Abstract
Mucoepidermoid carcinoma is the most common salivary gland malignancy. The majorities of these tumors arise in the parotid and minor salivary glands, but may rarely develop intraosseously. The latter is not uncommonly associated with diagnostic and management difficulties. We report an example of intraosseous mucoepidermoid carcinoma with positive TORC1/MAML2 gene fusion transcript and discuss the clinical implications.
Keywords: Central mucoepidermoid carcinoma, Salivary gland tumors, Fusion genes, Molecular analysis, Mandible, Intraosseous, Odontogenic cyst, TORC1/MAML2
Introduction
Mucoepidermoid carcinoma (MEC) is the most common malignant tumor of salivary glands [1]. Their intraosseous development is rare and not infrequently associated with early diagnostic and management difficulties [2]. The histogenesis of intraosseous MEC is uncertain, but an origin from ectopic salivary tissue or metaplastic transformation of odontogenic epithelium has been proposed [3–5].
Case reports and review articles of such an occurrence show that they typically present at relatively young age, more in female than male patients, with the most common symptoms being pain and swelling [2, 3, 5–7]. Radiologically, these lesions show unilocular or multilocular well-circumscribed radiolucency, though variable radiological patterns have been reported [8]. Histopathologically, tumors manifest cystic formation with conventional features of low to intermediate grade features [2, 8].
We present an additional example of this entity with emphasis on the role of the TORC1/MAML2 fusion gene detection on the differential diagnosis and biology of this cancer.
Case Report
The patient is a 20 year old male who presented at an outside hospital with history of pain, swelling and discharge in the left mandible of one and a half years duration.
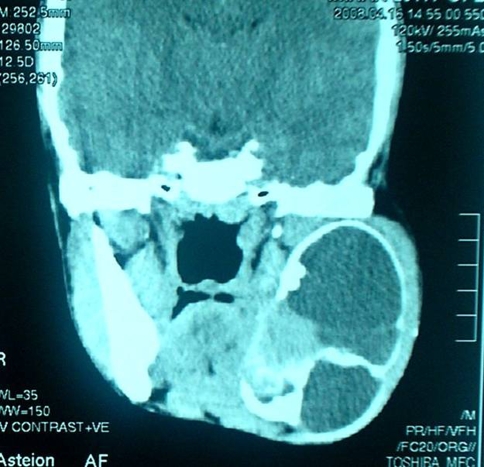
A CT scan showed a circumscribed intraosseous lytic lesion with solid and cystic components involving ramus and part of body of the left mandible. The lesion was displacing an unerrupted 3rd molar tooth, causing thickening of overlying bone and soft tissue. The differential diagnosis at this stage included odontogenic keratocyst and ameloblastoma (Fig. 1).
Fig. 1.
Radiologically a circumscribed intraosseous lytic lesion with solid and cystic components involving ramus and part of body of the left mandible
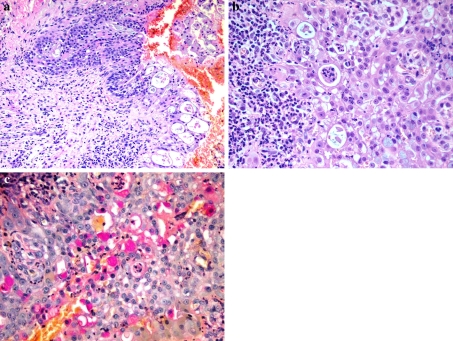
An incisional biopsy of the lesion was performed and consisted of multiple hemorrhagic soft tissue fragments. Microscopic examination showed epithelial lined cyst wall with infiltrating nests of squamoid and mucin producing cells (Fig. 2a, b). Presence of mucin was confirmed by mucicarmine stain (Fig. 2c). Cytokeratin AE1/AE3 positivity proved the epithelial nature of the tumor cells. The diagnosis of central mucoepidermoid carcinoma was made.
Fig. 2.
a Low power histologic image showing epithelial lined cyst wall with invasive tumor nest. (H&E stain, 200x). b High power histologic image showing squamoid and mucous cells. (H&E stain, 400x). c High power view, highlighting mucin production by some of the tumor cells. (Mucicarmine stain, 400x)
RNA was extracted from formalin-fixed paraffin-embedded tissue (10-μm paraffin sections) using a Recover All Nucleic Acid Isolation Kit (Ambion Diagnostics, Austin, TX) per the manufacturer’s instructions. We quantitated the optical density of the extracted RNA with a DU 60 spectrophotometer (Beckman Coulter, Fullerton, CA). For positive controls, we used RNA from one t(11;19)-positive cell line NCI-H292 and one mucoepidermoid carcinoma that had previously been identified as having the transcript. Cell blocks were prepared from the cell line. For the negative control, we used RNA from a mucoepidermoid carcinoma that had previously been shown to lacking the transcript. The control represented PCR reactions with the respective gene-specific primers but without the cDNA template.
One-tube RT–PCR (Promega Corp., Madison, WI) was performed using 0.5 μg of extracted RNA in a final mixture of 50 μl consisting of nuclease-free water, 2 μl of 25 mM MgSO4, 1 μl avian myeloblastosis viral RT (5 U/μl), 10 μl avian myeloblastosis virus/Tfl 5 × reaction buffer, 1 μl 10 mM deoxyribonucleotide triphosphate mix, 1 μl Tfl DNA polymerase (5 U/μl), and 50 pmol of both primers. Initial primers used for the one-tube RT–PCR were CRTC1 5′-AAGATCGCGCTGCACAATCA-3′ and MAML2 5′-GGTCGCTTGC TGTTGGCAGG-3′. The RT–PCR cycling consisted of 45°C for 45 min, 94°C for 2 min, followed by 40 cycles of 94°C for 30 s, 60°C for 45 s, 68°C for 60 s, and one final cycle of 68°C for 7 min.
All samples were subjected to nested PCR using 0.2 μg of amplified DNA from the initial RT–PCR product, using TaqGOLD DNA polymerase and 1.5 mmol/l MgCl2. The amplification conditions consisted of 94°C for 10 min followed by 35 cycles at 95°C for 30 s, 55°C for 30 s, 72°C for 30 s, and one final cycle of 72°C for 5 min. The primers used for the nested PCR for CRTC1/MAML2 were CRTC1 5′-GGAGGAGACGGCGGCCTTCG-3′ and MAML2 5′-TTGCTGTTGGCAGG AGATAG-3′. The final PCR product was 117 bp, which was detected by gel electrophoresis (2% Metaphor agarose; (FMC Bioproducts, Rockland, ME) and visualized with ethidium bromide staining under ultraviolet light. For determining specificity, we used counterpart sets of mismatched primers. We amplified the ubiquitously expressed beta actin mRNA fragment (190 bp) as the internal control for RNA quality, using previously published oligonucleotide primers.
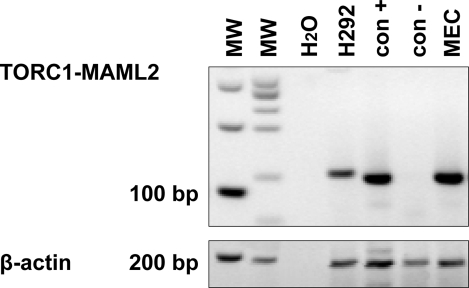
The CRTC1/MAML2 fusion transcript was detected in the mucoepidermoid carcinoma. The integrity and quality of the mRNA extracted from all samples was confirmed by concurrent amplification of beta actin as a housekeeping gene control (Fig. 3).
Fig. 3.
Nested PCR gel
Discussion
We present, for the first time, a central mucoepidermoid carcinoma of the mandible with a molecular alteration characteristic of their salivary counterparts. The patients presented with signs, symptoms and radiological findings similar to those previously published. In addition, the presumptive diagnosis in our case was initially believed to represent an intraosseous odontogenic neoplasm.
The detection of TORC1/MAML2 fusion gene in our tumor is the first documentation of this event in these tumors. Studies of mucoepidermoid carcinomas in salivary glands and at unconventional sites, such as breast, cervix, lung, thyroid and skin, have detected the same fusion gene in these tumors [9]. The TORC1/MAML2 fusion gene results from translocation of the long arm of chromosome 11 with the long arm of chromosome 19, where exon 1 of the TORC1 gene and exons 2–5 of the MAML2 are linked. This leads to altered Notch signaling which suggests a unique mechanism of tumorigenesis [10]. The true frequency of this fusion in MECs is unknown, but recent data suggests that it is fairly common and therefore can help as a diagnostic marker especially in poorly differentiated tumors [11]. This finding may allow for the potential application of this analysis in suspected cases of central MEC either on FNA or small tissue biopsy. RT–PCR is considered preferable to conventional cytogenetic analysis in this regard, but is hampered by poor tissue fixation in the paraffin embedded sections [12]. The translocation is more commonly observed in RNA obtained from snap-frozen samples as compared to formalin-fixed tissue which suggests that even adequately fixed specimens may show false negativity due to technical reasons [13]. The specimen in our case was properly fixed and did not need decalcification, however the need for decalcification in most of the central MECs may also similarly impinge on the results. Although fusion genes are attractive therapeutic targets, the therapeutic roles of this fusion gene in the entity are unknown. The sustained expression of Mect1-Maml2 is required for the growth of MEC tumor cell lines. This establishes its role as a molecular target and shows a promising future [14].
The prognosis of patients with intraosseous MEC is good [2, 7], especially for patients with radiologically cystic or rarefying type lesion [8]. Our patient follow up and outcome are in agreement with these findings. It remains to be seen the fusion positivity in these lesions may play a role in their biological evaluation. Studies of salivary MEC with fusion positive have also reported a better survival advantage [9, 10]. Whether similar findings hold true for intraosseous MECs must await additional cases and studies with long-term follow up.
References
- 1.Barnes L, Eveson JW, Reichart P, Sidransky D (eds.). World health organization classification of tumors. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005.
- 2.Kochaji N, Goossens A, Bottenberg P. Central Mucoepidermoid carcinoma: case report, literature review for missing and available information and guideline proposal for coming case reports. Oral Oncology Extra. 2004;40:95–105.
- 3.Alexander RW, Dupuis RH, Holton H. Central mucoepidermoid tumor (carcinoma) of the mandible. J Oral Surg. 1974;32:541–547. [PubMed] [Google Scholar]
- 4.Bouquot JE, Gnepp DR, Dardick I, et al. Intraosseous salivary tissue: jaw bone examples of choristomas, hamartomas, embryonic rests, and inflammatory entrapment: another histogenetic source for intraosseous adenocarcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:205–217. doi: 10.1067/moe.2000.107058. [DOI] [PubMed] [Google Scholar]
- 5.Eversole LR, Sabes WR, Rovin S. Aggressive growth and neoplastic potential of odontogenic cysts with special reference to central epidermoid and mucoepidermoid carcinomas. Cancer. 1975;35:270–282. doi: 10.1002/1097-0142(197501)35:1<270::AID-CNCR2820350134>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 6.Bhaskar SN. Central mucoepidermoid tumors of the mandible. Report of 2 cases. Cancer. 1963;16:721–726. doi: 10.1002/1097-0142(196306)16:6<721::AID-CNCR2820160606>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 7.Browand BC, Waldron CA. Central mucoepidermoid tumors of the jaws: report of nine cases and review of the literature. Oral Surg Oral Med Oral Pathol. 1975;40:631–643. doi: 10.1016/0030-4220(75)90373-4. [DOI] [PubMed] [Google Scholar]
- 8.Inagaki M, Yuasa K, Nakayama E, et al. Mucoepidermoid carcinoma in the mandible: findings of panoramic radiography and computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:613–618. doi: 10.1016/S1079-2104(98)90300-6. [DOI] [PubMed] [Google Scholar]
- 9.Tirado Y, Williams MD, Hanna EY, et al. CRTC1/MAML2 fusion transcript in high grade mucoepidermoid carcinomas of salivary and thyroid glands and Warthin’s tumors: implications for histogenesis and biologic behavior. Genes Chromosomes Cancer. 2007;46:708–715. doi: 10.1002/gcc.20458. [DOI] [PubMed] [Google Scholar]
- 10.Röser K, Jäkel KT, Bullerdiek J, et al. Significance of molecular-cytogenetic findings in mucoepidermoid carcinoma as an example of salivary gland tumors. Pathologe. 2005;26:359–366. doi: 10.1007/s00292-005-0778-x. [DOI] [PubMed] [Google Scholar]
- 11.Behboudi A, Enlund F, Winnes M, et al. Molecular classification of mucoepidermoid carcinomas-prognostic significance of the MECT1-MAML2 fusion oncogene. Genes Chromosomes Cancer. 2006;45:470–481. doi: 10.1002/gcc.20306. [DOI] [PubMed] [Google Scholar]
- 12.Martins C, Cavaco B, Tonon G, et al. A study of MECT1-MAML2 in mucoepidermoid carcinoma and Warthin’s tumor of salivary glands. J Mol Diagn. 2004;6:205–210. doi: 10.1016/S1525-1578(10)60511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaye FJ. Emerging biology of malignant salivary gland tumors offers new insights into the classification and treatment of mucoepidermoid cancer. Clin Cancer Res. 2006;12:3878–3881. doi: 10.1158/1078-0432.CCR-06-0791. [DOI] [PubMed] [Google Scholar]
- 14.Komiya T, Park Y, Modi S, Coxon AB, Oh H, Kaye FJ. Sustained expression of Mect1-Maml2 is essential for tumor cell growth in salivary gland cancers carrying the t(11;19) translocation. Oncogene. 2006;25:6128–6132. doi: 10.1038/sj.onc.1209627. [DOI] [PubMed] [Google Scholar]