Abstract
For many years, gingival tumors of what appear to be peripherally located intraosseous ameloblastoma (IA) arising from the alveolar bone surface have often been confused with peripheral ameloblastoma (PA) causing resorption of the underlying bone. We analyzed a series of five cases of ameloblastoma that demonstrated a combined PA and IA architecture. The tumor commonly involved the anterior-premolar area, mostly in the maxilla and mainly in middle-aged men. The clinical presentation was an exophytic gingival mass inferior to which was a small bone defect. The predominant extraosseous component showed a papillary gross surface, reflecting the histologic proof of fusion between the submucosal tumor and the surface epithelium. In addition to the PA-like growth pattern, common to all was the presence of neoplastic destruction of the alveolar process, corresponding to an associated radiolucent lesion. This restrained component was acceptable as IA. In two cases, recurrence was observed deep in the alveolar bone with no involvement of the gingiva. These tumors appear to be IA that arose from the marginal alveolar bone and grew preferentially in the gingiva, forming a PA-like appearance. From diagnostic, therapeutic and prognostic points of view, this type of IA should not be confused with PA.
Keywords: Ameloblastoma, Gingival tumor, Intraosseous type, Peripheral type
Introduction
Ameloblastoma can be encountered in any area of the jaws from the body of bone through the alveolar crest to the gingiva [1, 2], and between 1 and 10% of cases are reported to occur peripherally [3]. By definition, peripheral ameloblastoma (PA) does not spread beyond the gingival submucosa into the alveolar bone [1, 3]. Thus, the final diagnosis of PA always rests with exclusion of a recognizable intrabony lesion; however, the bone defect may occur to varying degrees, when large enough [4]. It is often confusing whether such lesions are primarily PA that erode into the underlying bone or if superficial intraosseous ameloblastomas (IA) that expand out into the overlying gingiva. Indeed, several reports allowed the diagnosis of PA in the presence of bone destruction indistinguishable from IA [4–12]. Although IA of the alveolar bone surface has been referred to as PA of central origin in Gold’s review [8], the most authors rejected this view and argued that these lesions should be classified as IA [4].
In this article, five cases of ameloblastoma in the alveolar process that demonstrated both central and peripheral involvements are presented. The important clinicopathologic features and the differential diagnosis, as well as a review of the literature, are described.
Materials and Methods
Five cases of ameloblastoma with a combination of PA and IA growth pattern were retrieved from the archives of Tsurumi and Meikai University Hospitals, one of which was previously reported as PA by us [4]. Available clinical records were retrospectively reviewed and the newly prepared hematoxylin and eosin-stained slides were thoroughly examined through sectioning at many levels. This work was approved by the Research Ethics Committees.
Results
Clinical and Radiographic Findings (Table 1 and Figs. 1, 2, 3, 4)
Table 1.
Clinical information
| Case number | Sex/age | Location | Size (cm) | Radiographic patterna | Treatment | Recurrence | Year of diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | M/48 | Mx (A) | 2.0 | Cup | SE + Cu | Yesb | 1995 |
| 2 | M/75 | Mx (P) | 1.5 | SR | SE + Cu | Yesb | 2000 |
| 34c | M/28 | Mn (A-P) | 2.5 | Cup | BR | No | 2000 |
| 4 | M/54 | Mx (A) | 1.4 | Cup | BR | No | 2001 |
| 5 | M/57 | Mx (M) | 3.0 | SR | BR | No | 2009 |
M male, Mx maxilla, Mn mandible, A anterior, P premolar, A-P anterior-premolar, M molar, Cup cupping, SR small radiolucency, SE simple excision, Cu curettage, BR block resection
a Plain radiographs
b Recurrence was treated by marginal resection
c Reference number
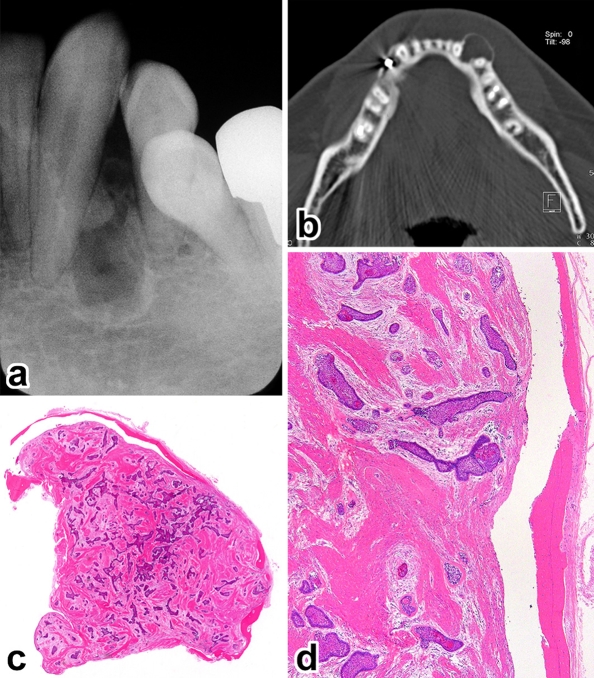
Fig. 1.
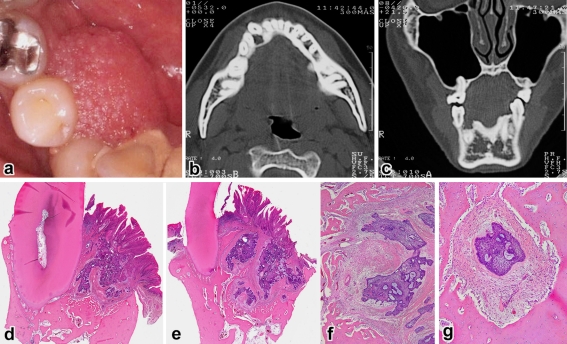
Case 3. a Pebbly surfaced mass, axial (b) and coronal (c) CT showing a pocket-shaped bone destruction, d gingival tumor showing multiple connections with the papillary surface epithelium and cupping of the underlying bone (H&E, ×6), e another area showing tumor growth far beyond the root tip (H&E, ×3), f deep-seated tumor nests (H&E, ×40), g isolated tumor follicle surrounded by the compact bone (H&E, ×100)
Fig. 2.
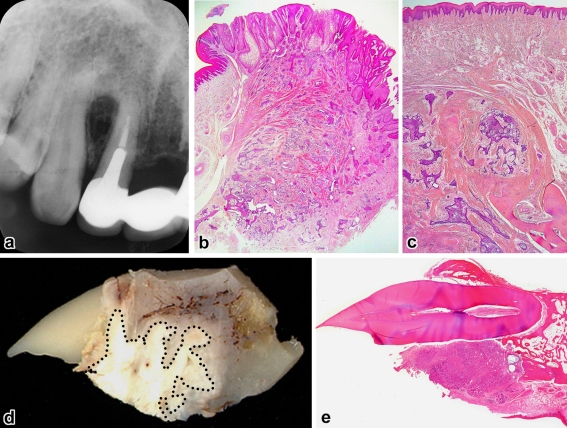
Cases 1 (a–c) and 4 (d and e). a Radiographic cupping of the interradicular bone, b gingival tumor showing scattered nests just near the excision edge (H&E, ×8), c recurrence in the bone without involving the surface epithelium (H&E, ×20), d decalcified gross specimen showing tumor location (dottedline), e surface ulceration and resorption of the palatal alveolar process (H&E, ×3)
Fig. 3.
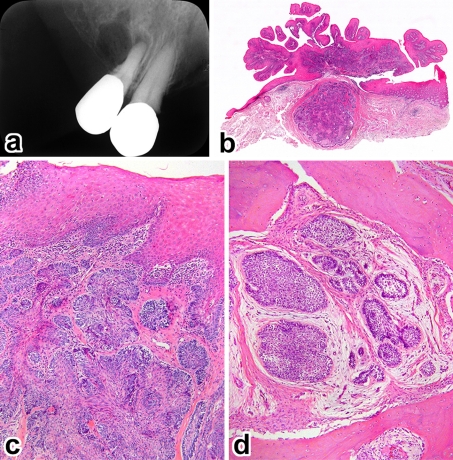
Case 2. a Superficial bone defect with a small daughter radiolucency, b papillary gingival tumor showing a discrete nodule (H&E, ×6), c submucosal tumor nests fusing with the surface epithelium (H&E, ×100), d recurrent tumor deep in the alveolar bone (H&E, ×200)
Fig. 4.
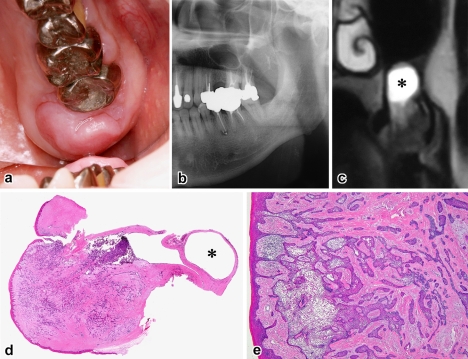
Case 5. a Exophytic nodular mass, b panoramic radiograph of bone destruction in the alveolar process, c T2-weighted MRI showing intraosseous tumor and mucosal cyst of the maxillary sinus (asterisk), d Scanning view of tumor and associated mucous retention cyst (asterisk) (H&E, ×3), e fusions of submucosal tumor with the gingival epithelium (H&E, ×40)
The patients ranged in age from 28 to 75 years with an average of 52 years and all were men. Four cases involved the maxilla and the remaining one was found in the mandible. With the exception of case 5, they had a distinct predilection for the anterior-premolar region, 2 of which involved the incisor area. The average size of tumors was 2.0 cm, ranging from 1.4 to 3.0 cm. Tumors extended in a palatal/lingual direction. The most common sign was a painless gingival swelling of several months’ duration. The lesion appeared as a broad-based, exophytic soft-tissue mass with a granular surface (Figs. 1a, 4a). A focal ulceration was recorded in case 4. Since bony expansion was minimal or none, the tumor was seen composed almost entirely of the extraosseous component which is clinically accepted as representative of the whole lesion.
On plain radiographs, three lesions showed a deep cup-like defect in the marginal alveolar bone (Fig. 2a), and an ill-defined, small radiolucency was found in cases 2 and 5 (Figs. 3a, 4b). Neither displacement nor resorption was visible in the teeth roots. In computed tomography and magnetic resonance imaging, a true invading defect was clearly demonstrated in cases 3 and 5 (Figs. 1b, c, 4c).
Treatment and Follow-up (Table 1)
Three cases were diagnosed preoperatively as ameloblastoma by incisional biopsy and managed by en bloc resection. At surgery, the breakup of the alveolar process was recorded. Although a follow-up period is variable, ranging from 6 months to 8 years, none of them recurred. In cases 1 and 2 that were treated by a combination of simple excision and curettage of tumor bed under the clinical diagnosis of papilloma, recurrence was evident within 2 years. After further marginal resection of the alveolar bone with the removal of lesional teeth, both patients were locally free of tumor for 7 years.
Pathologic Findings (Figs. 1, 2, 3, 4)
In a tissue section whole mount, extraosseous proliferations of ameloblastoma in the gingiva were predominant (Figs. 1d, 2b, 2e, 3b, 4d). All tumors fused with the surface epithelium over a wide area (Figs. 1d, 2b, 3b, 4e). Further examinations of the leading edge of tumor on multiple sections at different levels revealed destructive intraosseous proliferations, mostly at the apical root part of lesional teeth (Figs. 1e, 2d, 2e). Spread far beyond the apex in the surrounding alveolar bone was found in case 3 (Fig. 1e, f), and decalcified bone contained a few outlying tumor nests (Fig. 1g). This histomorphology was recognizable as IA. Cases 1 and 2 recurred in the bone, but the surface epithelium was intact with no neoplastic change (Figs. 2c, 3d).
Discussion
There are many indications in the literature concerning unsuspected small IA of the tooth-bearing part of the jawbone [2, 4, 13–19]. Given that the surrounding alveolar bone provides more resistance to tumor growth than does the gingiva, infiltrating IA has a tendency to extend into the overlying mucosa, either directly or along the periodontal ligament, once tumor nests penetrate the bony confine. As a result, such IA sometimes appears as a gingival mass lesion without causing any clinically visible expansion of the affected bone, thus producing the illusion of PA [8, 16, 20–23].
The co-existence of intraosseous and extraosseous tumors may cause differential diagnostic problems. Theoretically, IA would be within the bone whereas PA would be against the buccal or lingual bone. Critical to differentiating IA from PA is the identification of an intact cortical bone covering the tumor (Fig. 5). Unfortunately, this decisive finding may be obscured with time through complete loss of thinner cortical plates. As with the cases described here, new imaging modalities are more successful than plain radiographs in evaluating the actual extent of the tumor in the alveolar bone. The root divergence, one of the most characteristic radiographic signs of interradicular IA, is not marked in our cases, probably reflecting their unobtrusive intraosseous growth. Since recurrence is so likely to follow too conservative an operation, the removal of unaffected bone and involved teeth as a bloc is consequently advisable.
Fig. 5.
Intraosseous ameloblastoma. A 52-year-old man presented with a 2-cm, firm mass on the left mandible, a interradicular radiolucency similar to Fig. 2a, b axial CT showing a thin but intact cortical bone, c and d whole tissue mount showing a peripheral rim of residual bone (H&E, c, ×3; d, ×40)
With regard to the histogenesis, the rests of Malassez inside or outside the periodontal ligament space may be a potential candidate [2, 24–29]. Such epithelial residues, either in the alveolar bone surface or in the cervical or middle portion of the periodontal ligament, seem likely starting points of our cases. The previous observations that ameloblastomatoid rests appeared later in adult life and tended to increase with age lend credence to the occurrence in an older age range [2, 25–29].
To conclude, the present lesion combines an exophytic growth similar to PA with the presence of IA component ahead of the main gingival tumor. The distinction from PA may therefore become challenging [4, 8, 16, 20–23]. In view of the above summarized features, the diagnosis can not be determined by its extraosseous/intraosseous proportion. The practical approach for the surgeons is that an identifiable intrabony lesion is curious in PA [1, 3, 4], and if observed, either radiologically or clinically during surgery, should prompt consideration of IA. They also draw attention to the co-existence of insidious IA in what seems at first glance to be PA, when larger than 2 cm [4]. If, on histologic diagnosis of clinically presumed PA, there is any doubt that the tumor has proliferated from the gingiva into the underlying bone, the pathologists should re-examine the tumor-bone interface by extensive sampling of the entire specimen.
References
- 1.Gardner DG, Heikinheimo K, Shear M, Philipsen HP, Coleman H. Ameloblastomas. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 296–300. [Google Scholar]
- 2.Ide F, Mishima K, Yamada H, et al. Unsuspected small ameloblastoma in the alveolar bone: a collaborative study of 14 cases with discussion of their cellular sources. J Oral Pathol Med. 2008;37:221–227. doi: 10.1111/j.1600-0714.2007.00628.x. [DOI] [PubMed] [Google Scholar]
- 3.Jordan RCK, Speight PM. Current concepts of odontogenic tumours. Diagn Histopathol. 2009;15:303–310. doi: 10.1016/j.mpdhp.2009.03.002. [DOI] [Google Scholar]
- 4.Ide F, Kusama K, Tanaka A, Sakashita H. Peripheral ameloblastoma is not a hamartoma but rather more of a neoplasm. Oral Oncol. 2002;38:318–320. doi: 10.1016/S1368-8375(01)00124-5. [DOI] [PubMed] [Google Scholar]
- 5.Waal I, Rijcke TBM, Kwast WAM. Possible squamous odontogenic tumor: report of case. J Oral Surg. 1980;38:460–462. [PubMed] [Google Scholar]
- 6.Yamashita T, Enomoto M, Arao M, et al. Peripheral ameloblastoma: report of two cases [in Japanese with English abstract] Jpn J Oral Maxillofac Surg. 1987;33:76–82. [Google Scholar]
- 7.Iwai M, Furuta I, Ohki J, Kameyama Y. Peripheral ameloblastoma. Case report and review of the literature [in Japanese with English abstract] Jpn J Oral Maxillofac Surg. 1990;36:538–546. [Google Scholar]
- 8.Gold L. Biologic behavior of ameloblastoma. Oral Maxillofac Surg Clin N Am. 1991;3:21–71. [Google Scholar]
- 9.Ohuchida M, Tanaka S, Kusukawa J, Nagata A, Okina T, Kameyama T. A case of peripheral ameloblastoma arising in the maxilla [in Japanese] Jpn J Oral Maxillofac Surg. 1992;38:1437–1438. [Google Scholar]
- 10.Lopez-Jornet P, Bermejo-Fenoll A. Peripheral ameloblastoma of the gingiva: the importance of diagnosis. J Clin Periodontol. 2005;32:12–15. doi: 10.1111/j.1600-051X.2004.00627.x. [DOI] [PubMed] [Google Scholar]
- 11.LeCorn DW, Bhattacharyya I, Vertucci FJ. Peripheral ameloblastoma: a case report and review of the literature. J Endod. 2006;32:152–154. doi: 10.1016/j.joen.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 12.Vanoven BJ, Parker NP, Petruzzelli GJ. Peripheral ameloblastoma of the maxilla: a case report and literature review. Am J Otolaryngol. 2008;29:357–360. doi: 10.1016/j.amjoto.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Small IA, Waldron CA. Ameloblastomas of the jaws. Oral Surg Oral Med Oral Pathol. 1955;8:281–297. doi: 10.1016/0030-4220(55)90350-9. [DOI] [PubMed] [Google Scholar]
- 14.Colby RA. Odontogenic tumors. Dent Clin N Am. 1957; 709–719.
- 15.Kajiyama M, Dohjyo M, Onizuka K. Ameloblastoma of the mandibular anterior region: report of a case [in Japanese] Jpn J Oral Surg. 1974;20:462–468. doi: 10.5794/jjoms.20.462. [DOI] [PubMed] [Google Scholar]
- 16.Iida O, Ono T, Sugiyama Y, et al. Ameloblastoma located in the alveolar region: report of two cases [in Japanese] Jpn J Oral Maxillofac Surg. 1983;29:1600–1605. [Google Scholar]
- 17.Fowler CB. Benign and malignant neoplasms of the periodontium. Periodontology. 2000 1999; 21: 33–83. [DOI] [PubMed]
- 18.Faitaroni LA, Bueno MR, Carvalhosa AA, Ale KAB, Estrela C. Ameloblastoma suggesting large apical periodontitis. J Endod. 2008;34:216–219. doi: 10.1016/j.joen.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Ide F, Mishima K, Saito I, Kusama K. Diagnostically challenging epithelial odontogenic tumors: a selective review of 7 jawbone lesions. Head Neck Pathol. 2009;3:18–26. doi: 10.1007/s12105-009-0107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawai T, Shigeto M, Fukuma K, Izumi K, Katayama T. A case of maxillary ameloblastoma with a polyp-like appearance [author’s transl] Jpn J Oral Surg. 1959;5:353–356. [Google Scholar]
- 21.Stevenson ARL, Austin BW. A case of ameloblastoma presenting as an exophytic gingival lesion. J Periodontol. 1990;61:378–381. doi: 10.1902/jop.1990.61.6.378. [DOI] [PubMed] [Google Scholar]
- 22.Miyoshi N, Nishijima K, Mizukawa N, Mori T. A case of intraosseous ameloblastoma presenting clinically as peripheral ameloblastoma [in Japanese] Jpn J Oral Maxillofac Surg. 1992;38:1683–1684. [Google Scholar]
- 23.Yasui T, Onizawa K, Tanaka Y, Hyodo T, Kodaka R, Iwasaki R. A case of mandibular ameloblastoma with papillomatous proliferations in the gingiva [in Japanese with English abstract] Jpn J Oral Maxillofac Surg. 2009;55:419–423. [Google Scholar]
- 24.Suzuki I. Histopathological study of epithelial islands in reactive hyperplasia of the gingiva [in Japanese with English abstract] Jpn J Oral Biol. 1984;26:1278–1294. [Google Scholar]
- 25.Ide F, Obara K, Yamada H, et al. Hamartomatous proliferations of odontogenic epithelium within the jaws: a potential histogenetic source of intraosseous epithelial odontogenic tumors. J Oral Pathol Med. 2007;36:329–335. doi: 10.1111/j.1600-0714.2007.00488.x. [DOI] [PubMed] [Google Scholar]
- 26.Orban B. Einige besondere Epithelbefunde [in German] Schweiz Mschr Zahnheilk. 1926;36:628–634. [Google Scholar]
- 27.Higaki R. Besondere Befunde von Epithelnestern außerhalb des Periodontiums [in German] Dtsch Mschr Zahnheilk. 1932;50:337–341. [Google Scholar]
- 28.Masaki T, Kondo S. Distribution of the Mallassez’s epithelial nests at the regions outside of the periodontal space [in Japanese] J Jpn Odont Soc. 1933;30:285–294. [Google Scholar]
- 29.Masaki T. Welche klinische Beziehung hat der patho-histologische Bau der Kiefergeschwulst [in Japanese] Folia Odont Prac. 1939;11:229–265. [Google Scholar]