Abstract
The HSV-2 lifecycle involves virus spread in a circuit from the inoculation site to dorsal root ganglia and return. We evaluated the role of gE-2 in the virus lifecycle by deleting amino acids 124–495 (gE2-del virus). In the mouse retina infection model, gE2-del virus does not spread to nuclei in the brain, indicating a defect in anterograde (pre-synaptic to post-synaptic neurons) and retrograde (post-synaptic to pre-synaptic neurons) spread. Infection of neuronal cells in vitro demonstrates that gE-2 is required for targeting viral proteins from neuron cell bodies into axons, and for efficient virus spread from epithelial cells to axons. The mouse flank model confirms that gE2-del virus is defective in spread from epithelial cells to neurons. Therefore, we defined two steps in the virus lifecycle that involve gE-2, including efficient spread from epithelial cells to axons and targeting viral components from neuron cell bodies into axons.
Keywords: HSV-2, glycoprotein E, anterograde, retrograde, neuron, spread, Campenot chamber
Introduction
Herpes simplex virus type 2 (HSV-2) is the predominant cause of genital ulcer disease in humans, with a seroprevalence of 17% in the 14–49 year age group in the United States (Xu et al., 2006). HSV-2 genital ulcer disease contributes to the HIV pandemic, since the risk of acquiring HIV infection increases 2- to 3-fold among individuals with HSV-2 infection (Holmberg et al., 1988; Wald and Link, 2002). Transmission to neonates is another serious outcome of HSV-2 infection, often with devastating consequences (Brown et al., 1991). Virus spread from cell-to-cell and within neurons is essential for HSV-2 pathogenesis.
In this paper we evaluate the role of HSV-2 gE in mediating virus spread. We define virus spread as follows: Cell-to-cell spread involves spread between adjacent epithelial cells or between epithelial cells and neurons. Anterograde spread refers to spread across a synapse from pre-synaptic to post-synaptic neurons, while retrograde spread involves spread across a synapse from post-synaptic to pre-synaptic neurons. Anterograde axonal transport refers to movement of viral components within axons in the direction of the axon terminus, while retrograde axonal transport involves movement of viral components within axons in the direction of the neuron cell body. Targeting viral components into axons refers to the movement of viral proteins from the neuron cell body into axons.
HSV-2, like other members of the alphaherpesvirus family, first infects epithelial cells where it replicates and spreads from epithelial cells to axons that innervate these structures. The viral DNA, capsid proteins and some tegument proteins are transported to the neuron nucleus where the viral DNA establishes latency within neurons in the dorsal root ganglia (DRG). When viral DNA reactivates in the neuron nucleus, viral components spread from the neuron cell body along axons to the axon terminus and then to epithelial cells to cause recurrent infections (Luxton et al., 2005; Smith et al., 2004).
Alphaherpesvirus genes US7, US8 and Us9 encode the envelope proteins glycoprotein I (gI), glycoprotein E (gE) and Us9 respectively, that mediate spread from pre-synaptic to post-synaptic neurons (anterograde spread) of pseudorabies virus (PRV) (Babic et al., 1996; Brideau, Card, and Enquist, 2000; Brittle, Reynolds, and Enquist, 2004; Card et al., 1992; Ch’ng and Enquist, 2005; Husak, Kuo, and Enquist, 2000; Lyman, Curanovic, and Enquist, 2008; Whealy et al., 1993). HSV-1 gE and gI and bovine herpes virus (BHV) type 1 and type 5 gE and Us9 are also required for anterograde spread (Butchi et al., 2007; Chowdhury et al., 2000; Chowdhury et al., 2002; McGraw et al., 2009; Snyder, Polcicova, and Johnson, 2008; Wang et al., 2005). The mechanism by which these proteins mediate anterograde spread has not been resolved; however, a role of HSV-1 gE has been demonstrated in targeting viral proteins from the neuron cell body into the axon (Wang et al., 2005).
Interestingly, HSV-1 gE has a much greater impact on spread from post-synaptic to pre-synaptic neurons (retrograde spread) in the retina infection model than PRV gE (Brideau, Card, and Enquist, 2000; Curanovic et al., 2009; Wang et al., 2005). In embryonic rat SCG neuron cultures in vitro, deletion of gE or gI results in a complete defect in HSV-1 spread from neurons to epithelial cells, but only a partial defect in spread of PRV, indicating that differences exist in the functions mediated by these alphaherpesviruses glycoproteins (Ch’ng and Enquist, 2005; McGraw et al., 2009). Deletion of PRV Us9 results in a profound decrease in anterograde spread from the rat retina to the brain in vivo and spread from neurons to epithelial cells in vitro, while Us9 appears to play a much more limited role for HSV-1 (Brideau, Card, and Enquist, 2000; Ch’ng and Enquist, 2005; McGraw et al., 2009).
The role of gE-1 in virus spread from the retina to the brain, targeting of viral components from neuron cell bodies into axons and spread between epithelial cells and neurons has been extensively evaluated; however, no study has addressed the contribution of gE-2 (Dingwell et al., 1994; Dingwell, Doering, and Johnson, 1995; McGraw et al., 2009; McGraw and Friedman, 2009; Snyder, Polcicova, and Johnson, 2008; Wang et al., 2005). HSV-1 and HSV-2 gE share 72% identity at the amino acid level; therefore, we hypothesized that these proteins may share similar functions. To test this hypothesis, we deleted a large region of the gE-2 gene comparable to that deleted in previous studies of gE-1 (Nagashunmugam et al., 1998; Wang et al., 2005). The gE-2 deletion mutant was evaluated in neuronal cell cultures and in the mouse retina and flank infection models to define the contribution of gE-2 to virus spread.
Materials and Methods
Virus strains
HSV-2 strain 2.12 was isolated from a woman with genital ulcer disease and used at passage 8 to derive the mutant strain (Hook et al., 2006). HSV-2 WT gE contains 545 amino acids, while mutant strain 2.12gE-del(124-495gfp) (gE2-del virus) has a deletion of amino acids 124–495 that were replaced by the green fluorescent protein-2 (gfp2) cassette under the control of a CMV promoter. To introduce the CMV-gfp2 cassette into HSV-2 strain 2.12, 5′ and 3′ HSV-2 flanking sequences were linked to the cassette. The 5′ region contains 669 bp, including 300 bp upstream of the protein start site and 369 bp of the gE-2 protein. The primers used to amplify the 5′ flanking region were 5′- GACGGTACCGCCCCCATCGTG-3′ and 5′-GTTAAGCTTCTTTCCTGCGTTATCCC-3′. The 3′ flanking region has 541 bp, including 156 bp at the C-terminus of gE-2. The primers used to amplify the 3′ flanking region were 5′-GGCCTGCAGACAAATGGATCCGG-3′ and 5′-GTAGAGCTCCGCGGAAGGGCG-3′. The 5′ flanking region was inserted into pBluescript SK+ at Kpn1 and HindIII sites and the 3′ flanking region at Pst1 and Sac1 sites. The gfp2 cassette under a CMV promoter (pGFP2-N1, BioSignal Packard) was inserted between the flanking regions at the HindIII and Pst1 sites. The resulting construct was sequenced and co-transfected with HSV-2 2.12 DNA. Plaques were screened for green fluorescence, plaque purified 3 times, and then evaluated by Western blot for the gE-2 protein.
A rescue strain (rgE2-del) to restore wild-type (WT) gE-2 sequences was constructed by PCR amplification of DNA encompassing 300 bp 5′ of the translation start site to 385 bp 3′ of the protein stop codon using the same 5′ and 3′ primers as described above and inserted into pBluescript SK+ at Kpn1 and Sac1 sites that were incorporated into the construct. Rescue plaques were screened for the absence of green fluorescence, plaque purified 3 times, and then screened for gE-2 by Western blot.
Cells and antibodies
Vero cells (African green monkey kidney epithelial cells) and HaCaT cells (human keratinocytes) were grown in Dulbecco’s modified Eagle’s medium supplemented with 10 mM HEPES (pH 7.3), 2 mM L-glutamine (Invitrogen), 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen), and 10% heat-inactivated fetal bovine serum (Hyclone). Mouse monoclonal anti-HSV-1 gD (1D3) and rabbit polyclonal anti- HSV-1 VP5 (NC1), gD (R7), gE (UP575), Us9, and VP26 were previously described (Cohen et al., 1980; Desai, Akpa, and Person, 2003; Friedman et al., 1984; Hook et al., 2008; Isola et al., 1989; Lin, Lubinski, and Friedman, 2004; McGraw et al., 2009). Rabbit polyclonal anti-HSV-2 (Dako), rat anti-mouse Thy 1.2 (Pharmingen), chicken anti-neurofilament heavy chain (Abcam), Alexa Fluor 555 goat anti-mouse and anti-rabbit IgG, and Alexa Fluor 488 goat anti-chicken and donkey anti-rat IgG (Invitrogen) were purchased.
Western blotting
Vero cells were mock infected or infected with HSV-2 strain 2.12 (WT), rgE2-del, or gE2-del at a MOI of 2. The cells were lysed with cell culture lysis buffer (Promega) containing a cocktail of protein inhibitors (Roche) at 20 h post infection (hpi). Cell lysates were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis on a 4% to 15% gel, transferred to an Immobilon-P membrane, and probed with rabbit anti-HSV-1 VP5 (1:5,000), rabbit UP575 anti-HSV-1 gE (1:5,000), rabbit R7 anti-HSV-1 gD (1:100,000), or rabbit anti-HSV-1 Us9 (1:10,000).
Embryonic rat SCG neuronal cell cultures
Dissociated SCG from E17 Sprague-Dawley rat embryos (Charles River Laboratories) were plated into assembled Campenot chambers to contain approximately 5,000 neurons per chamber as previously described (McGraw and Friedman, 2009). The neurons were differentiated for 2 weeks before use. For anterograde transport studies, 3 × 104 Vero cells were seeded into the neurite (N) chamber 1 day prior to infection. For evaluation of virus spread from epithelial cells to axons, 3 × 104 HaCaT cells were allowed to polarize in the N chamber for 1 week prior to infection (McGraw and Friedman, 2009). The N or S chambers were infected with 105 PFU of virus, and at the time points indicated, the contents of the chambers were harvested and titered on Vero cell monolayers.
Single-step growth curves
Vero cell monolayers were infected with HSV-2 WT, rgE2-del, or gE2-del at a MOI of 2. An aliquot of the inoculum was frozen at −80°C and served as the time 0 harvest. After 1 h adsorption at 37°C, cells were treated with citrate buffer (pH 3.0) for 1 min followed by 2 washes with PBS (pH 7.4) to remove virus that had not yet entered cells. Cells and supernatants were harvested immediately after the citrate wash, which was considered the 1 h post infection (hpi) time point, and at 4, 8, 16 and 24 hpi, frozen, thawed once, sonicated and titered on Vero cell monolayers (McGraw and Friedman, 2009).
Approximately 5,000 neurons from half of a SCG were placed in a 12-well plate and infected with 2.5 x 105 PFU. A high titer inoculum was used to ensure infection of all the neurons since much of the inoculum remains attached to the matrix (McGraw and Friedman, 2009). After 1 h incubation at 37°C, virus that had not yet entered neurons was inactivated by a citrate buffer (pH 3.0) wash. At the time points indicated, the contents of the wells were harvested and titered on Vero cell monolayers (McGraw and Friedman, 2009).
Plaque size analysis
Confluent Vero cells were infected with 60 PFU/well of HSV-2 WT, rgE2-del, or gE2-del, and at 1 hpi overlaid with 0.6% low-melt agarose. At 96 hpi, the diameters of 30 plaques were measured with an optical micrometer in an inverted light microscope at 40X magnification, and the area calculated based on the size of 2 perpendicular diameters of each plaque (McGraw and Friedman, 2009; Weeks et al., 2000).
Eye injections and virus titers in retinas
The retinas of anesthetized eight-week old female BALB/c mice were infected by inoculation of the vitreous body with 4 × 105 PFU of HSV-2 WT, rgE2-del, or gE-del in 1μl as previously described (McGraw et al., 2009). Mice were anesthetized at the indicated times post infection, and perfused by intracardiac injection with PBS. Eyes were harvested and the lenses and corneas removed. The retinas were washed with PBS, frozen, thawed, homogenized in PBS, sonicated, and centrifuged at 1,500 x g at 4°C for 5 min, and supernatants were titered on Vero cells (Wang et al., 2005).
Immunofluorescence and immunohistochemistry of eye and brain tissues
Three to 8 days post infection (dpi) mice were anesthetized and perfused by intracardiac injection with 10ml of 4% paraformaldehyde in 0.1 M sodium phosphate buffer pH 7.4. Eyes were dissected leaving optic nerves attached to the retinas, while brains were separated from the optic nerves. Eyes and brains were fixed in 4% paraformaldehyde overnight at 4°C and then placed in 30% sucrose overnight at 4°C. Samples were embedded in TBS freezing medium (Triangle Biomedical Sciences). Eyes were cut into 10μm saggital sections onto Tissue Tack slides (Polysciences, Inc.). Brains were cut into 40μm coronal sections and placed in cryoprotectant solution (30% sucrose, 1% polyvinylpyrrolidone, and 30% ethylene glycol in 0.1 M PBS, pH 7.2) and stored at −20°C until staining (McGraw et al., 2009; Wang et al., 2005).
Retinas were stained with a rabbit polyclonal HSV-2 antibody to detect viral antigens and with rat anti-mouse Thy 1.2 to identify mouse axon fibers (McGraw and Friedman, 2009; Wang et al., 2005). Secondary antibodies included Alexa 555 goat anti-rabbit IgG and Alexa 488 donkey anti-rat IgG. Background staining was reduced by incubating in PBS and 1% horse serum, 1% BSA, and 0.05% Triton X-100 for 30 min. Primary antibodies (1:500) and secondary antibodies (1:200) were diluted in PBS with 0.3% Triton X-100. The primary antibodies were incubated for 2 h and the secondary antibodies for 1 h at room temperature. DAPI (4′-6′-diamidino-2-phenylindole) (Molecular Probes) was added with the secondary antibodies to stain nuclei.
Brain sections were stained by incubating with polyclonal rabbit anti-HSV-2 antibody (1:2,000) for 36 h at room temperature, followed by biotinylated goat anti-rabbit IgG (1:200) and avidin-biotin/peroxidase (1:100; Vector Laboratories, Inc.) each added for 90 min at room temperature (Wang et al., 2005). The color was developed using diaminobenzidine (DAB) and hydrogen peroxide (Sigma), and sections were mounted onto gelatin-coated slides.
Immunofluorescence of rat SCG neurons
Rat SCG neurons were harvested from E17 Sprague-Dawley rat embryos and plated in 35 mm tissue culture dishes on a glass cover slip. After 10 to 14 days, the SCG neurons were fixed and stained with primary and secondary antibodies as previously described (Wang et al., 2005). Pictures were taken on a Nikon Eclipse E1000 microscope using Phase 3 Imaging Systems.
Mouse flank model
Nine-week old BALB/c mice were flank inoculated with 5 × 105 PFU of HSV-2 WT, rgE2-del or gE2-del virus as previously described (Nagashunmugam et al., 1998). Mice were monitored for survival and scored for disease at the inoculation site on a scale of 0–4, where 0 is no disease, 1 is redness or swelling, 2 is skin erosion, 3 is ulcers and 4 is necrosis. Lesions outside the inoculation site were considered zosteriform disease, which was scored on a 0–4 scale, where 0 is no lesions, 1 is one or more discrete lesions, 2 is coalesced lesions, 3 is ulcerated lesions, and 4 is necrosis (Brittle et al., 2008). DRG were harvested at the indicated times and processed by mincing with scissors, pulverizing with a pestle, and titering on Vero cells (Brittle et al., 2008).
Mouse vaginal model
Replication of 5 × 105 PFU of HSV-2 WT and gE2-del viruses was compared in the vagina of 5–6 week-old BALB/c mice. Mice received a subcutaneous injection of 2 mg of medroxyprogesterone (Sicor Pharmaceuticals, Inc., Irvine, CA) in 0.9% NaCl and 10 mM Hepes 5 days prior to infection (Brittle et al., 2008). Swabs were performed at 1 hpi, and 1, 2, and 3 dpi and virus titers determined by plaque assay on Vero cells.
Statistics
GraphPad Prism software was used for all statistical analyses. One-way ANOVA with Tukey’s adjustment was used for comparison of 3 or more groups, while t tests were used for comparison of two groups. Survival analysis was performed using the Logrank (Mantel-Cox) test.
Results
gE2-del virus does not express gE-2 but retains expression of neighboring genes
We constructed gE2-del virus (2.12gE-del124–495gfp) in the background of a low passage clinical isolate, HSV-2 2.12 (Figure 1A). The Us8 deletion was designed based on a previously described HSV-1 gE mutant, NS-gEnull (Brittle et al., 2008; Nagashunmugam et al., 1998; Wang et al., 2005). A rescue virus, rgE2-del, was made by restoring the Us8 coding sequences and removing the gfp2 reporter gene.
Figure 1.
HSV-2 2.12gE-del124–495gfp (gE2-del) construction and characterization. A. Diagram of 2.12gE-del124–495gfp. 5′ flanking sequence: the white box represents sequences prior to the ATG protein coding start site, while the black box indicates the first 369 bp of the gE-2 protein coding sequence. 3′ flanking sequence: the black box represents 156 bp of the gE-2 protein coding sequence prior to the stop codon, while the white box indicates the remaining 3′ flanking sequences. The green fluorescent protein under the control of a CMV promoter was inserted into Us8 to replace the 1,116 deleted bp. B. Western blot of Vero cells infected with gE2-del, rgE2-del or WT virus probed with antibodies to VP5, gE, gD, and Us9.
Western blots were performed in Vero cells to verify the absence of gE-2 and that neighboring genes were unaffected (Figure 1B). Antibody to VP5 capsid antigen was used as a loading control. HSV-2 gE is expressed in cells infected with the WT and rescue viruses but not with the gE2-del strain. Blots for HSV-2 glycoprotein D (gD) and Us9 proteins showed comparable expression in cells infected with gE2-del, rgE2-del or WT virus, indicating that the Us8 deletion did not affect Us6 (gD) or Us9. We lack an antibody that detects HSV-2 gI; therefore, we sequenced the gE2-del Us7 gene to verify that the gene contains no unintended mutations (results not shown).
gE-2 is not required for intact single-step growth kinetics
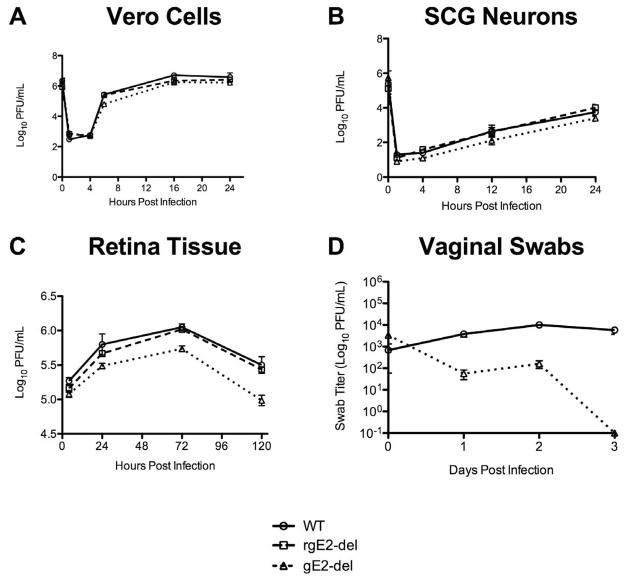
Vero cells were infected at a MOI of 2 with WT, rgE2-del or gE2-del virus and cells and supernatant fluids were collected at 0, 1, 4, 8, 16 and 24 hpi (Figure 2A). No statistically significant differences were detected comparing WT, rgE2-del or gE2-del at any time point (P=0.29 by 1-way ANOVA); therefore, gE-2 is not required for growth of HSV-2 in Vero cells.
Figure 2.
Single-step growth kinetics of WT, rgE2-del or gE2-del virus. A. Vero cells. Results are the mean of 2 wells per time point. B. Primary embryonic rat superior cervical ganglia neurons. Results are the mean of 4 wells per time point ± SEM. C. Virus replication in retina. Results are the mean of 2 to 3 retinas per time point. D. Virus replication in mice vagina. n=5 mice at each time point ± SEM.
Single-step growth experiments were performed in cultured embryonic rat SCG neurons (Figure 2B). Neurons were infected with WT, rgE2-del or gE2-del virus using 2.5 × 105 PFU per well. Cells and supernatants were collected for viral titers at 1, 4, 12 and 24 hpi. The growth curves for the 3 viruses were similar with no significant differences at any time point (P=0.89 by 1-way ANOVA), indicating that gE-2 is not required for replication in neurons.
Replication of gE2-del virus in vivo
To evaluate replication of the gE2-del virus in vivo, 4 × 105 PFU of WT, rgE2-del or gE2-del virus was inoculated into the vitreous fluid chamber of the eye of BALB/c mice. Retinas were titered at 4, 24, 72 or 120 hpi (Figure 2C). The gE2-del virus replicated in retinas based on an increase in titers from 4 to 72 hpi (P<0.001); however, the mutant strain replicated to significantly lower titers than WT or rgE2-del virus (P<0.001). The gE2-del virus also replicated to significantly lower titers than WT virus in the mouse vagina (P<0.001) (Figure 2D). The vaginal titers of the gE2-del virus were higher on day 2 than day 1, suggesting that the virus replicates in vaginal tissues, although the difference between day 1 and day 2 titers was not statistically significant (P=0.17).
gE-2 is required for efficient cell-to-cell spread in vitro
Vero cells were infected with WT, rgE2-del or gE2-del virus and at 96 hpi the diameters of plaques were measured and the average area of 30 plaques calculated (Figure 3). The gE2-del virus plaques were 6.5- to 6.9-fold reduced compared with rgE2-del and WT viruses, respectively (P< 0.001 for each virus), demonstrating a cell-to-cell spread defect for gE2-del virus.
Figure 3.

A. Plaque size of WT, rgE2-del or gE2-del virus in Vero cells. n=30 plaques per virus ± SEM.
gE-2 is required for virus spread from the eye to the brain in vivo
4 × 105 PFU of WT, rgE2-del or gE2-del virus was injected into the vitreous body of eight-week old female BALB/c mice. In this model, the neurons that are infected first are the ganglion cell neurons at the innermost layer of the retina. Virus then spreads to adjacent neurons and supporting cells in the retina and travels from the cell bodies of ganglion cell neurons into their axon fibers that form the optic nerve (targeting of viral components from neuron cell bodies into axons and anterograde axonal transport) and then to post-synaptic neurons in the brain (anterograde spread). Virus also spreads from the iris, ciliary body or extraocular muscles of the orbit to parasympathetic or motor nerve axons that innervate these structures (epithelial to axon spread), then to the neuron nucleus (retrograde axonal transport) and to pre-synaptic neurons in the brain (retrograde spread) (Figures 4A and 5A) (McGraw et al., 2009; Wang et al., 2005).
Figure 4.


A. Top figure: Model of mouse eye and surrounding orbital structures. Virus is injected into the vitreous body of the eye from which it infects retina ganglion cell neurons, iris, and ciliary body. During the inoculation, the needle penetrates the extra-ocular muscles, which often become infected. Middle figure: Enlargement of the retina: The innermost neurons of the retina are the ganglion cell neurons. Their axon fibers comprise the optic nerve. Movement of virus particles from cell bodies of ganglion cell neurons into the optic nerve represents targeting of viral components into axons and anterograde axonal transport. Bottom figure: Spread of virus from the ciliary body, iris or skeletal muscle to parasympathetic or motor nerve axons (epithelial to axon spread) and then to the neuron cell body (retrograde axonal transport). B. Retinas and optic nerves 3 or 5 dpi with 4 × 105 PFU of WT, rgE2-del or gE2-del virus. HSV-2 antigens are in red, axon fibers are in green and nuclei are in blue. The retinas are shown in saggital section demonstrating all cell layers of the retina with the innermost ganglion cell layer towards the bottom of the figure. The optic nerve is also shown in saggital section; however, the orientation of the retina is rotated clockwise 90° compared with the retina infection figures. White arrowheads point to infected cells, including ganglion cell neurons at the innermost layer of the retina. Thick white arrows point to the optic nerve, and thin white arrows point to viral antigens in the retina. Results shown are representative of 3 retinas and optic nerves evaluated for WT and rgE2-del viruses, and 6 retinas and optic nerves for gE2-del virus. Magnification is 200X for retina infections, and 100X for optic nerves.
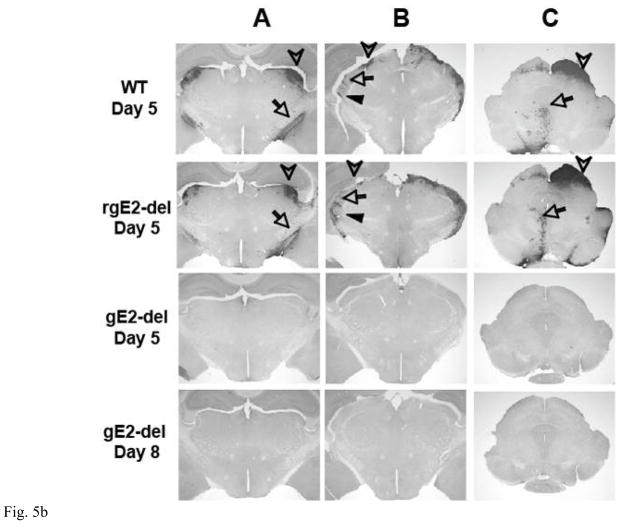
Figure 5.

A. The eye is shown at the bottom of the figure. Spread of viral antigens from the retina to the optic nerve and optic tract (OT) requires targeting of viral components from the neuron cell body into axons and anterograde axonal transport. Spread of antigen to the superior colliculus (SC), dorsal and ventral lateral geniculate nuclei (dLGN, vLGN) represents spread from pre-synaptic to post-synaptic neurons (anterograde spread). Spread of virus from the eye to the oculomotor nucleus (OMN) and Edinger-Westphal nucleus (EWN) requires spread from infected tissues in the eye to the innervating axons (epithelial to axon spread), retrograde axonal transport and spread from post-synaptic to pre-synaptic neurons (retrograde spread). B. Immunoperoxidase staining of mouse brains 5 or 8 dpi of the retina with 4 × 105 PFU of WT, rgE2-del or gE2-del virus. The location of antigen is indicated by dark precipitate. Column A: HSV-2 antigen appears in the optic tract (requires targeting of viral antigens from the neuron cell body into axons and anterograde axonal transport, arrow) and the dLGN (requires anterograde spread, arrowhead) in WT and rgE2-del virus infected mice by 5 dpi but not in the brains of mice infected with gE2-del at 5 or 8 dpi. Column B: HSV-2 antigen is present in the dLGN (requires anterograde spread, arrowhead), the vLGN (anterograde spread, filled arrowhead) and the intergeniculate leaflet of the LGN (retrograde spread, arrow) in the brains of WT and rgE2-del virus infected mice but not in the brains of mice infected with gE2-del virus. Column C: HSV-2 antigen is detected in the SC (requires anterograde spread, arrowhead), the OMN and EWN (requires retrograde spread, arrow) of mice infected with WT or rgE2-del virus but not in the brains of mice infected with gE2-del virus. Results shown are representative of 3 mouse brains evaluated for each virus at each time point. Magnification is 20X.
Retina infection by each of these viruses was assessed by immunofluorescence at 3 and 5 dpi (Figure 4B). Viral antigens were readily detected in the retinas infected with WT, rgE2-del or gE2-del on both days, although somewhat less antigen was present in gE2-del infected mice, particularly on day 5 pi, which is consistent with results shown in Figure 2C.
Optic nerves from mice inoculated with WT or rgE2-del virus contain abundant viral antigens 3 and 5 dpi, suggesting that viral antigen is transported from the ganglion neuron cell bodies into the axon fibers of these neurons (targeting of viral components from neuron cell bodies into axons and anterograde axonal transport). In contrast, no viral antigen was detected in the optic nerve of mice infected with gE2-del virus at 3 or 5 dpi, supporting the hypothesis that gE-2 is necessary for targeting viral proteins into axons and/or anterograde axonal transport of viral proteins.
gE-2 is required for viral antigens to reach the brain by anterograde or retrograde spread from the eye
The vitreous body was injected with 4 × 105 PFU of WT, rgE2-del or gE2-del virus as in Figure 4. Figure 5A shows a model of virus spread in the anterograde and retrograde directions to nuclei in the brain. Five and 8 (gE2-del virus only) dpi, brains were removed, sectioned and stained for HSV-2 antigens (Figure 5B). WT and rgE2-del viral antigens were detected in mouse brains 5 dpi. Antigens were present in neurons reached by anterograde spread from the retina, including the dorsal lateral geniculate nucleus (LGN), the ventral LGN, and the superior colliculus. Antigens were also detected in neurons reached by retrograde spread from structures of the eye, including the intergeniculate leaflet of the LGN and the Edinger-Westphal nucleus. In contrast, mouse brains infected with gE2-del virus had no viral antigen detected in any area of the brain at 5 and 8 dpi, indicating a defect in one or more steps required for anterograde spread (targeting viral components from ganglion cell bodies into axons, anterograde axonal transport, or spread from pre-synaptic to post-synaptic neurons) and retrograde spread (spread from iris, ciliary body or muscles to axons, retrograde axonal transport, or spread from post-synaptic to pre-synaptic neurons).
gE-2 is required for virus spread from neurons to epithelial cells in vitro
We used the Campenot chamber system and embryonic rat SCG neurons to assess spread of virus from neurons to epithelial cells (McGraw et al., 2009). SCG neurons were added to the soma (S) chamber and allowed to differentiate. Over 1–2 weeks, neurites grew into the middle (M) and neurite (N) (axon) chambers. The M chamber was filled with methylcellulose to prevent leakage of virus from one compartment to another. Vero cells were seeded into the N chamber 1 d prior to infection of neurons in the S chamber. The addition of Vero cells is based on observations with PRV that indicator cells are required in the N chamber to detect virus transported from the S to N chamber (Ch’ng and Enquist, 2005; Curanovic and Enquist, 2009). 105 PFU of WT, rgE2-del or gE2-del virus was added to the S chamber and at 48 hpi the contents of the S and N chambers were harvested and titers determined on Vero cells. WT, rgE2-del, and gE2-del viruses grew to equivalent titers in the S chamber (Figure 6A). WT and rgE2-del viruses spread to Vero cells in the N chamber by 48 hpi and reached titers of 3.7 log10 and 3.3 log10, respectively. In contrast, no gE2-del virus was detected in the N-chamber (Figure 6B) (P<0.001 compared with WT and rgE2-del). These results indicate that gE2-del virus has a defect in spread in one or more of the following: spread from the neuron cell body into axons, anterograde axonal transport, or spread from axons to epithelial cells.
Figure 6.
Virus spread from neuron cell bodies along axons and to epithelial cells in Campenot chambers. A and B. Vero cells were added to the N chamber one day prior to infection. 105 PFU of WT, gE2-del, or rgE2-del virus was added to the S chamber and at 48 hpi the contents of the S (A) and N (B) chambers were titered on Vero cells. The limit of detection for the titration is 1 PFU.
gE-2 targets viral proteins into axons
To further evaluate the site of the block in gE2-del virus spread, rat SCG neurons were infected with 105 PFU of WT, rgE2-del or gE2-del virus. The neurons were stained for envelop glycoprotein gD, capsid protein VP26 at 18 hpi (both shown in red), and neurofilaments to outline the neuron cell bodies and most importantly the axon filaments (shown in green). Viral gD and VP26 antigens were detected in neuron cell bodies and axons after infection with WT and rescue viruses (Figure 7). gE2-del gD and VP26 antigens were readily detected in neuron cell bodies; however, no viral antigen was detected in axons, indicating that gE-2 is required for targeting viral proteins from neuron cell bodies into axons. This result is consistent with the optic nerve findings described in Figure 4B.
Figure 7.

HSV-2 gE is required for targeting envelop and capsid proteins into axons. SCG neurons were infected with 105 PFU of WT, rgE2-del or gE2-del virus and at 18 h post infection, neurons were stained with antibodies to viral antigens gD (red, first column), VP26 (red, fourth column), to SCG neurofilaments (green, second and fifth columns), and as merged images (third and sixth columns). White arrows indicate viral antigens in axons. Most cells are infected as separate neurons; however, some neurons are in clusters (infected with WT or rgE2-del virus and stained for VP26). Note the total absence of gD or VP26 antigen (red) in SCG axons infected with gE2-del virus. Magnification is 400X.
gE-2 is required for efficient spread from epithelial cells to axons, but not for retrograde axonal transport in vitro
To further assess the retrograde spread phenotype of gE2-del virus, 105 PFU of WT, rgE2-del or gE2-del (MOI 3.3) was added to N chambers containing 3 × 104 confluent, polarized HaCaT cells that were seeded 1 week earlier on top of neurites. At 48 hpi the contents of the S and N chambers were collected and titered on Vero cells. The N chamber showed equivalent titers of the 3 viruses, indicating comparable replication in HaCaT cells (Figure 8A); however, S chamber titers were significantly different comparing the 3 viruses (Figure 8B). WT and rgE2-del virus titers were approximately 3 log10 higher than gE2-del virus (P<0.001), indicating that gE-2 is required for efficient spread from epithelial cells to axons (epithelial-to-axon spread) and/or spread from axons to neuron cell bodies (retrograde axonal transport).
Figure 8.

Spread of virus from epithelial cells to neuron cell bodies in Campenot chambers. A and B. HaCaT cells were seeded in the N chamber and allowed to polarize for 1 week prior to infection with 1 × 105 PFU of WT, gE2-del, or rgE2-del virus. At 48 hpi, contents of the S (A) and N (B) chamber were harvested and titered on Vero cells. C. Neurites in the N chamber were infected with 1 × 105 PFU of WT, gE2-del, or rgE2-del virus in the absence of HaCaT cells. At 24 hpi, contents of the S chamber were harvested and titered on Vero cells. n=5 chambers for each assay ± SEM.
To define the site of the defect in spread, WT, rgE2-del or gE2-del virus was added directly to the neurites in the N chamber. No HaCaT cells were present; therefore, virus did not first spread from epithelial cells to neurites. Under these conditions, no difference in S chamber titers was detected comparing the 3 viruses (Figure 8C), suggesting that gE-2 is required for spread from epithelial cells to axons, rather than for retrograde axonal transport.
gE-2 is required for virus spread from epithelial cells to neurons in the mouse flank model
Nine-week old female BALB/c mice were infected by scarification on denuded flank skin. In this model, the presence of zosteriform disease indicates that virus has spread from epithelial cells to axons, traveled by retrograde axonal transport to DRG, traveled by anterograde axonal transport back to the axon terminus and then spread from the axon terminus to epithelial cells (McGraw et al., 2009).
Mice were infected by flank scarification with 5 × 105 PFU WT, rgE2-del or gE2-del virus or were mock infected and monitored for survival (Figure 9A), inoculation site, and zosteriform site disease (Figure 9B–C). 100% of animals infected with WT or rgE2-del virus died by 10 and 13 dpi, respectively, while all animals infected with gE2-del virus survived (P<0.001 compared with WT or rgE2-del virus). WT and rgE2-del viruses resulted in severe inoculation site and zosteriform site disease that were not significantly different from one another (P=0.89). The gE2-del virus disease at the inoculation site was significantly less than WT or rgE2-del virus (P<0.001), but not significantly different from mock-infected animals (P=0.87). The gE2-del virus produced no zosteriform site disease and no infectious gE2-del virus was detected in DRG at 1, 3, 6, or 8 dpi compared with approximately 1.5 log10 PFU of WT virus (Figure 9D) (P= 0.02 at 6 dpi). Photographs of inoculation site and zosteriform site disease from representative mice taken at 7 dpi are shown (Figure 9E). These results indicate that in the absence of gE-2, HSV-2 causes little or no disease in the mouse flank model and is defective in spread from epithelial cells to neurons in DRG.
Figure 9.

Infection of the mouse flank with 5 × 105 PFU of WT, rgE2-del, or gE2-del virus, or mock-inoculation (A–C). A. Survival. B. Inoculation site disease scores ± SEM. C. Zosteriform site disease scores ± SEM. D. DRG titers comparing WT and gE2-del viruses ± SEM. E. Photographs taken of mock infected mice or mice infected with WT, rgE2-del or gE2-del virus at 7 dpi.
Discussion
We used in vitro and in vivo approaches to define the spread phenotype of an HSV-2 gE mutant strain. We modified gE-2 by introducing a large deletion from amino acids 124–495, which was based on our previous studies of HSV-1 gE that deleted amino acids 124–508 (Brittle et al., 2008; Nagashunmugam et al., 1998; Wang et al., 2005). Our results suggest that gE-1 and gE-2 mediate similar spread functions, since both gE-1 and gE-2 affect epithelial cell-to-cell spread, epithelial-to-axon spread, spread from skin to neurons in DRG, targeting of viral proteins from the neuron cell body into axons, and anterograde and retrograde spread from the mouse retina to the brain (Brittle et al., 2008; McGraw et al., 2009; McGraw and Friedman, 2009; Wang et al., 2005). We previously reported that HSV-1 gEnull virus is a candidate attenuated live HSV-1 vaccine. Based on comparable properties of the gE2-del virus we plan to evaluate this strain as an HSV-2 attenuated live virus vaccine.
The gE2-del virus defective spread from epithelial cells to neurons is likely the result of at least two abnormalities. First, gE2-del virus is defective in epithelial cell-to-cell spread, as measured by small plaque size in vitro and diminished inoculation site disease in vivo. The low epithelial cell titers limit the quantity of virus available to infect axons. Second, gE2-del virus is impaired in epithelial cell-to-axon (neurite) spread based on studies in Campenot chambers. When HaCaT cells in the N chamber were infected with gE2-del virus, we detected reduced S chamber titers; however, when neurites in the N chamber were infected in the absence of HaCaT cells, S chamber titers were not reduced. The epithelial-to-epithelial and the epithelial-to-neurite spread defects may both contribute to the lack of infectious gE2-del virus in DRG after flank inoculation. These same defects may also explain the absence of viral antigens in brain nuclei reached by retrograde spread.
The gE2-del virus replicates comparable to WT and rgE2-del viruses in SCG neurons in vitro; therefore, the spread defect from neurons to epithelial cells in Campenot chambers cannot be explained by lack of replication in neurons. In the retina infection model, the gE2-del virus replicates to lower titers than WT or rgE2-del virus (5.7 log10 versus 6 log10); however, these relatively small differences are unlikely to account for the striking absence of gE2-del antigens in the optic nerve. Defective targeting of viral components from the neuron cell body into axons clearly contributes to the spread defect detected in Campenot chambers and likely accounts for the lack of gE2-del antigen in the optic nerve, rather than a defect in anterograde axonal transport.
These studies represent an evaluation of the contributions of gE-2 to spread. HSV-2 Us9 and gI may also contribute to spread and studies are in progress to identify the functions of these proteins. Cellular binding partners for gE-2 have yet to be identified, which will aid in understanding the mechanisms by which gE-2 mediates epithelial-to-axon spread and targeting viral proteins from the neuron cell body into axons. A greater understanding of these interactions could lead to the design of inhibitors of neuroinvasion or to candidate HSV-2 vaccines.
Although the spread functions mediated by gE-1 and gE-2 are similar, it is not known whether gE-1 and gE-2 contribute comparably to immune evasion. HSV-1 gE forms a complex with gI on the virus envelope and infected cell surface to serve as an IgG Fc receptor. An important function of the HSV-1 Fc receptor is that it mediates antibody bipolar bridging. This term refers to the binding of the Fab domain of an antibody molecule that is targeting HSV-1 antigens, such as gD, and the binding of the Fc domain of the same antibody molecule to the viral Fc receptor (Frank and Friedman, 1989; Lin, Lubinski, and Friedman, 2004). Antibody bipolar bridging blocks functions mediated by the Fc domain, including complement activation and antibody dependent cellular cytotoxicity (Dubin et al., 1991; Frank and Friedman, 1989; Lin, Lubinski, and Friedman, 2004; Nagashunmugam et al., 1998).
In summary, HSV-2 is an important human pathogen that requires spread between epithelial cells and axons and viral transport within axons for its lifecycle in humans. Glycoprotein E of the related alphaherpesviruses HSV-1, PRV, BHV-1, and BHV-5 contributes significantly to neuroinvasion by mediating virus spread. Here we demonstrate an important role for HSV-2 gE in promoting virus spread from epithelial cells to axons and an essential role in targeting viral proteins from the neuron cell body into axons.
Acknowledgments
This work was supported by NIH grant AI 033063 and by a research grant from Merck and Co. Polyclonal rabbit anti-HSV-1 VP5 (NC1) and rabbit anti-HSV-1 gD (R7) were provided by Gary Cohen and Roselyn Eisenberg from the University of Pennsylvania. Polyclonal rabbit anti-HSV-1 VP26 was provided by Prashant Desai, Johns Hopkins University. We thank Helen Lazear (McGraw) for providing model figures 4A and 5A prepared while a graduate student in our laboratory, and Sarah J. Radcliffe from the University of Pennsylvania Department of Biostatistics and Epidemiology for advice on statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Fushan Wang, Email: fushanw18@hotmail.com.
Elizabeth E. Zumbrun, Email: ezumbrun@gmail.com.
Jialing Huang, Email: jialinghuang@hotmail.com.
Huaxin Si, Email: sihx@yahoo.com.
Lena Makaroun, Email: lena.makaroun@gmail.com.
Harvey M. Friedman, Email: hfriedma@mail.med.upenn.edu.
References
- Babic N, Klupp B, Brack A, Mettenleiter TC, Ugolini G, Flamand A. Deletion of glycoprotein gE reduces the propagation of pseudorabies virus in the nervous system of mice after intranasal inoculation. Virology. 1996;219(1):279–84. doi: 10.1006/viro.1996.0247. [DOI] [PubMed] [Google Scholar]
- Brideau AD, Card JP, Enquist LW. Role of pseudorabies virus Us9, a type II membrane protein, in infection of tissue culture cells and the rat nervous system. Journal of Virology. 2000;74(2):834–45. doi: 10.1128/jvi.74.2.834-845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittle EE, Reynolds AE, Enquist LW. Two modes of pseudorabies virus neuroinvasion and lethality in mice. J Virol. 2004;78(23):12951–63. doi: 10.1128/JVI.78.23.12951-12963.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittle EE, Wang F, Lubinski JM, Bunte RM, Friedman HM. A replication-competent, neuronal spread-defective, live attenuated herpes simplex virus type 1 vaccine. J Virol. 2008;82(17):8431–41. doi: 10.1128/JVI.00551-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ZA, Benedetti J, Ashley R, Burchett S, Selke S, Berry S, Vontver LA, Corey L. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. New England Journal of Medicine. 1991;324(18):1247–52. doi: 10.1056/NEJM199105023241804. [DOI] [PubMed] [Google Scholar]
- Butchi NB, Jones C, Perez S, Doster A, Chowdhury SI. Envelope protein Us9 is required for the anterograde transport of bovine herpesvirus type 1 from trigeminal ganglia to nose and eye upon reactivation. J Neurovirol. 2007;13(4):384–8. doi: 10.1080/13550280701375433. [DOI] [PubMed] [Google Scholar]
- Card JP, Whealy ME, Robbins AK, Enquist LW. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. Journal of Virology. 1992;66(5):3032–41. doi: 10.1128/jvi.66.5.3032-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch’ng TH, Enquist LW. Neuron-to-cell spread of pseudorabies virus in a compartmented neuronal culture system. J Virol. 2005;79(17):10875–89. doi: 10.1128/JVI.79.17.10875-10889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury SI, Lee BJ, Ozkul A, Weiss ML. Bovine herpesvirus 5 glycoprotein E is important for neuroinvasiveness and neurovirulence in the olfactory pathway of the rabbit. J Virol. 2000;74(5):2094–106. doi: 10.1128/jvi.74.5.2094-2106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury SI, Onderci M, Bhattacharjee PS, Al-Mubarak A, Weiss ML, Zhou Y. Bovine herpesvirus 5 (BHV-5) Us9 is essential for BHV-5 neuropathogenesis. Journal of Virology. 2002;76(8):3839–51. doi: 10.1128/JVI.76.8.3839-3851.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen GH, Ponce de Leon M, Diggelmann H, Lawrence WC, Vernon SK, Eisenberg RJ. Structural analysis of the capsid polypeptides of herpes simplex virus types 1 and 2. J Virol. 1980;34(2):521–31. doi: 10.1128/jvi.34.2.521-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curanovic D, Enquist LW. Virion-incorporated glycoprotein B mediates transneuronal spread of pseudorabies virus. J Virol. 2009;83(16):7796–804. doi: 10.1128/JVI.00745-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curanovic D, Lyman MG, Bou-Abboud C, Card JP, Enquist LW. Repair of the UL21 locus in pseudorabies virus Bartha enhances the kinetics of retrograde, transneuronal infection in vitro and in vivo. J Virol. 2009;83(3):1173–83. doi: 10.1128/JVI.02102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai P, Akpa JC, Person S. Residues of VP26 of herpes simplex virus type 1 that are required for its interaction with capsids. J Virol. 2003;77(1):391–404. doi: 10.1128/JVI.77.1.391-404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwell KS, Brunetti CR, Hendricks RL, Tang Q, Tang M, Rainbow AJ, Johnson DC. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. Journal of Virology. 1994;68(2):834–45. doi: 10.1128/jvi.68.2.834-845.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwell KS, Doering LC, Johnson DC. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. Journal of Virology. 1995;69(11):7087–98. doi: 10.1128/jvi.69.11.7087-7098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin G, Socolof E, Frank I, Friedman HM. Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. Journal of Virology. 1991;65(12):7046–50. doi: 10.1128/jvi.65.12.7046-7050.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank I, Friedman HM. A novel function of the herpes simplex virus type 1 Fc receptor: participation in bipolar bridging of antiviral immunoglobulin G. Journal of Virology. 1989;63(11):4479–88. doi: 10.1128/jvi.63.11.4479-4488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HM, Cohen GH, Eisenberg RJ, Seidel CA, Cines DB. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984;309(5969):633–5. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- Holmberg SD, Stewart JA, Gerber AR, Byers RH, Lee FK, O’Malley PM, Nahmias AJ. Prior herpes simplex virus type 2 infection as a risk factor for HIV infection. Jama. 1988;259(7):1048–50. [PubMed] [Google Scholar]
- Hook LM, Huang J, Jiang M, Hodinka R, Friedman HM. Blocking antibody access to neutralizing domains on glycoproteins involved in entry as a novel mechanism of immune evasion by herpes simplex virus type 1 glycoproteins C and E. J Virol. 2008;82(14):6935–41. doi: 10.1128/JVI.02599-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook LM, Lubinski JM, Jiang M, Pangburn MK, Friedman HM. Herpes simplex virus type 1 and 2 glycoprotein C prevents complement-mediated neutralization induced by natural immunoglobulin M antibody. J Virol. 2006;80(8):4038–46. doi: 10.1128/JVI.80.8.4038-4046.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husak PJ, Kuo T, Enquist LW. Pseudorabies virus membrane proteins gI and gE facilitate anterograde spread of infection in projection-specific neurons in the rat. Journal of Virology. 2000;74(23):10975–83. doi: 10.1128/jvi.74.23.10975-10983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isola VJ, Eisenberg RJ, Siebert GR, Heilman CJ, Wilcox WC, Cohen GH. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J Virol. 1989;63(5):2325–34. doi: 10.1128/jvi.63.5.2325-2334.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Lubinski JM, Friedman HM. Immunization strategies to block the herpes simplex virus type 1 immunoglobulin G Fc receptor. Journal of Virology. 2004;78(5):2562–71. doi: 10.1128/JVI.78.5.2562-2571.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxton GWG, Haverlock S, Coller KE, Antinone SE, Pincetic A, Smith GA. Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins. PNAS. 2005;102(16):5832–5837. doi: 10.1073/pnas.0500803102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman MG, Curanovic D, Enquist LW. Targeting of pseudorabies virus structural proteins to axons requires association of the viral Us9 protein with lipid rafts. PLoS Pathog. 2008;4(5):e1000065. doi: 10.1371/journal.ppat.1000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw HM, Awasthi S, Wojcechowskyj JA, Friedman HM. Anterograde spread of herpes simplex virus type 1 requires glycoprotein E and glycoprotein I but not Us9. J Virol. 2009;83(17):8315–26. doi: 10.1128/JVI.00633-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw HM, Friedman HM. Herpes simplex virus type 1 glycoprotein E mediates retrograde spread from epithelial cells to neurites. J Virol. 2009;83(10):4791–9. doi: 10.1128/JVI.02341-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashunmugam T, Lubinski J, Wang L, Goldstein LT, Weeks BS, Sundaresan P, Kang EH, Dubin G, Friedman HM. In vivo immune evasion mediated by the herpes simplex virus type 1 immunoglobulin G Fc receptor. Journal of Virology. 1998;72(7):5351–9. doi: 10.1128/jvi.72.7.5351-5359.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GA, Pomeranz L, Gross SP, Enquist LW. Local modulation of plus-end transport targets herpesvirus entry and egress in sensory axons. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(45):16034–9. doi: 10.1073/pnas.0404686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A, Polcicova K, Johnson DC. Herpes simplex virus gE/gI and US9 proteins promote transport of both capsids and virion glycoproteins in neuronal axons. J Virol. 2008;82(21):10613–24. doi: 10.1128/JVI.01241-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185(1):45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- Wang F, Tang W, McGraw HM, Bennett J, Enquist LW, Friedman HM. Herpes simplex virus type 1 glycoprotein E is required for axonal localization of capsid, tegument, and membrane glycoproteins. J Virol. 2005;79(21):13362–72. doi: 10.1128/JVI.79.21.13362-13372.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks BS, Ramchandran RS, Hopkins JJ, Friedman HM. Herpes simplex virus type-1 and -2 pathogenesis is restricted by the epidermal basement membrane. Archives of Virology. 2000;145(2):385–96. doi: 10.1007/s007050050030. [DOI] [PubMed] [Google Scholar]
- Whealy ME, Card JP, Robbins AK, Dubin JR, Rziha HJ, Enquist LW. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. Journal of Virology. 1993;67(7):3786–97. doi: 10.1128/jvi.67.7.3786-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. Jama. 2006;296(8):964–73. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]