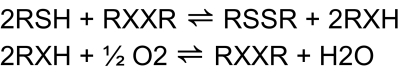Abstract
Ebselen (Ebs) and diphenyl diselenide [(PhSe)2] readily oxidize thiol groups. Here we studied mitochondrial swelling changes in mitochondrial potential (Δψm), NAD(P)H oxidation, reactive oxygen species production, protein aggregate formation, and oxygen consumption as ending points of their in vitro toxicity. Specifically, we tested the hypothesis that organochalchogens toxicity could be associated with mitochondrial dysfunction via oxidation of vicinal thiol groups that are known to be involved in the regulation of mitochondrial permeability (Petronilli et al. J. Biol. Chem., 269; 16638; 1994). Furthermore, we investigated the possible mechanism(s) by which these organochalchogens could disrupt liver mitochondrial function. Ebs and (PhSe)2 caused mitochondrial depolarization and swelling in a concentration-dependent manner. Furthermore, both organochalchogens caused rapid oxidation of the mitochondrial pyridine nucleotides (NAD(P)H) pool, likely reflecting the consequence and not the cause of increased mitochondrial permeability (Costantini, P., Chernyak, B. V., Petronilli, V., and Bernardi, P. (1996). Modulation of the mitochondrial permeability transition pore (PTP) by pyridine nucleotides and dithiol oxidation at two separate sites. J. Biol. Chem. 271, 6746–6751). The organochalchogens-induced mitochondrial dysfunction was prevented by the reducing agent dithiothreitol (DTT). Ebs- and (PhSe)2-induced mitochondrial depolarization and swelling were unchanged by ruthenium red (4μM), butylated hydroxytoluene (2.5μM), or cyclosporine A (1μM). N-ethylmaleimide enhanced the organochalchogens-induced mitochondrial depolarization, without affecting the magnitude of the swelling response. In contrast, iodoacetic acid did not modify the effects of Ebs or (PhSe)2 on the mitochondria. Additionally, Ebs and (PhSe)2 decreased the basal 2' 7' dichlorofluorescin diacetate (H2-DCFDA) oxidation and oxygen consumption rate in state 3 and increased it during the state 4 of oxidative phosphorylation and induced the formation of protein aggregates, which were prevented by DTT. However, DTT failed to reverse the formation of protein aggregates, when it was added after a preincubation of liver mitochondria with Ebs or (PhSe)2. Similarly, DTT did not reverse the Ebs- or (PhSe)2-induced Δψm collapse or swelling, when it was added after a preincubation period of mitochondria with chalcogenides. These results show that Ebs and (PhSe)2 can effectively induce mitochondrial dysfunction and suggest that effects of these compounds are associated with mitochondrial thiol groups oxidation. The inability of cyclosporine A to reverse the Ebs- and (PhSe)2-induced mitochondrial effects suggests that the redox-regulated mitochondrial permeability transition (MPT) pore was mechanistically regulated in a manner that is distinct from the classical MPT pore.
Keywords: mitochondrial dysfunction, Ebs, (PhSe)2, thiol oxidation
Mitochondria play an important role in the regulation of apoptotic or necrotic cell death, which can be preceded by the occurrence of mitochondrial permeability transition (MPT) (Javadov and Karmazyn, 2007; Kowaltowski et al., 2001; Norenberg and Rao, 2007). Consistent with this role, several agents, such as Ca2+, thiol oxidants, reactive oxygen species (ROS), and/or members of the Bcl-2 family of proteins can regulate cell death or survival by interference with MPT pore opening (Costantini et al., 1996; Crompton, 1999; Halestrap et al., 2004). Thus, factors potentially involved in regulating mitochondrial integrity are of considerable importance in cell biology because mitochondria are central to cell survival, and their dysfunction can lead to rapid cell death.
Selenium compounds are toxic to intact animals and cultured cells (Jung et al., 2001; Shen et al., 2001) primarily because of their ability to catalyze the oxidation of thiols with concomitant generation of superoxide anions (O2−) (Schiar et al., 2009; Yan and Spallholz, 1993). Thus, it is reasonable to suggest that selenium toxicity, at least in part, may be related to its effects on mitochondria, as this organelle represents the main source of intracellular ROS generation in mammalian cells (Morin et al., 2003; Zhao et al., 2006). Furthermore, organochalcogens, namely diselenides, can oxidize vicinal thiol groups of low molecular weight compounds and protein, apparently without generating ROS (see Nogueira et al., 2004 and references therein). Consequently, in view of the critical role of vicinal thiol groups in the regulation of MPT (Costantini et al., 1996; Petronilli et al., 1994), it is reasonable to suggest that these mitochondrial thiol groups represent important molecular targets for organochalcogens hepatotoxicity.
Ebselen (Ebs) is a seleno-organic drug with a variety of pharmacological and therapeutic properties (Nogueira et al., 2004), which have been linked to its glutathione peroxidase– and thioredoxin reductase–like activities (Muller et al., 1984; Zhao and Holmgren, 2002). Approximately 10 years ago, Ebs underwent clinical trials with modest success, indicating that other organochalcogens should be considered as potential antioxidant for the treatment of diseases associated with overproduction of oxidative stress (see Nogueira et al., 2004 and references therein). Diphenyl diselenide [(PhSe)2] is a potential candidate for counteracting oxidative stress because it shares with Ebs some chemical properties and has about twofold greater glutathione peroxidase–like activity and is also less toxic to rodents than Ebs (Nogueira et al., 2004). However, in spite of its well-documented pharmacological activities (Barbosa et al., 2008; Nogueira et al., 2004; Puntel et al., 2007), (PhSe)2 can be toxic to rodents (Brito et al., 2006; Nogueira et al., 2004; Rosa et al., 2007). The toxicology of organoselenium compounds is not completely understood, but the thiol oxidase activity (particularly the catalytic oxidation of vicinal thiols) may explain, at least in part, the toxic effect of diorganoyl diselenides (Nogueira et al., 2004).
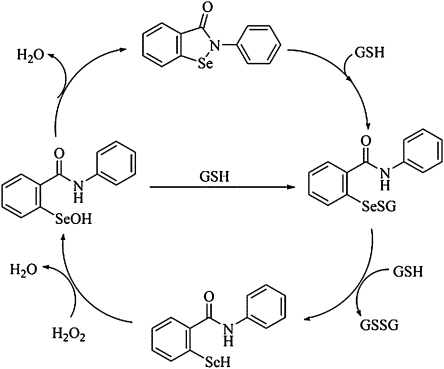
In fact, in order to reduce peroxides, these compounds must first form selenol/selenolate intermediates, a reaction which is accomplished via reduction of the Se moiety by different types of thiols (Nogueira et al., 2004; Wendel et al., 1984) (Fig. 1), including those in the mitochondrial membranes. Although the thiol-peroxidase– or thioredoxin-thiol-peroxidase–like activity of organochalcogens (Zhao and Holmgren, 2002; Zhao et al., 2002) can potentially be of biological and therapeutic significance via artificial modulation of the cellular levels of peroxides, the excessive oxidation of thiols by organochalcogens without a concomitant reduction of peroxides may be potentially toxic to living cells (thiol-oxidase activity, Fig. 2) (Nogueira et al., 2004). Thus, the exaggerated oxidation of thiols of biological importance, including those in mitochondrial membranes could be detrimental to the cell, because of the fact that mitochondrial dysfunction caused by thiol oxidation is closely related to the apoptotic cell death (Morin et al., 2003; Zhao et al., 2006). Accordingly, the organochalchogens should be considered as putative candidates for apoptotic cell death induction via mitochondrial dysfunction, which may explain, at least in part their pharmacological/toxicological action (Ardais et al., 2010; Nogueira et al., 2004).
FIG. 1.
Glutathione peroxidase cycle of ebselen. GSH represent a glutathione molecule. Different thiol groups (including that of mitochondrial membranes) could replace GSH in this figure.
FIG. 2.
Thiol oxidase futile cycle of diorganochalcogens (X = Se or Te). RSH can represent a low molecular endogenous molecule (glutathione, cysteine, etc.) or thiol-containing proteins (δ-ALA-D, Na+, K+-ATPase, mitochondrial proteins).
Recently, our group has shown that dietary (PhSe)2 extended the latency to tumor onset in rats (Barbosa et al., 2008), indicating a new pharmacological outcome for (PhSe)2. However, the exact mechanism involved in (PhSe)2 antitumoral activity was not investigated. These findings encouraged us to better explore the potential in vitro mitochondrial toxicity of (PhSe)2. In fact, it has been reported that selenium compounds could act as anticarcinogenic agents by altering mitochondrial function. Indeed, compounds that cause mitochondrial thiol oxidation are known to trigger apoptotic cell death (Jung et al., 2001; Morin et al., 2003; Shen et al., 2001; Zhao et al., 2006).
Although the organochalchogens' antioxidant properties have been extensively investigated, relatively few studies have focused on the mechanisms by which these compounds exert their pharmacological effects and/or toxicity. Given the chemical nature of these compounds (see above), it is reasonable to assume that at least some of their pharmacological and toxicological effects can be mediated via alterations in mitochondrial function. Thus, in this paper we tested the hypothesis that the pharmacological (anticancer) (Barbosa et al., 2008) activity of organochalchogens as well as the toxicological effects (Nogueira et al., 2004) are secondary to mitochondrial dysfunction. Hence, the objective of the present study was to evaluate the effects of Ebs and (PhSe)2 on liver mitochondrial swelling, ΔΨm (mitochondrial membrane potential), NAD(P)H oxidation, ROS production, protein aggregate formation, and oxygen consumption, in order to establish a mechanistic link between organochalchogens pharmacology/toxicity and mitochondrial dysfunction and putative mechanisms by which these compounds could modulate mitochondrial function.
MATERIALS AND METHODS
Chemicals.
Chemicals, including adenosine 5′-(trihydrogen diphosphate) (ADP), cyclosporine A, dithiothreitol (DTT), ethylene glycol tetra acetate (EGTA), 2,4 dinitrophenol (2,4 DNP), glutamic acid, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), rhodamine (Rho) 123, succinic acid, and ruthenium red (RR) were obtained from Sigma Chemical Company (St Louis, MO). 2' 7' Dichlorofluorescin diacetate (H2-DCFDA) and Amplex red were purchased from Molecular Probes (Eugene, OR). All other reagents were commercial products of the highest purity grade available.
Animals.
Adult male Wistar rats (250–350 g) from our own breeding colony were used in this study. The animals were housed in plastic cages with water and food ad libitum, at 22°C–23°C, 56% humidity, and 12-h light cycle. The nonpurified diet contained (in g/100 g) 52 carbohydrate, 20 crude protein, 5 fat, 6 crude fiber, 5 minerals, 11 moisture, 0.1 mg/kg of Se, and 30 IU/kg of vitamin E (for crude ingredients of nonpurified diet, complete mineral and vitamin constituents, see supplementary table 1). The animals were used in accordance to guidelines of the Committee on Care and use of Experimental Animal Resources, Federal University of Santa Maria, Brazil.
Isolation of rat liver mitochondria.
Rat liver mitochondria were isolated as previously described by Brustovetsky and Dubinsky (2000), with some modifications. Wistar rats were fasted overnight prior to euthanasia by decapitation. The livers were rapidly removed (within 1 min) and immersed in ice-cold “isolation buffer I” containing 225mM mannitol, 75mM sucrose, 1mM K+-EGTA, 0.1% bovine serum albumin, and 10mM K+-HEPES, pH 7.2. The tissue was minced using surgical scissors and then extensively washed. The tissue was then homogenized in a power-driven, tight-fitting Potter-Elvehjem homogenizer with Teflon pestle. The resulting suspension was centrifuged for 7 min at 2000 × g in a Hitachi CR 21E centrifuge. After centrifugation, the supernatant was recentrifuged for 10 min at 12,000 × g. The pellet was resuspended in “isolation buffer II” containing 225mM mannitol, 75mM sucrose, 1mM K+-EGTA, and 10mM K+-HEPES, pH 7.2, and recentrifuged at 12,000 × g for 10 min. The supernatant was decanted, and the final pellet was gently washed and resuspended in isolation buffer II without EGTA, to a protein concentration of 30–40 mg/ml.
Standard incubation procedure.
Measurements of mitochondrial transmembrane electrical potential (ΔΨm), mitochondrial swelling, determination of NAD(P) redox state, estimation of ROS production (see details below) were performed in a stirred cuvette mounted in a RF-5301 PC Shimadzu spectrofluorometer (Kyoto, Japan) at 30°C. Mitochondria (0.5-mg protein) were added to 3-ml standard incubation buffer containing 100mM sucrose, 65mM KCl, 10mM K+-HEPES buffer (pH 7.2), 50μM EGTA, 200μM ADP, 400μM MgCl2, 1mM Pi, 5mM glutamate, and 5mM succinate. Other additions are indicated in the figure legends. The results shown are representative of a series of three to six independent experiments, using independently isolated mitochondrial preparations. The results were reproducible within 10–20% variation.
Measurements of mitochondrial transmembrane electrical potential (ΔΨm).
Mitochondrial ΔΨm was estimated by fluorescence changes in Rho 123 (2μM) recorded by RF-5301 Shimadzu spectrofluorometer (Kyoto, Japan) operating at excitation and emission wavelengths of 495 and 535 nm, with slit widths of 1.5 nm (Guo et al., 1998). Data of mitochondrial transmembrane electrical potential (ΔΨm) in tables and figures are presented as arbitrary fluorescence units per second (AFU/s).
Mitochondrial swelling.
Measurement of mitochondrial swelling was performed in a RF-5301 Shimadzu spectrofluorometer at 600 nm (slit 1.5 nm for excitation and emission; Votyakova and Reynolds, 2005). Data for mitochondrial swelling are expressed as arbitrary absorbance units per second (AAU/s).
Determination of NAD(P) redox state.
The oxidation or reduction of pyridine nucleotides in the mitochondrial suspension was determined by a RF-5301 Shimadzu spectrofluorometer (Kyoto, Japan) operating at excitation and emission wavelengths of 365 (slit 3 nm) and 463 nm (slit 5 nm; Votyakova and Reynolds, 2005). Data for NAD(P) redox state on Figure 4 are presented as AFU/s.
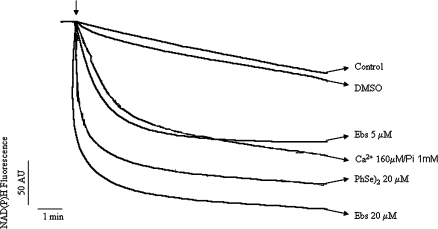
FIG. 4.
Effect of organochalchogens on mitochondrial NAD(P)H oxidation. Isolated rat liver mitochondria (0.5 mg) were incubated in standard medium, and the mitochondrial NAD(P)H oxidation was monitored as described in the Material and Methods. The Ebs, (PhSe)2, or Ca2+/Pi were added where indicated by the arrow. The traces are representative of three independent experiments.
Estimation of ROS production.
The mitochondrial generation of ROS was determined spectrofluorimetrically, using the membrane permeable fluorescent dye H2-DCFDA (1μM; Garcia-Ruiz et al., 1997). Fluorescence was determined at 488 nm for excitation and 525 nm for emission, with slit widths of 3 nm. The values are expressed as percent of control in the absence of the organochalchogens.
SDS-polyacrylamide slab gel electrophoresis.
After 1-h incubation under swelling conditions (in the presence or absence of DTT), mitochondrial samples were dissolved in the lyses buffer (4% SDS, 2mM EDTA, 500mM Tris/HCl, pH 6.8, 2mM Na3VO4, 200mM NaF, 0.1mM benzamidine, 0.1mM PMSF) and boiled for 10 min in the presence of 8% bromophenol. Samples (40 μg of proteins) were applied to the electrophoresis gel. Electrophoresis was performed by SDS-polyacrylamide gel electrophoresis in a discontinuous system as described by Laemmli (1970). The running and stacking gels consisted of 12 and 3% acrylamide, respectively, and the voltage was set at 120 mA. The gels were stained with Coomassie Brilliant Blue. Mitochondrial proteins were assayed by the Folin method (Lowry et al., 1951).
Oxygen uptake measurements.
Oxygen uptake was measured in an oxymeter fitted with a water-jacket Clark-type electrode (Yellow Springs Instruments Co., Model 5300 or Oxytherm, Hansatech). The isolated rat liver mitochondria (∼0.17 mg) were incubated with 1 ml of the standard respiration buffer as described above.
Synthesis of organochalcogens.
(PhSe)2 was synthesized using the method described by Paulmier (1986) and Ebs as described by Engman (1989). Solutions of organochalcogens were prepared freshly in dimethylsulfoxide (DMSO), and the final concentration of DMSO in all tubes was 3%.
Statistical analysis.
Data were analyzed by one-way ANOVA followed by Duncan's multiple range test when appropriated (Tables 1 and 2).
TABLE 1.
Calculated k (Rate for the Fast Phase) for Organochalchogens-Induced Mitochondrial Depolarization and Swelling
| ΔΨm (AFU/s) | Swelling (AAU/s) | % Depolarization (in %) | |
| Control | 0.05 | 0.006 | 0.1 |
| Ebs 1μM | 0.38 | 0.093 | 5 |
| Ebs 2μM | 1.025* | 0.125* | 30 |
| Ebs 4μM | — | 0.255* | — |
| Ebs 5μM | 6.43* | 0.485* | 80 |
| (PhSe)2 10μM | 0.35 | 0.027 | 5 |
| (PhSe)2 20μM | 0.97* | 0.114* | 25 |
| (PhSe)2 40μM | 2.088* | 0.443* | 50 |
Note. The values are expressed in AFU/s for ΔΨm or AAU/s for swelling. Data are expressed as mean (n = 3–6), and the SD not shown was less than 20%. Data were analyzed by one-way ANOVA followed by Duncan's multiple test.
p < 0.05 from control (without organochalchogens).
TABLE 2.
Calculated k (Rate for the Fast Phase) for Organochalchogens-Induced Mitochondrial Depolarization and Swelling in the Presence of Different Compounds
| ΔΨm (AFU/s) | Swelling (AAU/s) | % Depolarization (in %) | |
| Control | 0.09 | 0.007 | 0.1 |
| Ebs 5μM | 6.430a | 0.485a | 80 |
| Ebs 5μM + DTT 10μM | 2.680a,b | 0.012 | 42 |
| Ebs 5μM + CsA 1μM | 6.500a | 0.500a | 80 |
| Ebs 5μM + RR 4μM | 6.400a | 0.485a | 80 |
| Ebs 5μM + BHT 2.5μM | 6.520a | 0.498a | 80 |
| Ebs 5μM + IA 100μM | 6.650a | 0.472a | 78 |
| Ebs 5μM + NEM 100μM | 8.290a,b | 0.514a | 100 |
| Ebs 5μM + IA + NEM | 8.350a,b | 0.514a | 100 |
| (PhSe)2 40μM | 2.089a | 0.443a | 50 |
| (PhSe)2 40μM + DTT 10μM | 1.040a,b | 0.011 | 30 |
| (PhSe)2 40μM + CsA 1μM | 2.150a | 0.440a | 55 |
| (PhSe)2 40μM + RR 4μM | 2.115a | 0.433a | 50 |
| (PhSe)2 40μM + BHT 2.5μM | 2.200a | 0.420a | 56 |
| (PhSe)2 40μM + IA 100μM | 2.330a | 0.411a | 56 |
| (PhSe)2 40μM + NEM 100μM | 4.290a,b | 0.417a | 93 |
| (PhSe)2 40μM + IA + NEM | 4.286a,b | 0.439a | 93 |
Note. The values are expressed in AFU/s for ΔΨm or AAU/s for swelling. Data are expressed as mean (n = 3–6), and the SD not shown was less than 20%. Data were analyzed by one-way ANOVA followed by Duncan's multiple test.
p < 0.05 from control (without organochalchogens)
p < 0.05 from organochalchogens alone.
RESULTS
Effects of Organochalchogens on ΔΨm and on Mitochondrial Swelling
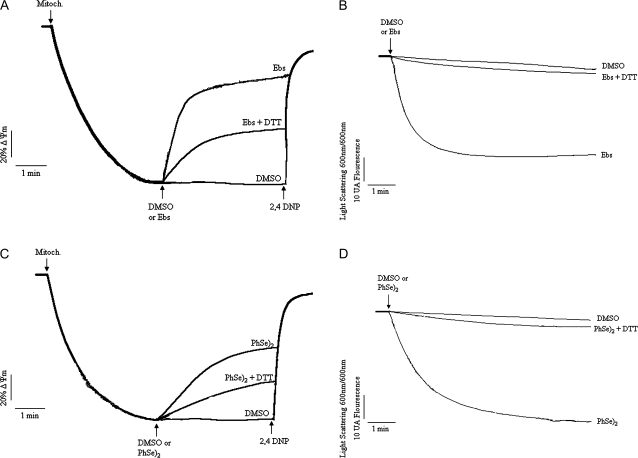
As shown Figure 3A, Ebs led to a collapse of the ΔΨm in a concentration-dependent manner (≥2μM). In fact, Ebs at 1μM did not cause mitochondrial depolarization, with a trace similar to control (∼5% depolarization when compared with 2,4 DNP 100%). At 2μM, Ebs induced a partial depolarization (∼30% depolarization), whereas 5μM rapidly depolarized the transmembrane potential (∼80% depolarization) (Fig. 3A). The initial velocity (“fast phase”) of Ebs-induced ΔΨm dissipation increased from 0.38 to 6.43 AFU/s as the concentration increased from 1 to 5μM (Table 1; p < 0.05). In parallel experiments (Fig. 3B), Ebs induced mitochondrial swelling in a concentration-dependent manner from 1 to 5μM, and the velocity of mitochondrial swelling increased from 0.093 (1μM) to 0.485 (AAU/s) (5μM) (Table 1; p < 0.05).
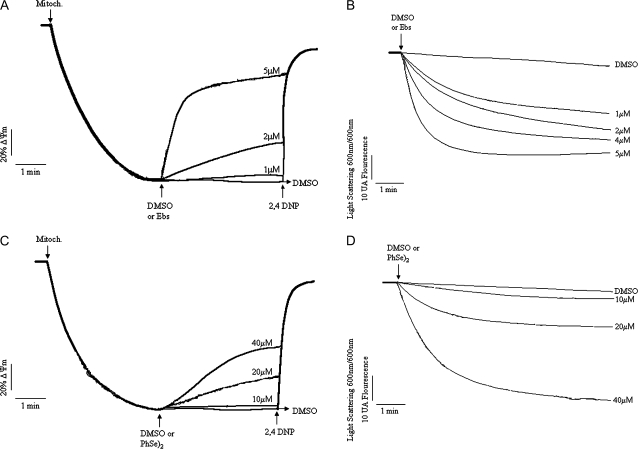
FIG. 3.
Effects of organochalchogens on ΔΨm and mitochondrial swelling. Isolated rat liver mitochondria (0.5 mg) were incubated in standard medium (see composition in the Materials and Methods), and the ΔΨm or swelling was monitored as described in the Material and Methods. (A) Effect of Ebs (1–5μM) on ΔΨm and (B) effect of Ebs (1–5μM) on mitochondrial swelling. (C) Effect of (PhSe)2 (10–40μM) on ΔΨm and (D) effect of (PhSe)2 (10–40μM) on mitochondrial swelling. The mitochondria (0.5 mg/ml), organochalchogens, or 2,4 DNP (100μM) were added where indicated by arrows. The traces are representative of three to five independent experiments.
Analogous to Ebs, (PhSe)2 caused a partial mitochondrial depolarization in a concentration-dependent manner (Fig. 3C), which was evident at 20μM (∼25% depolarization; p < 0.05) and 40μM (∼50% depolarization; p < 0.05). The calculated rate of depolarization induced by (PhSe)2 increased from 0.35 (10μM) to 2.088 AFU/s (40μM; p < 0.05). The (PhSe)2-induced mitochondrial swelling was apparent only at 20 and 40μM (Fig. 3D; p < 0.05 for both concentrations), with calculated rates of 0.114 and 0.443 AAU/s, respectively. Analogous to the effect observed on ΔΨm, where (PhSe)2 failed to depolarize the mitochondria, incubation with 10μM (PhSe)2 did not induce mitochondrial swelling (rate 0.027 AAU/s).
The control curves for these experiments (Figs. 3A–D) were carried out in the presence of DMSO (vehicle used to prepare the organochalchogens solutions). The control in the absence of DMSO was omitted because at this concentration (3%), the vehicle did not exert any effect per se (ΔΨm or swelling experiments, data not shown).
Effects of Organochalchogens on Mitochondrial NAD(P)H Oxidation
Ebs induced fast mitochondrial NAD(P)H oxidation in a concentration-dependent fashion (Fig. 4). Indeed, at 5μM the Ebs induced NAD(P)H oxidation that was comparable to that obtained with Ca2+ (160μM)/Pi (1mM). (PhSe)2 (20μM) also caused rapid mitochondrial NAD(P)H oxidation (Fig. 4). DMSO did not change NAD(P)H oxidation per se (trace similar to control). Addition of DTT to the incubation medium (simultaneously with Ebs or (PhSe)2) blocked NAD(P)H oxidation; however, DTT addition after NAD(P)H oxidation did not restore reduced pyridine nucleotide pool (data not shown).
Effects of DTT on Organochalchogens-Induced Mitochondrial Dysfunction
To determine if the organochalchogens-induced mitochondrial dysfunction was associated with oxidation of thiol groups, we carried out experiments in the presence of the reducing agent DTT. As shown in Figure 5A, Ebs (5μM)-induced mitochondrial depolarization (∼ 80% depolarization) was partially prevented by DTT (10μM, ∼42% depolarization; p < 0.05, when compared without DTT). The rate of mitochondrial depolarization was slower in the presence of DTT (2.68 AFU/s) than in its absence (6.43 AFU/s; p < 0.05; Table 2). In parallel experiments, Ebs (5μM)-induced mitochondrial swelling was completely prevented by 10μM of DTT (Fig. 5B). Indeed, the mitochondrial swelling rate induced by Ebs was decreased more than 40-fold by treatment with DTT (p < 0.05, when compared without DTT; Table 2).
FIG. 5.
Effect of DTT on organochalchogens-induced mitochondrial dysfunction. Effect of DTT (10μM) on Ebs (5μM)-induced mitochondrial depolarization (A) or swelling (B). Effect of DTT (80μM) on (PhSe)2 (20μM)-induced mitochondrial depolarization (C) or swelling (D). The mitochondria (0.5 mg/ml), organochalchogens, or 2,4 DNP (100μM) were added where indicated by arrows in medium containing DTT. The traces are representative of three independent experiments.
In a similar manner to Ebs, 20μM (PhSe)2-induced mitochondrial depolarization (∼50%) was partially prevented by DTT (80μM, ∼30% depolarization; p < 0.05, when compared without DTT; Fig. 5C and Table 2). The velocity of ΔΨm collapse caused by (PhSe)2 was decreased about twofold by DTT (Table 2). In contrast to the effect on ΔΨm, the (PhSe)2-induced mitochondrial swelling was completely prevented by DTT (80μM; Fig. 5D), and the velocity of mitochondrial swelling induced by (PhSe)2 was diminished by more than 40-fold by DTT (Table 2). The results presented above indicate that DTT can prevent the mitochondrial effects of (PhSe)2 and Ebs, likely as a consequence of reduction in the actual concentration of the oxidizing agents. To test the ability of chalcogenides to reverse mitochondrial dysfunction, we incubated liver mitochondria for 3 min with DMSO or 20μM (PhSe)2, then excess of (PhSe)2 was removed by gel filtration in sephadex G-50 column. Next, the mitochondria were incubated for 10 min with 1mM DTT, followed by measurement of ΔΨm. Notably, in contrast to control mitochondria, no polarization was observed in the mitochondria incubated with (PhSe)2 (data not shown). Similarly, addition of DTT at the end of the first initial (and rapid) phase of depolarization failed to attenuate or reverse the collapse in ΔΨm induced by either Ebs or (PhSe)2 (data not shown).
Effects of Cyclosporine A, RR, or Butylated Hydroxytoluene on Organochalchogens-Induced Mitochondrial Dysfunction
In order to rule out the dependence of organochalchogens-induced mitochondrial dysfunction on ROS generation and Ca2+ on, we tested the protective effects of butylated hydroxytoluene (BHT) and RR, respectively. To study the possible involvement of the classical MPT pore in organochalchogens-induced mitochondrial depolarization, experiments were conducted in the presence of cyclosporin A (CsA). Neither CsA nor RR or BHT reversed the Ebs-induced mitochondrial depolarization or swelling (for details, see Supplementary figs. 1a and 1b). Similarly, the (PhSe)2-induced mitochondrial depolarization or swelling was not affected by CsA, RR, or BHT (see Supplementary figs. 1c and 1d). In fact, as shown in Table 2, the organochalchogens-induced maximal depolarizations as well as the rate of organochalchogens-induced depolarization or swelling were unchanged by treatment with CsA, RR, or BHT (for comparisons, see Table 2).
Effects of Iodoacetic Acid or N-Ethylmaleimide on Organochalchogens-Induced Mitochondrial Dysfunction
In order to study the characteristics of the thiol groups involved in the organochalchogens-induced mitochondrial dysfunction, we carried out a series of experiments in the presence of iodoacetic acid (IA) and/or N-ethylmaleimide (NEM) and hydrophilic and hydrophobic thiol reagents, respectively. The mitochondrial depolarization induced by Ebs or (PhSe)2 was unchanged by IA (see Supplementary figs. 2a and 2c, respectively). In contrast, the addition of NEM to liver mitochondria augmented the organochalchogens effect on mitochondrial depolarization (p < 0.05; Table 2). Moreover, IA did not attenuate the NEM effect (see Supplementary figs. 2a and 2b). In fact, in the presence of NEM (alone or combined with IA), the organochalchogens-induced depolarization was ≥93% (Table 2).
Conversely, IA or NEM (separately or simultaneously) did not change the Ebs- or (PhSe)2-induced mitochondrial swelling (see Supplementary figs. 2b and 2d, respectively). In agreement with these observations, there was no appreciable change in the rate of organochalchogens-induced mitochondrial swelling when IA and/or NEM were used (Table 2). For both depolarization and swelling experiments, neither IA (100μM) nor NEM (100μM) when used alone exerted any effect per se at the studied concentrations (data not shown). For more details about the reactivity of thiols with organochalchogens in the presence of IA or NEM, see Supplementary figure 3(a–e).
Effects of Organochalchogens on ROS Generation
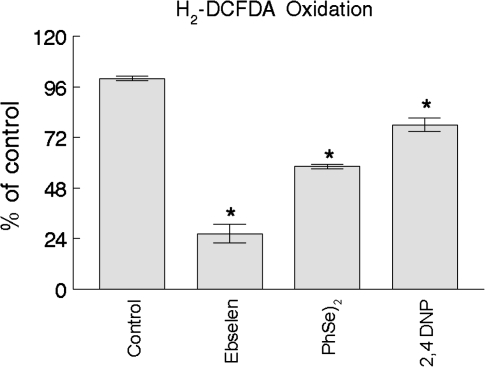
Ebs (5μM) and (PhSe)2 (20μM) caused a significant decrease in basal ROS generation, as measured by H2-DCFDA oxidation (p < 0.05 for both compounds; Fig. 6). The Ebs effect was more pronounced (∼75%) compared with that of (PhSe)2 (∼40%). The uncoupler 2,4 DNP (100μM) also caused a significant decrease in the basal ROS generation (∼20%).
FIG. 6.
Effect of organochalchogens on ROS generation. Isolated rat liver mitochondria (0.5 mg) were incubated in standard medium (see standard medium in the Materials and Methods) containing 1μM H2-DCFDA. Ebs (5μM), (PhSe)2 (20μM), or 2,4 DNP (100μM) were added, and the H2-DCFDA fluorescence was monitored during 10 min. The delta of fluorescence (final fluorescence – initial fluorescence) was used to do the calculations. The data are expressed as percent of control. Data represent the mean ± SE of three separate determinations performed in duplicates. *p < 0.05 compared with control by Duncan's multiple range test.
Effects of Organochalchogens on Oxygen Consumption
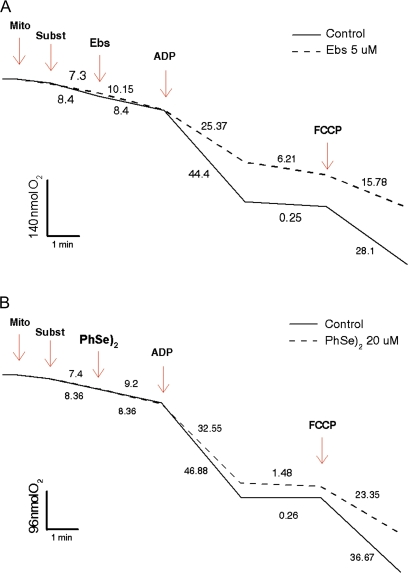
Ebs (5μM) or (PhSe)2 (20μM) (Figs. 7A and 7B, respectively) did not alter the oxygen consumption rate when they were added during the state 2 of respiration (after substrate addition). However, both compounds decreased the oxygen consumption rate during state 3 (after ADP stimulation) and increased the oxygen consumption rate during state 4 of the mitochondrial respiration. Consequently, both Ebs and (PhSe)2 caused a considerable decrease in the respiratory control ratio (RCR). In fact, the RCR in the control was ∼178, whereas in the presence of Ebs and (PhSe)2 the RCR was reduced to ∼4 and 22, respectively (p < 0.05 for both compounds). As expected, when carbonylcyanide p-trifluoromethoxyphenylhydrazone was added the oxygen consumption was rapidly increased, but it remained lower (almost 40%) than that observed in control trace.
FIG. 7.
Effect of organochalchogens on oxygen consumption. Isolated rat liver mitochondria (0.15 mg/ml) were incubated in standard medium, and the oxygen consumption was measured as described in the Materials and Methods. Effect of Ebs (5μM) (A) and (PhSe)2 (20μM) (B) on mitochondrial oxygen consumption. The arrows indicate sequential additions of Mito, 0.15 mg/ml mitochondria; Subst, 10mM succinate; Ebs, 5μM ebselen (A); 20μM (PhSe)2 (B); ADP, 0.2mM ADP; and FCCP, 5μM carbonylcyanide p-trifluoromethoxyphenylhydrazone. Similar results were obtained with at least two independent mitochondrial preparations.
Effects of Organochalchogens on Protein Aggregates Formation
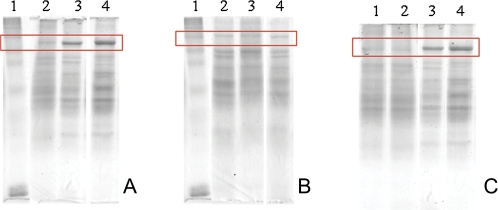
Ebs (5μM, lane 4) and (PhSe)2 (20μM, lane 3) caused mitochondrial protein aggregate formation when compared with the controls (DMSO, lane 2), as evidenced by the indicated band at the top of the gel (Fig. 8A). The control band (in the absence of DMSO) was omitted because DMSO did not exert any effect per se (data not shown). However, when DTT [10μM for Ebs or 80μM for (PhSe)2] was added to the medium during incubation with organochalchogens (1 h at 30°C), the protein aggregate formation was not apparent (Fig. 8B). In constrast, addition of DTT after incubation of mitochondria with Ebs or (PhSe)2 failed to reverse the protein aggregate formation (Fig. 8C). Similar results were obtained with the addition of 10mM beta-mercaptoethanol (a monothiol reducing agent, data not shown).
FIG. 8.
Effect of organochalchogens on protein aggregates formation. Representative SDS-polyacrylamide slab gel electrophoresis of membrane protein from rat liver mitochondria. In each lane, samples of 40 μg of protein were applied to a 12% acrylamide running gel after 1 h of incubation under conditions described in the Material and Methods in the absence (A) or presence (B) of DTT. (C) The mitochondria were incubated with organochalchogens in conditions described in (A) and after DTT was added for 10 min (10μM for Ebs and 80μM for (PhSe)2). Lane 1, molecular standard weight; Lane 2, control (DMSO); Lane 3, (PhSe)2 (20μM); and Lane 4, Ebs (5μM). Gels are representative from three to four different experiments.
DISCUSSION
The results presented indicate that the pharmacology (anticancer) (Barbosa et al., 2008) and toxicology of organochalchogens is, at least in part, mediated by mitochondrial dysfunction. Although no literature is available on the intracellular concentrations of these chalcogenides, previously we have observed that exposure to acute or chronic toxic doses of (PhSe)2 can increase selenium deposition in liver to levels as high as 100μM (see Nogueira et al., 2004 and references therein). Thus, it is reasonable to suggest that at high doses, mitochondria may play an important role in the toxicity of organochalchogens. However, more in vitro and in vivo studies are necessary to better understand the involvement of mitochondrial dysfunction in mediating the anticancerous activity of both compounds.
Ebs and (PhSe)2 induced mitochondrial dysfunction, as evidenced by mitochondrial depolarization, swelling, and endogenous NAD(P)H oxidation, likely reflecting mitochondrial protein aggregate formation (Beatrice et al., 1980; Costantini et al., 1996). Furthermore, Ebs and (PhSe)2 altered the oxygen consumption rate and caused a net decrease in the RCR ratio. The effects of both compounds seemed to be because of their interaction with critical mitochondrial protein thiols. This conclusion is based on the protective effect of a reducing agent, DTT, which could rescue the mitochondria from the aberrant effects of both Ebs and (PhSe)2.
In fact, the concentrations of DTT necessary to prevent mitochondrial dysfunction were different when in the presence of Ebs (10μM DTT) or (PhSe)2 (80μM DTT). We have selected these respective concentrations based on the selenol intermediates formed after a hypothetical complete reduction of these compounds by excess of thiol (here two times in a molar base). Indeed, Ebs (5μM) generates 5μM of selenol derivatives, whereas (PhSe)2 (20μM) generates 40μM of selenol intermediate.
The immediate effects of both Ebs and (PhSe)2 on mitochondrial function were Ca2+ and ROS independent because neither RR nor BHT afforded protection. Moreover, our results corroborate that Ebs-induced mitochondrial dysfunction was related to the oxidation of thiol groups (Morin et al., 2003). However, the major point to be considered is that contrary to Morin's findings, Ebs-induced mitochondrial dysfunction was Ca2+ independent and associated with pyridine nucleotide oxidation.
Despite some earlier observations that thiol oxidation by selenium compounds can produce ROS (Yan and Spallholz, 1993), we noted that (PhSe)2 and Ebs decreased basal ROS generation (Fig. 6), which may be related to mitochondrial depolarization. In fact, there are reports in the literature showing that the mitochondrial ROS generation is strictly dependent upon the ΔΨm (Brand et al., 2004; Votyakova and Reynolds, 2001). Our conclusion is further supported by the fact that a classical mitochondrial uncoupler (2,4 DNP) caused a similar decrease in ROS generation (Fig. 6). However, we cannot exclude the possibility that both the organochalchogens may have a scavenger effect on ROS formation or act along the electron transport chain, blocking electron leakage during oxidative phosphorylation. Thus, oxidation of mitochondrial thiols by Ebs and (PhSe)2 was not associated with ROS production, in agreement with previous results from our laboratory (Nogueira et al., 2004 and references therein).
Ebs and (PhSe)2 can catalytically oxidize thiols, and diselenide oxidizes vicinal thiols more efficiently than monothiols (Nogueira et al., 2004). Here we have obtained persuasive evidence that these chalcogens can oxidize mitochondrial thiols. This conclusion is further supported by the eminent protein aggregate formation evidenced by SDS-polyacrylamide slab gel electrophoresis (Fig. 8A); this effect was prevented but not reversed by DTT (Figs. 8B and 8C). Similar data were obtained for mitochondrial depolarization and swelling (Figs. 5A–D and data not shown), indicating that the organochalchogens mediate these effects at least in part by protein thiol cross linkage. In this scenario, the absence of protein aggregation, reduction in depolarization, and prevention of mitochondrial swelling in the presence of DTT may be attributed to the formation of Ebs and (PhSe)2 intermediates (Ebs selenol and phenyl selenol). In this case, DTT effectively prevented mitochondrial dysfunction by blocking the interaction of the selenocompouds with mitochondrial sulfhydryl groups. Here we have also demonstrated that Ebs was three- to fourfold more potent than (PhSe)2 in eliciting mitochondrial dysfunction. The putative explanation for these findings is likely related to the different reactivity of these selenocompounds with critical thiol groups of proteins (Nogueira et al., 2004). The rate for organochalchogens-induced depolarization in the presence of DTT was two- to threefolds lower than that in its absence, whereas the rate of swelling was decreased 20- to 40-fold by DTT. The fact that DTT only partially reestablished mitochondrial polarization and completely blocked mitochondrial chalcogen-induced swelling suggests that at least two groups of thiols with different reactivity toward DTT were oxidized by both Ebs and (PhSe)2. Thus, DTT likely prevents thiol oxidation in the proteins involved in the control of mitochondrial swelling, but not in the control of ΔΨm. In fact, because DTT is much more hydrophilic than Ebs and (PhSe)2, we suggest that thiols involved in regulating swelling are more accessible to water in the inner mitochondrial membrane. Furthermore, literature data indicate that the oxidation of both the pyridine nucleotide pool and dithiols tunes the pore opening probability at two separate sites (Costantini et al., 1996), which may explain the effects of Ebs and (PhSe)2, that is, the oxidation of mitochondrial thiols (both at S site and at a more cryptic site or less accessible critical thiol groups site; Costantini et al., 1996, 1998) by Ebs and (PhSe)2 could increase mitochondrial depolarization and permeability. The oxidation of NAD(P)H could be, at least in part, a consequence of PTP opening by Ebs and (PhSe)2 rather than the cause of pore opening per se (Beatrice et al., 1980; Costantini et al., 1996). Thus, although both Ebs and (PhSe)2 could also oxidize the mitochondrial thiol pool that is in equilibrium with the NAD(P)H pool, the increase in mitochondrial permeability induced by Ebs and (PhSe)2 may be better explained by a rapid oxidation of mitochondrial vicinal thiols, which depolarize and, sequentially, increase mitochondrial permeability depleting the NAD(P)H pool. This sequence of molecular events can help in explaining why DTT could only partially prevent the collapse of ΔΨm and could prevent more efficiently the mitochondrial swelling. Specifically, by decreasing the interaction of Ebs and (PhSe)2 with the mitochondrial thiol groups, DTT may decrease the probability of pore opening. We have also observed that DTT could prevent NAD(P)H pool oxidation when it added simultaneously with (PhSe)2 and Ebs (data not shown), corroborating the idea that the depletion in the mitochondrial pyridine nucleotide pool is secondary to PTP opening.
The addition NEM greatly potentiated the organochalchogens-induced mitochondrial depolarization, whereas the IA did not exert any effect per se (see Supplementary figs. 2a–d). The effects of high concentrations of NEM on mitochondrial function may depend on secondary oxidation of critical thiol groups (Costantini et al., 1998) and the synergic effect with Ebs and (PhSe)2 may indicate that NEM and chalcogens are oxidizing the same class of thiol groups.
The lack of CsA effect (see Supplementary figs. 2a–d; see also Table 2 that CsA did not change the rate of organochalchogens-induced depolarization and/or swelling) found in organochalchogens-treated mitochondria further supports the premise that the studied organocompounds did not induce classical MPT pore opening (Zoratti and Szabò, 1995), but rather, they induced an unregulated CsA-insensitive and Ca2+-independent form of the pore. Such an unregulated pore has been hypothesized to be the result of nonspecific protein aggregation in the mitochondrial membranes (He and Lemasters, 2002; Kim et al., 2003), which could be the result of the interaction between organocompounds and mitochondrial thiols. We propose that the fast mitochondrial depolarization induced by both organocompounds is triggered by oxidation of critical thiol groups that facilitate the mitochondrial swelling and mitochondrial protein thiol cross linkage, which is followed by pyridine nucleotide oxidation. Although the majority of these molecular events can be fitted to the classical PTP, the absence of modulation of organochalcogen-induced mitochondrial permeability by CsA indicates that the interaction of Ebs and (PhSe)2 with mitochondrial thiols which will culminate in protein aggregation and mitochondrial dysfunction is rather complex and possibly involves the oxidation of more than one class of thiol groups.
Future studies with Ebs and (PhSe)2 could be profitably used in carcinogenic cells to determine whether their ability to target mitochondria could enhance apoptotic and/or necrotic cell death, as a potential therapeutic modality. Furthermore, our findings can be helpful to explain, at least in part, mechanisms associated with the in vivo toxicity of high doses of organoselenium compounds.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
Universidade Federal de Santa Maria; Fundação de Amparo a Pesquisa do Estado do Rio Grande do Sul; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; Conselho Nacional de Desenvolvimento Científico e Tecnológico; Financiadora de Estudos e Projetos (Rede Instituto Brasileiro de Neurociência [IBN-Net] #01.06.0842-00); and Instituto Nacional de Ciência e Tecnologia em Excitotoxicidade e Neuroproteção. National Institute of Environmental Health Sciences (07331 to M.A.).
Supplementary Material
References
- Ardais AP, Viola GG, Costa MS, Nunes F, Behr GA, Klamt F, Moreira JC, Souza DO, Rocha JBT, Porciúncula LO. Acute treatment with diphenyl diselenide inhibits glutamate uptake into rat hippocampal slices and modifies glutamate transporters, SNAP-25, and GFAP immunocontent. Toxicol. Sci. 2010;113:434–443. doi: 10.1093/toxsci/kfp282. [DOI] [PubMed] [Google Scholar]
- Barbosa NBV, Rocha JBT, Soares JCM, Wondracek DC, Goncalves JF, Schetinger MRC, Nogueira CW. Dietary diphenyl diselenide reduces the STZ-induced toxicity. Food Chem. Toxicol. 2008;46:186–194. doi: 10.1016/j.fct.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Beatrice MC, Palmer JW, Pfeiffer DR. The relationship between mitochondrial membrane permeability, membrane potential, and the retention of Ca2+ by mitochondria. J. Biol. Chem. 1980;255:8663–8671. [PubMed] [Google Scholar]
- Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic. Biol. Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Brito VB, Folmer V, Puntel GO, Fachinetto R, Soares JCM, Zeni G, Nogueira CW, Rocha JBT. Diphenyl diselenide and 2,3-dimercaptopropanol increase the PTZ-induced chemical seizure and mortality in mice. Brain Res. Bull. 2006;68:414–418. doi: 10.1016/j.brainresbull.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Brustovetsky N, Dubinsky JM. Dual responses of CNS mitochondria to elevated calcium. J. Neurosci. 2000;20:103–113. doi: 10.1523/JNEUROSCI.20-01-00103.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini P, Chernyak BV, Petronilli V, Bernardi P. Modulation of the mitochondrial permeability transition pore by pyridine nucleotides and dithiol oxidation at two separate sites. J. Biol. Chem. 1996;271:6746–6751. doi: 10.1074/jbc.271.12.6746. [DOI] [PubMed] [Google Scholar]
- Costantini P, Colonna R, Bernardi P. Induction of the mitochondrial permeability transition by N-ethylmaleimide depends on secondary oxidation of critical thiol groups. Potentiation by copper-ortho-phenanthroline without dimerization of the adenine nucleotide translocase. Biochim. Biophys. Acta. 1998;1365:385–392. doi: 10.1016/s0005-2728(98)00090-5. [DOI] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- Engman LJ. Expedient synthesis of ebselen and related-compounds. J. Org. Chem. 1989;54:2964–2966. [Google Scholar]
- Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J. Biol. Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- Guo Q, Christakos S, Robinsen N. Calbindin D28k blocks the proapoptotic actions of mutant presenilin 1: reduced oxidative stress and preserved mitochondrial function. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3227–3232. doi: 10.1073/pnas.95.6.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion—a target for cardioprotection. Cardiovasc. Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett. 2002;512:1–7. doi: 10.1016/s0014-5793(01)03314-2. [DOI] [PubMed] [Google Scholar]
- Javadov S, Karmazyn M. Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardioprotection. Cell Physiol. Biochem. 2007;20:1–22. doi: 10.1159/000103747. [DOI] [PubMed] [Google Scholar]
- Jung U, Zheng X, Yoon S, Chung A. Se-methylselenocysteine induces apoptosis mediated by reactive oxygen species in HL-60 cells. Free Radic. Biol. Med. 2001;31:479–489. doi: 10.1016/s0891-5849(01)00604-9. [DOI] [PubMed] [Google Scholar]
- Kim JS, He L, Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem. Biophys. Res. Commun. 2003;304:463–470. doi: 10.1016/s0006-291x(03)00618-1. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Castilho RF, Vercesi AE. Mitochondrial permeability transition and oxidative stress. FEBS Lett. 2001;495:12–15. doi: 10.1016/s0014-5793(01)02316-x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Morin D, Zini R, Ligeret H, Neckameyer W, Labidalle L, Tillement JP. Dual effect of ebselen on mitochondrial permeability transition. Biochem. Pharmacol. 2003;65:1643–1651. doi: 10.1016/s0006-2952(03)00114-x. [DOI] [PubMed] [Google Scholar]
- Muller A, Cadenas E, Graf P, Sies H. A novel biologically-active organoselenium compound. 1. Glutathione peroxidase-like activity in vitro and antioxidant capacity of PZ-51 (ebselen) Biochem. Pharmacol. 1984;33:3235–3239. doi: 10.1016/0006-2952(84)90083-2. [DOI] [PubMed] [Google Scholar]
- Nogueira CW, Zeni G, Rocha JBT. Organoselenium and organotellurium compounds: toxicology and pharmacology. Chem. Rev. 2004;104:6255–6286. doi: 10.1021/cr0406559. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Rama Rao KV. The mitochondrial permeability transition in neurologic disease. Neurochem. Int. 2007;50:983–997. doi: 10.1016/j.neuint.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmier C. Selenium reagents and intermediates in organic synthesis. New York: Pergamon Press; 1986. Pergamon Press, Oxford. p. 463. [Google Scholar]
- Petronilli V, Nicolli A, Costantini P, Colonna R, Bernardi P. Regulation of the permeability transition pore, a voltage-dependent mitochondrial channel inhibited by cyclosporin A. Bioch. Biophys. Acta Bioen. 1994;1187:255–259. doi: 10.1016/0005-2728(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Puntel RL, Roos DH, Paixão MW, Braga AL, Zeni G, Nogueira CW, Rocha JBT. Oxalate modulates thiobarbituric acid reactive species (TBARS) production in supernatants of homogenates from rat brain, liver and kidney: effect of diphenyl diselenide and diphenyl ditelluride. Chem. Biol. Interact. 2007;165:87–98. doi: 10.1016/j.cbi.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Rosa RM, Roesler R, Braga AL, Saffi J, Henriques JA. Pharmacology and toxicology of diphenyl diselenide in several biological models. Braz. J. Med. Biol. Res. 2007;40:1287–1304. doi: 10.1590/s0100-879x2006005000171. [DOI] [PubMed] [Google Scholar]
- Schiar VP, Santos DB, Paixão MW, Nogueira CW, Rocha JBT, Zeni G. Human erythrocyte hemolysis induced by selenium and tellurium compounds increased by GSH or glucose: a possible involvement of reactive oxygen species. Chem. Biol. Interact. 2009;15:28–33. doi: 10.1016/j.cbi.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Shen HM, Yang CF, Ding WX, Liu J, Ong CN. Superoxide radical initiated apoptotic signalling pathway in selenite-treated HepG2 cells: mitochondria serve as the main target. Free Radic. Biol. Med. 2001;30:9–21. doi: 10.1016/s0891-5849(00)00421-4. [DOI] [PubMed] [Google Scholar]
- Votyakova TV, Reynolds IJ. DeltaPsi(m)-dependent and-independent production of reactive oxygen species by rat brain mitochondria. J. Neurochem. 2001;79:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- Votyakova TV, Reynolds IJ. Ca2+-induced permeabilization promotes free radical release from rat brain mitochondria with partially inhibited complex I. J. Neurochem. 2005;93:526–537. doi: 10.1111/j.1471-4159.2005.03042.x. [DOI] [PubMed] [Google Scholar]
- Wendel A, Fausel M, Safayi H, Otter R. A novel biologically active seleno-organic compound-II. Activity of PZ 51 in relation to glutathione peroxidase. Biochem. Pharmacol. 1984;33:3241–3245. doi: 10.1016/0006-2952(84)90084-4. [DOI] [PubMed] [Google Scholar]
- Yan L, Spallholz JE. Generation of reactive oxygen species from the reaction of selenium compounds with thiols and mammary tumor cells. Biochem. Pharmacol. 1993;45:429–437. [PubMed] [Google Scholar]
- Zhao R, Domann FE, Zhong W. Apoptosis induced by selenomethionine and methioninase is superoxide mediated and p53 dependent in human prostate cancer cells. Mol. Cancer Ther. 2006;5:3275–3284. doi: 10.1158/1535-7163.MCT-06-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Holmgren A. A novel antioxidant mechanism of ebselen involving selen diselenide, a substrate of mammalian thioredoxin and thioredoxin reductase. J. Biol. Chem. 2002;277:39456–39462. doi: 10.1074/jbc.M206452200. [DOI] [PubMed] [Google Scholar]
- Zhao R, Masayasu H, Holmgren A. Ebselen: a substrate for human thioredoxin reductase strongly stimulating its hydroperoxide reductase activity and a superfast thioredoxin oxidant. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8579–8584. doi: 10.1073/pnas.122061399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoratti M, Szabò I. The mitochondrial permeability transition. Biochim. Biophys. Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.