Clinical Presentation
A 37-year-old woman had a urinary tract infection in July 2009 that was successfully treated by antibiotics but resulted in diarrhea. She was placed on a probiotic diet and then became aware, during the same month, of persistent and widespread, bilateral oral lesions. The lesions on the tongue were somewhat painful and she was given miracle mouthwash for symptomatic relief. She denied dysphagia, odynophagia, neck swelling or masses, headaches, visual problems, skin lesions, or otalgia. She also denied tobacco or alcohol abuse.
Differential Diagnosis
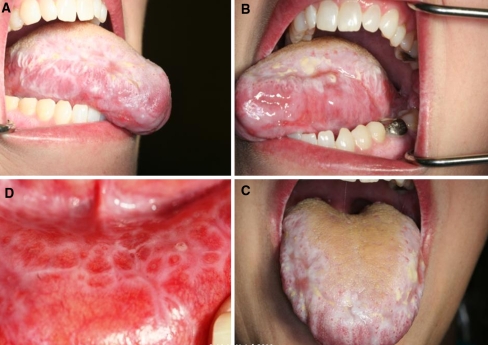
Clinical examination revealed diffuse involvement of the oral cavity with striated white lesions and areas of erosion and shallow ulceration. A distinct reticular pattern was noted on the lower labial mucosa. Erosive lichen planus was a prime consideration in this case due to the distribution and clinical appearance of the lesions. Lichen planus is a common condition of unknown etiology but believed to be immune mediated, can affect both the skin and oral mucosa. It develops primarily in middle-aged adults, and females are affected more often than males. Lesions can present bilaterally on the posterior buccal mucosa, but other oral sites such as the tongue, gingiva, lips, and palate may also be involved. The lesions of erosive lichen planus appear erythematous, often with central ulceration. Fine white striae are classically observed around the periphery of the ulcers.
Several other immunologic and allergic conditions can present with a clinical pattern similar to lichen planus including lupus erythematosus, chronic ulcerative stomatitis, erythema multiforme and graft-versus-host disease. The history of lesions developing while the patient was on a probiotic diet raises suspicion for the possibility of an allergic mucosal reaction to diet but it may also related to systemic antibiotics. Probiotics are commonly used to treat antibiotic-associated diarrhea which was the most probable scenario in the present case. They are available in the form of foods and dietary supplements and are not regulated by the Food and Drug Administration. Drug reactions of the oral mucosa can have a variety of clinical appearances, including lichenoid and lupus-like patterns. While probiotics have not been specifically implicated in mucosal drug reactions, the nature and composition of the patient’s diet were unknown. Resolution of lesions would be expected with discontinuation of the potentially offending agent.
Chronic ulcerative stomatitis (CUS) is a rare and recently-described immune-mediated condition that also deserves consideration in this case. Erosions and ulcerations of the tongue and buccal mucosa are common. Irregular red and white areas typically surround the ulcers, but defined striae may not be present. Similar to erosive lichen planus, CUS may also present as a desquamative gingivitis. The disease predominates among females in the sixth decade [1] which is a slightly older population when compared to lichen planus. Since CUS usually responds best to Plaquenil, an incisional biopsy with direct immunofluorescence is recommended for erosive lichenoid processes, especially cases that are unresponsive to corticosteroids.
Lupus erythematosus (LE) is a common, multisystem, immunologic condition that can present with a variety of oral manifestations. Systemic lupus erythematosus (SLE) is the most serious form of the disease due to the potential for kidney involvement, which can eventually progress to renal failure. Cutaneous, musculoskeletal, neurologic, and cardiac involvement are also common. Oral lesions may be seen in up to 40% of patients [1]. The average age at diagnosis is 31 and women are affected 8–10 times more often than men [1]. Chronic cutaneous lupus erythematosus (CCLE) is a much less severe form of LE that is usually limited to the skin and oral mucosa. A third form, subacute cutaneous lupus erythematosus (SCLE), has clinical overlap between SLE and CCLE. Oral lesions of LE may be lichenoid, granulomatous, or nonspecific in appearance. Typical anatomic locations affected include the buccal mucosa, palate, gingiva, and occasionally the lower lip. Given the age and sex of the patient, as well as the clinical appearance and distribution of the oral lesions, LE is another logical consideration in the differential diagnosis of this case.
Diagnosis and Discussion
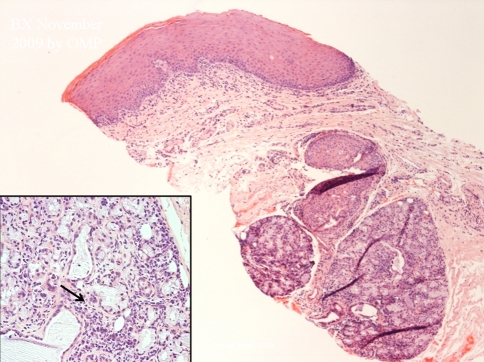
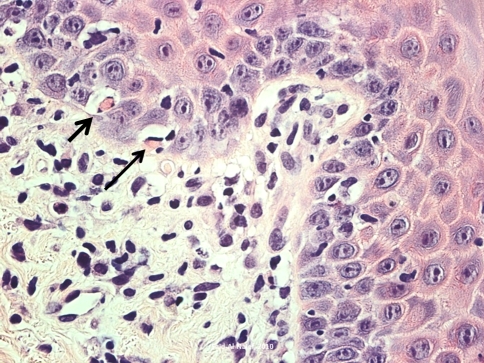
The patient in the current case was diagnosed with AML-M4 (acute myelogenous leukemia) in February of 2006 with normal cytogenetics. The leukemia was initially treated with induction chemotherapy followed by a HLA-matched BMT (human leukocyte antigen matched bone marrow transplant) from a sibling donor in July of 2006. She suffered from several opportunistic infections following the transplant, including Coag (−) Staphylococcus bacteremia, Acinetobacter bacteremia, AFB (acid fast bacillus) infection, Pseudomonas aeruginosa and Proteus mirabilis. The patient achieved remission following the transplant but developed graft-versus-host disease (GVHD) limited to the upper gastrointestinal tract area which was managed with photopheresis. She received re-induction chemotherapy with clofarabine and high dosage Ara-C after a relapse in the summer of 2007, which was followed by consolidation therapy with high dose Ara-C and idarubicin in September of 2007. This was then followed by a match-unrelated stem cell transplant, treatment with fludarabine and total body irradiation conditioning in November 2007. She again developed several opportunistic infections in July of 2009, including Coag (−) Staph bacteremia, cholecystitis, pancreatitis secondary to Diazide diuretics, and herpes labialis. The herpes labialis was initially resistant to Valtrex and acyclovir but was subsequently responsive to cidofovir. In July 2009, she developed generalized oral lesions with a lichenoid pattern on the gingiva, upper and lower labial mucosa, bilateral buccal mucosa, right and left lateral tongue, and posterior palate (Figs. 1, 2). Her oral lesions were erosive in some areas. The tongue, which also exhibited a reticular pattern, displayed erosive areas and the patient experienced prominent glossodynia. Cytology smears obtained for fungal identification, as well as fungal cultures, were persistently negative. There was only limited relief with topical antifungal and magic mouth rinses (comprised of 80 ml viscous lidocaine 2%, 80 ml Mylanta and 80 ml diphenhydramine 12.5 mg per 5 ml elixir). A superficial tongue biopsy performed in September 2009 revealed parakeratosis, but was essentially inconclusive since the sample did not contain any lamina propria. This was followed by a biopsy of the lower lip mucosa which was diagnostic of the patient’s condition, demonstrating mild keratosis with paucicellular interface mucositis and marked epitheliotropism. Scattered apoptotic bodies were detected at the basal layer of the epithelium. The lamina propria supported a patchy mononuclear inflammatory cell infiltrate including few macrophages. The histomorphological features were consistent with graft-versus-host disease (Figs. 3, 4). The patient was treated with prednisolone syrup 15 mg/5 ml with instructions to rinse and swallow for 2 min with 1 teaspoonful qid. The tongue symptoms resolved and her lesions showed significant improvement.
Fig. 1.
Generalized leukoplakia and shallow ulcerations were noted on the maxillary and mandibular gingiva (a–c) and hard palate (d) (Note the reticular pattern seen in the palatal lesions)
Fig. 2.
Striated white lesions and ulcerations were noted on the right and left lateral tongue (a, b). The dorsal tongue (c) was affected at the anterior and lateral aspects. A well-defined reticular pattern was also evident on the lower lip mucosa (d)
Fig. 3.
Biopsy of the lower lip mucosa revealed a paucicellular interface lichenoid inflammatory cell infiltrate. Periductal mononuclear inflammatory cell infiltrate was also seen in the labial minor salivary glands. (arrow, insert) (Hematoxylin and eosin stain original magnification 10×, insert original magnification 20×)
Fig. 4.
A high power view of the histomorphology of the lower lip biopsy showing prominent epitheliotropism and several apoptotic bodies identified in the basal layer of the epithelium (arrows). The lamina propria contained a mononuclear inflammatory cell infiltrate and scattered macrophages (Hematoxylin and eosin stain original magnification 40×)
GVHD is serious complication that occurs in recipients of allogenic hematopoietic stem cell transplantation, non-irradiated blood transfusions, or transplacental lymphocyte transfusion to an immunodeficient fetus. Bone marrow transplantation is a commonly implemented therapy for the management of hematologic malignancies, hemoglobinopathies, and selective autoimmune disorders, and GVHD is considered to be a major limitation to the success of the transplant procedure.
GVHD occurs as a result of the infusion of immunocompetent donor T cells into the patient who is immunocompromised as a result of reconditioning, total body irradiation, and high dose chemotherapy. This results in a cascade of complex processes that include CD8+ and CD4+ T cell differentiation and activation, recognition of major and minor histocompatibility antigens, and the liberation of a number of cytokines and interleukins in various stages including INF-α, IL1 and IL2 among others. This ultimately results in the formation of natural killer T cells and cytotoxic T cells, the latter being responsible for damage of many of the host’s internal organs including the liver, gastrointestinal tract, skin, and mucous membranes [2–5].
The acute type of GVHD is observed within the first 21 days to 3 months after BMT and is seen in approximately 75% of transplanted patients. Acute GVHD is characterized by TH-1 type cytokine production pattern with skin, liver, and gastrointestinal lesions, and is caused by cytotoxic T cells and NK cells. In comparison, the chronic form of GVHD is characterized by TH-2 type cytokine production with systemic manifestations and autoimmune disease type features and behavior. Chronic GVHD is seen in approximately 25% of BMT survivors and it usually takes place 3 months after the BMT. Acute and chronic GVHD also differ in their clinical and histomorphologic presentation [3, 6–9]. Acute GVHD may initially present with nonspecific signs and symptoms including fever, malaise, and faint facial rash that rapidly spreads as a morbilliform maculopapular rash to other areas, especially the palms and soles. This is accompanied by watery diarrhea, jaundice, and hepatomegaly with elevation of liver enzyme profiles. It can also present with more severe, toxic epidermal necrolysis—like features. Chronic GVHD, on the other hand, is usually characterized by systemic involvement of the eyes, esophagus, liver, muscle, genitalia, skin, as well as the central and peripheral nervous systems. Rarely seen are features similar to lichen planus, scleroderma and discoid lupus [2, 3, 5, 7, 9].
Tremendous overlap exists between the histomorphological presentation of acute and chronic GVHD, demonstrating lichen planus-like features with focal or diffuse basal cell hydropic change, apoptotic and dyskeratotic changes in all layers of epithelium, with closely associated lymphocytes and cytoid bodies. A paucicellular, predominantly chronic inflammatory cell infiltrate that may contain patchy neutrophilic and eosinophilic components is seen in the lamina propria. The acute lesions may also show, at least in early stages, lichen planus-like features but more scarring in the later or “sclerodermatous” phase of the disease [3, 5, 6].
With the advances in hematopoietic stem cell transplantation in the last two decades, the validity of the criteria of separation between chronic and acute GVHD was questioned since the separation was more or less arbitrary. The acute form may persist beyond 3 months in patients receiving reduced-intensity conditioning and the manifestations of acute and chronic GVHD can be present simultaneously. This generated the current consensus by the NIH that clinical manifestations, not the time of onset of symptoms following the transplant, is what determines if GVHD is acute or chronic [3, 5, 9].
Oral involvement is seen in 33–50% and 60–80% of acute and chronic GVHD, respectively. Lesions may vary in clinical presentation, spectrum, and severity, ranging from simple, punctate granular erythema with mucosal atrophy to superficial, widespread ulceration to reticular and erosive lichen planus-like and scleroderma—like features. The oral lesions of GVHD may also appear as bulla and vesicles and should therefore be distinguished from other entities that occur in this region. The salivary gland tissue also exhibits Sjogrens syndrome-like features histologically, such as periductal and perivascular lymphocytic or mixed mononuclear inflammatory cell aggregates. Careful and prolonged follow-up is necessary since there is a well-documented risk of transformation to oral squamous cell carcinoma with more than 64 cases reported in the last 30 years [2, 5, 6, 9].
In conclusion, the present case represents a well documented example of oral GVHD that occurred in a patient with AML. The exact time of onset of these lesions was difficult to determine but her oral complaints were brought to the attention of her clinicians after forty 8 months of the initial bone marrow transplant procedure. Currently, her oral lesions remain well controlled with topical prednisone rinse and she will also remain under careful clinical follow-up. Additional biopsies will be taken in 6 months to exclude any possible dysplastic changes which are well documented in oral GVHD lesions.
References
- 1.Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and maxillofacial pathology. 3. St. Louis, MO: Saunders; 2009. [Google Scholar]
- 2.Ratanatharathorn V, Ayash L, Lazarus HM, Fu J, Uberti JP. Chronic graft-versus-host disease; clinical manifestation and therapy. Mini review. Bone Marrow Transplant. 2001;28:121–129. doi: 10.1038/sj.bmt.1703111. [DOI] [PubMed] [Google Scholar]
- 3.De la Rosa Garcia E, Bologna Molina R, Jesus Vega Gonzalez MT. Graft-versus-host disease, an eight case report and literature review. Med Oral Patol Oral Cir Buccal. 2006;11:E486–E492. [PubMed] [Google Scholar]
- 4.McKee PH, Calonje E, Granter S. Pathology of the skin with clinical correlation. 3. Philadelphia, PA: Elsevier; 2005. [Google Scholar]
- 5.Filipovich AH, Weisdorf D, Pavletic S, et al. National institutes of health concensus development project on criteria for clinical trials in chronic graft-versus-host-disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–955. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Shubert MM, Sullivan KM, Morton TH, Izutu KT, Peterson DE, Flournoy N, et al. Oral manifestations of chronic graft-vs-host-disease. Arch Intern Med. 1984;144:1591–1595. doi: 10.1001/archinte.144.8.1591. [DOI] [PubMed] [Google Scholar]
- 7.Peterson DE. Oral toxicity of chemotherapeutic agents. Semin in Oncol. 1992;19:478–491. [PubMed] [Google Scholar]
- 8.Nicolatou-Galitis O, Kitra V, Vliet-Constantinidou C, Peristeri J, Goussetis E, Petropoulos D, et al. The oral manifestations of chronic graft-versus-host disease (cGVHD) in pediatric allogenic bone marrow transplant recipients. J Oral Pathol Med. 2001;30:148–153. doi: 10.1034/j.1600-0714.2001.300304.x. [DOI] [PubMed] [Google Scholar]
- 9.Del Pozo J, Garcia-Silva J, Yebra-Pimentel MT. Chronic graft-versus-host disease presenting as bullous lesions. Actas Dermosifiliogr. 2008;99:803–807. doi: 10.1016/S0001-7310(08)74961-5. [DOI] [PubMed] [Google Scholar]