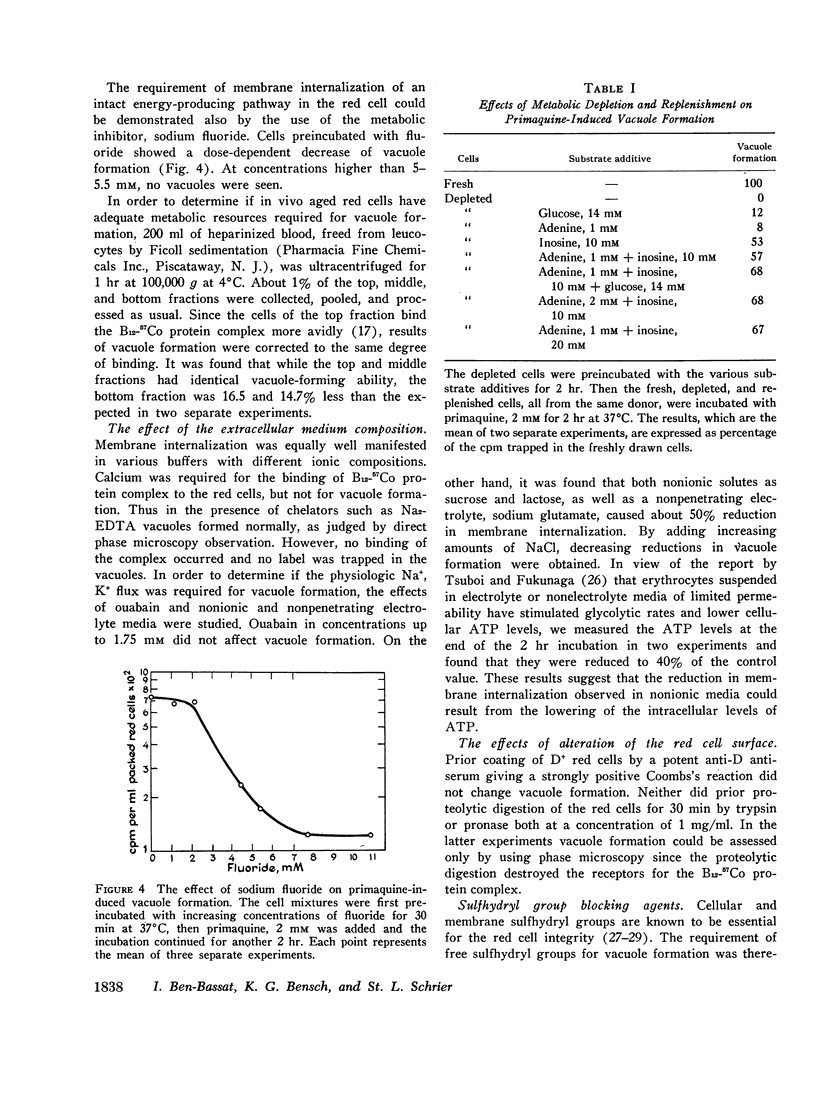
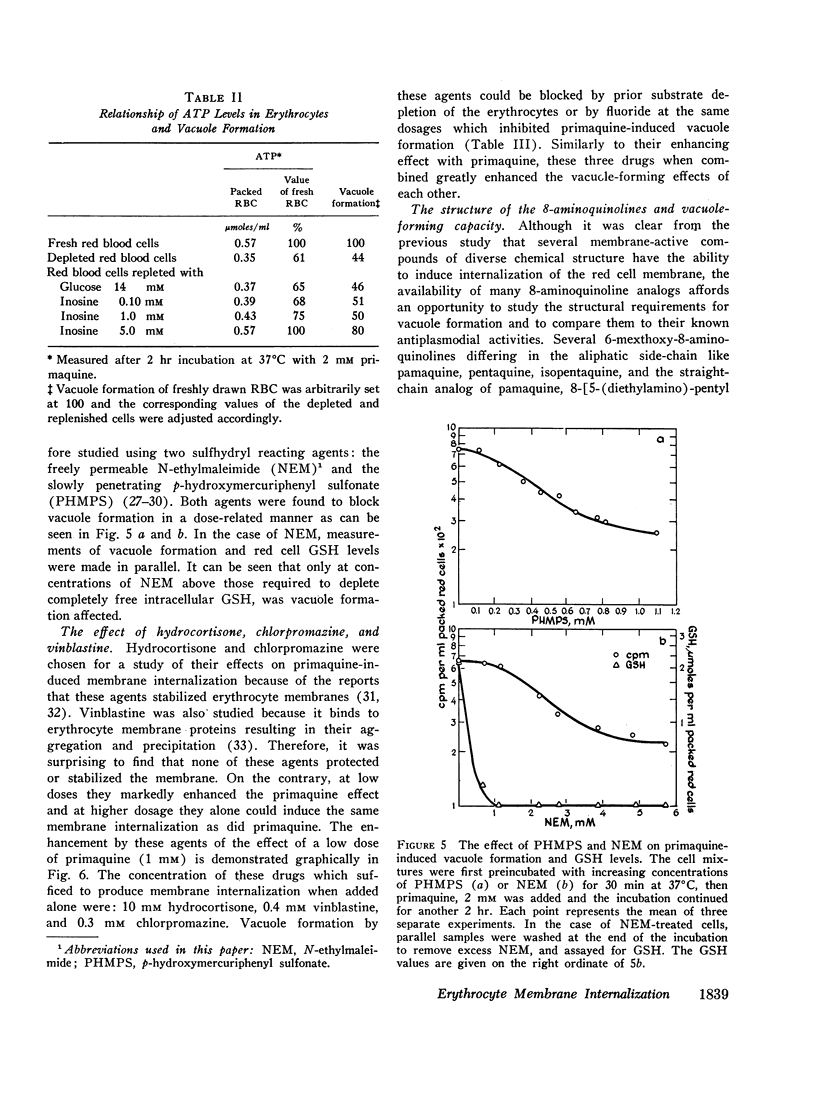
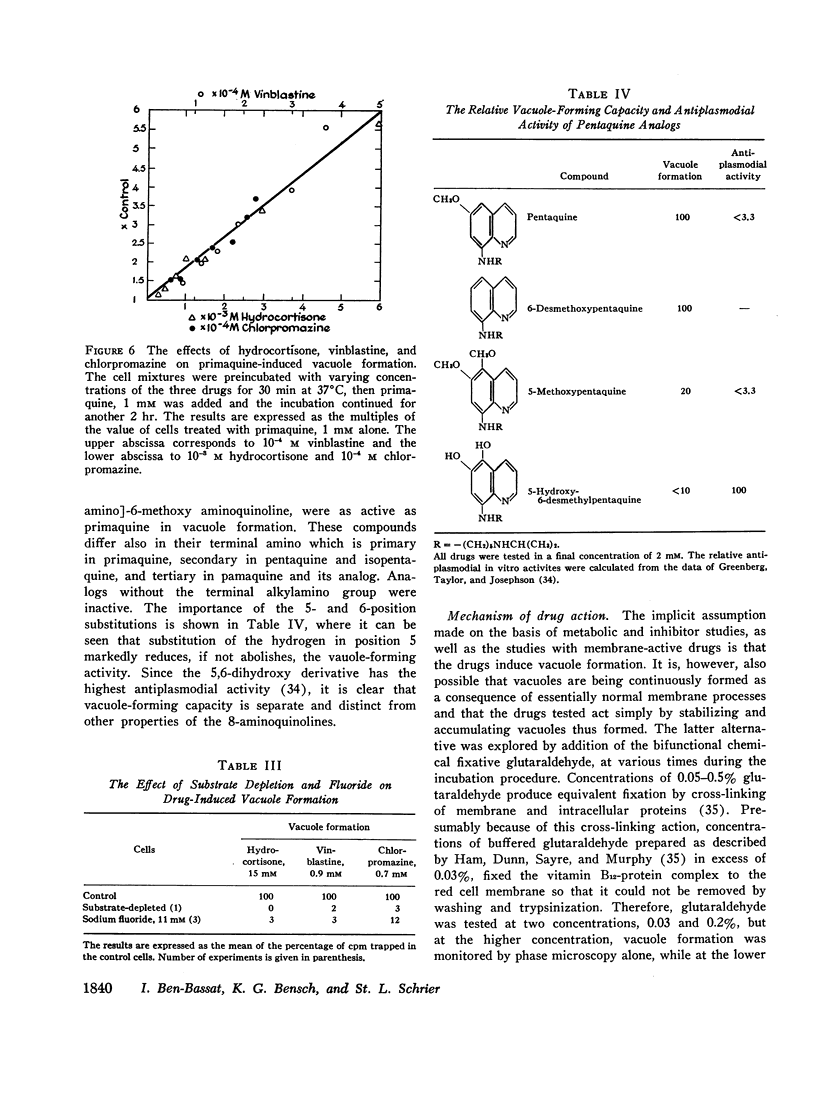
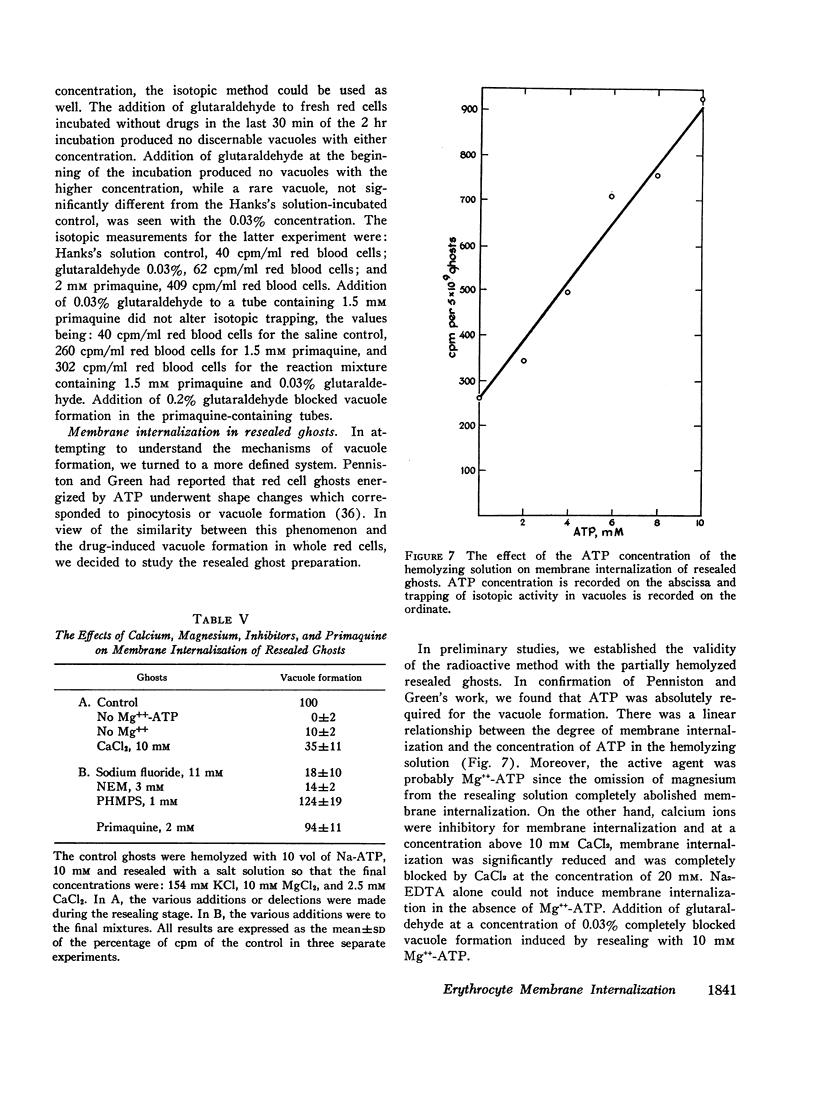
Abstract
In vitro erythrocyte membrane internalization, resulting in the formation of membrane-lined vacuoles, can be quantified by a radioisotopic method. A complex of 37Co-labeled vitamin B12 and its plasma protein binders is first adsorbed to the cell surface, and after vacuoles are formed, the noninternalized label is removed by washing and trypsin treatment. The residual radioactivity represents trapped label and can be used to measure the extent of membrane internalization.
Using this method, it was found that in addition to primaquine, a group of membrane-active drugs, specifically hydrocortisone, vinblastine, and chlorpromazine can induce membrane internalization in erythrocytes. This is a metabolic process dependent on drug concentration, temperature, and pH. Vacuole formation by all agents tested can be blocked by prior depletion of endogenous substrates or by poisoning the erythrocytes with sodium fluoride and sulfhydryl blocking agents. This phenomenon resembles in some respects the previously reported membrane internalization of energized erythrocyte ghosts. It is suggested that membrane internalization is dependent on an ATP-energized state and is influenced by the balance between the concentrations of magnesium and calcium in the membrane. This study provides a basis for proposing a unifying concept of the action of some membrane-active drugs, and for considering the role of erythrocyte membrane internalization in pathophysiologic events.
Full text
PDF


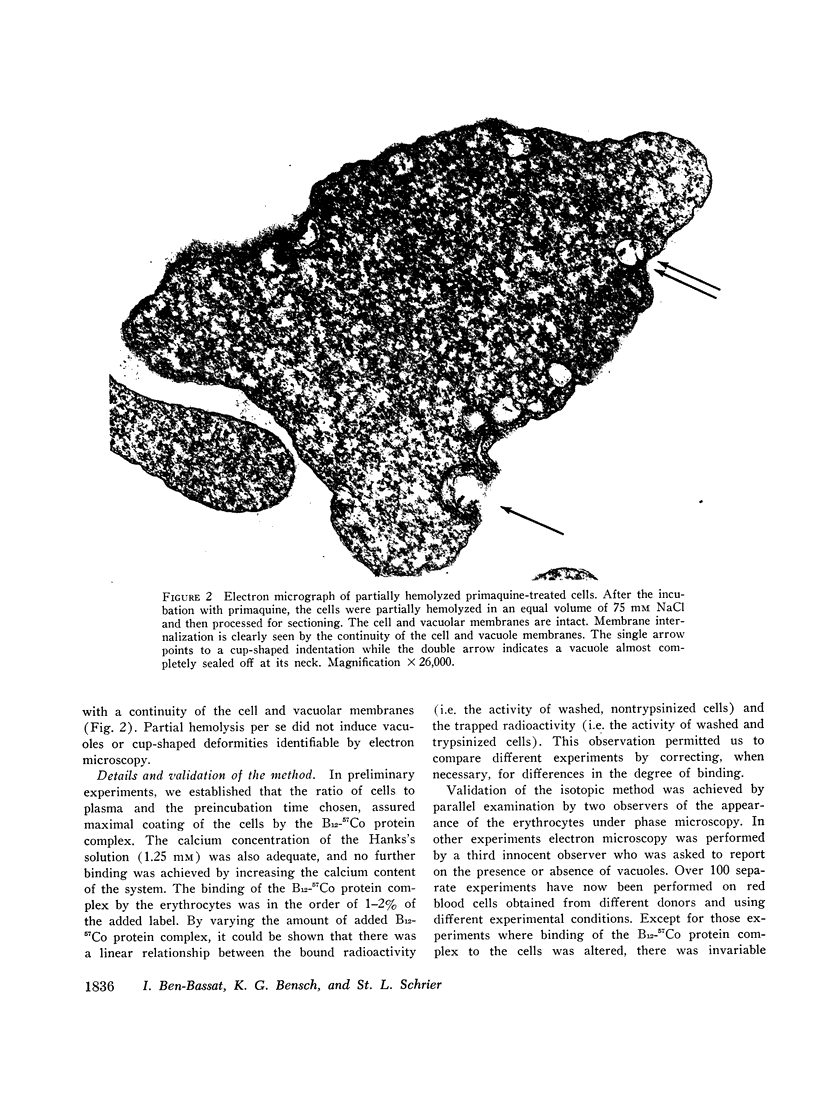
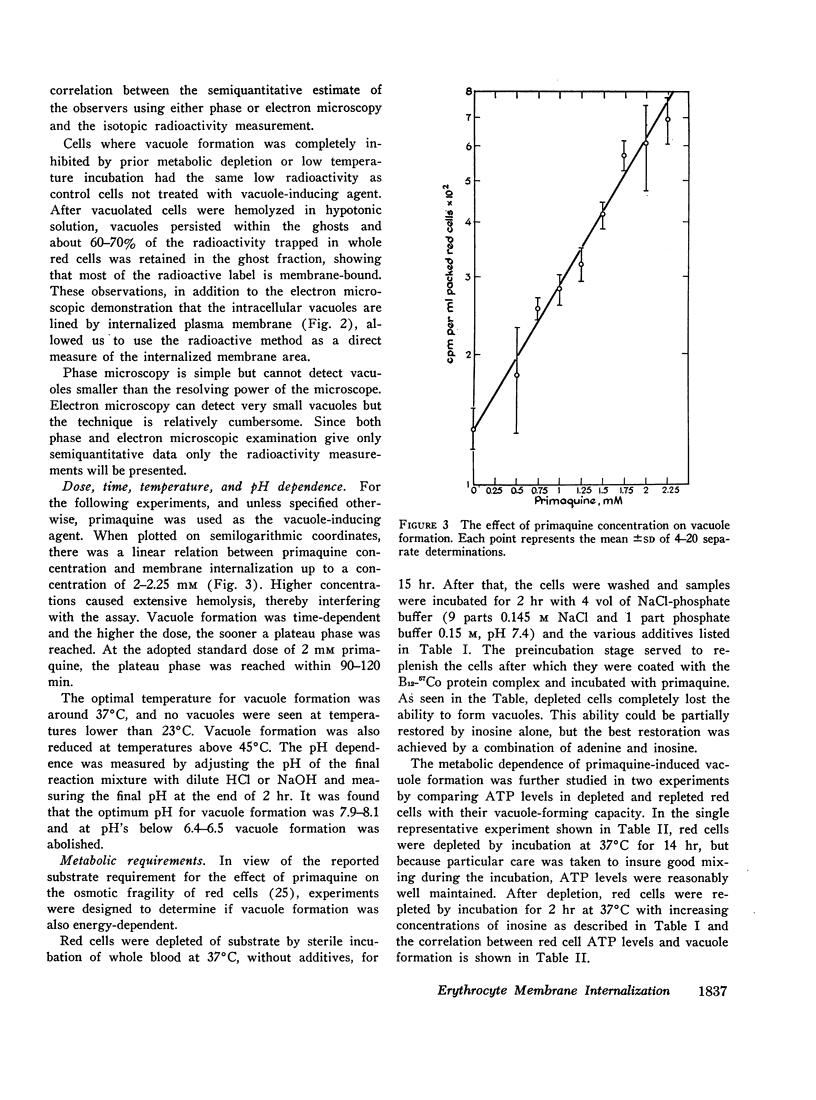



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEUTLER E., DURON O., KELLY B. M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963 May;61:882–888. [PubMed] [Google Scholar]
- Blanton P. L., Martin J., Haberman S. Pinocytotic response of circulating erythrocytes to specific blood grouping antibodies. J Cell Biol. 1968 Jun;37(3):716–728. doi: 10.1083/jcb.37.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN R. G., HENNESSEY M. A., WALTERSDORPH A. M., HUENNEKENS F. M., GABRIO B. W. Erythrocyte metabolism. V. Levels of glycolytic enzymes and regulation of glycolysis. J Clin Invest. 1962 Jun;41:1249–1256. doi: 10.1172/JCI104587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROSBY W. H. Normal functions of the spleen relative to red blood cells: a review. Blood. 1959 Apr;14(4):399–408. [PubMed] [Google Scholar]
- FREEMAN J. A., SPURLOCK B. O. A new epoxy embedment for electron microscopy. J Cell Biol. 1962 Jun;13:437–443. doi: 10.1083/jcb.13.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG J., TAYLOR D. J., JOSEPHSON E. S. Studies on Plasmodium gallinaceum in vitro II. The effects of some 8-aminoquinolines against the erythrocytic parasites. J Infect Dis. 1951 Mar-Apr;88(2):163–167. doi: 10.1093/infdis/88.2.163. [DOI] [PubMed] [Google Scholar]
- George J. N., O'Brien R. L., Pollack S., Crosby W. H. Studies of in vitro primaquine hemolysis: substrate requirement for erythrocyte membrane damage. J Clin Invest. 1966 Aug;45(8):1280–1289. doi: 10.1172/JCI105435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginn F. L., Hochstein P., Trump B. F. Membrane alterations in hemolysis: Internalization of plasmalemma induced by primaquine. Science. 1969 May 16;164(3881):843–845. doi: 10.1126/science.164.3881.843. [DOI] [PubMed] [Google Scholar]
- HOFFMAN J. F., TOSTESON D. C., WHITTAM R. Retention of potassium by human erythrocyte ghosts. Nature. 1960 Jan 16;185:186–187. doi: 10.1038/185186a0. [DOI] [PubMed] [Google Scholar]
- Ham T. H., Dunn R. F., Sayre R. W., Murphy J. R. Physical properties of red cells as related to effects in vivo. I. Increased rigidity of erythrocytes as measured by viscosity of cells altered by chemical fixation, sickling and hypertonicity. Blood. 1968 Dec;32(6):847–861. [PubMed] [Google Scholar]
- Holroyde C. P., Gardner F. H. Acquisition of autophagic vacuoles by human erythrocytes. Physiological role of the spleen. Blood. 1970 Nov;36(5):566–575. [PubMed] [Google Scholar]
- Holroyde C. P., Oski F. A., Gardner F. H. The "pocked" erythrocyte. Red-cell surface alterations in reticuloendothelial immaturity of the neonate. N Engl J Med. 1969 Sep 4;281(10):516–520. doi: 10.1056/NEJM196909042811002. [DOI] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. I. Mechanism of hemolysis. J Clin Invest. 1962 Apr;41:779–792. doi: 10.1172/JCI104536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. II. Studies in vivo. J Clin Invest. 1962 Jul;41:1514–1523. doi: 10.1172/JCI104607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H. LEAKY RED CELLS. Blood. 1965 Sep;26:367–382. [PubMed] [Google Scholar]
- Jacob H. S. Mechanisms of Heinz body formation and attachment to red cell membrane. Semin Hematol. 1970 Jul;7(3):341–354. [PubMed] [Google Scholar]
- Kent G., Minick O. T., Volini F. I., Orfei E. Autophagic vacuoles in human red cells. Am J Pathol. 1966 May;48(5):831–857. [PMC free article] [PubMed] [Google Scholar]
- Koyama S., Aoki S., Deguchi D. Electron microscopic observations of the splenic red pulp with special reference to the pitting function. Mie Med J. 1964 Sep;14(2):143–188. [PubMed] [Google Scholar]
- Kwant W. O., Seeman P. The displacement of membrane calcium by a local anesthetic (chlorpromazine). Biochim Biophys Acta. 1969;193(2):338–349. doi: 10.1016/0005-2736(69)90194-1. [DOI] [PubMed] [Google Scholar]
- Kwant W. O., Seeman P. The displacement of membrane calcium by a local anesthetic (chlorpromazine). Biochim Biophys Acta. 1969;193(2):338–349. doi: 10.1016/0005-2736(69)90194-1. [DOI] [PubMed] [Google Scholar]
- NAKAO M., NAKAO T., TATIBANA M., YOSHIKAWA H., ABE T. Effect of inosine and adenine on adenosine triphosphate regeneration and shape transformation in long-stored erythrocyts. Biochim Biophys Acta. 1959 Apr;32:564–565. doi: 10.1016/0006-3002(59)90640-7. [DOI] [PubMed] [Google Scholar]
- NAKAO M., NAKAO T., YAMAZOE S. Adenosine triphosphate and maintenance of shape of the human red cells. Nature. 1960 Sep 10;187:945–946. doi: 10.1038/187945a0. [DOI] [PubMed] [Google Scholar]
- PALADE G. E. A study of fixation for electron microscopy. J Exp Med. 1952 Mar;95(3):285–298. doi: 10.1084/jem.95.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penniston J. T., Green D. E. The conformational basis of energy transformations in membrane systems. IV. Energized states and pinocytosis in erythrocyte ghosts. Arch Biochem Biophys. 1968 Nov;128(2):339–350. doi: 10.1016/0003-9861(68)90040-4. [DOI] [PubMed] [Google Scholar]
- Rega A. F., Rothstein A., Weed R. I. Erythrocyte membrane sulfhydryl groups and the active transport of cations. J Cell Physiol. 1967 Aug;70(1):45–52. doi: 10.1002/jcp.1040700107. [DOI] [PubMed] [Google Scholar]
- Retief F. P., Gottlieb C. W., Herbert V. Mechanism of vitamin B12 uptake by erythocytes. J Clin Invest. 1966 Dec;45(12):1907–1915. doi: 10.1172/JCI105495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind R. A. Destruction of injured red cells in vivo. Am J Med. 1966 Nov;41(5):711–723. doi: 10.1016/0002-9343(66)90032-5. [DOI] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier S. L. ATP synthesis in human erythrocyte membranes. Biochim Biophys Acta. 1967 Sep 9;135(4):591–598. doi: 10.1016/0005-2736(67)90091-0. [DOI] [PubMed] [Google Scholar]
- Seeman P. Erythrocyte membrane stabilization by steroids and alcohols; a possible model for anesthesia. Biochem Pharmacol. 1966 Oct;15(10):1632–1637. doi: 10.1016/0006-2952(66)90214-0. [DOI] [PubMed] [Google Scholar]
- Shapiro B., Kollmann G., Martin D. The diversity of sulfhydryl groups in the human erythrocyte membrane. J Cell Physiol. 1970 Jun;75(3):281–291. doi: 10.1002/jcp.1040750304. [DOI] [PubMed] [Google Scholar]
- Simon E. R. Adenine and purine nucleosides in human red cell preservation: a review. Transfusion. 1967 Nov-Dec;7(6):395–400. doi: 10.1111/j.1537-2995.1967.tb04874.x. [DOI] [PubMed] [Google Scholar]
- Tsuboi K. K., Fukunaga K. Relationship of solute permeability to erythrocyte glycolysis. Biochim Biophys Acta. 1970;196(2):215–220. doi: 10.1016/0005-2736(70)90009-x. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voak D., Williams M. A. An explanation of the failure of the direct antiglobulin test to detect erythrocyte sensitization in ABO haemolytic disease of the newborn and observations on pinocytosis of IgG anti-A antibodies by infant (cord) red cells. Br J Haematol. 1971 Jan;20(1):9–23. doi: 10.1111/j.1365-2141.1971.tb00782.x. [DOI] [PubMed] [Google Scholar]
- Weed R. I., Bowdler A. J. Metabolic dependence of the critical hemolytic volume of human erythrocytes: relationship to osmotic fragility and autohemolysis in hereditary spherocytosis and normal red cells. J Clin Invest. 1966 Jul;45(7):1137–1149. doi: 10.1172/JCI105420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed R. I. Disorders of red cell membrane: history and perspectives. Semin Hematol. 1970 Jul;7(3):249–258. [PubMed] [Google Scholar]
- Weed R. I., LaCelle P. L., Merrill E. W. Metabolic dependence of red cell deformability. J Clin Invest. 1969 May;48(5):795–809. doi: 10.1172/JCI106038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed R. I., Reed C. F. Membrane alterations leading to red cell destruction. Am J Med. 1966 Nov;41(5):681–698. doi: 10.1016/0002-9343(66)90030-1. [DOI] [PubMed] [Google Scholar]
- Wilson L., Bryan J., Ruby A., Mazia D. Precipitation of proteins by vinblastine and calcium ions. Proc Natl Acad Sci U S A. 1970 Jul;66(3):807–814. doi: 10.1073/pnas.66.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittels B., Hochstein P. The effect of primaquine on lecithin metabolism in human erythrocytes. Biochim Biophys Acta. 1966 Dec 7;125(3):594–597. doi: 10.1016/0005-2760(66)90047-6. [DOI] [PubMed] [Google Scholar]
- Wittels B. Modification of phospholipid metabolism in human red cells by primaquine. A possible mechanism in drug-induced hemolysis. Biochim Biophys Acta. 1970 Jun 9;210(1):74–85. doi: 10.1016/0005-2760(70)90063-9. [DOI] [PubMed] [Google Scholar]