Abstract
The purpose of this study was to see whether the receptor for cardiac glycosides might be localized upon or within the plasma membrane of digitalis-sensitive cells. Ouabain and digoxin were joined covalently to several large protein molecules. These macromolecular conjugates are too large to enter intact cells; consequently, any pharmacologic or biochemical effects which they display should arise from interaction with a cell surface receptor. Conjugates were tested in several cardiac glycoside-sensitive systems: (a), contractility response of isolated cardiac muscle; (b), active 86Rb+ uptake by red cells; (c), enzymatic activity of isolated myocardial microsomal (Na+ + K+)-activated adenosine triphosphatase (ATPase); and (d), enzymatic activity of solubilized red cell (Na+ + K+)-activated ATPase. Results demonstrated that in all of these systems, the macromolecular-glycoside conjugates were 100- to 1000-fold less active than the free glycosides. Careful chromatographic examination of the various conjugates revealed that they contained a small but persistent free cardiac glycoside contaminant. The amount of this species ranged from 0.1 to 1.0% of the total macromolecule-bound glycoside, and its presence fully explains the levels of biologic activity observed with the conjugates.
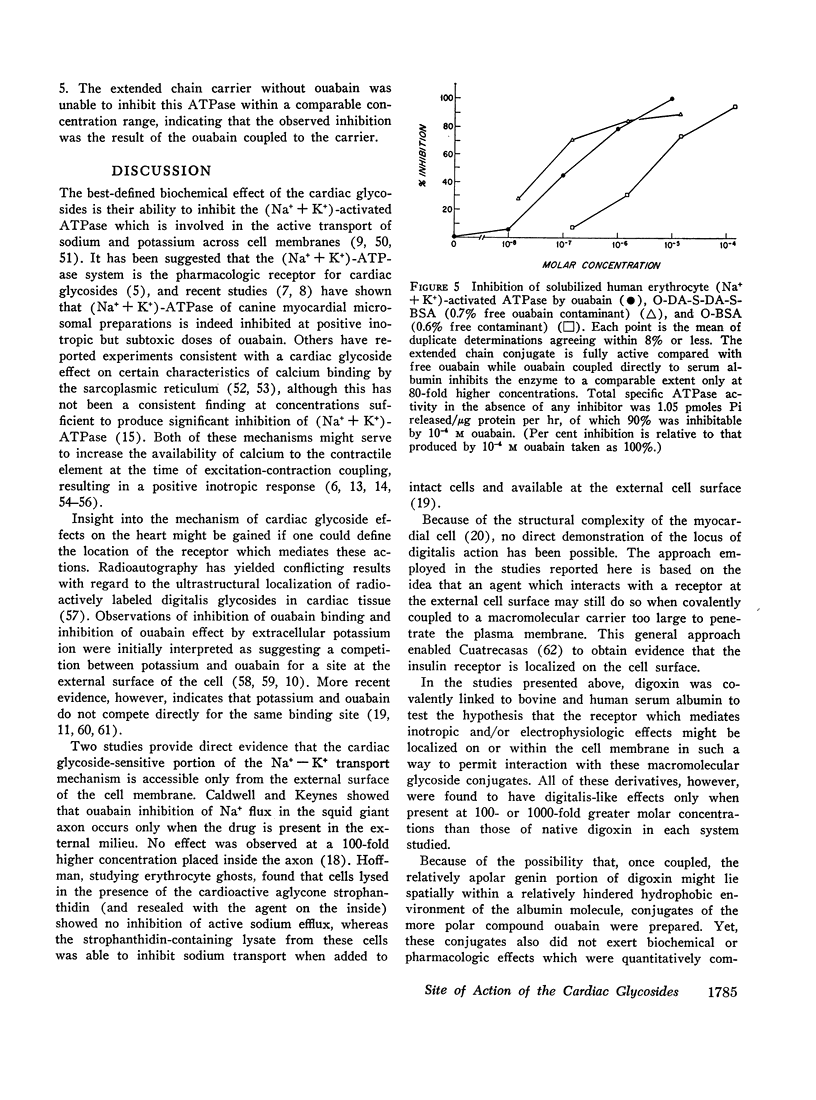
To try to minimize steric factors which could interfere with glycoside-receptor interaction, digoxin and ouabain were also coupled to macromolecule via long, flexible polyamide side-chains. These extended chain conjugates, in which the cardiac glycoside potentially lay some 30 A removed from the surface of the macromolecule, also exhibited negligible digitalis-like effects when tested upon isolated cardiac muscle, red cell 86Rb+ uptake, and enzymatic activity of cardiac microsomal (Na+ + K+)-ATPase. However, the extended chain conjugates were fully active when examined with the solubilized red cell (Na+ + K+)-ATPase system. To further ensure that the chemical reactions used to couple macromolecule to glycoside did not inactivate the drug, all conjugates were subjected to extensive proteolytic digests exhibited full pharmacologic activity. Digoxin was also coupled to the tripeptide alanylglycylglycine, and the resulting conjugate was fully active.
Taken together, these results suggest that if the receptor(s) for cardiac glycosides is associated with the plasma membrane, then it may lie deep within it.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANFINSEN C. B., SELA M., COOKE J. P. The reversible reduction of disulfide bonds in polyalanyl ribonuclease. J Biol Chem. 1962 Jun;237:1825–1831. [PubMed] [Google Scholar]
- Akera T., Brody T. M. Inhibition of brain sodium- and potassium-stimulated adenosine triphosphatase activity by chlorpromazine free radical. Mol Pharmacol. 1968 Nov;4(6):600–612. [PubMed] [Google Scholar]
- Akera T., Larsen F. S., Brody T. M. Correlation of cardiac sodium- and potassium-activated adenosine triphosphatase activity with ouabain-induced inotropic stimulation. J Pharmacol Exp Ther. 1970 May;173(1):145–151. [PubMed] [Google Scholar]
- Akera T., Larsen F. S., Brody T. M. The effect of ouabain on sodium- and potassium-activated adenosine triphosphatase from the hearts of several mammalian species. J Pharmacol Exp Ther. 1969 Nov;170(1):17–26. [PubMed] [Google Scholar]
- Albers R. W., Koval G. J., Siegel Studies on the interaction of ouabain and other cardio-active steroids with sodium-potassium-activated adenosine triphosphatase. Mol Pharmacol. 1968 Jul;4(4):324–336. [PubMed] [Google Scholar]
- Allen J. C., Schwartz A. Effects of potassium, temperature and time on ouabain interaction with the cardiac Na+, K+-ATPase: further evidence supporting an allosteric site. J Mol Cell Cardiol. 1970 Mar;1(1):39–45. doi: 10.1016/0022-2828(70)90027-1. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J. D., Young M. Rapid and complete purification of acetylcholinesterases of electric eel and erythrocyte by affinity chromatography. Proc Natl Acad Sci U S A. 1971 Feb;68(2):395–398. doi: 10.1073/pnas.68.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besch H. R., Jr, Allen J. C., Glick G., Schwartz A. Correlation between the inotropic action of ouabain and its effects on subcellular enzyme systems from canine myocardium. J Pharmacol Exp Ther. 1970 Jan;171(1):1–12. [PubMed] [Google Scholar]
- Blinks J. R. Convenient apparatus for recording contractions of isolated heart muscle. J Appl Physiol. 1965 Jul;20(4):755–757. doi: 10.1152/jappl.1965.20.4.755. [DOI] [PubMed] [Google Scholar]
- Blinks J. R. Field stimulation as a means of effecting the graded release of autonomic transmitters in isolated heart muscle. J Pharmacol Exp Ther. 1966 Feb;151(2):221–235. [PubMed] [Google Scholar]
- Brown D. M., Read A. P. Nucleotides. XLIX. The reduction of the adduct of periodate-oxidised adenosine-5' phosphate and methylamine. J Chem Soc Perkin 1. 1965 Sep;:5072–5074. doi: 10.1039/jr9650005072. [DOI] [PubMed] [Google Scholar]
- Butler V. P., Jr, Chen J. P. Digoxin-specific antibodies. Proc Natl Acad Sci U S A. 1967 Jan;57(1):71–78. doi: 10.1073/pnas.57.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Chipperfield D., Nayler W. G. The effect of ouabain on calcium in subcellular fractions of cardiac muscle. J Pharmacol Exp Ther. 1969 Dec;170(2):311–317. [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of insulin with the cell membrane: the primary action of insulin. Proc Natl Acad Sci U S A. 1969 Jun;63(2):450–457. doi: 10.1073/pnas.63.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Dunham P. B., Hoffman J. F. Active cation transport and ouabain binding in high potassium and low potassium red blood cells of sheep. J Gen Physiol. 1971 Jul;58(1):94–116. doi: 10.1085/jgp.58.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham P. B., Hoffman J. F. Partial purification of the ouabain-binding component and of Na,K-ATPase from human red cell membranes. Proc Natl Acad Sci U S A. 1970 Jul;66(3):936–943. doi: 10.1073/pnas.66.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERLANGER B. F., BEISER S. M. ANTIBODIES SPECIFIC FOR RIBONUCLEOSIDES AND RIBONUCLEOTIDES AND THEIR REACTION WITH DNA. Proc Natl Acad Sci U S A. 1964 Jul;52:68–74. doi: 10.1073/pnas.52.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett D. W., McNutt N. S. The ultrastructure of the cat myocardium. I. Ventricular papillary muscle. J Cell Biol. 1969 Jul;42(1):1–45. doi: 10.1083/jcb.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. THE ACTION OF CARDIAC GLYCOSIDES ON ION MOVEMENTS. Pharmacol Rev. 1964 Dec;16:381–407. [PubMed] [Google Scholar]
- GLYNN I. M. The action of cardiac glycosides on sodium and potassium movements in human red cells. J Physiol. 1957 Apr 3;136(1):148–173. doi: 10.1113/jphysiol.1957.sp005749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABEEB A. F., CASSIDY H. G., SINGER S. J. Molecular structural effects produced in proteins by reaction with succinic anhydride. Biochim Biophys Acta. 1958 Sep;29(3):587–593. doi: 10.1016/0006-3002(58)90016-7. [DOI] [PubMed] [Google Scholar]
- Hoffman J. F. The red cell membrane and the transport of sodium and potassium. Am J Med. 1966 Nov;41(5):666–680. doi: 10.1016/0002-9343(66)90029-5. [DOI] [PubMed] [Google Scholar]
- KHYM J. X. The reaction of methylamine with periodate-oxidized adenosine 5'-phosphate. Biochemistry. 1963 Mar-Apr;2:344–350. doi: 10.1021/bi00902a029. [DOI] [PubMed] [Google Scholar]
- KOCH-WESER J. Effect of rate changes on strength and time course of contraction of papillary muscle. Am J Physiol. 1963 Mar;204:451–457. doi: 10.1152/ajplegacy.1963.204.3.451. [DOI] [PubMed] [Google Scholar]
- Klaus W., Lee K. S. Influence of cardiac glycosides on calcium binding in muscle subcellular components. J Pharmacol Exp Ther. 1969 Mar;166(1):68–76. [PubMed] [Google Scholar]
- Koch-Weser J. Mechanism of digitalis action on the heart. N Engl J Med. 1967 Aug 31;277(9):469–concl. doi: 10.1056/NEJM196708312770905. [DOI] [PubMed] [Google Scholar]
- Koch-Weser J. Role of norepinephrine release in the interval-strength relationship of heart muscle. J Pharmacol Exp Ther. 1965 Nov;150(2):184–189. [PubMed] [Google Scholar]
- LOWENSTEIN J. M. A METHOD FOR MEASURING PLASMA LEVELS OF DIGITALIS GLYCOSIDES. Circulation. 1965 Feb;31:228–233. doi: 10.1161/01.cir.31.2.228. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langer G. A. Ion fluxes in cardiac excitation and contraction and their relation to myocardial contractility. Physiol Rev. 1968 Oct;48(4):708–757. doi: 10.1152/physrev.1968.48.4.708. [DOI] [PubMed] [Google Scholar]
- Langer G. A., Serena S. D. Effects of strophanthidin upon contraction and ionic exchange in rabbit ventricular myocardium: relation to control of active state. J Mol Cell Cardiol. 1970 Mar;1(1):65–90. doi: 10.1016/0022-2828(70)90029-5. [DOI] [PubMed] [Google Scholar]
- Langer G. A. The role of sodium ion in the regulation of myocardial contractility. J Mol Cell Cardiol. 1970 Sep;1(3):203–207. doi: 10.1016/0022-2828(70)90001-5. [DOI] [PubMed] [Google Scholar]
- Mason D. T., Spann J. F., Jr, Zelis R. New developments in the understanding of the actions of the digitalis glycosides. Prog Cardiovasc Dis. 1969 May;11(6):443–478. doi: 10.1016/0033-0620(69)90001-2. [DOI] [PubMed] [Google Scholar]
- PAGE E. Cat heart muscle in vitro. III. The extracellular space. J Gen Physiol. 1962 Nov;46:201–213. doi: 10.1085/jgp.46.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGE E. THE ACTIONS OF CARDIAC GLYCOSIDES ON HEART MUSCLE CELLS. Circulation. 1964 Aug;30:237–251. doi: 10.1161/01.cir.30.2.237. [DOI] [PubMed] [Google Scholar]
- Ryser H. J. Uptake of protein by mammalian cells: an underdeveloped area. The penetration of foreign proteins into mammalian cells can be measured and their functions explored. Science. 1968 Jan 26;159(3813):390–396. doi: 10.1126/science.159.3813.390. [DOI] [PubMed] [Google Scholar]
- SCHECHTER I., BAUMINGER S., SELA M., NACHTIGAL D., FELDMAN M. IMMUNE RESPONSE TO POLYPEPTIDYL PROTEINS IN RABBITS TOLERANT TO THE PROTEIN CARRIERS. Immunochemistry. 1964 Dec;1:249–265. doi: 10.1016/0019-2791(64)90026-6. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Matsui H., Laughter A. H. Tritiated digoxin binding to (Na+ + K+)-activated adenosine triphosphatase: possible allosteric site. Science. 1968 Apr 19;160(3825):323–325. doi: 10.1126/science.160.3825.323. [DOI] [PubMed] [Google Scholar]
- Smith T. W., Butler V. P., Jr, Haber E. Characterization of antibodies of high affinity and specificity for the digitalis glycoside digoxin. Biochemistry. 1970 Jan 20;9(2):331–337. doi: 10.1021/bi00804a020. [DOI] [PubMed] [Google Scholar]
- Smith T. W., Butler V. P., Jr, Haber E. Determination of therapeutic and toxic serum digoxin concentrations by radioimmunoassay. N Engl J Med. 1969 Nov 27;281(22):1212–1216. doi: 10.1056/NEJM196911272812203. [DOI] [PubMed] [Google Scholar]
- Smith T. W. Ouabain-specific antibodies: immunochemical properties and reversal of Na + , K + -activated adenosine triphosphatase inhibition. J Clin Invest. 1972 Jun;51(6):1583–1593. doi: 10.1172/JCI106956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALDI D. Eine neue systematische Analyse von Alkaloiden mit Hilfe der Papierchromatographie. Arch Pharm Ber Dtsch Pharm Ges. 1959 Apr;292(64):206–220. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Whittam R., Ager M. E. Vectorial aspects of adenosine-triphosphatase activity in erythrocyte membranes. Biochem J. 1964 Nov;93(2):337–348. doi: 10.1042/bj0930337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam R., Wheeler K. P. Transport across cell membranes. Annu Rev Physiol. 1970;32:21–60. doi: 10.1146/annurev.ph.32.030170.000321. [DOI] [PubMed] [Google Scholar]
- Wilson W. D., Foster J. F. Conformation-dependent limited proteolysis of bovine plasma albumin by an enzyme present in commercial albumin preparations. Biochemistry. 1971 May 11;10(10):1772–1780. doi: 10.1021/bi00786a007. [DOI] [PubMed] [Google Scholar]



