Abstract
Injury to growth plate cartilage in children can lead to bone bridge formation and result in bone growth deformities, a significant clinical problem currently lacking biological treatment. Mesenchymal stem/stromal cells (MSC) offer a promising therapeutic option for regeneration of damaged cartilage, due to their self renewing and multi-lineage differentiation attributes. Although some small animal model studies highlight the therapeutic potential of MSC for growth plate repair, translational research in large animal models, which more closely resemble the human condition, are lacking. Our laboratory has recently characterised MSCs derived from ovine bone marrow, and demonstrated these cells form cartilage-like tissue when transplanted within the gelatin sponge, Gelfoam, in vivo. In the current study, autologous bone marrow MSC were seeded into Gelfoam scaffold containing TGF-β1, and transplanted into a surgically created defect of the proximal ovine tibial growth plate. Examination of implants at 5 week post-operatively revealed transplanted autologous MSC failed to form new cartilage structure at the defect site, but contributed to an increase in formation of a dense fibrous tissue. Importantly, the extent of osteogenesis was diminished, and bone bridge formation was not accelerated due to transplantation of MSCs or the gelatin scaffold. The current study represents the first work that has utilised this ovine large animal model to investigate whether autologous bone marrow derived MSC can be used to initiate regeneration at the injured growth plate.
Keywords: Mesenchymal stem cell, bone marrow, ovine, growth plate, tissue engineering.
INTRODUCTION
The identification of self renewing multipotential progenitor cells from adult stromal tissues has stimulated significant interest and promise for the utilisation of these cells in regenerative medicine and tissue engineering. Since the discovery of putative mesenchymal stem/stromal cells (MSC) from bone marrow suspensions [1] with the ability to form adherent clonogenic clusters (CFU-F), and capacity to differentiate into a multitude of cell types in vitro and in vivo, MSCs have now been isolated from numerous connective tissues [2]. In addition, MSCs appear to share similar characteristics across species, which has facilitated the application of MSC in translational studies using animal models.
The growth plate is a unique cartilaginous tissue located at the proximal and distal ends of the long bones of children, and is responsible for longitudinal bone growth until closure at skeletal maturity. Due to the avascular and alymphatic nature of cartilage, once damaged, cartilage has a poor regenerative capacity. Injury to growth plate cartilage often results in an undesirable repair response mechanism at the site of injury, where ossification of the damaged tissue may lead to formation a bone bridge. Establishment of a bone bridge across the growth plate can have serious consequences in children, and may lead to limb length discrepancies and angular deformity. Current treatments of limb abnormalities include surgical correction of angularity or length discrepancy after manifestation of the deformity. To date, there is no cell-based biological based therapy to prevent bone bridge formation and regenerate the damaged growth plate cartilage in clinical practice [2].
Although there have been some recent investigations in rabbit or rodent models examining potential applications of MSCs in repairing injured growth plate with some success [3-6], the therapeutic potential of MSCs in growth plate regeneration remains to be investigated in a large animal model. The use of sheep as a large animal model for orthopaedic research continues to increase in popularity due to similarities with humans in weight, size, joint structure, bone/cartilage regenerative processes, and thus the potential in translational research.
Our laboratory has undertaken numerous studies employing a sheep model to investigate mechanisms of growth plate injury repair, techniques for the prevention of bone bridge formation, and reversal of skeletal deformity [7-13]. Several interpositional materials have been assessed for their potential to impede or prevent bone formation and regenerate the damaged cartilage. These include fat, cultured chondrocytes [7, 9], cartilage [11], periosteum [11], and a type 1 collagen paste [12].
We have recently described the functional and phenotypic properties of ovine bone marrow derived MSCs, including incidence, immunophenotype, proliferative response to mitogens, and differentiation potential both in vitro and in vivo [14]. In this pilot study the potential of autologous MSCs to repair damaged growth plate cartilage in an immature ovine model was investigated.
MATERIALS AND METHODS
Subjects and Cell Culture
All procedures were approved by the institutional animal ethics committee and performed under sterile conditions. Fresh bone marrow aspirates were obtained from the iliac crest of 5 lambs (approximately 8 weeks old) under general anaesthesia according to procedures approved by the ethics committee of the Women’s & Children’s Hospital, South Australia. Bone marrow mononuclear cells (BM MNC) were isolated by centrifugation of aspirates on a Lymphoprep™ density gradient (S.G. 1.077 g/ml) (Axis Shield or Nycomed, Oslo, Norway) as previously described [15, 16].
Ovine Primary Mesenchymal Stem Cell Cultures
Briefly, single cell suspensions of BM MNC were plated in monolayer in α-MEM media supplemented with penicillin (50 i.u./ml)/streptomycin sulphate (50 μg/ml), 10% (v/v) fetal calf serum (FCS; SAFC Biosciences, Lenexa, KS), 2 mM L-glutamine (SAFC Biosciences), 1 mM sodium pyruvate (SAFC Biosciences), and 100 μM L-ascorbate-2-phosphate (Asc-2-P, WAKO Pure Chemical Industries Ltd, Osaka, Japan) and incubated at 37°C in the presence of 5% CO2. After 3 days, non adherent cells were removed and fresh media replaced. Upon confluence, cells were detached by enzymatic digestion in 0.05% (w/v) trypsin (Gibco, Invitrogen, Carlsbad, CA, USA) and 0.5 mM EDTA (BDH AnalaR®, Merck) in sterile PBS.
Transplantation of Autologous MSC to Growth Plate Defect in Lambs
Four million lamb MSCs (P1) were resuspended in serum replete chondrogenic media containing growth factor TGF-β1 (10 ng/ml) and statically seeded onto Gelfoam sponge (Pharmacia & Upjohn) (approximately 10 x 8 mm squares). The MSC were consolidated within the Gelfoam sponge using a fibrin clot (gift from Mr. Rick Tocchetti, IMVS, Adelaide, Australia).
The procedure for creation of a small (10 mm deep x 10 mm wide x 5 mm high) growth plate defect at the proximal tibia of lambs has been established previously by Foster et al. [9, 13]. The defect site was immersed in 100 μl of 1 Unit/ml Chondroitinase ABC (Sigma-Aldrich), which was removed by aspiration and blotted dry after 5 mins. The Gelfoam scaffold with or without MSCs was placed within the defect site. Nine lambs were divided into two groups. One group (n=5) received a growth plate defect on both hind legs, with one defect on one limb being filled with Gelfoam scaffold in chondrogenic media containing no cells, with the defect on the contralateral limb receiving the Gelfoam scaffold seeded with 4 x 106 autologous MSC. The other group (n = 4) received an untreated defect on one limb and the contralateral limb was uninjured as a normal control. Two titanium Kirschener wires (K-wires) were inserted after wound closure perpendicular to the growth plate, in the epiphysis and diaphysis respectively, using a F-shaped template of 20 mm separation, in order to facilitate the precise measurement of limb growth following partial growth plate disturbance.
All animals were sacrificed five weeks post-operatively. In order to perfuse, fix, and collect tissue specimens, animals were sedated and general anaesthesia was induced as previously described [9, 13]. Following catheterisation the leg was perfused with 500ml of 1% sodium nitrite in 0.1 M phosphate buffer. During the sodium nitrite perfusion, the animal was euthanized by an overdose of sodium pentobarbitone (10ml, Lethabarb, Virbac, Milperra, NSW, Australia). Subsequently, each leg was perfused with 500 ml of 10% buffered formalin. The hind leg was removed and the portion (15 mm x 15 mm x 15 mm) containing the defect was excised using an Isomet Low Speed Saw (Buehler Ltd., Lake Bluff, IL, USA) and placed in a solution of 10% buffered formalin for 48 hours. Following fixation, the sample was decalcified in Immunocal (United Biosciences, Carindale, Qld, Australia) for 7 days and processed for paraffin embedding, from which 5 µm sections were cut and stained with Haematoxylin, Eosin, and 1% Alcian Blue.
Growth Plate Injury Repair Measurements and Statistical Analysis
Measurement of the area representative of different repair tissue types (mesenchymal, bone, cartilage, marrow, and fat) within lamb growth plate defects was performed using the Olysia BioReport software imaging system (Olympus Corporation, Tokyo, Japan). Measurements were taken within a defined area adjacent to the intact growth plate and calculated as the percentage of the total area measured [17]. The measurements for each group were combined and compared for statistical significance using either a paired t-test or for non parametric data, the Mann-Whitney test. Statistical significance was confirmed where p < 0.05.
RESULTS
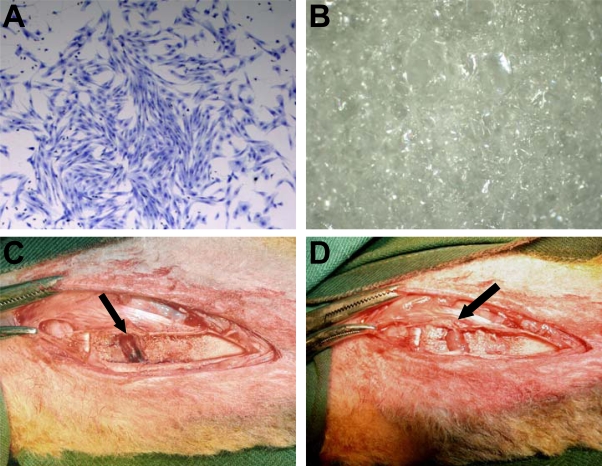
Morphologically, ovine MSC are a large adherent population exhibiting a heterogeneous phenotype including cells resembling fibroblasts, polygonal, stellate, and spindle shaped cells with long processes (Fig. 1A). Gelfoam, a medical device made from porcine skin gelatin and used clinically, was used as a scaffold carrier for MSC implantation in this study (Fig. 1B). Merino cross lambs (8 to 10 weeks old) were placed under general anaesthesia, and using a high speed dental drill and small dental burr (2 mm), a single partial peripheral growth plate defect was created of approximately 1 cm2 (Fig. 1C). In the instance of transplantation with scaffold or scaffold plus autologous MSC, the Gelfoam implant was easily placed and fit into the defect site, and due to the absorbent and spongy characteristic of the scaffold, was highly compatible with the topography of the defect (Fig. 1D).
Fig. (1).
Representative example of the morphological phenotype of ovine BM derived MSC (A). BM MNCs were cultured in vitro and stained with Toluidine Blue at day 14 (20x). Gelfoam is a naturally occurring scaffold derived from purified porcine gelatin, with a sponge– like appearance (B). Surgical creation of a growth plate defect and transplantation of Gelfoam scaffold. A medial longitudinal incision was made over the proximal tibia. The growth plate can be visualised as the thin white line running along the bottom of the defect. An approximately 1 cm2 excision was made to the growth plate (arrow) using a small dental burr (C). Transplanted Gelfoam scaffold within the growth plate defect (arrow) (D). As the scaffold is absorbent and pliable, it can easily be placed within, and completely fill the injury site.
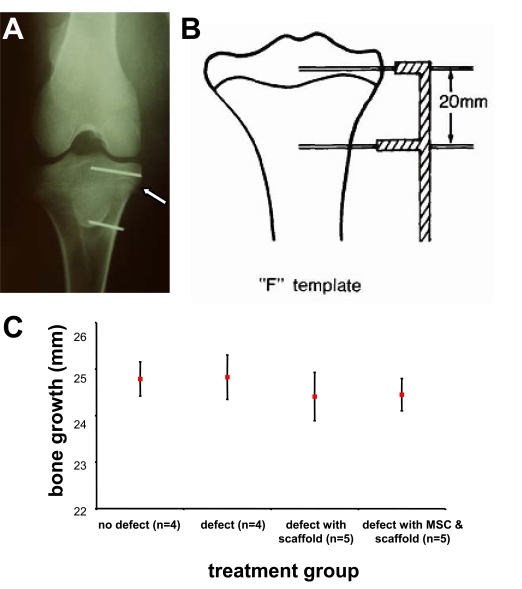
The insertion of K-wires proximally and distally to the growth plate (Fig. 2A) to measure longitudinal bone growth was performed utilising an F-shaped template (Fig. 2B). Comparison between the different treatment groups at 5 weeks post surgery showed there were no statistically significant differences in the rate of limb growth in comparison to the uninjured control (Fig. 2C).
Fig. (2).
Kirschner wire (K-wire) marker placement as an indicator of bone growth post operatively. Antero-posterial X-Ray of a lamb tibia indicating K-wire marker placement (A). The white arrow indicates the location of the growth plate. Epiphyseal and diaphyseal pins were placed 20 mm apart on the day of surgery using an F shaped template to ensure parallel and equidistant insertion of the K-wires (B). The distance between the pins was measured using callipers to determine the amount of post-operative limb growth in 5 weeks (C). Total limb growth is estimated by the 5-week differences of the distance (mm) of the two implanted K-wires below and above the defect (mean ± SEM), showing no statistically significant differences in the rates of limb growth between groups compared to non-injured control.
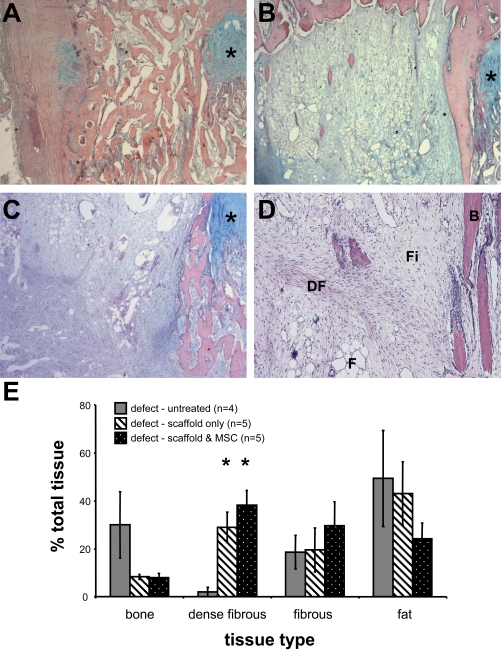
Histological examination of the untreated samples revealed extensive bone formation within the defects (Fig. 3A). Interposition of Gelfoam scaffold alone to the defect site, on the other hand, resulted in bone bridge-free repair comprised of dense fibrous tissue, fibrous tissue and fat (Fig. 3B). A bone spur adjacent to the growth plate was observed in nearly all samples examined, with some small trabecular bone deposits originating from the metaphysis and epiphysis. Examination of the tissue formed within the defect site following transplantation of Gelfoam scaffold loaded with autologous MSC revealed tissue deposits being comparable to those in defects treated with scaffold alone (Fig. 3C). Although fibrous tissue, fat, and some bone were also observed, overall the repair tissue was more cellular and extracellular tissue was denser. Although samples treated with autologous MSC exhibited a medial bone spur adjacent to the growth plate, the bony deposits originated from the periphery (mainly metaphyseal region) of the defect, and virtually no bone was detected centrally.
Fig. (3).
Surgical creation of a growth plate defect and analysis of repair tissues 5 weeks after untreated or treated with interpositional transplantation of Gelfoam scaffold alone or seeded with autologous BM MSC. Representative images of repair tissues on cross sections stained with Haematoxylin, Eosin, and Alcian Blue (A-C). The location of the growth plate is indicated by *. A representative example of extensive bone bridge formation is observed in the untreated defect (2x) (A). A representative example of growth plate defect treated with Gelfoam scaffold only (2x) (B), and defect treated with Gelfoam scaffold and autologous MSC (2x) (C). An example of different tissues observed within defect (4x) (D), which include bone (B), fat (F), fibrous tissue (Fi), and dense fibrous tissue (DF). Using Image Analysis software, the proportions of each repair tissue type were determined and are presented at the % mean area ± SEM of the total injury area for each group (E). Statistical significance between treatment groups is indicated by a * (Mann-Whitney, p < 0.05).
Quantitative histological analysis (Fig. 3D) of the proportions of repair tissues (including bone, dense fibrous tissue, fibrous tissue and fat) within the injury site show that the predominant tissue observed within the defect site of the untreated group was adipose-like tissue (49.4 ± 20.0 % of the total injury area), closely followed by bone (30.0 ± 13.8%) (Fig. 3E). In contrast, the tissue composition of defects treated with Gelfoam scaffold without cells was in the order of fat (43.1 ± 13.2%), dense fibrous tissue (29.0 ± 6.3%), fibrous tissue (20.0 ± 9.2%) and bone (8.3 ± 1.1%). Compared with the untreated control, there was approximately 72% reduction in amount of bone formation when the interpositional Gelfoam scaffold was applied. Moreover, a 14 fold increase in dense fibrous tissue was observed. The quantity of fibrous tissue remained consistent, with fat content decreasing slightly by approximately one tenth. Analysis of growth plate defects treated with Gelfoam scaffold and autologous MSC revealed a tissue composition consisting of dense fibrous (38.2 ± 6.1%), fibrous (29.6 ± 10.0%), fat (24.2 ± 6.6%) and bone (7.9 ± 1.8%). In comparison to the untreated control, the proportion of bone formation decreased by approximately three quarters following transplantation of autologous MSC seeded Gelfoam scaffold. In addition, a 19 fold increase in dense fibrous tissue was observed. The proportion of fibrous tissue was over 1.5 times that of the control group, while conversely, the proportion of fat was halved.
Several differences were observed between treatments with the scaffold alone (interpositional) and the scaffold seeded with autologous BM MSC. Whilst the proportion of bone was equivalent between the two groups, in the group treated with scaffold seeded with MSC, fibrous tissue was 1.5 times greater, and fat content was half that of the scaffold only group. Lastly, the amount of dense fibrous tissue in the scaffold only treatment was three quarters that of scaffold plus MSC.
DISCUSSION
Previous description of growth plate injury in animal models have reported bone bridge formation in rats [17-24], mice [25], rabbits [26], and dogs [27] 2 to 3 weeks following the surgical procedure. Previous ovine studies have determined an infiltration of inflammatory cells 4 days post surgery, and ossification of the defect site within 14 days [11-13], comparable to that observed in rats [19]. To date, successful repair of growth plate defects using MSC has been achieved using agarose, chitin, gelatin and Gelfoam composites, and fibrin glue [3-6, 28]. The aforementioned studies all utilised a rabbit model, however the therapeutic potential of MSCs for growth plate regeneration in larger animal models has not been documented.
Recently, we demonstrated that adult ovine bone marrow aspirates contain a proportion of CFU-F cells with phenotypic and functional similarities to human MSC capable of multipotential differentiation in vitro and in vivo [14]. Undifferentiated ovine BM MSC seeded in Gelfoam with TGF-β1 form cartilage-like tissue, but not osteogenic tissue, following subcutaneous transplantation in immuno-comprimised mice [14]. In the current study, Gelfoam scaffold was further selected for application in a model of growth plate injury for three additional reasons. Firstly, used clinically as a haemostatic agent, the intrinsic properties of Gelfoam may be beneficial in inhibiting the early inflammatory response that occurs following mechanical disruption of the growth plate, by physically impeding the infiltration of inflammatory cells. Secondly, the topography of Gelfoam renders it ideal for implantation in the defect created here using a lamb model. Thirdly, the characteristic of Gelfoam to withhold a high fluid content within its matrices may recapitulate cartilage tissue where the water content can extend to 80% of the wet weight. Gelfoam or gelatin sponges have been applied in studies examining chondrogenesis in vivo and cartilage repair, using both chondrocytes [29, 30] and MSC [3, 31-33].
The current study represents the first work that has utilised this ovine large animal model to investigate whether bone marrow derived MSC can be used to initiate regeneration at the injured growth plate. Results indicated that the application of MSC to the site of growth plate injury did not result in chondrogenic differentiation or regeneration of the damaged cartilage, but formation of dense fibrous tissue. The differentiation of cells to form more fibrous repair tissue may be attributed to the inflammatory response at the injury site, which may overwhelm signals imparted by the scaffold and the growth factor. Nonetheless, the undesirable bone formation was reduced and most importantly, not accelerated at the injury site. The application of scaffold without MSC reduced the proportion of bony tissue and the repair site contained fibrous tissue suggesting the infiltration of endogenous fibroblastic cells to the injury site. Taken together, the Gelfoam scaffold does not appear to be pro-osteogenic, which is desirable in studies addressing cartilage regeneration.
Questions to be addressed in the future may include 1) analysis of the repair tissue at longer intervals post-operatively to determine if the repair tissue is maintained and bone bridge is prevented long term, and thus whether different treatment groups are affecting reaction kinetics rather that overall outcome; 2) the potential of MSCs ‘pre-differentiated/committed’ to chondrogenic lineage in vitro prior to transplantation - to improve the possibility of chondrogenic tissue repair; 3) tracking of the introduced MSCs to determine their role in the repair response - whether MSCs directly contribute to the repair tissue or facilitate the recruitment of endogenous cells. Further study is required to evaluate whether MSC may be a viable therapeutic option for the biological regeneration of the growth plate following injury.
ACKNOWLEDGEMENTS
The authors with to thank Ms Jo Cool (Women’s & Children’s Hospital, South Australia) for her assistance in large animal surgery and tissue processing, Dr Tim Kuchel (IMVS, South Australia) and Ms Jodie Dier (IMVS) for bone marrow collection, Ms Lynn Scarman (WCH) for large animal co-ordination and assistance, and Bone Growth Foundation (BGF Australia) for providing The John Fitzgerald BGF PhD Scholarship (to RCM). This work is funded in part by NHMRC project grants (242804, 258700, 453443, and 453497) and funding from BGF.
REFERENCES
- 1.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 2.Xian CJ, Foster BK. Repair of injured articular and growth plate cartilage using mesenchymal stem cells and chondrogenic gene therapy. Curr Stem Cell Res Ther. 2006;1:213–29. doi: 10.2174/157488806776956904. [DOI] [PubMed] [Google Scholar]
- 3.Ahn JI, Terry Canale S, Butler SD, Hasty KA. Stem cell repair of physeal cartilage. J Orthop Res. 2004;22(6):1215–21. doi: 10.1016/j.orthres.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Hui JH, Li L, Teo YH, Ouyang HW, Lee EH. Comparative study of the ability of mesenchymal stem cells derived from bone marrow, periosteum, and adipose tissue in treatment of partial growth arrest in rabbit. Tissue Eng. 2005;11(5-6):904–12. doi: 10.1089/ten.2005.11.904. [DOI] [PubMed] [Google Scholar]
- 5.Chen F, Hui JH, Chan WK, Lee EH. Cultured mesenchymal stem cell transfers in the treatment of partial growth arrest. J Pediatr Orthop. 2003;23(4):425–9. [PubMed] [Google Scholar]
- 6.Planka L, Gal P, Kecova H, et al. Allogeneic and autogenous transplantations of MSCs in treatment of the physeal bone bridge in rabbits. BMC Biotechnol. 2008;8:70. doi: 10.1186/1472-6750-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen AL, Foster BK, Gibson GJ, Binns GF, Wiebkin OW, Hopwood JJ. Growth-plate chondrocyte cultures for reimplantation into growth-plate defects in sheep. Characterization of cultures. Clin Orthop. 1990;256:286–98. [PubMed] [Google Scholar]
- 8.Foster B. Epiphyseal plate repair using fat interposition to reverse physeal deformity [Doctor of Medicine] Adelaide: University of Adelaide. 1989.
- 9.Foster BK, Hansen AL, Gibson GJ, Hopwood JJ, Binns GF, Wiebkin OW. Reimplantation of growth plate chondrocytes into growth plate defects in sheep. J Orthop Res. 1990;8(4):555–64. doi: 10.1002/jor.1100080412. [DOI] [PubMed] [Google Scholar]
- 10.Foster B. Epiphyseal plate repair using fat interposition to reverse physeal deformity. An Experimental study. [M.D]. Adelaide: The University of Adelaide. 1989.
- 11.Wirth T, Byers S, Byard RW, Hopwood JJ, Foster BK. The implantation of cartilaginous and periosteal tissue into growth plate defects. Int Orthop. 1994;18(4):220–8. doi: 10.1007/BF00188326. [DOI] [PubMed] [Google Scholar]
- 12.Johnstone EW, McArthur M, Solly PB, Higginson K, Byers S, Foster BK. The effect of osteogenic protein-1 in an in vivo physeal injury model. Clin Orthop. 2002;395:234–40. doi: 10.1097/00003086-200202000-00028. [DOI] [PubMed] [Google Scholar]
- 13.Thomas BJ, Byers S, Johnstone EW, Foster BK. The effect of recombinant human osteogenic protein-1 on growth plate repair in a sheep model. J Orthop Res. 2005;23(6):1336–44. doi: 10.1016/j.orthres.2005.03.020.1100230615. [DOI] [PubMed] [Google Scholar]
- 14.McCarty RC, Gronthos S, Zannettino AC, Foster BK, Xian CJ. Characterisation and developmental potential of ovine bone marrow derived mesenchymal stem cells. J Cell Physiol. 2009;219(2):324–33. doi: 10.1002/jcp.21670. [DOI] [PubMed] [Google Scholar]
- 15.Gronthos S, Zannettino AC, Hay SJ, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116(Pt 9):1827–35. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 16.Gronthos S, Simmons PJ. The growth factor requirements of STRO-1-positive human bone marrow stromal precursors under serum-deprived conditions in vitro. Blood. 1995;85(4):929–40. [PubMed] [Google Scholar]
- 17.Zhou FH, Foster BK, Zhou XF, Cowin AJ, Xian CJ. TNF-alpha mediates p38 MAP kinase activation and negatively regulates bone formation at the injured growth plate in rats. J Bone Miner Res. 2006;21(7):1075–88. doi: 10.1359/jbmr.060410. [DOI] [PubMed] [Google Scholar]
- 18.Garces GL, Mugica-Garay I, Lopez-Gonzalez Coviella N, Guerado E. Growth-plate modifications after drilling. J Pediatr Orthop. 1994;14(2):225–8. doi: 10.1097/01241398-199403000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Xian CJ, Zhou FH, McCarty RC, Foster BK. Intramembranous ossification mechanism for bone bridge formation at the growth plate cartilage injury site. J Orthop Res. 2004;22(2):417–26. doi: 10.1016/j.orthres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Zhou FH, Foster BK, Sander G, Xian CJ. Expression of proinflammatory cytokines and growth factors at the injured growth plate cartilage in young rats. Bone. 2004;35(6):1307–15. doi: 10.1016/j.bone.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Arasapam G, Scherer M, Cool JC, Foster BK, Xian CJ. Roles of COX-2 and iNOS in the bony repair of the injured growth plate cartilage. J Cell Biochem. 2006;99(2):450–61. doi: 10.1002/jcb.20905. [DOI] [PubMed] [Google Scholar]
- 22.Chung R, Cool JC, Scherer MA, Foster BK, Xian CJ. Roles of neutrophil-mediated inflammatory response in the bony repair of injured growth plate cartilage in young rats. J Leukoc Biol. 2006;80(6):1272–80. doi: 10.1189/jlb.0606365. [DOI] [PubMed] [Google Scholar]
- 23.Ngo TQ, Scherer MA, Zhou FH, Foster BK, Xian CJ. Expression of bone morphogenic proteins and receptors at the injured growth plate cartilage in young rats. J Histochem Cytochem. 2006;54(8):945–54. doi: 10.1369/jhc.6A6939.2006. [DOI] [PubMed] [Google Scholar]
- 24.Chung R, Foster BK, Zannettino AC, Xian CJ. Potential roles of growth factor PDGF-BB in the bony repair of injured growth plate. Bone. 2009;44(5):878–85. doi: 10.1016/j.bone.2009.01.377. [DOI] [PubMed] [Google Scholar]
- 25.Lee MA, Nissen TP, Otsuka NY. Ultilization of a murine model to investigate the molecular process of transphyseal bone formation. J Pedeatr Orthop. 2000;20:802–6. doi: 10.1097/00004694-200011000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Jaramillo D, Shapiro F, Hoffer FA, et al. Posttraumatic growth-plate abnormalities: MR imaging of bony-bridge formation in rabbits. Radiology. 1990;175(3):767–73. doi: 10.1148/radiology.175.3.2343128. [DOI] [PubMed] [Google Scholar]
- 27.Stadelmaier DM, Arnoczky SP, Dodds J, Ross H. The effect of drilling and soft tissue grafting across open growth plates. A histologic study. Am J Sports Med. 1995;23(4):431–5. doi: 10.1177/036354659502300410. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Hui JH, Goh JC, Chen F, Lee EH. Chitin as a scaffold for mesenchymal stem cells transfers in the treatment of partial growth arrest. J Pediatr Orthop. 2004;24(2):205–10. doi: 10.1097/00004694-200403000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Hoshikawa A, Nakayama Y, Matsuda T, Oda H, Nakamura K, Mabuchi K. Encapsulation of chondrocytes in photopolymerizable styrenated gelatin for cartilage tissue engineering. Tissue Eng. 2006;12(8):2333–41. doi: 10.1089/ten.2006.12.2333. [DOI] [PubMed] [Google Scholar]
- 30.Chiang H, Kuo TF, Tsai CC, et al. Repair of porcine articular cartilage defect with autologous chondrocyte transplantation. J Orthop Res. 2005;23(3):584–93. doi: 10.1016/j.orthres.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Ponticiello MS, Schinagl RM, Kadiyala S, Barry FP. Gelatin-based resorbable sponge as a carrier matrix for human mesenchymal stem cells in cartilage regeneration therapy. J Biomed Mater Res. 2000;52(2):246–55. doi: 10.1002/1097-4636(200011)52:2<246::aid-jbm2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 32.Angele P, Kujat R, Nerlich M, Yoo J, Goldberg V, Johnstone B. Engineering of osteochondral tissue with bone marrow mesenchymal progenitor cells in a derivatized hyaluronan-gelatin composite sponge. Tissue Eng. 1999;5(6):545–54. doi: 10.1089/ten.1999.5.545. [DOI] [PubMed] [Google Scholar]
- 33.Quintavalla J, Uziel-Fusi S, Yin J, et al. Fluorescently labeled mesenchymal stem cells (MSCs) maintain multilineage potential and can be detected following implantation into articular cartilage defects. Biomaterials. 2002;23(1):109–19. doi: 10.1016/s0142-9612(01)00086-2. [DOI] [PubMed] [Google Scholar]