Abstract
Both the ahl allele of Cdh23 and the null mutation of Sod1 have been shown to contribute to age-related hearing loss (AHL) in mice, but mixed strain backgrounds have confounded analyses of their individual and combined effects. To test for the effects of Sod1 deficiency independently from those of Cdh23ahl, we produced mice with four digenic genotypes: Sod1+/+Cdh23ahl/ahl, Sod1+/+ Cdh23+/+, Sod1−/− Cdh23ahl/ahl, and Sod1−/− Cdh23+/+, all on a uniform C57BL/6J strain background. We assessed hearing loss by ABR threshold measurements and evaluated cochlear pathologies in age-matched mice of each digenic combination. ABR analysis showed that Sod1+/+ Cdh23+/+ mice retain normal hearing up to 15 months of age and that hearing loss of Sod1+/+ Cdh23ahl/ahl mice is more age and frequency dependent than that of Sod1−/− Cdh23+/+ mice. ABR results also showed that mice with both gene mutations (Sod1−/− Cdh23ahl/ahl) exhibit the earliest onset and most severe hearing loss, greater than predicted for strictly additive effects. Histological analysis of cochleas showed that hair cell lesions are most severe in Sod1−/− Cdh23ahl/ahl mice followed closely by Sod1+/+ Cdh23ahl/ahl mice and much smaller in Sod1−/− Cdh23+/+ and Sod1+/+ Cdh23+/+ mice. Despite extensive damage to cochlear hair cells, vestibular hair cells appeared remarkably normal in all strains. Although both Sod1−/− and Cdh23ahl/ahl genotypes had strong effects on hearing loss, the Cdh23ahl/ahl genotype was primarily responsible for the increase in hair cell loss, suggesting that the two mutations have different underlying mechanisms of pathology.
Keywords: age-related hearing loss, Sod1, Cdh23, ahl, C57BL/6J, inbred mouse strain, cochlear pathology, ABR thresholds
1. Introduction
Age-related hearing loss (AHL) or presbycusis is a major health concern because of its widespread occurrence among the elderly and its negative influence on their quality of life (Dalton et al., 2003). AHL can be exacerbated by a variety of environmental insults including exposures to loud noises and ototoxic drugs, but at its core is a significant genetic component (DeStefano et al., 2003). Recently, an extensive whole genome association study identified the first human gene mutation that confers susceptibility to AHL (Friedman et al., 2009), but genetic studies of complex traits in human populations remain difficult undertakings. Laboratory strains of mice offer important advantages for studying the genetic basis for AHL because of their short life span, well-defined genetics, and the ability to control their environment. The C57BL/6J strain of mice, in particular, has been used extensively as a model for AHL (Henry et al., 1980; Hequembourg et al., 2001; Mikaelian, 1979; Wang et al., 2008; Willott, 1986). Hearing loss in these mice is first detected in the high frequencies as early as 3–6 months of age (Spongr et al., 1997) and progresses to severe impairment by one year of age (Zheng et al., 1999).
To map genes that might underlie AHL in C57BL/6J (B6) mice, a linkage backcross was generated with mice of the wild-derived inbred strain CAST/EiJ (CAST), which retain normal hearing to old age. Analysis of this cross detected a locus on mid-Chromosome 10 that showed a highly significant linkage with the auditory brainstem response (ABR) thresholds of backcrossed mice (Johnson et al., 1997), and the recessive hearing loss susceptibility allele of this locus, derived from the B6 parent, was designated ahl. The same Chr 10 locus was shown to contribute to AHL in several other inbred mouse strains (Johnson et al., 2000), and subsequent evidence indicated that the strains share the same ahl allele, which is a splice variant of the cadherin 23 (Cdh23) gene (Noben-Trauth et al., 2003). To evaluate the isolated effect of this variant on AHL, a congenic strain, designated B6.CAST-Cdh23Ahl+/Kjn, was produced by backcrossing the CAST-derived AHL resistance allele (Ahl+) onto the B6 strain background. The congenic mice were shown to be protected from early onset hearing loss and basal turn hair cell degeneration, but older mice showed a moderate degree of hearing loss and ganglion cell degeneration, which was greater than that seen in CAST mice but less than that observed in B6 mice (Keithley et al., 2004). Cadherin 23 (CDH23), the protein encoded by Cdh23, later was shown to be a component of stereocilia tip links, which are thought to gate mechanotransduction channels in hair cells (Kazmierczak et al., 2007). The ahl variant of Cdh23, like a recently described ENU-induced missense mutation of this gene (Schwander et al., 2009), may encode a defective protein that weakens tip links or impedes their repair. The accumulation of broken tip links over time could explain the progressive hearing loss associated with these variant alleles.
Oxidative stress and free radical production have also been shown to contribute to AHL in inbred mouse strains (Jiang et al., 2007; McFadden et al., 2001; Staecker et al., 2001). Normal cellular respiration results in the production of the highly toxic superoxide radical that damages cells if it is not quickly eliminated. The superoxide radical is normally eliminated from cells by a family of superoxide dismutase (SOD) enzymes, the most abundant of which is Cu/Zn SOD. Cu/Zn SOD, coded by the Sod1 gene, is abundantly expressed in the cochlea and plays a key role in reducing oxidative stress. Inactivation of the Sod1 gene was reported to accelerate age-related hair cell loss and hearing loss in Sod1−/− mice that were developed on a 129/CD-1 background (McFadden et al., 1999a; McFadden et al., 1999b). The effects of the Sod1 gene deletion in these mice, however, were confounded by unknown contributions from segregating Cdh23 variants and by other strain differences. A subsequent study of Sod1−/− mice on a uniform B6 strain background (congenic strain B6.129S7-Sod1tm1Leb/DnJ) showed that the absence of SOD1 resulted in hearing loss at an earlier age than in wild-type mice (Keithley et al., 2005). Neither source of Sod1−/− mice analyzed in these previous studies, however, allowed for the assessment of the effects of Sod1−/− without the confounding effects of Cdh23ahl/ahl.
Here we report our efforts to test for the effects of Sod1 deficiency independently from those of the Cdh23ahl hearing loss susceptibility allele by producing and analyzing mice with four digenic genotypes: Sod1+/+Cdh23ahl/ahl, Sod1+/+ Cdh23+/+, Sod1−/− Cdh23ahl/ahl, and Sod1−/− Cdh23+/+, all on a uniform B6 strain background. We assessed hearing loss by ABR threshold analysis and evaluated cochlear pathologies in age-matched mice of each of the four digenic combinations. Our findings provide new insights into the specific roles played by tip link integrity (deduced from Cdh23 genotypic effects) and superoxide radicals (deduced from Sod1 genotypic effects) in age-related hearing loss and cochlear pathology.
2. Materials and methods
2.1 Mice
All mice examined in this study originated from The Jackson Laboratory (http://www.jax.org/). To generate C57BL/6J (B6) mice with the four experimental Cdh23 Sod1 digenic genotypes, we first mated B6.CAST-Cdh23Ahl+/Kjn strain mice (JAX stock # 2756; genotype Sod1+/+ Cdh23+/+) with B6.129S7-Sod1tm1Leb/DnJ strain mice (JAX stock # 3881: genotype Sod1−/− Cdh23ahl/ahl) to produce F1 hybrids (genotype Sod1+/− Cdh23+/ahl). The F1 hybrids were then interbred, and F2 intercross progeny with the following four digenic genotypes were selected for study: Sod1+/+ Cdh23+/+, Sod1+/+ Cdh23ahl/ahl, Sod1−/− Cdh23+/+, and Sod1−/− Cdh23ahl/ahl.
Experimental mice were housed in the Research Animal Facility of The Jackson Laboratory, and procedures involving their use were approved by the Institutional Animal Care and Use Committee. The Jackson Laboratory is accredited by the American Association for the Accreditation of Laboratory Animal Care.
2.2 Cdh23 and Sod1 genotyping
To genotype F2 mice obtained from the intercross of (B6.CAST-Cdh23Ahl+/Kjn X B6.129S7-Sod1tm1Leb/DnJ) F1 hybrids, we extracted DNA from tail tips for PCR analysis. To identify Cdh23 genotypes, we typed individual F2 progeny DNAs for microsatellite markers D10Mit130 and D10Mit108, which flank Cdh23 and differ in size between C57BL/6J and CAST/EiJ. If both flanking markers were of C57BL/6J origin, the Cdh23 genotype was presumed to be ahl/ahl, and if both flanking markers were of CAST/EiJ origin, the Cdh23 genotype was presumed to be +/+. For verification, a subsample of mice were genotyped directly by DNA sequence analysis of the Cdh23ahl splice site region (Noben-Trauth et al., 2003). The Jackson Laboratory’s Transgenic Genotyping Service was used to identify mice with Sod1+/+ and Sod1−/− genotypes by a quantitative PCR method described online at URL: http://jaxmice.jax.org/pub-cgi/protocols/protocols.sh?objtype=protocol&protocol_id=2047
2.3 Assessment of hearing
Hearing in mice was assessed by ABR threshold analysis, as previously described (Zheng et al., 1999). Stimulus presentation, ABR acquisition, equipment control and data management were coordinated using computerized equipment from Intelligent Hearing Systems (IHS; Miami, Florida). Briefly, the evoked brainstem responses of anesthetized mice were amplified and averaged and their wave patterns displayed on a computer screen. Broad-band clicks and 8, 16, and 32 kHz tone-bursts were respectively channeled through high frequency transducers into the animals’ ear canals. Auditory thresholds were obtained for each specific auditory stimulus by varying the sound pressure level (SPL) to identify the lowest level at which an ABR pattern could be recognized; 100 dB was the maximum SPL presented for all stimuli. With our testing system, average ABR thresholds (in dB SPL) for normal hearing mice are about 40 dB for click, 30 dB for 8 kHz, 20 dB for 16 kHz, and 45 dB for 32 kHz stimuli.
2.4 Cochlear histology
Cochleas were prepared and evaluated as previously described (Ding et al., 2001; Zheng et al., 2009). Mice were euthanized with CO2, decapitated, and their bullae quickly removed and opened to expose the inner ear. A small hole was carefully made in the round window through which 10% formalin fixative was gently perfused. The cochlea was then immersed in fixative and shipped to the University of Buffalo for analysis. Cochleas were stained with Ehrlich’s hematoxylin solution, the organ of Corti carefully microdissected out into two or three segments and mounted as a flat surface preparation in glycerin on glass slides. Surface preparations were examined with a light microscope (Zeiss Standard, 400X magnification). Inner hair cells (IHC) and outer hair cells (OHC) were counted along successive 0.12–0.24 mm intervals of the organ of Corti beginning at the apex. Hair cells were counted as present if the cell body and cuticular plate were intact. Cochleograms showing percent hair cells missing as a function of percent total distance from the apex were constructed for each animal. Percent hair cell losses were based on laboratory norms for young CBA/CaJ mice (Ding et al., 2001).
2.5 Vestibular histology
Our procedures for assessing the vestibular sensory epithelium have been described previously (Ding et al., 2001; Zheng et al., 2009). The horizontal, superior, and posterior portions of the vestibular cavities were opened by carefully removing the overlying bone. The saccule and utricle were separated from surrounding tissue and the otoconia of the saccule and utricle were removed to visualize the maculae. The ampullae were separated from each semicircular canal by sectioning the nerve fibers, blood vessels and connective tissue and the tissue containing cristae was removed. Specimens were stained with hematoxylin or toluidine blue and mounted in glycerin on glass slides. The surface preparations of each sensory epithelium were viewed with a light microscope (Axioskop, Carl Zeiss) at a magnification of 1000X as described in our earlier publications (Ding et al., 2001; Zheng et al., 2009). The numbers of canal ampullae and the numbers of maculae (either utricle or saccule) examined from each genotype, respectively, were: Sod1+/+ Cdh23+/+ (n = 6 and 2); Sod1+/+ Cdh23ahl/ahl (n = 6 and 4); Sod1−/− Cdh23+/+ (n = 4 and 4) and Sod1−/− Cdh23ahl/ahl (n = 6 and 2). Specimens were photographed with a digital camera (SPOT Insight, Diagnostic Instruments Inc), processed with imaging software (SPOT Software, version 4.6) and images assembled with Adobe Photoshop 5.5.
2.6 Statistical analyses
We used the JMP 7.0 interactive statistics and graphics software program (www.JMP.com) to analyze our data and display the results. Statistical significance of the differences between means was determined with the Tukey test, which corrects for multiple pair-wise comparisons. Analysis of variance (ANOVA) F-test statistics were used to determine the significance of the individual effects of Sod1 and Cdh23 genotypes and their interaction effects on ABR thresholds and percent hair cell loss.
3. Results
3.1 Age-related hearing loss
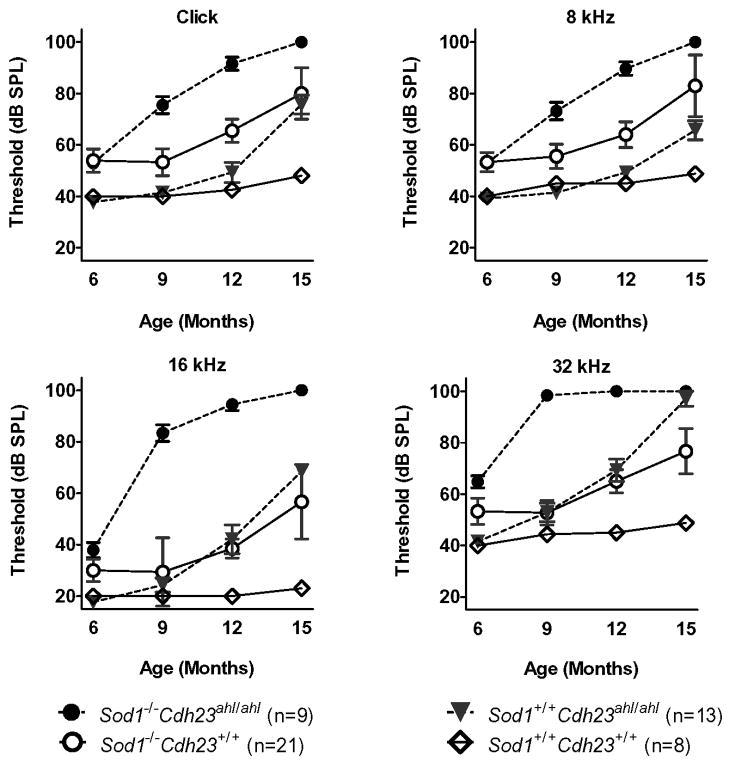
ABR thresholds were measured in all four genotypes at 6, 9, 12, and 15 months of age using clicks, and 8, 16, and 32 kHz tone bursts. Table 1 lists the mean ABR thresholds and their variance estimates (standard deviations) for each of the test groups for each test age and auditory stimulus and the numbers of mice tested in each group. The mean ABR thresholds over time among the four experimental groups (Cdh23 Sod1 digenic genotypes) are graphically illustrated in Figure 1 for each frequency. Mice with Sod1+/+ Cdh23+/+ genotypes retained normal ABR thresholds up to 15 months of age. The ABR thresholds of mice with Sod1+/+ Cdh23ahl/ahl genotypes remained near normal up to 9 months of age, but then increased rapidly at the higher frequencies so that by 15 months of age the 16 and 32 kHz thresholds exceeded those of Sod1−/− Cdh23+/+ mice. ABR thresholds for all frequencies were slightly above normal in Sod1−/− Cdh23+/+ mice at 6–9 months of age with moderate increases at 12 and 15 months of age. Mice with both gene mutations, genotype Sod1−/− Cdh23ahl/ahl, exhibited the earliest onset and most severe hearing loss of the four groups. ABR thresholds at the higher frequencies appeared greater than predicted for strictly additive effects. For example, at 9 months of age the mean 16 kHz thresholds of singly mutant mice, genotypes Sod1+/+ Cdh23ahl/ahl and Sod1−/− Cdh23+/+, are similar to each other and only about 10 dB above those of Sod1+/+ Cdh23+/+ mice, whereas thresholds of doubly mutant Sod1−/− Cdh23ahl/ahl mice are about 60 dB above those of Sod1+/+ Cdh23+/+ mice. A similar trend was evident at other frequencies and ages.
Table 1. Means and standard deviations (st dev) of ABR thresholds (dB SPL) for each genotype, test age, and auditory stimulus.
| genotype “A” | genotype “B” | genotype “C” | genotype “D” | ||
|---|---|---|---|---|---|
| Sod1+/+Cdh23+/+ | Sod1+/+Cdh23ahl/ahl | Sod1−/−Cdh23+/+ | Sod1−/−Cdh23ahl/ahl | ||
| 6 month test age | N = 8 | N = 13 | N = 9 | N = 21 | |
| click | mean | 39.4 | 37.7 | 53.9 | 52.9 |
| st dev | 1.8 | 3.9 | 13.6 | 8.5 | |
| 8 kHz | mean | 40.0 | 39.2 | 53.3 | 52.9 |
| st dev | 0.0 | 1.9 | 11..2 | 8.5 | |
| 16 kHz | mean | 20.0 | 17.7 | 30.0 | 37.9 |
| st dev | 0.0 | 3.3 | 13.0 | 13.1 | |
| 32 kHz | mean | 40.0 | 41.5 | 53.3 | 64.8 |
| st dev | 0.0 | 3.2 | 15.2 | 11.2 | |
| 9 month test age | N = 8 | N = 7 | N = 9 | N = 22 | |
| click | mean | 40.0 | 41.4 | 53.3 | 75.5 |
| st dev | 0.0 | 3.8 | 15.8 | 15.6 | |
| 8 kHz | mean | 45.0 | 41.4 | 55.6 | 73.2 |
| st dev | 5.3 | 3.8 | 14.2 | 15.9 | |
| 16 kHz | mean | 20.0 | 24.3 | 29.4 | 83.4 |
| st dev | 0.0 | 7.3 | 13.3 | 15.1 | |
| 32 kHz | mean | 44.4 | 52.9 | 52.8 | 98.4 |
| st dev | 5.0 | 9.5 | 14.4 | 5.6 | |
| 12 month test age | N = 8 | N = 7 | N = 10 | N = 17 | |
| click | mean | 42.5 | 49.3 | 65.5 | 91.6 |
| st dev | 4.8 | 10.2 | 14.2 | 10.8 | |
| 8 kHz | mean | 45.0 | 49.3 | 64.0 | 89.7 |
| st dev | 5.3 | 6.1 | 15.8 | 10.9 | |
| 16 kHz | mean | 20.0 | 42.1 | 38.5 | 94.5 |
| st dev | 0.0 | 14.7 | 11.6 | 9.6 | |
| 32 kHz | mean | 45.0 | 69.3 | 65.0 | 100 |
| st dev | 5.3 | 11.7 | 14.3 | 0.0 | |
| 15 month test age | N = 8 | N = 7 | N = 3 | N = 2 | |
| click | mean | 48.1 | 75.7 | 80.0 | 100 |
| st dev | 3.7 | 9.8 | 17.3 | 0.0 | |
| 8 kHz | mean | 48.8 | 65.7 | 83.3 | 100 |
| st dev | 3.5 | 9.8 | 20.8 | 0.0 | |
| 16 kHz | mean | 23.1 | 68.6 | 56.7 | 100 |
| st dev | 5.3 | 3.8 | 25.2 | 0.0 | |
| 32 kHz | mean | 48.8 | 97.1 | 76.7 | 100 |
| st dev | 3.5 | 7.6 | 15.3 | 0.0 | |
Figure 1. Effects of genotype, age and auditory stimulus on ABR thresholds.
ABR thresholds (means ± standard errors) are compared among mice with four different digenic genotypes of Cdh23 and Sod1, all on a uniform C57BL/6J background. Mice were tested at 6, 9, 12, and 15 months of age, and ABRs were recorded for broad-band click and 8 kHz, 16 kHz, and 32 kHz pure tone stimuli. The numbers of mice evaluated for each genotype and test age are given in Table 1.
Detailed comparisons of ABR threshold means and confidence intervals are illustrated in Supplementary Fig. 1. Those genotype pairs with statistically significant mean differences are listed in Table 2 and Supplementary Fig. 1 for each age and test stimulus. At 6 months of age, the Sod1 genotype had a significant effect on ABR thresholds for all test stimuli, but the Cdh23 genotype had a significant effect only on 32 kHz thresholds (Table 2). By 9 months of age, however, the individual Sod1 and Cdh23 genotypes and their interaction all had significant effects on ABR thresholds, which were seen most strongly at the higher 16 and 32 kHz test frequencies.
Table 2. Statistical analyses of ABR thresholds.
Analysis of variance (ANOVA) F-tests were used to evaluate the separate and interactive effects of Sod1 and Cdh23 genotypes on click, 8 kHz, 16 kHz, and 32 kHz thresholds of mice tested at 6, 9, 12, and 15 months of age. The probabilities of obtaining the calculated F ratios solely by chance are shown for each effect in each stimulus-age category. Statistically significant effects at the 0.05 alpha level are indicated by a single asterisk and at the 0.001 level by two asterisks. The digenic genotypes for the Tukey pair-wise tests of means are indicated by letters as follows: Sod1+/+ Cdh23+/+ (A), Sod1+/+ Cdh23ahl/ahl (B), Sod1−/− Cdh23+/+ (C), Sod1−/− Cdh23ahl/ahl (D). Only those genotype pairs with significant mean differences at the 0.05 alpha level are shown.
| 6 mo | 9 mo | 12 mo | 15 mo | |
|---|---|---|---|---|
| Click | ||||
| ANOVA for monogenic genotypes | ||||
| Sod1 main effect | <0.0001 ** | <0.0001 ** | <0.0001 ** | <0.0001 ** |
| Cdh23 main effect | 0.582 | 0.008 * | <0.0001 ** | <0.0001 ** |
| Sod1*Cdh23 interaction | 0.895 | 0.020 * | 0.009 * | 0.429 |
| Tukey pair-wise tests of digenic genotypes | ||||
| significant mean differences (p<0.05) | A-C, A-D, B-C, B-D | A-D, B-D, C-D | A-C, A-D, B-C, B-D, C-D | A-B, A-C, A-D, B-D |
| 8 kHz | ||||
| ANOVA for monogenic genotypes | ||||
| Sod1 main effect | <0.0001 ** | <0.0001 ** | <0.0001 ** | <0.0001 ** |
| Cdh23 main effect | 0.776 | 0.107 | 0.0001 ** | 0.0047 * |
| Sod1*Cdh23 interaction | 0.947 | 0.017 * | 0.0043 * | 0.977 |
| Tukey pair-wise tests of digenic genotypes | ||||
| significant mean differences (p<0.05) | A-C, A-D, B-C, B-D | A-D, B-D, C-D | A-C, A-D, B-C, B-D, C-D | A-B, A-C, A-D, B-D |
| 16 kHz | ||||
| ANOVA for monogenic genotypes | ||||
| Sod1 main effect | <0.0001 ** | <0.0001 ** | <0.0001 ** | <0.0001 ** |
| Cdh23 main effect | 0.371 | <0.0001 ** | <0.0001 ** | <0.0001 ** |
| Sod1*Cdh23 interaction | 0.104 | <0.0001 ** | <0.0001 ** | 0.840 |
| Tukey pair-wise tests of digenic genotypes | ||||
| significant mean differences (p<0.05) | A-D, B-C, B-D | A-D, B-D, C-D | A-B, A-C, A-D, B-D, C-D | A-B, A-C, A-D, B-D, C-D |
| 32 kHz | ||||
| ANOVA for monogenic genotypes | ||||
| Sod1 main effect | <0.0001 ** | <0.0001 ** | <0.0001 ** | 0.0012 * |
| Cdh23 main effect | 0.032 * | <0.0001 ** | <0.0001 ** | <0.0001 ** |
| Sod1*Cdh23 interaction | 0.099 | <0.0001 ** | 0.058 | 0.0057 * |
| Tukey pair-wise tests of digenic genotypes | ||||
| significant mean differences (p<0.05) | A-C, A-D, B-C, B-D, C-D | A-D, B-D, C-D | A-B, A-C, A-D, B-D, C-D | A-B, A-C, A-D, B-C, C-D |
3.2 Cochlear pathology
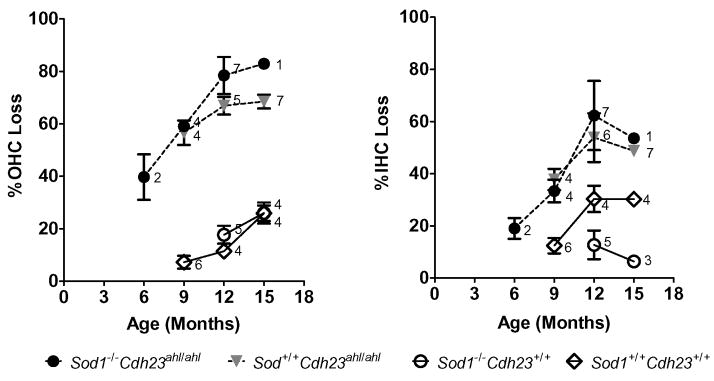
We prepared cytocochleograms from a total of 54 mice having all four experimental genotypes. The mice ranged in age from 6 to 15 months for Sod1−/− Cdh23+/+ and Sod1−/− Cdh23ahl/ahl, 9 to 15 months for Sod1+/+ Cdh23ahl/ahl, and 12 to 15 months for Sod1+/+ Cdh23+/+. Figure 2 shows the total percent OHC loss and the percent IHC loss along the entire length of the cochlea for each of the four genotypes. Mice with the Cdh23ahl/ahl genotype, regardless of Sod1 genotype, showed a large increase in both OHC and IHC loss between 6 and 15 months of age. This trend was most pronounced in Sod1−/− Cdh23ahl/ahl mice followed closely by Sod1+/+ Cdh23ahl/ahl mice; OHC and IHC lesions in these two strains were prominent at 9 months of age and tended to increase with advancing age. In contrast, mice with the Cdh23+/+ genotype, regardless of Sod1 genotype (Sod1−/− Cdh23+/+ and Sod1+/+ Cdh23+/+ mice), showed only a modest increase in OHC loss, which developed later in life between 12 and 15 month of age. Sod1−/− Cdh23+/+ mice showed little IHC loss even at 12–15 months of age, but surprisingly Sod1+/+ Cdh23+/+ mice showed approximately a 30% IHC loss at 12–15 months of age.
Figure 2. Effects of genotype and age on cochlear hair cell loss.
Mean percentage (± standard error) loss of OHC and IHC along the entire length of the cochlea is shown as a function of age for four different digenic genotypes of Cdh23 and Sod1, all on a uniform C57BL/6J genetic background. Numbers next to each symbol indicate sample size.
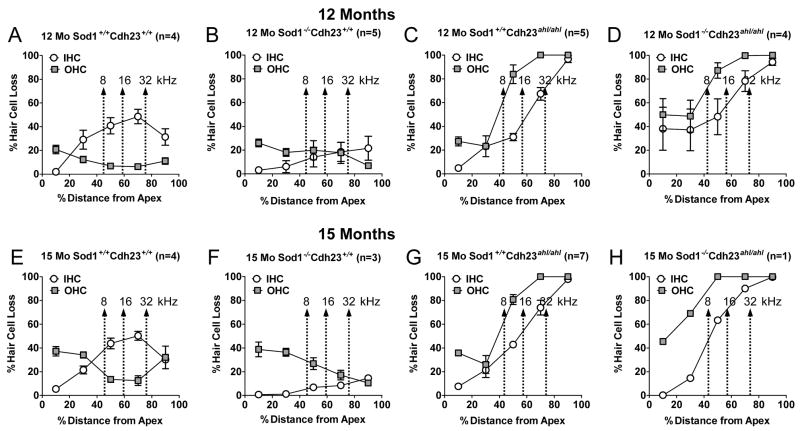
Figure 3 shows the mean IHC and OHC loss as a function of percent distance from the apex in 12-month-old and 15-month-old mice of each digenic genotype. The arrows show the relationship of 8, 16 and 32 kHz test frequencies to the cochlear place map (Muller et al., 2005). Each point represents the percent hair cell loss at 20% intervals along the cochlear duct. At both ages, Sod1−/− Cdh23ahl/ahl mice showed a large OHC and IHC loss, which was greatest near the base of the cochlea (100%) and decreased towards the apex. Sod1+/+ Cdh23ahl/ahl mice showed a similar pattern of IHC and OHC loss at both ages; however the degree of damage was slightly less than in Sod1−/− Cdh23ahl/ahl mice in the apical half of the cochlea. In contrast, the Sod1−/− Cdh23+/+ mice showed little IHC loss at 12 and 15 months; however, OHC loss in this genotype was atypical being greatest near the apex (30–40%) and declining towards the base. Unexpectedly, Sod1+/+ Cdh23+/+ mice exhibited 40–50% IHC loss near the middle of the cochlea at 12 and 15 months whereas OHC loss at 15 months was approximately 35% near the apex and 30% in the base of the cochlea.
Figure 3. Effects of genotype and cochlear location on hair cell loss.
Cochleograms show the means ± standard errors of the percent IHC and OHC loss in 12 and 15 month old mice for each of the four digenic genotypes of Cdh23 and Sod1, all on a uniform C57BL/6J genetic background. Each point represents the percent hair cell loss in 20% intervals along the cochlear duct. The arrows show the positions along the cochlear place map that correspond to the 8, 16 and 32 kHz test frequencies (Muller et al., 2005).
Detailed comparisons of means and confidence intervals for estimates of percent IHC and OHC loss in combined 12- and 15-month old mice are illustrated in Supplementary Fig. 2, for hair cell counts along the entire cochlea and for regions 30%, 50%, and 70% from the cochlear apex. Those digenic genotype pairs with statistically significant mean differences are listed in Table 3 and Supplementary Fig. 2. The Sod1 genotype by itself had a statistically significant effect only on IHC loss 70% from apex region and OHC loss 50% from the apex (Table 3). The Cdh23 genotype, in contrast, had a large effect on hair cell loss at the base to mid-region of the cochlea, but had little effect on hair cells in regions less than 40% from the apex. The Sod1*Cdh23 genotype interaction had no significant effect on OHC loss in any cochlear region; however a significant interaction occurred for IHC loss 50% and 70% from the cochlear apex.
Table 3. Statistical analyses of percent hair cell loss.
Mice of the 12- and 15-month age groups were combined for these analyses. Analysis of variance (ANOVA) F-tests were used to evaluate the separate and interactive effects of Sod1 and Cdh23 genotypes on the percent of inner and outer hair cell loss along the entire cochlea and in regions 30%, 50%, and 70% from the cochlear apex. The probabilities of obtaining the calculated F ratios solely by chance are shown for each effect in each cochlear region. Statistically significant effects at the 0.05 alpha level are indicated by a single asterisk and at the 0.001 level by two asterisks. The digenic genotypes for the Tukey pairwise tests of means are indicated by letters as follows: Sod1+/+ Cdh23+/+ (A), Sod1+/+ Cdh23ahl/ahl (B), Sod1−/− Cdh23+/+ (C), Sod1−/− Cdh23ahl/ahl (D). Only those genotype pairs with significant mean differences at the 0.05 alpha level are shown.
| entire cochlea | 30% from Apex | 50% from Apex | 70% from Apex | |
|---|---|---|---|---|
| Inner Hair Cells | ||||
| ANOVA for monogenic genotypes | ||||
| Sod1 main effect | 0.457 | 0.620 | 0.168 | 0.022 * |
| Cdh23 main effect | <0.0001 ** | 0.114 | 0.071 | <0.0001 ** |
| Sod1*Cdh23 interaction | 0.008 * | 0.055 | 0.024 * | 0.0004 ** |
| Tukey pairwise tests of digenic genotypes | ||||
| significant mean differences (p<0.05) | A-D, B-C, C-D | none | A-C, C-D | A-B, A-C, A-D, B-C, C-D |
| Outer Hair Cells | ||||
| ANOVA for monogenic genotypes | ||||
| Sod1 main effect | 0.101 | 0.072 | 0.048 * | 0.141 |
| Cdh23 main effect | <0.0001 ** | 0.073 | <0.0001 ** | <0.0001 ** |
| Sod1*Cdh23 interaction | 0.284 | 0.114 | 0.536 | 0.119 |
| Tukey pairwise tests of digenic genotypes | ||||
| significant mean differences (p<0.05) | A-B, A-D, B-C, C-D | none | A-B, A-D, B-C, C-D | A-B, A-D, B-C, C-D |
3.3 Vestibular Hair Cells
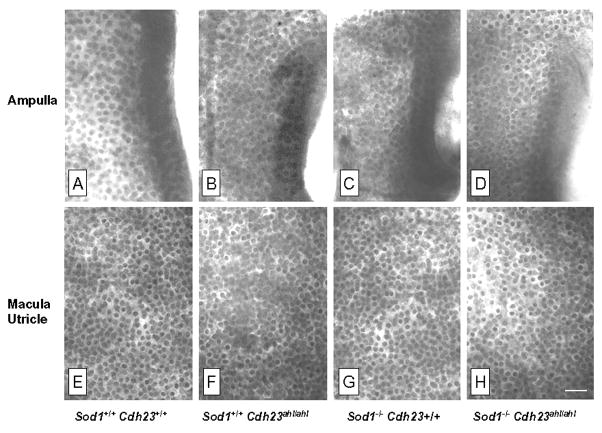
The protein encoded by Cdh23 is not only expressed in the cochlea, but is also expressed in the stereocilia of vestibular hair cells (Lagziel et al., 2005; Wilson et al., 2001). Since there was considerable age-related degeneration of cochlear hair cells in Cdh23ahl/ahl mice, we examined the hair cells in the macula of the saccule and utricle and in the ampulla of the semicircular canals. Figure 4 shows the status of these vestibular hair cells in 12-month-old mice of each digenic genotype. Despite extensive damage to cochlear hair cells, vestibular hair cell densities appeared remarkably normal and similar across the four genotypes, even in mice with both Sod1−/− and Cdh23ahl/ahl deficiencies.
Figure 4. Histology of vestibular hair cells.
Representative photomicrographs showing the density of vestibular hair cells in the ampulla (top row, A-D) and macula of the utricle (bottom row, E-H) in 12-month-old mice with the four different digenic genotypes of Cdh23 and Sod1. All panel images are shown at the same magnification. Scale bar 30 μm, shown in Panel H.
4. Discussion
Hearing loss progression is more rapid at the high frequencies in Sod1+/+ Cdh23ahl/ahl and Sod1−/− Cdh23ahl/ahl mice than in Sod1−/− Cdh23+/+ mice, which show a less frequency-dependent progression (Fig. 1; Table 1). The increase in hearing loss at high frequencies is consistent with the cochleograms of Sod1+/+ Cdh23ahl/ahl and Sod1−/− Cdh23ahl/ahl mice, which show the greatest IHC and OHC loss at the basal region of the cochlear duct (Fig. 3). The proposed role of CDH23 as a component of stereocilia tip links offers a potential explanation for these results (Schwander et al., 2009). Hair cells near the cochlear base have greater numbers of stereocilia than more apical hair cells, and OHCs have greater numbers of stereocilia than IHCs (Lim, 1986). Because OHCs near the base of the cochlea have the greatest number of stereocilia, they also have the greatest number of tip links and thus may be the most vulnerable to damage conferred by the detrimental ahl allelic form of CDH23. Because vestibular hair cells are subjected to much lower frequency stimulations than cochlear hair cells, tip links may be less detrimental, and this mechanism could explain why Cdh23ahl/ahl mice with profound hearing loss maintain normal balance function and lack vestibular hair cell damage (Fig. 4).
It is possible that the tip link abnormalities related to Cdh23ahl may cause an increase in the open probability of the mechanoelectrical transduction (MET) channel, and consequent leakage of cations may lead to a frequent state of depolarization, especially in hair cells with large numbers of stereocilia and MET channels (such as basal OHCs). This depolarization would open calcium channels in the basolateral membrane causing excessive entry of Ca2+ that might then lead to hair cell death. In support of this possible mechanism, Cdh23ahl has been shown to greatly exacerbate the effects of the deaf waddler (dfw) mutation of the Atp2b2 gene (Noben-Trauth et al., 2003), which encodes the PMCA2 calcium pump of hair cell stereocilia. A similar mechanism of Ca2+-related hair cell pathology has been proposed for the dfw mutation, in which defective PMCA2 decreases the Ca2+ concentration in the endolymph surrounding stereocilia tips and thereby causes a reduction in adaptation and an increase in the open probability of MET channels (Ficarella et al., 2007). Chronic depolarization causing Ca2+ influx through voltage-gated Ca2+ channels is also thought to underlie the selective degeneration of high frequency OHCs and progressive hearing loss in mice lacking KCNQ4-mediated K+ currents (Kharkovets et al., 2006). Calcium dysregulation also has been implicated in noise-induced hearing loss, NIHL (Fridberger et al., 1998; Shen et al., 2007), and calcium overload associated with NIHL has been shown to activate mitochondria-mediated cell death pathways in outer hair cells (Vicente-Torres et al., 2006).
Another explanation for the base-apex gradient of hair cell dysfunction is that hair cells in the base of the cochlea are more vulnerable to oxidative stress because the glutathione antioxidant enzyme is lower in the base of the cochlea than the apex (Sha et al., 2001). Thus, defects in CDH23 combined with reduced expression of glutathione would impose greater oxidative stress on hair cells in the base versus apex resulting in early onset, high-frequency hearing loss, consistent with our ABR data. Glutathione immunolabeling is slightly higher in vestibular hair cells than cochlear hair cells which may explain why vestibular hair cells are resistant to the ahl allelic form of CDH23 (Usami et al., 1996).
The degree of high frequency hearing loss in mice with both Sod1 and Cdh23 deficits is much greater than expected from additive effects of each gene (Fig. 1, Table 2) and indicates some type of synergistic interaction. Hair cells of Cdh23ahl/ahl mice are likely to become stressed from frequent tip link breakage and repair or calcium influx, and this increased stress would increase energy demands on mitochondria leading to an accumulation of ROS. A similar mechanism of ROS formation has been proposed for noise-induced hearing loss, which also includes broken tip links among the physical changes it induces (Henderson et al., 2006). A study of the expression levels of antioxidant enzymes in aging mouse cochleas showed much higher increases in B6 mice compared with B6.CAST-Cdh23Ahl+ congenic mice, which suggests that the Cdh23ahl variant may be associated with an increase in oxidative stress (Staecker et al., 2001). Calcium toxicity or increased levels of ROS caused by Cdh23ahl/ahl when combined with a decrease in antioxidant defense caused by Sod1−/− could have a severe effect on hair cell metabolism and viability in Sod1−/− Cdh23ahl/ahl mice resulting in a rapid progression of hearing loss (Fig. 1) and an increased loss of hair cells (Fig. 2).
OHC and IHC losses in Sod1+/+ Chd23ahl/ahl and Sod1−/− Cdh23ahl/ahl mice spread from the base to the apex with advancing age, but hair cell loss in the apical 40% of the cochlea was less severe in Sod1+/+ Cdh23ahl/ahl mice (Fig. 3C,G) than in Sod1−/− Cdh23ahl/ahl mice (Fig. 3G,H). The hearing loss at 32 kHz was severe in Sod1+/+ Cdh23ahl/ahl mice, presumably due to the complete loss of OHCs in the region (Fig. 3C,G). The 32 kHz losses, however, developed more slowly and tended to be less severe in these mice than in Sod1−/− Cdh23ahl/ahl mice (Fig. 1), presumably because acoustic energy from 32 kHz tone bursts was spilling over into the 8–16 kHz cochlear regions where there is less OHC loss (compare Figure 3G with 3H). A more accurate assessment of the threshold near the border of the hearing loss may require the use of notch noise or other masking paradigms as previously noted (Purdy et al., 2002). Since mouse ABR thresholds are normally lowest in the 16 kHz region, downward spectral splatter likely leads to an underestimate of 32 kHz ABR thresholds. Hearing loss in Sod1+/+ Chd23ahl/ahl mice is moderate at 16 kHz, which maps to the apical edge of the OHC lesion; again downward spectral splatter likely leads to an underestimate of 16 kHz thresholds in these mice. Hearing loss is minimal at 8 kHz where OHC loss was around 30%.
Hair cell losses in Sod1−/− Cdh23+/+ mice were minimal at 12 months of age, but at 15 months a peculiar, reverse apex-to-base gradient of OHC loss appeared. OHC losses were roughly 40%, 30%, and 20% in the 8, 16, and 32 kHz regions of the cochlea, respectively (Figure 3F). Between 9 and 15 months of age, ABR thresholds at 8, 16 and 32 kHz increased by 28, 27 and 24 dB, respectively (Table 1). In addition to the moderate OHC loss, other possible explanations for the ABR threshold shifts in Sod1−/− Cdh23+/+ mice include subtle hair cell pathologies that are not reflected in the cochleogram or damage to the fibrocytes in the spiral ligament (Hequembourg et al., 2001).
In Sod1+/+ Cdh23+/+ mice at 15 months of age, OHC losses in cochlear regions associated with 8, 16 and 32 kHz were roughly 35%, 20% and 10% respectively, and corresponding ABR thresholds increased less than 10 dB between 6 and 15 months of age. Surprisingly, IHC losses in cochlear regions associated with 16 and 32 kHz were quite large, on the order of 45–50%. Why were ABR thresholds unaffected by the IHC lesions? Previous studies have noted that auditory nerve fiber thresholds are essentially normal in animals with selective loss of IHC and type-I spiral ganglion neurons; however, neural thresholds increase significantly with selective OHC lesion (Dallos et al., 1978; Wang et al., 1997). Selective destruction of 30% of the IHC also has little effect on the compound action potential thresholds, although it reduces the amplitude of the compound action potential by approximately 30% (Qiu et al., 2000; Trautwein et al., 1996). The IHC loss in the middle of the cochlea was not observed in Sod1−/− Cdh23+/+ mice (Figure 3B, F), and we are at a loss to explain this unusual IHC lesion in the Sod1+/+ Cdh23+/+ mice other than the possibility of unknown environmental factors or genetic interactions.
Hair cell loss in the basal half of the cochlea was strongly associated with Cdh23 genotypes, but was little affected by Sod1 genotypes (Figure 3, Table 3). In contrast, ABR thresholds at 16 and 32 kHz frequencies, which map to the basal half of the cochlea, are influenced by genetic deficiencies at both loci. These results imply that Sod1 deficiency by itself impairs cochlear function without triggering hair cell apoptosis. Perhaps in the absence of the cellular stress caused by tip link breakage associated with the Cdh23ahl/ahl genotype, a moderate increase in the level of ROS caused by SOD1 deficiency in hair cells of Sod1−/− Cdh23+/+ mice may be enough to impair the physiological function of hair cells (or other cochlear structures) but not severe enough to activate signal transduction pathways initiating hair cell death. A recent study has provided evidence that OHC dysfunction, as indicated by perturbed mitochondrial metabolism, can occur well before cellular death in organ of Corti cultures subjected to oxidative stress induced by aminoglycoside exposure (Tiede et al., 2009). Non-lethal impairment of OHC function has also been proposed to explain some of the hearing loss exhibited by aged rats (Chen et al., 2009).
The Cdh23ahl/ahl (Davis et al., 2001) and Sod−/− (Ohlemiller et al., 1999) genotypes have been shown to increase the susceptibility of mice to noise-induced hearing loss (NIHL) as well as to AHL. That AHL and NIHL share these same genetic susceptibility factors suggests that aging and noise trauma may promote common cochlear injuries (Ohlemiller, 2006). It will be interesting to see if the specific Cdh23ahl/ahl and Sod−/− genotype combinations we report here for AHL have similar effects on NIHL and its associated cochlear pathologies.
In summary, we have taken a genetic approach to analyze individual and combined effects of Cdh23ahl and Sod1− mutations on age-related hearing loss and cochlear pathology in C57BL/6J mice. We show that mice homozygous for either gene mutation exhibit progressive hearing loss compared with non-mutant controls, but that hearing loss in Cdh23ahl/ahl mice is greater at high frequencies, while that of Sod−/− mice is less frequency-dependent. Mice homozygous for both mutations exhibit an accelerated high frequency hearing loss, which is greater than expected from solely additive effects. Mice homozygous for the Cdh23ahl mutation, whether alone or in combination with Sod1−, exhibit a progressive base to apex loss of cochlear hair cells but no loss of vestibular hair cells. Mice homozygous for the Sod1− mutation but without the Cdh23ahl mutation show little if any loss of cochlear hair cells, in contrast to their elevated ABR thresholds. Possible mechanisms that may underlie these results are discussed in the context of previously proposed effects of Cdh23ahl on stereocilia tip link integrity and Sod1− on cochlear vulnerability to ROS damage.
Supplementary Material
Acknowledgments
We thank Patsy Nishina and David Bergstrom for critical review of this manuscript. We also thank Sandra Gray for her skilled husbandry and management of inbred strain mice. This research was supported by R01 grants DC005827 (KRJ) and DC00630 (RJS) from the National Institutes of Health (NIH), National Institute on Deafness and Other Communication Disorders. The Jackson Laboratory institutional shared services are supported by NIH National Cancer Institute support grant CA34196.
Abbreviations
- ABR
auditory brainstem response
- AHL
age-related hearing loss
- NIHL
noise-induced hearing loss
- IHC
inner hair cell
- OHC
outer hair cell
- st dev
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chen GD, Li M, Tanaka C, Bielefeld EC, Hu BH, Kermany MH, Salvi R, Henderson D. Aging outer hair cells (OHCs) in the Fischer 344 rat cochlea: function and morphology. Hear Res. 2009;248:39–47. doi: 10.1016/j.heares.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Dallos P, Harris D. Properties of auditory nerve responses in absence of outer hair cells. J Neurophysiol. 1978;41:365–83. doi: 10.1152/jn.1978.41.2.365. [DOI] [PubMed] [Google Scholar]
- Dalton DS, Cruickshanks KJ, Klein BE, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43:661–8. doi: 10.1093/geront/43.5.661. [DOI] [PubMed] [Google Scholar]
- Davis RR, Newlander JK, Ling X, Cortopassi GA, Krieg EF, Erway LC. Genetic basis for susceptibility to noise-induced hearing loss in mice. Hear Res. 2001;155:82–90. doi: 10.1016/s0378-5955(01)00250-7. [DOI] [PubMed] [Google Scholar]
- DeStefano AL, Gates GA, Heard-Costa N, Myers RH, Baldwin CT. Genomewide linkage analysis to presbycusis in the Framingham Heart Study. Arch Otolaryngol Head Neck Surg. 2003;129:285–9. doi: 10.1001/archotol.129.3.285. [DOI] [PubMed] [Google Scholar]
- Ding D, McFadden SL, Salvi R. Cochlear Hair Cell Densities and Inner-Ear Staining Techniques. In: Willott JF, editor. Handbook of Mouse auditory Research from Behavior to Molecular Biology. CRC Press; Boca Raton: 2001. pp. 189–204. [Google Scholar]
- Ficarella R, Di Leva F, Bortolozzi M, Ortolano S, Donaudy F, Petrillo M, Melchionda S, Lelli A, Domi T, Fedrizzi L, Lim D, Shull GE, Gasparini P, Brini M, Mammano F, Carafoli E. A functional study of plasma-membrane calcium-pump isoform 2 mutants causing digenic deafness. Proc Natl Acad Sci U S A. 2007;104:1516–21. doi: 10.1073/pnas.0609775104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridberger A, Flock A, Ulfendahl M, Flock B. Acoustic overstimulation increases outer hair cell Ca2+ concentrations and causes dynamic contractions of the hearing organ. Proc Natl Acad Sci U S A. 1998;95:7127–32. doi: 10.1073/pnas.95.12.7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RA, Van Laer L, Huentelman MJ, Sheth SS, Van Eyken E, Corneveaux JJ, Tembe WD, Halperin RF, Thorburn AQ, Thys S, Bonneux S, Fransen E, Huyghe J, Pyykko I, Cremers CW, Kremer H, Dhooge I, Stephens D, Orzan E, Pfister M, Bille M, Parving A, Sorri M, Van de Heyning PH, Makmura L, Ohmen JD, Linthicum FH, Jr, Fayad JN, Pearson JV, Craig DW, Stephan DA, Van Camp G. GRM7 variants confer susceptibility to age-related hearing impairment. Hum Mol Genet. 2009;18:785–796. doi: 10.1093/hmg/ddn402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- Henry KR, Chole RA. Genotypic differences in behavioral, physiological and anatomical expressions of age-related hearing loss on the laboratory mouse. Audiology. 1980;19:369–383. doi: 10.3109/00206098009070071. [DOI] [PubMed] [Google Scholar]
- Hequembourg S, Liberman MC. Spiral ligament pathology: a major aspect of age-related cochlear degeneration in C57BL/6 mice. J Assoc Res Otolaryngol. 2001;2:118–29. doi: 10.1007/s101620010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Talaska AE, Schacht J, Sha SH. Oxidative imbalance in the aging inner ear. Neurobiol Aging. 2007;28:1605–12. doi: 10.1016/j.neurobiolaging.2006.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Erway LC. A major gene affecting age-related hearing loss is common to at least ten inbred strains of mice. Genomics. 2000;70:171–180. doi: 10.1006/geno.2000.6377. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Muller U, Kachar B. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449:87–91. doi: 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Canto C, Zheng QY, Fischel-Ghodsian N, Johnson KR. Age-related hearing loss and the ahl locus in mice. Hear Res. 2004;188:21–8. doi: 10.1016/S0378-5955(03)00365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithley EM, Canto C, Zheng QY, Wang X, Fischel-Ghodsian N, Johnson KR. Cu/Zn superoxide dismutase and age-related hearing loss. Hear Res. 2005;209:76–85. doi: 10.1016/j.heares.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharkovets T, Dedek K, Maier H, Schweizer M, Khimich D, Nouvian R, Vardanyan V, Leuwer R, Moser T, Jentsch TJ. Mice with altered KCNQ4 K(+) channels implicate sensory outer hair cells in human progressive deafness. EMBO J. 2006;25:642–652. doi: 10.1038/sj.emboj.7600951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagziel A, Ahmed ZM, Schultz JM, Morell RJ, Belyantseva IA, Friedman TB. Spatiotemporal pattern and isoforms of cadherin 23 in wild type and waltzer mice during inner ear hair cell development. Dev Biol. 2005;280:295–306. doi: 10.1016/j.ydbio.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Lim DJ. Functional structure of the organ of Corti: a review. Hear Res. 1986;22:117–146. doi: 10.1016/0378-5955(86)90089-4. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Salvi R. Anatomical, metabolic and genetic aspects of age-related hearing loss in mice. Audiology. 2001;40:313–21. [PubMed] [Google Scholar]
- McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol Aging. 1999a;20:1–8. doi: 10.1016/s0197-4580(99)00018-4. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Burkard RF, Jiang H, Reaume AG, Flood DG, Salvi RJ. Cu/Zn SOD deficiency potentiates hearing loss and cochlear pathology in aged 129,CD-1 mice. J Comp Neurol. 1999b;413:101–12. [PubMed] [Google Scholar]
- Mikaelian DO. Development and degeneration of hearing in the C57/bl6 mouse: relation of electrophysiologic responses from the round window and cochlear nucleus to cochlear anatomy and behavioral responses. Laryngoscopy. 1979;34:1–15. doi: 10.1288/00005537-197901000-00001. [DOI] [PubMed] [Google Scholar]
- Muller M, von Hunerbein K, Hoidis S, Smolders JW. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res. 2005;202:63–73. doi: 10.1016/j.heares.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet. 2003;35:21–23. doi: 10.1038/ng1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK. Contributions of mouse models to understanding of age- and noise-related hearing loss. Brain Res. 2006;1091:89–102. doi: 10.1016/j.brainres.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, McFadden SL, Ding DL, Flood DG, Reaume AG, Hoffman EK, Scott RW, Wright JS, Putcha GV, Salvi RJ. Targeted deletion of the cytosolic Cu/Zn-superoxide dismutase gene (Sod1) increases susceptibility to noise-induced hearing loss. Audiol Neurootol. 1999;4:237–46. doi: 10.1159/000013847. [DOI] [PubMed] [Google Scholar]
- Purdy SC, Abbas PJ. ABR thresholds to tonebursts gated with Blackman and linear windows in adults with high-frequency sensorineural hearing loss. Ear Hear. 2002;23:358–68. doi: 10.1097/00003446-200208000-00011. [DOI] [PubMed] [Google Scholar]
- Qiu C, Salvi R, Ding D, Burkard R. Inner hair cell loss leads to enhanced response amplitudes in auditory cortex of unanesthetized chinchillas: evidence for increased system gain. Hear Res. 2000;139:153–71. doi: 10.1016/s0378-5955(99)00171-9. [DOI] [PubMed] [Google Scholar]
- Schwander M, Xiong W, Tokita J, Lelli A, Elledge HM, Kazmierczak P, Sczaniecka A, Kolatkar A, Wiltshire T, Kuhn P, Holt JR, Kachar B, Tarantino L, Muller U. A mouse model for nonsyndromic deafness (DFNB12) links hearing loss to defects in tip links of mechanosensory hair cells. Proc Natl Acad Sci U S A. 2009;106:5252–7. doi: 10.1073/pnas.0900691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SH, Taylor R, Forge A, Schacht J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res. 2001;155:1–8. doi: 10.1016/s0378-5955(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Shen H, Zhang B, Shin J-H, Lei D, Du Y, Gao X, Wang Q, Ohlemiller KK, Piccirillo J, Bao J. Prophylactic and therapeutic functions of T-type calcium blockers against noise-induced hearing loss. Hear Res. 2007;226:52–60. doi: 10.1016/j.heares.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spongr VP, Flood DG, Frisina RD, Salvi RJ. Quantitative measures of hair cell loss in CBA and C57BL/6 mice throughout their life spans. J Acoust Soc Am. 1997;101:3546–53. doi: 10.1121/1.418315. [DOI] [PubMed] [Google Scholar]
- Staecker H, Zheng QY, Van De Water TR. Oxidative stress in aging in the C57B16/J mouse cochlea. Acta Otolaryngol. 2001;121:666–72. doi: 10.1080/00016480152583593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiede L, Steyger PS, Nichols MG, Hallworth R. Metabolic imaging of the organ of corti--a window on cochlea bioenergetics. Brain Res. 2009;1277:37–41. doi: 10.1016/j.brainres.2009.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein P, Hofstetter P, Wang J, Salvi R, Nostrant A. Selective inner hair cell loss does not alter distortion product otoacoustic emissions. Hear Res. 1996;96:71–82. doi: 10.1016/0378-5955(96)00040-8. [DOI] [PubMed] [Google Scholar]
- Usami S, Hjelle OP, Ottersen OP. Differential cellular distribution of glutathione--an endogenous antioxidant--in the guinea pig inner ear. Brain Res. 1996;743:337–40. doi: 10.1016/s0006-8993(96)01090-6. [DOI] [PubMed] [Google Scholar]
- Vicente-Torres MA, Schacht J. A BAD link to mitochondrial cell death in the cochlea of mice with noise-induced hearing loss. J Neurosci Res. 2006;83:1564–1572. doi: 10.1002/jnr.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Powers NL, Hofstetter P, Trautwein P, Ding D, Salvi R. Effects of selective inner hair cell loss on auditory nerve fiber threshold, tuning and spontaneous and driven discharge rate. Hear Res. 1997;107:67–82. doi: 10.1016/s0378-5955(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Wang J, Menchenton T, Yin S, Yu Z, Bance M, Morris DP, Moore CS, Korneluk RG, Robertson GS. Over-expression of X-linked inhibitor of apoptosis protein slows presbycusis in C57BL/6J mice. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Willott JF. Effects of aging, hearing loss and anatomical location on thresholds of inferior colliculus neurons in C57BL/6 and CBA mice. J Neurophysiol. 1986;56:391–408. doi: 10.1152/jn.1986.56.2.391. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Householder DB, Coppola V, Tessarollo L, Fritzsch B, Lee EC, Goss D, Carlson GA, Copeland NG, Jenkins NA. Mutations in Cdh23 cause nonsyndromic hearing loss in waltzer mice. Genomics. 2001;74:228–33. doi: 10.1006/geno.2001.6554. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QY, Ding D, Yu H, Salvi RJ, Johnson KR. A locus on distal chromosome 10 (ahl4) affecting age-related hearing loss in A/J mice. Neurobiol Aging. 2009;30:1693–1705. doi: 10.1016/j.neurobiolaging.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.