Abstract
The use of the peptidase neprilysin (NEP) as a therapeutic for lowering brain amyloid burden is receiving increasing attention. We have previously demonstrated that peripheral expression of NEP on the surface of hindlimb muscle lowers brain amyloid burden in a transgenic mouse model of Alzheimer’s disease. In this study we now show that using adeno-associated virus expressing a soluble secreted form of NEP (secNEP-AAV8), NEP secreted into plasma is effective in clearing brain Aβ. Soluble NEP expression in plasma was sustained over the 3-month time period it was measured. Secreted NEP decreased plasma Aβ by 30%, soluble brain Aβ by ~28%, insoluble brain Aβ by ~55%, and Aβ oligomers by 12%. This secNEP did not change plasma levels of substance P or bradykinin, nor did it alter blood pressure. No NEP was detected in CSF, nor did the AAV virus produce brain expression of NEP. Thus the lowering of brain Aβ was due to plasma NEP which altered blood-brain Aβ transport dynamics. Expressing NEP in plasma provides a convenient way to monitor enzyme activity during the course of its therapeutic testing.
Keywords: Neprilysin, Alzheimer’s disease, Gene therapy, adeno-associated virus, amyloid beta peptide
1. Introduction
The peptidase neprilysin (neutral endopeptidase 24.11, NEP) plays a major role in the clearance of amyloid beta peptides (Aβ) from the brain (Marr et al., 2004, Maruyama et al., 2005). Evidence is accumulating that the level of NEP declines with aging leading to decreased Aβ clearance. Since an accumulation of Aβ is considered to be a major factor in the etiology of Alzheimer’s disease (AD), the decline in NEP with aging is likely a contributing factor to the progression of AD (Miners et al., 2006). The use of NEP and other peptidases as a therapeutic for treating AD has attracted recent attention. It has been shown that increasing expression of NEP in the brain of mouse models of AD either through the use of lentivirus (Marr et al., 2003) or crossing transgenic mice overexpressing NEP with hAPP transgenic mice (Leissring et al., 2003) lowers brain Aβ levels.
Although NEP can clear Aβ from the brain, the problem of increasing CNS NEP in an AD patient is challenging. In an attempt to avoid having to penetrate the brain we considered the use of peripherally expressed NEP. A dynamic equilibrium between brain Aβ and plasma Aβ has been shown (Deane et al., 2004b, DeMattos et al., 2001, 2002) where LRP-1 transports Aβ from the brain into plasma, while RAGE transports Aβ from the plasma into the brain (Deane et al., 2003, 2004a). Disrupting this transport dynamics was exploited using passive immunization with Aβ antibodies to bind plasma Aβ in immune complexes, prevent reuptake into the brain, and decrease brain Aβ (DeMattos et al., 2001). Similarly Aβ-binding proteins, gelsolin and GM1, a soluble form of RAGE, and a soluble domain of LRP all altered the distribution of Aβ between brain and plasma (Deane et al., 2003, 2008, Matsuoka et al., 2003). Since these studies demonstrated that brain Aβ could be decreased by peripheral mechanisms we considered using NEP to clear plasma Aβ through hydrolysis as an alternative to introducing the enzyme into the brain.
We have demonstrated that NEP attached to red blood cells effectively degraded Aβ transported from the brain into plasma (Liu et al., 2007). We therefore tested NEP gene therapy as a means to delivery a therapeutic level of NEP to the periphery. We showed that NEP when expressed on leukocytes (Guan et al., 2009) or on the surface of hindlimb muscle cells (Liu et al., 2009) lowered brain Aβ in hAPP mice. These methods, although effective, either required bone marrow replacement or the need to sacrifice mice to assess the level of NEP expressed on hind limb muscle. In this study, we show that a soluble form of NEP secreted into plasma produces an easily quantifiable form of NEP that efficiently reduces Aβ burden in the brain.
2. Materials and Methods
2.1 Treatment of Mice
AAV8 expressing a secreted soluble form of mouse NEP was injected into the hindlimb muscle of 3XTg-AD mice (Oddo et al., 2003). Each mouse received 30 μl of NEP-AAV8 by injecting 5 μl into 6 sites in the hindlimb muscle as previously described (Liu et al., 2009). At 3 months post injection mice were sacrificed, brains were immediately removed, and placed on ice. One half brain was used for Aβ peptide measurement and the other half brain was frozen for tissue sectioning. Other tissues were taken for determination of virus integration. All procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Kentucky, KY, Institutional Animal Care and Use Committees.
2.2 Generation of AAV8-secNEP
A gene encoding the extracellular domain of mouse NEP linked to the pepsin secretion signal (Marr et al., 2003) was cloned into the vector pGFPNW. This form of mouse NEP (secNEP) is secreted from cells, retains full activity, and remains soluble. The pGFPNW expression vector contains the cytomegalovirus/chicken β-actin hybrid promoter flanked by AAV2 terminal repeats. The secNEP plasmid was cross-packaged into an AAV8 vector using an adenovirus helper plasmid and a packaging vector in HEK293-T cells (Klein et al., 2006). Virus was purified on a discontinuous iodixanol gradient (Kohlbrenner et al., 2005).
2.3 NEP activity measurements
Recombinant soluble mouse NEP was secreted from HEK293T cells transduced with lentivirus expressing the extracellular domain and purified by ion exchange chromatography on a Source15Q column (Amersham Bioscience) as previously described (Daily et al., 2006, Marr et al., 2003). NEP activity was measured using the substrate glutaryl-Ala-Ala-Phe-4-methoxy-2-naphthylamide (Sigma, St. Louis, MO) as previously described (Liu et al., 2009). Assay mixtures contained 100 μM glutaryl-Ala-Ala-Phe-4-methoxy-2-naphthylamide (GAAF-MNA), 5 μl of 10-fold diluted plasma, 1 μg of human puromycin sensitive aminopeptidase (Thompson et al., 2003), and 20 mM MES buffer, pH 6.5 in a total volume of 200 μl. Reactions were initiated by the addition of plasma and monitored at 37°C at an excitation of 340 nm and emission of 425 nm. Phosphoramidon (50 μM), an inhibitor of NEP, blocked the observed activity by more than 90%.
2.4 Isolation of plasma and CSF
To isolate plasma, 100 μl of mouse blood was collected into 5 units of heparin in PBS. The blood was centrifuged at 100g for 20 min and the supernatant used for determining NEP activity. Preliminary experiments established that heparin did not affect NEP activity. CSF was collected from the cisterna magna as described by DeMattos et al. (2001).
2.5 RT-PCR
Total RNA was isolated from tissues using the Qiagen RNeasy kit (Qiagen GmbH, Germany). cDNA was synthesized from 5 μg of RNA using the SuperScript III First Strand Synthesis System (Invitrogen, Carlsbad, CA). Forward and reverse primers used for detecting secreted mouse NEP were: 5′-TGC CAT TCA GGT CAT AGC CT-3′ and 5′-CTG CTA TCA ATA GCA GAC TC-3′, respectively. β-actin mRNA was measured using as forward and reverse primers 5′-TGT TTG AGA CCT TCA ACA CC-3′ and 5′-TAG GAG CCA GAG CAG TAA TC- 3′, respectively. PCR reactions were performed using 2 μl cDNA and 20, 25, 30 cycles. The expected PCR product sizes are 384 bp for secNEP and 600 bp for β-actin.
2.6 Blood pressure measurements
Systolic blood pressure was measured by the tail cuff method (BP-2000 Visitech Systems) (Cassis et al., 2005). Measurements were taken with conscious mice restrained on a 37°C heated stage for five consecutive days at the same time of day each time. We took ten preliminary measurements and then recorded the next ten measurements.
2.7 Aβ determination
Aβ levels were measured by sandwich ELISA using Ab9 (anti-Aβ1–16) (McGowan et al., 2005) as the capture antibody and 4G8 (anti-Aβ17–24) (Signet Laboratories, Dedham, MA) as the detection antibody. Aβ containing samples were prepared from mouse half-brain by homogenization in RIPA buffer (50 mM Tris-HCl, pH 8, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS, 150 mM NaCl, and a protein inhibitor cocktail) (Roche Diagnostics, Indianapolis, IN). Homogenates were centrifuged at 20,000g for 30 min to yield a supernatant containing detergent soluble Aβ. The detergent insoluble Aβ fraction was obtained by sonicating the pellet in 70% formic acid followed by centrifugation at 100,000g for 1 h. To measure Aβ oligomers, brain tissue was homogenized at 200 mg/ml PBS containing a protein inhibitor cocktail, (Roche Diagnostics, Indianapolis, IN), centrifuged at 20,000g for 30 min, and the supernatant used to measure buffer soluble oligomers. The pellet was sonicated in 2% SDS, centrifuged as above, and used to determine SDS soluble Aβ oligomers. Oligomers were measured using a single site epitope competition ELISA assay with anti- Aβ antibody 4G8/4G8-biotin (LeVine, 2004a). A standard curve was generated from synthetic oligomeric Aβ provided by Dr. H. Levine (University of Kentucky) with molecular weights ranging from 200 to 800 kDa.
2.8 Determination of APP expression
The RIPA homogenate was subjected to SDS-PAGE followed by Western blot analysis to determine APP expression. Rabbit anti-human APP (R8666) (obtained from Dr. Maria Kounnas, Torrey Pines Pharmaceutical Inc., La Jolla, CA) and anti-actin mouse monoclonal antibody (JLA20, Calbiochem, San Diego, CA) were used. Alexa Fluor 680-conjugated goat anti-rabbit antibody (Invitrogen, Carlsbad, CA) and IRDye 800-conjugated goat anti-mouse antibody (Invitrogen, Carlsbad, CA) were used for detection with a Li-COR Odyssey system (Biosciences, Lincoln, Nebraska). ImageQuant software (Molecular Devices, Pty, Ltd.) was used for quantitation and the APP to actin ratio calculated.
2.9 Peptide hormone measurements
One ml of blood was collected into 30 μl of PBS containing 1,000 U/ml of heparin and a protein inhibitor cocktail, (Roche Diagnostics, Indianapolis, IN). Plasma was collected as noted above and peptide levels determined with commercial ELISA kits for substance P (Assay Designs, MI) and bradykinin (Phoenix Pharmaceuticals, CA).
2.10 Immunohistochemistry
Immunohistochemistry was performed on cryostat coronal brain sections (16 μm) fixed in 3% paraformaldehyde in PBS. Prior to staining endogenous peroxidase activity was eliminated by treatment with 3% H2O2, sections were then blocked with 0.1% Triton X-100, 0.1% BSA, and 2% horse serum in PBS for 1 h at room temperature. For Congo red staining sections were incubated in 0.4% alkaline Congo red in 80% alcohol saturated with sodium chloride for 20 min at room temperature and rinsed in alcohol. Brain sections were then incubated at 4°C overnight in PBS containing rabbit anti-mouse glia fibriillary protein (GFAP, 1:100, Abcam Inc. Cambridge, MA). After washing, sections were incubated with goat anti-rabbit peroxidase secondary antibody (1:2000, Chemicon, Temecula, CA) for 1 h at room temperature. Stained sections were developed with Nickel-DAB (Vector Laboratories, Inc. Burlingame, CA) with images captured with an Olympus Provis AX80 microscope (B&B Microscopes, Ltd., Philadelphia, PA). Quantitative analysis was performed as described by Oddo et al. (2006a). Images of stained sections were imported into Image Pro Plus 6.2 software (Media Cybernetics, Inc., MD).
2.11 Thioflavin S staining
Serial sections of each brain (four sections per animal) were incubated in 0.5% solution of Thioflavine S (Sigma Aldrich) in 50% ethanol for 10 min. Sections were washed twice in 50% ethanol and water, respectively (Oddo et al., 2006b). Quantitative analysis of Aβ plaques was performed as described above.
2.12 Statistical analysis
Immunohistochemical and ELISA data were analyzed by the two-tailed unpaired student’s t test using GraphPad Prism 4 software (GraphPad Software, Inc., CA). Values were considered to be statistically significant with a p value of less than 0.05. Data are presented as mean ± SEM.
3. Results
3.1 Stability of soluble NEP in plasma
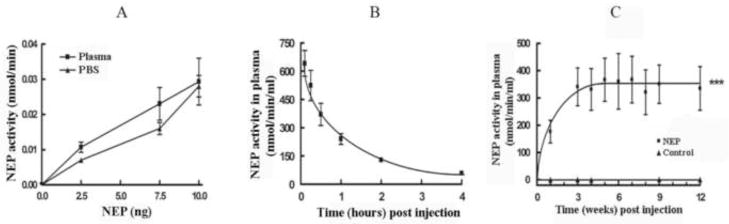
We have previously shown that NEP expressed on the surface of mouse hindlimb muscle can degrade plasma Aβ and lower brain Aβ (Liu et al., 2009). However in that study it was impossible to determine the level of NEP expression without sacrificing the mice. We therefore tested whether NEP, in the absence of viral expression, could be used peripherally to clear plasma Aβ. Soluble NEP was produced by secretion of its catalytically active extracellular domain fused to the pepsin secretion signal (Marr et al., 2003) from HEK293T cells and purified on a Source 15Q ion exchange chromatography column. The secNEP was fully active in mouse plasma, Figure 1A, indicating the absence of inhibitors and resistance to proteolysis by plasma proteases. We next determined the in vivo stability of secNEP by injecting purified enzyme into mouse tail vein, and sampling plasma at various times. As shown in Figure 1B the secNEP produced in HEK293 cells was rapidly cleared with half the activity lost after ~1 hr.
Figure 1.
A. Stability of soluble NEP plasma.
The indicated amounts of purified soluble NEP were diluted into fresh plasma or into PBS and then assayed for activity using glutaryl-Ala-Ala-Phe-methoxynaphthylamide as substrate. The data is presented as the mean ± SEM, n=3.
B. Clearance of purified secreted NEP in vivo.
Purified NEP (50 μl) was injected into mice intravascularly and blood collected at the indicated time points post injection. Plasma was assayed for NEP activity as described above. Data is presented as the mean ± SEM. n=3.
C. Stability of expression of mouse soluble NEP in plasma.
Nine-month old 3X-Tg-AD mice were injected with 2×1011 secNEP-AAV8 viral genomes (vg) into the hindlimb. Injections were 5 μl per site using 6 sites. At the indicated times post injection blood was collected, plasma isolated and assayed for NEP activity as above. Control mice were injected with GFP-AAV8. Data are presented as the mean ± SEM (n = 3). *** p=<0.001, AAV8-msecNEP 2×1011 vg vs. control mice.
3.2 Gene Delivery of soluble NEP to plasma
As an alternative to direct injection of secNEP into plasma we constructed an AAV8 expression vector encoding secreted NEP, secNEP-AAV8. A single dose of 2×1011 viral genomes (vg) of secNEP-AAV8 was injected intramuscularly into 3X-Tg-AD mice. At various times post injection, blood was collected, plasma isolated and NEP activity determined. One week after intramuscular injection of secNEP-AAV8 activity was detected in plasma and reached a maximum level of ~350 nmol/min/ml plasma, Figure 1C. This plasma NEP was sustained for the entire 3 months it was followed, while no plasma NEP was detectable in untreated control mice. We did not detect any increase in NEP activity in CSF 6 weeks post intramuscular injection, while NEP activity in plasma differed dramatically from control plasma or from CSF (p<0.001).
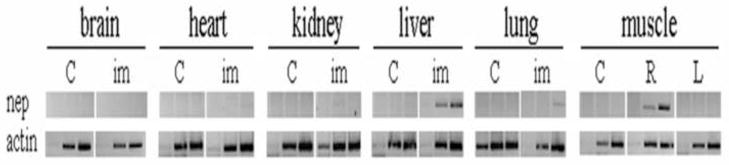
AAV8 has been reported to be able to penetrate the blood vessel barrier, enter the blood, and be taken up by other tissues (Wang et al., 2005). Therefore we tested for the incorporation of secNEP-AAV virus into other tissues. Since the NEP is secreted we could not test for enzymatic activity in tissues, but instead we tested for tissue uptake of secNEP-AAV8 virus by measuring secNEP mRNA. We used RT-PCR employing a forward primer corresponding to the pepsin signal peptide linked to NEP and a reverse NEP specific primer so that only NEP mRNA for secNEP derived from AAV would be detected. Figure 2 shows that indeed some of the secNEP-AAV injected into muscle went through the blood vessel barrier and transduced cells in the liver and to a much lesser extent lung. Viral transduction was not detectable in any other tissue, particularly brain, nor was it found in the contralateral muscle.
Figure 2.
Tissue distribution of secNEP mRNA 6 weeks post injection.
Nine-month old 3X-Tg-AD mice were injected with 2×1011 secNEP-AAV8 viral genomes as described in figure 1. Six weeks post injection mice were sacrificed, tissues dissected, and RNA extracted. RT-PCR was performed using 20, 25, and 30 cycles. The PCR product sizes are ~384 bp for secNEP and ~600 bp for β-actin.
C = control mice, im = mice receiving intramuscular injection of AAV8-secNEP.
3.3 Plasma NEP decreases Aβ levels
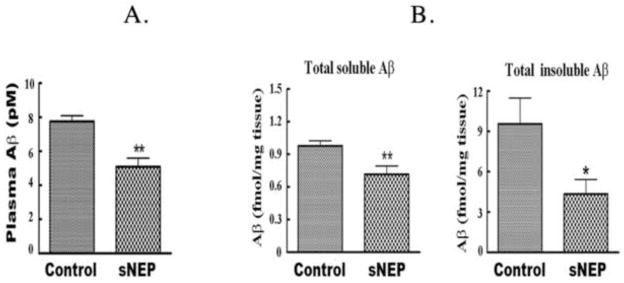
To determine if plasma secNEP can lower plasma Aβ levels and consequently lower brain Aβ levels, we studied ~9 month old 3XTg-AD mice as they best represent an early to middle stage of AD. Mice were injected with 2×1011 vg of secNEP-AAV into the hindlimb muscle and sacrificed three months latter. Plasma Aβ was found to be decreased 30% in secNEP expressing mice compared to untreated control mice, Figure 3A.
Figure 3.
Effect of plasma NEP expression on brain and plasma Aβ.
A. Effect of secNEP on plasma Aβ.
Plasma was isolated as described in methods and assayed for Aβ by sandwich ELISA. Data is presented as the mean ± SEM (n=5 for NEP group and 9 for control group). **p=0.004 for the NEP group vs. the control group.
B. Soluble Aβ and insoluble Aβ in mouse brain 3 months post intramuscular injection of secNEP-AAV8.
The level of Aβ in brain was determined by sandwich ELISA. Data are presented as the mean ± SEM (n=9 for NEP group and control group). * p=0.005 for soluble Aβ for the NEP treated group vs. the control group, p= 0.04 for insoluble Aβ between the NEP treated group vs. the control group.
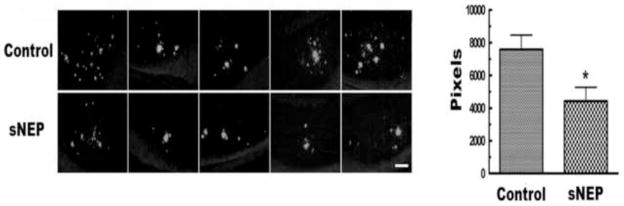
We next determined if peripheral secNEP could lower brain Aβ. Compared to the control group, secNEP lowered both soluble and insoluble brain Aβ levels by ~28% (p=0.005) and ~55% (p=0.04), respectively, Figure 3B. In agreement with the ELISA data, Thioflavin S staining showed a ~42% decrease in Aβ deposits (p = 0.02), Figure 4. In contrast the APP level in the brain showed no difference between treated mice and control as determined by Western blot analysis.
Figure 4.
Thioflavin S staining of Aβ in the brain of mice expressing NEP in plasma.
Brain sections were stained with Thioflavin S as described in Methods and quantitated using Image Pro Plus. (p=0.0235, secNEP vs. Control, n=9). Shown are representative images from five of the 9 mice studied with each lane showing a different mouse. The scale bar is 100 μm.
We examined Aβ oligomer levels using a single epitope ELISA assay (LeVine, 2004b). Although buffer soluble Aβ oligomers were barely detectable, the SDS soluble Aβ oligomer fraction showed a small but statistically significant decrease in the secNEP-expressing mice (p=0.03). Aqueous buffers typically extract only highly soluble, low molecular weight species of Aβ that are not deposited as insoluble fibrils or plaques in the brain (Murphy et al, 2007), whereas SDS extracts the less soluble forms. The single site 4G8/4G8 assay only detects oligomeric species of the peptide with a minimum molecular weight of 40–50 kDa (Levine, 2004b), indicating that there are no detectable soluble oligomers above 40–50 kDa in the PBS fraction in these mice.
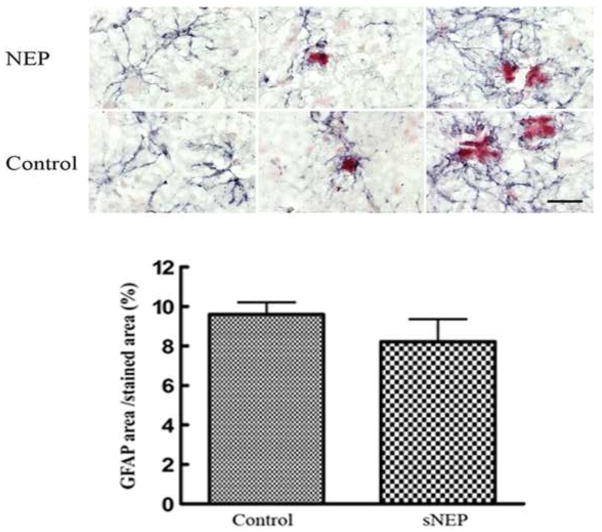
Hirko et al (2007) showed plasma-expressed recombinant gelsolin reduced brain Aβ pathology and led to an increase in activated astrocytes in the brain. To determine whether plasma secNEP might also activate astrocytes, we stained brain sections with an anti-glial fibrillary acidic protein (GFAP) antibody. We also stained with Congo red to identify amyloid deposits, since astrocytes are known to surround amyloid deposits. Similar to treatment with gelsolin (Hirko et al., 2007) we did not observe an increase in the number of GFAP–positive astrocytes. Although Hirko et al. (2007) observed that in recombinant gelsolin expressing mice the GFAP–positive astrocytes appeared thicker and contained more numerous processes, we did we see any difference in the appearance of GFAP–positive astrocytes in secNEP expressing mice, Figure 5.
Figure 5.
The effect of peripheral NEP expression on brain astrocytes.
Shown in grey are anti-glial fibrillary acidic protein stained astrocytes in the brains of control and NEP expressing 3XTg-AD mice, while Congo red stained amyloid deposits are shown in red. Scale bar = 20 μm
3.4 Plasma NEP does not affect blood pressure or peptide hormones
NEP is known to hydrolyze peptides that regulate blood pressure including angiotensin and atrial natriuretic peptide. We thus tested the effect of secNEP expression on systolic blood pressure at 3 months post injection of 2×1011 vg secNEP-AAV8 and found no effect. We also directly measured the effect of peripheral secreted NEP expression on plasma bradykinin and substance P levels, since these peptides are known substrates for the enzyme. After 3 months of expression of secNEP, although there was a trend toward lower peptide levels, the difference did not reach statistical significance. This is in contrast to the decreased plasma Aβ level shown in figure 3
4. Discussion
Increasing NEP levels in the brain has been shown to lower brain Aβ levels (Hemming et al., 2007, Marr et al., 2003) and thus NEP has received attention as a therapeutic target for Alzheimer’s disease. We have shown that NEP expressed peripherally either on the surface of leukocytes or on the surface of hindlimb muscle lowers brain Aβ levels. In this study we first tested the direct injection of the soluble active domain of NEP derived from HEK cells as a way to achieve plasma NEP, however the enzyme was rapidly cleared. This may reflect either the normal clearance rate for soluble NEP or a low sialic acid content of the recombinantly produced enzyme (Bork et al., 2009) and rapid clearance by liver asialo-glycoprotein receptor. On the other hand secreting NEP from mouse hindlimb via secNEP-AAV8 produced a sustained level of enzyme activity that likely reflects a steady state between its synthesis and clearance. This sustained level of NEP may reflect expression of a more stable fully sialylated enzyme or a high rate of production of rapidly cleared enzyme. Although some of the injected virus entered the bloodstream and was taken up by the liver and to a lesser extent lung, this is not a problem since both liver and lung already have a high content of NEP, albeit the membrane associated form.
NEP in plasma is effective in lowering brain Aβ levels by about 30%. That this secNEP-dependent decrease in brain Aβ is due to increased Aβ clearance is evidenced by the finding that APP levels remained unaffected. Plasma NEP also produced a small but statistically significant reduction in SDS extractable Aβ oligomers. The reason that this decrease was relatively small is not clear, but might be explained by a fraction of these oligomers being secreted from cells and not derived from extracellular monomeric Aβ. Since the LRP receptor poorly transports Aβ dimers (Ito et al., 2007) these cell derived oligomers represent a separate Aβ pool refractory to transport from brain into plasma and thus inaccessible to plasma NEP.
Recently Meilandt et al (2009) reported that NEP over-expression in brain reduced amyloid deposition, but did not reduce Aβ oligomers, and did not reduce cognitive deficits. This seems surprising in view of reports showing NEP over-expression in brain decreasing memory impairment in hAPP mice (Poirier et al., 2006, Spencer et al., 2008, Leissring et al., 2003, El-Amouri et al, 2008). The variance between these studies was suggested by Meilandt et al. to be attributable to several factors including different expression levels and different patterns of cellular transgene expression. For example higher NEP expression levels were achieved in both the Leissring et al. and Poirier et al. studies. Meilandt et al also noted different times and different patterns of transgene expression. NEP was expressed late postnatal and predominantly in excitatory neurons in the forebrain in their study compared to expression at E13.5 and in additional neuronal cells in the study of Poirier et al. This issue will clearly need to be resolved by additional studies.
The finding that secNEP-AAV8 injected into muscle did not produce secNEP mRNA in brain shows that the mechanism whereby NEP lowers brain Aβ is due to peripheral expression, and not brain expression of NEP. Due to the rather large size of NEP the impermeability of the blood brain barrier would prevent secNEP in plasma from entering the brain. We suggest that plasma-born secNEP, by hydrolyzing plasma Aβ disrupts brain-blood Aβ transport between LRP-1 mediated Aβ efflux out of the brain into plasma and RAGE mediated influx from plasma into the brain. Although it is unlikely that hydrolysis of plasma Aβ would affect efflux, it would certainly prevent Aβ transport into the brain. The net effect would be a unidirectional transport of Aβ out of the brain into plasma where it would hydrolyzed by secNEP.
In studies using peripherally expressed plasma gelsolin, astrocyte activation was observed (Hirko et al., 2007). This was suggested to be accounted for by either plasma gelsolin crossing the blood brain barrier and acting in the brain or by plasma gelsolin selectively activating the immune system. With expression of peripheral secNEP, astrocyte activation was not seen providing additional evidence that NEP lowers brain Aβ by hydrolyzing plasma born Aβ.
We found that plasma levels of other neuropeptides, such as bradykinin and substance P, although being substrates for NEP, were unaffected by plasma NEP. This is somewhat surprising and suggests that bradykinin and substance P may not be as good a physiological substrate for NEP as Aβ. Alternatively, there are other peptidases in plasma, such as aminopeptidases and carboxypeptidases, that degrade neuropeptides more efficiently than NEP, but don’t cleave Aβ, and therefore increasing plasma NEP has little effect on their concentration.
In conclusion we show here that a soluble secreted form of NEP when circulating in plasma can effectively lower brain Aβ levels and prevent Aβ deposition. This opens yet another therapeutic approach for treating Alzheimer’s disease. The use of soluble secreted NEP has the distinct advantage that the level of plasma enzyme can be easily determined and monitored at any time.
Acknowledgments
This study was supported by NIH grants DA 02243 from the National Institute on Drug Abuse, AG 24899 from the National Institute on Aging, P20RR02017 from the National Center for Research Resources (NCRR), and a grant from the Kentucky Science and Engineering Foundation, KSTC-144-401-08-027.
Biographies
Yinxing Liu
Dr. Liu received a B.Sc. in biochemistry from the Shenyang Pharmaceutical University, and a Ph.D. in Pharmacology from the Peking Union Medical College in China. Dr. Liu received a B.Sc. in biochemistry from the Shenyang Pharmaceutical University, and a Ph.D. in Pharmacology from the Peking Union Medical College in China. She joined the Department of Molecular and Cellular Biochemistry of the University of Kentucky in 2004, where she is currently a Research Associate. Dr. Liu’s research focuses on the neuropeptidase neprilysin and its use in AD.
Christa Studzinski
Dr. Studzinski received her B.Sc. in Psychology and Neuroscience (2002) and Ph.D. in Pharmacology (2007) at the University of Toronto. Dr. Studzinski was a Myositis Association Fellow at the University of Kentucky in the laboratory of Dr. M. Paul Murphy before moving to the Center for Research in Neurodegenerative Diseases at the University of Toronto (2008). Dr. Studzinksi’s research interests cover metabolic dysfunction, Alzheimer’s and Parkinson’s Disease.
Tina Beckett
Ms. Beckett received her H.B.Sc. in Biology and Neuroscience (2000) at the University of Toronto. Ms. Beckett joined the laboratory of Dr. M. Paul Murphy as a research scientist in the Department of Molecular and Cellular Biochemistry and the Sanders-Brown Center on Aging in 2005. Ms. Beckett’s research interests include amyloid diseases, such as inclusion body myositis and Alzheimer’s disease.
M. Paul Murphy
Dr. Murphy received his B.Sc. in Biochemistry (1991), M.A. in Psychology (1993) and Ph.D. in Neuroscience (1998) at the University of Toronto. Dr. Murphy was a French Foundation and Robert and Clarice Smith Fellow at the Mayo Clinic Jacksonville before moving to the University of Kentucky in 2005 as an Assistant Professor in the Department of Molecular and Cellular Biochemistry and the Sanders-Brown Center on Aging. His laboratory studies the amyloid-β peptides and the enzymes involved in their production.
Ronald L. Klein
Ronald Klein received his undergraduate degree at Hamilton College, Clinton, NY, before receiving a Ph.D. in Pharmacology from the University of Colorado in 1995. Post-doctoral studies were at the University of Florida, under Edwin Meyer. Dr. Klein joined the LSU medical center in Shreveport, LA in 2002. Dr. Klein’s research strives to improve the mimicry of neurodegenerative diseases via viral vector gene transfer methods He was involved in early successes achieving gene transfer to the brain, functional neurotrophic factor gene delivery, and models for neurodegenenerative diseases relevant to alpha-synuclein, tau, and TDP-43.
Louis B. Hersh
Dr. Hersh received his B.Sc. from Drexel University (1962) and a Ph.D. from Brandeis University in 1966. After postdoctoral training at the National Heart Institute, he joined the faculty at UT Southwestern (1968). In 1992 he went to the University of Kentucky College of Medicine as chair of the Molecular and Cellular Biochemistry department. Dr. Hersh’s research has most recently focused on the enzymology of neuropeptidases and their application to the treatment of human disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bork K, Horstkorte R, Weidemann W. Increasing the sialylation of therapeutic glycoproteins: the potential of the sialic acid biosynthetic pathway. J Pharm Sci. 2009;98:3499–3508. doi: 10.1002/jps.21684. [DOI] [PubMed] [Google Scholar]
- Cassis LA, Helton MJ, Howatt DA, King VL, Daugherty A. Aldosterone does not mediate angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Br J Pharmacol. 2005;144:443–448. doi: 10.1038/sj.bjp.0706098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily A, Nath A, Hersh LB. Tat peptides inhibit neprilysin. J Neurovirol. 2006;12:153–160. doi: 10.1080/13550280600760677. [DOI] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- Deane R, Sagare A, Zlokovic BV. The role of the cell surface LRP and soluble LRP in blood-brain barrier Abeta clearance in Alzheimer’s disease. Curr Pharm Des. 2008;14:1601–1605. doi: 10.2174/138161208784705487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004a;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Deane R, Wu Z, Zlokovic BV. RAGE (yin) versus LRP (yang) balance regulates alzheimer amyloid beta-peptide clearance through transport across the blood-brain barrier. Stroke. 2004b;35:2628–2631. doi: 10.1161/01.STR.0000143452.85382.d1. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science. 2002;295:2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer’s disease model. Nat Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- El-Amouri SS, Zhu H, Yu J, Marr R, Verma IM, Kindy MS. Neprilysin an enzyme candidate to slow the progression of Alzheimer’s disease. Am J Path. 2008;172:1342–1353. doi: 10.2353/ajpath.2008.070620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H, Liu Y, Daily A, Police S, Kim MH, Oddo S, LaFerla FM, Pauly JR, Murphy MP, Hersh LB. Peripherally expressed neprilysin reduces brain amyloid burden: a novel approach for treating Alzheimer’s disease. J Neurosci Res. 2009;87:1462–1473. doi: 10.1002/jnr.21944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming ML, Patterson M, Reske-Nielsen C, Lin L, Isacson O, Selkoe DJ. Reducing amyloid plaque burden via ex vivo gene delivery of an Abeta-degrading protease: a novel therapeutic approach to Alzheimer disease. PLoS Med. 2007;4:e262. doi: 10.1371/journal.pmed.0040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirko AC, Meyer EM, King MA, Hughes JA. Peripheral transgene expression of plasma gelsolin reduces amyloid in transgenic mouse models of Alzheimer’s disease. Mol Ther. 2007;15:1623–1629. doi: 10.1038/sj.mt.6300253. [DOI] [PubMed] [Google Scholar]
- Ito S, Ohtsuki S, Kamiie J, Nezu Y, Terasaki T. Cerebral clearance of human amyloid-beta peptide (1–40) across the blood-brain barrier is reduced by self-aggregation and formation of low-density lipoprotein receptor-related protein-1 ligand complexes. J Neurochem. 2007;103:2482–2490. doi: 10.1111/j.1471-4159.2007.04938.x. [DOI] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Leidenheimer NJ, Jansen K, Golde TE, Zweig RM. Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of tau or green fluorescent proteins. Mol Ther. 2006;13:517–527. doi: 10.1016/j.ymthe.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlbrenner E, Aslanidi G, Nash K, Shklyaev S, Campbell-Thompson M, Byrne BJ, Snyder RO, Muzyczka N, Warrington KH, Jr, Zolotukhin S. Successful production of pseudotyped rAAV vectors using a modified baculovirus expression system. Mol Ther. 2005;12:1217–1225. doi: 10.1016/j.ymthe.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, Frosch MP, Selkoe DJ. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003;40:1087–1093. doi: 10.1016/s0896-6273(03)00787-6. [DOI] [PubMed] [Google Scholar]
- LeVine H., 3rd The Amyloid Hypothesis and the clearance and degradation of Alzheimer’s beta-peptide. J Alzheimers Dis. 2004a;6:303–314. doi: 10.3233/jad-2004-6311. [DOI] [PubMed] [Google Scholar]
- LeVine H., 3rd Alzheimer’s beta-peptide oligomer formation at physiologic concentrations. Anal Biochem. 2004b;335:81–90. doi: 10.1016/j.ab.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Liu Y, Guan H, Beckett TL, Juliano MA, Juliano L, Song ES, Chow KM, Murphy MP, Hersh LB. In vitro and in vivo degradation of Abeta peptide by peptidases coupled to erythrocytes. Peptides. 2007;28:2348–2355. doi: 10.1016/j.peptides.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Studzinski C, Beckett T, Guan H, Hersh MA, Murphy MP, Klein R, Hersh LB. Expression of neprilysin in skeletal muscle reduces amyloid burden in a transgenic mouse model of Alzheimer disease. Mol Ther. 2009;17:1381–1386. doi: 10.1038/mt.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr RA, Guan H, Rockenstein E, Kindy M, Gage FH, Verma I, Masliah E, Hersh LB. Neprilysin regulates amyloid Beta peptide levels. J Mol Neurosci. 2004;22:5–11. doi: 10.1385/JMN:22:1-2:5. [DOI] [PubMed] [Google Scholar]
- Marr RA, Rockenstein E, Mukherjee A, Kindy MS, Hersh LB, Gage FH, Verma IM, Masliah E. Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J Neurosci. 2003;23:1992–1996. doi: 10.1523/JNEUROSCI.23-06-01992.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama M, Higuchi M, Takaki Y, Matsuba Y, Tanji H, Nemoto M, Tomita N, Matsui T, Iwata N, Mizukami H, Muramatsu S, Ozawa K, Saido TC, Arai H, Sasaki H. Cerebrospinal fluid neprilysin is reduced in prodromal Alzheimer’s disease. Annals of neurology. 2005;57:832–842. doi: 10.1002/ana.20494. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Saito M, LaFrancois J, Gaynor K, Olm V, Wang L, Casey E, Lu Y, Shiratori C, Lemere C, Duff K. Novel therapeutic approach for the treatment of Alzheimer’s disease by peripheral administration of agents with an affinity to beta-amyloid. J Neurosci. 2003;23:29–33. doi: 10.1523/JNEUROSCI.23-01-00029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, Jansen K, Delucia M, Lin WL, Dolios G, Wang R, Eckman CB, Dickson DW, Hutton M, Hardy J, Golde T. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilandt WJ, Cisse M, Ho K, Wu T, Esposito LA, Scearce-Levie K, Cheng IH, Yu GQ, Mucke L. Neprilysin overexpression inhibits plaque formation but fails to reduce pathogenic Abeta oligomers and associated cognitive deficits in human amyloid precursor protein transgenic mice. J Neurosci. 2009;29:1977–1986. doi: 10.1523/JNEUROSCI.2984-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miners JS, Van Helmond Z, Chalmers K, Wilcock G, Love S, Kehoe PG. Decreased expression and activity of neprilysin in Alzheimer disease are associated with cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2006;65:1012–1021. doi: 10.1097/01.jnen.0000240463.87886.9a. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Beckett TL, Ding Q, Patel E, Markesbery WR, St Clair DK, LeVine H, 3rd, Keller JN. Abeta solubility and deposition during AD progression and in APPxPS-1 knock-in mice. Neurobiol Dis. 2007;27:301–311. doi: 10.1016/j.nbd.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Smith IF, Green KN, LaFerla FM. A dynamic relationship between intracellular and extracellular pools of Abeta. Am J Pathol. 2006a;168:184–194. doi: 10.2353/ajpath.2006.050593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, LaFerla FM. Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem. 2006b;281:39413–39423. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- Park JH, Widi GA, Gimbel DA, Harel NY, Lee DH, Strittmatter SM. Subcutaneous Nogo receptor removes brain amyloid-beta and improves spatial memory in Alzheimer’s transgenic mice. J Neurosci. 2006;26:13279–13286. doi: 10.1523/JNEUROSCI.4504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier R, Wolfer DP, Welzl H, Tracy J, Galsworthy MJ, Nitsch RM, Mohajeri MH. Neuronal neprilysin overexpression is associated with attenuation of Abeta-related spatial memory deficit. Neurobiol Dis. 2006;24:475–483. doi: 10.1016/j.nbd.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, Goate A, Mayo K, Perlmutter D, Coma M, Zhong Z, Zlokovic BV. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, Marr RA, Rockenstein E, Crews L, Adame A, Potkar R, Patrick C, Gage FH, Verma IM, Masliah E. Long-term neprilysin gene transfer is associated with reduced levels of intracellular Abeta and behavioral improvement in APP transgenic mice. BMC Neurosci. 2008;9:109. doi: 10.1186/1471-2202-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MW, Govindaswami M, Hersh LB. Mutation of active site residues of the puromycin-sensitive aminopeptidase: conversion of the enzyme into a catalytically inactive binding protein. Arch Biochem Biophys. 2003;413:236–242. doi: 10.1016/s0003-9861(03)00123-1. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, Chen C, Li J, Xiao X. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nature biotechnology. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]