Abstract
Infrasonic sounds are generated internally in the body (by respiration, heartbeat, coughing, etc) and by external sources, such as air conditioning systems, inside vehicles, some industrial processes and, now becoming increasingly prevalent, wind turbines. It is widely assumed that infrasound presented at an amplitude below what is audible has no influence on the ear. In this review, we consider possible ways that low frequency sounds, at levels that may or may not be heard, could influence the function of the ear. The inner ear has elaborate mechanisms to attenuate low frequency sound components before they are transmitted to the brain. The auditory portion of the ear, the cochlea, has two types of sensory cells, inner hair cells (IHC) and outer hair cells (OHC), of which the IHC are coupled to the afferent fibers that transmit “hearing” to the brain. The sensory stereocilia (“hairs”) on the IHC are “fluid coupled” to mechanical stimuli, so their responses depend on stimulus velocity and their sensitivity decreases as sound frequency is lowered. In contrast, the OHC are directly coupled to mechanical stimuli, so their input remains greater than for IHC at low frequencies. At very low frequencies the OHC are stimulated by sounds at levels below those that are heard. Although the hair cells in other sensory structures such as the saccule may be tuned to infrasonic frequencies, auditory stimulus coupling to these structures is inefficient so that they are unlikely to be influenced by airborne infrasound. Structures that are involved in endolymph volume regulation are also known to be influenced by infrasound, but their sensitivity is also thought to be low. There are, however, abnormal states in which the ear becomes hypersensitive to infrasound. In most cases, the inner ear’s responses to infrasound can be considered normal, but they could be associated with unfamiliar sensations or subtle changes in physiology. This raises the possibility that exposure to the infrasound component of wind turbine noise could influence the physiology of the ear.
Keywords: Noise, infrasound, windmill, wind turbine syndrome
Introduction
The increasing use of wind turbines as a “green” form of energy generation is an impressive technological achievement. Over time, there have been rapid increases in the size of the towers, blades, and generator capacity of wind turbines, as well as a dramatic increase in their numbers. Associated with the deployment of wind turbines, however, has been a rather unexpected development. Some people are very upset by the noise that some wind turbines produce. Wind turbine noise becomes annoying at substantially lower levels than other forms of transportation noise, with the exception of railroad shunting yards (Pederson and Persson Wayne, 2004; Pederson and Persson Wayne, 2007; Pedersen et al, 2009). Some people with wind turbines located close to their homes have reported a variety of clinical symptoms that in rare cases are severe enough to force them to move away. These symptoms include sleep disturbance, headaches, difficulty concentrating, irritability and fatigue, but also include a number of otologic symptoms including dizziness or vertigo, tinnitus and the sensation of aural pain or pressure (Harry, 2007; Pierpont, 2009). The symptom group has been colloquially termed “wind turbine syndrome” and speculated to result from the low-frequency sounds that wind turbines generate (Pierpont, 2009). Similar symptoms resulting from low frequency sound emissions from non-wind turbine sources have also been reported (Feldmann and Pitten, 2004).
On the other hand, engineers associated with the wind industry maintain that infrasound from wind turbines is of no consequence if it is below the audible threshold. The British Wind Energy Association (2010), states that sounds from wind turbines are in the 30–50 dBA range, a level they correctly describe as difficult to discern above the rustling of trees [i.e. leaves].
This begs the question of why there is such an enormous discrepancy between subjective reactions to wind turbines and the measured sound levels. Many people live without problems near noisy intersections, airports and factories where sound levels are higher. The answer may lie in the high infrasound component of the sound generated by wind turbines. A detailed review of the effects of low frequency noise on the body was provided by Leventhall (2009). Although it is widely believed that infrasound from wind turbines cannot affect the ear, this view fails to recognize the complex physiology that underlies the ear’s response to low frequency sounds. This review considers the factors that influence how different components of the ear respond to low frequency stimulation and specifically whether different sensory cell types of the inner ear could be stimulated by infrasound at the levels typically experienced in the vicinity of wind turbines.
The Physics of Infrasound
Sounds represent fluctuating pressure changes superimposed on the normal ambient pressure, and can be defined by their spectral frequency components. Sounds with frequencies ranging from 20 Hz to 20 kHz represent those typically heard by humans and are designated as falling within the audible range. Sounds with frequencies below the audible range are termed infrasound. The boundary between the two is arbitrary and there is no physical distinction between infrasound and sounds in the audible range other than their frequency. Indeed, infrasound becomes perceptible if presented at high enough level.
The level of a sound is normally defined in terms of the magnitude of the pressure changes it represents, which can be measured and which does not depend on the frequency of the sound. In contrast, for sounds of constant pressure, the displacement of the medium is inversely proportional to frequency, with displacements increasing as frequency is reduced. This phenomenon can be observed as the difference in vibration amplitude between a subwoofer generating a low frequency tone and a tweeter generating a high frequency tone at the same pressure level. The speaker cone of the subwoofer is visibly displaced while the displacement of the tweeter cone is imperceptible. As a result of this phenomenon, vibration amplitudes to infrasound are larger than those to sounds in the auditory range at the same level, with displacements at 1 Hz being 1000 times those at 1 kHz when presented at the same pressure level. This corresponds to an increase in displacement at a rate of 6 dB/octave as frequency is lowered.
Overview of the anatomy of the ear
The auditory part of the inner ear, the cochlea, consists of a series of fluid-filled tubes, spiraling around the auditory nerve. A section through the middle of a human cochlea is shown in Fig 1A. The anatomy of each turn is characterized by three fluid-filled spaces (Fig 1B): scala tympani (ST) and scala vestibuli (SV) containing perilymph (yellow), separated by the endolymphatic space (ELS)(blue). The two perilymphatic compartments are connected together at the apex of the cochlea through an opening called the helicotrema. Perilymph is similar in ionic composition to most other extracellular fluids (high Na+, low K+) while endolymph has a unique composition for an extracellular fluid in the body, being high in K+ and low in both Na+ and Ca2+. It is also electrically polarized by about +80 mV with respect to perilymph, which is called the endocochlear potential (EP). The main sensory organ of the cochlea (Figs 1C,1D,1E, and shown colored green in Fig 1D) lies on the basilar membrane between the ELS and the perilymph of ST and is called the organ of Corti. The organ of Corti, seen here in cross section, contains one row of inner hair cells (IHC) and three rows of outer hair cells (OHC) along the spiral length of the cochlea. As shown schematically in Fig 1F, the sensory hairs (stereocilia) of the OHC have a gradation in length, with the tallest stereocilia embedded in the gelatinous tectorial membrane (TeM) which overlies the organ of Corti in the endolymphatic space (Kimura 1975). This arrangement allows sound-evoked displacements of the organ of Corti to be converted to a lateral displacement of OHC stereocilia. In contrast, the stereocilia of the IHC do not contact the tectorial membrane, but remain within the fluid of the subtectorial space (Kimura 1975, Lim 1986). Because of this difference in how the hair cell stereocilia interact with the TeM, the two types of hair cell respond differently to mechanical stimuli. At low frequencies, the IHC respond according to the velocity of basilar membrane displacement, while OHC respond to the displacement itself (Russell and Sellick, 1983; Dallos, 1984).
Figure 1.
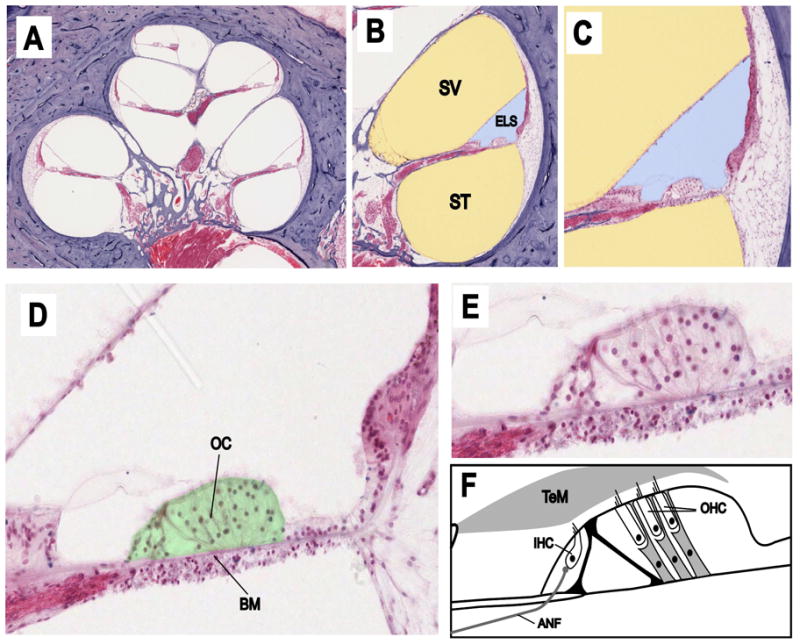
Panels A–E Cross section through the human cochlea shown with progressively increasing magnification. Panels B and C The fluid spaces containing perilymph have been colored yellow and endolymph blue. Panel D The sensory structure of the cochlea, the organ of Corti, is colored green. Panel F Schematic showing the anatomy of the main components of the organ of Corti. Abbreviations are: SV: scala vestibuli; ST: scala tympani; ELS: endolymphatic space; OC: organ of Corti; BM: basilar membrane; TeM: tectorial membrane; IHC: inner hair cell; OHC: outer hair cell; ANF: afferent nerve fiber. Original histological images courtesy of Saumil Merchant, MD, Otopathology Laboratory, Massachusetts Eye and Ear Infirmary and Harvard Medical School, Boston.
The two types of hair cells also contact different types of afferent nerve fibers, sending information to the brain (Spoendlin, 1972; Santi and Tsuprun, 2001). Each IHC is innervated by multiple Type I afferent fibers, with each fiber innervating only a single IHC. The Type I afferents represent the vast majority (95%) of the fibers transmitting information to the brain and as a result it is generally believed that mammals hear with their IHC (Dallos 2008). In contrast, the OHC contact Type II afferent fibers, which are unmyelinated and make synaptic contacts with a number of OHC. Type II afferents fibers are believed to be unresponsive to sounds and may signal the static position of the organ of Corti (Brown, 1994; Robertson et al., 1999). The OHC also receive substantial efferent innervation (from the brain) while the IHC receive no direct efferent innervation (Spoendlin, 1972).
Mechanics of low frequency stimulation
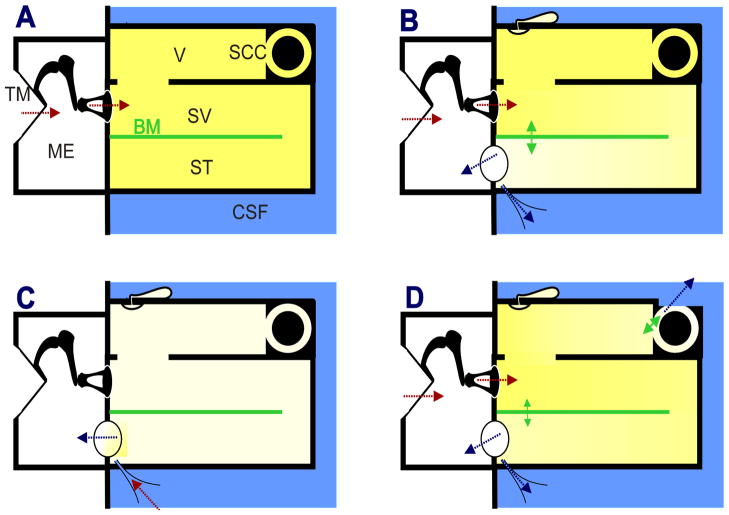
Infrasound entering the ear through the ossicular chain is likely to have a greater effect on the structures of the inner ear than is sound generated internally. The basic principles underlying stimulation of the inner ear by low frequency sounds are illustrated in Figure 2. Panel A shows the compartments of a simplified, uncoiled cochlea bounded by solid walls with two parallel fluid spaces representing SV and ST respectively that are separated by a distensible membrane representing the basilar membrane and organ of Corti. It is generally agreed that the differential pressure between SV and ST across the basilar membrane is the important factor driving the motion of the basilar membrane (von Békésy 1960; Dancer and Franke, 1980; Nakajima et al., 2008; Merchant and Rosowski, 2008). In example A, all the boundaries of the inner ear are solid and noncompliant with the exception of the stapes. In this non-physiologic situation, the stapes applies pressures to SV (indicated by the red arrows) but as the fluid can be considered incompressible, pressures are instantaneously distributed throughout both fluid spaces and pressure gradients across the basilar membrane will be small. In panel B, the round window (RW) and the cochlear aqueduct (CA) have been added to the base of ST. For frequencies below 300 Hz the RW provides a compliance between perilymph and the middle ear (Nakajima et al., 2008) and the CA provides fluid communication between perilymph and the cerebrospinal fluid (CSF). Under this condition, pressures applied by the stapes induce small volume flows between the stapes and the site(s) of compliance (blue arrows) which requires a pressure gradient to exist along the system, as indicated by the shading. The pressure differential across the basilar membrane will displace it, causing stimulation of the IHC and OHC. This is the situation for external sounds entering the normal cochlea via the ossicular chain. In panel C the situation is compared for sounds originating in the CSF and entering the system through the CA. In this case, the compliant RW is situated close to the location of aqueduct entry, so the major fluid flows and pressure gradients occur locally between these structures. As the stapes and other boundaries in scala vestibuli and the vestibule are relatively noncompliant, pressure gradients across the basilar membrane will be lower than with an equivalent pressure applied by the stapes. For infrasonic frequencies, it was shown that responses to 1 Hz pressure oscillation applied to the fluid in the basal turn of ST were substantially increased when the wall of SV was perforated thereby providing greater compliance in that scala (Salt and DeMott, 1999).
Figure 2.
Schematic representation of the uncoiled inner ear for four different mechanical conditions with low frequency stimulation. Red arrows indicate applied pressure and blue arrows indicate loss to compliant structures. A: indicates a hypothetical condition where the fluid space is rigidly bounded with no “windows” providing compliance. Sound pressure applied by the stapes causes uniform pressures (indicated by color shading) throughout the fluid space, so pressure difference across the basilar membrane and therefore stimulation is minimal. B: The normal situation with compliances provided by the round window and cochlear aqueduct at the base of scala tympani. Pressure differentials cause movement of fluid towards the compliant regions, a including a pressure differential across the basilar membrane causing stimulation. C: Situation where low frequency enters scala tympani through the cochlear aqueduct. The main compliant structure is located nearby so pressure gradients across the basilar membrane are small, limiting the amount of stimulation. Infrasound entering through the cochlear aqueduct (such as from respiration and body movements) therefore does not provide the same degree of stimulation as that entering via the stapes. D: Situation with compromised otic capsule, such as superior canal dehiscence. As pressure gradients occur both along the cochlea and through the vestibule and semi-circular canal, the sensory structures in the semi-circular canal will be stimulated. Abbreviations: BM: basilar membrane; CA: cochlear aqueduct; CSF: cerebrospinal fluid; ES: endolymphatic duct and sac; ME: middle ear; RW: round window; SCC: semi circular canal; ST: scala tympani, SV: scala vestibuli, TM: tympanic membrane; V:vestibule. The endolymphatic duct and sac is not an open pathway but is closed by the tissues of the sac, so it is not considered a significant compliance.
The final condition in Figure 2D shows the consequences of a “third window” on the SV/vestibule side of the cochlear partition. This causes an increased “air-bone gap” (i.e. an increase in sensitivity to bone conducted vibration and a decreased sensitivity to air conducted sounds, primarily at low frequencies; Merchant and Rosowski, 2008). It may also produce an abnormal sound-induced stimulation of other receptors in the inner ear, such as the hair cells in the ampulla of the semicircular canal. This is the basis of the Tullio phenomenon, in which externally or internally generated sounds, such as voice, induce dizziness.
Receptors in other organs of the inner ear, specifically both the saccule and the utricle also respond to airborne sounds delivered by the stapes, as discussed in more detail below. The mechanism of hair cell stimulation of these organs is less certain, but is believed to be related to pressure gradients through the sensory epithelium (Sohmer 2006).
Physiologic responses of the ear to low frequency stimuli
i) Cochlear Hair Cells
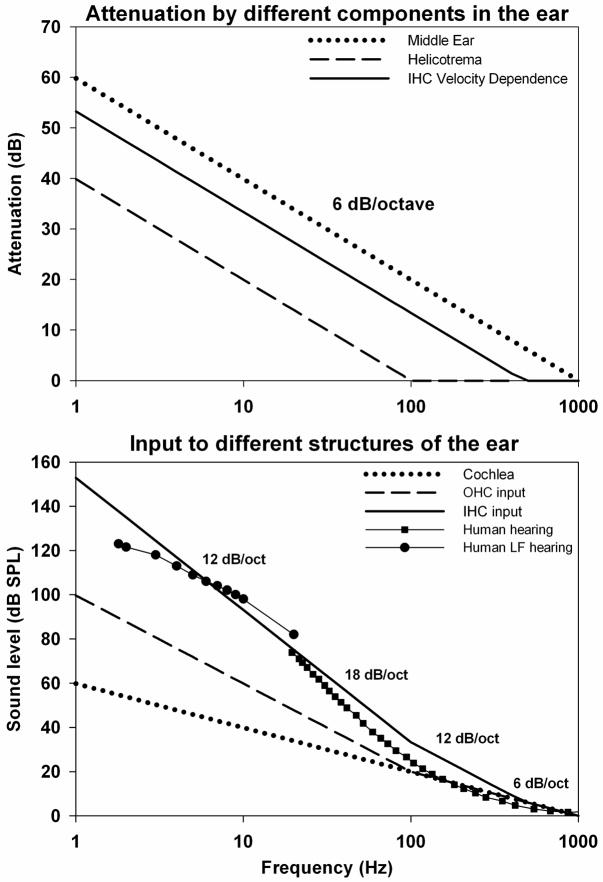
When airborne sounds enter the ear, to be transduced into an electrical signal by the cochlear hair cells, they are subjected to a number of mechanical and physiologic transformations, some of which vary systematically with frequency. The main processes involved were established in many studies and were summarized by Cheatham and Dallos (2001). A summary of the components are shown in Figure 3. There are three major processes influencing the sensitivity of the ear to low frequencies. The first arises from the transmission characteristics of sounds through the ossicular structures of the middle ear, which have been shown to attenuate signals at a rate of 6 dB/octave for frequencies below 1000 Hz (Dallos, 1973). As the vibration amplitude in air increases at 6 dB/octave as frequency is lowered, this attenuation characteristic of middle ear transmission results in the displacement of middle ear structures remaining almost constant across frequency for sounds of constant pressure level. A second process attenuating low frequency sounds is the fluid shunting between ST and SV through the helicotrema. The helicotrema has been shown to attenuate frequencies below 100 Hz by 6 dB/octave (Dallos 1970). The third filter arises from the demonstrated dependence of the IHC on stimulus velocity, rather than displacement (Dallos, 1984). This results in an attenuation of 6 dB/octave for frequencies below approximately 470 Hz for the IHC, and causes a 90° phase difference between IHC and OHC responses (Dallos, 1984). The combined results of these processes are compared with the measured sensitivity of human hearing (ISO 226:2003) in Fig 3B. The three processes combine to produce the steep decline of sensitivity (up to 18 dB/octave) in human hearing for frequencies between 100 and 20 Hz. This steep cutoff means that to hear a stimulus at 5 Hz it must be presented at 105 dB higher level than one at 500 Hz. This reflects the fact that the predominant, type I afferent fibers are stimulated by the IHC and that mammals hear with their IHC (Dallos 2008). However, an important consequence of this underlying mechanism is that the OHC and IHC differ markedly in their responses to low frequency stimuli. As the OHC respond to displacement, rather than velocity, they are not subject to the 6dB/octave attenuation seen by IHC, so at low frequencies they are stimulated by lower sound levels than the IHC. In theory, the difference between IHC and OHC responses will increase as frequency decreases (becoming over 50 dB at 1 Hz), but in practice, there is interaction between the two types of hair cells which limits the difference as discussed below.
Figure 3.
Upper panel: Estimated properties of high pass filter functions associated with cochlear signal processing (based on Cheatham and Dallos, 2001). The curves show the low frequency attenuation provided by the middle ear (6 dB/octave below 1000 Hz), by the helicotrema (6 dB/octave below 100 Hz) and by the fluid coupling of the inner hair cells (IHC) resulting in the IHC dependence on stimulus velocity (6 dB/Octave below 470 Hz). Lower panel: Combination of the three processes above into threshold curves demonstrating: input to the cochlea (dotted) as a result of middle ear attenuation; input to the outer hair cells (OHC) as a result of additional filtering by the helicotrema; and input to the IHC as a result of their velocity dependence. Shown for comparison is the sensitivity of human hearing in the audible range (ISO226:2003) and the sensitivity of humans to infrasounds (Møller and Pederson, 2004). The summed filter functions account for the steep (18 dB/octave) decrease in sensitivity below 100 Hz.
The measured response phase of OHC, IHC and auditory nerve fibers is consistent with the above processes. The cochlear microphonics (CM) recorded in the organ of Corti with low frequency stimuli are in phase with the intracellular potentials of the OHC. This supports the view that the low-frequency CM is dominated by OHC-generated potentials, which follow the displacement of the basilar membrane (Dallos et al., 1972). In contrast, intracellular responses from the IHC lead the organ of Corti CM response by an amount which approaches 90° as frequency is reduced to 100 Hz (Dallos, 1984) corresponding to maximal basilar membrane velocity towards SV (Nuttall et al., 1981). As frequency is lowered, the intracellular potentials of IHC and afferent fiber responses show phase changes consistent with the IHC no longer responding to the increasingly attenuated velocity stimulus, but instead responding to the extracellular potentials generated by the OHC (Sellick et al, 1982, Cheatham and Dallos 1997). A similar change of phase as frequency is lowered was reported in human psychophysical measurements (Zwicker 1977) with masking patterns differing by approximately 90° for frequencies above and below 40 Hz. This transition from a response originating from mechanical stimulation of the IHC, to one originating from electrical stimulation of the IHC by large extracellular responses from the OHC may account for the transition of low frequency sensitivity in humans from 18 dB/octave above 20 Hz to 12 dB/octave below 10 Hz (Møller and Pederson, 2004) (Fig 3B). Near 10 Hz the IHC transition to become primarily stimulated by the more sensitive OHC responses. It can be inferred that if extracellular voltages generated by the OHC are large enough to electrically stimulate the IHC at a specific frequency and level, then the lowest level that the OHC respond to at that frequency must be substantially lower. Based on this understanding of how the sensitivity of the ear arises, one conclusion is that at low frequencies the OHC are responding to infrasound at levels well below those that are heard. On the basis of the calculated input to OHC in Figure 3b, it is possible that for frequencies around 5 Hz, the OHC could be stimulated at levels up to 40 dB below those that stimulate the IHC. Although the OHC at 1 kHz are approximately 12 dB less sensitive than IHC (Dallos 1986), this difference declines as frequency is lowered and differences in hair cell sensitivity at very low frequencies (below 200 Hz) have not been measured.
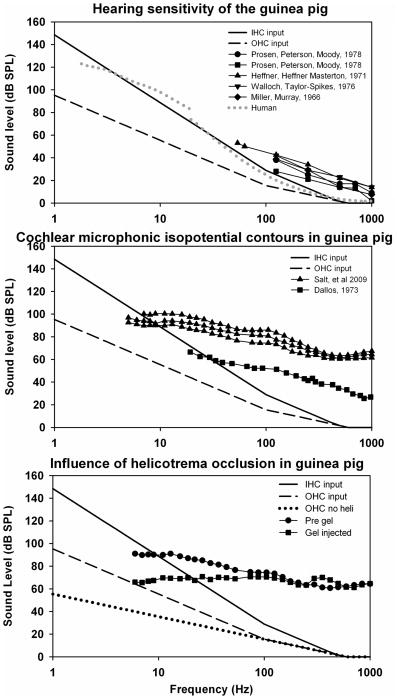
Much of the work understanding how the ear responds to low frequency sounds is based on measurements performed in animals. Although low frequency hearing sensitivity depends on many factors including the mechanical properties of the middle ear, low frequency hearing sensitivity has been shown to be correlated with cochlear length for many species with non-specialized cochleas, including humans and guinea pigs (West, 1985; Echteler et al., 1994). The thresholds of guinea pig hearing have been measured with stimulus frequencies as low as 50 Hz, as shown in Fig 4A. The average sensitivity at 125 Hz for five groups in four studies (Heffner et al., 1971; Miller and Murray, 1976; Walloch and Taylor-Spikes, 1976; Prosen et al, 1978, Fay, 1988) was 37.9 dB SPL, which is 17.6 dB less sensitive than the human at the same frequency and is consistent with the shorter cochlea of guinea pigs. In the absence of data to the contrary, it is therefore reasonable to assume that if low frequency responses are present in the guinea pig at a specific level, then they will be present in the human at a similar or lower stimulus level.
Figure 4.
Upper panel: Similar filter functions as Fig 3, with parameters appropriate for the guinea pig, and compared with measures of guinea pig hearing. At 125 Hz the guinea pig is approximately 18 dB less sensitive than the human (shown dotted for comparison). Middle panel: Cochlear microphonic isopotential contours in the guinea pig show no steep cutoff below 100 Hz, consistent with input to the OHC being maintained at lower levels than the IHC for low frequencies. Lower panel: Influence of helicotrema occlusion in the guinea pig, produced by injecting 2 μL of hyaluronate gel into the cochlear apex, on the CM isopotential function. Also shown for comparison is the estimated input sensitivity for the OHC with the attenuation by the helicotrema excluded. CM sensitivity curves both have lower slopes than their predicted functions, but the change caused by helicotrema occlusion is comparable.
ii) Cochlear microphonic measurements
Cochlear microphonics (CM) to low frequency tones originate primarily from the OHC (Dallos et al., 1972; Dallos and Cheatham, 1976). The sensitivity of CM as frequency is varied is typically shown by CM isopotential contours, made by tracking a specified CM amplitude as frequency is varied. Figure 4B shows low frequency CM sensitivity with two different criteria (Dallos 1973: 3 μV; Salt at al, 2009: 500 μV). The decrease in CM sensitivity as frequency is lowered notably follows a far lower slope than that of human hearing over the comparable frequency range, In the data from Salt et al., (2009), the stimulus level differences between 5 Hz and 500 Hz average only 34 dB (5.2 dB/octave), compared to the 105 dB difference (15.8 dB/octave) for human hearing over the same range. Although these are suprathreshold, extracellular responses, based on an arbitrary amplitude criterion, these findings are consistent with the OHC having a lower rate of cutoff with frequency than the IHC, and therefore responding to lower level stimuli at very low frequencies.
The measured change in CM sensitivity with frequency may include other components, such as a contribution from transducer adaptation at the level of the OHC stereocilia (Kros, 1996). Kennedy et al. (2003) have suggested that adaptation of the mechanoelectrical transducer channels is common to all hair cells and contributes to driving active motion of the hair cell bundle. Based on their measurements in cells isolated from the apical turns of neonatal rats, they estimated that adaptation caused high-pass filtering with a low frequency cutoff frequency of 2/3 of the best frequency for the cochlear location. This type of adaptation, however, does not appear to provide additional attenuation at very low frequencies, as inferred from CM sensitivity curves measured down to 5 Hz. On the contrary, the CM sensitivity curve appears to flatten below 10 Hz, a phenomenon which is currently under investigation in our laboratory.
Figure 4C shows the influence of plugging the helicotrema with gel on CM sensitivity with frequency, recorded from the basal turn of a guinea pig with a 500 μV criterion (Salt et al., 2009). These relative sensitivity changes, combined with a 90° phase shift in responses, replicate those of Franke and Dancer (1982) and demonstrate the contribution to attenuation provided by the helicotrema for frequencies below approximately 100 Hz. This contrasts with a prior suggestion that the helicotrema of the guinea pig was less effective than that of other species (Dallos, 1970). While the above CM measurements were made with the bulla open, measurements made in both the bulla open/closed conditions with closed sound-field stimulation suggest there is no pronounced frequency-dependence of the difference between these conditions below 300 Hz although there may be a level difference of 5–15 dB (Dallos 1973, Wilson & Johnstone 1975).
iii) Low frequency biasing, operating point, and distortion generation
As a result of the saturating, nonlinear transducer characteristic of cochlear hair cells (Russell and Sellick, 1983, Kros 1996), the fidelity of cochlear transduction depends highly on the so-called operating point of the cochlear transducer, which can be derived by Boltzmann analysis of the CM waveform (Patuzzi and Moleirinho 1998; Patuzzi and O’Beirne 1999). The operating point can be regarded as the resting position of the organ of Corti or its position during zero crossings of an applied stimulus (which may not be identical, as stimulation can itself influence operating point). Small displacements of operating point have a dramatic influence on even-order distortions generated by the cochlea (2f, f2–f1) while having little influence on odd-order distortions (3f, 2f1–f2) until displacements are large (Frank and Kössl 1996; Sirjani et al, 2004). Low frequency sounds (so called bias tones) have been shown to modulate distortion generated by the ear by their displacement of the operating point of the organ of Corti (Brown et al., 2009). In normal guinea pigs, 4.8 Hz bias tones at levels of 85 dB SPL have been shown to modulate measures of operating point derived from an analysis of CM waveforms (Brown et al, 2009; Salt et al, 2009). This is a level that is substantially below the expected hearing threshold of the guinea pig at 4.8 Hz. In animals where the helicotremea was occluded by injection of gel into the perilymphatic space at the cochlear apex, even lower bias levels (down to 60 dB SPL) modulate operating point measures (Salt et al., 2009). These findings are again consistent with the OHC being the origin of the signals measured and the OHC being more responsive to low frequency sounds than the IHC. A similar hypersensitivity to 4.8 Hz bias tones was also found in animals with surgically-induced endolymphatic hydrops (Salt et al., 2009). This was thought to be related to the occlusion of the helicotrema by the displaced membranous structures bounding the hydropic endolymphatic space in the apical turn. In some cases of severe hydrops, Reissner’s membrane was seen to herniate into ST. As endolymphatic hydrops is present both in patients with Meniere’s disease and in a significant number of asymptomatic patients (Merchant et al., 2005), the possibility exists that some individuals may be more sensitive to infrasound due the presence of endolymphatic hydrops.
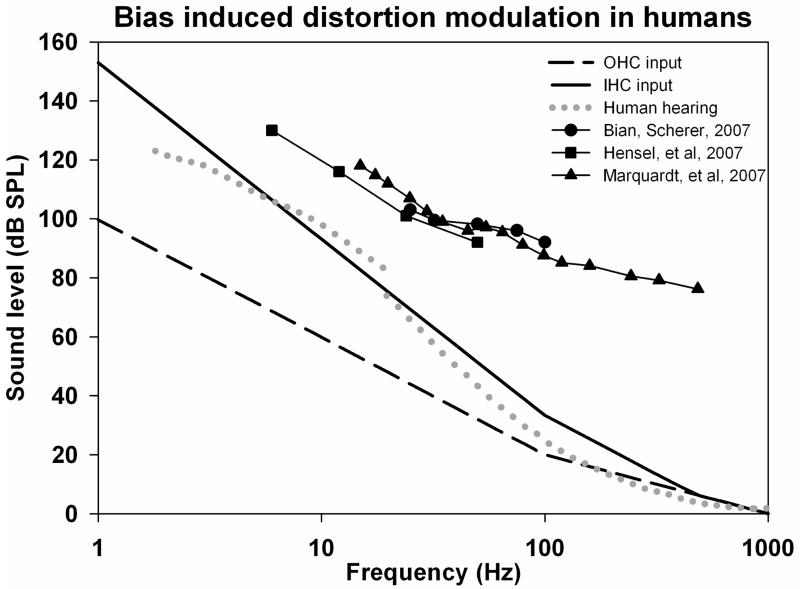
In the human ear, most studies have focused on the 2f1–f2 distortion product, as even-order distortions are difficult to record in humans. The 2f1–f2 component has been demonstrated to be less sensitive to operating point change (Sirjani et al., 2004, Brown et al, 2009). Using different criteria of bias-induced distortion modulation, the dependence on bias frequency was systematically studied in humans for frequencies down to 25 Hz, 6 Hz and 15 Hz respectively (Bian and Scherer, 2007, Hensel et al, 2007, Marquardt et al, 2007). In each of these studies, the bias levels required were above those that are heard by humans, but in all of them the change of sensitivity with frequency followed a substantially lower slope than the hearing sensitivity change as shown in Figure 5. Again this may reflect the OHC origins of acoustic emissions, possibly combined with the processes responsible for the flattening of equal loudness contours for higher level stimuli, since the acoustic emissions methods are using probe stimuli considerably above threshold. Although in some regions, slopes of 9–12 dB/octave were found, all showed slopes of 6 dB/octave around the 20 Hz region where human hearing falls most steeply at 18 dB/octave. It should also be emphasized that each of these studies selected a robust modulation criterion and was not specifically directed at establishing a threshold for the modulation response at each frequency. Indeed, in the data of Bian and Scherer (2007) (their Figure 3), significant modulation can be seen at levels down to 80 dB SPL at some of the test frequencies. In one of the studies (Marquardt et al, 2007) equivalent measurements were performed in guinea pigs. Although somewhat lower slopes were observed in guinea pigs it is remarkable that stimulus levels required for modulation of distortion were within 5–10 dB of each other for guinea pigs and humans across most of the frequency range. In this case the guinea pig required lower levels than the human. Although the threshold of sensitivity cannot be established from these studies, it is worth noting that for distortion product measurements in the audible range, “thresholds” typically require stimulus levels in the 35–45 dB SPL range (Lonsbury-Martin et al, 1990). In the Marquardt study, the bias tone level required at 500 Hz is over 60 dB above hearing threshold at that frequency.
Figure 5.
Frequency dependence of low frequency bias induced modulation of the 2f1–f2 distortion product measured in the external ear canal of humans in three studies, compared with estimated input functions and human hearing sensitivity. Below 100 Hz the sensitivity to bias falls off at a much lower slope than human hearing, consistent with the response originating from OHC with a lower cutoff slope.
iv) Feedback mechanisms stabilizing operating point
The OHC not only transduce mechanical stimuli to electrical responses, but also respond mechanically to electrical stimulation (reviewed by Dallos 2008) in a manner that provides mechanical amplification. This “active tuning” primarily enhances responses to high stimulus frequencies and is thought to provide little or no active gain with stimuli below approximately 1 kHz (Sellick et al., 2006). For low frequency stimulation, however, basilar membrane modulation by the low frequency tone does have a major influence on the mechanics at the best frequency of high frequency tones i.e. on the active tuning process. (Patuzzi et al., 1984). It has been suggested that slow mechanical movements of the OHC may play a part in stabilizing the operating point of the transducer (LePage 1987; LePage 1989) so the OHC may participate in an active cancellation of low frequency sounds. In models of the cochlear transducer, it was proposed that negative feedback occurred at low frequencies (in which the OHC opposed movements of the basilar membrane), which becomes a positive feedback at the best frequency for the region (Mountain et al., 1983). Chan and Hudspeth (2005) have also suggested OHC motility may be exploited to maintain the operating point of a fast amplifier in the hair cell bundle. However, this possibility has recently been questioned (Dallos 2010) for a number of reasons, one of which is the somatic motor protein, prestin, has an extremely fast response capability. So the interrelationships between hair cell motility and transduction, and between OHC and IHC remain an intense focus of current research. For low frequencies, it has been shown that an out-of phase motion exists between the IHC reticular lamina and the overlying TM so that electromechanical action of the OHC may stimulate the IHC directly, without involvement of the basilar membrane (Nowotny and Gummer, 2006). The possible roles of the OHC and efferent systems are made more complex by recent findings of reciprocal synapses between OHC and their efferent terminals, seen as afferent and efferent synapses on the same fiber (Thiers et al. 2008). One explanation for this system is that the synapses may locally (without involvement of the central nervous system) coordinate the responses of the OHC population so that optimum operating point is maintained for high frequency transduction. There is some evidence for active regulation of operating point based on the biasing of acoustic emission amplitudes by low frequency tones in which a “hysteresis” was observed (Bian et al, 2004). The hysteresis was thought to result from active motor elements, either in the stereocilia or the lateral wall of the OHC, shifting the transducer function in the direction of the bias. A similar hysteresis was also reported by Lukashkin and Russell (2005) who proposed that a feedback loop was present during the bias that keeps the operating point at its most sensitive region, shifting it in opposite directions during compression and rarefaction phase of the bias tone thereby partially counteracting its effects.
If there are systems in the cochlea to control operating point as an integral component of the amplification process, they would undoubtedly be stimulated in the presence of external infrasound.
v) Vestibular function
The otolith organs, comprising of the saccule and utricle, respond to linear accelerations of the head (Uzun-Coruhlu et al, 2007) and the semi-circular canals respond to angular acceleration. These receptors contribute to the maintenance of balance and equilibrium. In contrast to the hair cells of the cochlea the hair cells of the vestibular organs are tuned to very low frequencies, typically below 30 Hz (Grossman et al, 1988). Frequency tuning in vestibular hair cells results from the electrochemical properties of the cell membranes (Manley, 2000; Art and Fettiplace, 1987) and may also involve active mechanical amplification of their stereociliary input (Hudspeth, 2008; Rabbit et al., 2010). Although vestibular hair cells are maximally sensitive to low frequencies, they typically do not respond to airborne infrasound. Rather, they normally respond to mechanical inputs resulting from head movements and positional changes with their output controlling muscle reflexes to maintain posture and eye position. At the level of the hair cell stereocilia, although vibrations originating from head movements and low frequency sound would be indistinguishable, the difference in sensitivity lies in the coupling between the source stimulus and the hair cell bundle. Head movements are efficiently coupled to the hair cell bundle, while acoustic stimuli are inefficiently coupled due middle ear characteristics and the limited pressure gradients induced within the structure with sound stimuli (Sohmer 2006). In a similar manner to cochlear hair cells, which respond passively (i..e. without active amplification) to stimuli outside their best frequency range, vestibular hair cells respond passively to stimuli outside their best frequency range. The otolith organs have been shown to respond to higher, acoustic frequencies delivered in the form of airborne sounds or vibration. This has been demonstrated in afferent nerve fiber recordings from vestibular nerves (Young et al., 1977; McCue and Guinan, 1994; Curthoys et al., 2006) and has recently gained popularity as a clinical test of otolith function in the form of vestibular-evoked myogenic potential (VEMP) testing (Todd et al, 2003; Zhou and Cox, 2004; Curthoys, 2010). These responses arise because higher frequency stimuli are more effectively coupled to the otolithic hair cells. But as sound or vibration frequency is reduced, its ability to stimulate the vestibular organs diminishes (Murofushi et al., 1999; Hullar et al., 2005; Todd et al, 2008). So for very low frequencies, even though the hair cell sensitivity is increasing as active tuning is invoked, mechanical input is being attenuated. While there have been many studies of vestibular responses to physiologic stimuli (i.e. head accelerations, rotations, etc) comprising of infrasonic frequency components, we are unaware of any studies that have directly investigated vestibular responses to airborne infrasound of similar frequency composition. As people do not become unsteady and the visual field does not blur when exposed to high level infrasound, it can be concluded that sensitivity is extremely low.
In some pathologic conditions, coupling of external infrasound may be greater. It is known that “third window” defects, such as superior canal dehiscence increase the sensitivity of labyrinthine receptors to sounds (Wit et al, 1985; Watson et al., 2000; Carey et al., 2004), and are exhibited as the Tullio phenomenon (see earlier section). To our knowledge, the sensitivity of such patients to controlled levels of infrasound has never been evaluated. In this respect, it needs to be considered that vestibular responses to stimulation could occur at levels below those that are perceptible to the patient. (Todd et al., 2008).
vi) Inner ear fluids changes
Some aspects of cochlear fluids homeostasis have been shown to be sensitive to low frequency pressure fluctuations in the ear. The endolymphatic sinus is a small structure between the saccule and the endolymphatic duct which has been implicated as playing a pivotal role in endolymph volume regulation (Salt 2005). The sinus has been shown to act as a valve, limiting the volume of endolymph driven into the endolymphatic sac by pressure differences across the endolymphatic duct (Salt and Rask-Andersen, 2004). The entrance of saccular endolymph into the endolymphatic sac can be detected either by measuring the K+ concentration in the sac (as saccular endolymph has substantially higher K+ concentration) or by measuring hydrostatic pressure. The application of a sustained pressure to the vestibule did not cause K+ elevation or pressure increase in the sac, confirming that under this condition, flow was prevented by the membrane of the sinus acting as a valve. In contrast, the application of 5 cycles at 0.3 Hz to the external ear canal, caused a K+ increase in the sac, confirming that oscillation of pressure applied to the sinus allowed pulses of endolymph to be driven from the sinus into the endolymphatic sac. The pressure changes driving these pulses was large, comparable to those produced by contractions of the tensor tympani muscle, as occurs during swallowing. Tensor tympani contractions produce displacements of the stapes towards the vestibule for a duration of approximately 0.5 s (~ 2 Hz), which induce large EP changes and longitudinal movements of endolymph within the cochlea (Salt and DeMott, 1999). The lowest sound level that drives endolymph movements is currently unknown.
A therapeutic device (the Meniett: www.meniett.com; Odkvist et al, 2000) that delivers infrasound to the inner ear is widely used to treat Meniere’s disease in humans (a disease characterized by endolymphatic hydrops). The infrasonic stimulus (6 Hz or 9 Hz) is delivered by the device in conjunction with sustained positive pressure in the external canal. An important aspect of this therapy, however, is that a tympanostomy tube is placed in the tympanic membrane before the device is used. The tympanostomy tube provides an open perforation of the tympanic membrane which shunts pressure across the structure, so that ossicular movements (and cochlear stimulation) are minimized, and the pressures are applied directly to the round window membrane. Nevertheless, the therapeutic value of this device is based on infrasound stimulation influencing endolymph volume regulation in the ear.
As presented above, endolymphatic hydrops, by occluding the perilymph communication pathway through the helicotrema, makes the ear more sensitive to infrasound (Salt et al, 2009). It has also been shown that non-damaging low frequency sounds in the acoustic range may themselves cause a transient endolymphatic hydrops (Flock and Flock, 2000; Salt 2004). The mechanism underlying this volume change has not been established and it has never been tested whether stimuli in the infrasound range cause endolymphatic hydrops.
Although infrasound at high levels apparently does not cause direct mechanical damage to the ear (Westin 1975, Jauchem and Cook, 2007) in animal studies it has been found to exacerbate functional and hair cell losses resulting from high level exposures of sounds in the audible range (Harding et al., 2007). This was explained as possibly resulting from increased mixture of endolymph and perilymph around noise induced lesion sites in the presence of infrasound.
Wind turbine Noise
Demonstrating an accurate frequency spectrum of the sound generated by wind turbines creates a number of technical problems. One major factor that makes understanding the effects of wind turbine noise on the ear more difficult is the widespread use of A-weighting to document sound levels. A-weighting shapes the measured spectrum according to the sensitivity of human hearing, corresponding to the IHC responses. As we know the sensitivity for many other elements of inner ear related to the OHC do not decline at the steep slope seen for human hearing, then A-weighting considerably underestimates the likely influence of wind turbine noise on the ear. In this respect, it is notable that in none of the physiological studies in the extensive literature reporting cochlear function at low frequencies were the sound stimuli A-weighted. This is because scientists in these fields realize that shaping sound levels according to what the brain perceives is not relevant to understanding peripheral processes in the ear. A-weighting is also performed for technical reasons, because measuring unweighted spectra of wind turbine noise is technically challenging and suitable instrumentation is not widely available. Most common approaches to document noise levels (conventional sound level meters, video cameras, devices using moving coil microphones, etc) are typically insensitive to the infrasound component. Using appropriate instrumentation, Van den Berg (2006) showed that wind turbine noise was dominated by infrasound components, with energy increasing between 1000 Hz and 1 Hz (the lowest frequency that was measured) at a rate of approximately 5.5 dB/octave, reaching levels of approximately 90 dB SPL near 1 Hz. Sugimoto et al. (2008) reported a dominant spectral peak at 2 Hz with levels monitored over time reaching up to 100 dB SPL. Jung and Cheung (2008) reported a major peak near 1 Hz at a level of approximately 97 dB SPL. In most studies of wind turbine noise, this high level, low frequency noise is dismissed on the basis that the sound is not perceptible. This fails to take into account the fact that the OHC are stimulated at levels that are not heard.
Conclusions
The fact that some inner ear components (such as the OHC) may respond to infrasound at the frequencies and levels generated by wind turbines does not necessarily mean that they will be perceived or disturb function in any way. On the contrary though, if infrasound is affecting cells and structures at levels that cannot be heard this leads to the possibility that wind turbine noise could be influencing function or causing unfamiliar sensations. Long term stimulation of position-stabilizing or fluid homeostasis systems could result in changes that disturb the individual in some way that remains to be established. We realize that some individuals (such as fighter pilots) can be exposed to far higher levels of infrasound without undue adverse effects. In this review, we have confined our discussion to the possible direct influence of infrasound on the body mediated by receptors or homeostatic processes in the inner ear. This does not exclude the possibility that other receptor systems, elsewhere in the body could contribute to the symptoms of some individuals.
The main points of our analysis can be summarized as follows:
Hearing perception, mediated by the inner hair cells of the cochlea, is remarkably insensitive to infrasound.
Other sensory cells or structures in the inner ear, such as the outer hair cells, are more sensitive to infrasound than the inner hair cells and can be stimulated by low frequency sounds at levels below those that are heard. The concept that an infrasonic sound that cannot heard can have no influence on inner ear physiology is incorrect.
Under some clinical conditions, such as Meniere’s disease, superior canal dehiscence, or even asymptomatic cases of endolymphatic hydrops, individuals may be hypersensitive to infrasound.
A-weighting wind turbine sounds underestimates the likely influence of the sound on the ear. A greater effort should be made to document the infrasound component of wind turbine sounds under different conditions.
Based on our understanding of how low frequency sound is processed in the ear, and on reports indicating that wind turbine noise causes greater annoyance than other sounds of similar level and affects the quality of life in sensitive individuals, there is an urgent need for more research directly addressing the physiologic consequences of long-term, low level infrasound exposures on humans.
Acknowledgments
This work was supported by research grant RO1 DC01368 (2005–2010) and KO8 DC 006869 (2004–2010) from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health.
Abbreviations
- CA
cochlear aqueduct
- CM
cochlear microphonic
- CSF
cerebrospinal fluid
- cVEMP
cervical vestibular evoked myogenic potential
- EP
endocochlear potential
- IHC
inner hair cell(s)
- oVEMP
ocular vestibular evoked myogenic potential
- OHC
outer hair cell(s)
- RW
round window
- ST
scala tympani
- SV
scala vestibuli
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Art JJ, Fettiplace R. Variation of membrane properties in hair cells isolated from the turtle cochlea. J Physiol. 1987;385:207–242. doi: 10.1113/jphysiol.1987.sp016492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian L, Linhardt EE, Chertoff ME. Cochlear hysteresis: observation with low-frequency modulated distortion product otoacoustic emissions. J Acoust Soc Am. 2004;115:2159–2172. doi: 10.1121/1.1690081. [DOI] [PubMed] [Google Scholar]
- Bian L, Scherrer NM. Low-frequency modulation of distortion product otoacoustic emissions in humans. J Acoust Soc Am. 2007;122:1681–1692. doi: 10.1121/1.2764467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Wind Energy Association. 2010 http://www.bwea.com/ref/noise.html.
- Brown MC. Antidromic responses of single units from the spiral ganglion. J Neurophysiol. 1994;71:1835–1847. doi: 10.1152/jn.1994.71.5.1835. [DOI] [PubMed] [Google Scholar]
- Brown DJ, Hartsock JJ, Gill RM, Fitzgerald HE, Salt AN. Estimating the operating point of the cochlear transducer using low-frequency biased distortion products. J Acoust Soc Am. 2009;125:2129–2145. doi: 10.1121/1.3083228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JP, Hirvonen TP, Hullar TE, Minor LB. Acoustic responses of vestibular afferents in a model of superior canal dehiscence. Otol Neurotol. 2004;25:345–352. doi: 10.1097/00129492-200405000-00024. [DOI] [PubMed] [Google Scholar]
- Chan DK, Hudspeth AJ. Ca2+ current-driven nonlinear amplification by the mammalian cochlea in vitro. Nat Neurosci. 2005;8:149–155. doi: 10.1038/nn1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatham MA, Dallos P. Low-frequency modulation of inner hair cell and organ of Corti responses in the guinea pig cochlea. Hear Res. 1997;108:191–212. doi: 10.1016/s0378-5955(97)00032-4. [DOI] [PubMed] [Google Scholar]
- Cheatham MA, Dallos P. Inner hair cell response patterns: implications for low-frequency hearing. J Acoust Soc Am. 2001;110:2034–2044. doi: 10.1121/1.1397357. [DOI] [PubMed] [Google Scholar]
- Curthoys IS, Kim J, McPhedran SK, Camp AJ. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res. 2006;175:256–267. doi: 10.1007/s00221-006-0544-1. [DOI] [PubMed] [Google Scholar]
- Curthoys IS. A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin Neurophysiol. 2010;121:132–144. doi: 10.1016/j.clinph.2009.09.027. [DOI] [PubMed] [Google Scholar]
- Dallos P. Low-frequency auditory characteristics: Species dependence. J Acoust Soc Am. 1970;48:489–499. doi: 10.1121/1.1912163. [DOI] [PubMed] [Google Scholar]
- Dallos P, Billone MC, Durrant JD, Wang C, Raynor S. Cochlear inner and outer hair cells: functional differences. Science. 1972;177:356–358. doi: 10.1126/science.177.4046.356. [DOI] [PubMed] [Google Scholar]
- Dallos P. The Auditory Periphery. Academic Press; NY: 1973. pp. 83–126. [Google Scholar]
- Dallos P, Cheatham MA. Production of cochlear potentials by inner and outer hair cells. J Acoust Soc Am. 1976;60:510–512. doi: 10.1121/1.381086. [DOI] [PubMed] [Google Scholar]
- Dallos P. Some electrical circuit properties of the organ of Corti. II. Analysis including reactive elements. Hear Res. 1984;14:281–291. doi: 10.1016/0378-5955(84)90055-8. [DOI] [PubMed] [Google Scholar]
- Dallos P. Cochlear amplification, outer hair cells and prestin. Curr Opin Neurobiol. 2008;18:370–376. doi: 10.1016/j.conb.2008.08.016. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P. Feedback in the cochlea. Hear Res. 2010 Jan 30; doi: 10.1016/j.heares.2009.12.009. [Epub ahead of print] PMID: 20123056. [DOI] [PubMed] [Google Scholar]
- Dancer A, Franke R. Intracochlear sound pressure measurements in guinea pigs. Hear Res. 1980;2:191–205. doi: 10.1016/0378-5955(80)90057-x. [DOI] [PubMed] [Google Scholar]
- Echteler SM, Fay RR, Popper AN. The influence of cochlear shape on low-frequency hearing. In: Fay RR, Popper AN, editors. Comparative Hearing: Mammals. New York: Springer; 1994. pp. 134–171. [Google Scholar]
- Fay RR. Hearing in Vertebrates: A psychophysics databook. Hill-Fay Associates; 1988. pp. 375–378. [Google Scholar]
- Feldmann J, Pitten FA. Effects of low frequency noise on man--a case study. Noise Health. 2004;7:23–28. [PubMed] [Google Scholar]
- Flock A, Flock B. Hydrops in the cochlea can be induced by sound as well as by static pressure. Hear Res. 2000;150:175–188. doi: 10.1016/s0378-5955(00)00198-2. [DOI] [PubMed] [Google Scholar]
- Franke R, Dancer A. Cochlear mechanisms at low frequencies in the guinea pig. Arch Otorhinolaryngol. 1982;234:213–218. doi: 10.1007/BF00453634. [DOI] [PubMed] [Google Scholar]
- Frank G, Kössl M. The acoustic two-tone distortions 2f1–f2 and f2–f1 and their possible relation to changes in the operating point of the cochlear amplifier. Hear Res. 1996;98:104–115. doi: 10.1016/0378-5955(96)00083-4. [DOI] [PubMed] [Google Scholar]
- Grossman GE, Leigh RJ, Abel LA, Lanska DJ, Thurston SE. Frequency and velocity of rotational head perturbations during locomotion. Exp Brain Res. 1988;70:470–476. doi: 10.1007/BF00247595. [DOI] [PubMed] [Google Scholar]
- Harding GW, Bohne BA, Lee SC, Salt AN. Effect of infrasound on cochlear damage from exposure to a 4 kHz octave band of noise. Hear Res. 2007;225:128–138. doi: 10.1016/j.heares.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry A. Wind turbines, noise and health. 2007 www.windturbinenoisehealthhumanrights.com/wtnoise_health_2007_a_barry.pdf.
- Heffner R, Heffner H, Masterton B. Behavioral measurements of absolute and frequency-difference thresholds in guinea pig. J Acoust Soc Am. 1971;49:1888–1895. doi: 10.1121/1.1912596. [DOI] [PubMed] [Google Scholar]
- Hensel J, Scholz G, Hurttig U, Mrowinski D, Janssen T. Impact of infrasound on the human cochlea. Hear Res. 2007;233:67–76. doi: 10.1016/j.heares.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ. Making an effort to listen: mechanical amplification in the ear. Neuron. 2008;59:530–545. doi: 10.1016/j.neuron.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullar TE, Della Santina CC, Hirvonen T, Lasker DM, Carey JP, Minor LB. Responses of irregularly discharging chinchilla semicircular canal vestibular-nerve afferents during high-frequency head rotations. J Neurophysiol. 2005;93:2777–2786. doi: 10.1152/jn.01002.2004. [DOI] [PubMed] [Google Scholar]
- ISO226:2003 Normal equal loudness level contours. International Standards Organization; Genéve: [Google Scholar]
- Jauchem JR, Cook MC. High-intensity acoustics for military nonlethal applications: a lack of useful systems. Mil Med. 2007;172:182–189. doi: 10.7205/milmed.172.2.182. [DOI] [PubMed] [Google Scholar]
- Jung SS, Cheung W. Experimental identification of acoustic emission characteristics of large wind turbines with emphasis on infrasound and low-frequency noise. J Korean Physic Soc. 2008;53:1897–1905. [Google Scholar]
- Kennedy HJ, Evans MG, Crawford AC, Fettiplace R. Fast adaptation of mechanoelectrical transducer channels in mammalian cochlear hair cells. Nat Neurosci. 2003;6:832–836. doi: 10.1038/nn1089. [DOI] [PubMed] [Google Scholar]
- Kimura RS. The ultrastructure of the organ of Corti. Int Rev Cytol. 1975;42:173–222. doi: 10.1016/s0074-7696(08)60981-x. [DOI] [PubMed] [Google Scholar]
- Kros CJ. Physiology of mammalian cochlear hair cells. In: EDs Dallos P, Popper AN, Fay RR, editors. The Cochlea. Springer Press; New York: 1996. pp. 318–385. [Google Scholar]
- LePage EL. Frequency-dependent self-induced bias of the basilar membrane and its potential for controlling sensitivity and tuning in the mammalian cochlea. J Acoust Soc Am. 1987;82:139–154. doi: 10.1121/1.395557. [DOI] [PubMed] [Google Scholar]
- LePage EL. Functional role of the olivo-cochlear bundle: a motor unit control system in the mammalian cochlea. Hear Res. 1989;38:177–198. doi: 10.1016/0378-5955(89)90064-6. [DOI] [PubMed] [Google Scholar]
- Leventhall HG. Low frequency noise. What we know, what we do not know and what we would like to know. Low Frequency Noise, Vibration and Active Control. 2009;28:79–104. [Google Scholar]
- Lim DJ. Functional structure of the organ of Corti:a reviw. Hear Res. 1986;22:117–146. doi: 10.1016/0378-5955(86)90089-4. [DOI] [PubMed] [Google Scholar]
- Lonsbury-Martin BL, Harris FP, Stagner BB, Hawkins MD, Martin GK. Distortion product emissions in humans. I. Basic properties in normally hearing subjects. Ann Otol Rhinol Laryngol Suppl. 1990;147:3–14. [PubMed] [Google Scholar]
- Lukashkin AN, Russell IJ. Dependence of the DPOAE amplitude pattern on acoustical biasing of the cochlear partition. Hear Res. 2005;203:45–53. doi: 10.1016/j.heares.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Manley GA. Cochlear mechanisms from a phylogenetic viewpoint. Proc Natl Acad Sci U S A. 2000;97:11736–11743. doi: 10.1073/pnas.97.22.11736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T, Hensel J, Mrowinski D, Scholz G. Low-frequency characteristics of human and guinea pig cochleae. J Acoust Soc Am. 2007;121:3628–3638. doi: 10.1121/1.2722506. [DOI] [PubMed] [Google Scholar]
- McCue MP, Guinan JJ., Jr Acoustically responsive fibers in the vestibular nerve of the cat. J Neurosci. 1994;14:6058–600. doi: 10.1523/JNEUROSCI.14-10-06058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant SN, Adams JC, Nadol JB., Jr Pathophysiology of Meniere’s syndrome: are symptoms caused by endolymphatic hydrops? Otol Neurotol. 2005;26:74–81. doi: 10.1097/00129492-200501000-00013. [DOI] [PubMed] [Google Scholar]
- Merchant SN, Rosowski JJ. Conductive hearing loss caused by third-window lesions of the inner ear. Otol Neurotol. 2008;29:282–289. doi: 10.1097/mao.0b013e318161ab24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Murray FS. Guinea pig’s immobility response to sound: threshold and habituation. J Comp Physiol Psychol. 1966;61:227–233. doi: 10.1037/h0023135. [DOI] [PubMed] [Google Scholar]
- Møller H, Pederson CS. Hearing at low and infrasonic frequencies. Noise and Health. 2004;6:37–57. [PubMed] [Google Scholar]
- Mountain DC, Hubbard AE, McMullen TA. Electromechanical processes in the cochlea. In: de Boer E, Viergever MA, editors. Mechanics of Hearing. Delft University press; Delft, The Netherlands: 1983. pp. 119–126. [Google Scholar]
- Murofushi T, Matsuzaki M, Wu CH. Short tone burst-evoked myogenic potentials on the sternocleidomastoid muscle: are these potentials also of vestibular origin? Arch Otolaryngol Head Neck Surg. 1999;125:660–664. doi: 10.1001/archotol.125.6.660. [DOI] [PubMed] [Google Scholar]
- Nakajima HH, Dong W, Olson S, Merchant SN, Ravicz ME, Rosowski JJ. Differential intracochlear sound pressure measurements in normal human temporal bones. J Assoc Res Otolaryngol. 2008;10:23–36. doi: 10.1007/s10162-008-0150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny M, Gummer AW. Nanomechanics of the subtectorial space caused by electromechanics of cochlear outer hair cells. Proc Natl Acad Sci U S A. 2006;103:2120–2125. doi: 10.1073/pnas.0511125103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall AL, Brown MC, Masta RI, Lawrence M. Inner hair cell responses to the velocity of basilar membrane motion in the guinea pig. Brain Res. 1981;211:171–174. doi: 10.1016/0006-8993(81)90078-0. [DOI] [PubMed] [Google Scholar]
- Odkvist LM, Arlinger S, Billermark E, Densert B, Lindholm S, Wallqvist J. Effects of middle ear pressure changes on clinical symptoms in patients with Ménière’s disease--a clinical multicentre placebo-controlled study. Acta Otolaryngol Suppl. 2000;543:99–101. [PubMed] [Google Scholar]
- Patuzzi R, Sellick PM, Johnstone BM. The modulation of the sensitivity of the mammalian cochlea by low frequency tones. III. Basilar membrane motion. Hear Res. 1984;13:19–27. doi: 10.1016/0378-5955(84)90091-1. [DOI] [PubMed] [Google Scholar]
- Patuzzi R, Moleirinho A. Automatic monitoring of mechano-electrical transduction in the guinea pig cochlea. Hear Res. 1998;125(1–2):1–16. doi: 10.1016/s0378-5955(98)00125-7. [DOI] [PubMed] [Google Scholar]
- Patuzzi RB, O’Beirne GA. Boltzmann analysis of CM waveforms using virtual instrument software. Hear Res. 1999;133:155–159. doi: 10.1016/s0378-5955(99)00067-2. [DOI] [PubMed] [Google Scholar]
- Pedersen E, van den Berg F, Bakker R, Bouma J. Response to noise from modern wind farms in The Netherlands. J Acoust Soc Am. 2009;126:634–643. doi: 10.1121/1.3160293. [DOI] [PubMed] [Google Scholar]
- Pedersen E, Waye KP. Perception and annoyance due to wind turbine noise--a dose-response relationship. J Acoust Soc Am. 2004;116:3460–3470. doi: 10.1121/1.1815091. [DOI] [PubMed] [Google Scholar]
- Pedersen A, Persson Waye K. Wind turbine noise, annoyance and self-reported health and well-being in different living environments. Occup Environ Med. 2007;64:480–486. doi: 10.1136/oem.2006.031039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpont N. Wind turbine syndrome. K-selected books. 2009 http://www.kselected.com/?page_id=6560.
- Prosen CA, Petersen MR, Moody DB, Stebbins WC. Auditory thresholds and kanamycin-induced hearing loss in the guinea pig assessed by a positive reinforcement procedure. J Acoust Soc Am. 1978;63:559–566. doi: 10.1121/1.381754. [DOI] [PubMed] [Google Scholar]
- Rabbitt RD, Boyle R, Highstein SM. Mechanical amplification by hair cells in the semicircular canals. Proc Natl Acad Sci U S A. 2010;107:3864–3869. doi: 10.1073/pnas.0906765107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Sellick PM, Patuzzi R. The continuing search for outer hair cell afferents in the guinea pig spiral ganglion. Hear Res. 1999;136:151–158. doi: 10.1016/s0378-5955(99)00120-3. [DOI] [PubMed] [Google Scholar]
- Russell IJ, Sellick PM. Low-frequency characteristics of intracellularly recorded receptor potentials in guinea-pig cochlear hair cells. J Physiol. 1983;338:179–206. doi: 10.1113/jphysiol.1983.sp014668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt AN, DeMott JE. Longitudinal endolymph movements and endocochlear potential changes induced by stimulation at infrasonic frequencies. J Acoust Soc Am. 1999;106:847–856. doi: 10.1121/1.427101. [DOI] [PubMed] [Google Scholar]
- Salt AN, Rask-Andersen H. Responses of the endolymphatic sac to perilymphatic injections and withdrawals: evidence for the presence of a one-way valve. Hear Res. 2004;191:90–100. doi: 10.1016/j.heares.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Salt AN. Low frequency pressure changes may participate in endolymph volume regulation. In: Lim DJ, editor. Meniere’s Disease and Inner Ear Homeostasis Disorders. House Ear Institute Press; Los Angeles: 2005. pp. 27–29. [Google Scholar]
- Salt AN. Acute endolymphatic hydrops generated by exposure of the ear to non-traumatic low frequency tone. J Assoc Res Otolaryngol. 2004;5:203–214. doi: 10.1007/s10162-003-4032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt AN, Brown DJ, Hartsock JJ, Plontke SK. Displacements of the organ of Corti by gel injections into the cochlear apex. Hear Res. 2009;250:63–75. doi: 10.1016/j.heares.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi PA, Tsuprun VL. Cochlear microanatomy and ultrastructure. In: Jahn AF, Santos-Sacchi J, editors. Physiology of the Ear. 2. Singular; 2001. pp. 257–283. [Google Scholar]
- Sellick PM, Patuzzi R, Johnstone BM. Modulation of responses of spiral ganglion cells in the guinea pig cochlea by low frequency sound. Hear Res. 1982;7:199–221. doi: 10.1016/0378-5955(82)90014-4. [DOI] [PubMed] [Google Scholar]
- Sellick PM, Robertson D, Patuzzi R. The effect of BAPTA and 4AP in scala media on transduction and cochlear gain. Hear Res. 2006;211:7–15. doi: 10.1016/j.heares.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Sirjani DB, Salt AN, Gill RM, Hale SA. The influence of transducer operating point on distortion generation in the cochlea. J Acoust Soc Amer. 2004;115:1219–1229. doi: 10.1121/1.1647479. [DOI] [PubMed] [Google Scholar]
- Sohmer H. Sound induced fluid pressures directly activate vestibular hair cells: implications for activation of the cochlea. Clin Neurophysiol. 2006;117:933–934. doi: 10.1016/j.clinph.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Innervation Densities of the Cochlea Acta Oto-laryngologica. 1972;73:235–248. doi: 10.3109/00016487209138937. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Koyama K, Kurihara Y, Watanabe K. Measurement of infrasound generated by wind turbine generator. Proc. SICE Conference; 2008. pp. 5–8. [Google Scholar]
- Thiers FA, Nadol JB, Jr, Liberman MC. Reciprocal synapses between outer hair cells and their afferent terminals: evidence for a local neural network in the mammalian cochlea. J Assoc Res Otolaryngol. 2008;9:477–489. doi: 10.1007/s10162-008-0135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd NP, Rosengren SM, Colebatch JG. A short latency vestibular evoked potential (VsEP) produced by bone-conducted acoustic stimulation. J Acoust Soc Am. 2003;114:3264–3272. doi: 10.1121/1.1628249. [DOI] [PubMed] [Google Scholar]
- Todd NP, Rosengren SM, Colebatch JG. Tuning and sensitivity of the human vestibular system to low-frequency vibration. Neurosci Lett. 2008;444:36–41. doi: 10.1016/j.neulet.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Uzun-Coruhlu H, Curthoys IS, Jones AS. Attachment of the utricular and saccular maculae to the temporal bone. Hear Res. 2007;233:77–85. doi: 10.1016/j.heares.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Van den Berg GP. PhD Dissertation. University of Groningen; Netherlands: The sound of high winds: the effect of atmospheric stability on wind turbine sound and microphone noise. http://dissertations.ub.rug.nl/faculties/science/2006/g.p.van.den.berg/ [Google Scholar]
- Von Békésy G. Experiments in Hearing. McGraw-Hill; New York: 1960. [Google Scholar]
- Walloch RA, Taylor-Spikes M. Auditory thresholds in the guinea pig: a preliminary report of a behavioral technique employing a food reward. Laryngoscope. 1976;86:1699–1705. doi: 10.1288/00005537-197611000-00012. [DOI] [PubMed] [Google Scholar]
- Watson SR, Halmagyi GM, Colebatch JG. Vestibular hypersensitivity to sound (Tullio phenomenon): structural and functional assessment. Neurology. 2000;54:722–728. doi: 10.1212/wnl.54.3.722. [DOI] [PubMed] [Google Scholar]
- West CD. The relationship of the spiral turns of the cochlea and the length of the basilar membrane to the range of audible frequencies in ground dwelling mammals. J Acoust Soc Am. 1985;77:1091–1101. doi: 10.1121/1.392227. [DOI] [PubMed] [Google Scholar]
- Westin JB. Infrasound: A short review of effects on man. Aviat Space Environ Med. 1975;46:1135–1143. [PubMed] [Google Scholar]
- Wilson JP, Johnstone JR. Basilar membrane and middle-ear vibration in guinea pig measured by capacitive probe. J Acoust Soc Am. 1975;57:705–723. doi: 10.1121/1.380472. [DOI] [PubMed] [Google Scholar]
- Wit HP, Scheurink AJ, Bleeker JD. Hearing thresholds of normal and fenestrated deaf pigeons. A behavioural study on hearing with the vestibular organ. Acta Otolaryngol. 1985;100:36–41. doi: 10.3109/00016488509108585. [DOI] [PubMed] [Google Scholar]
- Young ED, Fernández C, Goldberg JM. Responses of squirrel monkey vestibular neurons to audio-frequency sound and head vibration. Acta Otolaryngol. 1977;84:352–360. doi: 10.3109/00016487709123977. [DOI] [PubMed] [Google Scholar]
- Zhou G, Cox LC. Vestibular evoked myogenic potentials: history and overview. Am J Audiol. 2004;13:135–143. doi: 10.1044/1059-0889(2004/018). [DOI] [PubMed] [Google Scholar]
- Zwicker E. Masking-period patterns produced by very-low-frequency maskers and their possible relation to basilar-membrane displacement. J Acoust Soc Am. 1977;61:1031–1040. doi: 10.1121/1.381387. [DOI] [PubMed] [Google Scholar]