Abstract
Irradiation interrupts spermatogenesis and causes prolonged sterility in male mammals. Hormonal suppression treatment with gonadotropin-releasing hormone (GnRH) analogues has restored spermatogenesis in irradiated rats, but similar attempts were unsuccessful in irradiated mice, monkeys, and humans. In this study, we tested a stronger hormonal suppression regimen (the GnRH antagonist, acyline, and plus flutamide) for efficacy both in restoring endogenous spermatogenesis and in enhancing colonization of transplanted stem spermatogonia in mouse testes irradiated with a total doses between 10.5 and 13.5 Gy. A 4-week hormonal suppression treatment, given immediately after irradiation, increased endogenous spermatogenic recovery 1.5-fold, and 11-week hormonal suppression produced twofold increases compared with sham-treated irradiated controls. Furthermore, 10-week hormonal suppression restored fertility from endogenous surviving spermatogonial stem cells in 90% of 10.5-Gy irradiated mice, whereas only 10% were fertile without hormonal suppression. Four- and 11-week hormonal suppression also enhanced spermatogenic development from transplanted stem spermatogonia in irradiated recipient mice, by 3.1- and 4.8-fold, respectively, compared with those not given hormonal treatment. Moreover, the 10-week hormonal suppression regimen, but not a sham treatment, restored fertility of some 13.5-Gy irradiated recipient mice from donor-derived spermatogonial stem cells. This is the first report of hormonal suppression inducing recovery of endogenous spermatogenesis and fertility in a mouse model treated with anticancer agents. The combination of spermatogonial transplantation with hormonal suppression should be investigated as a treatment to restore fertility in young men after cytotoxic cancer therapy.
Keywords: irradiation, spermatogenesis, spermatogonial transplantation, fertility, hormonal suppression, mice
Radiation and chemotherapy, as testicular toxicants, can lead to temporary or permanent sterility in mammals. Indeed, cancer therapy has induced prolonged or permanent azoospermia in many thousands of men (Meistrich et al., 2005). The continued increase in long-term survival and cure following cancer treatment makes the preservation and restoration of reproductive function of increasing importance (Meistrich et al., 2005).
The prolonged depletion of mature germ cells by radiation or chemotherapy is generally believed to be because of the killing of stem spermatogonia. Although a small number of surviving stem spermatogonia could regenerate spermatogenesis, it usually takes long times for spontaneous recovery to the level required for fertility (Meistrich et al., 1978; Pryzant et al., 1993).
Although testosterone is necessary for normal sperm production, it appears to be associated with the failure of recovery of spermatogenesis from surviving stem cells in some pathological situations (Meistrich and Shetty, 2003, Review). Consequently, transient hormonal suppression has been employed in attempts to protect the testis and/or stimulate recovery of spermatogenesis following radiation or chemotherapy-induced germinal damage (Meistrich et al., 2005). It has been demonstrated repeatedly in rats that the suppression of intratesticular testosterone levels induced by treatment with steroids or gonadotropin-releasing hormone (GnRH) analogues protects against prolonged damage to spermatogenesis if given before radiation or chemotherapy or stimulates recovery if given after the cytotoxic damage; as a consequence, subsequent fertility is increased (Meistrich and Kangasniemi, 1997; Meistrich et al., 2001; Udagawa et al., 2001). Suppression of testosterone has also been shown to enhance the recovery of rat spermatogenesis after damage induced by numerous environmental male reproductive toxicants (Meistrich and Shetty, 2003, Review).
However, the results differ between species (Shetty et al., forthcoming). Although the treatments improve fertility in rats, previous attempts using hormonal suppression to protect or simulate recovery of spermatogenesis in men (Meistrich and Shetty, 2008, Review) and primate model systems (Boekelheide et al., 2005; Kamischke et al., 2003) treated with irradiation and/or cytotoxic drugs have been unsuccessful, with the exception of one report in humans (Masala et al., 1997). In mice, pretreatment reductions of gonadotropins with GnRH analogues or genetic mutations also failed to protect against the radiation- or chemotherapy-induced disruption of spermatogenesis (Crawford et al., 1998; da Cunha et al., 1987; Kangasniemi et al., 1996a; Nonomura et al., 1991), and no study has been performed to examine the stimulation of recovery by posttreatment hormonal suppression.
The studies in rats have also shown that after cytotoxic exposure, a significant population of surviving stem spermatogonia are blocked in their differentiation (Kangasniemi et al., 1996b; Meistrich and Shetty, 2003). But in human (Kreuser et al., 1989) or monkey (Boekelheide et al., 2005; van Alphen et al., 1988) testis, such a radiation or chemotherapy-induced block in spermatogonial differentiation is only transient or rare. In mice, the spermatogonia that survive irradiation actively proliferate to produce colonies containing differentiating cells, and very few of the atrophic tubules contain undifferentiated spermatogonia (Kangasniemi et al., 1996a). Because the pathophysiological profile in the irradiated mouse testis is more similar to primates than is that of the rat, stimulation of spermatogenic recovery in the mouse by hormonal suppression may be a more appropriate model than rat for future applications to human.
To overcome the loss of stem spermatogonia resulting from cytotoxic therapies, spermatogonial transplantation may also be used to supplement this cell population. When donor stem spermatogonia are introduced into germ cell–depleted seminiferous tubules of host testes, they are able to colonize and undergo complete spermatogenesis. Furthermore, hormonal suppression significantly enhanced spermatogenesis from transplanted spermatogonia in recipient rat testes treated with irradiation (Zhang et al., 2007) or busulfan (Ogawa et al., 1999) and in recipient mouse testes (Dobrinski et al., 2001; Kanatsu-Shinohara et al., 2004; Ogawa et al., 1998; Ohmura et al., 2003). Although hormonal suppression's ability to improve the success of spermatogonial transplantation was dramatic in rat testes, the effects in mice were only moderate and variable from different studies and seemed to be strongly associated with the timing of treatment.
We hypothesized that a more effective hormonal suppression regimen, such as prolonged suppression using both a GnRH antagonist (GnRH-ant), which is more effective than GnRH agonists, and an antiandrogen can efficiently stimulate spermatogenesis from transplanted spermatogonia in mice. Moreover, we examined whether this treatment regimen could also promote the recovery of endogenous spermatogenesis and fertility in irradiated mice.
MATERIALS AND METHODS
Animals.
Adult C57BL/6Law male mice at 8–12 weeks of age, bred at The University of Texas, M. D. Anderson Cancer Center, were used in irradiation experiments and as transplantation recipients. Donor mice were obtained by breeding C57BL/6-Tg(CAG-EGFP)1Osb/J mice ubiquitously expressing green fluorescent protein (GFP) (Jackson Laboratory, Bar Harbor, ME) with C57BL/6Law mice. The animals were maintained on a 12-h light 12-h dark cycle and were allowed food and water ad libitum. All animal procedures were approved by The University of Texas M. D. Anderson Cancer Center Animal Care and Use Committee.
Experimental design.
Four experiments were conducted as outlined in Figure 1. The radiation doses and timing of assays used were based on earlier studies in which recovery of spermatogenesis in mice was measured (Meistrich et al., 1978). Total doses of 9–12 Gy resulted in gradual recoveries of sperm counts over the course of 45 weeks, with the mice regaining fertility at about 28 weeks after 9 Gy and failing to recover after 12 Gy. The durations of hormone-suppressive treatments were based on studies in rats, which showed that 4 weeks of GnRH-ant treatment, given after irradiation, with or without flutamide, was able to stimulate spermatogenic recovery (Shetty et al., 2000), 10 weeks of GnRH-ant treatment was able to stimulate both recovery of spermatogenesis and fertility (Meistrich et al., 2001), and that 13 weeks of suppression stimulated differentiation of transplanted spermatogonia (Zhang et al., 2007). In experiment (Exp.) 1, we examined effects of hormonal suppression regimens with GnRH-ant given for different time periods on spermatogenic recovery in mice treated with three different irradiation doses. In Exp. 2, we determined the effect of hormonal suppression on differentiation of endogenous stem cells and colonization of transplanted stem cells in the same irradiated mice with two different irradiation doses. In Exp. 3, we further examined whether hormonal suppression was able to restore fertility by improving recovery of endogenous spermatogenesis after a total dose of 10.5 Gy, the irradiation dose that demonstrated favorable response to hormonal suppression treatment in Exp. 1. In Exp. 4, we used a higher dose of irradiation (13.5 Gy) to destroy nearly all the endogenous spermatogenesis and primarily examined whether hormonal suppression could enhance donor cell colonization and donor-derived spermatogenesis and thereby restore fertility.
FIG. 1.
Schematics of the four experimental protocols used. Mice were irradiated at week 0 with total doses as indicated. Hormonal suppression treatment was started immediately after irradiation and continued for 4, 10, or 11 weeks. In Exps 2 and 4, transplantation was performed at 3 weeks after irradiation.
Irradiation.
Mice were restrained in plastic chambers and then placed into a metal shield module with a 3-cm diameter hole, so that only the lower abdominal and scrotal area of the animal was irradiated by a 137Cs gamma-ray unit. The radiation was delivered as an initial 1.5-Gy dose and followed by a variable second dose given 24 h later as indicated in Figure 1. The fractionated radiation regimen has been shown to be more effective than a single dose in depleting germ cells and produce less unwanted adverse effects (Creemers et al., 2002). The radiation doses are presented as the total dose of the two fractions throughout the text. Doses were chosen based on the recoveries of spermatogenesis and fertility after different single doses of irradiation (Meistrich et al., 1978).
Hormonal suppression treatment.
Hormonal suppression treatments were initiated immediately after irradiation and maintained for 4, 10, or 11 weeks in different experiments, as indicated in Figure 1. The GnRH-ant, acyline (obtained from the Contraceptive Development Branch of National Institute of Child Health and Human Development, North Bethesda, MD), was prepared in sterile water and sc injected at an initial dose of 20 mg/kg body weight and followed by maintenance doses of 10 mg/kg body weight given every other week. For Exps 3 and 4, in which fertility tests were performed, a lower dose of 6 mg acyline/kg body weight was given in the last injection at week 8 to allow quicker recovery of hormonal levels. Flutamide, an androgen receptor antagonist, was delivered by implanting two 2-cm Silastic brand silicone capsules filled with the drug. We used two 2-cm length flutamide capsules based on our previous experiments that a total length of 4-cm flutamide is effective in suppressing the testosterone action on the normal testis (Shetty et al., 2006b). The effect was similar to that observed previously with pellets releasing 1.2 mg of flutamide/day (Kangasniemi et al., 1996a). The flutamide capsules were implanted right after completion of irradiation (within 30 min) and were removed after 4, 10, or 11 weeks for 4-week, 10-week, or 11-week treatment groups, respectively. The controls were sham treated by injection of sterile water and implantation of empty capsules. In Exp. 1, the flutamide implants were found lost because of the sealing staples not being fastened well after implantation and were removed from the housing cages within the first few days. We thus considered the hormonal-suppressive treatment to be GnRH-ant only in that study.
Transplantation.
Immature heterozygous GFP mice at 14–17 days of age were used as donors, except for 12-Gy group in Exp. 2, in which 19- to 27-day-old mice were used. The stem cell spermatogonia donor cells were prepared as previously reported (Zhang et al., 2006). Briefly, after the tunica was removed, testicular tissue was sequentially digested, first with 0.05% type IV collagenase for 20 min and then with combined 0.05% type IV collagenase and 0.05% hyaluronidase for 20 min in modified Dulbecco's Modified Eagle Medium (DMEM)/F12 solution containing 100 μg/ml DNase at 35°C in a shaking water bath. The tubules were washed in Dulbecco's PBS (GIBCO, Carlsbad, CA) and then incubated in 0.1% trypsin in D-PBS containing magnesium, 100 μg DNase/ml, and 1mM ethylene glycol tetraacetic acid. After neutralization of trypsin with serum and filtration through a 35-μm nylon screen, the cell suspensions were centrifuged and resuspended in DMEM/F12 solution containing 100 μg DNase/ml. Trypan blue (Invitrogen, Grand Island, NY) was added to a final concentration of 0.04%, and the cell suspension was kept on ice until transplantation. The average of viability of cells was 93%.
Mice irradiated with 12 or 13.5 Gy, as indicated in Figure 1, were used as recipients for spermatogonial transplantation 3 weeks after irradiation. The lower abdomen was opened, and the testis was withdrawn from the body cavity. The efferent duct was identified, and surrounding fat was dissected away under a microscope. The donor cells were transplanted into seminiferous tubules through efferent duct injection using a glass micropipette controlled by a FemtoJet microinjector (Brinkmann Instruments Inc., Westbury, NY). The average injection volume was 8.2 μl, and an average of 3.1 × 105 cells per testis was injected. The success of the injection was monitored by observing the distribution of the Trypan blue dye. In Exp. 4, the transplantation control groups were injected with media instead of cells.
Evaluation of spermatogenesis.
The mice were euthanized at different times after irradiation as indicated in Figure 1. The weights of the body, testis, and seminal vesicle (SV) were recorded in all experiments, and in Exps 3 and 4, the epididymis weights were also recorded.
For histological evaluation of endogenous spermatogenic recovery, testes were fixed in Bouin's solution (both testes in Exp. 1, left testis in Exp. 3, and in sham-transplanted groups of Exp. 4), embedded in paraffin, and sectioned at 5-μm thickness. The testicular cross sections were stained with hematoxylin and periodic acid-Schiff reagent and then examined under light microscopy. Spermatogenic recovery was evaluated as previously described (Shetty et al., 2001) by the tubule differentiation index (TDI), which is defined as the percentage of tubules that contain three or more differentiating germ cells at the B spermatogonial stage or beyond.
For evaluation of spermatogenic recovery in the recipient testes (Exp. 2 and transplanted groups of Exp. 4), both testes were removed and fixed in 4% paraformaldehyde at 4°C for up to 24 h and embedded in paraffin. After deparaffinization and rehydration, the testicular sections were subjected to antigen retrieval and nonspecific antibody-binding blocking. The sections were then incubated with rabbit monoclonal anti-GFP (Cell Signaling, Danvers, MA) at 1:300 dilution at 4°C overnight, followed by a biotinylated anti-rabbit immunoglobulin G and an avidin-biotin-peroxidase complex reagent (Vectastain Elite kit, Vector Laboratories, Burlingame, CA). The immunoreactivity was visualized by incubation with the peroxidase substrate diaminobenzidine (Vector Laboratories). The slides were counterstained with hematoxylin.
The progress of spermatogenesis in the recipient testes was evaluated by the TDI as described above. GFP staining was used to differentiate whether the germ cells originated from the donor cells (GFP positive) or from the endogenous spermatogonia (GFP negative). The TDI for donor cells was corrected for injection of different cell numbers by normalization to the average cell numbers injected in each experiment.
The lengths of the donor cell colonies were measured in the 12-Gy study of Exp. 2. After removal of the tunica from one testis of three or four mice from each treatment group, the seminiferous tubules were separated using a fine forceps under a dissecting microscope. GFP-positive colonies were identified and imaged under a fluorescence microscope. The length of the colonies was measured by using the image processing software Axiovision version 4.6 (Carl Zeiss Microimaging, Inc., Göttingen, Germany).
To assess the recovery of sperm production, sperm heads were counted in the testes and sperm were counted in the cauda epididymis. The tunica albuginea was removed from one testis of a mouse, and the testis was weighed, homogenized, and sonicated. The sperm heads were counted in a hemacytometer (Meistrich and van Beek, 1993). For epididymal sperm counts, both cauda epididymis were minced in 1 ml PBS and incubated at 37°C for 30 min, and the suspension was passed though a 80-μm pore size metal filter. Sperm were counted using a hemacytometer.
Serum testosterone measurement.
Blood was collected from the axillary vein of mice under anesthesia at euthanasia. The serum was separated by centrifugation and stored at −20°C until measurement of testosterone. Serum testosterones were determined as described earlier (Shetty et al., 2000) by using a coated tube radioimmunoassay kit (DSL-4000, Diagnostic Systems Laboratories, Webster, TX).
Fertility test.
The fertility of mice was tested starting at 11 weeks after irradiation. Each male was housed with two ND4 Swiss Webster virgin females (Harlan Laboratories, Indianapolis, IN) until the time assigned for euthanasia of the males. The recovery of fertility for each mouse was defined as the date of conception of first litter, 20 days prior to the birth date. The number and size of litters were recorded. A group of unirradiated adult male mice were used as positive controls for the fertility test. The pups from the transplanted mice were examined for the expression of the GFP transgene to determine if the return of fertility was from endogenous stem spermatogonia or from donor cells. Because GFP heterozygous donors were used, we expected that half of pups would be GFP positive if the sperm were derived from donor stem cells.
Statistical analysis.
Simple comparisons of tissue weights, sperm counts, and TDI among groups were performed using two sample t-tests (for two group comparison) or ANOVA with Student-Newman-Keuls post hoc pairwise comparisons (for three or more groups). Statistical analysis of testicular sperm count data was performed on log-transformed data because the transformed distributions are closer to normal. In a few cases that the TDI data were not normally distributed, nonparametric analyses were performed, as indicated in the figure legends, using the Wilcoxon-Mann-Whitney test for two group comparisons and the Kruskal Wallis test for three or more group comparisons. Time to fertility recovery was analyzed by the Kaplan-Meier estimator. Difference between treatment groups was considered significant when p < 0.05. These statistical analyses were performed using the SPSS version 16 statistical software package (SPSS Inc., Chicago, IL).
We used linear mixed models (Verbeke and Molenberghs, 2000) to examine the effects of hormone suppression treatment, source of stem cells, radiation level, stem cell transplantation, and sacrifice time on the TDI. For this analysis, the TDI of the hormonal-suppressed groups was normalized against the no-hormone–treated controls by dividing all their TDI values by the mean TDI of the mice that received no hormonal suppression with the same combination of radiation dose, source of stem cells, and sacrifice time point, and is referred to as the TDI ratio. To assess whether the effect of 4-week hormonal suppression differs from that of 11-week hormonal suppression and whether the differences between the two treatment regimens are dependent on the source of stem cells, we fitted the linear mixed model on all data points from Exps 1 and 2. The model included fixed effects of hormone suppression (11 weeks vs. 4 weeks), source of stem cells (endogenous vs. donor), the interaction between hormone suppression and source of stem cell, transplantation, and radiation nested within transplantation, and a random effect of mice (Model 1). To assess whether the effect of 10 weeks of hormone suppression on endogenous TDI irradiated with 10.5 Gy varies over time, we fitted a linear model with a fixed effect of sacrifice time (four levels: 16, 21, 31, and 46 weeks) on all the data points from Exp. 3 (Model 2). Finally, to assess whether the effect of 10-week hormone suppression on TDI is greater toward donor cells than endogenous cells and whether the effect is time dependent, we fitted a linear mixed model on the data points from the mice receiving stem cell transplantation from Exp. 4, with fixed effects of source of stem cells (endogenous vs. donor), sacrifice time (five levels: 11, 16, 21, 31, and 46 weeks), the interaction between source of stem cell and sacrifice time, and a random effect of animal (Model 3). All linear models were performed in SAS v 9.1 (SAS Institute, Cary, NC).
RESULTS
Hormonal Suppression (Exp. 1 and Exp. 2)
In Exp. 1, mice were irradiated with total radiation doses of 10.5, 11.5, or 12.5 Gy followed by 4- or 11-week treatments with GnRH-ant (acyline) and euthanized at 11 weeks after irradiation. Body weights were slightly, but reversibly, reduced in the mice under hormonal suppression treatment (Table 1). There was a marked decrease in testis weights, SV weights, and serum testosterone concentration with the 11-week treatment. The reduction in SV weight with 11-week treatment clearly confirms that testosterone activity was suppressed during the treatment.
TABLE 1.
Effects of Hormone Suppression on Weights and Testosterone Levels
| Experiment and irradiation dose | Hormonal suppression | Analysis time (weeks after irradiation) | Body weight (g) | Testis weight (mg) | SV weight (mg) | Serum testosterone concentration (ng/ml) |
| Exp. 1 | ||||||
| 10.5 Gy | Sham | 11 | 29 ± 1a | 25 ± 1a | 300 ± 12a | 2.8 ± 0.8a |
| 11.5 Gy | 4 weeks GnRH-ant | 11 | 27 ± 1a | 24 ± 1a | 260 ± 7b | 3.2 ± 0.9a |
| 12.5 Gy | 11 weeks GnRH-ant | 11 | 25 ± 1b | 14 ± 1b | 22 ± 2c | 0.5 ± 0.1b |
| Exp. 2 | Sham | 11 | 28 ± 0d | 29 ± 1d | 256 ± 12d | 1.2 ± 0.3d |
| 12 Gy | 4 weeks GnRH-ant + flutamide | 4 | 25 ± 0e | 14 ± 0e | 21 ± 3e | 2 ± 0.9d |
| 13.5 Gy | 4 weeks GnRH-ant + flutamide | 11 | 27 ± 0d | 27 ± 1d | 241 ± 7d | 0.9 ± 0.2d |
| 11 weeks GnRH-ant + flutamide | 11 | 25 ± 0e | 11 ± 0f | 10 ± 1e | 0.1 ± 0.0e |
Note. The data of three irradiation doses from Exp. 1 were pooled because ANOVA analysis showed no significant difference among them. The data from the two irradiation doses from Exp. 2 were pooled because t-test analysis showed no significant difference between them except for the SV weights of 11-week treatments (9 ± 0 mg at 12 Gy vs. 13 ± 2 mg at 13.5 Gy), both of which were dramatically lower than those in control mice. N was between 9 and 29 for weights and between 5 and 12 for serum testosterone concentrations. For a given parameter, values that are significantly different from each other (p < 0.05) within each experiment are indicated by different letters.
In a subsequent experiment (Exp. 2), mice were irradiated with total radiation doses of 12 or 13.5 Gy followed by 4- or 11-week treatments with GnRH-ant (acyline) plus antiandrogen and spermatogonial transplantation. Alterations of body and tissue weights were similar to those in Exp. 1. In addition, in a group of mice euthanized at the end of the 4-week treatment time, both testis and SV weights were reduced (Table 1). Although the concentration of serum testosterone was not suppressed after 4-week treatment, the action of testosterone was clearly reduced in those mice at 4 weeks because the SV weights were significantly decreased. The reduced SV weights in the absence of serum testosterone level reductions at 4-week treatment is likely because of the effect of antiandrogen flutamide that blocks the action of testosterone. Note that the tissue weights in these mice returned to nearly the levels of control mice at 11 weeks, suggesting that the suppression of testosterone was reversible after the treatment ceased. With 11-week treatment, both serum testosterone levels and SV weights were significantly reduced, showing that both testosterone level and action were effectively suppressed in those animals.
Effect of Hormonal Suppression on Recovery of Endogenous Spermatogenesis (Exp. 1 and Exp. 2)
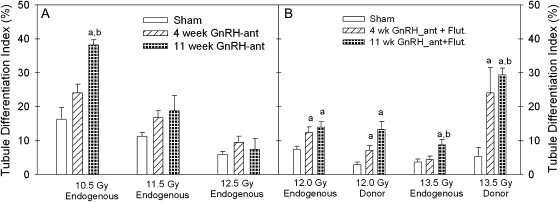
The hormonal suppression treatment stimulated the recovery of endogenous spermatogenesis. In mice irradiated with 10.5 Gy, there was a significant increase in the percentages of tubules with differentiated germ cells after 11-week hormonal suppression, as compared with sham-treated mice (Fig. 2A). Four-week treatment also showed a consistent trend of an increase compared with controls, but the effects were generally less than those with the 11-week treatment. Similar results were observed with the recovery of endogenous spermatogenesis in mice irradiated with 12 and 13.5 Gy and then also subjected to spermatogonial transplantation, with significant increases in recovery after the 11-week hormonal suppression and a significant increase of spermatogenic recovery with the 4-week treatment in mice irradiated with 12 Gy (Fig. 2B).
FIG. 2.
Hormonal suppression improved spermatogenic recovery from both endogenous and donor-derived stem spermatogonia in irradiated mouse testis in Exp. 1 (A) and Exp. 2 (B). The TDI is the percentage of tubule cross sections with more than three differentiated germ cells. “Endogenous” indicates the differentiated germ cells that were derived from surviving stem spermatogonia of the recipient. “Donor” indicates the differentiated germ cells that were derived from GFP-positive transplanted stem spermatogonia. The statistical analyses of 12- and 13.5-Gy donor TDI data in panel (B) were conducted by a nonparametric method because the data are not normally distributed. N = 3–4 (panel A) and N = 4–18 (panel B). “a” and “b”, p < 0.05 versus the sham- or 4-week–treated groups, respectively.
The results from Model 1 revealed that there were no significant effects of transplantation (p = 0.61) or radiation dose (nested within transplantation) (p = 0.43), though the stimulatory effect of hormone suppression was marginally lower in Exp. 1, in which the flutamide capsules were lost, than in Exp. 2. The 4-week hormone suppression treatment appeared to increase the TDI of endogenous cells over that observed with no hormone suppression by 1.5-fold (p = 0.10), whereas the 11-week treatment significantly increased the TDI by twofold (p = 0.025). Although the overall effect of the 11-week hormone suppression appeared greater than that of the 4-week treatment, the difference was not statistically significant (p = 0.25).
Effect of Hormonal Suppression on Colony Development from Transplanted Stem Spermatogonia (Exp. 2)
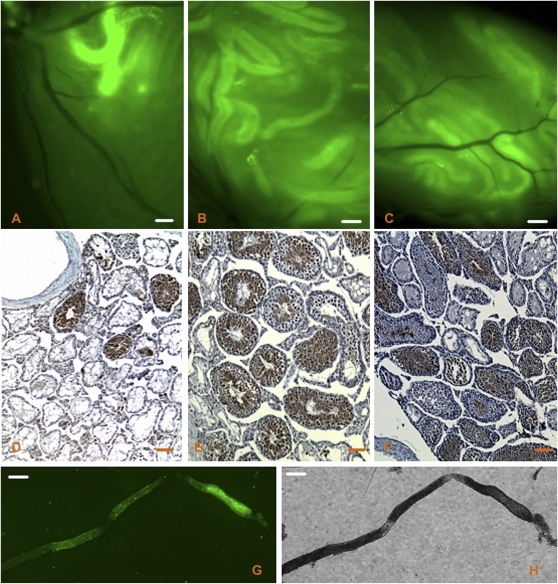
Gross examination of recipient testes under fluorescence microscopy showed that transplanted spermatogonia successfully colonized the testes in all treatment groups (Figs. 3A–C). Immunohistochemical staining for GFP was then performed on tissue sections (Figs. 3D–F) to determine which tubules were repopulated with spermatogenic cells derived from donor versus endogenous stem cells. We noted that the overall efficiency of donor-derived stem cell colonization was lower in the 12-Gy study than that in the 13.5-Gy study (Fig. 2B). This discrepancy is attributable to the older age of the donor animals used in the 12-Gy study as previous observations showed that the efficiency of colonization by cells from 28-day-old donors was only half that of cells from 12-day-old donors (McLean et al., 2003).
FIG. 3.
Evaluation of the recipient testes. GFP-expressing donor cell colonization was visualized at 11 weeks after transplantation by fluorescence microscopy in irradiated recipient testes that were sham treated (A) or given 4-week GnRH-ant plus flutamide (B) or 11-week GnRH-ant plus flutamide (C) treatments. Immunohistochemical staining for GFP in fixed tissue cross sections from (D) sham-treated mice, (E) 4-week GnRH-ant plus flutamide treatment, or (F) 11-week GnRH-ant plus flutamide treatment was performed to distinguish tubules with donor-derived spermatogenesis (brown) from those with endogenous spermatogenesis (blue). Note that there is some nonspecific staining of cytoplasm of the late spermatids. GFP-positive tubules were separated from recipient testis and imaged by fluorescence (G) for measurement of colony length or by bright field (H) to clearly show the tubule. Bars, 200 μm (A, B, C, G, and H) and 100 μm (D, E, and F).
When testosterone was suppressed for either 4 or 11 weeks in the irradiated recipient mouse testes, the recovery of spermatogenesis from transplanted stem spermatogonia was consistently improved in two separate studies with different doses of irradiation (Fig. 2B). The results from Model 1 showed that the 4-week hormonal suppression treatment significantly (p < 0.0001) increased the TDI of donor cells over that observed with no hormonal suppression by 3.1-fold, whereas the 11-week treatment significantly (p < 0.0001) increased the TDI of donor cells by 4.8-fold. Furthermore, the increase in TDI with 11-week therapy was greater than that with the 4-week treatment (p = 0.002). The effects of hormonal suppression appeared to be greater toward donor than toward endogenous stem cells (p for interaction term = 0.07), and the fold increases in TDI with both 4- and 11-week hormonal suppressions were greater toward the donor than toward the endogenous cells (p = 0.002 and p < 0.0001 for 4- and 11-week hormonal suppression, respectively).
Because the relationship between the TDI, which is the percentage of tubule cross sections with differentiating germ cells, and the actual number of developing donor germ cell colonies is dependent on the length of those colonies, we measured the colony lengths (Fig. 3G) after the different hormonal treatments. The length of donor cell colonies (12-Gy dose, Exp. 2) was 512 ± 12 μm (n = 9 colonies from three testes) in sham-treated mice, 498 ± 14 μm (n = 36 from four testes) in mice receiving 4-week hormonal suppression, and 464 ± 34 μm (n = 26 from four testes) in mice receiving 11-week hormonal suppression. These values were not significantly different from each other. These results demonstrate that the TDI values were proportional to numbers of colonies and that the higher TDI in the 11-week hormonal suppression group was actually because of increased colony numbers.
Effect of Hormonal Suppression on Regeneration of Reproductive Tissues and Spermatogenesis (Exp. 3 and Exp. 4)
Because hormonal suppression was able to stimulate the initiation of spermatogenesis from both endogenous and donor-derived stem spermatogonia, we tested whether the spermatogenic recovery was maintained after treatment and whether spermatozoa were produced.
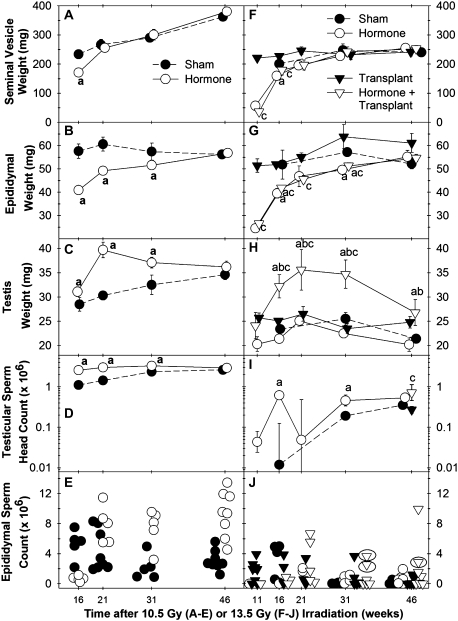
We first examined the time course of the recovery of the androgen-dependent reproductive accessory organs after either 10.5-Gy irradiation (Exp. 3) or 13.5-Gy irradiation (Exp. 4). Because androgen is required for the development of spermatozoa and mating behavior, we monitored the reversibility of testosterone suppression by SV weights. In the mice receiving GnRH-ant (acyline) and flutamide for the first 10 weeks after irradiation, the SV weights remained low at 16 weeks but returned to levels observed in the non–hormone-suppressed mice by 21 weeks (Figs. 4A and 4F). Note that the epididymal weights, which were reduced primarily by hormonal suppression, although sperm numbers may also have some effect, took between 31 and 46 weeks to recover to the levels observed in the irradiated mice that did not receive hormonal suppression (Figs. 4B and 4G).
FIG. 4.
Time course of accessory sexual organ or spermatogenic recovery in mice irradiated with 10.5 Gy (A–E) from Exp. 3 or 13.5 Gy (F–J) from Exp. 4, as measured by SV weight (A and F), epididymal weight (B and G), testis weight (C and H), testicular sperm head count (note log scale) (D and I), and cauda epididymal sperm count (E and J). Groups are designated as Sham, Hormone (GnRH-ant + flutamide only, no transplantation), Transplant (transplantation only), Hormone + Transplant (GnRH + flutamide and spermatogonial transplantation). N = 10 for 46-week time point data and 5 for all other time points for both irradiation doses. “a,” “b,” and “c”, p < 0.05 versus values in the sham group, hormone group, and transplant group, respectively. Ellipses in (K) identify those mice that were fertile.
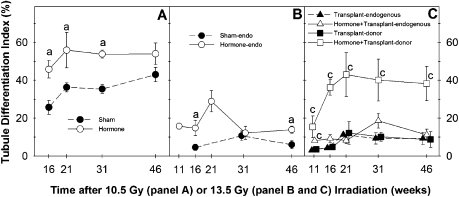
Spermatogenesis gradually recovered in 10.5-Gy irradiated testes not receiving hormonal suppression treatment, as indicated by gradual increases in testis weights, testicular sperm head counts, and TDIs (Figs. 4C and 4D and 5A). The suppression of testosterone significantly improved the progress of spermatogenic recovery, as shown by the significant increases of testis weights (Fig. 4C), testicular sperm head count (Fig. 4D), and TDI (Fig. 5A) at the 16-, 21-, and 31-week postirradiation time points. Model 2 showed that the TDI ratio was overall significantly greater than 1 (p < 0.0001); it appeared to be highest at early time points (week 16, 1.78-fold) and showed a downward trend (p = 0.08) with time. The cauda epididymal sperm counts were lower in the hormone-suppressed mice than in controls at week 16 as a result of the residual effect of the hormonal suppression. However, they were significantly increased in the previously hormone-suppressed group by week 21 and were significantly higher than those in the sham-treated controls on weeks 31 and 46 (Fig. 4E). However, it should be noted that epididymal sperm counts are more variable than testicular sperm head counts, can be affected by sperm storage or hormone levels because of changes in transport rate, and show an attenuated response to changes after irradiation (Meistrich and Samuels, 1985).
FIG. 5.
TDIs in testes of mice irradiated with 10.5 Gy (A) from Exp. 3 or 13.5 Gy (B, endogenous and C, transplanted) from Exp. 4, with and without hormonal suppression. Furthermore, in the transplanted testes, the TDI of the endogenous cells and donor cells were scored separately. N = 10 for 46-week time point group and 5 for all other time point groups for both irradiation doses. The statistical analysis of 46-week TDI data in panel (B) was conducted by a nonparametric method because the data are not normally distributed. “a” and “c”, p < 0.05 versus values in the sham group and transplant-only group, respectively.
We then examined the time course of restoration of spermatogenesis in mice irradiated with a higher dose (13.5 Gy) to deplete endogenous spermatogenesis and subjected to the hormonal suppression treatment and/or germ cell transplantation (Exp. 4); four treatment groups: sham, hormonal suppression only, transplantation only, and the combination of hormonal suppression and transplantation were analyzed. In 13.5-Gy irradiated testes in which only sham transplantation was performed, hormonal suppression improved the spermatogenic recovery from endogenous spermatogonia, as indicated by elevated sperm head count in the testis (Fig. 4I) and increased endogenous TDIs (Figs. 5B and C) at nearly all time points, with the differences being statistically significant at several of these time points.
Transplantation of germ cells alone, without hormonal suppression, resulted in the formation of donor colonies in up to 10% of the tubules at the 21-, 31-, and 46-week time points (filled squares, Fig. 5C), but these donor colonies did not produce enough cells to significantly increase testis weights (filled inverted triangles, Fig. 4H). However, in these transplanted mice, 10-week suppression of testosterone markedly enhanced recovery of spermatogenesis as measured by significant increases in testis weights during weeks 16–31 after irradiation (open inverted triangles, Figs. 4H and 4I). Testicular sperm head counts, which were only measured at week 46, were also increased (triangles, Fig. 4I). This was clearly a result of enhanced spermatogenesis from transplanted spermatogonia, compared with mice receiving transplantation but not hormonal suppression, as shown by significant increases of donor TDI (open squares, Fig. 5C) at all time points.
Model 3 showed that the 10-week hormone suppression significantly increased the TDI ratio for both donor and endogenous stem cells in this experiment and that the TDI ratio for donor stem cells was greater than for endogenous stem cells (p = 0.0003). Although effect of time of assessment was not statistically significant, the increases in donor TDI ratios were between 4.7-fold (at the 11-week time point) and approximately sevenfold (later time points).
In general, the spermatozoa numbers in cauda epididymis in all treatment groups were relatively low and variable from animal to animal (Fig. 4J). However, during the 21- to 46-week time period, most of the mice with the highest sperm counts were those treated with both hormonal suppression and germ cell transplantation.
Effect of Hormonal Suppression on Restoration of Fertility (Exp. 3 and Exp. 4)
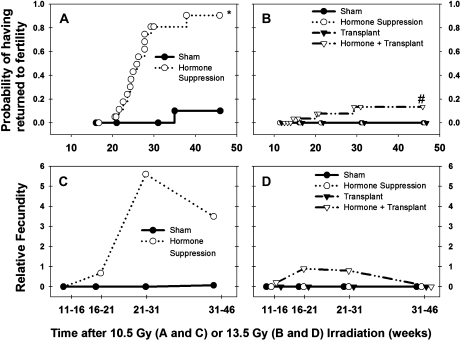
The recovery of spermatogenesis by hormonal suppression treatment observed in Exp. 3 was indeed translated into function as fertility was restored in 90% of the treated mice during 20–40 weeks after irradiation. This was significantly higher than the recovery of fertility in the mice without hormonal suppression, in which only 10% recovered (p < 0.001) and the recovery did not occur until week 37 (Fig. 6A). Although a high level of fertility was restored in the irradiated, hormonally suppressed mice, their fecundity was only at most a quarter of that of unirradiated, non-hormonally treated control mice (Fig. 6C). During the 21- to 46-week postirradiation time periods, only 83% of the treated males were fertile, litter size was 5.2 compared with 8.9 in normal controls, and the number of litters they produced per 5-week period was only half that of the controls.
FIG. 6.
Time course of fertility recovery expressed as probability of having returned to fertility for male mice irradiated with 10.5 Gy with or without hormone suppression (A) or irradiated with 13.5 Gy with or without hormone suppression or spermatogonial transplantation (B) by Kaplan-Meier survival analysis. Relative fecundity (fraction of males that were fertile × litters per fertile male per 5-week time period × average litter size) for male mice irradiated with 10.5 Gy, with or without hormone suppression (C), or irradiated with 13.5 Gy, with or without hormone suppression or spermatogonial transplantation (D). Note, for comparison, that the calculated Relative fecundity for our unirradiated, non-hormonally treated control mice was 21.4. Symbols in panels (A and B) represent times at which individual mouse became fertile or were censored (i.e., the time of euthanasia before they became fertile). *p < 0.05 compared with values of sham-treated group. #p < 0.05 compared with values of sham, hormonal suppression, or transplant groups.
Although mice irradiated with 13.5 Gy (Exp. 4), even if they were treated with either hormonal suppression or transplantation, were sterile up to 46 weeks after irradiation, combined treatment with hormonal suppression and transplantation successfully restored fertility of three mice between 15 and 30 weeks after irradiation (Fig. 6B) (p = 0.09). However, it is not clear why there was a trend of declining fecundity and also testis weights (Fig. 4H) at week 46, but not decreases in donor cell TDI (Fig. 5C). In the hormonally treated, transplanted mice, the fecundity was only 5% of controls (Fig. 6D), as at most 20% of mice were fertile during any time period; average litter sizes were only 6.9; and the frequency of litters was 40% of that in controls.
In every litter produced by the transplanted, hormonally suppressed mice, both GFP-positive and GFP-negative pups were present, and of the total of 76 pups from 11 litters, 43 were GFP positive. Because the donor animals we used were hemizygous for GFP, we expected that half of spermatozoa from donor stem spermatogonia would carry the GFP transgene. Thus, nearly all the spermatozoa in those fertile mice must have developed from transplanted stem spermatogonia.
DISCUSSION
In the current study, for the first time, we demonstrated that hormonal suppression given after irradiation successfully accelerated the recovery of spermatogenesis and significantly shortened the time to return of fertility in irradiated mice. Moreover, the suppression regimen also enhanced the efficiency of transplanted cell colonization in irradiated mouse recipient testes and resulted in the production of progeny mice derived from the donor cells.
The duration, timing, and degree of hormonal suppression all appeared to be important for successful recovery from spermatogenic injury in the irradiated mouse testis. In previous attempts to stimulate spermatogenic recovery following cytotoxic damage in mice, hormonal suppression was generally given prior to or during irradiation or chemotherapy (da Cunha et al., 1987; Kangasniemi et al., 1996a; Nonomura et al., 1991). The durations of hormonal suppression treatment were generally short, being less than 4 weeks in these studies. Two studies (da Cunha et al., 1987; Nonomura et al., 1991) used treatment with a GnRH agonist, which is not as effective as GnRH-ant at hormonal suppression (Meistrich et al., 2001). In a third study, hypogonadal mice with a null mutation in the GnRH gene were used (Crawford et al., 1998), but there was still some basal production of testosterone from the Leydig cells in these mice (Singh et al., 1995). Thus, the androgen ablation in most of the studies was incomplete. Only one of the studies employed GnRH-ant and antiandrogen to produce total androgen ablation, but the duration of treatment was only 2 weeks (Kangasniemi et al., 1996a). The success of our current treatment strategy is likely attributable to the suppression of testosterone immediately in the postirradiation period, a relatively prolonged treatment period, and perhaps the use of an antiandrogen to completely inhibit the action of the residual testosterone.
In comparison to the less than twofold stimulation of endogenous colony formation observed here in the mouse, hormonal suppression in the rat using GnRH-ant alone for as short as 4 weeks (Shetty et al., 2000) or GnRH-ant plus flutamide for 2 weeks, either prior to or after cytotoxic therapy (Kangasniemi et al., 1995; Shetty et al., 2006a), produced much greater stimulation of endogenous colony formation. Because hormonal suppression stimulates recovery of spermatogenesis in irradiated rats by reversing the block to spermatogonial differentiation (Meistrich and Shetty, 2003; Meistrich et al., 2000), this interspecies difference in stimulation appears to be related to differences in the magnitude of the differentiation block that is produced by the cytotoxic injury.
The duration, timing, and degree of hormonal suppression are also of importance in enhancing colonization of transplanted spermatogonia in recipient mouse testes. In the present study, the hormonal suppression was always started 3 weeks before transplantation. When the hormonal suppression was continued for only 1 week more after transplantation, there was an average of a 3.1-fold stimulation of the TDI (Exp. 2), and when it was continued for 7 or 8 weeks (Exp. 4 or Exp. 2, respectively) after transplantation, the TDI was increased significantly to 4.8-fold. Analysis of three previous studies using hormonal suppression with GnRH agonists (Dobrinski et al., 2001; Ogawa et al., 1998; Ohmura et al., 2003) given for 4 weeks indicates a consistent enhancement of donor spermatogenesis by about 1.9-fold. GnRH agonist suppressive treatments lasting 8–10 weeks stimulated increases in colonization by about threefold (Dobrinski et al., 2001; Kanatsu-Shinohara et al., 2004). By comparison, our hormonal suppression strategy with the GnRH-ant and antiandrogen appears to be somewhat more effective.
Several processes are needed for the development of spermatogenesis from transplanted stem spermatogonia. Homing involves moving to the basement membrane of seminiferous tubules to already existing niches or formation of new niches. Individual stem cells must then proliferate, undergoing self-renewing divisions to increase their numbers (de Rooij, 2001). Finally, the stem cells must reach a steady state of self-renewal and differentiation divisions to produce later spermatogenic cells while maintaining their populations. The use of two different hormonal suppression times and the evaluation of both endogenous and donor-derived spermatogenesis in the same animals allowed us to determine the relative effect of hormonal suppression on homing versus proliferation/differentiation.
The greater stimulation of tubule repopulation by transplanted cells, which were introduced after 3 weeks of hormonal suppression, than by endogenous stem cells must have been due primarily to an increase in the homing efficiency of the donor cells. The 3.1-fold stimulation by the 4-week hormonal suppression treatment was primarily because of enhancement of the homing step. The additional stimulation to 4.8-fold from continuing the treatment to week 11 can be attributed to effects on proliferation/differentiation. Previous observations showing that hormonal suppression prior to but not after transplantation stimulates recovery (Dobrinski et al., 2001) are consistent with the effect on the homing step but not the additional stimulation we observed with continuing the hormone suppression after transplantation.
The enhancement of the abilities of stem cells to both produce differentiated cells and maintain their numbers, independent of the homing process, by hormonal suppression must be responsible for the stimulatory effect of the 4-week suppression on the endogenous TDI and the greater effectiveness of the longer than the shorter treatment on both endogenous and donor TDI. Previously, a 1.8-fold increase in donor cell colony number was observed even when the hormonal-suppressive treatment was not started until 4 weeks after transplantation (Ohmura et al., 2003).
The molecular and cellular mechanisms by which hormonal suppression facilitates homing of transplanted stem cell spermatogonia and enhances their proliferation and/or differentiation are unclear. Because cellular alterations produced by irradiation that inhibit the differentiation of both endogenous and transplanted spermatogonia in the rat are in the somatic environment (Zhang et al., 2007), which contains the cells that have the receptors for testosterone and the gonadotropic hormones, it is most likely that hormonal suppression enhances germ cell development from spermatogonial stem cells by action on the supporting somatic environment. Recently, β1-integrin, expressed in both the stem spermatogonia and the Sertoli cells, has been identified as an essential adhesion receptor for the homing of mouse transplanted stem spermatogonia (Kanatsu-Shinohara et al., 2008). However, analysis of gene expression changes in irradiated rat testes (Zhou et al., 2010) and SCARKO mouse testes (Wang et al., 2009) (Zhou, Wang, Small, Liu, Weng, Yang, Griswold, and Meistrich, submitted) showed that neither β1-integrin nor its targets, the various laminin genes, is upregulated by hormonal suppression or abrogation of androgen action on Sertoli cells and therefore do not appear to be involved in the enhancement of homing. Further studies of the hormonal regulation of stem spermatogonial homing and proliferation/differentiation are necessary to understand this phenomenon.
The combined treatment of hormonal suppression and spermatogonial transplantation appears to be necessary for promoting recovery of spermatogenesis and fertility, especially after relatively high radiation doses. Although hormonal suppression alone restored fertility after 10.5-Gy irradiation, it did not increase endogenous spermatogenesis to the level necessary for fertility in 13.5-Gy irradiated mice. Spermatogonial transplantation supplemented the recipient testes with stem spermatogonia, providing an additional source for repopulation of the testes.
Our results demonstrated that functional spermatozoa developed after hormonal suppression and/or transplantation. The fecundity was low compared with wild-type mice, as expected because the testicular sperm counts were below or barely above the cutoff of 2 × 106 (15% of control) necessary for recovery of fertility after irradiation (Meistrich et al., 1978). This result also illustrates the interspecies difference, as in irradiated rats hormonal suppression for 10 weeks restored endogenous testicular sperm production to 86% of control and nearly completely restored fecundity (Meistrich et al., 2001).
The application of the hormonal suppression treatment and spermatogonial transplantation can be considered as treatment for men exposed to testicular toxicants. Although clinical trials of hormonal suppression alone have not been very successful, it is likely in several of these studies that the gonadal damage was so severe that there were too few stem cells to yield significant recovery (Meistrich and Shetty, 2008). Combined treatment of hormonal suppression and spermatogonial transplantation should be more promising because hormonal suppression more significantly stimulated homing of the transplanted germ cells than it did recovery of endogenous spermatogenesis. The development of methods for improvement of transplantation efficiency are most important for fertility preservation in prepubertal boys who are subjected to gonadal damage from a cytotoxic cancer treatment but are too young to be able to produce sperm for storage. The mouse may be a better model than the rat for preclinical trials because the stimulation of recovery occurs without reversal of a major block of spermatogonial differentiation as occurs in the rat.
FUNDING
National Institutes of Health (Grant numbers R01 HD-040397 and R01 ES-008075 to M.L.M.); Cancer Center Support (Grant number CA-016672 to M. D. Anderson Cancer Center).
Acknowledgments
The authors are very grateful to Mr Kuriakose Abraham for his help with the histological preparations, Dr Susan Tucker for guidance in statistical analysis, and Mr Walter Pagel for his editorial assistance.
References
- Boekelheide K, Schoenfeld H, Hall SJ, Weng CCY, Shetty G, Leith J, Harper J, Sigman M, Hess DL, Meistrich ML. Gonadotropin-releasing hormone antagonist (cetrorelix) therapy fails to protect non-human primates (macaca arctoides) from radiation-induced spermatogenic failure. J. Androl. 2005;26:222–234. doi: 10.1002/j.1939-4640.2005.tb01089.x. [DOI] [PubMed] [Google Scholar]
- Crawford BA, Spaliviero JA, Simpson JM, Handelsman DJ. Testing the gonadal regression-cytoprotection hypothesis. Cancer Res. 1998;58:5105–5109. [PubMed] [Google Scholar]
- Creemers LB, Meng X, Den Ouden K, Van Pelt AM, Izadyar F, Santoro M, Sariola H, De Rooij DG. Transplantation of germ cells from glial cell line-derived neurotrophic factor-overexpressing mice to host testes depleted of endogenous spermatogenesis by fractionated irradiation. Biol. Reprod. 2002;66:1579–1584. doi: 10.1095/biolreprod66.6.1579. [DOI] [PubMed] [Google Scholar]
- da Cunha MF, Meistrich ML, Nader S. Absence of testicular protection by a gonadotropin releasing hormone analog against cyclophosphamide-induced testicular cytotoxicity in the mouse. Cancer Res. 1987;47:1093–1097. [PubMed] [Google Scholar]
- de Rooij DG. Proliferation and differentiation of spermatogonial stem cells. Reproduction. 2001;121:347–354. doi: 10.1530/rep.0.1210347. [DOI] [PubMed] [Google Scholar]
- Dobrinski I, Ogawa T, Avarbock MR, Brinster RL. Effect of the GnRH-agonist leuprolide on colonization of recipient testes by donor spermatogonial stem cells after transplantation in mice. Tissue Cell. 2001;33:200–207. doi: 10.1054/tice.2001.0177. [DOI] [PubMed] [Google Scholar]
- Kamischke A, Kuhlmann M, Weinbauer GF, Luetjens M, Yeung C-H, Kronholz HL, Nieschlag E. Gonadal protection from radiation by GnRH antagonist or recombinant human FSH: a controlled trial in a male nonhuman primate (Macaca fascicularis) J. Endocrinol. 2003;179:183–194. doi: 10.1677/joe.0.1790183. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Morimoto T, Toyokuni S, Shinohara T. Regulation of mouse spermatogonial stem cell self-renewing division by the pituitary gland. Biol. Reprod. 2004;70:1731–1737. doi: 10.1095/biolreprod.103.025668. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Takehashi M, Takashima S, Lee J, Morimoto H, Chuma S, Raducanu A, Nakatsuji N, Fassler R, Shinohara T. Homing of mouse spermatogonial stem cells to germline niche depends on beta1-integrin. Cell Stem Cell. 2008;3:533–542. doi: 10.1016/j.stem.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Kangasniemi M, Dodge K, Pemberton AE, Huhtaniemi I, Meistrich ML. Suppression of mouse spermatogenesis by a gonadotropin-releasing hormone antagonist and antiandrogen: failure to protect against radiation-induced gonadal damage. Endocrinology. 1996a;137:949–955. doi: 10.1210/endo.137.3.8603608. [DOI] [PubMed] [Google Scholar]
- Kangasniemi M, Huhtaniemi I, Meistrich ML. Failure of spermatogenesis to recover despite the presence of A spermatogonia in the irradiated LBNF1 rat. Biol. Reprod. 1996b;54:1200–1208. doi: 10.1095/biolreprod54.6.1200. [DOI] [PubMed] [Google Scholar]
- Kangasniemi M, Wilson G, Parchuri N, Huhtaniemi I, Meistrich ML. Rapid protection of rat spermatogenic stem cells against procarbazine by treatment with a gonadotropin-releasing hormone antagonist (Nal-Glu) and an antiandrogen (flutamide) Endocrinology. 1995;136:2881–2888. doi: 10.1210/endo.136.7.7789313. [DOI] [PubMed] [Google Scholar]
- Kreuser ED, Kurrle E, Hetzel WD, Heymer B, Porzsolt R, Hautmann R, Gaus W, Schlipf U, Pfeiffer EF, Heimpel H. Reversible germ cell toxicity after aggressive chemotherapy in patients with testicular cancer: results of a prospective study. Klin. Wochenschr. 1989;67:367–378. doi: 10.1007/BF01711264. [DOI] [PubMed] [Google Scholar]
- Masala A, Faedda R, Alagna S, Satta A, Chiarelli G, Rovasio PP, Ivaldi R, Taras MS, Lai E, Bartoli E. Use of testosterone to prevent cyclophosphamide-induced azoospermia. Ann. Intern. Med. 1997;126:292–295. doi: 10.7326/0003-4819-126-4-199702150-00005. [DOI] [PubMed] [Google Scholar]
- McLean DJ, Friel PJ, Johnston DS, Griswold MD. Characterization of spermatogonial stem cell maturation and differentiation in neonatal mice. Biol. Reprod. 2003;69:2085–2091. doi: 10.1095/biolreprod.103.017020. [DOI] [PubMed] [Google Scholar]
- Meistrich M, Shetty G. Hormonal suppression for fertility preservation in males and females. Reproduction. 2008;136:691–701. doi: 10.1530/REP-08-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meistrich ML, Hunter N, Suzuki N, Trostle PK, Withers HR. Gradual regeneration of mouse testicular stem cells after ionizing radiation. Radiat. Res. 1978;74:349–362. [PubMed] [Google Scholar]
- Meistrich ML, Kangasniemi M. Hormone treatment after irradiation stimulates recovery of rat spermatogenesis from surviving spermatogonia. J. Androl. 1997;18:80–87. [PubMed] [Google Scholar]
- Meistrich ML, Samuels RC. Reduction in sperm levels after testicular irradiation of the mouse. A comparison with man. Radiat. Res. 1985;102:138–147. [PubMed] [Google Scholar]
- Meistrich ML, Shetty G. Inhibition of spermatogonial differentiation by testosterone. J. Androl. 2003;24:135–148. doi: 10.1002/j.1939-4640.2003.tb02652.x. [DOI] [PubMed] [Google Scholar]
- Meistrich ML, van Beek MEAB. Spermatogonial stem cells: assessing their survival and ability to produce differentiated cells. In: Chapin RE, Heindel J, editors. Methods in Toxicology. Vol. 3A. New York: Academic Press; 1993. pp. 106–123. [Google Scholar]
- Meistrich ML, Vassilopoulou-Sellin R, Lipshultz LI. Gonadal dysfunction. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 2560–2574. [Google Scholar]
- Meistrich ML, Wilson G, Kangasniemi M, Huhtaniemi I. Mechanism of protection of rat spermatogenesis by hormonal pretreatment: stimulation of spermatogonial differentiation after irradiation. J. Androl. 2000;21:464–469. [PubMed] [Google Scholar]
- Meistrich ML, Wilson G, Shuttlesworth G, Huhtaniemi I, Reissmann T. GnRH agonists and antagonists stimulate recovery of fertility in irradiated LBNF1 rats. J. Androl. 2001;22:809–817. [PubMed] [Google Scholar]
- Nonomura M, Okada K, Hida S, Yoshida O. Does a gonadotropin-releasing hormone analogue prevent cisplatin-induced spermatogenic impairment? An experimental study in the mouse. Urol. Res. 1991;19:135–140. doi: 10.1007/BF00368192. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Leuprolide, a gonadotropin-releasing hormone agonist, enhances colonization after spermatogonial transplantation into mouse testes. Tissue Cell. 1998;30:583–588. doi: 10.1016/s0040-8166(98)80039-6. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Dobrinski I, Brinster RL. Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell. 1999;31:461–472. doi: 10.1054/tice.1999.0060. [DOI] [PubMed] [Google Scholar]
- Ohmura M, Ogawa T, Ono M, Dezawa M, Hosaka M, Kubota Y, Sawada H. Increment of murine spermatogonial cell number by gonadotropin-releasing hormone analogue is independent of stem cell factor c-kit signal. Biol. Reprod. 2003;68:2304–2313. doi: 10.1095/biolreprod.102.013276. [DOI] [PubMed] [Google Scholar]
- Pryzant RM, Meistrich ML, Wilson E, Brown B, McLaughlin P. Long-term reduction in sperm count after chemotherapy with and without radiation therapy for non-Hodgkin's lymphomas. J. Clin. Oncol. 1993;11:239–247. doi: 10.1200/JCO.1993.11.2.239. [DOI] [PubMed] [Google Scholar]
- Shetty G, Wang G, Meistrich ML. Regenerative potential of spermatogenesis after gonadotoxic therapies. In: Orwig KE, Hermann BP, editors. Male Germline Stem Cells: Developmental and Regenerative Potential (Forthcoming) Springer/Humana Press, New York. [Google Scholar]
- Shetty G, Weng CC, Meachem SJ, Bolden-Tiller OU, Zhang Z, Pakarinen P, Huhtaniemi I, Meistrich ML. Both testosterone and FSH independently inhibit spermatogonial differentiation in irradiated rats. Endocrinology. 2006a;147:472–482. doi: 10.1210/en.2005-0984. [DOI] [PubMed] [Google Scholar]
- Shetty G, Weng CC, Porter KL, Zhang Z, Pakarinen P, Kumar TR, Meistrich ML. Spermatogonial differentiation in juvenile spermatogonial depletion (jsd) mice with androgen receptor or follicle stimulating hormone mutations. Endocrinology. 2006b;147:3563–3570. doi: 10.1210/en.2006-0159. [DOI] [PubMed] [Google Scholar]
- Shetty G, Wilson G, Huhtaniemi I, Boettger-Tong H, Meistrich ML. Testosterone inhibits spermatogonial differentiation in juvenile spermatogonial depletion mice. Endocrinology. 2001;142:2789–2795. doi: 10.1210/endo.142.7.8237. [DOI] [PubMed] [Google Scholar]
- Shetty G, Wilson G, Huhtaniemi I, Shuttlesworth GA, Reissmann T, Meistrich ML. Gonadotropin-releasing hormone analogs stimulate and testosterone inhibits the recovery of spermatogenesis in irradiated rats. Endocrinology. 2000;141:1735–1745. doi: 10.1210/endo.141.5.7446. [DOI] [PubMed] [Google Scholar]
- Singh J, O'Neill C, Handelsman DJ. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology. 1995;136:5311–5321. doi: 10.1210/endo.136.12.7588276. [DOI] [PubMed] [Google Scholar]
- Udagawa K, Ogawa T, Watanabe T, Yumura Y, Takeda M, Hosaka M. GnRH analog, leuprorelin acetate, promotes regeneration of rat spermatogenesis after severe chemical damage. Int. J. Urol. 2001;8:615–622. doi: 10.1046/j.1442-2042.2001.00382.x. [DOI] [PubMed] [Google Scholar]
- van Alphen MMA, van de Kant HJG, de Rooij DG. Repopulation of the seminiferous epithelium of the rhesus monkey after x irradiation. Radiat. Res. 1988;113:487–500. [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York, NY: Springer; 2000. [Google Scholar]
- Wang G, Weng CC, Shao SH, Zhou W, De Gendt K, Braun RE, Verhoeven G, Meistich ML. Androgen receptor in Sertoli cells is not required for testosterone-induced suppression of spermatogenesis, but contributes to Sertoli cell organization in UTP14bisd mice. J. Androl. 2009;30:338–348. doi: 10.2164/jandrol.108.006890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Shao S, Meistrich M. The radiation-induced block in spermatogonial differentiation is due to damage to the somatic environment, not the germ cells. J. Cell Physiol. 2007;211:149–158. doi: 10.1002/jcp.20910. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Shao S, Meistrich ML. Irradiated mouse testes efficiently support spermatogenesis derived from donor germ cells of mice and rats. J. Androl. 2006;27:365–375. doi: 10.2164/jandrol.05179. [DOI] [PubMed] [Google Scholar]
- Zhou W, Bolden-Tiller OU, Shetty G, Shao SH, Weng CC, Pakarinen P, Liu Z, Stivers DN, Meistrich ML. Changes in gene expression in somatic cells of rat testes resulting from hormonal modulation and radiation-induced germ cell depletion. Biol. Reprod. 2010;82:54–65. doi: 10.1095/biolreprod.109.078048. [DOI] [PMC free article] [PubMed] [Google Scholar]