Abstract
Cytosolic phospholipase A2 (cPLA2) is the rate-limiting enzyme responsible for the generation of prostaglandins (PGs), which are bioactive lipids that play critical roles in maintaining gastrointestinal (GI) homeostasis. There has been a long-standing association between administration of cyclooxygenase (COX) inhibitors and GI toxicity. GI injury is thought to be induced by suppressed production of GI-protective PGs as well as direct injury to enterocytes. The present study sought to determine how pan-suppression of PG production via a genetic deletion of cPLA2 impacts the susceptibility to COX inhibitor–induced GI injury. A panel of COX inhibitors including celecoxib, rofecoxib, sulindac, and aspirin were administered via diet to cPLA2− / − and cPLA2+ / + littermates. Administration of celecoxib, rofecoxib, and sulindac, but not aspirin, resulted in acute lethality (within 2 weeks) in cPLA2− / − mice, but not in wild-type littermates. Histomorphological analysis revealed severe GI damage following celecoxib exposure associated with acute bacteremia and sepsis. Intestinal PG levels were reduced equivalently in both genotypes following celecoxib exposure, indicating that PG production was not likely responsible for the differential sensitivity. Gene expression profiling in the small intestines of mice identified drug-related changes among a panel of genes including those involved in mitochondrial function in cPLA2− / − mice. Further analysis of enterocytic mitochondria showed abnormal morphology as well as impaired ATP production in the intestines from celecoxib-exposed cPLA2− / − mice. Our data demonstrate that cPLA2 appears to be an important component in conferring protection against COX inhibitor–induced enteropathy, which may be mediated through affects on enterocytic mitochondria.
Keywords: cytosolic phospholipase A2, COX inhibitor, mitochondria, intestine
Cytosolic phospholipase A2 (cPLA2) is the rate-limiting enzyme in the generation of arachidonic acid (AA), which is further metabolized by cyclooxygenase (COX) enzymes to generate prostaglandins (PGs). PGs are bioactive lipids that have been shown to be important in maintaining gastrointestinal (GI) homeostasis (Dey et al., 2006). They have been shown to play roles in regulating epithelial cell proliferation and apoptosis as well as in maintaining intestinal mucin production and vascular integrity (Dey et al., 2006; Wang and Dubois, 2006; Wang et al., 2005). The exposure of intestinal tissue to COX inhibitors reduces basal levels of PGs and thus can disrupt their ability to confer GI protection (Thiefin and Beaugerie, 2005; Whittle, 2004).
Adverse GI events in patients have been associated with the long-term use of nonselective COX inhibitors (Bjarnason et al., 1993; Davies et al., 2000; Thiefin and Beaugerie, 2005; Whittle, 2004). The damage profiles found in the small intestine of patients include ulceration, bleeding, stricture formation, increased intestinal permeability, and, in extreme cases, intestinal perforation that leads to peritonitis and sepsis (Davies et al., 2000). The development of selective COX-2 inhibitors such as celecoxib largely circumvented GI toxicity; however, there is a subpopulation that still experiences adverse events when exposed to this drug class (Bombardier et al., 2000; Juni et al., 2002; Reuben and Steinberg, 1999; Silverstein et al., 2000).
Non-steroidal anti-inflammatory drug (NSAID) toxicity to the GI mucosa is generally attributed to two underlying mechanisms: (1) compromised production of key PGs (e.g., PGE2) that are important in maintaining gut epithelial integrity and (2) a “topical” effect resulting from direct toxicity. The topical effect is associated with inhibition of enterocytic oxidative phosphorylation with accompanying mitochondrial vacuolization and decreased ATP production (Krause et al., 2003; Somasundaram et al., 1997, 2000). These effects can reduce cellular integrity and lead to increased intestinal permeability (Krause et al., 2003; Somasundaram et al., 1997, 2000). In the present study, we have evaluated the effects of NSAID exposure on mice with a compromised ability to generate prostanoids.
During the course of these studies, we observed a remarkable acute COX inhibitor–induced lethality that was dependent upon cPLA2 genotype and limited to exposure of celecoxib, rofecoxib, and sulindac. Further examination using celecoxib revealed that lethality was associated with severe GI damage resulting in bacteremia and sepsis. Surprisingly, injury was unrelated to PG status as levels were equivalently reduced after drug exposure regardless of cPLA2 status. Morphological and functional studies showed abnormalities in intestinal mitochondria after celecoxib exposure in cPLA2− / − mice. Our results suggest that in the absence of cPLA2, COX inhibitors induce severe GI injury that may be related to impairment of enterocytic mitochondria.
MATERIALS AND METHODS
Administration of COX inhibitors.
To determine the effects of COX inhibitors on mouse viability, cPLA2− / − and cPLA2+ / + (n = 100) littermates were administered either control diet (5001 LabDiet, Purina Mills, St Louis, MO) or diet incorporated with one of the following drugs: 0.0075% rofecoxib (Merck & Co., Whitehouse Station, NJ), 0.15% celecoxib (Pfizer Inc., La Jolla, CA), 0.015% sulindac (Sigma-Aldrich, St Louis, MO), or 0.05% aspirin (Bayer, West Haven, CT) at clinically relevant doses previously reported in the literature (Gupta et al., 2004; Oshima et al., 2001; Perkins et al., 2003). For studies examining microsomal PGE2 synthase 1 (mPGES-1), a total of five mPGES-1−/− mice were administered 0.15% celecoxib-incorporated chow or control chow for a period of 2 weeks. Mice were housed in a ventilated, temperature-controlled (23°C ± 1°C), and Association for Assessment and Accreditation of Laboratory Animal Care-approved facility with a 12-h light/dark cycle and were allowed access to standard or modified chow and water ad libitum. Genotyping and PCR analysis were performed on tail DNA as previously described (Ilsley et al., 2005). All animal procedures were approved by the Institutional Animal Care Committee.
For necropsy, cPLA2+ / + and cPLA2− / − mice (n = 10) aged 12–14 weeks were fed either control diet or a diet containing 0.15% celecoxib until sacrifice by carbon dioxide (CO2) asphyxiation between 3 and 9 days later. Upon necropsy, the heart, lungs, thymus, GI tract, liver, kidney, and spleen were harvested, analyzed grossly, and photographed. After analysis, tissues were formalin fixed and paraffin embedded for subsequent histopathological analysis.
Cytokine measurements and bacterial culture.
In order to determine whether sepsis or bacteremia were occurring in celecoxib-fed mice, cPLA2+ / + and cPLA2− / − mice (n = 3) were fed either control chow or diet containing 0.15% celecoxib. At the earliest signs of weight loss (5–9 days), mice were euthanized by CO2 asphyxiation. Blood was immediately collected by cardiac puncture and allowed to coagulate for 20 min. Coagulated blood was centrifuged at 10,000 × g for 10 min to extract serum and stored at −20°C until analysis. Serum samples were examined to determine the levels of interleukin (IL) 10, IL-6, and macrophage chemoattractant protein (MCP) 1 by ELISA using the Inflammation Assay Kit (BD Biosciences, Palo Alto, CA) per manufacturer's protocol.
For bacterial culture, mice were treated as above and at sacrifice the thoracic region of the mouse was shaved and wiped down with betadine and 70% ethanol. Blood was collected by cardiac puncture in a sterile needle and syringe and immediately transferred to a Bactec Peds Plus/F bacterial culture bottle containing bacterial growth broth (BD Biosciences). Prior to peritoneal lavage, the skin of the abdominal region was cut away leaving the muscle layer intact. A sterile needle was inserted into the abdominal cavity, and sterile 1× PBS was injected and aspirated immediately before transfer to a bacterial culture bottle. All bottles were sent to the Medical Microbiology Department of the University of Connecticut Health Center for bacterial culturing and classification.
Measurement of cardiac function in heart preparations.
cPLA2+ / + and cPLA2− / − mice (n = 6) were administered either control or celecoxib chow for a total of 3 days to examine whether cardiac abnormalities were induced by celecoxib administration. After intraperitoneal injection of heparin sodium (500 U/kg) and Nembutal (150 mg/kg), hearts were removed and analyzed for cardiac function using a working heart model as described previously (Chowdari et al., 2001; Hu et al., 2001). Briefly, the aorta was cannulated with a 20-gauge catheter, and retrograde perfusion via the aorta was started immediately with a column of Krebs-Henseleit solution (KHS) to provide a constant coronary perfusion pressure of 55 mm Hg. The opening of the pulmonary vein was connected via a 20-gauge catheter to a reservoir of KHS buffer that maintained a “venous return” flow into the left atrium of ∼5 ml/min. The venous return was maintained by a constant level of hydrostatic pressure (6 mm Hg) and yielded a steady rate of flow. The KHS buffer was then switched from retrograde to antegrade perfusion and produced a work-performing heart preparation. A 25-gauge catheter was inserted into the left ventricle, and its distal end was connected to a pressure transducer to record the following endpoints: left ventricular developed pressure (difference between systolic and diastolic pressure), heart rate (number of heart beats per minute), cardiac output (sum of aortic flow and coronary flow), and contraction and relaxation (change in pressure within heart over time).
PG measurements.
cPLA2+ / + and cPLA2− / − mice (n = 4–6) were given either control or diet containing 0.15% celecoxib or 0.05% aspirin for 3 days prior to sacrifice in order to determine the effects of COX inhibitor exposure on the generation of intestinal PGs. All tissue samples were free of gross pathology to ensure no artificial alterations as a result of tissue injury. Small intestines were excised and flushed with ice-cold PBS and snap frozen in liquid nitrogen. Intestinal tissue was homogenized in phosphate buffer (2M potassium chloride and 0.5M potassium phosphate) and 2 volumes of methanol were added to the homogenates. Deuterated internal standards for respective metabolites (2 ng each) were then added to each sample. Samples were centrifuged at 2500 rpm for 10 min. Supernatants were diluted with water to adjust the final methanol concentration below 15%, and samples were extracted using solid-phase extraction cartridges (Strata C18-E, 100 mg/1 ml, Phenomenex, Torrance, CA) preconditioned with 1 ml of methanol and 1 ml of water. The eluate (1 ml of methanol) was dried down and solubilized in 40 μl high pressure liquid chromatography (HPLC) solvent A (8.3mM acetic acid buffered to pH 5.7 with ammonium hydroxide) plus 20 μl HPLC solvent B (acetonitrile:methanol 65:35, vol/vol). An aliquot of each sample (25 μl) was injected into a C-18 HPLC column (Gemini 150 × 2 mm, 5 μm, Phenomenex) and eluted at a flow rate of 200 μl/min with a linear gradient from 45 to 98% of HPLC solvent B, which was increased from 45 to 75% in 12 min, to 98% in 2 min, and held at 98% for further 11 min. The HPLC system was directly interfaced into the electrospray source of a triple quadrupole mass spectrometer (Sciex API 3000, PE-Sciex, Thornhill, Ontario, Canada) where mass spectrometric analysis was performed in the negative ion mode using multiple reaction monitoring of the specific transitions (mass/charge).
AA measurements.
cPLA2+ / + and cPLA2− / − mice (n = 5) were given either control or diet containing 0.15% celecoxib for 3 days prior to sacrifice in order to determine the effects of COX inhibitor exposure on the generation of intestinal AA. All tissue samples were free of gross pathology to ensure no artificial alterations as a result of tissue injury. Free AA was extracted from whole intestinal tissue in a manner similar to PG extraction with the buffer consisting of methanol, 1mM EDTA, and 500 ng deuterated AA standard (Cayman Chemicals). After two chloroform extractions, samples were dried under nitrogen gas and dissolved in 30 μl acetonitrile (Fisher Scientific, Suwanee, GA), 10 μl diisopropylethylamine (Sigma-Aldrich), and 10 μl 35% pentafluorobenzyl bromide (Pierce Biotechnology) dissolved in acetonitrile. Samples were incubated for 20 min at 40°C and then dried under N2 gas. The residue was treated with 50 μl hexanes and then transferred for gas chromatography/mass spectroscopy (GC/MS) analysis. GC/MS was carried out on a Hewlett-Packard 5890-gas chromatograph interfaced with a 5988A-mass spectrometer. Fatty acid (FA) derivative samples were applied to an SPB-1 (12 m × 0.2 mm, 0.33 mm film thickness, Supelco, Inc.) column held at 150°C. Samples were analyzed using a temperature program of 10°C/min from 150°C to 250°C followed by 5°C/min to 280°C with the final temperature maintained for 2 min. The injector block was held at 260°C, and the transfer tube was maintained at 280°C. AA was detected using electron capture negative chemical ionization, and methane was used as reagent gas maintained at 0.5 Torr in the ion source. AA levels were quantified using selected ion monitoring for the characteristic base peak ions of the deuterated and authentic metabolites.
Electron microscopy.
In order to determine whether morphological abnormalities occur within intestinal mitochondria, 8 h after celecoxib-incorporated or control chow administration, a 5-cm piece of proximal small intestines from cPLA2− / − and cPLA2+ / + mice (n = 2) were excised and flushed with PBS and incubated in fixative comprised of 2.5% glutaraldehyde in 0.1M sodium cacodylate buffer, pH 7.4, for 3 h. Fixed tissues were then transferred to 0.1M cacodylate buffer. Small pieces of the intestinal mucosa were cut from proximal, middle, and distal portions, rinsed again with 0.1M cacodylate buffer, postfixed for 2 h in 1% osmium tetroxide/0.8% potassium ferricyanide in 0.1M cacodylate, and stained in block with 1% aqueous uranyl acetate. The samples were then dehydrated with graded concentrations of ethanol, embedded in Polybed epoxy resin (Polysciences, Warrington, PA), and polymerized at 60°C. One-micron sections were cut with a glass knife using a Reichert Ultracut E ultramicrotome, collected on glass slides, stained with methylene blue-azure II, and observed under a light microscope. Thin sections (∼70 nm) were cut from selected areas with a diamond knife, collected on Formvar-carbon–coated 200-mesh nickel grids, stained with uranyl acetate and lead citrate, and observed in a Philips CM10 transmission electron microscope (TEM). Criteria for abnormal mitochondria include swelling, paling of the matrix, disrupted cristae, or altered shapes.
Gene expression profiling and data analysis.
Following 3 days of celecoxib or control treatment, intestines from cPLA2+ / + and cPLA2− / − mice (n = 4) were removed, and RNA was extracted using Trizol Reagent (Invitrogen, Carlsbad, CA) to examine gene expression changes induced by celecoxib exposure. All tissue samples were free of gross pathology to ensure no artificial alterations as a result of tissue injury. RNA samples were analyzed on an Agilent 2100 Bioanalyzer, and only those samples with an RNA integrity number greater or equal to 7 and a 28S:18S ratio greater than 1.7 were used for the arrays. The RNA was amplified and labeled with biotin using the Illumina TotalPrep RNA Amplification Kit (Ambion, Austin, TX). Biotinylated complementary RNA (750 ng) was mixed with 10 μl of the Illumina hybridization mix, and the samples were hybridized to MouseRef-8 v1.1 Sentrix BeadChip Array at 58°C for 16 h. The BeadChips were then washed, stained with streptavidin-cyanine3, and scanned on the Illumina BeadArray Reader. Images were then analyzed with the Illumina Bead Studio software, and values were reported as average signal for each gene. All data were then normalized using the rank invariant method. False signals were filtered out by eliminating all signals with a detection p value greater than 0.001. Significant differences in gene expression were determined by performing one-way ANOVA, and genes considered for analysis had a p value of ≤ 0.05. Significantly altered genes were entered into GeneSight 4.1.6 software (BioDiscovery, Inc., El Segundo, CA), and heat maps indicating gene signal intensity were generated by K-means clustering algorithm to determine differentially expressed gene patterns.
Mitochondrial isolation and ATP measurement.
cPLA2+ / + and cPLA2− / − mice (n = 6) were given either control diet or diet containing 0.15% celecoxib or 0.05% aspirin for 3 days prior to sacrifice in order to measure ATP production in intestinal mitochondria. All tissue samples were free of gross pathology to ensure no artificial alterations as a result of tissue injury. Upon sacrifice, small intestines were excised and enterocytic mitochondria were isolated as previously described (de Talamoni et al., 1985). Briefly, intestinal mucosa was scraped and homogenized in a dounce homogenizer containing Buffer A (0.23M mannitol, 0.07M sucrose, 0.1M EDTA, and 0.1M Tris-HCl). Homogenates were subjected to differential centrifugation to isolate mitochondria and resuspended in Buffer B (Buffer A without EDTA). ATP levels were measured using the ATP determination kit (Invitrogen) as per manufacturer's instructions. Sample concentrations were calculated in relation to a standard curve using purified ATP. ATP concentrations were normalized to mitochondrial protein concentration determined by the Bradford method (Bradford, 1976).
Statistical analysis.
One-way ANOVA and two-tailed, unpaired t-tests were performed to determine statistical significance by the probability of difference between means as indicated in each figure. A p value equal to or less than 0.05 was considered statistically significant. Values in the figures are expressed as the means plus or minus standard errors of the mean.
RESULTS
COX Inhibitors Induce Acute Mortality in cPLA2− / − Mice
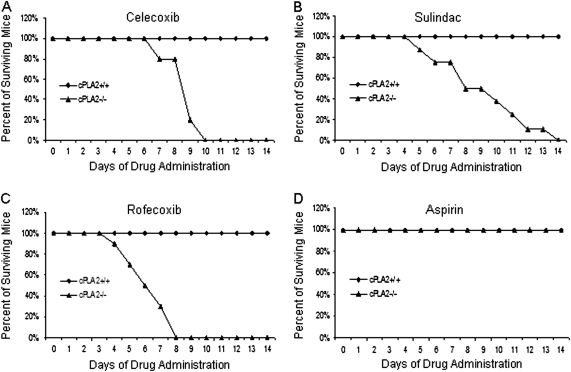
In order to test the impact of cPLA2 deletion on sensitivity to COX inhibitors, we administered a panel of COX inhibitors, including celecoxib, rofecoxib, sulindac, and aspirin to cPLA2− / − and cPLA2+ / + littermates at clinically relevant doses as previously reported in the literature (Gupta et al., 2004; Oshima et al., 2001; Perkins et al., 2003). Administration of celecoxib, rofecoxib, and sulindac, but not aspirin, produced the rapid onset of lethality (within 2 weeks) in cPLA2− / − mice but had no effect on cPLA2+ / + littermates (Figs. 1A–D). cPLA2− / − mice began to show signs of morbidity within 5 days (weight loss and lethargy) and remained moribund throughout the study period.
FIG. 1.
Survival curve of mice treated with COX inhibitors. cPLA2+ / + and cPLA2− / − mice were placed on diets containing celecoxib (0.15%), rofecoxib (0.0075%), sulindac (0.015%), or aspirin (0.05%), and mortality was recorded. The graph shows the percentage of surviving mice following the start of administration (day 0) of (A) celecoxib, (B) sulindac, (C) rofecoxib, or (D) aspirin. The data represent a minimum of 10 mice per group.
To establish the specificity of this effect, celecoxib-incorporated chow (0.15%) was administered to mice with a genetic deletion of mPGES-1 (n = 5), the rate-limiting enzyme in PGE2 production, for 2 weeks. Neither morbidity nor mortality was observed throughout the experimental period (data not shown).
Celecoxib Induces Damage to the GI Tract of cPLA2− / − Mice
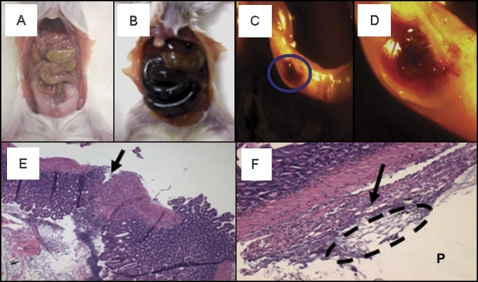
In order to determine the cause of COX inhibitor–induced death in cPLA2− / − mice, cPLA2− / − and cPLA2+ / + littermates were administered 0.15% celecoxib (celecoxib was used as a representative drug for all subsequent studies) and mice were sacrificed immediately upon evidence of toxicity (weight loss exceeding 10%, lethargy, and dehydration determined by “tenting”), typically occurring between 5 and 9 days after the start of drug treatment. At necropsy, severe GI damage, including black intestinal content and marked distention of the small bowel, was observed only in cPLA2− / − mice (Figs. 2A and B). This gross pathology extended from the stomach to the ileocecal junction in a fairly even distribution, sparing the colon that was devoid of luminal contents. The observation that damage was limited to the small intestine may result from extensive drug absorption in the upper GI tract. The small intestine was fragile and upon closer inspection revealed multiple strictures and perforations (Figs. 2C and D). Histological analysis of the small intestine revealed areas of severe ulceration, peritonitis, and fecal material on the peritoneal side of the intestine, indicating that intestinal material had leaked out of the lumen and into the peritoneum (Figs. 2E and F). In addition, we observed thymic atrophy and splenomegaly that was related to an expansion of the white pulp (data not shown). In contrast, there were neither adverse effects observed in the cPLA2+ / + mice treated with celecoxib nor was there evidence of intestinal damage in untreated mice of either cPLA2 genotype.
FIG. 2.
Effects of celecoxib exposure on small intestinal pathology. Representative photomicrographs are shown of the abdominal cavities of a (A) cPLA2+ / + mouse and a (B) cPLA2− / − mouse after celecoxib exposure (0.15% incorporated in chow for 8 days). Closer examination of cPLA2− / − intestines showed perforations throughout the intestinal wall (C, circle [×1], D [×3] [magnification of C]). Histological evaluation of hematoxylin and eosin sections shows ulceration with near rupture (arrow) within the intestinal wall (E) (×100); Peritonitis (arrow) and fecal material (dashed circle) were observed on the peritoneal side (P) of the small intestine (F) (×100).
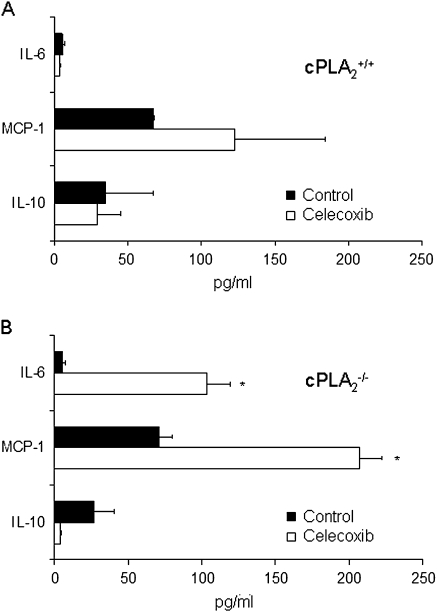
The observed damage to the small intestine raised the possibility that lethality may occur as the direct result of translocation of bacterial species into the peritoneum. Thus, bacterial cultures of both the blood and the peritoneum were prepared from celecoxib-treated cPLA2− / − and cPLA2+ / + mice. Bacteremia and peritonitis were identified only in the celecoxib-treated cPLA2− / − group. As shown in Table 1, the spectrum of pathogens that were recovered from the peritoneum and blood suggest their intestinal origin, which included Escherichia coli, Enterococcus gallinarum, Streptococcus, and Clostridium perfringens. Bacterial cultures for control or celecoxib-administered cPLA2+ / + mice were negative (Table 1). The identification of these species outside of the intestines indicated a dramatic increase in intestinal permeability. The occurrence of sepsis was investigated in celecoxib-administered mice by the measurement of blood serum cytokine levels using ELISA. These analyses showed that whereas administration of celecoxib to wild-type mice had no effect on cytokine levels (Fig. 3A), significant elevation of the proinflammatory cytokines, MCP-1 and IL-6, and a trend for a reduction in the anti-inflammatory cytokine IL-10 were observed in cPLA2− / − administered celecoxib for 5–9 days relative to the control diet group (Fig. 3B).
TABLE 1.
Bacterial Species Cultured in Blood and Peritoneal Fluid
| Species | cPLA2+ / + | cPLA2− / − |
| Escherichia coli | − | + |
| Enterococcus gallinarum | − | + |
| Streptococcus | − | + |
| Clostridium perfringens | − | + |
Note. Blood and peritoneal fluid were cultured from cPLA2− / − and cPLA2− / − mice following celecoxib administration (5–9 days) to determine whether bacterial species were present as described under Materials and Methods section.
FIG. 3.
Effects of celecoxib exposure on serum cytokine levels. Cytokine levels were measured in the serum of (A) cPLA2+ / + and (B) cPLA2− / − mice fed control or celecoxib-incorporated chow for 5–9 days. *p < 0.05 as compared with control samples for each cytokine as determined by unpaired t-tests. Data represent the means ± standard errors of the mean of three mice per group.
As cardiovascular toxicity is an important adverse effect of COX-2–selective inhibitors, we examined whether celecoxib-induced mortality was exacerbated by cardiovascular injury in cPLA2− / − mice (Breyer, 2005; Grosser et al., 2006). Measurement of cardiac function using a working heart model as an indicator of myocardial infarction was tested in cPLA2+ / + and cPLA2− / − mice before and after celecoxib administration. No differences were found among genotypes in the panel of heart function indices that were examined (Supplementary table 1). Thus, the acute lethality observed was likely to be independent of direct damage to cardiac tissue.
cPLA2 Status Affects AA Production after Celecoxib Exposure
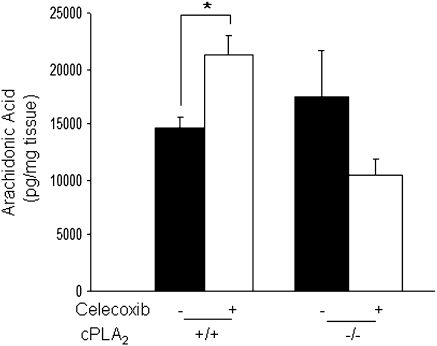
cPLA2 is the rate-limiting enzyme in the release of free AA; therefore, we determined how genetic deletion of cPLA2 would impact AA production in mice. AA levels were measured by GC/MS in the intestines of cPLA2+ / + and cPLA2− / − mice with or without celecoxib exposure for 3 days. All tissue samples were free of gross pathology to ensure no artificial alterations as a result of tissue injury. As shown in Figure 4, under control conditions, cPLA2 status did not impact intestinal AA production. However, after celecoxib exposure, there was a marked increase in AA levels by ∼30% in cPLA2+ / + mice, an effect that was absent in cPLA2− / − mice.
FIG. 4.
Measurement of intestinal AA production. AA levels were measured by GC/MS in the small intestines of cPLA2+ / + and cPLA2− / − mice under control conditions and after 3 days of celecoxib administration. Data represent the means ± standard errors of the mean of six mice per group. *p < 0.05 as determined by one-way ANOVA followed by unpaired t-tests.
Celecoxib Treatment Reduces PG Levels Independent of cPLA2 Status
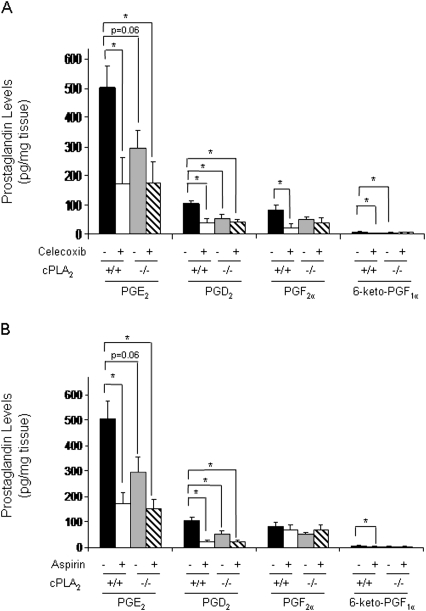
The important roles of COX-derived PGs in the physiological function and maintenance of the intestinal epithelium have been well documented (Stenson, 2007; Wang et al., 2005). To provide further insight into underlying mechanisms of celecoxib-induced GI damage, we compared PG levels within the intestinal tissue of cPLA2+ / + and cPLA2− / − mice before and after drug administration. All tissue samples were free of gross pathology to ensure no artificial alterations as a result of tissue injury. In the absence of drug, the levels of PGD2 and PGE2 were significantly higher in the cPLA2+ / + mice compared with cPLA2− / − littermates (Fig. 5A). In addition, the levels in both prostaglandin F2α and 6-keto-PGF1α trended higher in the cPLA2+ / + intestines (Fig. 5A). Upon administration with celecoxib, however, intestinal PG levels were reduced to a comparable extent regardless of cPLA2 genotype (Fig. 5A).
FIG. 5.
The effects of COX inhibitor administration on PG levels in the small intestine. (A) PG levels were measured by liquid chromatography/MS in the small intestines of cPLA2+ / + and cPLA2− / − mice fed control or celecoxib-incorporated chow for 3 days. (B) PG levels were measured as described in (A), but aspirin was given in place of celecoxib. Statistical analysis was performed by one-way ANOVA followed by post-hoc analysis using unpaired t-tests. Data represent the means ± standard errors of the mean of four to six mice per group. A p value ≤ 0.05 is considered statistically significant.
To eliminate the possibility that sulfonamide-induced suppression of PG formation may underlie the drug-induced GI damage, PG levels were compared in mice administered aspirin as well. Whereas aspirin causes no apparent GI damage or lethality in these mice (Fig. 1D), previous studies have shown that aspirin reduces PG levels in the intestinal mucosa (Frieboes et al., 2001; Krishnan et al., 2001) and can thus serve as an appropriate comparison. Consistent with these earlier studies, liquid chromatography/mass spectrometry analysis revealed that aspirin was nearly as effective as celecoxib in reducing PG levels within the intestinal mucosa in both cPLA2− / − and cPLA2+ / + mice (Fig. 5B). Thus, the acute sensitivity of the cPLA2− / − mice cannot be entirely explained by drug-related suppression of PG production in the intestines.
Effects of Celecoxib Exposure on Intestinal Gene Expression Profiles
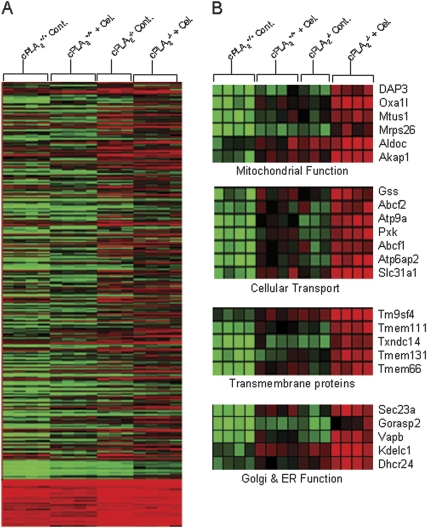
In the following experiment, global gene expression profiling was performed on intestinal tissue isolated from mice of both genotypes (cPLA2+ / + and cPLA2− / −) at 3 days after celecoxib exposure. A control group of mice fed regular diet was used for comparison. Analysis of heat maps generated by Genesight software showed 1579 genes that were significantly different when comparing the four groups as determined by one-way ANOVA (Fig. 6A). Further analysis of the 1579 genes identified a subset of 23 genes that were upregulated to a greater extent after drug exposure in cPLA2− / − mice as compared with cPLA2+ / + mice (Fig. 6B). Many of these genes encode for proteins involved in mitochondrial function including oxidase (cytochrome c) assembly 1-like (Oxa1l) and aldolase C, fructose-bisphosphate (Aldoc) as well as other cellular processes including cellular transport and Golgi and endoplasmic reticulum (ER) function as shown in Figure 6B.
FIG. 6.
Global gene expression analysis in intestinal tissues. (A) Gene expression patterns were examined by microarray analysis in the small intestines of cPLA2+ / + and cPLA2− / − mice fed control or celecoxib-incorporated chow for a total of 3 days. Signal intensities were determined using the K-means clustering algorithm as described under Materials and Methods section. A total of 1579 genes were determined to be significantly different (p ≤ 0.05) between groups using one-way ANOVA. (B) Genes found to be more markedly altered after celecoxib exposure in cPLA2− / − mice were categorized by function and shown as a heat map representing gene signal intensities.
Mitochondrial Abnormalities Are Induced after Celecoxib Exposure in cPLA2− / − Mice
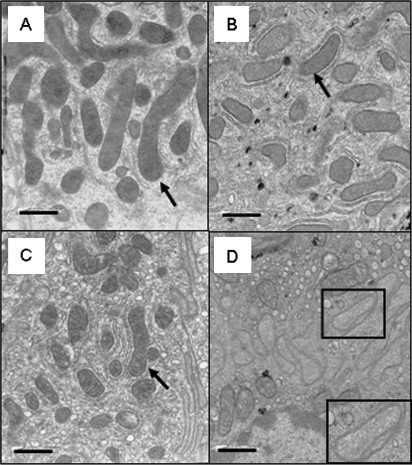
As we observed differential expression of genes involved in mitochondrial function between genotypes and COX inhibitors have been shown to induce enterocytic mitochondrial abnormalities after short-term exposure, we determined whether morphological changes were occurring in the mitochondria. In order to examine early mitochondrial changes in the intestines of mice, we exposed cPLA2+ / + and cPLA2− / − mice to celecoxib-incorporated or control chow for 8 h and analyzed morphology by TEM. As shown in Figures 7A and 7B, enterocytes from cPLA2+ / + mice exhibited normal appearing mitochondria both under control conditions and after celecoxib exposure. Mitochondria from enterocytes of untreated cPLA2− / − mice appear to have slight abnormalities, which may represent an underlying susceptibility to drug-induced damage. However, mitochondria from celecoxib-treated mice frequently exhibited abnormal mitochondria, including swelling and paling of the matrix, as well as disrupted cristae or altered shapes (criteria are described in detail under Materials and Methods section) (Figs. 7C and D).
FIG. 7.
Effects of celecoxib exposure on mitochondrial morphology. Representative photomicrographs of mitochondria as visualized by electron microscopy within enterocytes from (A) cPLA2+ / + mice fed control chow or (B) celecoxib-incorporated chow for 8 h and (C) cPLA2− / − mice fed control chow or (D) celecoxib-incorporated chow for 8 h are shown. Mitochondria were examined for morphological abnormalities as described under Materials and Methods section. Mitochondria are indicated by arrows. A representative mitochondrion showing evidence of matrix paling and disrupted cristae is indicated by a box (D, inset). (Magnification: ×16,525, scale bar = 1 μm).
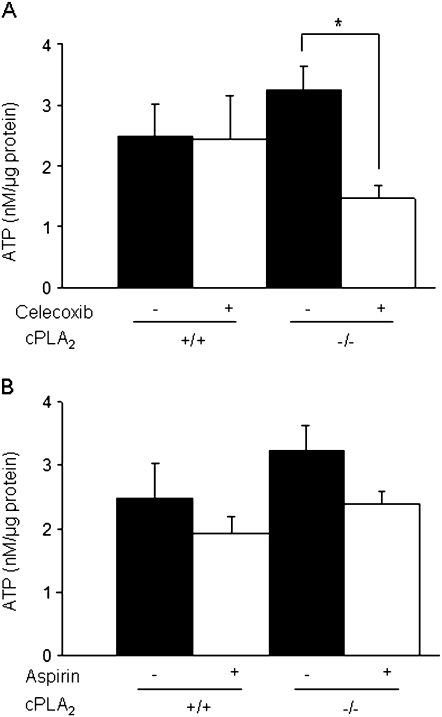
We next sought to determine whether early morphological changes in mitochondria were associated with impaired mitochondrial function at a later time point of drug exposure. To test this possibility, mitochondria were isolated from intestinal enterocytes after 3 days of celecoxib exposure and the rate of ATP production was determined in vitro. All tissue samples were free of gross pathology to ensure no artificial alterations as a result of tissue injury. As shown in Figure 8A, celecoxib exposure to cPLA2− / − mice resulted in an ∼60% reduction in ATP production, whereas drug treatment had no effect on ATP generation in mitochondria isolated from of wild-type littermates (Fig. 8A). No effect on ATP generation was found in either genotype after aspirin administration (Fig. 8B).
FIG. 8.
Effects of celecoxib exposure on intestinal mitochondrial ATP production. (A) ATP production was measured in isolated mitochondria from enterocytes as described under Materials and Methods section in cPLA2+ / + and cPLA2− / − mice fed control or celecoxib-incorporated chow for 3 days. (B) ATP production was measured in the same groups as described in (A), but mice were given aspirin in place of celecoxib. Data were compared by one-way ANOVA followed by unpaired t-tests and a p value ≤ 0.05 was considered statistically significant. Data represent the means ± standard errors of the mean of six mice per group.
DISCUSSION
The present study demonstrates that genetic deletion of cPLA2 results in a lethal susceptibility to certain COX inhibitors including celecoxib. This lethality was associated with severe GI damage associated with bacteremia and sepsis. Gene expression analysis in the intestines showed a number of differentially expressed genes including a set associated with mitochondrial function after celecoxib exposure. Further examination of intestinal mitochondria revealed abnormalities in morphology and function after celecoxib exposure in cPLA2− / − mice.
Previous studies have shown nonselective COX inhibitors to induce direct damage to enterocytic mitochondria when orally or directly administered to the intestines of rats, known as a topical effect (Somasundaram et al., 1997, 2000). Analysis of mitochondrial morphology by electron microscopy revealed vacuolization and ballooning of the organelle with a concomitant uncoupling of oxidative phosphorylation determined by measuring oxygen consumption as a marker for respiration in isolated mitochondria (Somasundaram et al., 1997, 2000). These studies also demonstrated that orally administered aspirin did not induce this type of mitochondrial injury. Aspirin and other NSAIDs differ largely in their structure and metabolism. Many NSAIDs, including those examined in the present study, have a sulfur group as part of their structure, whereas aspirin does not. It is believed that this drug class can act as protonophores, which are classic uncouplers of oxidative phosphorylation and accumulate at high concentrations within the mitochondrial membrane thus disturbing the critical balance of the proton gradient (Krause et al., 2003). Additionally, unlike aspirin, a number of these inhibitors have been shown to undergo enterohepatic recirculation, thereby prolonging exposure of the small intestine to drug damage (Huntjens et al., 2008). Our study is the first to demonstrate this type of injury to the rodent intestine using a selective COX-2 inhibitor. As we have observed a very similar pattern of damage with resulting deficiencies in mitochondrial function, including a reduction in ATP generation, we speculate that the selective COX-2 inhibitor, celecoxib, is acting as a classic uncoupler of oxidative phosphorylation in the mouse intestine in the absence of cPLA2. These previous studies indicate likely non–COX-related effects of these drugs on the intestinal mucosa. Given our findings of mitochondrial injury as well as the observation that there is an equivalent reduction of PG levels in both genotypes after drug exposure (Fig. 5), we suspect that the phenotype observed in cPLA2− / − mice could be independent of COX-related activity.
ATP production is a critical component to normal cellular function and integrity. Disturbances in mitochondrial membrane permeability are important steps leading to cell death (Lemasters, 2005). Mitochondrial function in intestinal epithelial cells is of particular importance given their role in maintaining gut barrier function (Nazli et al., 2004; Somasundaram et al., 2000). A number of studies have shown that perturbations in ATP generation can lead to impaired cellular respiration and loss of gut barrier function. For example, in vivo exposure of the intestinal mucosa to 2,4-dinitrophenol, an uncoupler of oxidative phosphorylation, resulted in increased junction permeability determined by ion conductance and transepithelial flux of horseradish peroxidase or 51CrEDTA (Nazli et al., 2004; Somasundaram et al., 2000). Given that we observe impairment in ATP generation after celecoxib exposure in those mice experiencing GI damage, it is possible that a reduction in ATP production leads to loss of gut integrity in our model.
The exact mechanism by which cPLA2 deletion confers susceptibility to COX inhibitors is not clear. Given that mitochondria have been shown to utilize FAs to generate ATP, especially under pharmacological stress, it is possible that the inability to generate FAs impair proper mitochondrial function in our model (Hagen et al., 2002; Virmani et al., 2003, 2005). It has been demonstrated that L-carnitine, a carrier molecule that is critical for the transport and oxidation of FAs into mitochondria, elicits a protective effect in neuronal cells following exposure to drugs which impair mitochondrial enzyme function (Hagen et al., 2002; Virmani et al., 2003, 2005). These studies suggest that a shift occurs in the source for energy generation from glucose to FAs under pharmacological stress.
Given the important role of cPLA2 in releasing AA, we examined AA production in mouse intestines. Under control conditions, cPLA2 status did not impact basal AA levels. This finding indicates that cPLA2 might not be critical to generate AA in the steady state. However, after drug challenge, we observed impaired AA production in cPLA2− / − mice. This observation suggests that the enzymatic activity of cPLA2 is most critical when the gut is exposed to exogenous insult. Additionally, it could be reasoned that other PLA2s such as the secretory PLA2s could have compensatory enzymatic actions to generate AA under basal conditions but are not sufficient to release AA upon drug challenge. Although we did not find a difference in the levels of AA under control conditions, we did find impaired intestinal PG production in cPLA2− / − mice. Studies have demonstrated that upon stimulation, cPLA2 and COX-2 colocalize at organelle membranes to generate PGs (Bidgood et al., 2000; Grewal et al., 2005). Loss of cPLA2 in our model would not allow for colocalization of these two enzymes and might account for the impaired PG production regardless of AA levels.
Global gene expression profiling of intestinal tissue identified a large number of expression changes induced by celecoxib exposure. Many of these genes are found to be involved in mitochondrial function including Oxa1l and Aldoc, which are known to be important mediators of energy generation via mitochondrial respiration (Jian et al., 2010; Stiburek et al., 2007). These genes were all found to be more markedly upregulated after drug exposure in cPLA2− / − mice, which might reflect a tissue response to attempt to increase rates of mitochondrial respiration. However, there was impaired ATP production in these same groups; therefore, it is possible that in the absence of cPLA2, other steps in the energy generation pathway are defective. Although in this study we have focused primarily on celecoxib-induced alterations in mitochondrial function, it is possible that celecoxib may also affect other key signaling pathways that may contribute to the exaggerated lethality observed in the cPLA2− / − mice. A number of genes encoding proteins that are involved in cellular transport processes, Golgi and ER function, as well as a number of transmembrane proteins (Tmems), were all found to be induced after celecoxib exposure to a greater extent in the cPLA2− / − mice. These differentially expressed genes include ATP-binding cassette transporter class f (Abcf) and solute carrier family 31 that control small molecule transport and cellular ion flux (Dassa and Bouige, 2001; Dassa and Schneider, 2001; Molloy and Kaplan, 2009). Additionally, we found sec23a, Golgi reassembly stacking protein 2 and vesicle-associated membrane protein-associated protein B, to be differentially expressed. These genes play varying roles in protein transport, assembly of Golgi apparatus, and protein accumulation in the ER, respectively (Kanekura et al., 2009; Saito et al., 2009; Xiang and Wang, 2010). Additionally, genes encoding several Tmems were also found to be more highly expressed in the cPLA2− / − mice. These genes include Tmem 111 and Tmem66, a set of proteins that play a role in organelle stress response as well as in maintenance of the cellular redox environment (Nayak et al., 2009; Romanuik et al., 2009). Each of these processes may be critical in maintaining cellular homeostasis, and the toxicological outcome of their dysregulation as a result of COX inhibitor administration is likely exaggerated by the absence of cPLA2.
Although the use of coxibs has largely circumvented the GI toxicity associated with COX inhibition, a subset of patients have continued to experience adverse GI events, suggesting the possibility of an underlying genetic susceptibility (Bombardier et al., 2000; Juni et al., 2002; Reuben and Steinberg, 1999; Silverstein et al., 2000). The basis to this differential response to coxibs is unknown but could involve dysregulation of genes within the AA cascade, including the COXs and PLA2 isoforms. cPLA2 appears to be the most critical PLA2 in the release of AA and, positioned upstream of the COX enzymes, directly controls the release of AA substrate. Several studies have identified the presence of genetic polymorphisms on the cPLA2 gene within human populations, including CA and poly A repeats within the promoter region, although these genetic variants were not shown to lead to any adverse clinical events (Chowdari et al., 2001; Frieboes et al., 2001; Pae et al., 2004; Tay et al., 1995). More recently, a case was reported of a patient who had diminished basal cPLA2 expression as a result of two heterozygous single basepair mutations on the cPLA2 gene. This defect was associated with spontaneous small intestinal ulcerations (Adler et al., 2008). Based in part on these recent data, we postulate that a deficiency in cPLA2 might partially explain individual susceptibility to drug-related GI toxicity.
Our findings have demonstrated that certain COX inhibitors induce severe damage to the GI tract of cPLA2− / − mice, leading to death. We show that in the absence of cPLA2, intestines are susceptible to a number of genetic alterations including those involved in mitochondrial function. These gene changes are associated with abnormalities of mitochondrial morphology and ATP generation. We propose that impaired mitochondrial function leads to reduced cellular integrity and as a result produces septicemia with concomitant acute lethality. Susceptibility to COX inhibitor–induced GI injury in mice with a genetic deletion in cPLA2 might serve as a representative model for the human subpopulation described.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (“Altered Arachidonic Acid Balance and Colon Cancer” R01CA114635).
Supplementary Material
References
- Adler DH, Cogan JD, Phillips JA, III, Schnetz-Boutaud N, Milne GL, Iverson T, Stein JA, Brenner DA, Morrow JD, Boutaud O, et al. Inherited human cPLA(2alpha) deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. J. Clin. Invest. 2008;118:2121–2131. doi: 10.1172/JCI30473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidgood MJ, Jamal OS, Cunningham AM, Brooks PM, Scott KF. Type IIA secretory phospholipase A2 up-regulates cyclooxygenase-2 and amplifies cytokine-mediated prostaglandin production in human rheumatoid synoviocytes. J. Immunol. 2000;165:2790–2797. doi: 10.4049/jimmunol.165.5.2790. [DOI] [PubMed] [Google Scholar]
- Bjarnason I, Hayllar J, MacPherson AJ, Russell AS. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology. 1993;104:1832–1847. doi: 10.1016/0016-5085(93)90667-2. [DOI] [PubMed] [Google Scholar]
- Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N. Engl. J. Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. 2 p following 1528. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breyer MD. Getting to the heart of COX-2 inhibition. Cell Metab. 2005;2:149–150. doi: 10.1016/j.cmet.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Chowdari KV, Brandstaetter B, Semwal P, Bhatia T, Deshpande S, Reddy R, Wood J, Weinberg CR, Thelma BK, Nimgaonkar VL. Association studies of cytosolic phospholipase A2 polymorphisms and schizophrenia among two independent family-based samples. Psychiatr. Genet. 2001;11:207–212. doi: 10.1097/00041444-200112000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassa E, Bouige P. The ABC of ABCS: a phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol. 2001;152:211–229. doi: 10.1016/s0923-2508(01)01194-9. [DOI] [PubMed] [Google Scholar]
- Dassa E, Schneider E. The rise of a protein family: ATP-binding cassette systems. Res. Microbiol. 2001;152:203. doi: 10.1016/s0923-2508(01)01214-1. [DOI] [PubMed] [Google Scholar]
- Davies NM, Saleh JY, Skjodt NM. Detection and prevention of NSAID-induced enteropathy. J. Pharm. Pharm. Sci. 2000;3:137–155. [PubMed] [Google Scholar]
- de Talamoni NT, Pereira R, de Bronia DH, Moreno J, Canas F. Phospholipids and sialic acid changes produced by vitamin D3 on intestinal mitochondria. Metabolism. 1985;34:1007–1011. doi: 10.1016/0026-0495(85)90071-x. [DOI] [PubMed] [Google Scholar]
- Dey I, Lejeune M, Chadee K. Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br. J. Pharmacol. 2006;149:611–623. doi: 10.1038/sj.bjp.0706923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieboes RM, Moises HW, Gattaz WF, Yang L, Li T, Liu X, Vetter P, Macciardi F, Hwu HG, Henn F. Lack of association between schizophrenia and the phospholipase-A(2) genes cPLA2 and sPLA2. Am. J. Med. Genet. 2001;105:246–249. [PubMed] [Google Scholar]
- Grewal S, Herbert SP, Ponnambalam S, Walker JH. Cytosolic phospholipase A2-alpha and cyclooxygenase-2 localize to intracellular membranes of EA.hy.926 endothelial cells that are distinct from the endoplasmic reticulum and the Golgi apparatus. FEBS J. 2005;272:1278–1290. doi: 10.1111/j.1742-4658.2005.04565.x. [DOI] [PubMed] [Google Scholar]
- Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J. Clin. Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Adhami VM, Subbarayan M, MacLennan GT, Lewin JS, Hafeli UO, Fu P, Mukhtar H. Suppression of prostate carcinogenesis by dietary supplementation of celecoxib in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2004;64:3334–3343. doi: 10.1158/0008-5472.can-03-2422. [DOI] [PubMed] [Google Scholar]
- Hagen TM, Moreau R, Suh JH, Visioli F. Mitochondrial decay in the aging rat heart: evidence for improvement by dietary supplementation with acetyl-L-carnitine and/or lipoic acid. Ann. N. Y. Acad. Sci. 2002;959:491–507. doi: 10.1111/j.1749-6632.2002.tb02119.x. [DOI] [PubMed] [Google Scholar]
- Hu B, Mei QB, Yao XJ, Smith E, Barry WH, Liang BT. A novel contractile phenotype with cardiac transgenic expression of the human P2X4 receptor. FASEB J. 2001;15:2739–2741. doi: 10.1096/fj.01-0445fje. [DOI] [PubMed] [Google Scholar]
- Huntjens DR, Strougo A, Chain A, Metcalf A, Summerfield S, Spalding DJ, Danhof M, Della Pasqua O. Population pharmacokinetic modelling of the enterohepatic recirculation of diclofenac and rofecoxib in rats. Br. J. Pharmacol. 2008;153:1072–1084. doi: 10.1038/sj.bjp.0707643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilsley JN, Nakanishi M, Flynn C, Belinsky GS, De Guise S, Adib JN, Dobrowsky RT, Bonventre JV, Rosenberg DW. Cytoplasmic phospholipase A2 deletion enhances colon tumorigenesis. Cancer Res. 2005;65:2636–2643. doi: 10.1158/0008-5472.CAN-04-3446. [DOI] [PubMed] [Google Scholar]
- Jian B, Wang D, Chen D, Voss J, Chaudry I, Raju R. Hypoxia induced alteration of mitochondrial genes in cardiomyocytes—role of Bnip3 and Pdk1. Shock. 2010 doi: 10.1097/SHK.0b013e3181cffe7d. Advance Access published on February 10, 2010; doi: 10.1097/SHK.0b013e3181cffe7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni P, Rutjes AW, Dieppe PA. Are selective COX 2 inhibitors superior to traditional nonsteroidal anti-inflammatory drugs? BMJ. 2002;324:1287–1288. doi: 10.1136/bmj.324.7349.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekura K, Suzuki H, Aiso S, Matsuoka M. ER stress and unfolded protein response in amyotrophic lateral sclerosis. Mol. Neurobiol. 2009;39:81–89. doi: 10.1007/s12035-009-8054-3. [DOI] [PubMed] [Google Scholar]
- Krause MM, Brand MD, Krauss S, Meisel C, Vergin H, Burmester GR, Buttgereit F. Nonsteroidal antiinflammatory drugs and a selective cyclooxygenase 2 inhibitor uncouple mitochondria in intact cells. Arthritis Rheum. 2003;48:1438–1444. doi: 10.1002/art.10969. [DOI] [PubMed] [Google Scholar]
- Krishnan K, Ruffin MT, Normolle D, Shureiqi I, Burney K, Bailey J, Peters-Golden M, Rock CL, Boland CR, Brenner DE. Colonic mucosal prostaglandin E2 and cyclooxygenase expression before and after low aspirin doses in subjects at high risk or at normal risk for colorectal cancer. Cancer Epidemiol. Biomarkers Prev. 2001;10:447–453. [PubMed] [Google Scholar]
- Lemasters JJ. Dying a thousand deaths: redundant pathways from different organelles to apoptosis and necrosis. Gastroenterology. 2005;129:351–360. doi: 10.1053/j.gastro.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Molloy SA, Kaplan JH. Copper-dependent recycling of hCTR1, the human high affinity copper transporter. J. Biol. Chem. 2009;284:29704–29713. doi: 10.1074/jbc.M109.000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak RR, Kearns M, Spielman RS, Cheung VG. Coexpression network based on natural variation in human gene expression reveals gene interactions and functions. Genome Res. 2009;19:1953–1962. doi: 10.1101/gr.097600.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazli A, Yang PC, Jury J, Howe K, Watson JL, Soderholm JD, Sherman PM, Perdue MH, McKay DM. Epithelia under metabolic stress perceive commensal bacteria as a threat. Am. J. Pathol. 2004;164:947–957. doi: 10.1016/S0002-9440(10)63182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima M, Murai N, Kargman S, Arguello M, Luk P, Kwong E, Taketo MM, Evans JF. Chemoprevention of intestinal polyposis in the Apcdelta716 mouse by rofecoxib, a specific cyclooxygenase-2 inhibitor. Cancer Res. 2001;61:1733–1740. [PubMed] [Google Scholar]
- Pae CU, Yu HS, Kim JJ, Lee CU, Lee SJ, Lee KU, Jun TY, Paik IH, Serretti A, Lee C. BanI polymorphism of the cytosolic phospholipase A2 gene and mood disorders in the Korean population. Neuropsychobiology. 2004;49:185–188. doi: 10.1159/000077364. [DOI] [PubMed] [Google Scholar]
- Perkins S, Clarke AR, Steward W, Gescher A. Age-related difference in susceptibility of Apc(Min/+) mice towards the chemopreventive efficacy of dietary aspirin and curcumin. Br. J. Cancer. 2003;88:1480–1483. doi: 10.1038/sj.bjc.6600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben SS, Steinberg R. Gastric perforation associated with the use of celecoxib. Anesthesiology. 1999;91:1548–1549. doi: 10.1097/00000542-199911000-00055. [DOI] [PubMed] [Google Scholar]
- Romanuik TL, Wang G, Holt RA, Jones SJ, Marra MA, Sadar MD. Identification of novel androgen-responsive genes by sequencing of LongSAGE libraries. BMC Genomics. 2009;10:476. doi: 10.1186/1471-2164-10-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Hino S, Murakami T, Kanemoto S, Kondo S, Saitoh M, Nishimura R, Yoneda T, Furuichi T, Ikegawa S, et al. Regulation of endoplasmic reticulum stress response by a BBF2H7-mediated Sec23a pathway is essential for chondrogenesis. Nat. Cell Biol. 2009;11:1197–1204. doi: 10.1038/ncb1962. [DOI] [PubMed] [Google Scholar]
- Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- Somasundaram S, Rafi S, Hayllar J, Sigthorsson G, Jacob M, Price AB, Macpherson A, Mahmod T, Scott D, Wrigglesworth JM, et al. Mitochondrial damage: a possible mechanism of the “topical” phase of NSAID induced injury to the rat intestine. Gut. 1997;41:344–353. doi: 10.1136/gut.41.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaram S, Sigthorsson G, Simpson RJ, Watts J, Jacob M, Tavares IA, Rafi S, Roseth A, Foster R, Price AB, et al. Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAID-enteropathy in the rat. Aliment. Pharmacol. Ther. 2000;14:639–650. doi: 10.1046/j.1365-2036.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- Stenson WF. Prostaglandins and epithelial response to injury. Curr. Opin. Gastroenterol. 2007;23:107–110. doi: 10.1097/MOG.0b013e3280143cb6. [DOI] [PubMed] [Google Scholar]
- Stiburek L, Fornuskova D, Wenchich L, Pejznochova M, Hansikova H, Zeman J. Knockdown of human Oxa1l impairs the biogenesis of F1Fo-ATP synthase and NADH:ubiquinone oxidoreductase. J. Mol. Biol. 2007;374:506–516. doi: 10.1016/j.jmb.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Tay A, Simon JS, Squire J, Hamel K, Jacob HJ, Skorecki K. Cytosolic phospholipase A2 gene in human and rat: chromosomal localization and polymorphic markers. Genomics. 1995;26:138–141. doi: 10.1016/0888-7543(95)80093-2. [DOI] [PubMed] [Google Scholar]
- Thiefin G, Beaugerie L. Toxic effects of nonsteroidal antiinflammatory drugs on the small bowel, colon, and rectum. Joint Bone Spine. 2005;72:286–294. doi: 10.1016/j.jbspin.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Virmani A, Gaetani F, Binienda Z. Effects of metabolic modifiers such as carnitines, coenzyme Q10, and PUFAs against different forms of neurotoxic insults: metabolic inhibitors, MPTP, and methamphetamine. Ann. N. Y. Acad. Sci. 2005;1053:183–191. doi: 10.1196/annals.1344.016. [DOI] [PubMed] [Google Scholar]
- Virmani A, Gaetani F, Imam S, Binienda Z, Ali S. Possible mechanism for the neuroprotective effects of L-carnitine on methamphetamine-evoked neurotoxicity. Ann. N. Y. Acad. Sci. 2003;993:197–207. doi: 10.1111/j.1749-6632.2003.tb07530.x. discussion 287–288. [DOI] [PubMed] [Google Scholar]
- Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Mann JR, DuBois RN. The role of prostaglandins and other eicosanoids in the gastrointestinal tract. Gastroenterology. 2005;128:1445–1461. doi: 10.1053/j.gastro.2004.09.080. [DOI] [PubMed] [Google Scholar]
- Whittle BJ. Mechanisms underlying intestinal injury induced by anti-inflammatory COX inhibitors. Eur. J. Pharmacol. 2004;500:427–439. doi: 10.1016/j.ejphar.2004.07.042. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Wang Y. GRASP55 and GRASP65 play complementary and essential roles in Golgi cisternal stacking. J. Cell Biol. 2010;188:237–251. doi: 10.1083/jcb.200907132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.