Abstract
Polychlorinated biphenyls (PCBs) are known to reduce serum thyroxine (T4) in rats, but the relative effects of individual PCB congeners on thyroid hormones are not known. Thus, male Sprague-Dawley rats were administered Aroclor 1254, Aroclor 1242 (4, 8, 16, or 32 mg/kg/day), PCB 95 (2,2′,3,5′,6-pentachlorobiphenyl), PCB 99 (2,2′,4,4′,5-pentachlorobiphenyl), PCB 118 (2,3′,4,4′,5-pentachlorobiphenyl) (2, 4, 8, or 16 mg/kg/day), PCB 126 (3,3′4,4′,5-pentachlorobiphenyl) (2.5, 5, 10, 20, or 40 μg/kg/day), TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) (0.14, 0.43, 1.3, or 3.9 μg/kg/day), or corn oil via oral gavage for 7 days. Rats were necropsied 24 h after the last dose. Serum thyroid hormone levels were evaluated by radioimmunoassay, and induction of hepatic Cyp1a (a TCDD-inducible protein) and Cyp2b (a phenobarbital [PB]-inducible protein) activity was determined by ethoxyresorufin-O-deethylase and pentoxyresorufin-O-deethylase assays, respectively. Significant increases in Cyp1a activity occurred in response to PCBs, except PCB 95 and PCB 99. Aroclor 1254, PCB 99, and PCB 118 significantly induced Cyp2b activity. Serum total T4 and free T4 were dramatically reduced in response to each of the seven treatments in a dose-dependent manner. The marked T4 reductions occurred in response to Aroclor 1254, PCB 99 (a PB-type congener), and PCB 118 (a mixed-type congener). In contrast, reductions in serum triiodothyronine (total and free) were variable and mild, and serum thyroid-stimulating hormone was not significantly affected by any of the compounds. These data indicate that the PB and mixed-type PCB congeners are more effective than the TCDD-type PCB congeners at reducing serum T4.
Keywords: PCB congeners, thyroid hormones, liver, cytochrome P450, dose-response
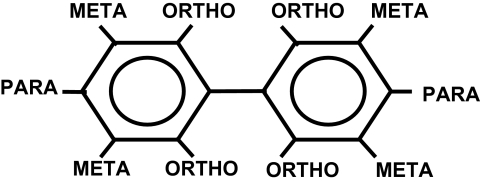
Polychlorinated biphenyls (PCBs) decrease circulating levels of thyroxine (T4) in rats (Barter and Klaassen, 1994; Hood et al., 1999; Liu et al., 1995; Li and Hansen, 1997), but the mechanisms by which this occurs are not clearly understood. Most of these studies were conducted with commercially available mixtures of PCBs, like Aroclor 1254. Aroclor 1254 was produced by Monsanto Chemical Corporation until the late 1970's and is so named for the 12 positions on the biphenyl rings and the fact that the mixture contains 54% chlorine by weight (Safe, 1984). PCB mixtures, like Aroclor 1254, are produced by chlorination of the biphenyl rings using various catalysts and synthetic conditions (De Voogt and Brinkman, 1989). The biphenyl molecule has 10 positions available for chlorination: 2 para, 4 meta, and 4 ortho (Fig. 1). Because these 10 positions are randomly chlorinated in the production process, it is theoretically possible for the Aroclor mixture to be composed of 209 different PCB congeners (De Voogt and Brinkman, 1989; Frame et al., 1996). The congeners range from mono to decachlorobiphenyls, and each mixture contains differing amounts of each congener (Frame et al., 1996). The coplanar PCB congeners, which have no chlorine substitutions in the ortho positions, have affinity for the aryl hydrocarbon receptor (AhR), induce cytochrome P450 1A (Cyp1a), and are referred to as “TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin)-type congeners”. The noncoplanar PCB congeners, which have at least two chlorine substitutions in the ortho positions, have low affinity for the AhR, induce Cyp2b, and are referred to as “phenobarbital (PB)-type congeners”. The mono-ortho coplanar PCB congeners have one chlorine substitution in an ortho position, which reduces, but does not abolish, affinity for the AhR. These congeners induce both Cyp1a and Cyp2b in rats and are referred to as “mixed-type congeners” (Safe et al., 1985).
FIG. 1.
Chemical structure of PCB with Cl-binding positions.
In general, the most toxic congeners are those that are coplanar or TCDD types (Bager et al., 1995; Safe, 1984, 1994), but not much is known about the effects of any of the three types of individual congeners on thyroid hormone status in rats. Indeed, some of the adverse effects associated with TCDD exposure (induction of Cyp1a, hepatotoxicity, chloracne, dermal lesions, and immunotoxicity) are also observed with administration of the coplanar PCBs and are most likely mediated through the AhR (Li and Hansen, 1997). However, additional effects observed following PCB exposure, such as induction of Cyp2b, estrogenicity, and neurotoxicity, cannot be fully explained by interaction of PCBs with the AhR (Li and Hansen, 1997; Safe, 1994) and are probably the result of the two additional types (PB and mixed) of congeners present within the mixture. In fact, each of the three types of PCB congeners has been linked with a broad spectrum of toxic effects (Fischer et al., 1998; Li and Hansen, 1997; Safe, 1994).
It could thus be presumed that the effects of PCBs on thyroid hormones could be the result of any one or all three types of PCB congeners. A few studies have addressed the potential disruption of thyroid hormone homeostasis by individual PCB congeners, such as the TCDD-type congeners PCB 77 and PCB 126 (3,3′4,4′,5-pentachlorobiphenyl) (Alvarez-Lloret et al., 2009; Craft et al., 2002; Fisher et al., 2006; Seo et al., 1995); the PB-type congeners PCB 28, PCB 95 (2,2′,3,5′,6-pentachlorobiphenyl), PCB 99 (2,2′,4,4′,5-pentachlorobiphenyl), PCB 101, and PCB 153 (Bager et al., 1995; Braathen et al., 2004; Desaulniers et al., 1999; Khan et al., 2002); and the mixed-type congeners PCB 118 (2,3′,4,4′,5-pentachlorobiphenyl), Aroclor 1242, and Aroclor 1254 (Braathen et al., 2004; Mayes et al., 1998; Ness et al., 1993; National Toxicology Program, 2006). However, none of these studies compares the relative ability of the three types of PCB congeners to produce reductions in circulating levels of thyroid hormones or has shown a direct comparison with PCB mixtures.
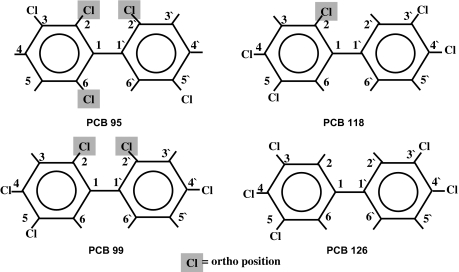
In this study, we chose to evaluate Aroclor 1254 along with four different pentachlorobiphenyl congeners with various degrees of planarity (PCBs 95, 99, 118, and 126; see Fig. 2) to determine whether the effects on circulating thyroid hormone status associated with administration of Aroclor 1254 could be attributed to a particular class of PCB congener. PCB 126 is a coplanar TCDD-type congener, PCB 95 and PCB 99 are noncoplanar PB-type congeners, and PCB 118 is a mono-ortho coplanar mixed-type congener. Aroclor 1242 was also evaluated to examine the effects of a PCB mixture with a lower (42% by weight) degree of chlorination. TCDD was evaluated as the prototypical AhR ligand for comparison with PCB 126.
FIG. 2.
Structures of PCB 95, PCB 99, PCB 118, and PCB 126.
MATERIALS AND METHODS
Chemicals and reagents.
Aroclor 1254 and Aroclor 1242 were kindly provided by Dr Lawrence Hansen (University of Illinois at Urbana). TCDD was a gift from Dr Karl Rozman (University of Kansas Medical Center, Kansas City, KS). PCB 95, PCB 99, PCB 118, and PCB 126 were obtained from AccuStandard (New Haven, CT). Radioimmunoassay kits for assay of serum thyroid hormones were obtained from Diagnostics Products Corporation (Los Angeles, CA). Radioimmunoassay kits for evaluation of serum thyroid-stimulating hormone (TSH) were obtained from Amersham Life Sciences (Piscataway, NJ). Resorufin, ethoxyresorufin, and pentoxyresorufin were obtained from Sigma Chemical Co. (St Louis, MO). All remaining reagents were obtained from Sigma Chemical Co.
Animals and treatments.
The Institutional Animal Care and Use Committee approved all protocols prior to initiation. Male Sprague-Dawley rats (Sasco, Wilmington, MA) weighing 225–250 g were individually housed in hanging wire-bottomed cages and maintained at ∼21°C on a 12-h light/dark cycle. All compounds were dissolved in corn oil (Mazola; Best Foods, Englewood Cliffs, NJ). Rats (six per treatment group) were administered the corn oil solutions of Aroclor 1254, Aroclor 1242 (4, 8, 16, or 32 mg/kg/day), PCB 95, PCB 99, PCB 118 (2, 4, 8, or 16 mg/kg/day), PCB 126 (2.5, 5, 10, 20, or 40 μg/kg/day), or TCDD (0.14, 0.43, 1.3, or 3.9 μg/kg/day) via gavage for seven consecutive days in a dose volume of 5 ml/kg. Control rats were administered corn oil (5 ml/kg) for seven consecutive days. The dose selections were based on the literature and preliminary studies. All rats had ad libitum access to feed (Purina Rodent Chow, 5001) and water for the 7-day dosing period. Body weights were recorded daily.
Sampling.
Approximately 24 h after the last dose (day 7), each rat was anesthetized with CO2 and decapitated. Approximately 5 ml of trunk blood was collected. Serum was prepared by allowing the blood to clot for ∼2 h at 4°C, followed by centrifugation, and the supernate was collected and stored at −70°C. Livers were harvested, weighed, immediately frozen in liquid nitrogen, and stored at −70°C.
Determination of serum T4, triiodothyronine, and TSH.
The concentrations of total (representing both free and protein-bound) and free T4 and triiodothyronine (T3), as well as TSH in serum were determined by radioimmunoassay. The limits of detection of these kits were 0.25 μg/dl, 0.01 ng/dl, 7 ng/dl, 0.2 pg/ml, and 1 ng/ml, respectively.
Microsome preparation.
Cytochrome P450 activity was determined in liver microsomes. Liver microsomes were prepared as previously described (Hood and Klaassen, 2000). Briefly, livers were homogenized in 2 volumes of ice-cold buffer containing 50mM Tris and 150mM potassium chloride (pH 7.5). Homogenates were centrifuged at 860 × g for 15 min at 4°C, and the pellet was discarded. The supernate was centrifuged at 23,300 × g for 25 min at 4°C, and the pellet was again discarded. This second supernate was decanted into ultracentrifuge tubes and centrifuged at 105,000 × g for 1 h. The resulting pellet was removed and homogenized in 1.15% potassium chloride containing 10mM neutralized EDTA. The homogenate was centrifuged at 105,000 × g for 1 h. The supernate was discarded, and the microsomal pellet was homogenized in 0.25M sucrose (0.4 ml/g tissue) and stored at −70°C. Protein concentrations in microsomes were determined according to the Bradford method using a Bio-Rad protein assay kit (Richmond, CA) with bovine serum albumin as the standard.
Cytochrome P450 activity assays.
Markers for Cyp1a and Cyp2b activity, pentoxyresorufin-O-deethylase (PROD) activity, and ethoxyresorufin-O-deethylase (EROD) activity, respectively, were determined spectrofluorometrically based on the dynamic assay methods described previously. Briefly, the reaction mixture contained 0.1M KPO4 buffer with bovine serum albumin and substrate (ethoxyresorufin or pentoxyresorufin) at pH 7.5. Diluted liver microsomes were added, and the reaction mixture was preincubated for 4 min at 37°C. The reaction was initiated by adding nicotinamide adenine dinucleotide phosphate (NADPH) and was carried out at 37°C for 2–3 min. Resorufin production was monitored fluorometrically (Model RF-5301; Shimadzu, Columbia, MD).
Statistics.
Differences between control and treated animals were determined using ANOVA followed by the Duncan's multiple range post hoc test. Asterisks in the figures indicate significant differences between treated and control groups (p < 0.05). Statistical analyses were performed using STATISTICA 4.5 (Statsoft, Inc., Tulsa, OK).
RESULTS
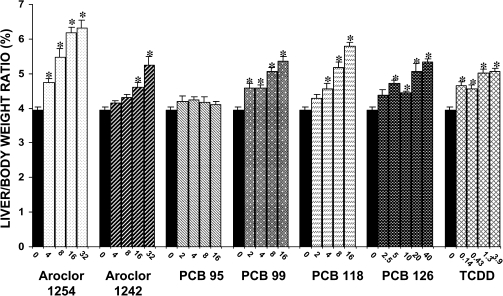
None of the treatments produced significant changes in body weight during the 7-day dosage period. Significant, and generally dose-dependent, increases in liver to body weight ratios were produced by repeated administration of each PCB mixture, congener, and TCDD, with the exception of PCB 95 (Fig. 3). Aroclor 1254 and PCB 118 caused the greatest increases, with the high doses of Aroclor 1254 (32 mg/kg/day) and PCB 118 (16 mg/kg/day) producing increases of 59 and 46%, respectively, compared with the control group.
FIG. 3.
Effect of PCBs and TCDD on liver weight. Each value represents the mean ± SEM of 6–12 rats. *Significantly different from control (p < 0.05). Dose units are mg/kg/day for all compounds except PCB 126 and TCDD, for which the dose units are μg/kg/day.
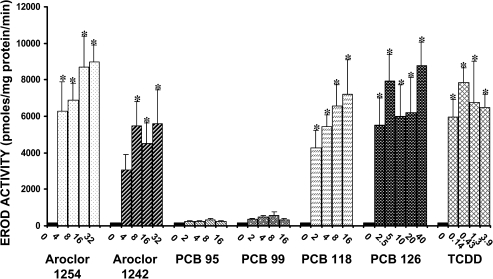
The effect of PCBs and TCDD on the induction of Cyp1a activity (as quantified by EROD activity) in liver microsomes is shown in Figure 4. Significant increases in Cyp1a activity occurred in response to administration of each of these compounds, with the exception of PCB 95 and PCB 99. Aroclor 1254, PCB 118, PCB 126, and TCDD tended to produce the largest induction in activity. The highest doses (32 mg/kg/day, 16 mg/kg/day, 40 μg/kg/day, and 3.9 μg/kg/day, respectively) of these compounds caused 40- to 56-fold increases in activity. Aroclor 1242 also significantly increased Cyp1a activity, but to a lesser extent, with the highest dose producing a 35-fold increase.
FIG. 4.
Effect of PCBs and TCDD on the activity of Cyp1a in liver microsomes. Each value represents the mean ± SEM of 6–12 rats. *Significantly different from control (p < 0.05). Dose units are mg/kg/day for all compounds except PCB 126 and TCDD, for which the dose units are μg/kg/day.
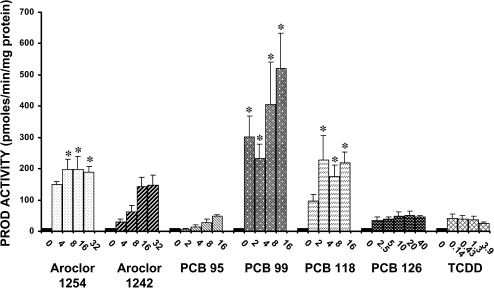
Administration of Aroclor 1254, PCB 99, and PCB 118 significantly induced Cyp2b activity (as quantified by PROD activity) in liver microsomes (Fig. 5). PCB 99 produced the largest induction in Cyp2b activity, with the highest dose (16 mg/kg/day) causing a 60-fold increase in activity compared with the control group. Aroclor 1254 and PCB 118 produced 17- to 26-fold increases in Cyp2b activity. The remaining compounds (Aroclor 1242, PCB 95, PCB 126, and TCDD) did not significantly increase Cyp2b activity.
FIG. 5.
Effect of PCBs and TCDD on the activity of Cyp2b in liver microsomes. Each value represents the mean ± SEM of 6–12 rats. *Significantly different from control (p < 0.05). Dose units are mg/kg/day for all compounds except PCB 126 and TCDD, for which the dose units are μg/kg/day.
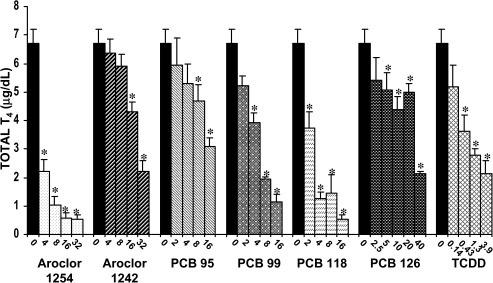
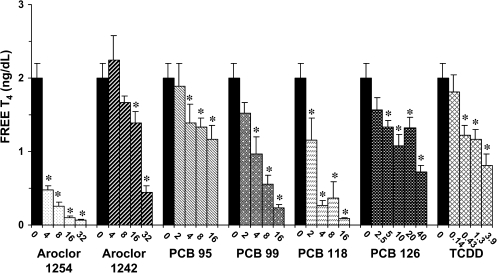
Circulating levels of T4 were dramatically reduced after seven consecutive days of treatment with each of these compounds, and the reductions generally occurred in a dose-dependent manner (Figs. 6 and 7). The most marked reductions in serum total and free T4 occurred in response to Aroclor 1254, PCB 118, and PCB 99. At the highest dose of each of these compounds, total T4 was reduced by 93, 92, and 83%, respectively. Serum total T4 was reduced up to 67% by Aroclor 1242, 54% by PCB 95, 68% by PCB 126, and 32% by TCDD at the highest doses tested. The effects of these compounds on serum-free T4 parallel those described for serum total T4, with the most marked reductions occurring in response to Aroclor 1254, PCB 118, and PCB 99. In the high-dose groups of each of these compounds, reductions in serum-free T4 were 97, 95, and 86%, respectively. The reductions in serum levels of free T4 in response to administration of the high doses of Aroclor 1242, PCB 126, TCDD, and PCB 95 were 77, 64, 59, and 41%, respectively.
FIG. 6.
Effect of PCBs and TCDD on serum total T4. Each value represents the mean ± SEM of 6–12 rats. *Significantly different from control (p < 0.05). Dose units are mg/kg/day for all compounds except PCB 126 and TCDD, for which the dose units are μg/kg/day.
FIG. 7.
Effect of PCBs and TCDD on serum-free T4. Each value represents the mean ± SEM of 6–12 rats. *Significantly different from control (p < 0.05). Dose units are mg/kg/day for all compounds except PCB 126 and TCDD, for which the dose units are μg/kg/day.
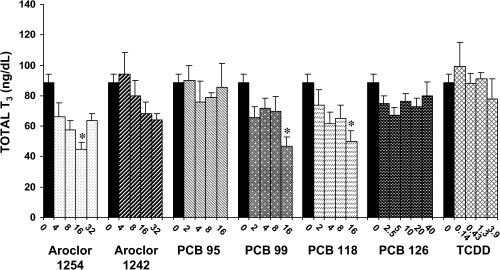
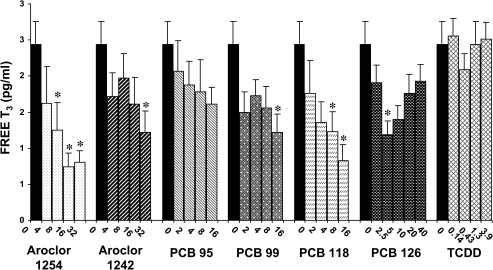
Circulating levels of T3 were not as markedly reduced by repeated administration of PCBs and TCDD as the levels of serum T4 (Figs. 8 and 9). However, high dose of Aroclor 1254 (16 mg/kg/day), PCB 99 (16 mg/kg/day), and PCB 118 (16 mg/kg/day) led to significant decreases in serum total T3. Aroclor 1242, PCB 95, PCB 126, and TCDD did not significantly affect serum total T3. Free T3 levels were decreased to a greater degree than total T3. Significant reductions in free T3 occurred in response to three doses of Aroclor 1254 (8, 16, and 32 mg/kg/day), highest dose of Aroclor 1242 (32 mg/kg/day) and of PCB 99 (16 mg/kg/day), two high doses of PCB 118 (8 and 16 mg/kg/day), and one middle dose of PCB 126 (5 μg/kg/day). These decreases ranged from 49 to 70%.
FIG. 8.
Effect of PCBs and TCDD on serum total T3. Each value represents the mean ± SEM of 6–12 rats. *Significantly different from control (p < 0.05). Dose units are mg/kg/day for all compounds except PCB 126 and TCDD, for which the dose units are μg/kg/day.
FIG. 9.
Effect of PCBs and TCDD on serum-free T3. Each value represents the mean ± SEM of 6–12 rats. *Significantly different from control (p < 0.05). Dose units are mg/kg/day for all compounds except PCB 126 and TCDD, for which the dose units are μg/kg/day.
Serum TSH was not significantly affected by any of the compounds administered (data not shown).
DISCUSSION
In the present study, the effects of the three different classes of PCB congeners (TCDD type, PB type, and mixed type) on thyroid hormone status in male rats in comparison with PCB mixtures (Aroclor 1242 and Aroclor 1254) and TCDD have been investigated. It was essential to first evaluate the induction of Cyp1a and Cyp2b as markers of TCDD-like and PB-like activity, respectively, to confirm that the PCB congeners were producing the expected induction of Cyp1a and/or Cyp2b. The pattern of induction of Cyp1a and Cyp2b activity was as predicted for each compound (Chu et al., 1995; Connor et al., 1995; Craft et al., 2002; Harris et al., 1993). TCDD, Aroclor 1242, and the TCDD-type congener, PCB 126, induced Cyp1a activity; the PB-type congener, PCB 99, induced Cyp2b activity; and Aroclor 1254 and the mixed-type congener, PCB 118, induced both Cyp1a and Cyp2b activity. The lack of a significant induction of Cyp2b by Aroclor 1242, the PCB mixture with a lower degree of chlorination and a lower percentage (by weight) of potent PB-type congeners like PCB 99 and PCB 153, has been reported previously (Burgin et al., 2001; Craft et al., 2002; Frame et al., 1996; Harris et al., 1993). PCB 95, a PB-type congener, did not significantly induce either Cyp1a or Cyp2b activity, although there was a tendency toward a dose-dependent increase in Cyp2b activity. This lack of a robust increase in Cyp2b activity by a PB-type congener probably reflects the more labile structure of PCB 95, which has unsubstituted meta and para positions, leaving the molecule more susceptible to oxidative metabolism (Matthews and Dedrick, 1984). The lack of an increase in liver weight in response to PCB 95 correlates with the lack of increase in liver enzyme activity.
This study demonstrates that repeated administration of each of the three types of congeners, PCB mixtures, and TCDD produces significant, and generally dose-dependent, reductions in serum total and free T4 (Figs. 6 and 7). Aroclor 1254, the mixed-type congener (PCB 118), and one of the PB-type congeners (PCB 99) produced the most dramatic reductions in serum levels of T4. The effects on T3 were inconsistent and much less dramatic (Figs. 8 and 9); however, the same three compounds (Aroclor 1254, PCB 99, and PCB 118) produced the largest reductions in T3. Serum TSH was not significantly increased by any of the compounds (data not shown) in response to the depressed serum levels of T4 and/or T3.
The dramatic reductions in serum T4, the variable effects on T3, and the lack of an increase in TSH observed in response to administration of Aroclor 1254 are consistent with previous reports (Hood et al., 1999; Liu et al., 1995; Khan et al., 2002; Vansell and Klaassen, 2002; Vansell et al., 2004). The variable reductions in serum T3 levels could be related to the fact that Aroclor 1254 differentially induces UDP-glucuronosyltransferase (UGT) activity toward T4 and T3 in liver microsomes from male rats (Hood and Klaassen, 2000). In this aforementioned study, Aroclor 1254 increased UGT activity toward T4 as much as 430% but did not have any appreciable effect on UGT activity toward T3. These results support the view that T3 and T4 are glucuronidated by different isoforms of UGT (Beetstra et al., 1991; Hood and Klaassen, 2000; Visser et al., 1993). It is also suggested that the lack of a TSH response in PCB-treated rats is related to the lack of induction of UGT activity toward T3. Compounds like pregnenolone-16α-carbonitrile and PB, which increase TSH in male rats, increase UGT activity toward T3 in liver microsomes and increase the biliary excretion of T3-glucuronide in vivo (Hood and Klaassen, 2000, Vansell and Klaassen, 2002) but are not as effective at reducing serum levels of T4 as is Aroclor 1254 (Hood et al., 1999). The lack of induction of UGT activity toward T3 by Aroclor 1254 is further verified by the fact that Aroclor 1254 does not increase the biliary excretion of T3-glucuronide in vivo (Vansell and Klaassen, 2002).
The less dramatic reductions in thyroid hormones in response to Aroclor 1242, compared with Aroclor 1254, were not unexpected. Aroclor 1242 contains a lower percentage of chlorine, and the congeners present in the mixture are mostly trichlorobiphenyls and tetrachlorobiphenyls (Frame et al., 1996), which are more readily metabolized than the more highly chlorinated congeners present in Aroclor 1254 (Safe, 1984, 1994). Aroclor 1242 also contains a much lower percentage (by weight) of the mixed-type congener PCB 118 and the PB-type congener PCB 99 (Frame et al., 1996) than Aroclor 1254, which in this study were shown to be highly effective at reducing serum levels of T4. The fact that Aroclor 1254 is more potent than Aroclor 1242 in reducing serum T4 has also been shown in neonatal rats (Cooke et al., 1996). The induction of UGTs and biliary excretion of thyroid hormone conjugates in response to Aroclor 1242 have not been investigated, but such a study might further explain these results.
The effect of TCCD on thyroid hormones (reduced serum levels of total and free T4, with little or no effect on T3 or TSH) was as expected, based on previous published accounts (Schuur et al., 1997; Seo et al., 1995; van der Plas et al., 2001), and the similar effects produced by PCB 126 verify the TCDD-like nature of this congener. Whereas PCBs appear to affect multiple mechanisms (Collins and Capen, 1980; Li and Hansen, 1997; Klaassen and Hood, 2001), it is proposed that induction of the UGT isoform that glucuronidates T4 is the sole mechanism responsible for TCDD-induced hypothyroidism (Kohn, 2000).
In the present study, the effects of PCB 99 and PCB 118 on serum T4 are essentially identical to those produced in response to Aroclor 1254. Aroclor 1254 contains relatively large amounts of these two congeners and less of the coplanar TCDD type (Frame et al., 1996). But the potential importance of the TCDD-type (coplanar) congeners cannot be ignored. Pure soil extracts contaminated with PCBs induce UGT activity toward 4-nitrophenol (4-NP) (Li and Hansen, 1997), a marker substrate for the UGT isoform that reportedly glucuronidates T4 (Beetstra et al., 1991; Visser et al., 1993). However, when the soil extract is filtered through charcoal to remove the coplanar (TCDD-type) congeners, the induction of UGT activity toward 4-NP is abolished. Curiously, the soil extract and the charcoal-filtered soil caused similar reductions in serum T4 in female rats, indicating that there are mechanisms in addition to induction of UGTs responsible for the reduction in serum thyroid hormones produced by PCB mixtures. Like Aroclor 1254, neither the pure soil extract nor the charcoal-filtered version induced activity of the UGT toward phenolphthalein, a marker for activity of the UGT isoform reportedly responsible for the glucuronidation of T3 (Visser et al., 1993). Because deiodination of T4 produces the majority of circulating T3 (Kohrle, 2000; Viluksela et al., 2004), the moderate reductions in T3 that are observed could be related to the decreased availability of T4 for deiodination to T3.
PCBs can be metabolized to hydroxylated PCBs in the liver, possibly by P450 enzymes (Liu et al., 2006). Hydroxylated PCBs have been shown to interfere with thyroid hormone transport and to inhibit thyroid hormone sulfation, thereby potentially disrupting thyroid homeostasis (Wang and James, 2006). Hydroxylated PCBs also interfere with the binding of T4 to transthyretin in rodents, humans, and polar bears (Gutleb et al., 2010), which could be one of the potential mechanisms for reduction of total serum T4.
In conclusion, these data suggest that the reductions in circulating levels of T4 in male rats produced by Aroclor 1254 cannot be attributed to an individual type of congener. However, the dramatic effects produced by this PCB mixture on serum T4 may be predominantly in response to the mixed-type and PB-type congeners within the mixture because they were the most effective individually and are the predominant congeners present in Aroclor 1254.
FUNDING
National Institutes of Health Research Grant (ES-013714, ES-09716, DK-081461, RR-021940, ES-05022) and National Institutes of Health Training Grant (ES-07079, ES-07148).
References
- Alvarez-Lloret P, Lind PM, Nyberg I, Orberg J, Rodríguez-Navarro AB. Effects of 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) on vertebral bone mineralization and on thyroxin and vitamin D levels in Sprague-Dawley rats. Toxicol. Lett. 2009;187:63–68. doi: 10.1016/j.toxlet.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Bager Y, Hemming H, Flodstrom S, Ahlborg UG, Warngard L. Interaction of 3,4,5,3′,4′-pentachlorobiphenyl and 2,4,5,2′,4′,5′-hexachlorobiphenyl in promotion of altered hepatic foci in rats. Pharmacol. Toxicol. 1995;77:149–154. doi: 10.1111/j.1600-0773.1995.tb01004.x. [DOI] [PubMed] [Google Scholar]
- Barter RA, Klaassen CD. Reduction of thyroid hormone levels and alteration of thyroid function by four representative UDP-glucuronosyltransferase inducers in rats. Toxicol. Appl. Pharmacol. 1994;128:9–17. doi: 10.1006/taap.1994.1174. [DOI] [PubMed] [Google Scholar]
- Beetstra JB, van Engelen JG, Karels P, van der Hoek HJ, de Jong M, Docter R, Krenning EP, Hennemann G, Brouwer A, Visser TJ. Thyroxine and 3,3′,5-triiodothyronine are glucuronidated in rat liver by different uridine diphosphate-glucuronyltransferases. Endocrinology. 1991;128:741–746. doi: 10.1210/endo-128-2-741. [DOI] [PubMed] [Google Scholar]
- Braathen M, Derocher AE, Wiig Ø, Sørmo EG, Lie E, Skaare JU, Jenssen BM. Relationships between PCBs and thyroid hormones and retinol in female and male polar bears. Environ. Health Perspect. 2004;112:826–833. doi: 10.1289/ehp.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgin DE, Diliberto JJ, Derr-Yellin EC, Kannan N, Kodavanti PR, Birnbaum LS. Differential effects of two lots of aroclor 1254 on enzyme induction, thyroid hormones, and oxidative stress. Environ. Health Perspect. 2001;109:1163–1168. doi: 10.1289/ehp.011091163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu I, Villeneuve DC, Yagminas A, Lecavalier P, Hakansson H, Ahlborg UG, Valli VE, Kennedy SW, Bergman A, Seegal RF, et al. Toxicity of PCB 77 (3,3′,4,4′-tetrachlorobiphenyl) and PCB 118 (2,3′,4,4′5-pentachlorobiphenyl) in the rat following subchronic dietary exposure. Fundam. Appl. Toxicol. 1995;26:282–292. doi: 10.1006/faat.1995.1099. [DOI] [PubMed] [Google Scholar]
- Collins WT, Jr, Capen CC. Biliary excretion of 125I-thyroxine and fine structural alterations in the thyroid glands of Gunn rats fed polychlorinated biphenyls (PCB) Lab. Invest. 1980;43:158–164. [PubMed] [Google Scholar]
- Connor K, Safe S, Jefcoate CR, Larsen M. Structure-dependent induction of CYP2B by polychlorinated biphenyl congeners in female Sprague-Dawley rats. Biochem. Pharmacol. 1995;50:1913–1920. doi: 10.1016/0006-2952(95)02087-x. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Zhao YD, Hansen LG. Neonatal polychlorinated biphenyl treatment increases adult testis size and sperm production in the rat. Toxicol. Appl. Pharmacol. 1996;136:112–117. doi: 10.1006/taap.1996.0013. [DOI] [PubMed] [Google Scholar]
- Craft ES, DeVito MJ, Crofton KM. Comparative responsiveness of hypothyroxinemia and hepatic enzyme induction in Long-Evans rats versus C57BL/6J mice exposed to TCDD-like and phenobarbital-like polychlorinated biphenyl congeners. Toxicol. Sci. 2002;68:372–380. doi: 10.1093/toxsci/68.2.372. [DOI] [PubMed] [Google Scholar]
- De Voogt P, Brinkman AT. Production properties and usage of polychlorinated biphenyls. In: Kimbrough RD, Jensen AA, editors. Halogenated Biphenyls, Terphenyls, Naphthalenes and Dibenzodioxins and Related Products. New York: Elsevier; 1989. [Google Scholar]
- Desaulniers D, Leingartner K, Wade M, Fintelman E, Yagminas A, Foster WG. Effects of acute exposure to PCBs 126 and 153 on anterior pituitary and thyroid hormones and FSH isoforms in adult Sprague Dawley male rats. Toxicol. Sci. 1999;47:158–169. doi: 10.1093/toxsci/47.2.158. [DOI] [PubMed] [Google Scholar]
- Fischer LJ, Seegal RF, Ganey PE, Pessah IN, Kodavanti PR. Symposium overview: toxicity of non-coplanar PCBs. Toxicol. Sci. 1998;41:49–61. doi: 10.1006/toxs.1997.2386. [DOI] [PubMed] [Google Scholar]
- Fisher JW, Campbell J, Muralidhara S, Bruckner JV, Ferguson D, Mumtaz M, Harmon B, Hedge JM, Crofton KM, et al. Effect of PCB 126 on hepatic metabolism of thyroxine and perturbations in the hypothalamic-pituitary-thyroid axis in the rat. Toxicol. Sci. 2006;90:87–95. doi: 10.1093/toxsci/kfj069. [DOI] [PubMed] [Google Scholar]
- Frame GM, Wagner RM, Carnahan JC, Brown JF, Jr, May RJ, Smullen LA, Bedard DL. Comprehensive, quantitative, congener specific analyses of eight aroclors and complete PCB congener assignments on DB-1 capillary GC columns. Chemosphere. 1996;33:603–623. [Google Scholar]
- Gutleb AC, Cenijn P, Velzen M, Lie E, Ropstad E, Skaare JU, Malmberg T, Bergman A, Gabrielsen GW, Legler J. In vitro assay shows that PCB metabolites completely saturate thyroid hormone transport capacity in blood of wild polar bears (Ursus maritimus) Environ. Sci. Technol. 2010;44:3149–3154. doi: 10.1021/es903029j. [DOI] [PubMed] [Google Scholar]
- Harris M, Zacharewski T, Safe S. Comparative potencies of Aroclors 1232, 1242, 1248, 1254, and 1260 in male Wistar rats–assessment of the toxic equivalency factor (TEF) approach for polychlorinated biphenyls (PCBs) Fundam. Appl. Toxicol. 1993;20:456–463. doi: 10.1006/faat.1993.1056. [DOI] [PubMed] [Google Scholar]
- Hood A, Hashmi R, Klaassen CD. Effects of microsomal enzyme inducers on thyroid-follicular cell proliferation, hyperplasia, and hypertrophy. Toxicol. Appl. Pharmacol. 1999;160:163–170. doi: 10.1006/taap.1999.8752. [DOI] [PubMed] [Google Scholar]
- Hood A, Klaassen CD. Differential effects of microsomal enzyme inducers on in vitro thyroxine (T4) and triiodothyronine (T3) glucuronidation. Toxicol. Sci. 2000;55:78–84. doi: 10.1093/toxsci/55.1.78. [DOI] [PubMed] [Google Scholar]
- Khan MA, Lichtensteiger CA, Faroon O, Mumtaz M, Schaeffer DJ, Hansen LG. The hypothalamo-pituitary-thyroid (HPT) axis: a target of nonpersistent ortho-substituted PCB congeners. Toxicol. Sci. 2002;65:52–61. doi: 10.1093/toxsci/65.1.52. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Hood AM. Effects of microsomal enzyme inducers on thyroid follicular cell proliferation and thyroid hormone metabolism. Toxicol. Pathol. 2001;29:34–40. doi: 10.1080/019262301301418838. [DOI] [PubMed] [Google Scholar]
- Kohn MC. Effects of TCDD on thyroid hormone homeostasis in the rat. Drug Chem. Toxicol. 2000;23:259–277. doi: 10.1081/dct-100100114. [DOI] [PubMed] [Google Scholar]
- Kohrle J. The deiodinase family: selenoenzymes regulating thyroid hormone availability and action. Cell. Mol. Life Sci. 2000;57:1853–1863. doi: 10.1007/PL00000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MH, Hansen LG. Consideration of enzyme and endocrine interactions in the risk assessment of PCBs. Rev. Toxicol. 1997;1:71–156. [Google Scholar]
- Liu J, Liu Y, Barter RA, Klaassen CD. Alteration of thyroid homeostasis by UDP-glucuronosyltransferase inducers in rats: A dose-response study. J. Pharmacol. Exp. Ther. 1995;273:977–985. [PubMed] [Google Scholar]
- Liu Y, Apak TI, Lehmler HJ, Robertson LW, Duffel MW. Hydroxylated polychlorinated biphenyls are substrates and inhibitors of human hydroxysteroid sulfotransferase SULT2A1. Chem. Res. Toxicol. 2006;19:1420–1425. doi: 10.1021/tx060160+. [DOI] [PubMed] [Google Scholar]
- Matthews HB, Dedrick RL. Pharmacokinetics of PCBs. Annu. Rev. Pharmacol. Toxicol. 1984;24:85–103. doi: 10.1146/annurev.pa.24.040184.000505. [DOI] [PubMed] [Google Scholar]
- Mayes BA, McConnell EE, Neal BH, Brunner MJ, Hamilton SB, Sullivan TM, Peters AC, Ryan MJ, Toft JD, Singer AW, et al. Comparative carcinogenicity in Sprague-Dawley rats of the polychlorinated biphenyl mixtures Aroclors 1016, 1242, 1254, and 1260. Toxicol. Sci. 1998;41:62–76. doi: 10.1093/toxsci/41.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of a Mixture of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) (CAS No. 1746-01-6), 2,3,4,7,8-Pentachlorodibenzofuran (PeCDF) (CAS No. 57117-31-4), and 3,3′,4,4′,5-Pentachlorobiphenyl (PCB 126) (CAS No. 57465-28-8) in Female Harlan Sprague-Dawley Rats (Gavage Studies) Natl. Toxicol. Program Tech. Rep. Ser. 2006;526:1–180. [PubMed] [Google Scholar]
- Ness DK, Schantz SL, Moshtaghian J, Hansen LG. Effects of perinatal exposure to specific PCB congeners on thyroid hormone concentrations and thyroid histology in the rat. Toxicol. Lett. 1993;68:311–323. doi: 10.1016/0378-4274(93)90023-q. [DOI] [PubMed] [Google Scholar]
- Safe S. Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs): biochemistry, toxicology, and mechanism of action. Crit. Rev. Toxicol. 1984;13:319–395. doi: 10.3109/10408448409023762. [DOI] [PubMed] [Google Scholar]
- Safe S, Bandiera S, Sawyer T, Robertson L, Safe L, Parkinson A, Thomas PE, Ryan DE, Reik LM, Levin W, et al. PCBs: structure-function relationships and mechanism of action. Environ. Health Perspect. 1985;60:47–56. doi: 10.1289/ehp.856047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe SH. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit. Rev. Toxicol. 1994;24:87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- Schuur AG, Boekhorst FM, Brouwer A, Visser TJ. Extrathyroidal effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on thyroid hormone turnover in male Sprague-Dawley rats. Endocrinology. 1997;138:3727–3734. doi: 10.1210/endo.138.9.5386. [DOI] [PubMed] [Google Scholar]
- Seo BW, Li MH, Hansen LG, Moore RW, Peterson RE, Schantz SL. Effects of gestational and lactational exposure to coplanar polychlorinated biphenyl (PCB) congeners or 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on thyroid hormone concentrations in weanling rats. Toxicol. Lett. 1995;78:253–262. doi: 10.1016/0378-4274(95)03329-j. [DOI] [PubMed] [Google Scholar]
- van der Plas SA, Lutkeschipholt I, Spenkelink B, Brouwer A. Effects of subchronic exposure to complex mixtures of dioxin-like and non-dioxin-like polyhalogenated aromatic compounds on thyroid hormone and vitamin A levels in female Sprague-Dawley rats. Toxicol. Sci. 2001;59:92–100. doi: 10.1093/toxsci/59.1.92. [DOI] [PubMed] [Google Scholar]
- Vansell NR, Klaassen CD. Effect of microsomal enzyme inducers on the biliary excretion of triiodothyronine (T3) and its metabolites. Toxicol. Sci. 2002;65:184–191. doi: 10.1093/toxsci/65.2.184. [DOI] [PubMed] [Google Scholar]
- Vansell NR, Muppidi JR, Habeebu SM, Klaassen CD. Promotion. of thyroid tumors in rats by pregnenolone-16alpha-carbonitrile (PCN) and polychlorinated biphenyl (PCB) Toxicol. Sci. 2004;81:50–59. doi: 10.1093/toxsci/kfh197. [DOI] [PubMed] [Google Scholar]
- Viluksela M, Raasmaja A, Lebofsky M, Stahl BU, Rozman KK. Tissue-specific effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on the activity of 5′-deiodinases I and II in rats. Toxicol. Lett. 2004;147:133–142. doi: 10.1016/j.toxlet.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Visser TJ, Kaptein E, Gijzel AL, de Herder WW, Ebner T, Burchell B. Glucuronidation of thyroid hormone by human bilirubin and phenol UDP-glucuronyltransferase isoenzymes. FEBS Lett. 1993;324:358–360. doi: 10.1016/0014-5793(93)80151-j. [DOI] [PubMed] [Google Scholar]
- Wang LQ, James MO. Inhibition of sulfotransferases by xenobiotics. Curr. Drug Metab. 2006;7:83–104. doi: 10.2174/138920006774832596. [DOI] [PubMed] [Google Scholar]