Abstract
Background & Aims
Although abnormal hepatic methionine metabolism plays a central role in the pathogenesis of experimental alcoholic liver disease (ALD), its relationship to the risk and severity of clinical ALD is not known. The aim of this clinical study was to determine the relationship between serum levels of methionine metabolites in chronic alcoholics and the risk and pathological severity of ALD.
Methods
Serum levels of liver function biochemical markers, vitamin B6, vitamin B12, folate, homocysteine, methionine, S-adenosylmethionine, S-adenosylhomocysteine, cystathionine, cysteine, α-aminobutyrate, glycine, serine, and dimethylglycine were measured in 40 ALD patients, of whom 24 had liver biopsies, 26 were active drinkers without liver disease, and 28 were healthy subjects.
Results
Serum homocysteine was elevated in all alcoholics, whereas ALD patients had low vitamin B6 with elevated cystathionine and decreased α-aminobutyrate/cystathionine ratios, consistent with decreased activity of vitamin B6 dependent cystathionase. The α-aminobutyrate/cystathionine ratio predicted the presence of ALD, while cystathionine correlated with the stage of fibrosis in all ALD patients.
Conclusions
The predictive role of the α- aminobutyrate/cystathionine ratio for the presence of ALD and the correlation between cystathionine serum levels with the severity of fibrosis point to the importance of the homocysteine transsulfuration pathway in ALD and may have important diagnostic and therapeutic implications.
Keywords: alcohol, methionine, cystathionine, vitamin B6
INTRODUCTION
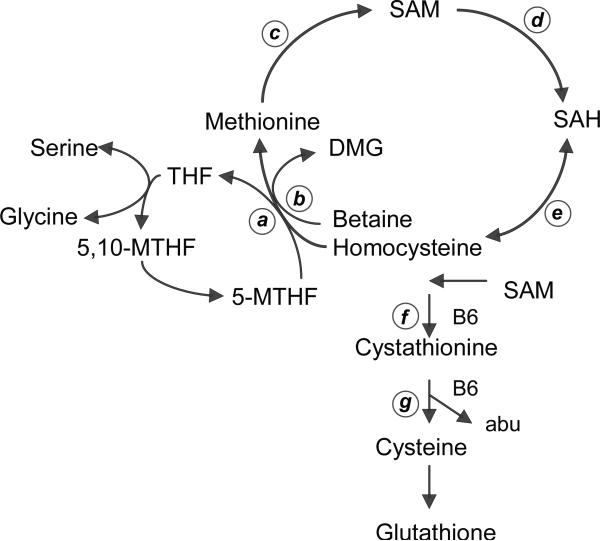
Multiple interacting pathways that include aberrant methionine metabolism contribute to the development and progression of alcoholic liver disease (ALD) (Fig. 1). Chronic ethanol administration to experimental animals increases levels of plasma homocysteine (HCY), reduces liver S-adenosylmethionine (SAM) and increases S-adenosylhomocysteine (SAH) [1]. Acetaldehyde induced inhibition of methionine synthase (MS) activity is associated with increased activity of betaine homocysteine methyltransferase (BHMT), which uses betaine as a substrate to methylate HCY to methionine and dimethylglycine (DMG) [2]. Reactive oxygen species generated by ethanol metabolism impairs the expression of methionine adenosyl transferase (MAT), with consequent reduced levels of SAM [3], which is the universal substrate for methyltransferase reactions that generates SAH, an inhibitor of methylation. SAH is also a substrate for the bidirectional enzyme SAH hydrolase (SAHH) that can both regenerate HCY, or can enhance levels of SAH when HCY is elevated [1]. HCY is mainly reduced through the transsulfuration pathway that includes two vitamin B6 dependent enzymes, cystathionine-β-synthase (CβS) and γ-cystathionase, ultimately producing the antioxidant glutathione. α-Aminobutyrate (ABU) is produced during the conversion of cystathionine (CYSTAT) to cysteine as a byproduct of the transsulfuration pathway [1]. Since SAM facilitates the CβS reaction, reduced liver SAM levels are associated with decreased production of glutathione [4] [5]. Reduced SAM and elevated SAH predictably reduce the potential for methylation reactions, potentially contributing to the activation of many genes relevant to liver injury [5]. Ethanol-induced increases in hepatic SAH also potentiates pro-inflammatory cytokine TNFα and cell death in ethanol-fed mice [6]. The central role of aberrant methionine metabolism in the pathogenesis of alcoholic steatohepatitis was underscored by findings that its pathology and many of its mechanisms can be prevented by the addition of SAM to folate deficient and ethanol containing diets in a micropig model [7,8].
Fig. 1. Methionine metabolism.
Homocysteine in the liver is converted to methionine by two reactions: methionine synthase (MS) (a) and betaine homocysteine methionine transferase (BHMT) (b). 5-methyltetrahydrofolate (MTHF) is a substrate for MS which produces tetrahydrofolate (THF). BHMT uses betaine as a substrate with production of dimethylglycine (DMG). Methionine is converted by methionine adenosyltransferase (MAT) (c) to S-adenosylmethionine (SAM), which is irreversibly converted to S-adenosylhomocysteine (SAH) by donating its methyl moiety to DNA methyltransferases (DNMTs) (d). SAH hydrolase (e) regulates the bi-directional reaction that leads to the synthesis of homocysteine from SAH or vice versa. The transsulfuration pathway is regulated by the vitamin B6-dependent enzymes cystathionine β synthase (f) and cystathionase (g) which both catalyze irreversible reactions. Alpha-aminobutyric acid (abu) is the collateral product of the reaction catalyzed by cystathionase.
The goal of our study was to identify changes in serum methionine metabolites predictive of clinical ALD. We hypothesized that profiles of serum methionine metabolites are influenced by alcohol consumption and may predict and correlate with the severity of hepatic histopathology in ALD. We compared serum parameters of hepatic methionine metabolism in ALD patients, in active drinkers without evidence of liver disease, and in healthy subjects.
PATIENTS AND METHODS
Patients
Forty patients with clinical and biochemical features of ALD provided data for the present study, including 24 who had liver biopsies within one week of enrollment. The reasons for inability to obtain the liver biopsy in 16 patients included ascites or coagulopathy (8 cases) and patient refusal (8 cases). The enrolled patients were gender matched with 26 active drinkers (AD) without clinical evidence of liver disease and were age- and gender-matched with 28 healthy subjects (HS). The diagnosis of chronic alcoholism was based on criteria from the Diagnostic and Statistical Manual of Mental Disorders (fourth edition) and the World Health Organization [9]. Patients were categorized according to the Child score (based on levels of bilirubin, albumin, international normalized ratio (INR), severity of ascites and hepatic encephalopathy) [10] and to the model for end-stage liver disease (MELD) score (based on levels of INR, bilirubin, and creatinine) [11]. Exclusion criteria included Child C cirrhosis, insufficient history for alcohol abuse, and laboratory testing consistent with viral or autoimmune hepatitis, Wilson disease, or hemochromatosis. The entry criteria for AD subjects included a history of chronic alcoholism without clinical or biochemical stigmata of chronic liver disease or other significant illness. Inclusion in the HS group required drinking on average less than two drinks per day (men) or one drink per day (women), normal liver biochemistry, and absence of significant illness. All subjects were enrolled from the University of California Davis Health Systems Hepatology clinic, Emergency Department, Primary Care Network clinics, or by flyers posted in the community. The study was performed at the University of California Davis Clinical and Translational Science Center (CTSC) Clinical Research Center (CCRC). The Institutional Review Board approved the protocol (# 200311168-7), and all patients provided written informed consent. The study was conducted according to the guidelines of the Declaration of Helsinki under provisions of “Good Clinical Practices” as defined in the U.S. Code of Federal Regulations on the Protection of Human Subjects for the United States.
Laboratory evaluations
Fasting venous blood was used for determinations of complete blood count, serum AST, ALT, bilirubin, alkaline phosphatase, albumin, INR, creatinine, folate and vitamin B12. Vitamin B6 was measured as pyridoxal-5’-phosphate by co-author J.F.G. as the semicarbazone-derivative by reverse-phase HPLC with fluorescence detection [12]. Cytokines TNFα, Interleukin 6 (IL-6), and IL-10 were measured using high sensitivity cytokine ELISA reactions (R&D Systems, Minneapolis, Minnesota). Patients were screened for alcohol use by the modified measurement of serum carbohydrate deficient transferrin (%CDT) turbidimetric immunoassay (Bio-Rad Laboratories, Inc. Hercules, California) [13]. For measurements of serum methionine metabolites, about 4 cc of blood were clotted in a serum separator tube for 15 minutes at room temperature, then placed in ice until centrifugation at 3,000 rpm for 15 minutes within 30 minutes of venipuncture. Serum was removed and stored at –80°C, until sent to Metabolite Laboratories, University of Colorado Health Sciences Center for measurements by co-author S.P.S. of serum SAM, SAH, HCY, cystathionine, cysteine, methionine, ABU, DMG, serine, and glycine using stable isotope dilution gas or liquid chromatography/mass spectrometry [14].
Liver histopathology
Liver biopsies were obtained percutaneously from ALD patients using a 16G Jamshidi needle and ultrasound guidance. Safety criteria required INR <1.5 and a platelet count >50,000/mm3. Biopsies were considered valid for the analysis if they were >15 mm in length, 1.4 mm wide, and contained more than five portal tracts. Each biopsy specimen was fixed in formalin and shipped to co-author S.W.F. for scoring. The liver biopsy grading was blinded using a Nikon E400 microscope equipped with a digital camera and Nikon Metomorph computer software (Molecular Devices Corporation, Downingtown, Pennsylvania) to make quantitative morphometric measurements of abnormalities per square millimeter of biopsy surface. Using published criteria [15] a quantitative score was applied to each of the following parameters: steatosis (four-point scale: grade 0: steatosis involving <10% of hepatocytes; grade 1: steatosis up to 30%; grade 2: steatosis between 30 and 60%; and grade 3: steatosis >60%) , fibrosis by Sirius red stain (five-point scale, stage 0: no fibrosis; stage 1: zone 3 perisinusoidal fibrosis; stage 2: perisinusoidal fibrosis and periportal fibrosis; stage 3: focal or extensive bridging fibrosis; stage 4: cirrhosis), mononuclear and polymorphonuclear inflammation (four-point scale based on inflammatory foci per 20x: grade 0; 1 with 1-2 foci; 2 with 3-4 foci; 3 with > 4 foci). Mallory-Denk hyaline bodies were counted in 10 fields (× 20 magnification) to determine the average number per field.
Statistical analysis
Descriptive characteristics were calculated for all variables for ALD patients, AD, and HS groups. Variables that were not normally distributed were transformed as appropriate prior to analysis. If no suitable transformation was found, non-parametric analysis was used. The data from all groups were analyzed for differences using analysis of variance (ANOVA). Correlations were made among biochemical parameters, clinical Child and MELD scores, and histology scores using multiple variable analysis. Logistic regression was used to identify predictors of liver disease among all alcoholics with and without liver disease. All analyses were conducted with SAS v. 8.2 or higher (Cary, North Carolina). With 40 ALD, more than 20 AD, and more than 20 HS evaluable subjects, the study would have at least an 80% power at an α-value of 0.05 (two-tailed) to detect a significant difference in methionine metabolites varying between groups.
RESULTS
Clinical features
As shown in Table 1, AD subjects were younger than ALD and HS. The prevalent ethnicity was Caucasian, followed by African American, Hispanic, Asian, and Native American. The mean body mass index (BMI) (weight in kg per height in m2) of all subjects was similar in the three groups. Twenty-one ALD patients were actively drinking at the time of the enrollment with the last drink 1 to 7 days prior to enrollment, and the remainder had been sober for more than 8 days. The weekly historical average of drinks for patients with ALD was 59.6 ± 44.1 (mean ± SD) and for AD was 40.2 ± 12.9, corresponding to 99.6 ± 75 and 69 ± 22 grams of daily alcohol, respectively (n.s.). Seventeen (42.5%) ALD patients used over the counter multivitamins, 10 (25%) used folic acid, and 5 (12.5%) thiamine supplements.
Table 1. Demographic features of patients with alcoholic liver disease (ALD), active drinkers without liver disease (AD), and healthy subjects (HS).
In this and all subsequent tables, the data are presented as median (range) or percentage. Values without a common letter are significantly different (for example: a vs a or b vs b: not statistically different; a vs b: statistically different; a, b vs a or a, b vs b: not statistically different).
| Alcoholic Liver Disease (ALD) (n = 40) | Active drinkers w/o liver disease (AD) (n = 26) | Healthy Subjects (HS) (n = 28) | Overall p value | |
|---|---|---|---|---|
| Age | 46.5 (34-67)a | 41 (23-66)b | 45.5 (31-59)a | < .05 |
| Gender (F/M) | 12/28 | 4/22 | 10/18 | |
| BMI (kg/m2) | 25.8 (29-17.8) | 26.4 (21-30) | 26.6 (20-28) | 0.8 |
| Duration of abstinence (days) | 8 (0-447) | 1 (0-7) | n/a | 0.09 |
| Age at onset of heavy drinking | 20 (10-62) | 17 (13-30)b | n/a | < .05 |
| Duration of drinking (years) | 23.5 (2-48) | 25 (2-40) | n/a | 0.4 |
| AUDIT score | 15.5 (8-24) | 13 (8-21) | n/a | 0.2 |
| Ethnicity | ||||
|---|---|---|---|---|
| Caucasian | 27 (67.5%) | 15 (57.7%) | 16 (57.1%) | 0.1 |
| African-American | 4 (10%) | 8 (30.7%) | 2 (7.1%) | |
| Hispanic | 6 (15%) | 2 (7.6%) | 5 (18%) | |
| Asian | 2 (5%) | 0 | 3 (10.7%) | |
| Native American | 1 (2.5%) | 1 (4%) | 2 (7.1%) | |
Clinical parameters
There were no differences in biochemical parameters, vitamin levels, or methionine metabolite levels between ALD patients who did or did not undergo liver biopsies. Serum AST, ALT, bilirubin, alkaline phosphatase, and INR were higher in the ALD group than the other groups (Table 2), and the median AST/ALT ratio in the ALD patients was 1.72. ALD patients had a mean MELD score of 11 ± 5.2 and Child score of 6.7 ± 1.6, distributed as 19 patients in Child class A and 21 in Child class B. The serum % CDT, an index of recent alcohol consumption, was similar and elevated in all ALD patients and in AD compared to HS, and was similar in ALD subjects who were actively drinking and who were abstinent at the time of the enrollment, confirming its limited value in the presence of ALD [16]. Serum folate levels were within normal range, but lower in ALD and AD compared to HS. Vitamin B12 levels were higher in ALD than in AD and HS. Vitamin B6 levels were lower in ALD than in AD and HS.
Table 2. Clinical laboratory parameters.
INR: International Normalized ratio; % CDT: percentage of carbohydrate deficient transferrin.
| Alcoholic Liver Disease (ALD) (n = 40) | Active drinkers w/o liver disease (AD) (n = 26) | Healthy Subjects (HS) (n = 28) | Overall p value | |
|---|---|---|---|---|
| WBC K/mm3 [4.5-11] | 6.3 (3.6-15.4) | 6.1 (4-9.5) | 5.7 (4.5-10.4) | 0.06 |
| Hb mg/dL [12-16] | 13 (9.2-17.2)a | 14 (11-16.5)b | 14.5 (11.8-17.3)b | <.02 |
| Platelets K/mm3 [130-400] | 206 (55-403)a | 254 (167-422)b | 242 (167-449)a, b | <.02 |
| AST U/L [15-37] | 70.5 (47-350)a | 28 (15-37)b | 26 (14-37)b | <.0001 |
| ALT U/L [5-37] | 38 (14-211)a | 24 (14-33)b | 26 (12-36)b | <.0001 |
| Total bilirubin mg/dL [0.3-1.3] | 1.4 (0.5-7.3)a | 0.7 (0.5-1.3)b | 1 (0.6-1.3)b | <.0001 |
| Alkaline Phosphatase U/L [35-115] | 121 (52-425)a | 62 (44-89)b | 62 (34-99)b | <.0001 |
| Albumin g/dL [3.2-4.6] | 3.4 (2.4-4.3)a | 4 (3.4-4.7)b | 4 (3.1-4.5)b | <.0001 |
| INR [0.75-1.19] | 1.1 (0.9-1.8)a | 0.9 (0.8-1.1)b | 1 (0.9-1.1)b | <.0001 |
| Creatinine mg/dL [0.44-1.4] | 0.8 (0.5-1.39) | 0.9 (0.62-1.2) | 0.9 (0.6-1.2) | 0.06 |
| %CDT | 3.9 (1.9-8.7)a | 3.8 (1.6-6.9)a | 2.6 (1.4-3.8)b | <.02 |
| Folate ng/mL [3.5-16.1] | 16.5 (0.4-20)a | 14.8 (4.8-20)a | 20 (9-20)b | <.05 |
| Vitamin B12 pg/mL [200-600] | 667 (288-1733)a | 473 (220-2000)b | 505 (322-1850)b | <.02 |
| Vitamin B6 nmol/L [15-120] | 44.5 (8.6-187.4)a | 71.7 (22.4-195.8)b | 83.6 (15-299)b | <.02 |
Serum methionine metabolites
Serum HCY levels were similar in ALD and AD and higher than in HS, while SAH levels were higher in ALD patients than in HS (Table 3). Values for HCY and SAH levels were positively correlated among all subjects (r = 0.29, p = 0.005). Serum SAM levels were higher in ALD patients compared to AD and HS, and there were no differences in the SAM/SAH ratio among the groups. Serum methionine and cysteine were marginally increased in ALD and serine and glycine were similar in all groups. Serum CYSTAT, the substrate for cystathionase (Fig. 1), was increased by more than 2-fold in ALD, while ABU was moderately decreased and the ABU/CYSTAT ratio was decreased by more than 3-fold in ALD, consistent with reduction in cystathionase activity. DMG, a byproduct of BHMT (Fig. 1), was increased in ALD patients, consistent with activation of the BHMT alternate pathway of HCY transmethylation.
Table 3. Serum methionine metabolites and vitamin levels.
HCY: homocysteine; SAH: S-adenosylhomocysteine; SAM: S-adenosylmethionine; CYSTAT: cystathionine; ABU: α-aminobutyrate; DMG: dimethylglycine.
| Alcoholic Liver Disease (ALD) (n = 40) | Active drinkers w/o liver disease (AD) (n = 26) | Healthy Subjects (HS) (n = 28) | Overall p value | |
|---|---|---|---|---|
| HCY μmol/L | 10.2 (5.4-58.3)a | 8.8 (5.8-23)a | 6.4 (4.1-10)b | <.001 |
| SAH nmol/L | 33 (15-142)a | 26 (13-74)a, b | 20 (14-64)b | <.02 |
| SAM nmol/L | 120 (63-328)a | 96 (49-341)b | 88.5 (72-160)b | <.02 |
| SAM/SAH | 4.2 (0.4-9.2) | 4 (1.2-10.3) | 4.1 (1.2-7) | 0.9 |
| Methionine μmol/L | 30 (11.4-77.2)a | 24 (10.7-45.8)b | 25.1 (17-35.4)b | <.02 |
| CYSTAT nmol/L | 300 (88-1786)a | 111 (72-944)b | 135 (86-538)b | <.001 |
| Cysteine μmol/L | 352 (231-528)a | 318 (259-404)a, b | 319 (255-383)b | <.05 |
| ABU μmol/L | 11.3 (3.9-38.2)a | 20.4 (6.1-67.8)b | 16.1 (7.4-31.8)b | <.05 |
| ABU/CYSTAT | 0.03 (0.005-0.22)a | 0.13 (0.01-0.5)b | 0.1 (0.02-0.2)b | <.001 |
| DMG μmol/L | 4.2 (2.3-89.4)a | 3.5 (1.7-5.6)b | 3.1 (1.7-7.6)b | <.001 |
| Serine μmol/L | 100 (51-168) | 94 (59-119) | 96.5 (64-134) | 0.43 |
| Glycine μmol/L | 200 (120-341) | 202 (115-305) | 200 (158-314) | 0.73 |
Multivariate analysis indicated these metabolite levels were not influenced by use of supplemental multivitamins containing vitamin B6 or folic acid.
HCY and SAH levels were positively correlated with AST (r = 0.44, p <0.0001 and r = 0.27, p = 0.01, respectively) and alkaline phosphatase levels (r = 0.37, p = 0.0002 and r = 0.4, p = 0.0003, respectively). CYSTAT levels were positively correlated with AST (r = 0.4, p <0.0001), bilirubin (r = 0.57, p <0.0001), alkaline phosphatase (r = 0.57, p <0.0001), and INR (r = 0.53, p <0.0001), as well as MELD and Child scores (r = 0.66, p <0.0001, and r = 0.6, p <0.0001). Levels of vitamin B6 correlated negatively with levels of serum AST (r = -0.37, p <0.006) and correlated negatively with CYSTAT levels (r = -0.24, p = 0.02) and positively with the ABU/CYSTAT ratio (r = 0.37, p = 0.0006).
Cytokine levels
There were no differences among the three groups in values for IL-6 and TNFα, while IL-10 levels were lower in ALD (Table 4).
Table 4.
Serum cytokine levels.
| Alcoholic Liver Disease (ALD) (n = 40) | Active drinkers w/o liver disease (AD) (n = 26) | Healthy Subjects (HS) (n = 28) | Overall p value | |
|---|---|---|---|---|
| IL-6 pg/mL | 2.9 (0.07-20.8) | 1.3 (0.1-4.5) | 0.7 (0.05-24.3) | 0.08 |
| TNFα pg/mL | 5.8 (1.15-28.4) | 4.7 (0.04-21.7) | 3.44 (0.2-19.7) | 0.4 |
| IL-10 pg/mL | 2.4 (0.01-27)a | 3.5 (0.01-31.6)b | 3.21 (0.01-27)b | < .02 |
Liver histopathology
Liver biopsies were performed in 24 ALD patients, including 15 ALD actively drinking and 9 ALD non-drinking at the time of enrollment. The mean time elapsed between the enrollment and the liver biopsy was 7.9 ± 4.4 days (range 2-22 days). The ALD actively drinking group continued to drink during this interval, except for two who had an interval of 7.5 ± 0.7 days between the enrollment and the liver biopsy. Actively drinking ALD subjects had a higher degree of steatosis and lower stage of fibrosis compared to non active ALD (Table 5). In the entire group of ALD patients, the stages of fibrosis and steatosis were inversely correlated (r = -0.46, p = 0.021), while the grades of inflammation and steatosis were positively correlated (r = 0.4, p = 0.046). The duration of abstinence from alcohol was negatively associated with the grade of steatosis (r = -0.47, p = 0.021) and was positively associated with the stage of fibrosis (r = 0.41, p = 0.047). AST was positively correlated with the grade of steatosis, inflammation, and with the frequency of Mallory-Denk bodies (Table 6). Among the parameters of liver function, INR, MELD, and Child scores were all positively correlated with the stage of fibrosis. The average number of Mallory-Denk bodies was positively correlated with the grade of steatosis (r = 0.53, p = 0.008) and inflammation (r = 0.52, p = 0.01).
Table 5. Hepatic histopathological parameters.
Steatosis in actively drinking ALD vs non actively drinking ALD, p = 0.006; fibrosis in actively drinking ALD vs non actively drinking ALD, p = 0.003.
| (mean ± SD) | Steatosis | Inflammation | Necrosis | Mallory Bodies | Fibrosis |
|---|---|---|---|---|---|
| Overall | 2 (0-4) | 1(0-3) | 0 (0-3) | 0.5 (0-4) | 3 (0-4) |
| ALD-active | 4 (1-4)a | 1 (0-3) | 0 (0-3) | 1 (0-4) | 2 (0-4)a |
| ALD-non active | 0 (1-4)b | 1 (1-4) | 0 (1-4) | 0 (1-4) | 4 (1-4)b |
Table 6. Correlations among liver biochemistry, methionine metabolites and histopathogy.
Data are reported as correlation coefficient (r) and p value.
| Steatosis | Inflammation | Mallory-Denk Bodies | Fibrosis | |
|---|---|---|---|---|
| AST | 0.57 p = 0.003 | 0.48 p = 0.010 | 0.53 p = 0.008 | 0.17 p =0.40 |
| ALT | -0.58 p = 0.002 | 0.28 p =0.17 | 0.16 p =0.45 | -0.45 p = 0.020 |
| INR | -0.56 p = 0.006 | 0.02 p =0.92 | 0.16 p =0.44 | 0.61 p = 0.002 |
| MELD | 0.38 p =0.08 | 0.06 p =0.77 | -0.07 p =0.71 | 0.5 p = 0.020 |
| Child | 0.004 p =0.98 | 0.08 p =0.08 | -0.33 p =0.11 | 0.42 p = 0.041 |
| Cystathionine | 0.39 p =0.05 | -0.33 p =0.10 | 0.18 p =0.39 | 0.44 p = 0.028 |
| ABU/Cystathionine | 0.25 p =0.22 | 0.27 p =0.19 | 0.04 p =0.84 | -0.46 p = 0.022 |
Regressions
According to logistic regression analysis of data from all three alcoholic groups, the ABU/CYSTAT ratio was a predictor of the likelihood of ALD (r = -0.38, p <0.0001). However, although CYSTAT and ABU/CYSTAT correlated with the stage of fibrosis (Table 6), the small number of liver biopsies did not allow for the determination of predictors of the severity of fibrosis.
DISCUSSION
Although others related changes in serum methionine metabolite profiles to abnormal biochemistry in ALD patients [17], our study is the first to correlate serum methionine metabolite levels with hepatic histopathology. The population of patients was characterized by a long history of heavy drinking with typical clinical features of ALD including elevated AST, MELD, and Child scores. Heavy drinking was demonstrated by elevated %CDT levels in all alcoholics, regardless of the duration of sobriety in the ALD group (Table 2). Ours is the first report on serum folate levels in an alcoholic population after the introduction of the 1998 US governmental mandate for supplemental dietary folic acid. Folate deficiency was established as a feature of chronic alcoholism over four decades ago [18] and is attributable to combinations of poor diet, impaired folate absorption, and increased urinary folate excretion [19]. Although folate levels were in the normal range in all groups, the lower levels in ALD and AD subjects confirm the risk of chronic alcoholism for lower folate levels [18]. The finding of increased serum vitamin B12 levels in ALD patients can be attributed to impaired hepatic uptake or retention, or increased release of this vitamin by injured hepatocytes [20]. Relatively lower circulating levels of vitamin B6 were previously described in alcoholics and can be attributed to dietary deficiency, malabsorption [21], or to displacement of the vitamin from its protein carrier by acetaldehyde with subsequent degradation by phosphatases [22]. The variability of IL-6 and TNFα levels could be ascribed to differences in the duration of abstinence [23], the severity of liver disease [24], and/or to genetic polymorphisms [25]. IL-10 was significantly lower in the ALD patients compared to the other 2 groups, contrasting with results of others [26,27], including a study showing a higher prevalence of polymorphisms in the IL-10 promoter in association with increased risk of advanced liver disease [28].
The observed elevations in plasma HCY levels among ALD and AD subjects (Table 3) is consistent with prior data [17,29,30], and can be attributed to inhibitory effects of acetaldehyde on MS activity [2], although a block in transsulfuration could play an additional role (Fig. 1). Elevated HCY can contribute to the development of histopathological damage in the liver through induction of oxidative liver injury [7] and by ER stress pathways of steatosis and apoptosis [8]. Whereas serum HCY correlated with AST, it did not correlate with the histopathologic score or to the likelihood of liver disease among all chronic alcoholics. Although MS is reduced with chronic alcohol exposure, the observed maintenance of serum methionine levels and increase in serum DMG levels are consistent with a compensatory increase in BHMT activity in experimental models of ALD (Table 3) [1]. HCY can also be converted to SAH by the reversible enzyme SAHH [1], as indicated by the correlation between HCY and SAH levels.
Surprisingly, we observed higher serum SAM levels in ALD patients than in AD and HS subjects (Table 3). This novel finding is inconsistent with findings of reduced hepatic levels of SAM in various experimental animal models of ALD [1,5,8] and in liver biopsies of patients with alcoholic hepatitis [29]. Due to the small size of clinical liver biopsies, we could not make comparisons of serum and liver levels of SAM and SAH. We propose that levels of SAM in the serum of patients with ALD do not reflect their concentrations in the liver, but probably reflect reduced retention of SAM by injured hepatocytes. An animal model with simultaneous severe depletion of liver SAM with unchanged serum SAM levels has been reported, suggesting that changes in serum do not parallel those in the liver [31].
HCY levels are regulated by two intersecting pathways of transmethylation and transsulfuration (Fig. 1). While transmethylation of HCY is controlled by both MS and BHMT, transsulfuration is regulated by two vitamin B6 dependent enzymes, CβS and cystathionase [1]. A previous study of liver biopsies from ALD patients and control subjects found reduced expressions of genes relevant to methionine metabolism including CβS [32]. Previous studies of diet-induced vitamin B6 deficiency found that HCY levels were unaffected while CYSTAT levels were increased [33] suggesting a greater effect of deficiency of vitamin B6 on cystathionase than on CβS. Clinical Vitamin B6 deficiency was associated with increased CYSTAT and reduced ABU [34]. This finding was confirmed in experimental vitamin B6- deficient rats [35]. The observed two-fold increase in CYSTAT and the 3-fold reduction in ABU/CYSTAT ratio in ALD patients (Table 3) are consistent with impairment of the vitamin B6 dependent cystathionase reaction. An alternate attribution of more than two-fold increased serum CYSTAT levels in ALD to export from injured hepatocytes is less likely in view of minimal to no increase in serum levels of several other methionine metabolites, serine and glycine. Furthermore, the consistency of elevated CYSTAT with the reduced ABU/CYSTAT substrate to product ratio is indicative of altered metabolism of CYSTAT in the ALD patients. The moderately increased cysteine levels observed in this study could be attributed to reduction of the downstream activity of cysteine dioxygenase which catabolizes cysteine to cysteine sulfinic acid [36].
The findings of positive correlations between serum CYSTAT with parameters of liver function, including albumin, INR, and the MELD score, confirms results from a previous study in ALD patients [17]. Since vitamin B6 is a co-factor for cystathionase, the findings of lower vitamin B6 levels in the ALD group (Table 2) and the correlation of vitamin B6 levels with the observed increase in CYSTAT and reduced ABU and ABU/CYSTAT ratio and AST levels in all ALD patients (Table 3) suggest a primary role for vitamin B6 deficiency in disturbing the transsulfuration pathway in this group. The significant effect of vitamin B6 deficiency in the present study is supported by the prior observation that vitamin B6 deficiency promotes ALD in ethanol fed rats [37]. Furthermore, our study linked abnormal transsulfuration to the presence and severity of ALD by finding a relationship between elevated CYSTAT with AST levels and the stage of fibrosis in the ALD group, and that the ABU/CYSTAT ratio was a valid predictor of ALD among all chronic alcoholics. The reduced ABU/CYSTAT ratio in ALD and its predictive value of the presence of liver disease among alcoholics is a new finding that points to the potential importance of vitamin B6 deficiency in the pathogenesis of ALD, as underscored by the positive correlation between B6 levels and both the MELD and Child scores. The pathophysiological significance of the ABU/CYSTAT ratio relies on its connection with glutathione synthesis and consequently impaired antioxidant mechanisms leading to liver injury. Moreover, the ratio could potentially be used to guide the decision to perform the liver biopsy in subjects at risk of ALD. The cross-sectional design of our study does not allow us to achieve conclusive results on the cause-effect relation between alcohol, methionine metabolism, and ALD. However, the correlation between aberrant transsulfuration pathway metabolites and parameters of liver function as well as with histological parameters points to a central role of this pathway in the development of chronic liver injury related to alcohol use.
AKNOWLEDGEMENT
The authors are grateful to Theresa Tonjes, University of California Davis, for the technical support provided for % CDT measurement.
Financial Support
Supported by R01AA14562 (to C.H.H.), R01AG09834 (to S.P.S.), R01AA11999 and NIAAAPA50 10011 (to S.W.F.), R01DK072398 (to J.F.G.), and UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Abbreviations
- ALD
alcoholic liver disease
- HCY
homocysteine
- SAM
S-adenosylmethionine
- SAH
S-adenosylhomocysteine
- MS
methionine synthase
- BHMT
betaine homocysteine methyltransferase
- DMG
dimethylglycine
- MAT
methionine adenosyl transferase
- SAHH
S-adenosylhomocysteine hydrolase
- CβS
cystathionine-β-synthase
- ABU
α-aminobutyrate
- CYSTAT
cystathionine
- AD
active drinkers
- HS
healthy subjects
- INR
international normalized ratio
- MELD
model for end-stage liver disease
- IL
Interleukin
- %CDT
% carbohydrate deficient transferrin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kharbanda KK. Alcoholic liver disease and methionine metabolism. Semin Liver Dis. 2009;29:155–65. doi: 10.1055/s-0029-1214371. [DOI] [PubMed] [Google Scholar]
- 2.Kenyon SH, Nicolaou A, Gibbons WA. The effect of ethanol and its metabolites upon methionine synthase activity in vitro. Alcohol. 1998;15:305–9. doi: 10.1016/s0741-8329(97)00134-1. [DOI] [PubMed] [Google Scholar]
- 3.Avila MA, Carretero MV, Rodriguez EN, Mato JM. Regulation by hypoxia of methionine adenosyltransferase activity and gene expression in rat hepatocytes. Gastroenterology. 1998;114:364–71. doi: 10.1016/s0016-5085(98)70489-5. [DOI] [PubMed] [Google Scholar]
- 4.Halsted CH, Villanueva JA, Devlin AM, Niemelä O, Parkkila S, Garrow TA, et al. Folate deficiency disturbs hepatic methionine metabolism and promotes liver injury in the ethanol-fed micropig. Proc Natl Acad Sci U S A. 2002;99:10072–7. doi: 10.1073/pnas.112336399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esfandiari F, Medici V, Wong DH, Jose S, Dolatshahi M, Quinlivan E, et al. Epigenetic Regulation of Hepatic Endoplasmic Reticulum Stress Pathways in the Ethanol-fed Cystathionine βeta Synthase Deficient Mouse. Hepatology. 2010 doi: 10.1002/hep.23382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song Z, Zhou Z, Uriarte S, Wang L, Kang YJ, Chen T, et al. S-adenosylhomocysteine sensitizes to TNF-alpha hepatotoxicity in mice and liver cells: a possible etiological factor in alcoholic liver disease. Hepatology. 2004;40:989–97. doi: 10.1002/hep.20412. [DOI] [PubMed] [Google Scholar]
- 7.Villanueva JA, Esfandiari F, White ME, Devaraj S, French SW, Halsted CH. S-adenosylmethionine attenuates oxidative liver injury in micropigs fed ethanol with a folate-deficient diet. Alcohol Clin Exp Res. 2007;31:1934–43. doi: 10.1111/j.1530-0277.2007.00511.x. [DOI] [PubMed] [Google Scholar]
- 8.Esfandiari F, You M, Villanueva JA, Wong DH, French SW, Halsted CH. S-adenosylmethionine attenuates hepatic lipid synthesis in micropigs fed ethanol with a folate-deficient diet. Alcohol Clin Exp Res. 2007;31:1231–9. doi: 10.1111/j.1530-0277.2007.00407.x. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor PG, Schottenfeld RS. Patients with alcohol problems. N Engl J Med. 1998;338:592–602. doi: 10.1056/NEJM199802263380907. [DOI] [PubMed] [Google Scholar]
- 10.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–9. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 11.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 12.Ubbink JB, Serfontein WJ, de Villiers LS. Stability of pyridoxal-5-phosphate semicarbazone: applications in plasma vitamin B6 analysis and population surveys of vitamin B6 nutritional status. J Chromatogr. 1985;342:277–84. doi: 10.1016/s0378-4347(00)84518-1. [DOI] [PubMed] [Google Scholar]
- 13.Myrick H, Henderson S, Anton RF. Utility of a new assay for carbohydrate-deficient transferrin (Biorad %CDT TIA) to monitor abstinence during a treatment outcome study. Alcohol Clin Exp Res. 2001;25:1330–4. [PubMed] [Google Scholar]
- 14.Stabler SP, Allen RH. Quantification of serum and urinary S-adenosylmethionine and S-adenosylhomocysteine by stable isotope dilution liquid chromatography mass spectrometry. Clin Chem. 2004;50:365–372. doi: 10.1373/clinchem.2003.026252. [DOI] [PubMed] [Google Scholar]
- 15.French SW, Nash J, Shitabata P, Kachi K, Hara C, Chedid A, et al. Pathology of alcoholic liver disease. VA Cooperative Study Group 119. Semin Liver Dis. 1993;13:154–69. doi: 10.1055/s-2007-1007346. [DOI] [PubMed] [Google Scholar]
- 16.Berlakovich GA, Soliman T, Freundorfer E, Windhager T, Bodingbauer M, Wamser P, et al. Pretransplant screening of sobriety with carbohydrate-deficient transferrin in patients suffering from alcoholic cirrhosis. Transpl Int. 2004;17:617–21. doi: 10.1007/s00147-004-0765-9. [DOI] [PubMed] [Google Scholar]
- 17.Look MP, Riezler R, Reichel C, Brensing KA, Rockstroh JK, Stabler SP, et al. Is the increase in serum cystathionine levels in patients with liver cirrhosis a consequence of impaired homocysteine transsulfuration at the level of gamma-cystathionase? Scand J Gastroenterol. 2000;35:866–72. doi: 10.1080/003655200750023255. [DOI] [PubMed] [Google Scholar]
- 18.Herbert V, Zalusky R, Davidson CS. Correlation of folate deficiency with alcoholism and associated macrocystosis, anemia, and liver disease. Ann Intern Med. 1963;58:977–88. doi: 10.7326/0003-4819-58-6-977. [DOI] [PubMed] [Google Scholar]
- 19.Halsted CH, Medici V, Esfandiari F. Influence of alcohol on folate status and methionine metabolism in relation to alcoholic liver disease. In: Bailey L, editor. Folate in Health and Disease. 2nd ed. CRC Press; Boca Raton, FL: 2010. pp. 429–48. [Google Scholar]
- 20.Kanazawa S, Herbert V. Total corrinoid, cobalamin (vitamin B12), and cobalamin analogue levels may be normal in serum despite cobalamin in liver depletion in patients with alcoholism. Lab Invest. 1985;53:108–10. [PubMed] [Google Scholar]
- 21.Baker H, Frank O, Zetterman RK, Rajan KS, ten Hove W, Leevy CM. Inability of chronic alcoholics with liver disease to use food as a source of folates, thiamin and vitamin B6. Am J Clin Nutr. 1975;28:1377–80. doi: 10.1093/ajcn/28.12.1377. [DOI] [PubMed] [Google Scholar]
- 22.Lumeng L. The role of acetaldehyde in mediating the deleterious effect of ethanol on pyridoxal 5'-phosphate. J Clin Invest. 1978;62:286–93. doi: 10.1172/JCI109128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.González-Quintela A, Dominguez-Santalla MJ, Pérez LF, Vidal C, Lojo S, Barrio E. Influence of acute alcohol intake and alcohol withdrawal on circulating levels of IL-6, IL-8, IL-10 and IL-12. Cytokine. 2000;12:1437–40. doi: 10.1006/cyto.2000.0715. [DOI] [PubMed] [Google Scholar]
- 24.Sheron N, Bird G, Goka J, Alexander G, Williams R. Elevated plasma interleukin-6 and increased severity and mortality in alcoholic hepatitis. Clin Exp Immunol. 1991;84:449–53. [PMC free article] [PubMed] [Google Scholar]
- 25.Marcos M, Gómez-Munuera M, Pastor I, González-Sarmiento R, Laso FJ. Tumor necrosis factor polymorphisms and alcoholic liver disease: a HuGE review and meta-analysis. Am J Epidemiol. 2009;170:948–56. doi: 10.1093/aje/kwp236. [DOI] [PubMed] [Google Scholar]
- 26.Naveau S, Balian A, Capron F, Raynard B, Fallik D, Agostini H, et al. Balance between pro and anti-inflammatory cytokines in patients with acute alcoholic hepatitis. Gastroenterol Clin Biol. 2005;29:269–74. doi: 10.1016/s0399-8320(05)80760-2. [DOI] [PubMed] [Google Scholar]
- 27.von Baehr V, Döcke WD, Plauth M, Liebenthal C, Küpferling S, Lochs H, et al. Mechanisms of endotoxin tolerance in patients with alcoholic liver cirrhosis: role of interleukin 10, interleukin 1 receptor antagonist, and soluble tumour necrosis factor receptors as well as effector cell desensitisation. Gut. 2000;47:281–7. doi: 10.1136/gut.47.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grove J, Daly AK, Bassendine MF, Gilvarry E, Day CP. Interleukin 10 promoter region polymorphisms and susceptibility to advanced alcoholic liver disease. Gut. 2000;46:540–5. doi: 10.1136/gut.46.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee TD, Sadda MR, Mendler MH, Bottiglieri T, Kanel G, Mato JM, et al. Abnormal hepatic methionine and glutathione metabolism in patients with alcoholic hepatitis. Alcohol Clin Exp Res. 2004;28:173–181. doi: 10.1097/01.ALC.0000108654.77178.03. [DOI] [PubMed] [Google Scholar]
- 30.Blasco C, Caballería J, Deulofeu R, Lligoña A, Parés A, Lluis JM, et al. Prevalence and mechanisms of hyperhomocysteinemia in chronic alcoholics. Alcohol Clin Exp Res. 2005;29:1044–8. doi: 10.1097/01.alc.0000169265.36440.ee. [DOI] [PubMed] [Google Scholar]
- 31.Stabler SP, Sekhar J, Allen RH, O'Neill HC, White CW. Alpha-lipoic acid induces elevated S-adenosylhomocysteine and depletes S-adenosylmethionine. Free Radic Biol Med. 2009;47:1147–53. doi: 10.1016/j.freeradbiomed.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avila MA, Berasain C, Torres L, Martín-Duce A, Corrales FJ, Yang H, et al. Reduced mRNA abundance of the main enzymes involved in methionine metabolism in human liver cirrhosis and hepatocellular carcinoma. J Hepatol. 2000;33:907–14. doi: 10.1016/s0168-8278(00)80122-1. [DOI] [PubMed] [Google Scholar]
- 33.Miller JW, Ribaya-Mercado JD, Russell RM, Shepard DC, Morrow FD, Cochary EF, et al. Effect of vitamin B-6 deficiency on fasting plasma homocysteine concentrations. Am J Clin Nutr. 1992;55:1154–60. doi: 10.1093/ajcn/55.6.1154. [DOI] [PubMed] [Google Scholar]
- 34.Ubbink JB, van der Merwe A, Delport R, Allen RH, Stabler SP, Riezler R, et al. The effect of a subnormal vitamin B-6 status on homocysteine metabolism. J Clin Invest. 1996;98:177–84. doi: 10.1172/JCI118763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stabler SP, Sampson DA, Wang LP, Allen RH. Elevations of serum cystathionine and total homocysteine in pyridoxine-, folate-, and cobalamin-deficient rats. J Nutr Biochem. 1997;8:279–89. [Google Scholar]
- 36.Stipanuk MH, Ueki I, Dominy JE, Jr, Simmons CR, Hirschberger LL. Cysteine dioxygenase: a robust system for regulation of cellular cysteine levels. Amino Acids. 2009;37:55–63. doi: 10.1007/s00726-008-0202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.French SW. Effect of chronic ethanol ingestion on liver enzyme changes induced by thiamine, riboflavin, pyridoxine, or choline deficiency. J Nutrition. 1966;88:291–302. doi: 10.1093/jn/88.3.291. [DOI] [PubMed] [Google Scholar]