Abstract
Introduction
Salivary lysozyme (SLZ) is a proteolytic enzyme secreted by oral leukocytes and contains a domain that has an affinity to advanced glycation end products (AGE). Thus, we hypothesized that SLZ would be associated with metabolic syndrome (metS), a pro-inflammatory state.
Methods
Utilizing cross-sectional data from 250 coronary artery disease (CAD) and 250 non-CAD patients, the association of SLZ with metS was tested by logistic regression analyses controlling for age, sex, smoking, total cholesterol and CRP levels. The analyses were stratified by CAD status to control for the possible effects of CAD.
Results
MetS was found in 122 persons. The adjusted Odds Ratio (OR) for metS associated with the highest quartile of SLZ was 1.95 with 95% confidence interval (CI) 1.20 - 3.12, p-value = 0.007, compared with the lower three quartiles combined. Among the 40 subjects with metS but without CAD, the OR was 1.63 (CI: 0.64 - 4.15, p=0.31), while in the CAD group, SLZ was significantly associated with metS [OR=1.96 (1.09 - 3.52), p= 0.02]. In both subgroups, CRP was not significantly associated with metS.
Conclusion
Salivary lysozyme was significantly associated with metS (OR=1.95) independent of CRP level. Future longitudinal research is warranted.
Keywords: inflammation, metabolic syndrome, Salivary lysozyme, C-reactive protein, atherosclerosis
Salivary lysozyme (SLZ) is a proteolytic enzyme expressed by neutrophil leukocytes in response to infection (Klempner and Malech, 1998) and is capable of cleaving the glycosidic linkage on the bacterial cell wall resulting in an anti-infective action. It also contains a domain that has a strong affinity for advanced glycation endproducts (AGEs) (Karima et al., 2005) and has been used for the removal of AGEs in diabetic patients.(Zheng et al., 2001)
Although oral infections, including periodontitis, have been presumed to contribute to systemic inflammation, hyperglycemia is also known to be a powerful source of inflammatory response.(Liu and Willett, 2002; Pickup, 2004) Because SLZ is closely related to both infection and impaired glucose metabolism, we postulated that SLZ might provide a measure of pro-inflammatory processes involved in metabolic syndrome (metS) and diabetes.(Janket et al., 2008a) Previous studies evaluating the relationship of C-reactive protein (CRP) and metabolic syndrome(Conen et al., 2009; Ridker et al., 2003) were conducted in apparently healthy women and did not adjust for the multitude of other factors affecting CRP levels.(Kushner et al., 2006) Although Drs. Ridker and Silvertown acknowledged the close relationship of oral infection/inflammation systemic inflammation, (Ridker and Silvertown, 2008) the three-way relationship assessment (oral inflammation, systemic inflammation and vascular inflammation) has never been conducted. The objective of this study was to test the hypothesis that “salivary lysozyme is associated with metS, independent of systemic inflammation” in a population with high prevalence of coronary artery disease.
The present cohort of the Kuopio Oral Health and Heart (KOHH) Study consists of 250 (50%) of subjects with diagnosed CAD and 250 (50%) without CAD. The aims of this study were: (1) to evaluate whether salivary lysozyme is associated with metabolic syndrome; (2) to assess if adjusting systemic inflammation changes the relationship of SLZ to metabolic syndrome; (3) Finally, if (2) is proven true, then, to determine which inflammatory factor (local or systemic) has a stronger association with metabolic syndrome.
Materials and methods
Ethical and human subjects' protection
This is a secondary data analysis of the Kuopio Oral Health and Heart (KOHH) Study. The Joint Ethical Committee of the Kuopio University Hospital and the University of Kuopio approved the study protocol. All participants signed a written informed consent and the KOHH Study adhered to the guidelines set forth by the Declaration of Helsinki and the Belmont Accord to assure the safety of human research subjects.
Study population
Kuopio Oral Health and Heart (KOHH) study was initiated in 1996 to investigate the relationship between oral health and coronary artery disease (CAD) in Kuopio, Finland. We recruited 250 consecutive cardiac patients at Kuopio University Hospital who were referred for coronary angiography and confirmed as having CAD determined by the presence of at least 50% stenosis in one of the coronary arteries. Potential subjects were excluded if they took antibiotics during the previous 30 days or had chronic infection other than dental disease. Also recruited were 250 age- and gender-matched controls who were admitted to the general surgery or otorhinolaryngology (ORL) departments at the same hospital for an elective surgery. They were considered as not having heart disease based on the medical history and pre-admission electrocardiogram (ECG) and were the representative of the population of the same catchment area where the cases arose. The same exclusion and inclusion criteria were applied to non-cardiac patients. Additional exclusion criteria were: (1) those who needed emergency coronary by-pass surgery or valvular replacement surgery; (2) those whose disease status was so grave that a dental examination or dental x-ray could not be performed safely; (3) those who received antibiotic prophylaxis prior to periodontal probing. Further details regarding this cohort have been published elsewhere. (Janket et al., 2004; Janket et al., 2006; Qvarnstrom et al., 2008)
Main exposure
Oral examinations were conducted by a single examiner (MQ) and the results were published previously.(Janket et al., 2004; Meurman et al., 2003a; Meurman et al., 2003b) To avoid diurnal fluctuation, saliva samples were collected from the subjects between 7 and 9 a.m. Subjects had been advised not to eat or smoke 1 hour before saliva collection. Using the free flow method, saliva was collected into a 10 mL test tube for 5 minutes after initial swallowing. Whenever possible, fresh saliva was centrifuged (10 min, 12,000g) and analyzed immediately. SLZ was quantified at the Kuopio University research laboratory by the modified lysoplate method utilizing Micrococcus lysodeikticus (Sigma Chemical Co., St. Louis, Mo.) and human milk lysozyme (Sigma Chemical Co.) and bovine serum albumin (Sigma Chemical Co.) as standards according to the methods previously used (Rudney and Smith, 1985). The reported Coefficient of Variation (CV) of lysozyme was between 4.2% and10%.(Desai et al., 2006; Sato et al., 2001).
Other covariates assessed
Age in years, smoking in three categories; never-smokers, current smokers and past smokers were assessed. Body mass index (BMI) was calculated by weight in kg divided by squared height in meters. C-reactive protein (CRP) was measured by high-sensitivity immuno-turbidometry assay utilizing the Hitachi 717 analyzer. The reported Coefficient of Variation (CV) for CRP assay was between 8.1 -11.4%.(Aziz et al., 2003; Sung et al., 2002) All blood samples were analyzed immediately in the hospital laboratory. The analyses were performed in batches including both cases and controls to evenly distribute any potential environmental changes and measurement variability. Total cholesterol (TC), triglyceride (TG), and high-density lipoprotein cholesterol (HDL) were measured by automated enzymatic technique. Hypertension (HT) and diabetes were ascertained by medical record review by one of the authors (MQ). Subjects were categorized as hypertensive or diabetic if their medical records documented these diagnoses or their treatments.
Ascertainment of metabolic syndrome
From the laboratory measures, if a person satisfied 3 out of 5 National Cholesterol Education Program (NCEP) criteria for metabolic syndrome (Grundy et al., 2004), s/he was deemed to have metabolic syndrome.
NCEP criteria include (Grundy et al., 2004):
Central obesity waist circumference > 102 cm (male) or 88 cm (female)
Fasting blood glucose > 110 mg/dl (6.1 mmol/L) or having diabetes
Systolic blood pressure ≥ 130 mm Hg Diastolic blood pressure ≥ 85 mmHg
Triglyceride ≥ 150 mg/dL (1.69 mmol/L)
High density lipoprotein cholesterol < 40 mg/dL (1.04 mmol/L in male) < 50 mg/dL (1.29 mmol/L in female)
We modified NCEP criteria by substituting waist circumference with BMI > 28, more stringent criterion than the International Diabetes Federation (IDF) criteria following the precedence (Ridker et al., 2003).
Statistical analysis
Using Statistical Analysis System (SAS) version 9.1, the basic characteristics such as mean age, sex, smoking status, body mass index, number of teeth, SLZ and cholesterol levels were compared between those with and without metS in univariate analyses. We used t-tests, chi square tests or Wilcoxon non-parametric tests depending on the normality of the variable distributions. In multivariate analyses, the relationship between the probability of having metS who belong in the top 25% of SLZ levels was examined utilizing logistic regression methods. All p-values were calculated as two-tailed, and all confidence intervals were computed at the 95 percent level. Initially, we compared the odds of having metS in quartiles of SLZ levels. Since there appeared to be a threshold at the top quartile, we dichotomized the data at the top 25% to better capture this trend. To control for the effect of systemic inflammation, we dichotomized CRP at 2 mg/L or 3 mg/L as they were considered a marker for moderate risk for cardiovascular disease.(Yeh and Willerson, 2003) However, in this cohort with high vascular inflammation, these cutoffs did not generate a valid maximum likelihood estimates suggesting that both compared groups have CRP levels above these cutoffs. Therefore, we used log-transformed CRP. To compare the robustness in the relationship to metS, we fitted three separate models: one SLZ as the predictor, CRP as the predictor and the third with both SLZ and CRP as the predictors.
Results
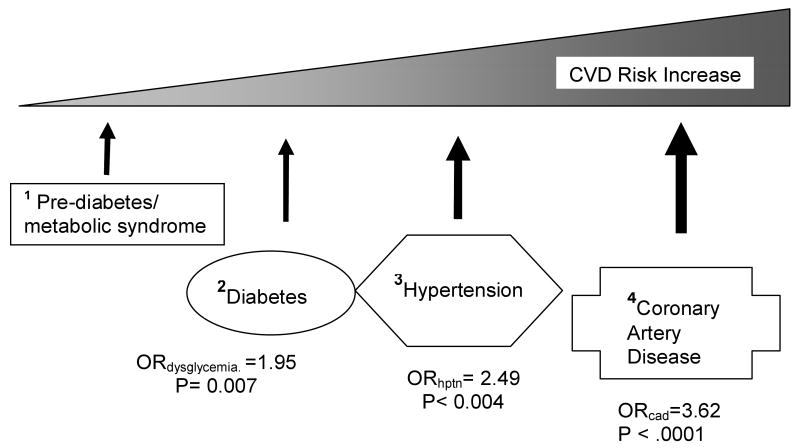
There were 122 subjects with metabolic syndrome or diabetes. High triglyceride and low HDL levels were prominent features in the patients with metS. The frequencies of each component of metS subgroups are presented in Table 1. Salivary lysozyme levels appear to be highly correlated to cholesterol subtypes and to a lesser degree with diabetes. However, SLZ did not appear to be associated with BMI (Table 1B), which was significantly associated with periodontitis in another study. (Offenbacher et al., 2009) In Table 2, the baseline characteristics, age, and sex, were not statistically different between the groups. CRP levels appeared to differ according to the CAD status rather than to the metS status. In other words, CRP levels were distinctly higher in the CAD group than in the non-CAD group regardless of metS status. On the contrary, SLZ displayed a dose-response relationship consistent with the hypothesis with increasing atherogenicity beginning from metS/diabetes, hypertension to CAD. This hypothesis is illustrated in Figure 1.
Table 1.
Frequencies of metabolic syndrome components according to NCEP criteria
| Metabolic syndrome component | frequency |
|---|---|
| Trig*, obese†, and low_HDL‡ | 36 |
| Trig, Obese, and Hypertension | 23 |
| Trig, low_HDL, and Hypertension | 67 |
| Hypertension, obese and low_HDL | 19 |
| Both MetS§ and DM‖ in the same person | 23 |
| Total Metabolic syndrome (metS) | 122 |
| Table 1B. Salivary lysozyme levels related to each component of metabolic syndrome | |||
|---|---|---|---|
| Criterion absent | Criterion present | P-value | |
| Mean S. lysozyme (SD) μg/ml | Mean S. lysozyme (SD) μg/ml | ||
| NCEP_obesity | 31.5 (35.4) | 33.0 (37.1) | 0.76 |
| NCEP_triglyceride | 28.4 (32.7) | 34.9 (37.1) | 0.04 |
| NCEP_HDL | 27.6 (31.8) | 37.2 (38.3) | 0.001 |
| Hypertension | 28.3 (32.8) | 39.4 (40.3) | 0.0008 |
| Diabetes | 31.8 (35.9) | 35.6 (36.0) | 0.10 |
Trig: high triglyceride according to NCEP criterion (≥1.69 mmol/L)
Obese: body mass index >=28
HDL: high density lipoprotein cholesterol (<1.04 mmol/L in male, 1.29 mmol/L in female)
MetS: metabolic syndrome
DM: diabetes mellitus
NCEP: National cholesterol education program
Table 2. General characteristics of the cohort.
| Without CAD (N=250) |
With CAD (N=250) |
|||||
|---|---|---|---|---|---|---|
| Without metS N=212 |
With metS N=38 |
p-value | Without metS N=152 |
With metS N=81 |
p-value | |
| Mean age (S.D.) | 58.9 (9.8) | 61.0 (9.2) | 0.29 | 59.6 (9.3) | 60.1 (8.8) | 0.49 |
| Sex (N, %) | ||||||
| • Men | 137(65.2%) | 22 (55.0%) | 0.46 | 97 (63.8%) | 50(61.7%) | 0.78 |
| • Women | 75 (34.8%) | 16 (45.0%) | 55 (36.2%) | 31(38.3%) | ||
| Body Mass Index (BMI) mean (SD) |
25.4 (3.4) | 28.9(4.1) | <.0001 | 23.3(2.8) | 25.6 (3.6) | <.0001 |
| Salivary lysozyme (mg/L)# | ||||||
| Median (inter- quartile range) |
11.5 (4.94-30.7) | 18.8 (8.3-36.2) | 0.08 | 26.9 (10.1-54.8) | 36.2 (12.0-56.7) | 0.09 |
| Proportion with > 12 years of education N (%) | 85 (40.5%) | 13 (32.5%) | 0.59 | 37 (24.3%) | 17(21.0%) | 0.62 |
| Edentulism (N, %) | 27 (12.9%) | 9 (22.5%) | 0.08 | 47 (30.9%) | 35 (43.2%) | 0.06 |
| Total cholesterol (mmol/L) mean (SD) |
5.17 (0.97) | 5.77 (1.03) | 0.03 | 5.60 (1.01) | 5.70 (1.03) | 0.51 |
| Triglyceride (mmol/L) mean (SD) |
1.67 (0.96) | 2.21 (0.84) | 0.0003 | 1.81(0.89) | 2.69 (1.16) | <.0001 |
| HDL cholesterol (mmol/L) mean (SD) |
1.34 (0.32) | 0.95(0.27) | <.0001 | 1.18 (0.30) | 1.01 (0.24) | <.0001 |
| Hypertension (N, %) |
29 (13.9%) | 23 (57.5%) | 0.0001 | 50 (32.9%) | 66 (81.5%) | <.0001 |
| CRP (mg/L)** Median (inter-quartile range) |
4.0 (2.0 – 5.0) | 4.0 (2.0 – 5.0) | 0.57 | 9.0 (8.0 – 20.0) | 10.0 (9.0- 21) | 0.54 |
| Smoking N (%) | ||||||
| • Never smoker | 172 (81.9%) | 31 (77.5%) | 0.67 | 80 (52.6%) | 41 (50.6%) | 0.63 |
| • Current smoker | 19 (9.1 %) | 5 (12.5%) | 18 (11.8%) | 7 (8.6%) | ||
| • Past smoker | 19 (9.1%) | 4 (10.0%) | 54 (35.5%) | 33 (40.7%) | ||
• # SLZ and **CRP were compared using non-parametric, median Test due non-normal distribution.
• Due to missing values in dependent, explanatory and confounding variables, the actual analyses included < 500 observations.
Figure 1.
CVD Risk Increase associated with Salivary lysozyme
References: 1,2 : the current study
3: Qvarnstrom M, Janket S, Jones JA, Nuutinen P, Baird AE, Nunn ME, Van Dyke TE, Meurman JH: Salivary lysozyme and prevalent hypertension. Journal of Dental Research 87:480-484, 2008
4: Janket SJ, Meurman JH, Nuutinen P, Qvarnstrom M, Nunn ME, Baird AE, Van Dyke TE, Jones JA: Salivary lysozyme and prevalent coronary heart disease: possible effects of oral health on endothelial dysfunction. Arteriosclerosis, Thrombosis & Vascular Biology 26:433-434, 2006
In a multivariate analysis where age, sex, education, total cholesterol and logCRP were controlled for, the ORs for each quartile of SLZ were 1.00, 2.12, 1.49, and 2.75 (p-value for trend = 0.007). It appeared that the odds for having metS spiked at the top quartile. To capture this trend better we dichotomized SLZ at the top 25% and lower 3 quartiles combined. Since adjusting smoking by categorical variable or linear trend did not materially change the main parameter estimate, we used smoking as a linear trend to save the degree of freedom. Adjusting total cholesterol did not change the parameter estimate of SLZ (1.93 vs. 1.92) and for the reason of parsimony, we removed it from the model in some analyses. As shown in Table 3, being in the highest quartile of SLZ was associated with a nearly two-fold increase in the OR of having metS with OR= 1.95, (95% confidence interval: 1.20 - 3.12) p= 0.007.
Table 3.
Multivariate models to assess the association of salivary lysozyme and metabolic syndrome
| Main predictors | Odds ratio (OR) (95% confidence interval) | p-values | |
|---|---|---|---|
| Model 1. Whole cohort (N= 500) |
Lysozyme quartiles (mg/L) | 0.007** (for trend) | |
| Q1 (0 - 7.70) | 1.00 (reference) | ||
| Q2 (7.71 - 20.43) | 2.12 (1.12 - 4.03) | ||
| Q3 (20.44 - 44.56) | 1.49 (0.77 - 2.89) | ||
| Q4 (> 44.56) | 2.75 (1.45 - 5.20) | ||
| Model 2. Whole cohort (N= 500) |
Dichotomy of SLZ | 0.007** | |
| • Lower 3 quartiles of SLZ | 1.00 (reference) | ||
| • 4th quartile of SLZ | 1.95 (1.20 - 3.12) | ||
All models adjusted for Age, Sex, Smoking (in 3 categories), Education, Total cholesterol (log-transformed), and CRP(log-transformed).
To control for the effect modification by CAD, we conducted stratified analyses. Among 250 persons without CAD, both SLZ and CRP were not significantly associated with metS. Considering the small number of individual with metS (N=38), non-significant p-values were expected. Among 250 persons with CAD, high SLZ was significantly associated with having metS (N=81), OR=1.96 (1.09 - 3.52), p-value= 0.02. However, CRP was not significantly associated with metS. These results are presented in Table 4.
Table 4.
Multivariate adjusted association of salivary lysozyme with metabolic syndrome stratified by CAD
| Main predictors | Odds ratio (OR) (95% confidence interval) | p-values | |
|---|---|---|---|
| Model 3. Subgroup without CAD (N= 250) |
• Lower 3 quartiles of SLZ | 1.00 (reference) | 0.31 |
| • 4th quartile of SLZ | 1.63 (0.64 - 4.15) | ||
| Model 4. Subgroup with CAD (N= 250) |
• Lower three quartiles of SLZ | 1.00 (reference) | 0.02 |
| • 4th quartile of SLZ | 1.97 (1.09 - 3.56) | ||
• Model 3 is adjusted for age, sex, smoking (in 3 categories), education, and log-transformed CRP.
• Model 4 is adjusted for Age, Sex, Smoking (in 3 categories), Education, Total cholesterol (log-transformed) and CRP (log-transformed).
To make a comparison, we fitted either top 25% of SLZ or the same of CRP or both in the model (model 5, 6 and 7) as given in Table 5. The 4th quartile of CRP was not significantly associated with metS while the 4th quartile of SLZ was. When SLZ was added to the CRP model, CRP became significant suggesting contribution of SLZ to CRP. It should be noted, however, that adding CRP in the model did not change the point estimate or the p-value of SLZ.
Table 5. Comparison of explanatory ability of Salivary lysozyme and CRP.
| Main predictor(s) | Odds ratio (OR) (95% confidence interval) | p-values | |
|---|---|---|---|
| Model 5 Subgroup with CAD (N= 250) |
• 4th quartile of S. lysozyme level (≥ 44.6 mg/L) | 1.81(1.02 - 3.21)) | 0.04*** |
| • reference | 1.00 | ||
| Model 6 Subgroup with CAD (N= 250) |
• 4th quartile of CRP level (>10 mg/L) | 1.68 (0.96 - 2.93) | 0.07 (n.s.) |
| • reference | 1.00 | ||
| Model 7 Subgroup with CAD (N= 250) |
Both S. lysozyme and CRP In the same model | ||
| • 4th quartile of SLZ | 2.00 (1.10 - 3.61) | 0.02*** | |
| • 4th quartile of CRP | 1.84 (1.03 - 3.26) | 0.04*** | |
• Model 5,6, and 7 were adjusted for Age, Sex, Smoking (in 3 categories), Education, Total cholesterol (log-transformed).
significant at the α-level of 0.05
Discussion
In this cohort with high prevalence of inflammatory vascular disease, salivary lysozyme was significantly associated with metabolic syndrome, independent of CRP. This finding is consistent with our previous observation that approximately 26% of CRP can be explained by oral infection (Janket et al., 2010) and further confirms that SLZ has been significantly associated with every step in the continuum of the inflammatory cardiovascular disease risk, called the “common soil” of atherogenesis (Stern, 1995) as illustrated in Figure 1. Increasing level of SLZ coincided with increasing intensity of postulated atherogenicity (in Figure 1 and Table 1) while CRP showed distinct threshold effect (Table 1). As shown in Table 2, SLZ was nearly significantly associated with metS in both subgroups (p= ∼0.08) with or without CAD. On the contrary, CRP was not significantly associated with metS in either group (p=∼0.54). These subtle differences may not be obvious in large cohorts because significant p-value is a function of sample size.(Gardner and Altman, 1986; Panagiotakos, 2008)
After the JUPITER results publication, dispensation of rosuvastatin has been approved by the U.S. FDA in individuals without clinically evident coronary heart disease but with an increased risk of cardiovascular disease (CVD) based on age (men ≥50 and women ≥60), CRP ≥ 2 mg/L, and the presence of at least one additional CVD risk factor.(FDA, 2010) The median CRP levels and mean age of our cohort are similar to those of JUPITER cohort (Ridker et al., 2009). CRP alone was not a significant predictor of met S in the group with CAD in the current study. Only when SLZ was added to the model CRP became significant. This suggests that SLZ and CRP are confounders (Table 5). As reported in the previous publication, CRP appeared to be more strongly associated with the BMI and diabetes components (Ridker et al., 2003) while SLZ was more closely associated with the hypertension, triglyceride and HDL cholesterol components of metS.(Table 1B in the current study) This is in agreement with the latest report that hypertension and cholesterol levels were more powerful markers of atherosclerosis than dysglycemia (Sarwar et al., 2010) and supports our hypothesis illustrating the echelon of dysglycemia and hypertension on the continuum of the atherosclerosis pathway (Figure 1). Furthermore, a newly published report demonstrated that arterial lysozyme was a more powerful predictor of atherosclerosis than CRP and indirectly supports our observation presented herein.(Abdul-Salam et al., 2010) Considering our results together with the fact that high proportions of current smokers (16%) and of individuals with metabolic syndrome (41%) in the JUPITER cohort (Ridker et al., 2008), we find that remediating the underlying factors contributing to elevated CRP such as oral inflammation, smoking or metabolic syndrome may be a more prudent approach than statin administration.
Although lysozymes are expressed in various mucosal surfaces through out the human body, e.g., in the ophthalmic, respiratory and digestive mucosae, its local production is not affected by the expression in other locations.(Wagner and Wagnerova, 1989) The variability of SLZ and CRP assays being similar (at approximately 10%), the differences we observed in SLZ and CRP in relation to metS could be measurement errors. It, however, could also be due to a non-specific nature of CRP, which has been noted by several researchers. (Kushner et al., 2006; Levinson and Elin, 2002)
Poor oral health is a restricting factor for a healthy diet. Edentulous persons have been shown to have lower intake of fruits and vegetables, (Nowjack-Raymer and Sheiham, 2003) which is a significant predictor for future diabetes.(Liu et al., 2004) The mastication difficulties force the edentulous persons to favor soft, easily chewable carbohydrates with high glycemic index.(Liu and Willett, 2002) This impact of oral health on diet has been largely ignored by nutrition research and is often attributed to dietary effects. Nonetheless, reverse causation cannot be completely ruled out due to our cross-sectional study design.
A major strength of the present study is that we explicitly adjusted for CRP to control for the systemic inflammation. Many reports involving CRP have not adjusted for the multitude of other potential contributors to systemic inflammation (Kushner et al., 2006) including oral infection.
Several limitations are also noted. The cross-sectional study design is one of them. Therefore, our results may not be interpreted in a causal context. However, the significant association of SLZ with every early stage on the pathway to CAD suggests that the relationship of SLZ and atherosclerosis may be longitudinal (Figure 1). However, whether the relationship between SLZ and metS is causal or not must be determined from future studies. Also it should be noted that not all significant risk factors identified in longitudinal studies have causal relationship.(Janket et al., 2008b; Wang, 2008)
Another limitation is that we did not have information regarding alcohol consumption or fasting glucose levels. Thus, some participants in non-metS group actually might have metS, and our results might have under-estimated the true association of SLZ and metS.
In summary, our results suggest an association of salivary lysozyme with metabolic syndrome above and beyond CRP. Future longitudinal studies may establish this novel marker as a risk factor for metabolic syndrome.
Clinical relevance.
Scientific rationale
Unlike previous studies, we examined the relationship between oral inflammation measured by salivary lysozyme and metabolic syndrome in a population with high prevalence of inflammatory vascular disease.
Principal Findings
Salivary lysozyme was significantly associated with metabolic syndrome (metS) controlling for C-reactive protein (CRP) and other risk factors.
Practical Implications
The positive results of the JUPITER trial brought greater public attention to CRP, a non-specific inflammatory marker. Additionally, U.S. Food and Drug Administration (FDA) approved the administration of rosuvastatin based on CRP levels along with one additional risk factor in men aged 50 and older, and women aged 60 and older. However, considering the pleiotropic nature of statins and the non-specific characteristic of CRP, rosuvastatin dispensation based on CRP level and one unspecified risk factor leaves many unanswered questions. The present study is consistent with our previous observation that oral infection explains approximately 26% of CRP and reducing oral inflammation prior to statin administration may be prudent.
Acknowledgments
Funding Sources: The funding sources listed below had no influence in our results.
1. This study is supported by a grant from the American Heart Association # 0635351N to Dr. Sok-Ja Janket.
2. Dr. Meurman is supported by grant TYH 3245 from the Helsinki University Central Hospital, Helsinki, Finland and a grant from the Finnish Medical Society.
3. Dr. Jones is supported by NIH K24 DE018211 award from National Institute of Health.
4. Dr. Van Dyke is supported by NIH grant DE DE16191 and USPHS grant DE15566.
5. Dr. Garcia is supported by NIH grant K24 DE000419 and R01 DE019833.
Footnotes
Declaration of Competing Interests: Nothing to declare
Abstract of this study has been presented at The 3rd International Metabolic Syndrome Congress in Nice, France in April, 2009.
References
- Abdul-Salam VB, Ramrakha P, Krishnan U, Owen DR, Shalhoub J, Davies AH, et al. Identification and assessment of plasma lysozyme as a putative biomarker of atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30(5):1027–33. doi: 10.1161/ATVBAHA.109.199810. [DOI] [PubMed] [Google Scholar]
- Aziz N, Fahey JL, Detels R, Butch AW. Analytical performance of a highly sensitive C-reactive protein-based immunoassay and the effects of laboratory variables on levels of protein in blood. Clin Diagn Lab Immunol. 2003;10(4):652–7. doi: 10.1128/CDLI.10.4.652-657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conen D, Rexrode KM, Creager MA, Ridker PM, Pradhan AD. Metabolic syndrome, inflammation, and risk of symptomatic peripheral artery disease in women: a prospective study. Circulation. 2009;120(12):1041–7. doi: 10.1161/CIRCULATIONAHA.109.863092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A, Lee C, Sharma L, Sharma A. Lysozyme refolding with cyclodextrins: structure-activity relationship. Biochimie. 2006;88(10):1435–45. doi: 10.1016/j.biochi.2006.05.008. [DOI] [PubMed] [Google Scholar]
- FDA. Center for Drug Evaluation and Research. Silver Spring, MD, U.S. Food and Drug Administration; 2010. New Indication for Crestor. pp. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm199891.htm. [Google Scholar]
- Gardner MJ, Altman DG. Confidence intervals rather than P values: estimation rather than hypothesis testing. British Medical Journal Clinical Research Ed. 1986;292(6522):746–50. doi: 10.1136/bmj.292.6522.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, American Heart A, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. see comment. [DOI] [PubMed] [Google Scholar]
- Janket SJ, Jones JA, Meurman JH, Baird AE, Van Dyke TE. Oral infection, hyperglycemia, and endothelial dysfunction. Oral Surgery Oral Medicine Oral Pathology Oral Radiology & Endodontics. 2008a;105(2):173–9. doi: 10.1016/j.tripleo.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janket SJ, Shen Y, Baird AE. Why must new cardiovascular risk factors be carefully re-assessed prior to clinical application? Eur Heart J. 2008b;29(10):1336–7. doi: 10.1093/eurheartj/ehn151. comment. author reply 1337. [DOI] [PubMed] [Google Scholar]
- Janket S, Meurman JH, Baird AE, Qvarnstrom M, Nuutinen P, Ackerson LK, et al. Salivary immunoglobulins and prevalent coronary artery disease. J Dent Res. 2010;89(4):389–94. doi: 10.1177/0022034509359884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janket SJ, Qvarnstrom M, Meurman JH, Baird AE, Nuutinen P, Jones JA. Asymptotic dental score and prevalent coronary heart disease. Circulation. 2004;109(9):1095–100. doi: 10.1161/01.CIR.0000118497.44961.1E. see comment. [DOI] [PubMed] [Google Scholar]
- Janket SJ, Meurman JH, Nuutinen P, Qvarnstrom M, Nunn ME, Baird AE, et al. Salivary lysozyme and prevalent coronary heart disease: possible effects of oral health on endothelial dysfunction. Arteriosclerosis, Thrombosis & Vascular Biology. 2006;26(2):433–4. doi: 10.1161/01.ATV.0000198249.67996.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karima M, Kantarci A, Ohira T, Hasturk H, Jones VL, Nam BH, et al. Enhanced superoxide release and elevated protein kinase C activity in neutrophils from diabetic patients: association with periodontitis. J Leukoc Biol. 2005;78(4):862–70. doi: 10.1189/jlb.1004583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempner MS, Malech HL. Phagocytes: normal and abnormal neutrophil host defenses. In: Gorbach SL, Bartlett JG, Blacklow NR, editors. Infectious diseases. Philadelphia: Saunders; 1998. pp. 41–47. [Google Scholar]
- Kushner I, Rzewnicki D, Samols D. What Does Minor elevation of C-reactive protein signify? The American Journal of Medicine. 2006;119(2):166.e17–166.e28. doi: 10.1016/j.amjmed.2005.06.057. [DOI] [PubMed] [Google Scholar]
- Levinson S, Elin R. What is C-reactive protein telling us about coronary artery disease? Arch Intern Med. 2002;162:389–392. doi: 10.1001/archinte.162.4.389. [DOI] [PubMed] [Google Scholar]
- Liu S, Willett WC. Dietary glycemic load and atherothrombotic risk. Current Atherosclerosis Reports. 2002;4(6):454–61. doi: 10.1007/s11883-002-0050-2. [DOI] [PubMed] [Google Scholar]
- Liu S, Serdula M, Janket SJ, Cook NR, Sesso HD, Willett WC, et al. A prospective study of fruit and vegetable intake and the risk of type 2 diabetes in women. Diabetes Care. 2004;27(12):2993–6. doi: 10.2337/diacare.27.12.2993. [DOI] [PubMed] [Google Scholar]
- Meurman JH, Janket SJ, Qvarnstrom M, Nuutinen P. Dental infections and serum inflammatory markers in patients with and without severe heart disease. Oral Surgery Oral Medicine Oral Pathology Oral Radiology & Endodontics. 2003a;96(6):695–700. doi: 10.1016/j.tripleo.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Meurman JH, Qvarnstrom M, Janket SJ, Nuutinen P. Oral health and health behavior in patients referred for open-heart surgery. Oral Surgery Oral Medicine Oral Pathology Oral Radiology & Endodontics. 2003b;95(3):300–7. doi: 10.1067/moe.2003.22. see comment. [DOI] [PubMed] [Google Scholar]
- Nowjack-Raymer RE, Sheiham A. Association of Edentulism and Diet and Nutrition in US adults. J Dent Res. 2003;82(2):123–126. doi: 10.1177/154405910308200209. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Beck JD, Moss K, Mendoza L, Paquette DW, Barrow DA, et al. Results from the Periodontitis and Vascular Events (PAVE) Study: a pilot multicentered, randomized, controlled trial to study effects of periodontal therapy in a secondary prevention model of cardiovascular disease. J Periodontol. 2009;80(2):190–201. doi: 10.1902/jop.2009.080007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotakos DB. The Value of p-Value in Biomedical Research. The Open Cardiovascular Medicine Journal. 2008;2:97–99. doi: 10.2174/1874192400802010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27(3):813–23. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- Qvarnstrom M, Janket S, Jones JA, Nuutinen P, Baird AE, Nunn ME, et al. Salivary lysozyme and prevalent hypertension. J Dent Res. 2008;87(5):480–4. doi: 10.1177/154405910808700507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107(3):391–7. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Jr, Kastelein JJP, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207. doi: 10.1056/NEJMoa0807646. see comment. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Silvertown JD. Inflammation, C-reactive protein, and atherothrombosis. J Periodontol. 2008;79(8 Suppl):1544–51. doi: 10.1902/jop.2008.080249. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373(9670):1175–82. doi: 10.1016/S0140-6736(09)60447-5. see comment. [DOI] [PubMed] [Google Scholar]
- Rudney JD, Smith QT. Relationships between levels of lysozyme, lactoferrin, salivary peroxidase, and secretory immunoglobulin A in stimulated parotid saliva. Infect Immun. 1985;49(3):469–75. doi: 10.1128/iai.49.3.469-475.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar N, Aspelund T, Eiriksdottir G, Reeta G, Seshasa S, Forouhi NG, et al. Markers of dysglycemia and risk of CHD in people without diabetes: Reykjavik prospective study and systematic review. PLoS Medicine. 2010;7(5):1–11. doi: 10.1371/journal.pmed.1000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato R, Takeyama H, Tanaka T, Matsunaga T. Development of high-performance and rapid immunoassay for model food allergen lysozyme using antibody-conjugated bacterial magnetic particles and fully automated system. Appl Biochem Biotechnol. 2001;91-93:109–16. doi: 10.1385/abab:91-93:1-9:109. [DOI] [PubMed] [Google Scholar]
- Stern MP. Diabetes and cardiovascular disease. The “common soil” hypothesis. Diabetes. 1995;44(4):369–74. doi: 10.2337/diab.44.4.369. [DOI] [PubMed] [Google Scholar]
- Sung HJ, Kim JH, Park R, Lee KR, Kwon OH. Evaluation of Denka-Seiken turbidimetric high-sensitivity C-reactive protein assay. Clin Chem Lab Med. 2002;40(8):840–5. doi: 10.1515/CCLM.2002.146. [DOI] [PubMed] [Google Scholar]
- Wagner V, Wagnerova M. Lack of correlation between serum and salivary concentration levels of immunoglobulin A and lysozyme (muramidase) J Hyg Epidemiol Microbiol Immunol. 1989;33(3):353–6. [PubMed] [Google Scholar]
- Wang T. New cardiovascular risk factors exist, but are they clinically useful? Eur Heart J. 2008;29(4):441–444. doi: 10.1093/eurheartj/ehm644. [DOI] [PubMed] [Google Scholar]
- Yeh ET, Willerson JT. Coming of age of C-reactive protein: using inflammation markers in cardiology. Circulation. 2003;107(3):370–1. doi: 10.1161/01.cir.0000053731.05365.5a. [DOI] [PubMed] [Google Scholar]
- Zheng F, Cai W, Mitsuhashi T, Vlassara H. Lysozyme enhances renal excretion of advanced glycation endproducts in vivo and suppresses adverse age-mediated cellular effects in vitro: a potential AGE sequestration therapy for diabetic nephropathy? Mol Med. 2001;7(11):737–47. [PMC free article] [PubMed] [Google Scholar]