Abstract
Open channel block (OCB) is a process by which ions bind to the inside of a channel pore and block the flow of ions through that channel. Repulsion of the blocking ions by membrane depolarization is a known mechanism for open channel block removal. For the N-methyl-D-aspartate (NMDA) channel, this mechanism is necessary for channel activation and is involved in neuronal plasticity. Several types of Transient Receptor Potential (TRP) channels, including the Drosophila TRP and TRP-Like (TRPL) channels, also exhibit open channel block. For the Drosophila TRP and TRPL channels, removal of open channel block is necessary for the production of the physiological response to light. Recently, we have shown that lipids such as polyunsaturated fatty acids (PUFAs), represented by linoleic acid (LA), alleviate OCB under physiological conditions, from the Drosophila TRP and TRPL channels and from the mammalian NMDA channel. Here we show that OCB removal by LA is not confined to the Drosophila TRPs but also applies to mammalian TRPs such as the heat activated TRPV3 channel. TRPV3 shows OCB alleviation by LA, although it shares little amino acid sequence homology with the Drosophila TRPs. Strikingly, LA inhibits the heat-activated TRPV1 and the cold temperature-activated TRPM8 channels, which are intrinsic voltage sensitive channels and do not show OCB. Together, our findings further support the notion that lipids do not act as second messengers by direct binding to a specific site of the channels but rather act indirectly by affecting the channel-plasma membrane interface.
Keywords: TRP channels, open channel block, divalent cations, lipids, membrane
Open channel block (OCB) is a process by which ions have access to intra-channel binding sites inside the pore of an ion channel and block the flow of ions through that channel. The known mechanism of OCB removal is repulsion of the blocking ion by depolarization. Activation of the NMDA channel is a notable example for OCB and its removal in brain neurons. In the case of the ligand-gated NMDA channel, ligand binding by itself is not sufficient to allow cationic influx through the channel's pore at resting membrane potential because of the OCB by Mg2+. Removal of this Mg2+ OCB by depolarization1 allows activation of the NMDA channel, and serves as a coincident detector of pre and post synaptic activities in the brain2. The Drosophila transient receptor potential (TRP) and TRP-like (TRPL) channels are also subject to OCB. However, puzzlingly, these channels mediate light-induced current in the absence of previous membrane depolarization. Recently we have reported on a novel mechanism of OCB alleviation by lipids. OCB causes an outwardly rectifying current voltage relationship (I–V curve) typical for the TRPL and NMDA channels. Application of lipids such as the polyunsaturated fatty acid linoleic acid (LA), results in a linearization of the I–V curve for both TRPL and NMDA channels in the presence of blocking cations. We have shown that lipids alleviate OCB from both NMDA and TRPL channels by facilitating the passage rate of the blocking cations in the channels' pore. Furthermore, we have suggested that the effect of lipids is indirect and operates by modulating the interactions between the membrane lipids and the channels. Thus, lipids do not affect the TRPL channel as second messengers but rather as modifiers of membrane lipid-channel interactions.
The TRP superfamily is evolutionary conserved and plays important roles in signal transduction of many cells types 3–9. Experimental evidence has suggested that several mammalian members of the TRP family that show outward rectification (e.g. TRPC2 10, TRPC6 11, TRPV3 12, TRPM6 13 and TRPM7 14) undergo OCB. However, the physiological mechanism underlying the alleviation of OCB in these channels is still unknown. In contrast, some other TRP channels reveal outward rectification which is not due to OCB. Rather, these TRP channels are thought to have intrinsic voltage sensitivity. Examples for extensively studied TRP channels in which their voltage dependence does not arise from OCB are the heat-activated TRPV1 and the cold temperature-activated TRPM8 channels 9.
If alleviation of OCB by lipids is a general phenomenon, it is expected that lipid application will cause linearization of the I–V curve of mammalian TRP channels that reveal OCB but that have not been examined in our previous study 15. To test this possibility we examined in the present study the effect of LA on I–V curve of the mammalian TRPV3 channel, which shows divalent open channel block16. TRPV3 expressed in S2 cells was slightly activated by application of 2-aminoethoxydiphenylborate (2-APB12), which produced an outwardly rectifying current as measured by whole-cell patch recordings (Fig 1, green curve, 2). Application of LA greatly enhanced both inward and outward currents (Fig 1, purple curve, 4) resulting in a virtually linear I–V curve (Fig 1, black curve, 5). Our previous study on the TRPL and NMDA channels revealed that lipids have a faster effect at positive than at negative membrane potentials. Figure 1 shows, in a similar manner, that application of LA to S2 cell expressing TRPV3 enhanced the outward currents, faster than the inward currents. In the previous study we further showed that an increased concentration of the blocking cation (Ca2+ for the TRPL and Mg2+ for the NMDA channels) in the presence of LA restored the outwardly rectifying I–V curve 15. Similarly, application of Ca2+ (not shown) or La3+ (Fig. 1 violet curve, 6) restored the outwardly rectifying I–V curve of the TRPV3 channel in the presence of LA.
Figure 1. LA removed divalent open channel block from the TRPV3 channel.
LA affected the mammalian TRPV3 channel in a similar manner to the effect on the previously reported TRPL channel. Representative I–V curves measured from S2 cells expressing the TRPV3 channel by whole cell patch clamp recordings, using voltage ramps from −100mV to 100mV in 1s. The channel was activated by application of 2-APB (100µM, green curve, 2), in a similar manner to a previous report 12. Application of 40µM LA resulted in a change from an outwardly rectifying to an almost linear I–V curve (black curve 5) with a faster change at positive membrane potentials relative to negative membrane potentials. The outwardly rectifying I–V curve was restored upon application of 2mM La3+ (violet curve 6, n=6). In control experiments when no LA was applied, no linearization was observed (data not shown)
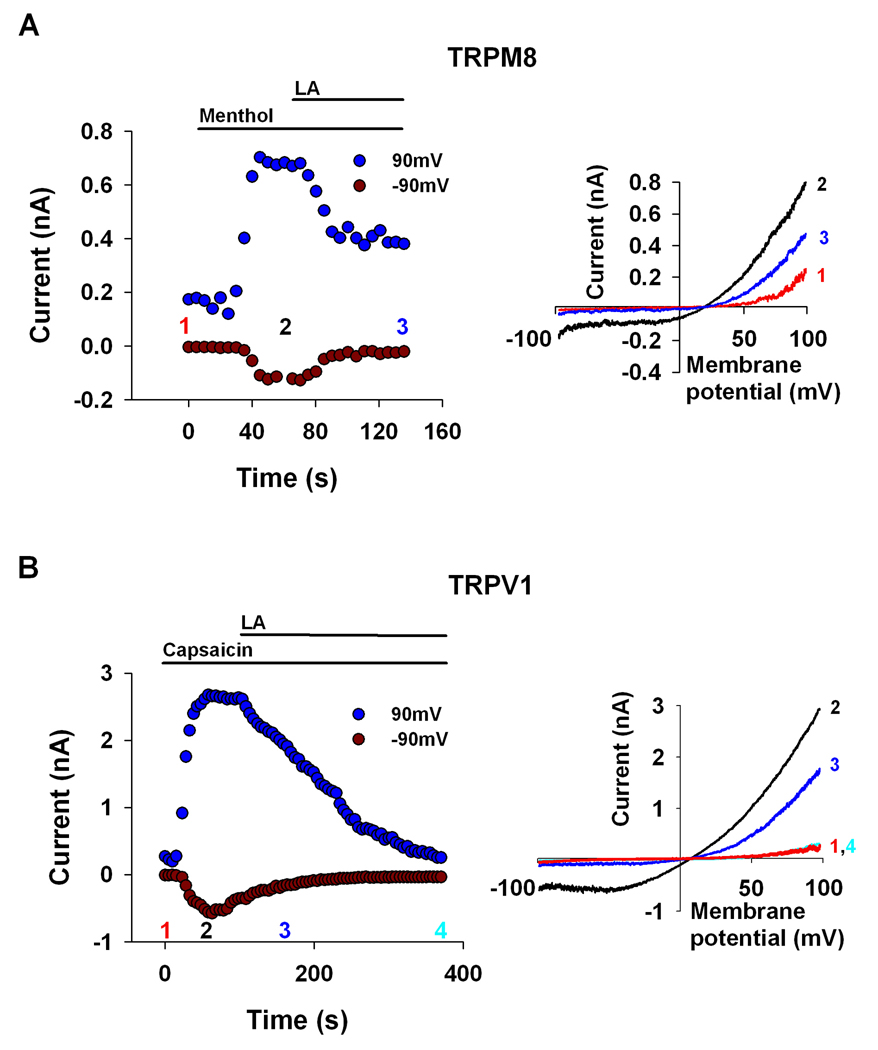
If some TRP channels show outwardly rectifying I–V curves because of intrinsic voltage sensitivity and not due to OCB, we expect that application of LA will not cause linearization of their I–V curve. To test this notion we applied LA to the mammalian TRPM8 and TRPV1 channels, which do not exhibit OCB but rather exhibit an intrinsic voltage sensitivity 9, 17. Application of menthol18 and capsaicin19, (under low Ca2+ conditions to prevent Ca2+ dependent inactivation of the channels) activated continuously, without desensitization, the TRPM8 and TRPV1 channels, respectively, during ~ 1min of whole cell recordings (Fig. 2, A, B, respectively). Indeed, in contrast to channels that exhibit OCB, the menthol and capsaicin-induced currents were inhibited rather than enhanced by LA (Fig. 2). Similar inhibition by PUFAs of capsaicin activated TRPV1 channels expressed in HEK 293 cells was previously reported 20.
Figure 2. LA inhibits the voltage sensitive TRPM8 and TRPV1 channels.
A. LA inhibited rather than activated the mammalian TRPM8, which is considered to be an intrinsic voltage sensitive channel. The paradigm of Fig. 1 was repeated. Application of 40µM LA to S2 cells expressing the TRPM8 channel, which were initially activated by menthol (100µM) decreased current at both negative and positive membrane potentials (n=4). In control experiments when no LA was applied, no decline of the current was observed as there was no desensitization due to Ca2+ cations 22.
B. The mammalian TRPV1 channel is also considered to be an intrinsic voltage sensitive channel. The paradigm of Fig. 1 was repeated. Application of 40µM LA to S2 cells expressing the TRPV1 channel, which were initially activated by capsaicin (1µM), decreased channel activity, at both negative and positive membrane potentials (n=4). In control experiments when no LA was applied, no decline of the current was observed as there was no desensitization due to Ca2+ cations 23 (see above)
TRPV3 shares little amino acid sequence homology with the TRPL channel, and this is obviously true for the NMDA channel. It is therefore unlikely that LA acts as a second messenger and affects these channels by a direct binding to a specific site, in all of these channel proteins. Rather, it is more likely that alteration of the channel-membrane lipid interface underlies the effect of LA 15, 21. The common denominator of all these channels, with regard to the effect of LA is OCB alleviation and hence current enhancement. In contrast, the intrinsically voltage sensitive, TRPV1 and TRPM8 do not exhibit OCB and are inhibited by LA. These channels also share little amino acid sequence homology with the channels that exhibit OCB or with each other, further supporting an indirect effect of LA. The fact that LA inhibits rather than enhances the activity of TRPV1 and TRPM8 further supports the notion that the enhanced TRPL, NMDA and TRPV3 currents due to LA is not a general effect on all channels, but is related to the removal of the OCB. Together, the present findings further support our notion that lipids do not act as second messengers but rather act indirectly by affecting the channel-plasma membrane interface.
Acknowledgments
We thank Shaya Lev for very useful comments and critical reading of the manuscript. This research was supported by grants from the National Institute of Health (EY 03529), the Israel Science Foundation (ISF), the German Israeli Foundation (GIF) and the Moscona Foundation and the Minerva Foundation.
Footnotes
Addendum to: Parnas M., Katz B., Lev S., Tzarfaty V., Dadon D., Gordon-Shaag A., Metzner H., Yaka R.and Minke B. Membrane lipid modulations remove divalent open channel block from TRPL and NMDA channels. J. Neurosci. 2009; 29:2371–83
References
- 1.Kandel ER. Synaptic integration. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of neural science. 4. Vol. 4. New York: McGraw-Hill, Health Professions Division; 2000. pp. 212–214. [Google Scholar]
- 2.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 3.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 4.Dhaka A, Viswanath V, Patapoutian A. TRP Ion Channels and Temperature Sensation. Annu Rev Neurosci. 2006:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- 5.Hardie RC. TRP channels and lipids: from Drosophila to mammalian physiology. J Physiol. 2007;578:9–24. doi: 10.1113/jphysiol.2006.118372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordt SE, McKemy DD, Julius D. Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr Opin Neurobiol. 2003;13:487–492. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 7.Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- 8.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 10.Lucas P, Ukhanov K, Leinders-Zufall T, Zufall F. A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: mechanism of pheromone transduction. Neuron. 2003;40:551–561. doi: 10.1016/s0896-6273(03)00675-5. [DOI] [PubMed] [Google Scholar]
- 11.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci U S A. 2006;103:16586–16591. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu HZ, Xiao R, Wang C, Gao N, Colton CK, Wood JD, Zhu MX. Potentiation of TRPV3 channel function by unsaturated fatty acids. J Cell Physiol. 2006;208:201–212. doi: 10.1002/jcp.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topala CN, Groenestege WT, Thebault S, van den BD, Nilius B, Hoenderop JG, Bindels RJ. Molecular determinants of permeation through the cation channel TRPM6. Cell Calcium. 2007;41:513–523. doi: 10.1016/j.ceca.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 15.Parnas M, Katz B, Lev S, Tzarfaty V, Dadon D, Gordon-Shaag A, Metzner H, Yaka R, Minke B. Membrane lipid modulations remove divalent open channel block from TRP-like and NMDA channels. J Neurosci. 2009;29:2371–2383. doi: 10.1523/JNEUROSCI.4280-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- 17.Brauchi S, Orio P, Latorre R. Clues to understanding cold sensation: thermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proc Natl Acad Sci U S A. 2004;101:15494–15499. doi: 10.1073/pnas.0406773101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 19.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 20.Matta JA, Miyares RL, Ahern GP. TRPV1 is a novel target for omega-3 polyunsaturated fatty acids. J Physiol. 2007;578:397–411. doi: 10.1113/jphysiol.2006.121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janmey PA, Kinnunen PK. Biophysical properties of lipids and dynamic membranes. Trends Cell Biol. 2006;16:538–546. doi: 10.1016/j.tcb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Abe J, Hosokawa H, Sawada Y, Matsumura K, Kobayashi S. Ca2+-dependent PKC activation mediates menthol-induced desensitization of transient receptor potential M8. Neurosci Lett. 2006;397:140–144. doi: 10.1016/j.neulet.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol. 2004;123:53–62. doi: 10.1085/jgp.200308906. [DOI] [PMC free article] [PubMed] [Google Scholar]