Abstract
The small GTPase RhoA and its downstream effectors, ROCK1 and ROCK2, regulate a number of cellular processes, including cell motility, proliferation, survival, and permeability. Pharmacological inhibitors of the Rho pathway reportedly block angiogenesis; however, the molecular details of this inhibition are largely unknown. We demonstrate that vascular endothelial growth factor-A (VEGF) rapidly induces RhoA activation in endothelial cells (ECs). Moreover, the pharmacological inhibition of ROCK1/2 using 10 μM Y-27632 (the IC50 for this compound in ECs) strongly disrupts vasculogenesis in pluripotent embryonic stem cell cultures, VEGF-mediated regenerative angiogenesis in ex vivo retinal explants, and VEGF-mediated in vitro EC tube formation. Furthermore, using small interfering RNA knockdown and mouse heterozygote knockouts of ROCK1 and ROCK2, we provide data indicating that VEGF-driven angiogenesis is largely mediated through ROCK2. These data demonstrate that Rho/ROCK signaling is an important mediator in a number of angiogenic processes, including EC migration, survival, and cell permeability, and suggest that Rho/ROCK inhibition may prove useful for the treatment of angiogenesis-related disorders.—Bryan, B. A., Dennstedt, E., Mitchell, D. C., Walshe, T. E., Noma, K., Loureiro, R., Saint-Geniez, M., Campaigniac, J.-P., Liao, J. K., D’Amore, P. A. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis.
Keywords: endothelial cells, Y-27632, Rho kinase, GTPase, vascular endothelial growth factor
Vascular endothelial growth factor-A (VEGF) is the master regulatory growth factor orchestrating both vasculogenesis (the de novo formation of the embryonic circulatory system) and angiogenesis (the growth of blood vessels from preexisting vasculature). VEGF exerts its biological effects primarily on endothelial cells (ECs; refs. 1, 2); however, a number of VEGF-mediated effects have been reported for non-EC types (3, 4). The complex organization of events necessary for the formation of new blood vessels, including the modulation of vascular permeability, extracellular matrix (ECM) degradation, migration, proliferation, and survival, are each regulated through VEGF-induced signaling cascades. Since the discovery of VEGF as an angiogenic factor, multiple downstream signaling pathways have been implicated in the modulation of VEGF-dependent effects, including the PI3K/AKT pathway in the regulation of cell survival, the Ras/MAPK pathway in the regulation of gene expression and cell proliferation, the PLCγ pathway in the control of cell proliferation and vascular permeability, and the FAK/paxillin pathway in cytoskeletal rearrangement and cell migration (5, 6).
The Rho family of small GTPases controls a diverse array of cellular processes, including cytoskeletal dynamics, cell polarity, membrane transport, and gene expression (7). The Rho proteins, whose prototypical member is RhoA, are molecular switches that respond to cell surface receptors for various cytokines, growth factors, adhesion molecules, and G-protein-coupled receptors by cycling between an inactive guanosine diphosphate (GDP)-bound and an active guanosine triphosphate (GTP)-bound form. Rho-associated kinases (ROCK1 and ROCK2) are the most extensively studied RhoA effector proteins and regulate actomyosin contractility via a direct phosphorylation of myosin light chain and phosphorylation and inactivation of the myosin-binding subunit of myosin phosphatase (8). Moreover, ROCK proteins are reported to phosphorylate LIM-kinase, leading to phosphorylation of the actin-regulatory protein cofilin, which contributes to Rho-induced reorganization of the actin cytoskeleton (8). While both ROCK1 and ROCK2 are important regulators of the actin cytoskeleton assembly across many cell types, a handful of studies have described differential signaling between these 2 RhoA effectors. For instance, ROCK1, but not ROCK2, is cleaved by caspase-3 during apoptosis and ROCK1, but not ROCK2, is negatively regulated by Rnd3/RhoE and Gem (9, 10). Smooth muscle-specific basic calponin is phophorylated only by ROCK2 (11). Furthermore, ROCK1 is expressed ubiquitously, whereas ROCK2 is preferentially expressed in cardiovascular and brain tissues (12, 13). Homozygous deletion of ROCK1 (ROCK1−/−) leads to embryonic and postnatal lethality and failure of eyelid and ventral body wall closure (14,15,16), while homozygous ROCK2-knockout (ROCK2−/−) mice die embryonically due to placental dysfunction and intrauterine growth retardation (17, 18). These data suggest that the specific expression and unique roles of each ROCK paralog may play a very important role in EC function during angiogenesis.
Rho signaling is reportedly essential for VEGF-dependent in vivo angiogenesis and in vitro capillary formation (19,20,21). Although implicated in the regulation of angiogenesis, little has been described regarding RhoA control of the complex multistep processes involved in blood vessel formation. While the Rho pathway may control cellular processes such as migration in ECs, a comprehensive analysis of the specific mechanisms by which Rho/ROCK signaling mediates VEGF-induced angiogenesis is lacking. In this study, we block the RhoA/ROCK pathway with the pharmacological inhibitor Y-27632 and analyze the biological effects using ex vivo and in vitro angiogenic assays and in cell-based assays monitoring vascular permeability, matrix metalloproteinases (MMP) expression, migration, proliferation, and survival. Moreover, we examine the ROCK paralog-specific roles in VEGF signaling to determine their contribution to angiogenesis.
MATERIALS AND METHODS
Cell culture and mouse model
Wild-type mouse pluripotent embryonic stem cells (generous gift from Andras Nagy, Mount Sinai Hospital, New York, NY, USA) were cultured as described previously (22). Bovine retinal ECs (BRECs) were previously isolated and cultured as described previously (23). MS1 mouse pancreatic ECs [CRL-2279; American Type Culture Collection (ATCC), Manassas, VA, USA] were maintained according to ATCC recommendations.
Rock1+/− and Rock2+/− mice were previously generated (24, 25).
Cell treatments
Recombinant human VEGF165 (obtained from National Cancer Institute, Bethesda, MD, USA; http://www.cancer.gov) was used at a concentration of 2.5 ng/ml (unless otherwise noted). Y-27632 (Sigma, St. Louis, MO, USA) was used at a concentration of 10 μM. Control treatments for all experiments were sterile vehicle.
All experiments in this study were performed ≥3 independent times in triplicate.
Immunofluorescent microscopy
For platelet endothelial cell adhesion molecule (PECAM) staining, cells grown on coverslips or cryosectioned mouse lung slides were fixed in ice-cold methanol/acetone (1:1) for 10 min. Reactions were blocked for 60 min with PBS containing 0.2% BSA, followed by 60 min of incubation with an anti-PCAM antibody (1:100; BD Pharmingen, San Jose, CA, USA). Fluorescein-conjugated secondary antibody was added for 30 min (1:1000; R&D Systems, Minneapolis, MN, USA). Where indicated, the nucleus was stained with a 10 min incubation with DAPI. Fluorescence images were captured on a Leica TSC-SP2 upright confocal laser scanning microscope(Leica Microsystems, Wetzlar, Germany).
For actin staining, cells were fixed in 4% paraformaldehyde for 10 min and permeabilized with 0.02% Triton X-100 for 5 min. Reactions were blocked for 30 min with PBS containing 0.2% BSA, followed by 20 min of incubation with FITC-labeled phalloidin (Sigma). Fluorescence images were captured on a Nikon Eclipse E400 fluorescent microscope (Nikon, Tokyo, Japan).
Western blotting
Western blotting was performed as described previously (3). Anti-RhoA (sc-179; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-ROCK1 (sc-17794; Santa Cruz Biotechnology), anti-ROCK2 (sc-5561; Santa Cruz Biotechnology), and anti-actin (sc-8432; Santa Cruz Biotechnology) were utilized for protein detection.
Collagen and matrigel in vitro angiogenesis assay
The BREC tube formation assay was performed as described previously (26). After 24 h, 3 different fields per well were randomly chosen and photographed on a SPOT camera attached to a Nikon Eclipse TE2000-S inverted microscope.
The matrigel cord formation assay was performed as described previously (27) with the exception that MS1 cells were used. Wells were photographed on a SPOT camera attached to a Nikon Eclipse TE2000-S inverted microscope. Total cord length was quantified using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA).
Retinal explants
Retinal explants were isolated and cultured as described previously (26). The explants were incubated at 37°C for 30 d, during which time they were periodically photographed through a SPOT camera attached to a Nikon Eclipse TE2000-S inverted microscope. Capillaries grew out of the explants in 3 dimensions, making it impossible to obtain images in a single plane of focus. Thus, representative 2-dimensional prototypical drawings of the explants are presented.
RhoA activation assay
RhoA activation assays were performed as described previously (28).
Proliferation assay
BRECs were plated in 100-mm dishes at 104 cells/well, serum starved overnight, and allowed to proliferate for 96 h in the indicated treatments. Proliferation was measured using an MTT assay (Cayman Chemicals, Ann Arbor, MI, USA), according to the manufacturer’s directions.
Apoptosis assay
BRECs were cultured in 0.1% FBS for 3 d in the presence of the indicated treatments. Apoptosis was measured using the fluorescent in situ cell death detection kit (Roche, Indianapolis, IN, USA) according to the manufacturer’s instructions. Detection of fluorescein-labeled dUTP was performed using a Nikon Eclipse TE2000-S inverted microscope.
Migration/invasion assay
BRECs were seeded onto 6-well plates, grown to 100% confluence, serum starved overnight, and wounded with a sterile pipette tip to remove cells by 2 perpendicular linear scratches. The progress of migration was digitally photographed immediately following injury and at 12 h after wounding with a SPOT camera attached to a Nikon Eclipse TE2000-S inverted microscope.
Semiquantitative RT-PCR
Semiquantitative RT-PCR was performed as described previously (3). Quantitation of band intensity was performed using ImageJ software.
Transwell permeability assay
Permeability across a monolayer of BRECs was studied in a Transwell system (Corning, Corning, NY, USA), with membranes that are 12 mm in diameter and 0.4 μm in pore size. BRECs were seeded into the upper chamber at 1 × 105 cells/ml and grown to 100% confluence. One hour after the indicated treatment, 20 μg/ml of FITC-labeled dextran was added into the upper chamber and incubated at 37° for up to 240 min. The amount of fluorescent dextran that diffused through the cell monolayer into the lower chamber was measured with the use of a Biotek Synergy-2 fluorescent plate reader (Biotek Instruments Inc., Winooski, VT, USA) as the index of EC monolayer permeability.
Small interfering RNA (siRNA) knockdown of ROCK1 and ROCK2
MS1 cells were transfected with siRNA pools of mouse ROCK1 and ROCK2 or scrambled siRNA controls (Dharmacon, Lafayette, CO, USA) using Dharmafect transfection reagent (Dharmacon), according to the manufacturer’s instructions specific for human umbilical vein ECs. The peak decrease in protein levels was observed 72 h post-transfection; therefore, assays using siRNA-transfected cells cumulated at the 72 h time point.
RESULTS
VEGF stimulation of ECs leads to RhoA activation
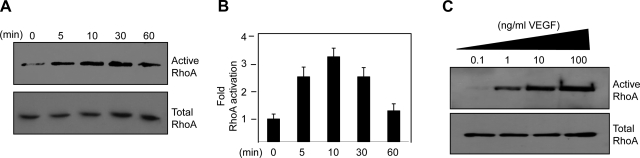
To test whether VEGF treatment of BRECs results in alterations in the activation status of RhoA, GTPase activation assays were performed to examine the levels of the active GTP-bound forms of RhoA over a time course of VEGF stimulation of BRECs. Active RhoA was affinity purified with GST bead-bound Rhotekin, which only binds RhoA in its active GTP-bound form. To detect the active forms from the GST fusion protein pulldown assays as well as the total GTPase levels from the cell lysate, Western blots were performed with a RhoA-specific antibody. Within 5 min of VEGF stimulation, RhoA activity increased by >2-fold and subsequently returned to approximately normal levels after 60 min (Fig. 1A, B). Similar results were observed using porcine aortic ECs stably overexpressing VEGFR2 (data not shown). Moreover, RhoA activation in BRECs directly correlated with increasing doses of VEGF (Fig. 1C), indicating a classic dose curve response.
Figure 1.
VEGF induces cytoskeletal alterations and RhoA activation in ECs. A) BRECs were treated with 2.5 ng/ml VEGF, and lysates were collected over a time course for 60 min. Amount of activated RhoA was determined by affinity pulldown assays using GST-Rhotekin. Active RhoA (top panel) and total RhoA (bottom panel) were visualized by Western blot analysis using a Rho-specific antibody. B) Quantification of RhoA activation following VEGF stimulation. Data are means ± sd of 3 experiments. C) BRECs were treated with a dose curve of VEGF, and lysates were collected after 5 min. Amount of activated RhoA was determined by affinity pulldown assays using GST-Rhotekin. Active RhoA (top panel) and total (bottom panel) RhoA were visualized by Western blot analysis using a Rho-specific antibody.
Disruption of RhoA/ROCK signaling inhibits VEGF-mediated alterations to cytoskeletal morphology
To determine the effective inhibitory concentration of Y-27632 on ECs, subconfluent MS1 ECs were treated with a dose curve of Y-27632 (ranging from 0 to 200 μM). As ROCK inhibition has extensively been shown to promote the formation of cellular projections from the cell body in a number of cell types, we utilized projection length as a measure of the effective biological response for Y-27632. Based on changes in projection length, the IC50 for Y-27632 on MS1 ECs is ∼10 μM (Supplemental Fig. 1A).
To directly examine cytoskeletal reorganization induced by blocking VEGF-mediated RhoA/ROCK signaling, MS1 ECs were treated for 24 h with control, VEGF, Y-27632, or VEGF plus Y-27632, and fluorescently labeled with FITC-phalloidin to stain actin filaments within the cell. As observed in Supplemental Fig. 1B, control ECs maintained their typical cobblestone appearance. In contrast, VEGF treatment resulted in increased stress fiber formation coupled with elongation and polarization of the cells. Y-27632 treatment resulted in marked disorganization of the cytoskeleton compared with the control and, importantly, blocked the VEGF-mediated cell elongation and cytoskeletal alterations.
Y-27632 inhibits vasculogenesis in embryonic stem cell cultures
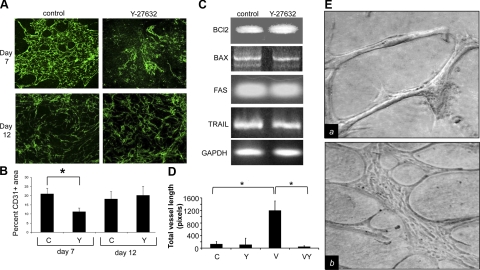
Utilizing the cystic embryoid body (CEB) model, we compared vascularization in control vs. Y-27632-treated CEBs. At d 7 of differentiation, immunofluorescent detection of vascular networks with the endothelial-specific marker PECAM (CD31) revealed an ∼50% reduction in PECAM positive staining in Y-27632-treated cultures vs. control cultures (Fig. 2A, B; d 7). PECAM-positive ECs in the Y-27632 treatment failed to assemble into recognizable vessel-like structures and remained largely dispersed. To assess whether inhibition of Rho/ROCK signaling disrupted established vascular networks, CEBs were allowed to differentiate for 9 d (allowing vascular network assembly and stabilization) and subsequently treated with Y-27632 or vehicle for 3 d. Immunofluorescent detection of PECAM levels and vascular organization was not statistically different between control or Y-27632-treated cultures (Fig. 2A, B; d 12), suggesting that Rho/ROCK signaling is essential for the formation of vascular networks but may not be necessary for the maintenance of relatively mature vessels. To address the possibility that changes in PECAM staining in CEB cultures treated with Y-27632 were due to ROCK inhibition-mediated alterations in cell viability, we examined the expression of the intrinsic (Bcl2 and Bax) and extrinsic (Fas and Trail) apoptotic regulators in control and Y-27632-treated differentiating embryonic stem cells. No differences in the expression of these genes were observed between treatments, suggesting that Y-27632 did not induce apoptosis in this assay (Fig. 2C).
Figure 2.
Rho/ROCK inhibition disrupts vasculogenesis, ex vivo angiogenesis, and in vitro angiogenesis. A) Top panel: CEBs were treated with either 10 μM Y-27632 or control before the onset of vasculogenesis (treatment began at d 4 of differentiation). Cells were collected after 7 d of differentiation. PECAM expression was detected using immunofluorescent staining. Bottom: CEBs were treated with either 10 μM Y-27632 or control subsequent to the onset of vasculogenesis (treatment began at d 9 of differentiation). Cells were collected after 12 d of differentiation. PECAM expression was detected using immunofluorescent staining. B) Quantification of PECAM positive area per field. Immunofluorescent staining of ≥3 fields was analyzed and reported as mean ± sd percentage of PECAM positive area per field. C, control; Y, Y-27632. C) Semiquantitative RT-PCR for steady-state RNA levels of intrinsic and extrinsic apoptotic regulators was performed on cDNA collected from sham or Y-27632-treated CEB cultures on d 7 of differentiation (cultures were treated as indicated for A, top panel). GAPDH expression was utilized as a control. D) Retinal explants (1-mm2 pieces) were collected and embedded within a collagen matrix and treated with VEGF, Y-27632, VEGF plus Y-27632, or vehicle. Total capillary vessel outgrowth of ≥3 explants was measured after 30 d and reported as mean ± sd vessel length. V, VEGF; VY, VEGF plus Y-27632. E) Phase contrast images of BRECs after 2 d embedded in a collagen matrix treated with VEGF (a) or VEGF plus Y-27632 (b). *P < 0.05; Student’s t test.
Y-27632 disrupts ex vivo and in vitro angiogenesis
To address the general relevance of our observations in the CEB model, we tested the effect of Y-27632 on vessel formation in an ex vivo setting. Retinal explants (1-mm2 pieces) were obtained from wild-type mice and embedded within a collagen sandwich gel. The explants were then treated with VEGF, Y-27632, VEGF plus Y-27632, or vehicle, and total capillary vessel outgrowth length was measured after 30 d (Fig. 2D). No significant difference was observed between control and Y-27632-treated explants. VEGF treatment of retinal explants led to a significantly enhanced vessel outgrowth compared with control explants, and addition of Y-27632 completely abrogated VEGF-induced vessel outgrowths.
To corroborate our ex vivo findings, we utilized an in vitro angiogenesis assay in which BRECs plated between 2 layers of collagen have been shown to form lumen-containing capillary-like structures (26). Similar to previous reports in a number of EC lines, robust vessel formation was observed in VEGF-treated bovine retinal ECs (Fig. 2E). While treatment of BRECs with control or Y-27632 failed to induce any significant vessel network formation (data not shown), the combination of VEGF and Y-27632 resulted in a greatly enhanced cord formation over the VEGF alone; however, these networks appeared morphologically distinct from VEGF-treated tubes and consisted largely of broadly flattened monolayers of cords, indicative of immaturely formed vessels.
Rho/ROCK signaling is essential for multiple VEGF-mediated EC processes
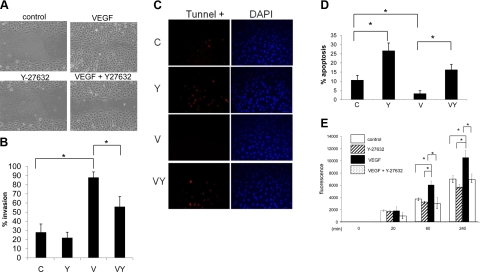
Previous conflicting reports have indicated that RhoA signaling either promotes VEGF-mediated EC migration (29,30,31) or has no effect on VEGF-mediated EC migration (20). To clarify the effect of Rho/ROCK signaling in the regulation of VEGF-mediated EC migration, we performed migration assays in which confluent monolayers of BRECs were scratch wounded and allowed to migrate. ECs will migrate in order to close the scratch wound within a matter of several hours, largely eliminating the contribution of proliferation to the wound closure. Immediately following injury, serum-starved cells were treated with control, Y-27632, VEGF, or VEGF plus Y-27632. VEGF treatment of BRECs led to near complete wound closure; however addition of Y-27632 plus VEGF to ECs led to a significant blockage of the VEGF-induced migration (Fig. 3A, B). The level of wound closure in control or Y-27632-treated cells did not significantly differ, as expected under serum-starved conditions.
Figure 3.
Rho/ROCK signaling is essential for VEGF activation of ECs. A) BRECs were grown to 100% confluence, serum starved overnight, wounded with a sterile pipette tip to remove cells, and treated with control (a), Y-27632 (b), VEGF (c), or VEGF plus Y-27632 (d). Photographs (×40) were taken at 8 h after injury. B) Wound closure of ≥3 wells was quantified and is reported as mean ± sd percentage invasion. C) Fluorescence tunnel analysis of BRECs after 3 d of treatment at 100% confluence in low-serum medium. D) Quantification of tunnel-positive cells as an indicator of the apoptotic index. E) BRECs were grown to confluent monolayers in the top layer of Transwells and treated with VEGF, Y-27632, VEGF plus Y-27632, or vehicle. One hour after treatment, FITC-labeled dextran was added to the top chamber. Amount of fluorescent dextran that diffused through the cell monolayer into the bottom chamber was measured over 240 min. *P < 0.05; Student’s t test.
The role of RhoA-signaling in cell survival has been examined in a number of non-EC types. Some studies (32,33,34,35) report that inhibition of Rho signaling leads to apoptosis via alterations in cell adhesion and induction of p53 and other proapoptotic proteins, whereas others (36, 37) report that Rho signaling induces apoptosis via ceramide up-regulation, leading to caspase cleavage and subsequent activation. As no detectable changes in the expression of the apoptotic regulator Bcl2 were observed in Y-27632-treated ECs compared with control cells grown in normal growth (serum rich) conditions (Supplemental Fig. 2A), we sought to determine the role of Rho/ROCK signaling in EC survival by challenging BRECs for 3 d in serum-starvation conditions at 100% confluence in the presence of Y-27632, VEGF, a combination of VEGF and Y-27632, or vehicle. Apoptosis was measured using tunnel staining. Y-27632-treated cells exhibited an ∼2.6-fold increase in apoptosis compared with control cells (Fig. 3C, D). VEGF treatment resulted in a significant reduction in the apoptotic index compared with the control. Interestingly, the combination treatment of Y-27632 and VEGF led to a marked increase in apoptosis compared with VEGF-treated alone, abrogating the VEGF-mediated increase in cell survival.
Pharmacological inhibition of Rho/ROCK signaling in ECs has been reported to reduce cell permeability by inhibiting the ROCK-dependent formation of transcellular gaps, vesiculo-vacuolar organelles, and fenestrations (38, 39); however, it is unknown whether this signaling pathway is responsible for VEGF-induced cell barrier loss and enhanced cell permeability. To test this possibility, cell permeability was assessed in confluent monolayers of BRECs grown in Transwells treated with Y-27632, VEGF, a combination of VEGF and Y-27632, or vehicle. No significant difference in permeability was observed in Y-27632-treated cells compared with the control; however, there was a marked increase in permeability after 60 min in VEGF-treated cells (Fig. 3E). Inclusion of Y-27632 with VEGF reduced permeability measurements to the control levels.
Both VEGF and the Rho pathway have been shown to regulate cell proliferation in various cell types (40,41,42,43,44); however, the role of the Rho/ROCK signaling pathway in VEGF-stimulated EC proliferation is unknown. To determine its role, proliferation assays were performed on serum-starved BRECs treated with Y-27632, VEGF, the combination of VEGF and Y-27632, or vehicle. Treatment with Y-27632 did not significantly affect BREC proliferation compared with the control (Supplemental Fig. 2B). Moreover, the addition of Y-27632 did not reduce VEGF-mediated proliferation, indicating that under these conditions Rho/ROCK signaling is not required for VEGF-mediated proliferation.
In ECs, both overexpression of activated RhoA as well as overexpression of VEGF up-regulate MMP expression (45, 46); however, no reports to date have investigated RhoA as a downstream effector of VEGF-mediated MMP expression. To examine this possibility, BRECs were treated with Y-27632, VEGF, a combination of VEGF and Y-27632, or vehicle, and the levels of MMP-1 and MMP-9 mRNA were determined by semiquantitative RT-PCR 24 h post-treatment. Y-27632 treatment resulted in a marked reduction in MMP-1 and MMP-9 transcript expression compared with the control (Supplemental Fig. 2C); however, Y-27632 failed to suppress VEGF-induced MMP expression, indicating that Rho/ROCK signaling, while essential for MMP expression in unstimulated EC cultures, is not required for VEGF-mediated MMP expression.
VEGF-mediated activation of ECs is largely dependent on ROCK2 signaling
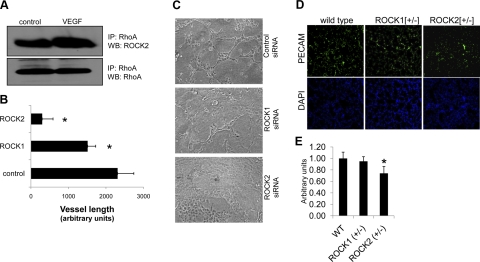
While ROCK1 and ROCK2 are homologous protein paralogs and have similar roles in regulating the cytoskeleton, few reports have determined the unique roles and differential regulation of these proteins. Our data indicate that both ROCK proteins are expressed across multiple EC cell lines (Supplemental Fig. 3A), and therefore, we sought to examine the individual contributions of these proteins to angiogenesis. VEGF-mediated physical association of RhoA/ROCK protein complexes in MS1 cells was analyzed using immunoprecipitation experiments. As indicated in Fig. 4A, physical association of RhoA to ROCK2 is increased ∼2-fold in response to VEGF stimulation.
Figure 4.
In vivo and in vitro blood vessel formation is dependent primarily on ROCK2 signaling. A) MS1 cell lysates from cells treated with control or VEGF for 5 min were immunoprecipitated with a RhoA specific antibody, and subsequent detection was performed using Western blotting. IP, antibody used as bait; WB, antibody used in Western blotting. B, C) Control siRNA, ROCK1 siRNA, or ROCK2 siRNA MS1 ECs were subjected to matrigel cord formation assays (C). Total vessel length of each condition was quantified using Image J and is represented as arbitrary units corresponding to pixels detected (B). *P < 0.05; Student’s t test. D) Cryosections of lungs collected from haploinsufficient Rock1 (Rock1+/−) and Rock2 (Rock2+/−) mice were analyzed using immunofluorescent detection of PECAM expression as a measure of vascular density in tissue sections (green, PECAM; blue, DAPI; 4 mice/genotype; ≥3 sections/lung). E) Quantification of PECAM staining using Image J software of wild-type (WT) and haploinsufficient Rock1 and Rock2 lungs (n=4 mice/condition; ≥4 lung sections/animal).
To test the paralog-dependent contribution of the ROCK proteins to VEGF-mediated angiogenesis, we utilized siRNA knockdown of ROCK1 and ROCK2 in MS1 cells, resulting in an ∼95 and 75% reduction in protein expression, respectively, after 72 h (Supplemental Fig. 3B). Using MS1 EC matrigel cord formation assays, we observed a drastic reduction in vascular cord formation in ROCK2-deficient cells compared with control siRNA, which was characterized by broadly flattened monolayers of cords similar to those observed in Y-27632 treatment of BRECs (Fig. 4B, C). A modest, but significant, reduction in cord formation was observed in ROCK1-deficient ECs compared with wild type; however, ROCK1-deficient cords more closely resembled the phenotype of those seen in control siRNA conditions.
To address the paralog-specific role of ROCK proteins in blood vessel formation in vivo, we cryosectioned lungs collected from Rock1+/− and Rock2+/− mice. Lung vascularity was compared between wild-type and ROCK heterozyote mice via fluorescent detection of PECAM expression as a measure of vascular density (Fig. 4D, E). No significant difference in PECAM density was observed in the lungs from ROCK1+/− mice compared with the control; however, there was a modest, yet significant, reduction in PECAM staining in ROCK2+/− lungs, reflecting a decrease in blood vessel density.
DISCUSSION
MAPK, PI3K, and PLCγ signaling pathways are reported to be the major downstream effectors in VEGF signaling. In this report, we examine the role of the Rho/ROCK signaling in VEGF-driven angiogenesis utilizing Y-27632, a pharmacological inhibitor of ROCK that competitively inhibits the ATP-binding domain of ROCK1 and ROCK2. Our results suggest that the Rho/ROCK pathway is involved in numerous aspects of the angiogenic process and that it is essential for VEGF-mediated migration, survival, and permeability.
Y-27632 disruption of vasculogenesis and angiogenesis
VEGF stimulation of human umbilical vein ECs reportedly promotes the active form of RhoA (29). We describe similar VEGF-dependent activation of RhoA in other EC lines, suggesting that this signaling response is likely uniform across ECs. Using CEB, an in vitro model of vasculogenesis, we demonstrated that inhibition of Rho/ROCK signaling resulted in the failure of differentiated ECs to assemble into vessel-like structures and instead remained dispersed, a finding that is similar to our previous observations in CEB formed from VEGF-null embryonic stem cells (22). However, Rho/ROCK signaling was nonessential for specification into the EC lineage, as evidenced by a reduced, but still substantial, number of CD31-positive cells in Y-27632-treated cultures. We utilized ex vivo retinal explant cultures and in vitro EC tube formation assays to assess the effects of Rho/ROCK inhibition on angiogenesis. Retinal explants clearly demonstrated that VEGF strongly promotes vessel outgrowth, an effect that was effectively abrogated by the addition of Y-27632. Using EC tube formation assays, we demonstrated that addition of Y-27632 disrupted VEGF-mediated tube morphogenesis, resulting in a vascular network consisting of flattened multicellular structures vs. lumen-containing tube-like structures as reported in VEGF-treated cultures.
Similar to our data, others have reported a disruption of VEGF-mediated EC activation on inhibition of the Rho pathway using in vivo angiogenesis and in vitro tube-formation assays (20, 21, 47, 48). One recent study (49) suggested that tumor-derived ECs display an enhanced ability to organize into tubular networks in vitro, and these behaviors correlate with a constitutively high level of RhoA/ROCK signaling, which on disruption normalized tumor EC tube formation ability to levels seen in nontumor ECs. In contrast, other groups have reported the opposite finding. One report demonstrated that pharmacological inhibition of ROCK proteins with H-1152 leads to increased retinal neovascularization and sprouting angiogenesis (50), and it has been suggested that the Rho/ROCK pathway promotes vessel regression and is antagonized by src-family kinases (51). Transient inhibition of Rho/ROCK signaling has been shown to result in increased blood vessel sprouting and length (52). Our observations, in a number of angiogenesis-related assays, including in vitro tube formation assays, vasculogenesis in the CEB model and retinal angiogenesis in ex vivo cultures, suggest that Rho inhibition blocks VEGF-driven blood vessel formation.
Y-27632 inhibition of VEGF-mediated EC activation
While a handful of reports have examined singular aspects of VEGF-mediated EC activation through the RhoA/ROCK pathway (i.e., migration or proliferation), a comprehensive analysis of the cellular effects of RhoA/ROCK inhibition in ECs is lacking. VEGF has been shown to modulate migration in ECs (53), angioblasts (54), and extensively in cells other than ECs (55,56,57). Given the volume of literature supporting a role for Rho proteins in controlling cell movement (58,59,60), it was not surprising that our data demonstrated that inhibition of Rho signaling blocks EC migration in response to VEGF stimulation. Indeed, we demonstrate that in addition to mediating VEGF-induced migration, Rho/ROCK signaling also regulates VEGF-mediated cell permeability and survival. Independent of VEGF signaling, thrombin has been shown to induce EC permeability via activation of Rho/ROCK through p115Rho-GEF and GEFH1, and PKC-mediated inactivation of the RhoA-inhibitor Rho-GDI, which leads to the destabilization of adherens and tight junctions (61,62,63). Inhibition of protein prenylation (which blocks RhoA activation) in ECs reportedly reduces RhoA activation and its subsequent membrane translocation, resulting in reduction in EC survival, migration, and cell adhesion (64). RhoA inhibition in EC-fibroblast tube forming assays before vessel regression was reported to lead to enhanced vessel stability (52). Although it cannot be determined whether the effects of Rho inhibition on EC survival are direct or indirect through changes in fibroblasts, this result suggests that Rho-mediated survival may function in a context-dependent manner.
Contribution of ROCK paralogs to angiogenesis
The established chemical inhibitors of ROCK (Y-27632, H-1152, Wf-536, fasudil, and hydroxyfasudil) do not distinguish between ROCK1 and ROCK2 and exhibit some nonspecific inhibition of other protein kinases; therefore, it is not clear how ROCK1 and ROCK2 differ in their regulation and function. The ROCK proteins share 65% identity with the highest degree of similarity in their kinase domains (92% identity), and differences in their activity, expression, and regulation have been reported (8,9,10,11,12,13,14,15,16,17,18). Our data using siRNA-mediated gene knockdown and knockout mouse models suggest that ROCK2 performs the major role in VEGF-mediated RhoA/ROCK signaling during angiogenesis. Future experiments utilizing gene targeting of ROCK1 and ROCK2 are necessary to provide more direct evidence for the role of these proteins in regulating biological processes in the endothelium. Given the recent advances in cancer treatment with antiangiogenic drugs and the effective clinical use of hydroxyl-fasudil in Japan (with relatively low side effects) for cardiovascular disease and cerebral vasospasm, a better understanding of the differences between ROCK1 and ROCK2 may lead to the development of more specific and effective therapeutics.
Supplementary Material
Acknowledgments
This study was supported by U. S. National Institutes of Health grants EY-05318, EY-015435, and CA-45548 to P.A.D; HL-052233 to J.K.L.; and HL-098931 to B.A.B. P.A.D is a Research to Prevent Blindness Senior Scientific Investigator. The authors thank Dr. M. Liu (Texas A&M University Health Science Center, Houston, TX, USA) for the use of the Rhotekin-GST expression plasmid.
References
- Ferrara N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog Horm Res. 2000;55:15–35. discussion 35–16. [PubMed] [Google Scholar]
- Lee S, Chen T T, Barber C L, Jordan M C, Murdock J, Desai S, Ferrara N, Nagy A, Roos K P, Iruela-Arispe M L. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan B A, Walshe T E, Mitchell D C, Havumaki J S, Saint-Geniez M, Maharaj A S, Maldonado A E, D'Amore P A. Coordinated vascular endothelial growth factor expression and signaling during skeletal myogenic differentiation. Mol Biol Cell. 2008;19:994–1006. doi: 10.1091/mbc.E07-09-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao X O, Xie L, Greenberg D A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Gerber H P, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Zachary I. VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans. 2003;31:1171–1177. doi: 10.1042/bst0311171. [DOI] [PubMed] [Google Scholar]
- Jaffe A B, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Zhao Z S, Manser E. PAK and other Rho-associated kinases–effectors with surprisingly diverse mechanisms of regulation. Biochem J. 2005;386:201–214. doi: 10.1042/BJ20041638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M L, Sahai E A, Yeo M, Bosch M, Dewar A, Olson M F. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3:339–345. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346–352. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Amano M, Maeda A, Goto H, Takahashi K, Ito M, Kaibuchi K. Identification of calponin as a novel substrate of Rho-kinase. Biochem Biophys Res Commun. 2000;273:110–116. doi: 10.1006/bbrc.2000.2901. [DOI] [PubMed] [Google Scholar]
- Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392:189–193. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- Wei L, Roberts W, Wang L, Yamada M, Zhang S, Zhao Z, Rivkees S A, Schwartz R J, Imanaka-Yoshida K. Rho kinases play an obligatory role in vertebrate embryonic organogenesis. Development. 2001;128:2953–2962. doi: 10.1242/dev.128.15.2953. [DOI] [PubMed] [Google Scholar]
- Rikitake Y, Oyama N, Wang C Y, Noma K, Satoh M, Kim H H, Liao J K. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/− haploinsufficient mice. Circulation. 2005;112:2959–2965. doi: 10.1161/CIRCULATIONAHA.105.584623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, Noda Y, Matsumura F, Taketo M M, Narumiya S. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol. 2005;168:941–953. doi: 10.1083/jcb.200411179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y M, Bo J, Taffet G E, Chang J, Shi J, Reddy A K, Michael L H, Schneider M D, Entman M L, Schwartz R J, Wei L. Targeted deletion of ROCK1 protects the heart against pressure overload by inhibiting reactive fibrosis. FASEB J. 2006;20:916–925. doi: 10.1096/fj.05-5129com. [DOI] [PubMed] [Google Scholar]
- Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, Oshima M, Taketo M M, Narumiya S. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol. 2003;23:5043–5055. doi: 10.1128/MCB.23.14.5043-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumkeo D, Shimizu Y, Sakamoto S, Yamada S, Narumiya S. ROCK-I and ROCK-II cooperatively regulate closure of eyelid and ventral body wall in mouse embryo. Genes Cells. 2005;10:825–834. doi: 10.1111/j.1365-2443.2005.00882.x. [DOI] [PubMed] [Google Scholar]
- Connolly J O, Simpson N, Hewlett L, Hall A. Rac regulates endothelial morphogenesis and capillary assembly. Mol Biol Cell. 2002;13:2474–2485. doi: 10.1091/mbc.E02-01-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang M V, Whelan M C, Senger D R. Rho activity critically and selectively regulates endothelial cell organization during angiogenesis. Proc Natl Acad Sci U S A. 2004;101:1874–1879. doi: 10.1073/pnas.0308525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H J, Kong D, Iruela-Arispe L, Begley U, Tang D, Galper J B. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors interfere with angiogenesis by inhibiting the geranylgeranylation of RhoA. Circ Res. 2002;91:143–150. doi: 10.1161/01.res.0000028149.15986.4c. [DOI] [PubMed] [Google Scholar]
- Ng Y S, Ramsauer M, Loureiro R M, D'Amore P A. Identification of genes involved in VEGF-mediated vascular morphogenesis using embryonic stem cell-derived cystic embryoid bodies. Lab Invest. 2004;84:1209–1218. doi: 10.1038/labinvest.3700150. [DOI] [PubMed] [Google Scholar]
- Ramsauer M, D'Amore P A. Contextual role for angiopoietins and TGFbeta1 in blood vessel stabilization. J Cell Sci. 2007;120:1810–1817. doi: 10.1242/jcs.003533. [DOI] [PubMed] [Google Scholar]
- Noma K, Rikitake Y, Oyama N, Yan G, Alcaide P, Liu P Y, Wang H, Ahl D, Sawada N, Okamoto R, Hiroi Y, Shimizu K, Luscinskas F W, Sun J, Liao J K. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Invest. 2008;118:1632–1644. doi: 10.1172/JCI29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikitake Y, Kim H H, Huang Z, Seto M, Yano K, Asano T, Moskowitz M A, Liao J K. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36:2251–2257. doi: 10.1161/01.STR.0000181077.84981.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im E, Venkatakrishnan A, Kazlauskas A. Cathepsin B regulates the intrinsic angiogenic threshold of endothelial cells. Mol Biol Cell. 2005;16:3488–3500. doi: 10.1091/mbc.E04-11-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darland D C, D'Amore P A. TGF beta is required for the formation of capillary-like structures in three-dimensional cocultures of 10T1/2 and endothelial cells. Angiogenesis. 2001;4:11–20. doi: 10.1023/a:1016611824696. [DOI] [PubMed] [Google Scholar]
- Bryan B A, Li D, Wu X, Liu M. The Rho family of small GTPases: crucial regulators of skeletal myogenesis. Cell Mol Life Sci. 2005;62:1547–1555. doi: 10.1007/s00018-005-5029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nieuw Amerongen G P, Koolwijk P, Versteilen A, van Hinsbergh V W. Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arterioscler Thromb Vasc Biol. 2003;23:211–217. doi: 10.1161/01.atv.0000054198.68894.88. [DOI] [PubMed] [Google Scholar]
- Vincent L, Chen W, Hong L, Mirshahi F, Mishal Z, Mirshahi-Khorassani T, Vannier J P, Soria J, Soria C. Inhibition of endothelial cell migration by cerivastatin, an HMG-CoA reductase inhibitor: contribution to its anti-angiogenic effect. FEBS Lett. 2001;495:159–166. doi: 10.1016/s0014-5793(01)02337-7. [DOI] [PubMed] [Google Scholar]
- Zeng H, Zhao D, Mukhopadhyay D. KDR stimulates endothelial cell migration through heterotrimeric G protein Gq/11-mediated activation of a small GTPase RhoA. J Biol Chem. 2002;277:46791–46798. doi: 10.1074/jbc.M206133200. [DOI] [PubMed] [Google Scholar]
- Bobak D, Moorman J, Guanzon A, Gilmer L, Hahn C. Inactivation of the small GTPase Rho disrupts cellular attachment and induces adhesion-dependent and adhesion-independent apoptosis. Oncogene. 1997;15:2179–2189. doi: 10.1038/sj.onc.1201396. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Fabbri A, Flatau G, Donelli G, Matarrese P, Lemichez E, Falzano L, Boquet P. Escherichia coli cytotoxic necrotizing factor 1 (CNF1), a toxin that activates the Rho GTPase. J Biol Chem. 1997;272:19532–19537. doi: 10.1074/jbc.272.31.19532. [DOI] [PubMed] [Google Scholar]
- Giry M, Popoff M R, von Eichel-Streiber C, Boquet P. Transient expression of RhoA, -B, and -C GTPases in HeLa cells potentiates resistance to Clostridium difficile toxins A and B but not to Clostridium sordellii lethal toxin. Infect Immun. 1995;63:4063–4071. doi: 10.1128/iai.63.10.4063-4071.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning S W, Galandrini R, Hall A, Cantrell D A. The GTPase Rho has a critical regulatory role in thymus development. EMBO J. 1997;16:2397–2407. doi: 10.1093/emboj/16.9.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez B, Arends M, Esteve P, Perona R, Sanchez R, Ramon y Cajal S, Wyllie A, Lacal J C. Induction of apoptosis in NIH3T3 cells after serum deprivation by overexpression of rho-p21, a GTPase protein of the ras superfamily. Oncogene. 1995;10:811–816. [PubMed] [Google Scholar]
- Petrache I, Crow M T, Neuss M, Garcia J G. Central involvement of Rho family GTPases in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. Biochem Biophys Res Commun. 2003;306:244–249. doi: 10.1016/s0006-291x(03)00945-8. [DOI] [PubMed] [Google Scholar]
- Lum H, Malik A B. Mechanisms of increased endothelial permeability. Can J Physiol Pharmacol. 1996;74:787–800. doi: 10.1139/y96-081. [DOI] [PubMed] [Google Scholar]
- Zeng L, Xu H, Chew T L, Eng E, Sadeghi M M, Adler S, Kanwar Y S, Danesh F R. HMG CoA reductase inhibition modulates VEGF-induced endothelial cell hyperpermeability by preventing RhoA activation and myosin regulatory light chain phosphorylation. FASEB J. 2005;19:1845–1847. doi: 10.1096/fj.05-4240fje. [DOI] [PubMed] [Google Scholar]
- Croft D R, Olson M F. The Rho GTPase effector ROCK regulates cyclin A, cyclin D1, and p27Kip1 levels by distinct mechanisms. Mol Cell Biol. 2006;26:4612–4627. doi: 10.1128/MCB.02061-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pille J Y, Denoyelle C, Varet J, Bertrand J R, Soria J, Opolon P, Lu H, Pritchard L L, Vannier J P, Malvy C, Soria C, Li H. Anti-RhoA and anti-RhoC siRNAs inhibit the proliferation and invasiveness of MDA-MB-231 breast cancer cells in vitro and in vivo. Mol Ther. 2005;11:267–274. doi: 10.1016/j.ymthe.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Kroll J, Waltenberger J. The vascular endothelial growth factor receptor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells. J Biol Chem. 1997;272:32521–32527. doi: 10.1074/jbc.272.51.32521. [DOI] [PubMed] [Google Scholar]
- Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- McLaughlin A P, De Vries G W. Role of PLCgamma and Ca2+ in VEGF- and FGF-induced choroidal endothelial cell proliferation. Am J Physiol Cell Physiol. 2001;281:C1448–C1456. doi: 10.1152/ajpcell.2001.281.5.C1448. [DOI] [PubMed] [Google Scholar]
- Abecassis I, Olofsson B, Schmid M, Zalcman G, Karniguian A. RhoA induces MMP-9 expression at CD44 lamellipodial focal complexes and promotes HMEC-1 cell invasion. Exp Cell Res. 2003;291:363–376. doi: 10.1016/j.yexcr.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Wang H, Keiser J A. Vascular endothelial growth factor upregulates the expression of matrix metalloproteinases in vascular smooth muscle cells: role of flt-1. Circ Res. 1998;83:832–840. doi: 10.1161/01.res.83.8.832. [DOI] [PubMed] [Google Scholar]
- Yin L, Morishige K, Takahashi T, Hashimoto K, Ogata S, Tsutsumi S, Takata K, Ohta T, Kawagoe J, Takahashi K, Kurachi H. Fasudil inhibits vascular endothelial growth factor-induced angiogensis in vitro and in vivo. Mol Cancer Ther. 2007;6:1517–1525. doi: 10.1158/1535-7163.MCT-06-0689. [DOI] [PubMed] [Google Scholar]
- Hata Y, Miura M, Nakao S, Kawahara S, Kita T, Ishibashi T. Antiangiogenic properties of fasudil, a potent Rho-Kinase inhibitor. Jpn J Ophthalmol. 2008;52:16–23. doi: 10.1007/s10384-007-0487-5. [DOI] [PubMed] [Google Scholar]
- Ghosh K, Thodeti C K, Dudley A C, Mammoto A, Klagsbrun M, Ingber D E. Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc Natl Acad Sci U S A. 2008;105:11305–11310. doi: 10.1073/pnas.0800835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll J, Epting D, Kern K, Dietz C T, Feng Y, Hammes H P, Weiland T, Augustin H G. Inhibition of Rho-dependent kinases ROCK 1/II activates VEGF-driven retinal neovascularization and sprouting angiogenesis. Am J Physiol Heart Circ Physiol. 2009;269:893–899. doi: 10.1152/ajpheart.01038.2008. [DOI] [PubMed] [Google Scholar]
- Im E, Kazlauskas A. Src family kinases promote vessel stability by antagonizing the Rho/ROCK pathway. J Biol Chem. 2007;282:29122–29129. doi: 10.1074/jbc.M702637200. [DOI] [PubMed] [Google Scholar]
- Mavria G, Vercoulen Y, Yeo M, Paterson H, Karasarides M, Marais R, Bird D, Marshall C J. ERK-MAPK signaling opposes Rho-kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer Cell. 2006;9:33–44. doi: 10.1016/j.ccr.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Anand-Apte B, Zetter B R. Differential endothelial migration and proliferation to basic fibroblast growth factor and vascular endothelial growth factor. Growth Factors. 1996;13:57–64. doi: 10.3109/08977199609034566. [DOI] [PubMed] [Google Scholar]
- Cleaver O, Krieg P A. VEGF mediates angioblast migration during development of the dorsal aorta in Xenopus. Development. 1998;125:3905–3914. doi: 10.1242/dev.125.19.3905. [DOI] [PubMed] [Google Scholar]
- Bryan B A, D'Amore P A. What tangled webs they weave: Rho-GTPase control of angiogenesis. Cell Mol Life Sci. 2007;64:2053–2065. doi: 10.1007/s00018-007-7008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss M, Gerlach M, Gerlach H, Brett J, Wang F, Familletti P C, Pan Y C, Olander J V, Connolly D T, Stern D. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. 1990;172:1535–1545. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajska A, Torry R J, Kitten G T, Kolker S J, Tomanek R J. Modulation of cell migration and vessel formation by vascular endothelial growth factor and basic fibroblast growth factor in cultured embryonic heart. Dev Dyn. 1995;203:399–407. doi: 10.1002/aja.1002030403. [DOI] [PubMed] [Google Scholar]
- Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Titus B, Schwartz M A, Theodorescu D. Rho proteins in cell migration and metastasis. Crit Rev Eukaryot Gene Expr. 2005;15:103–114. doi: 10.1615/critreveukaryotgeneexpr.v15.i2.20. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Noritake J, Kaibuchi K. Regulation of microtubules in cell migration. Trends Cell Biol. 2005;15:76–83. doi: 10.1016/j.tcb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Birukova A A, Adyshev D, Gorshkov B, Bokoch G M, Birukov K G, Verin A D. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2006;290:L540–548. doi: 10.1152/ajplung.00259.2005. [DOI] [PubMed] [Google Scholar]
- Holinstat M, Mehta D, Kozasa T, Minshall R D, Malik A B. Protein kinase Calpha-induced p115RhoGEF phosphorylation signals endothelial cytoskeletal rearrangement. J Biol Chem. 2003;278:28793–28798. doi: 10.1074/jbc.M303900200. [DOI] [PubMed] [Google Scholar]
- Mehta D, Rahman A, Malik A B. Protein kinase C-alpha signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J Biol Chem. 2001;276:22614–22620. doi: 10.1074/jbc.M101927200. [DOI] [PubMed] [Google Scholar]
- Hasmim M, Bieler G, Ruegg C. Zoledronate inhibits endothelial cell adhesion, migration and survival through the suppression of multiple, prenylation-dependent signaling pathways. J Thromb Haemost. 2007;5:166–173. doi: 10.1111/j.1538-7836.2006.02259.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.