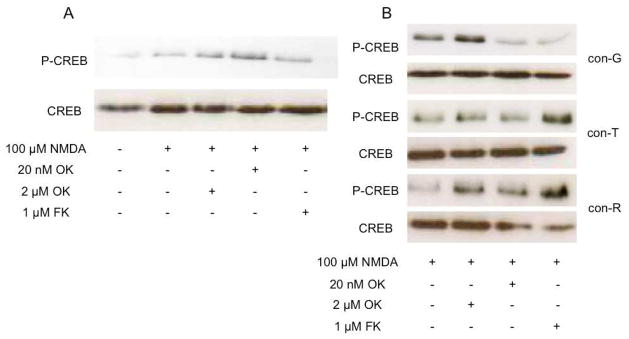
In DIV 16 neurons NMDA-induced CREB phosphorylation was slightly increased in the presence of 2 αM and 20 nM OA (Figure 6A). At least two studies have demonstrated that inhibition of PP1 led to increased levels of P-CREB in mature neurons (Bito et al., 1996; Sala et al., 2000). Levels of P-CREB co-stimulated by NMDA/con-G and similarly, with NMDA/MK-801 (not shown) in DIV 16 neurons were increased in the presence of 2 αM OA, compared to incubations with 20 nM OA or 1 αM FK506 (Figure 6B). On the other hand, levels of P-CREB co-stimulated with NMDA/con-R[1-17] and NMDA/con-T were increased when incubated in the presence of 1 αM FK506 (Figure 6B). Our data indicate that conantokins affected NMDAR-mediated activation of CREB differentially in a developmentally-dependent manner. Furthermore, the extent of sustained CREB phosphorylation in mature neurons by conantokins and MK-801was governed differentially by different phosphatases. Inhibition of PP1 enhanced P-CREB levels stimulated by NMDA/con-G (and NMDA/MK-801), whereas inhibition of PP2B increased P-CREB levels stimulated by NMDA/con-T and NMDA/con-R[1-17].
Fig. 6.
Effects of NMDAR antagonists on levels of P-CREB. Neurons at DIV 16 were treated with 100 αM NMDA (A) or 100 αM NMDA/conantokins (B) for 5 min, and then treated with the indicated phosphatase inhibitors for 20 min. Total cell lysates were immunoblotted for P-CREB and CREB.
2.5 Effect of con-G on the subcellular localization of NR2B
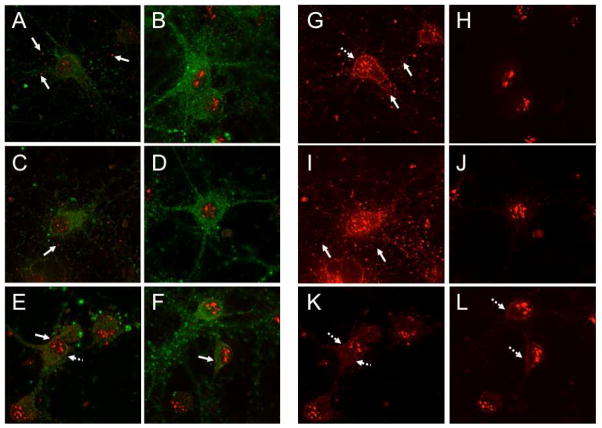
Localization of the NR subunits is important in understanding the manner in which excitotoxic events are mechanistically regulated. However, current knowledge regarding NMDAR complex formation at the neuron synapse is not comprehensive. It has been established that C-terminal residues of the NR subunits are bound to signaling proteins, such as Ca2+/calmodulin-dependent protein kinase II (CaMKII) and PSD-95 (Gardoni et al., 1998; Strack and Colbran, 1998), thus aiding in regulation of synaptic transmission and induction of long-term potentiation (Barria and Malinow, 2005). Furthermore, redistribution of the NR2B subunit between the synaptic and extrasynaptic membranes demonstrates perturbations of the ionotropic synapse leading to disturbances of LTP induction (Gardoni et al., 2009). Since CamKII acts as a signaling molecule and binds at an order magnitude higher to NR2B than to NR1 or NR2A (Strack and Colbran, 1998), it was relevant to understand how the presence of NMDA, in the absence or presence of con-G particularly (since it is considered to be NR2B-specific), would affect localization of the NR2B subunit. Therefore, the subcellular distribution of the NR2B subunit (red) and CaMKII (green) was evaluated in treated and untreated neurons at DIV 9 and DIV 16 (Fig. 7). CaMKII staining was employed as an aid in cell morphology, allowing identification of dendrites and the cell bodies so that the NR2B staining was better localized. The data show that in control cells at DIV 9, some NR2B is detected in the dendrites (Fig. 7A, G), which is not distinctively observed in these cells at DIV 16 (Fig. 7B, H). Treatment of neurons with 100 αM NMDA resulted in an increase of dendrite-localized NR2B in DIV 9 (Fig. 7C, I) neurons as compared to untreated DIV 9 cells (Fig. 7A, G). The NR2B subunit is not clearly detected in the dendrites of untreated DIV 16 neurons (Fig. 7H), or those treated with NMDA (Fig. 7J). In the presence of NMDA and con-G, NR2B is mostly absent in the extended dendritic areas of DIV 9 (Fig. 7E, K) and DIV 16 neurons (Fig. 7F, L), but is visible in the cell bodies of neurons at both stages of development and in dendrite areas proximal to the cell bodies in DIV 9 neurons (Fig. 7E, K).
Fig. 7.
Subcellular localization of NR2B in cultured hippocampal neurons after treatment with NMDA and NMDA/con-G at DIV 9 and DIV 16. The neurons were fixed and immunolabeled for NR2B (red) and CaMKII (green). (A, B). Control neurons at DIV 9 (A) and DIV 16 (B) pretreated with TTX, CNQX, and nifedipine. (C, D). DIV 9 (C) and DIV 16 (D) neurons treated with 100 αM NMDA for 20 min. (E, F). DIV 9 (E) and DIV 16 (F) neurons treated with 100 αM NMDA/40 αM con-G for 20 min. Panels (G-L) show only the NR2B staining (red) of images A-G. (G, H). Control neurons at DIV 9 (G) and DIV 16 (H). (I, J). DIV 9 (I) and DIV 16 (J) neurons treated with 100 αM NMDA for 20 min. (K, L). DIV 9 (K) and DIV 16 (L) neurons treated with 100 αM NMDA/40 αM con-G for 20 min. The solid white arrows show examples of NR2B staining in the dendrites and broken white arrows indicate examples of staining of NR2B in cell bodies. The intense red staining around the nucleus of the cells is an antibody artifact observed in many studies of this type.
Discussion
One aim of this study was to assess the comparative effects of con-G, con-R[1-17], and con-T on their abilities to inhibit ion flow through whole cell and synaptic NMDARs in developing hippocampal neurons and to assess mechanistic consequences of their activities on the cells. Con-G is a NR2B subunit-selective NMDAR inhibitor, with inhibition efficacy decreasing in receptors containing NR1/NR2B > NR1/NR2A/NR2B ≫ NR1/NR2A, whereas con-R[1-17] and con-T potently inhibit both NR2A and NR2B-containing NMDARs. Thus, this study also provides an assessment of the importance of ion flow in specific subunit combinations of NMDARs in the neuron population under development.
We initially confirmed, both in whole cells and in synaptic locations, that the NR2B/NR2A ratio is higher in immature neurons than in mature neurons. Thus, con-G, con-R[1-17], and con-T should be effective inhibitors at all developmental stages. Using whole cell recordings, we offer evidence that con-T and con-R[1-17], which inhibit both NR2A and NR2B-containing NMDARs, are more effective inhibitors of current flow than the NR2B-selective con-G at all stages of development. The differences in efficacy between con-T/con-R[1-17] and con-G become larger as the neurons age because of the developmental increases in the NR2A/NR2B ratio, resulting in relative decreases of inhibition by con-G. This is also the case in the synaptic NMDARs, wherein con-T and con-R[1-17] more effectively inhibited the amplitudes of sEPSCs, relative to con-G, DIV 19 neurons, as compared to DIV 13 neurons. Our data are consistent with other work (Alex et al., 2006; Barton et al., 2004l), and demonstrate that inhibition of NMDA-evoked currents and NMDAR-mediated sEPSCs by con-G is dampened in mature neurons.
Developmental changes in efficacy of the conantokins are also noted in the ability of these conantokins to inhibit inward flow of Ca2+ into neurons, with stronger inhibition of Ca2+ influx by con-R[1-17] and con-T than con-G at all developmental stages, except in the oldest neurons, where the inhibitory strengths equalized. These data suggest that comparative inhibition by con-G, con-R[1-17], and con-T can be employed to assess functional contribution of NR2B and NR2A in neurons.
The phosphorylation/dephosphorylation of the transcription factor, CREB, is one of the upstream events that regulates Ca2+-mediated signaling events, e.g., neuronal apoptosis. Also, events that increase P-CREB levels in neurons enhance pro-survival genes and neuronal plasticity (Sassone-Corsi et al., 1988; Sauerwald et al., 1990). Our data show that activation of NMDARs is one of the initiating events leading to P-CREB, since addition of con-G, con-R[1-17], or con-T, to DIV 9 neurons leads to severe attenuation of Ca2+ flow into these cells, coupled with the lack of appearance of P-CREB, thus linking Ca2+ influx via the NMDAR to P-CREB formation. From previous studies (Sala et al., 2000), and from our observations, it is apparent that phosphorylation/dephosphorylation of CREB in neurons is related to the developmental stage of the neurons. Diminished levels of P-CREB in DIV 14 neurons in the presence of NMDA has been correlated to NMDAR-dependent stimulation of a dephosphorylation pathway involving phosphatase PP1 (Cho et al., 1992; Sala et al., 2000). In the presence of an NMDAR inhibitor, antagonism of this PP1 pathway may be "shut-off" allowing for enhanced P-CREB levels. Our original hypothesis linking iCa2+ to downstream signaling events was confirmed in immature neurons, where inhibition of CREB phosphorylation paralleled inhibition of iCa2+ influx. However, this linkage did not hold true in DIV 16 neurons where robust inhibition of Ca2+ influx by conantokins did not correlate with diminishment of P-CREB. It has been shown that high levels of iCa2+ in postsynaptic neurons activates the calmodulin-CaMKII axis, which, in-turn, could phosphorylate CREB, whereas lower levels of iCa2+ results in calmodulin-induced activation of one of its targets PP2B, which potentiates the PP1 pathway (Bito et al., 1996; Groth et al., 2003), perhaps ultimately leading to dephosphorylation of CREB, or another protein necessary for CREB phosphorylation. Thus, while it may be speculated that diminishment of iCa2+ influx by conantokins in immature neurons may be triggering activation of phosphatases, thus allowing for CREB dephosphorylation, an event that does not occur in mature neurons, we find that levels of the relevant and ubiquitous eukaryotic protein phosphatases, e.g., PP1, PP2A, and PP2B, are not affected by conantokin treatments in either DIV 9 and DIV 16 neurons. Similarly, levels of activated (P-Thr286)CaMKII and (P-Thr196)CaMKIV, main regulators of neuronal CREB phosphorylation, are also not affected by conantokins, despite their ability to inhibit Ca2+ influx. A direct correlation between Ca2+ influx and P-CREB has been published (Deisseroth et al., 1996), wherein it was observed that spatial location of Ca2+ entry is an important determinant of CREB phosphorylation. Emigrated Ca2+ binds with high affinity to calmodulin at the synaptic sub-membrane, resulting in CREB activation. In Figure 3, it is observed that the levels of iCa2+ in DIV 7 and in DIV 16 neurons are similar. Therefore, the difference in P-CREB levels in DIV 9 and DIV 16 neurons could be due to developmental regulation of the Ca2+ threshold for CREB phosphorylation.
Our studies indicate that NMDA-induced P-CREB levels in immature neurons were robust and that the NMDAR-specific antagonists, conantokins and MK-801, attenuated phosphorylation of CREB. In these DIV 9 neurons, inhibition of phosphatases PP1, PP2A, and PP2B did not prevent deactivation of CREB when co-treated with the NMDA/NMDAR antagonists. This suggests that in immature neurons PP1, PP2A, and PP2B are not involved in modification of CREB activation in the presence of these inhibitors, although in another study it was reported that in other cAMP-responsive cells maintenance of P-CREB levels is controlled by its phosphatases (Bito et al., 1996; Hagiwara et al., 1992). In mature neurons, differential effects on P-CREB were observed that were dependent on the type of conantokin utilized. According to the immunoblots of Figure 6B, selective inhibition of PP1 allows for sustained activation of CREB when con-G was incubated with the neurons. In neurons treated with con-G, inhibition of PP2A and PP2B dramatically decreased P-CREB levels. On the other hand, inhibition of PP2B in neurons co-treated with NMDA/con-R[1-17] and NMDA/con-T caused increased levels of P-CREB in DIV 16 neurons, indicating that while the end-effects of various NMDAR antagonists are the same, i.e., enhanced P-CREB levels in mature neurons, the phosphatases that gate the dephosphorylation of CREB are different.
Our observations further indicate that treatment of DIV 9 neurons with NMDA/con-G increased NR2B at extrasynaptic sites, as observed by their presence in the neuron bodies. It has been shown that reduction of NR2B at synaptic sites (dendrites), relative to extrasynaptic sites, after inhibition of CaMKII effectively diminished CREB phosphorylation (Gardoni et al., 2009). Additionally, glutamate- or hypoxia-induced Ca2+ entry via extrasynaptic NMDARs triggered dephosphorylation of CREB, whereas Ca2+ influx through the synaptic complex promoted CREB phosphorylation (Hardingham and Bading, 2002). Herein, we show that although NMDA-stimulated iCa2+ influx is decreased in both immature and mature neurons by con-G, accompanying changes in the cellular location of the NR2B subunit depends on neuron maturity. Increased levels of NR2B subunits in the extrasynaptic sites of neuron bodies in DIV 9 neurons in the presence of NMDA/con-G implies that extrasynaptic activation of the NMDAR is leading to a CREB shut-off pathway. However, treatment of DIV 16 neurons with NMDA/con-G does not lead to an increase in NR2B subunit in neuron bodies, as observed in DIV neurons, thus allowing CREB phosphorylation to occur, implying sustained synaptic stimulation in more mature neurons.
In conclusion, we show conantokin-mediated inhibition of synaptic current flow by NMDARs in developing neurons reflects the subunit composition of these receptors and that inhibition of current flow in NMDARs in immature and mature neurons is directly linked to Ca2+ mobilization via these channels and to signaling events that occur downstream of P-CREB generation. Thus, conantokins can be employed as non-channel blocking alternative pharmacological and, potentially, therapeutic tools to mechanistically isolate extrasynaptic and synaptic events in neurons that relate to NMDAR channels of specific subunit composition.
4. Experimental methods
4.1 Conantokin synthesis
The amino acid sequences of the conantokins that were chemically synthesized for these studies are provided below. The methods used for the syntheses have been published earlier (Prorok et al., 1996). A C-terminal truncated analogue of con-R was used (con-R[1-17]), which has been previously shown to contain essentially all of the NMDAR activity of the 27 residue native peptide (Blandl et al., 2001).
con-G: GEγγL5QγNQγ10LIRγK15SN(NH2)
con-R[1-17]: GEγγVAKMAAγLARγNI(NH2)
con-T: GEγγY5QKMLγ10NLRγA15EVKKN20A(NH2)
Solid phase peptide synthesis was employed using an Applied Biosystems (Foster City, CA) Model 433A peptide synthesizer.
4.2 Primary cultures of rat hippocampal neurons
Hippocampal neuron cultures were prepared from embryonic day-18 Sprague-Dawley rat embryos (average of 12–17 embryos/rat). Hippocampal neurons were dissociated with 2 mg/ml papain dissolved in Hibernate E (Brain Bits, Springfield, IL) for 30 min at 37 °C, and then mechanically dissociated using a fire-polished Pasteur pipette. The cells were plated on plastic or glass-bottomed plates that were precoated with poly-L-lysine (1 mg/ml in 100 mM borate buffer, pH 8.5) in Neurobasal medium (Invitrogen, Carlsbad, CA), supplemented with 2% B27 (Invitrogen) and 1% L-glutamine. The plating densities of the neurons depended on the type of experiment planned. Cell cultures were maintained at 37º C in a humidified atmosphere with 5% CO2. One-half of the medium was exchanged with fresh medium once weekly. The plates were directly used for electrophysiological recordings, Ca2+ imaging, and Western analysis at DIV 7-22.
Animal handling and experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Notre Dame.
4.3 NMDA-evoked current recordings
NMDA-stimulated currents were recorded from hippocampal neurons maintained in culture for DIV 7-22, as described (Sheng et al., 2007). Neurons were placed in extracellular medium containing 140 mM NaCl/2.5 mM KCl/2.0 mM CaCl2/10 mM Hepes/10 mM D-glucose, pH 7.4. Mg2+ was omitted to prevent the voltage-dependent block of the NMDAR ion channels (Mayer et al., 1984). Tetrodotoxin (TTX; Sigma, St. Louis, MO) was added to the extracellular solution at a concentration of 1.0 μM to block Na+ channel activities. The intracellular solution contained 110 mM Cs-gluconate/20 mM CsCl/10 mM Hepes/10 mM EGTA/4 mM Mg-ATP/0.4 mM Na-GTP, pH 7.3 (adjusted with CsOH). In order to record whole cell responses of NMDARs, the coagonists, NMDA (differing concentrations specified in the particular experiment) and glycine (10 μM), were applied to the neurons. Recordings of 11-18 neurons were averaged for NMDA-evoked currents at different development days. In some cases, [(R)-[(S)-1-(4-bromo-phenyl)-ethylamino]-2,3-dioxo-1,2,3,4-tetrahydro-quinoxalin-5yl)-methyl]-phosphonic acid (NVP-AAM077; from Y.P. Auberson, Novartis Pharma, Basel, Switzerland) and ifenprodil (Sigma) was used as selective pharmacological blocks of NR2A- and NR2B-containing ion channels, respectively.
NMDA-mediated spontaneous excitatory postsynaptic currents (sEPSCs) were recorded using hippocampal neurons at various ages in culture. The bath solution included the coagonist, glycine (10 μM), bicuculline methiodide (20 μM) to antagonize GABAA receptors, strychnine-HCl (1μM) to block glycine channels, and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 20 μM) to block AMPA/kainate channels. In some experiments, 2-amino-5-phosphopentanoic acid (AP-5; 50 μM) was added to block NMDAR channel responses. Membrane impermeable N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium bromide (QX314; 2 mM) was added to the intracellular solution to block spiking and the fast voltage-gated Na+ currents of the neurons under study. Consecutive recordings were made in the gap-free mode with an Axopatch 200 B amplifier and Clampfit 8.2 (Axon Instruments). The data were digitized for off-line analysis using the MiniAnalysis Program 6.0 (Synaptosoft, Fort Lee, NJ). The responses of 6-11 neurons in 3 different preparations were recorded for inhibition experiments.
4.4 Calcium imaging
For Ca2+ imaging, the neurons were seeded at a density of 2.5 x 105 cells/ml on poly-L-lysine-coated 14 mm glass-bottom microwell dishes (Mat Tek, Ashland, MA). The cells were washed 3X with ACSF (artificial cerebrospinal fluid: 140 mM NaCl/5 mM KCl/2 mM CaCl2/10 mM Hepes/24 mM glucose, pH 7.2) and incubated with 1 αM fura-2-acetoxymethyl ester (Fura-2/AM, Invitrogen) at room temperature for 30 min. After this time, the cells were washed 3X in ACSF and the neurons were incubated for 15–30 min in the same solution to allow de-esterification of intracellular AM esters. The dish was mounted onto an imaging chamber and placed on the stage of a Nikon Eclipse TE 2000-S microscope (Nikon Instruments, Melville, NY). Application of ACSF, or stimulation with 50 μM NMDA/10 μM glycine or 50 μM NMDA/10 μM glycine/40 μM conantokins, was performed using a ValveBankII perfusion system (AutoMate Scientific, Berkeley, CA) at a manually controlled flow rate of 1 ml/min. The neurons were exposed to alternating 340 nm and 380 nm light from a xenon lamp via a shutter (Lambda LS). The resulting images were captured with a Cascade II 512 camera (Photometrics, Tucson, AZ) and acquired at 2 sec intervals, for 60 sec, before stimulation to obtain a steady baseline. The neurons were then stimulated with 50 μM NMDA/10 μM glycine until a plateau in signal was reached. The 50 μM NMDA/10 μM glycine was washed from the cells with ACSF and the cells were stimulated with 50 μM NMDA/10 μM glycine/40 μM con-G, con-R[1-17], or con-T until the signal reached a plateau, then washed as above.
Ratiometric traces were generated using NIS-Elements AR 3.0 software (Nikon) software. The peak of NMDA/glycine- or NMDA/glycine/conantokin-evoked increase in [iCa2+] was calculated by subtracting the basal value from the peak value and plotted as increase above basal Ca2+ levels. Changes in [iCa2+] responses induced by NMDA alone were then compared to responses elicited by NMDA in the presence of the conantokins.
4.5 Cell stimulation for phospho(P)-CREB and total CREB analysis
Experiments to determine the effects of NMDA and NMDA/conantokin on CREB phosphorylation at Ser133 were performed as described earlier, with operational modifications (Sala et al., 2000). At 15 hr prior to NMDA stimulation, one-half of the medium was exchanged with fresh Neurobasal medium without the B27 supplement and 1 αM TTX was added to the culture. The neurons (DIV 9 and DIV 16) were then pretreated with 40 αM CNQX and 5 αM nifedipine for 20–30 min before stimulation with 100 αM NMDA or 100 αM NMDA/40 αM conantokins for 20 min. In order to determine the effects of inhibition of phosphatases, PP1, PP2A, and calcineurin (PP2B), on CREB phosphorylation in the presence of NMDA and NMDA/conantokins were performed utilizing the cytotoxic polyether, okadaic acid (OA; Sigma), and the immunosuppressive drug, tacrolimus (FK506; Sigma). The neurons were treated with 100 αM NMDA or 100 αM NMDA/40 αM conantokins for 5 min and then treated with 2 αM OA, 20 nM OA, or 1 αM FK506 for 20 min. After this step, cell lysates were obtained by first washing the neurons with 3X with PBS and lysing directly utilizing 60 αl of SDS gel loading buffer. The fractionated samples were probed for phospho(P)-CREB(Ser133) using rabbit-anti-P-CREB (Cell Signaling Technology) and total CREB (loading control) using rabbit-anti-CREB (48H2, Cell Signaling Technology). The bands were visualized by chemiluminescence.
Densitometric analyses of P-CREB(Ser133) and total-CREB were performed utilizing Image Scope software (v10.0.36.1805) (Aperio, Vista, CA). The intensities of the bands were read as percent strong positive, utilizing the Color Deconvolution algorithm that was modified for the red, blue, and green channels. The values obtained for percent strong positive were used to calculate the ratio of P-CREB(Ser133)/CREB.
Similarly, cell lysates obtained were utilized to measure the effects of NMDA and NMDA/conantokins on the phosphatases, PP1, PP2B-Aα, PP2B-Aβ, as well as kinases, CaMKII and CamKIV. The antibodies utilized were rabbit-anti-phospho CaMKII, rabbit-anti-P-CaMKIV, mouse-anti-PP-1, mouse-anti-PP-2B α subunit, and goat-anti-PP-2B β subunit (Santa Cruz Biotechnology).
4.6 Immunofluorescence labeling and image acquisition of NR2B and CamKII
Neurons at DIV 9 and DIV 16 were treated with NMDA and the antagonists described above, and fixed in 100% chilled methanol at −20 °C for 10 min. The neurons were then incubated in 10% normal donkey serum/PBS at room temperature for 20 min. The primary antibodies against peptides from human proteins were goat-anti-human NMDAε2 (NR2B) and rabbit-anti-human CamKII (Santa Cruz Biotechnology). These antibodies were applied to the cells in the presence of 1.5% normal donkey serum/PBS. After washing, the secondary antibodies, AlexaFluor 488- or AlexaFluor 594-donkey-anti-goat IgG (Invitrogen, Molecular Probes) were applied. Fluorescence images were acquired using a 40X oil objective on an Olympus IX-71 Inverted Microscope. The operating software used was softWoRx version 3.6.0 (Delta Vision, Issaquah, WA).
4.7 Data analysis
Data are presented as mean ± S.E.M. The Student’s t-test was employed to examine the statistical significance of the differences between groups of data. Significance was assigned at P < 0.05.
Acknowledgments
We thank Ms. Allison Carmony for preparation of the hippocampal neurons.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AP-5
2-amino-5-phosphopentanoic acid)
- CamKII
calcium/calmodulin-dependent kinase II
- con
conantokin
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- CREB
cAMP response element-binding protein
- DIV
days in vitro
- Gla
γ-carboxyglutamate
- LTD
long-term depression
- LTP
long-term potentiation
- NMDA
N-methyl-D-aspartate
- NMDAR
N-methyl-D-aspartate receptor
- NVP-AAM077
[(R)-[(S)-1-(4-bromo-phenyl)-ethylamino]-2,3-dioxo-1,2,3,4-tetrahydro-quinoxalin-5yl)-methyl]-phosphonic acid
- OA
okadaic acid, QX314, N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium bromide
- sEPSCs
spontaneous excitatory postsynaptic currents
- TTX
tetrodotoxin
Footnotes
Conflict of interest statement
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alex AB, Baucum AJ, Wilcox KS. Effect of Conantokin G on NMDA receptor-mediated spontaneous EPSCs in cultured cortical neurons. J Neurophysiol. 2006;96:1084–1092. doi: 10.1152/jn.01325.2005. [DOI] [PubMed] [Google Scholar]
- Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 5-phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett. 2002;12:1099–1102. doi: 10.1016/s0960-894x(02)00074-4. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Barton ME, White HS, Wilcox KS. The effect of CGX-1007 and CI-1041, novel NMDA receptor antagonists, on NMDA receptor-mediated EPSCs. Epilepsy Res. 2004;59:13–24. doi: 10.1016/j.eplepsyres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Bialojan C, Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988;256:283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;27:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- Bito H, Takemoto-Kimura S. Ca(2+)/CREB/CBP-dependent gene regulation: a shared mechanism critical in long-term synaptic plasticity and neuronal survival. Cell Calcium. 2003;34:425–430. doi: 10.1016/s0143-4160(03)00140-4. [DOI] [PubMed] [Google Scholar]
- Blandl T, Zajicek J, Prorok M, Castellino FJ. Sequence requirements for the N-methyl-D-aspartate receptor antagonist activity of conantokin-R. J Biol Chem. 2001;276:7391–7396. doi: 10.1074/jbc.M006648200. [DOI] [PubMed] [Google Scholar]
- Borza I, Domány G. NR2B selective NMDA antagonists: the evolution of the ifenprodil-type pharmacophore. Curr Top Med Chem. 2006;6:687–695. doi: 10.2174/156802606776894456. [DOI] [PubMed] [Google Scholar]
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui JK, Tu SC, Kevin ASK, Nakanishi N, Tong G, Lipton SA, Zhang DX. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415:793–798. doi: 10.1038/nature715. [DOI] [PubMed] [Google Scholar]
- Chazot PL. The NMDA receptor NR2B subunit: a valid therapeutic target for multiple CNS pathologies. Curr Med Chem. 2004;11:389–396. doi: 10.2174/0929867043456061. [DOI] [PubMed] [Google Scholar]
- Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- Donevan SD, McCabe RT. Conantokin G is an NR2B-selective competitive antagonist of N-methyl-D-aspartate receptors. Mol Pharmacol. 2000;58:614–623. doi: 10.1124/mol.58.3.614. [DOI] [PubMed] [Google Scholar]
- Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005;438:185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- Gardoni F, Caputi A, Cimino M, Pastorino L, Cattabeni F, Di Luca M. Calcium/calmodulin-dependent protein kinase II is associated with NR2A/B subunits of NMDA receptor in postsynaptic densities. J Neurochem. 1998;71:1733–1741. doi: 10.1046/j.1471-4159.1998.71041733.x. [DOI] [PubMed] [Google Scholar]
- Gardoni F, Mauceri D, Malinverno M, Polli F, Costa C, Tozzi A, Siliquini S, Picconi B, Cattabeni F, Calabresi P, Di Luca M. Decreased NR2B subunit synaptic levels cause impaired long-term potentiation but not long-term depression. J Neurosci. 2009;29:669–677. doi: 10.1523/JNEUROSCI.3921-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowd KH, Twede V, Watkins M, Krishnan KS, Teichert RW, Bulaj G, Olivera BM. Conantokin-P, an unusual conantokin with a long disulfide loop. Toxicon. 2008;52:203–213. doi: 10.1016/j.toxicon.2008.04.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth RD, Dunbar RL, Mermelstein PG. Calcineurin regulation of neuronal plasticity. Biochem Biophys Res Commun. 2003;311:1159–1171. doi: 10.1016/j.bbrc.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Haack JA, Rivier J, Parks TN, Mena EE, Cruz LJ, Olivera BM. Conantokin-T. A γ-carboxyglutamate-containing peptide with N-methyl-D-aspartate antagonist activity. J Biol Chem. 1990;265:6025–6029. [PubMed] [Google Scholar]
- Hagiwara M, Alberts A, Brindle P, Meinkoth J, Feramisco J, Deng T, Karin M, Shenolikar S, Montminy M. Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell. 1992;70:105–113. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Coupling of extrasynaptic NMDA receptors to a CREB shut-off pathway is developmentally regulated. Biochim Biophys Acta. 2002;1600:148–153. doi: 10.1016/s1570-9639(02)00455-7. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hovinga CA. Novel anticonvulsant medications in development. Expert Opin Investig Drugs. 2002;11:1387–1406. doi: 10.1517/13543784.11.10.1387. [DOI] [PubMed] [Google Scholar]
- Jimenez EC, Donevan S, Walker C, Zhou LM, Nielsen J, Cruz LJ, Armstrong H, White HS, Olivera BM. Conantokin-L, a new NMDA receptor antagonist: determinants for anticonvulsant potency. Epilepsy Res. 2002;51:73–80. doi: 10.1016/s0920-1211(02)00101-8. [DOI] [PubMed] [Google Scholar]
- Klein RC, Galdzicki Z, Castellino FJ. Inhibition of NMDA-induced currents by conantokin-G and conantokin-T in cultured embryonic murine hippocampal neurons. Neuropharmacology. 1999;38:1819–1829. doi: 10.1016/s0028-3908(99)00065-9. [DOI] [PubMed] [Google Scholar]
- Klein RC, Prorok M, Galdzicki Z, Castellino FJ. The amino acid residue at sequence position 5 in the conantokin peptides partially governs subunit-selective antagonism of recombinant N-methyl-D-aspartate receptors. J Biol Chem. 2001;276:26860–26867. doi: 10.1074/jbc.M102428200. [DOI] [PubMed] [Google Scholar]
- Kopnisky KL, Chalecka-Franaszek E, Gonzalez-Zulueta M, Chuang DM. Chronic lithium treatment antagonizes glutamate-induced decrease of phosphorylated CREB in neurons via reducing protein phosphatase 1 and increasing MEK activities. Neuroscience. 2003;116:425–435. doi: 10.1016/s0306-4522(02)00573-0. [DOI] [PubMed] [Google Scholar]
- Laube B, Hirai H, Sturgess M, Betz H, Kuhse J. Molecular determinants of agonist discrimination by NMDA receptor subunits: Analysis of the glutamate binding site on the NR2B subunit. Neuron. 1997;18:493–503. doi: 10.1016/s0896-6273(00)81249-0. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 1993;16:521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Gilbert H, McCabe RT, Basbaum AI. Powerful antinociceptive effects of the cone snail venom-derived subtype-selective NMDA receptor antagonists conantokins G and T. Pain. 2003;101:109–116. doi: 10.1016/s0304-3959(02)00303-2. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Mayer ML. N-methyl-D-aspartic acid receptor structure and function. Physiol Rev. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- McIntosh J, Olivera BM, Cruz L, Gray W. γ-Carboxyglutamate in a neuroactive toxin. J Biol Chem. 1984;259:14343–14346. [PubMed] [Google Scholar]
- Muramatsu M, Lapiz MD, Tanaka E, Grenhoff J. Serotonin inhibits synaptic glutamate currents in rat nucleus accumbens neurons via presynaptic 5-HT1B receptors. Eur J Neurosci. 1998;10:2371–2379. doi: 10.1046/j.1460-9568.1998.00248.x. [DOI] [PubMed] [Google Scholar]
- Nikam SS, Meltzer LT. NR2B selective NMDA receptor antagonists. Curr Pharm Des. 2002;8:845–855. doi: 10.2174/1381612024607072. [DOI] [PubMed] [Google Scholar]
- Parsons JN, Wiederrecht GJ, Salowe S, Burbaum JJ, Rokosz LL, Kincaid RL, O'Keefe SJ. Regulation of calcineurin phosphatase activity and interaction with the FK-506. FK-506 binding protein complex. J Biol Chem. 1994;269:19610–19616. [PubMed] [Google Scholar]
- Prorok M, Geng JP, Warder SE, Castellino FJ. The entire gamma-carboxyglutamic acid- and helical stack-domains of human coagulation factor IX are required for optimal binding to its endothelial cell receptor. Int J Pept Protein Res. 1996;48:281–285. doi: 10.1111/j.1399-3011.1996.tb00842.x. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Feltz A, Westbrook GL. Synaptic NMDA receptor channels have a low open probability. J Neurosci. 1995;15:2788–2795. doi: 10.1523/JNEUROSCI.15-04-02788.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala C, Rudolph-Correia S, Sheng M. Developmentally regulated NMDA receptor-dependent dephosphorylation of cAMP response element-binding protein (CREB) in hippocampal neurons. J Neurosci. 2000;20:3529–3536. doi: 10.1523/JNEUROSCI.20-10-03529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P, Visvader J, Ferland L, Mellon PL, Verma IM. Induction of proto-oncogene fos transcription through the adenylate cyclase pathway: characterization of a cAMP-responsive element. Genes Dev. 1988;12:1529–1538. doi: 10.1101/gad.2.12a.1529. [DOI] [PubMed] [Google Scholar]
- Sauerwald A, Hoesche C, Oschwald R, Kilimann MW. The 5'-flanking region of the synapsin I gene. A G+C-rich, TATA- and CAAT-less, phylogenetically conserved sequence with cell type-specific promoter function. J Biol Chem. 1990;265:14932–14937. [PubMed] [Google Scholar]
- Schneggenburger R, López-Barneo J, Konnerth A. Excitatory and inhibitory synaptic currents and receptors in rat medial septal neurones. J Physiol. 1992;445:261–276. doi: 10.1113/jphysiol.1992.sp018923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Sheng Z, Dai Q, Prorok M, Castellino FJ. Subtype-selective antagonism of N-methyl-D-aspartate receptor ion channels by synthetic conantokin peptides. Neuropharmacology. 2007;53:145–156. doi: 10.1016/j.neuropharm.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Owens S, Forsythe ID. Characterisation of inhibitory and excitatory postsynaptic currents of the rat medial superior olive. J Physiol. 2000;529:681–698. doi: 10.1111/j.1469-7793.2000.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack S, Colbran RJ. Autophosphorylation-dependent targeting of calcium/calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 1998;273:20689–20692. doi: 10.1074/jbc.273.33.20689. [DOI] [PubMed] [Google Scholar]
- Teichert RW, Jimenez EC, Twede V, Watkins M, Hollmann M, Bulaj G, Olivera BM. Novel conantokins from conus parius venom are specific antagonists of NMDA receptors. J Biol Chem. 2007;282:36905–36913. doi: 10.1074/jbc.M706611200. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron. 2002;34:253–264. doi: 10.1016/s0896-6273(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtype mediated bidirectional control of p38 mitogen-activated protein kinase. J Biol Chem. 2005;280:29322–29333. doi: 10.1074/jbc.M502080200. [DOI] [PubMed] [Google Scholar]
- Wei F, Wang GD, Kerchner GA, Kim SJ, Xu HM, Chen ZF, Zhuo M. Genetic enhancement of inflammatory pain by forebrain NR2B overexpression. Nature Neurosci. 2001;4:164–169. doi: 10.1038/83993. [DOI] [PubMed] [Google Scholar]
- Wei J, Dong M, Xiao C, Jiang F, Castellino FJ, Prorok M, Dai Q. Conantokins and variants derived from cone snail venom inhibit naloxone-induced withdrawal jumping in morphine-dependent mice. Neurosci Lett. 2006;405:137–141. doi: 10.1016/j.neulet.2006.06.040. [DOI] [PubMed] [Google Scholar]
- White HS, McCabe RY, Armstrong H, Donevan SD, Cruz LJ, Abogadie FC, Torres J, Rivier JE, Paarmann I, Hollmann M, Olivera BM. In vitro and in vivo characterization of conantokin-R, a selective NMDA receptor antagonist isolated from the venom of the fish-hunting snail Conus radiatus. J Pharmacol Exp Ther. 2000;292:425–432. [PubMed] [Google Scholar]
- Williams AJ, Dave JR, Lu XM, Ling G, Tortella FC. Selective NR2B NMDA receptor antagonists are protective against staurosporine-induced apoptosis. Eur J Pharmacol. 2002a;452:135–136. doi: 10.1016/s0014-2999(02)02327-0. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Ling G, McCabe RT, Tortella FC. Intrathecal CGX-1007 is neuroprotective in a rat model of focal cerebral ischemia. Neuroreport. 2002b;1e3:821–824. doi: 10.1097/00001756-200205070-00017. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Wu LJ, Xu H, Ren M, Cao X, Zhuo M. Pharmacological isolation of postsynaptic currents mediated by NR2A- and NR2B-containing NMDA receptors in the anterior cingulate cortex. Mol Pain. 2007;3:11. doi: 10.1186/1744-8069-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Huang Y, Dong M, Hu J, Hou S, Castellino FJ, Prorok M, Dai Q. NR2B-selective conantokin peptide inhibitors of the NMDA receptor display enhanced antinociceptive properties compared to non-selective conantokins. Neuropeptides. 2008;42:601–609. doi: 10.1016/j.npep.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]