Abstract
One of the goals of evolutionary developmental biology is to link specific adaptations to changes in developmental pathways. The dentition of cypriniform fishes, which in contrast to many other teleost fish species possess pharyngeal teeth but lack oral teeth, provides a suitable model to study the development of feeding adaptations. Here, we have examined the involvement of retinoic acid (RA) in tooth development and show that RA is specifically required to induce the pharyngeal tooth developmental program in zebrafish. Perturbation of RA signaling at this stage abolished tooth induction without affecting the development of tooth-associated ceratobranchial bones. We show that this inductive event is dependent on RA synthesis from aldh1a2 in the ventral posterior pharynx. Fibroblast growth factor (FGF) signaling has been shown to be critical for tooth induction in zebrafish, and its loss has been associated with oral tooth loss in cypriniform fishes. Pharmacological treatments targeting the RA and FGF pathways revealed that both pathways act independently during tooth induction. In contrast, we find that in Mexican tetra and medaka, species that also possess oral teeth, both oral and pharyngeal teeth are induced independently of RA. Our analyses suggest an evolutionary scenario in which the gene network controlling tooth development obtained RA dependency in the lineage leading to the cypriniforms. The loss of pharyngeal teeth in this group was cancelled out through a shift in aldh1a2 expression, while oral teeth might have been lost ultimately due to deficient RA signaling in the oral cavity.—Gibert, Y., Bernard, L., Debiais-Thibaud, M., Bourrat, F., Joly, J.-S., Pottin, K., Meyer, A., Retaux, S., Stock, D. W., Jackman, W. R., Seritrakul, P., Begemann, G., Laudet, V. Formation of oral and pharyngeal dentition in teleosts depends on differential recruitment of retinoic acid signaling.
Keywords: tooth, Astyanax, FGF, zebrafish
Teeth are a vertebrate innovation that not only display a huge variability in terms of localization, number, shape, and size but also exhibit a conserved mode of development in most vertebrates (1). It is believed that the teeth of teleost fish have the same dual epithelio-mesenchymal origin as mammals and that neural crest cells (NCCs) are the source of the mesenchyme implicated in tooth development (2).
The function and expression of genes required for tooth development have been intensively studied in the mouse (3, 4). In contrast to mammals, the study of actinopterygian fish allows a better understanding of the general mechanisms controlling the development of teeth at additional sites within the oral cavity (reviewed in ref. 5). In actinopterygian fishes, there is extensive variation in tooth location, i.e., teeth may be present in upper and lower jaw margins, palate, floor of the mouth, as well as upper and lower pharyngeal surfaces (5). The dentition of actinopterygian fish therefore provides a unique model system to decipher the mechanisms controlling tooth location and its variation during evolution. For example, the 3 main developmental models for tooth development (the zebrafish Danio rerio, a cypriniform; the Mexican tetra Astyanax mexicanus, a characiform that is closely related to cypriniforms inside the Otophysi; and the medaka Oryzias latipes, a beloniform that is located in a less basal position in the actinopterygian tree, inside the percomorph) bear teeth in different locations (6).
The adult zebrafish, like all cypriniforms, lacks teeth in the oral cavity but retains a set of 11 teeth on each side of the fifth ceratobranchial arch (7). The precise pattern by which teeth are formed in the pharyngeal cavity and are then replaced has been described in zebrafish (8). All cypriniforms lack an upper pharyngeal jaw. While other fish species posses both upper and pharyngeal jaws, in cypriniforms the teeth grind against a chewing pad on the base of the skull (7). The first pair of pharyngeal teeth (called 4V1) is induced ∼48 hours postfertilization (hpf) in zebrafish and rapidly starts to undergo differentiation events, such as matrix deposition, and becomes attached to the fifth ceratobranchial arch at 3 days postfertilization (dpf). Research on mutants that lack posterior pharyngeal arches, yet develop the first pair of teeth (i.e., howler), has shown that tooth development in zebrafish is independent of pharyngeal arch formation (9). Furthermore, an fgf3 morphant develops the first pharyngeal teeth despite the absence of the posterior-most ceratobranchials (10).
In contrast to the zebrafish, the medaka and the Mexican tetra possess both oral and pharyngeal teeth (6, 11). The nature of the differences in inductive signals that explain the loss of oral teeth in cypriniforms remains unknown. Given that oral teeth have never been regained in the ∼3000 species of cypriniforms (12), it has been proposed that a developmental constraint in the ability to form teeth in the oral cavity exists in these fishes, as a consequence of a complex series of genetic modifications (6).
In zebrafish, depletion of fibroblast growth factor (FGF) signaling by the use of SU5402, an inhibitor of Fgf receptors, abolishes the induction of the first pair of pharyngeal teeth (10). Interestingly, inhibition of FGF signaling in A. mexicanus produces a partial phenocopy of the zebrafish oral cavity, in that oral teeth and the expression of markers of the tooth epithelium are absent (6). Therefore, it has been hypothesized that the specific loss of FGF signaling in the oral epithelium of cypriniforms led to the associated loss of oral teeth (6).
Given the numerous examples where signaling through Fgfs is linked to or antagonized by retinoic acid (RA) signaling during development (13,14,15,16,17) and since, in zebrafish, teeth are localized in a pharyngeal region that is patterned by RA, we scrutinized the role of the RA-signaling pathway during pharyngeal tooth development. RA is derived from vitamin A and acts as a signaling molecule in a variety of processes during vertebrate embryonic development (18). Its effect is transduced by RA receptors (RARs) encoded by 3 different genes in mammals, the product of which regulates the expression of specific target genes (reviewed in refs. 19, 20). In zebrafish mutants for aldh1a2, the main RA-producing enzyme during mouse development (21) have been identified, and loss of RA signaling during gastrulation leads to apoptosis of postotic NCCs, resulting in a near complete loss of pharyngeal arches 3–7 (22,23,24). Similarly, Aldh1a2−/− mice fail to form pharyngeal arch 3 (21). A lack of RA signaling during gastrulation leads to the absence of all branchial arches (22, 23). During somitogenesis, a lack of RA results in the failure of the formation of the posterior pharyngeal pouches leading to the absence of the posterior-most pharyngeal arches (24). Furthermore, a lack of RA during somitogenesis leads to a loss of the 4 posterior most branchial arches by massive cell death of NCC derivatives in the ventral posterior pharynx (24). However, a late role of RA signaling in the development of pharyngeal dentition, after NCC migration and pharyngeal arch development, has not been addressed until now.
Here, we show that RA signaling is required during pharyngeal tooth development in the zebrafish and characterize the underlying signaling components. We demonstrate that tooth development in other teleosts such as medaka and Astyanax that possess oral teeth in addition to the pharyngeal ones is independent of RA signaling, and we propose an evolutionary scenario for the loss of oral teeth in cypriniforms.
MATERIALS AND METHODS
Fish husbandry
Zebrafish, medaka, and Astyanax were reared and staged as described previously (25,26,27). Zebrafish strains of AB/Tü and Konstanz wild-type, nlsi26 (22), were reared and staged at 28.5°C according to Kimmel et al. (27). Medaka embryos of the Carbio strain were raised at 28.5°C and staged according to Iwamatsu (25). Astyanax embryos were obtained from surface fish as described previously (26), reared at 22.5°C, and staged by comparing their development to the zebrafish embryo (27). Incubating Astyanax embryos at 22.5°C led to a much slower developmental rate than what authors reported where their Astyanax embryos are incubated at 25°C or higher (6). This temperature difference explains the difference of timing in teeth formation (e.g., onset of dlx2b expression in the first pharyngeal teeth at 60 hpf in our case and at 42 hpf in Stock et al. (6).
Pharmacological treatments
Embryos were incubated in the dark at 28.5°C in all-trans RA (Sigma, St. Louis, MO, USA) at various concentrations (from 10−9 M to 10−6 M) diluted with embryo medium from a 10−4 M stock solution in ethanol. The pan-RA receptor antagonist BMS493 (a kind gift of Hinrich Gronemeyer; Institut Génétique Biologie Moléculaire Cellulaire, Illkirch, France) was diluted to 5 × 10−6 M from a 10−2 M stock solution in ethanol. 4-Diethylaminobenzaldehyde (DEAB; Fluka, Buchs, Switzerland) was applied at a concentration of 10−5 M from a 10−2 M stock in DMSO, without shielding from daylight. The RARα and RARγ antagonists and agonists (BMS614, CD2665, BMS641, and BMS961, respectively; a kind gift of Pierre Germain; Centre de Biochimie Structurale, Montpellier, France) were used at a concentration of 5 × 10−7 M, derived from a 10−2 M stock solution in DMSO. SU5402, an Fgfr inhibitor (Sigma), was diluted to 5 × 10−7 M from a 2.5 × 10−3 M stock in DMSO. As controls, wild-type embryos were treated with equivalent concentrations of ethanol or DMSO.
For pulse treatments using DEAB, embryos were washed 3 times in E3 medium after treatment to remove DEAB by washing out. Embryos where then allowed to develop under normal condition in E3 medium until fixation.
Injection of an inducible zebrafish fgf10 construct
A heat shock-inducible DNA construct (hsp70:fgf10a-GFP) was used to activate zebrafish fgf10a expression during development. This construct is composed of the zebrafish hsp70 promoter (28), the coding sequence of zebrafish fgf10a with GFP fused to the C-terminal end, and the SV40 polyadenylation signal, with all of these flanked by I-SceI homing endonuclease sequences. Details of the construction will be described elsewhere. Before injection, plasmid DNA was digested with I-SceI meganuclease (New England Biolabs, Beverly, MA, USA) for 1 h at 37°C, mixed 1:1 with 0.2 M KCl and 0.2% phenol red, and stored at −20°C. One-cell-stage embryos were injected with ∼1 nl (25 pg) of the digested DNA solution. Heat shocks of 30 min at 40°C were performed once or several times during development to induce hsp70:fgf10a-GFP expression.
In situ hybridization
Whole-mount in situ hybridization was performed as described previously for aldh1a2 (23), using the following additional probes: dlx2a, dlx2b, dlx3b (29), pitx2a (10), and eve1 (30) for zebrafish; dlx3b (11) for the medaka; and dlx2b (6) and aldh1a2 (this study) for the Mexican tetra.
Histology
Cartilage staining with Alcian blue was performed as described by Schilling et al. (9) at 82 or 112 hpf. Mineralized tissue staining with Alizarin red was performed as described (31) at 120 hpf for zebrafish and 12 dpf for medaka. For sectioning, embryos were embedded in paraffin and sectioned at 7 μm.
Cloning of A. mexicanus aldh1a2
RT-PCR was carried out on total cellular RNA isolated from A. mexicanus surface fish 24 hpf embryos. RT-PCR was carried out on total cellular RNA isolated from A. mexicanus surface fish 24 hpf embryos using the following degenerated primers 5′-ATCAARGAGGCTGGITTTCCACC-3′ and 5′-CCATTGCCRGACATTTTGWAICC-3′ (PCR condition: 94°C for 30 s and 58°C for 30 s 72°C for 2 min with 35 cycles). The resulting PCR product was cloned into PCRII-Topo (Invitrogen, Carlsbad, CA, USA) and sequenced. The partial sequence of the A. mexicanus aldh1a2 gene has been deposited in the NCBI database under accension no. FJ360898.
RESULTS
RA signaling during late development is necessary for tooth induction
RA plays a crucial role in pharyngeal patterning, and a lack of RA signaling during early development leads to major deficiencies in the development of the posterior pharynx (21,22,23,24). It was therefore possible that RA might also be required for the development of additional structures, such as the pharyngeal dentition, that emerge from the posterior pharyngeal region. To first examine whether RA-deficient zebrafish embryos develop teeth in the absence of posterior pharyngeal arches, we assessed whether teeth formed in nls mutant larvae, which are devoid of a functional aldh1a2 enzyme throughout gastrulation. Initiation of tooth buds is characterized by the expression of pitx2a in the dental epithelium and dlx2a and dlx2b in the dental epithelium and mesenchyme (10, 32), as well as eve1 expression in the dental epithelium at 56 hpf (30). In wild-type embryos at 56 hpf, the first pair of pharyngeal teeth already expresses pitx2a (Fig. 1A). In contrast, pitx2a expression is not detected in the ventral posterior pharynx of nls larvae (Fig. 1B). More generally, neither of the other tooth markers mentioned above (dlx2a, dlx2b, or eve1) are expressed in the ventral posterior pharynx in nls at 56 hpf but are present in the tooth buds of wild types (not shown). To avoid dealing with developmental delay, nls embryos were able to develop until 72 hpf and tooth development was visualized using dlx2b staining. Although the first tooth bud was still detected in control embryo, nls embryos never express dlx2b in the tooth bud even at 72 hpf (not shown). Moreover, teeth are never detected by Alcian blue or Alizarin red staining in nls mutant embryos at 120 hpf (not shown; refs. 22, 23). This confirms that the loss of early RA signaling, starting with zygotic expression, which has pleiotropic defects on pharyngeal development (24), leads to a failure of pharyngeal tooth induction.
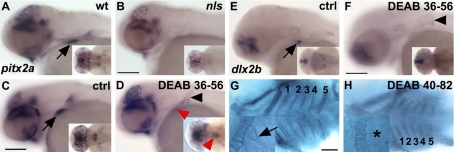
Figure 1.
RA is required for pharyngeal tooth induction. A, B) pitx2a expression is absent in the ventral posterior pharynx in nls/aldh1a2 at 56 hpf (B) as compared with wild types (WT; A). C–H) Wild-type embryos treated with DEAB from 36 hpf (C–F) or 40 hpf (G, H) onward fixed at 56 hpf (C–F) or 82 hpf (G, H). In DEAB-treated embryos, pitx2a is faintly detected in the pharyngeal epithelium (D, red arrowhead) and is located in a group of cells at the midline (D, inset, red arrowhead). dlx2b expression is not detected in tooth buds in DEAB-treated embryos at 56 hpf (F). Alcian blue staining at 82 hpf of a control embryo (G) shows all branchial arches numbered from 1 to 5, including teeth (G, inset). In DEAB-treated embryos, all ceratobranchial arches are present (H), but teeth are absent. Asterisk marks the absence of tooth. Black arrowheads denote the presence of the pectoral fin that is present when DEAB is applied at late stage (later than 13 hpf; ref. 36). Scale bars = 100 μm.
To study whether RA plays a role specifically during pharyngeal tooth induction, we blocked RA signaling by 2 complementary strategies, i.e., inhibiting RA synthesis and blocking the signaling pathway at the level of RARs. We treated wild-type embryos with 10−5 M DEAB, a competitive, reversible inhibitor of retinaldehyde dehydrogenases (33), or with 5 × 10−6 M BMS493, a specific antagonist that inhibits the activation of all RARs. Embryos were treated from different developmental stages onward and assayed for molecular markers of tooth induction (Table 1). Overall, the expression of pitx2a was always abolished when embryos were incubated before 36 hpf and left in DEAB or BMS 493 until fixation at 56 hpf. In contrast, when treatment commenced within this developmental window (36–56 hpf), faint pitx2a expression was detected (Fig. 1D, red arrowhead). pitx2a is the earliest known indicator of zebrafish tooth development and starting at 36 hpf is expressed in the pharyngeal epithelium of wild-type embryo, with the strongest expression prefiguring the first tooth germ (10). Therefore, when RA signaling is inhibited from 36 hpf onward, a low level of pitx2a expression remains in the pharyngeal epithelium (Fig. 1D). Of note, the later RA signaling is inhibited, the stronger pitx2a expression remains (not shown). Interestingly, its expression is confined to 1 patch of cells at the midline rather than to the paired but distinct tooth buds of wild types (Fig. 1C, D). dlx2b expression in the tooth epithelium, however, is absent from embryos treated with DEAB from 36 hpf onward, until fixation at 56 hpf (Fig. 1F). This also holds true for dlx2a and eve1, which are not detected in DEAB-treated embryos (not shown). Here again to avoid a simple developmental tooth induction delay induced by DEAB treatment, we treated wild-type embryos with DEAB form 36 hpf onward and fixed them at 72 hpf. DEAB treatments commencing at 36 hpf display very little developmental delay compare with the nls mutant. DEAB-treated embryos from 36 to 72 hpf do not express dlx2b in the posterior ventral pharynx (Supplemental Fig. S1B).
TABLE 1.
Timing of RA and FGF for the induction of the first pair of teeth in zebrafish
| Time of treatment (hpf) | pitx2a | dlx2a | dlx2b | eve1 | |
|---|---|---|---|---|---|
| DEAB 32-56 | Absent (100%, n=6) | Absent (100%, n=6) | Absent (100%, n=6) | Absent (100%, n=6) | |
| BMS 493 32-56 | Absent (100%, n=6) | Absent (100%, n=6) | Absent (100%, n=6) | Absent (100%, n=6) | |
| DEAB 36-56 | Present (100%, n=6) | Absent (100%, n=6) | Absent (100%, n=6) | Absent (100%, n=6) | |
| BMS 493 36-56 | Present (100%, n=6) | Absent (100%, n=6) | Absent (100%, n=6) | Absent (100%, n=6) | |
| DEAB 38-56 | Present (100%, n=6) | Absent (100%, n=6) | Absent (100%, n=6) | Absent (100%, n=6) | |
| DEAB 40-56 | Present (100%, n=6) | Absent (100%, n=6) | Absent (100%, n=6) | NA | |
| DEAB 42-56 | Present (100%, n=6) | Absent (100%, n=6) | Absent (100%, n=6) | NA | |
| DEAB 43-56 | Present (100%, n=6) | Absent (85%, n=14) | Absent (83%, n=6) | NA | |
| DEAB 44-56 | Present (100%, n=6) | Present (85%, n=14) | Present (100%, n=6) | NA | |
| DEAB 45-56 | Present (100%, n=6) | Present (100%, n=6) | Present (100%, n=6) | NA | |
| SU5402 32-56 | Present (100%, n=6) | Absent (100%, n=6) | Absent (100%, n=6) | NA | |
| SU5402 36-56 | NA | Absent (100%, n=6) | Absent (100%, n=6) | NA | |
| SU5402 40-56 | NA | Absent (100%, n=6) | Absent (100%, n=6) | NA | |
| SU5402 44-56 | NA | Absent (100%, n=6) | Absent (100%, n=6) | NA | |
| SU5402 47-56 | NA | Absent (100%, n=6) | Absent (100%, n=6) | NA | |
| SU5402 48-56 | NA | Present (100%, n=12) | Present (100%, n=6) | NA | |
| SU5402 49-56 | NA | Present (100%, n=6) | Present (100%, n=6) | NA |
DEAB, BMS 493, or SU5402 was applied at different time points in development. Tooth induction was scored by the presence or absence of specific tooth markers in the tooth bud. NA, nonapplicable.
To confirm that late DEAB treatment does not interfere with pharyngeal patterning and, consequently, with the development of posterior branchial arches, we blocked RA synthesis from 40 hpf onward, after pitx2a is detected in DEAB-treated embryos, fixed the embryos at 82 hpf, and found that the cartilaginous skeleton of all 5 branchial arches developed normally (Fig. 1H). At the same time, this DEAB treatment regime was effective at specifically inhibiting pharyngeal tooth induction, as judged by Alcian blue staining (Fig. 1H, inset).
To find the developmental timing at which RA is not required anymore to induce the first pair of teeth, we performed DEAB treatment starting at different times postfertilization. All treatments that commenced before 43 hpf resulted in the absence of tooth induction as monitored by either dlx2a or dlx2b expression (Table 1). To confirm that 43 hpf is a crucial time in RA requirement for tooth induction, we treated wild-type embryos with DEAB at either 40 hpf (before the crucial time point) or 45 hpf (after the crucial time point) and monitored tooth presence by Alcian blue at 112 hpf or Alizarin red at 120 hpf. In all embryos treated at 40 hpf, teeth are always absent, while teeth are always present when treatment starts at 45 hpf (Supplemental Fig. S1C–F). This is true with both staining methods used for tooth visualization. We further confirmed on transverse sections that no expression can be found for dlx2b in the tooth anlage when DEAB is applied before 43 hpf (Supplemental Fig. S2E). In summary, these pharmacological treatments convincingly show that RA signaling is required during pharyngeal tooth induction up to 43 hpf in zebrafish.
aldh1a2 expression is required for the induction of the first pair of pharyngeal teeth
To determine the location and source of RA required for pharyngeal tooth induction, we examined the expression of the RA-synthesizing enzymes known in zebrafish. The last enzymatic step of RA production is catalyzed by the retinaldahyde dehydrogenases of the Aldha1 protein family, which in mammals are encoded by 3 genes, aldh1a1–3. Two orthologs of the mammalian aldh1a2 and aldh1a3 genes have been characterized in zebrafish (22, 23, 34, 35), whereas the aldh1a1 homologue is absent from the genomes of fish (35, 36). Another gene thought to be a retinaldehyde dehydrogenase, originally named raldh4, was identified and found to belong to a different subfamily and is now referenced as aldh8a1 (36). We studied the expression of these 3 genes at 43 hpf. At that stage, aldh1a2 is expressed in the pectoral fins and in 2 patches of cells in the ventral posterior pharynx (Fig. 2A; see Supplemental Fig. S2 for sections); aldh1a3 is expressed in the otic vesicle (Fig. 2B) and aldh8a1 is expressed asymmetrically in the gut and liver (Fig. 2C). Since tooth induction is bilateral in the posterior pharynx and since aldh8a1 is unlikely to have retinaldehyde dehydrogenase activity, we conclude that this enzyme is unlikely to be the source of RA for induction of the first pair of teeth. Furthermore, aldh1a3 expression in this region is exclusive to the otic vesicle and therefore probably too far removed from the posterior pharynx for paracrine signaling to occur.
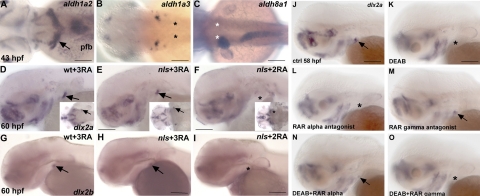
Figure 2.
RA, provided by aldh1a2, serves as a signal for tooth induction that is transduced by raraa receptors. A–C) Expression of the zebrafish aldh1a2, aldh1a3, and aldh8a1 genes at 43 hpf in dorsal views. A) aldh1a2 is mainly detected in the dorsal spinal cord, the pectoral fin mesenchyme, and 2 patches of cells in the ventral posterior pharynx engulfing the fifth ceratobranchial arch (arrow). B) aldh1a3 is expressed in the otic vesicle. C) aldh8a1 is expressed in the liver and the anterior gut. Asterisks in B, C mark the location where teeth develop. D–I) nls embryos exposed to exogenous RA during gastrulation and somitogenesis fail to restore tooth induction (F, I). Asterisk in F marks lack of teeth. +3 RA indicates that exogenous RA was added during gastrulation, somitogenesis and for tooth induction. +2 RA indicates that exogenous RA was added during gastrulation and somitogenesis only. All in situ hybridizations were performed at 60 hpf. J–O) Tooth induction is mediated by raraa subtypes. Inhibition of rara subtypes blocks tooth induction, as marked by absence of dlx2a expression (L), while activating raras in embryos lacking RA signaling restores tooth induction (N). The rarg subtypes play no role during tooth induction (M, O). Asterisks in K, L, O denote the absence of tooth bud expression (pfb, pectoral fin bud). Scale bars = 100 μm.
To follow up on a possible involvement of aldh1a2 in this process and to investigate whether the induction of the first pair of pharyngeal teeth is dependent on aldh1a2, we supplied the nls/aldh1a2 mutant with exogenous RA during the developmental stage when RA is required for the migration and the maintenance of NCCs, i.e., during gastrulation (from 5 to 10 hpf), during the end of somitogenesis (from 18 to 26 hpf) (24), and from 36 hpf onward for teeth induction. This treatment rescued the induction of the first pair of teeth as marked by dlx2a (Fig. 2E) and dlx2b (Fig. 2H) expression in the tooth bud. In contrast, application of RA from 5 to 10 hpf, and from 18 to 26 hpf only, failed to induce pharyngeal teeth in nls (Fig. 2F, I). These results demonstrate that aldh1a2 is required to induce the first pair of pharyngeal teeth at 43 hpf.
At 43 hpf, aldh1a2 is strongly expressed in 2 patches of cells in the ventral posterior pharynx (Fig. 2A, arrow, and Supplemental Fig. S2A) but also in the pectoral fin bud mesenchyme (Supplemental Fig. S3A, B; arrowhead; ref. 37). Due to its proximity with the tooth bud, we examined the possibility that the pectoral fin could be a source of RA for the induction of the first pair of teeth. To this end, we analyzed pharyngeal tooth formation in embryos that lack pectoral fins, in spadetail/no tail double mutants (37), and in tbx5 morphants (38). We found that the absence of pectoral fin buds does not prevent the induction of the first pair of teeth (Supplemental Fig. S3C–H). Given the expression pattern of aldh1a2 and the rescue of pharyngeal teeth in nls/aldh1a2 mutants by RA, it is therefore most likely that the source of RA must be the 2 groups of cells located in the ventral posterior pharynx (Supplemental Fig. S2A). A transverse section of a 43 hpf wild-type embryo stained for aldh1a2 showed that the cells expressing aldh1a2 at this stage coincide with the expression of the tooth bud as marked by pitx2a at 56 hpf (Supplemental Fig. S2).
RA acts via the rara receptors for the induction of the first pair of teeth
RA regulates gene expression through ligand-induced activation of RARs, which in zebrafish are encoded by 4 different genes: 2 rara paralogues (raraa and -b) and 2 rarg paralogues (rarga and -b). Orthologs of mammalian RARβ have been shown to be missing from the zebrafish genome (39). At 43 hpf, expression of all RAR transcripts can be detected in the ventral pharynx (39, 40). To determine which RARs are required for the induction of the first pair of pharyngeal teeth, we used a pharmacological approach with compounds whose selectivity for zebrafish RARs had been characaterized in vitro (41). First, we selectively blocked the rara subtypes from 36 hpf onward until fixation at 58 hpf with the selective antagonist BMS614 (42) and assayed for dlx2a expression as an indication of tooth induction at 58 hpf. Inhibition of the raras resulted in a failure of tooth induction (Fig. 2L), whereas specific inhibition of the rarg subtypes using the selective antagonist CD2665 did not inhibit the induction of dlx2a expression in the tooth bud at 58 hpf (Fig. 2M). To confirm that tooth induction requires rara genes, we simultaneously blocked RA biosynthesis with DEAB before tooth induction (from 36 hpf onward) and activated the rara or rarg subtypes with selective agonists. We found that in the absence of endogenous RA activation of the raras using the selective agonist BMS641 was sufficient to rescue the induction of the first pair of teeth (Fig. 2N), while activation of the rargs using the selective agonist BMS961 cannot restore dlx2a expression in DEAB-treated embryos (Fig. 2O). These results strongly suggest that activation of raras is required and sufficient for the induction of the first pair of pharyngeal teeth. Due to the unavailability of paralogue-specific compounds, it was not possible to discriminate between the 2 rara paralogues in zebrafish. At the time of the induction of the first pair of teeth, raraa is expressed specifically in the posterior most branchial arches, while rarab is found in a more diffuse pattern along the entire pharyngeal arches (39, 43). At the same time of development, rarga is strongly expressed in the entire pharynx, while rargb is strongly detected in all but the last pharyngeal arch (39, 43). The expression patterns of raraa and raraab thus do not provide a clear indication about which of these genes is responsible for mediating the effect of RA on tooth induction, as they are both detected in the ventral posterior pharynx at the time of tooth induction by RA. Given that morpholino knockdown experiments have shown that rargs are necessary for pharyngeal patterning (44), our data showing that it is rather the rara paralogs that are critical for pharyngeal tooth induction further reinforce the notion that pharyngeal patterning and pharyngeal tooth induction are unlinked events during zebrafish development.
RA and FGF signaling act independently of each other during tooth induction
The induction of the first pair of pharyngeal teeth is FGF dependent in teleosts, but a lack of FGF signaling does not impair pitx2a expression in the tooth bud (10). To understand the relationship between FGF and RA signaling in the process of tooth induction, we determined the developmental time during which FGF signaling is required to induce the pharyngeal teeth by treating wild-type embryos with SU5402, a compound that has been shown to bind to FGF receptors and to concomitantly block signaling (45, 46), for different periods of development. We observed that blocking FGF signaling at any stage before 48 hpf abolished tooth induction, while later treatments had no effect on the onset of dlx2a and dlx2b expression in the tooth bud (Table 1). To investigate the epistatic relationship between the RA and the FGF signaling pathways, we concomitantly activated the RA pathway and inhibited the FGF signaling pathway. We found that exogenous RA, applied from 32 to 56 hpf, fails to rescue the expression of dlx2 genes in the tooth buds at 56 hpf when FGF signaling has been inhibited during the same developmental interval (Fig. 3A–F).
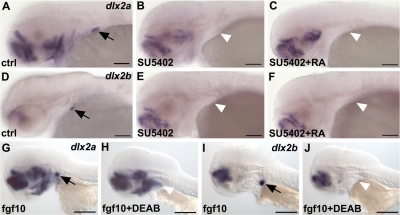
Figure 3.
RA and FGF signaling act independently during tooth induction. A–F) Exogenous RA applied at 32 hpf does not restore dlx2a (C) or dlx2b (F) expression when Fgf signaling has been blocked at 32 hpf. G–J) Ectopic activation of FGF signaling using an inducible fgf10a construct does not restore dlx2a (H) or dlx2b (J) expression in RA-depleted embryos. White arrowheads indicate absence of tooth induction. All in situ hybridizations were performed at 56 hpf. Scale bars = 100 μm.
Expression of fgf10a, using a heat-inducible construct (hsp70:fgf10a-GFP), leads to widespread formation of ectopic teeth along the fifth ceratobranchial arch. However, ectopic teeth are never observed more anteriorly under fgf10a ectopic expression (e.g., on ceratobranchial arch 4 or more anteriorly; unpublished results). To study whether exogenous FGF signaling can restore tooth induction in the absence of RA, we injected wild-type zebrafish at the 1-cell stage with hsp70:fgf10a-GFP DNA, blocked RA signaling at 38 hpf using DEAB, and subsequently induced fgf10a expression by applying a heat shock at 40 hpf. These experiments failed to restore expression of dlx2a or dlx2b in the tooth bud (Fig. 3H, J), and mineralized teeth could not be detected at 100 hpf (not shown). We conclude from these experiments that RA and fgf are both required in independent pathways to induce teeth in zebrafish.
Induction of oral and pharyngeal teeth in medaka and Astyanax is independent of RA signaling
Having shown that pharyngeal teeth are RA-signaling dependent in zebrafish, we next asked whether oral teeth are also RA dependent in other species, such as the medaka (O. latipes) and the Mexican tetra (A. mexicanus). First, we tested the efficiency of RA-signaling inhibition through DEAB in these species. To confirm that DEAB actively affects pharyngeal development, we treated medaka embryos with DEAB from stage 20 (4-somite stage) to stage 32 (somite completion stage; ref. 25) and allowed the embryos to develop until 12 dpf at 28.5°C. As in zebrafish, a lack of RA signaling during somitogenesis abolishes the induction of pharyngeal teeth in medaka as monitored by Alizarin red skeletal staining (Fig. 4B) and dlx3b in situ hybridization (not shown). In our hand, dlx3b staining in medaka embryos gives a much clearer result than dlx2b; therefore, for this species we used dlx3b as tooth marker, while we used dlx2b for the 2 other species. Similar treatments in Astyanax embryos also compromised tooth induction when DEAB was applied during somitogenesis (Table 2), confirming that DEAB treatment efficiently blocks RA-dependent developmental processes in both species.
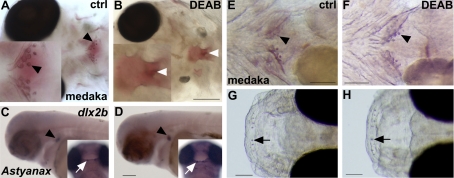
Figure 4.
RA is not required for oral and pharyngeal tooth induction in noncypriniform teleosts. A, B) Alizarin red staining of medaka embryos treated with DEAB during somitogenesis shows lack of pharyngeal teeth (white arrowhead in B) and the most posterior ceratobranchial arch (B), confirming the potency of DEAB in these species (ventral views). C, D) A. mexicanus control embryo (C) and DEAB-treated embryo from 30 hpf onward (D), showing dlx2b expression in oral (white arrow) and pharyngeal (black arrowhead) teeth (lateral views). E–H) Alizarin red staining of medaka embryos treated with DEAB from 3–7 dpf shows pharyngeal (F, arrowhead) and upper oral teeth (H, arrow; lateral views). Scale bars = 100 μm.
TABLE 2.
DEAB treatment in zebrafish, medaka, and Mexican tetra at different time points
| Species | DEAB treatment | Tooth induction |
|---|---|---|
| D. rerio | 5–11 hpf (5 μm) | − |
| D. rerio | 12–24 hpf (10 μm) | + |
| D. rerio | 18–30 hpf (10 μm) | − |
| D. rerio | 23–30 hpf (10 μm) | − |
| D. rerio | 20–28 hpf (10 μm) | − |
| D. rerio | 28–32 hpf (10 μm) | + |
| D. rerio | 40–56 hpf (10 μm) | − |
| O. latipes | Stage 20–stage 32 (30 μm) | − |
| O. latipes | 4–8 dpf (30 μm) | + |
| O. latipes | 3–7 dpf (60 μm) | + |
| O. latipes | 5–8 dpf (60 μm) | + |
| O. latipes | 4–7 dpf (100 μm) | + |
| O. latipes | 4–8 dpf (120 μm) | + |
| O. latipes | 4–8 dpf (160 μm) | + |
| A. mexicanus | 18–75 hpf (20 μm) | − |
| A. mexicanus | 24–75 hpf (20 μm) | − |
| A. mexicanus | 27–75 hpf (50 μm) | +/− |
| A. mexicanus | 30–75 hpf (50 μm) | + |
| A. mexicanus | 30–75 hpf (75 μm) | + |
| A. mexicanus | 40–75 hpf (50 μm) | + |
The presence of teeth was monitored by in situ hybridization with dlx2a and or dlx2b for the zebrafish and the Mexican tetra and by Alizarin red staining and dlx3b for the medaka.
We next examined whether RA is required for oral tooth induction. We blocked RA synthesis with DEAB at the end of somitogenesis and allowed the embryos to develop until 12 dpf for the medaka and 75 hpf for Astyanax, when oral tooth formation is well under way according to our observations and a previous study (11). Given that the timing is critical in these experiments, we adjusted the antagonist treatments using published developmental data (5, 47) to the precise timing of known morphological and gene expression events linked to tooth formation (Supplemental Fig. S4). A lack of RA signaling from the end of somitogenesis onward does not prevent the induction of oral teeth in both medaka and Astyanax [Fig. 4D (inset), H]. We conclude that oral tooth induction is independent of RA signaling in these teleosts.
To examine whether RA is required for pharyngeal tooth induction, medaka and Astyanax embryos were treated with DEAB from the end of somitogenesis onward, i.e., after RA is required for the maintenance of NCC derivates. In these experiments, where RA inhibition commences before tooth marker gene expression, induction of pharyngeal teeth surprisingly was not compromised in either species (Fig. 4D, F). Because we did not anticipate a difference in RA requirements for pharyngeal dentition between teleosts species, we examined whether higher doses of DEAB, up to 160 μM for the medaka and 75 μM for Astyananx at different developmental stages, would affect pharyngeal tooth development. Neither of these treatments led to an absence of pharyngeal teeth (Table 2). However, earlier treatments lead to an absence of pharyngeal tooth development: for example, when DEAB was applied at 24 hpf in Astyanax, no pharyngeal teeth were visible at 75 hpf (n=8), while 50% of embryos had teeth when DEAB was applied at 27 hpf (n=8), and all embryos developed teeth when DEAB was added at 30 hpf or later (n=16) (Table 2). However, a lack of RA during early stage of embryonic development (i.e., 24 hpf in Astyanax) will affect the maintenance of posterior NCC derivates (24), leading to an absence of posterior structure in the pharynx including teeth. In our experimental settings, pharyngeal teeth are induced at ∼60 hpf in Astyanax (Supplemental Fig. S4), meaning that DEAB treatment from 30 hpf onward will definitively target this developmental stage. We believe that 27 hpf is the end of RA requirement for maintenance of the posterior NCCs in Astyanax; that is why DEAB treatments starting at this stage or earlier prevent pharyngeal tooth induction by suppressing posterior NCC derivatives, while later treatments (after 27 hpf) only target pharyngeal tooth induction without any effect. Notably, typical defects that accompany the loss of RA signaling, like pericardial edema and a failure of swim bladder inflation, were always observed after DEAB treatments at the higher doses. The inhibition experiments showed that pharyngeal tooth induction is not dependent on RA in medaka and Astyanax, even though in zebrafish teeth clearly fail to be induced in the absence of RA signaling.
Differential requirements for RA signaling in the posterior pharynx correlate with distinct aldh1a2 expression patterns in teleosts
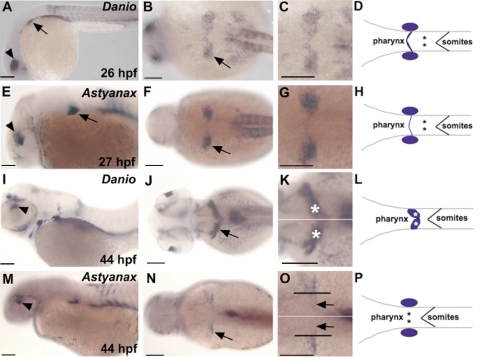
To examine whether the lack of RA requirement for pharyngeal tooth induction in medaka and Astyanax might be linked to differences in RA synthesis, we compared aldh1a2 expression with that in zebrafish. Since heterochronic shifts in pharyngeal tooth induction are more pronounced between zebrafish and medaka (Supplemental Fig. S4), we compared the gene expression patterns of aldh1a2 orthologues of Astyanax and zebrafish, which develop at more similar rates. At 26 hpf in zebrafish, aldh1a2 is detected in the dorsal retina, the caudal part of the branchial arch primordium, and in the somitic mesoderm (Fig. 5A–C; ref. 23). A similar pattern is observed for the Astyanax orthologue of aldh1a2 (Fig. 5E–G), suggesting an overall evolutionary conservation of aldh1a2 gene expression in Ostariophysi, the taxon containing the orders Characiformes and Cypriniformes.
Figure 5.
aldh1a2 expression in zebrafish and Mexican tetra embryos. A–H) aldh1a2 expression in embryos at 26 hpf for zebrafish and 27 hpf for Mexian tetra. Arrows indicate expression in the caudal branchial arch; arrowheads denote expression in the dorsal retina. I–P) aldh1a2 expression at 44 hpf. Arrows indicate the 2 patches of cells in the ventral posterior pharynx in zebrafish (J) and the expression in the caudal branchial arch in Astyanax (N). White lines (K, O) demarcate the midline of the embryo; black lines (O) the limit of aldh1a2 expression in the pharynx toward the midline. Asterisks (D, H, K, L, P) mark the putative location of the induction of the first tooth. Note that this location is within the aldh1a2-positive cells for the zebrafish (K) but not for the Astyanax as marked by the black arrows (O). Scale bars = 100 μm.
At the time of pharyngeal tooth induction, however, aldh1a2 expression in the pharyngeal region differs markedly between zebrafish and Astyanax. In zebrafish, expression is detected in 2 patches of cells in the ventral posterior pharynx engulfing the fifth ceratobranchial arch (Fig. 5I–K). While the overall Astyanax aldh1a2 expression resembles the expression pattern observed in zebrafish (e.g., expression in dorsal retina and the dorsal anterior spinal cord; see Fig. 5M–O; see also Supplemental Fig. S5 for aldh1a2 expression at 44 hpf), expression in the pharyngeal region shows distinct differences between the 2 species. In Astyanax, aldh1a2-expressing cells are located further laterally than in zebrafish and exclude the pharyngeal region (Fig. 5J–L, N–P). In contrast, in zebrafish aldh1a2 expression is located in the ventral posterior pharynx reaching the midline. Based on dlx2b expression in the tooth bud at 56 hpf in zebrafish and 75 hpf in Astyanax, the tooth primordia in zebrafish are located in a domain overlapping with aldh1a2 expression, whereas in Astyanax induction of the first teeth (marked by arrows in Fig. 5O) takes place outside the aldh1a2 expression domain.
Based on RA inhibition experiments in medaka and Astyanax and on the expression pattern of aldh1a2 in Astyanax, we conclude that the tooth primordia are not in close proximity to RA at the stage when tooth induction occurs. In Astyanax and medaka, therefore, the induction of both oral and pharyngeal dentition is independent of RA signaling. Because oral tooth loss in zebrafish is thought to be a derived trait (5), this suggests that primitively in teleost fishes tooth induction was RA independent and that cypriniform fish developed a RA-dependent mode of tooth development. The apparent shift of pharyngeal aldh1a2 expression toward the midline in zebrafish, when compared with Astyanax, puts forward a possible scenario how RA dependency might have been obtained and exemplifies an unexpected evolutionary change in the deployment of a developmental pathway during tooth development.
DISCUSSION
RA is required for induction of pharyngeal teeth in zebrafish
RA has been shown to regulate patterning and development of the pharynx in chordates (22,23,24, 48, 49). However, possible later roles for RA in tooth development have so far not been investigated. Using pharmacological compounds that block or selectively activate the rara and rarg genes, we demonstrated that tooth induction is mediated by the rara subtypes (Fig. 2). Accordingly, NCCs that form the pharyngeal skeleton have been shown to express both raraa and rarab (44). Recently, Linville et al. (44), using antisense morpholinos targeting raraa and rarab, showed that neither of these genes is involved in pharyngeal arch development, while morpholino-mediated knockdown of rarga phenocopied the pharyngeal defects observed in the nls/aldh1a2 mutant. This suggests that tooth loss accompanying rara-selective inhibitor treatment is not a consequence of defects in pharyngeal arch development but reflects a true dependence on rara subtypes for pharyngeal tooth induction.
RA and FGF signaling act in parallel during tooth induction
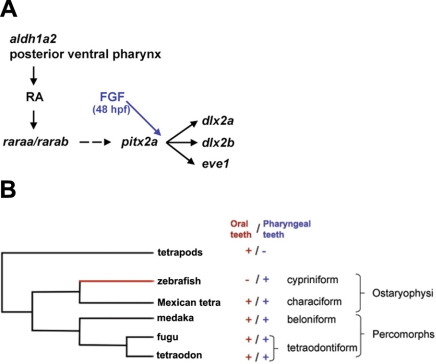
Fgf signaling has been shown to be required for the induction of pharyngeal teeth in zebrafish (10) and for pharyngeal and oral tooth induction in Astyanax (6). We have shown that in zebrafish RA signaling is required in addition to FGF signaling for tooth induction. The temporal requirements for both signaling pathways appear to be slightly distinct, RA signaling is only required before 43 hpf to induce tooth markers, while inhibition of Fgf signaling up to 48 hpf results in loss of dlx gene expression at 56 hpf (Table 1). However, we were not able to derive a clear epistatic relationship between both pathways, as exogenous RA was unable to rescue early tooth markers in the absence of FGF signaling, and overexpression of fgf10 alone, which is sufficient to induce ectopic teeth on the fifth ceratobranchial arches (unpublished results), was ineffective at rescuing tooth development when RA signaling had been blocked. Jackman et al. (10) showed that inhibition of FGF signaling does not impair the expression of pitx2a, the earliest known gene expressed in pharyngeal teeth. Our finding that a lack of RA signaling reduces and changes the expression domain of pitx2a in the dental epithelium suggests that RA and Fgf signaling both participate in the tooth developmental program. RA is required before 36 hpf for induction of pitx2a, whose expression is normally induced at 36 hpf in the epithelium of the first pharyngeal teeth, and up to 43 hpf for continued expression of pitx2a. In contrast, Fgf signaling is neither required for induction nor maintenance of pitx2a expression but is necessary to induce dlx gene expression. Since RA cannot overcome a lack of Fgf signaling, Fgf might play a more specific role in tooth formation after the dental epithelium has been specified. Given that we observe a misplacement of the pitx2a expression domains when RA signaling is reduced after pitx2a induction, it is likely that RA is also involved in patterning events at the midline of the ventral pharynx. We conclude from these observations that RA and FGF signaling are required in parallel for successive events during tooth induction (Fig. 6).
Figure 6.
Proposed model of genetic pathways involved in pharyngeal tooth induction in zebrafish. A) aldh1a2 from the posterior ventral pharynx generates RA. This signal activates the RAR subtype α. RA signaling is required for a proper expression of pitx2a; however, in absence of this signal, pitx2a is still detected at a lower level as a spot at the midline. Dotted lines represent an RA requirement for proper expression of pitx2a. pitx2a, in turn, regulates the expression of dlx2a, dlx2b, and eve1. FGF signaling is required after RA signaling (48 vs. 43 hpf). Moreover we know from previous study that a lack of FGF signaling does not abolish pitx2a expression in the tooth bud (10). B) Phylogenetic tree of the main fish models showing the presence of absence of a particular set of teeth (oral vs. pharyngeal). Red branch represents the hijack of RA for tooth induction in zebrafish.
RA is not involved in tooth induction in other teleosts
Our experiments in medaka and Astyanax show that RA is dispensable for oral tooth induction and, more surprisingly, suggest that RA signaling is also not required for the induction of pharyngeal teeth. In addition, expression of aldh1a2 in the pharynx of zebrafish extends more toward the midline and thus toward the region of pharyngeal tooth formation than in Astyanax at the time of tooth induction. To gather these observations into a coherent model, it is important to recall that cypriniforms have lost oral teeth early on during evolution and that this event has not been reverted in any of the 3000 known species in this order (5, 6). Thus, medaka and Astyanax probably provide a better glimpse on the ancestral situation occurring in fishes, whereas the zebrafish corresponds to the derived situation (Fig. 6B). Our data suggest that pharyngeal teeth in cypriniforms obtained RA regulation for induction, while in other taxa this inductive phase is RA independent. It is tempting to correlate this RA dependency of tooth induction with the observed shift in aldh1a2 expression, suggesting that a relocation of RA synthesis that occurred specifically in cypriniforms acted as a precondition for this event. It is likely that dependency on RA arose at the level of a specific enhancer of one of the early genes controlling the tooth induction process. One candidate gene could be pitx2a, as it is the only gene in the tooth development cascade whose expression is affected by RA but not Fgf signaling.
Once pharyngeal tooth induction became RA dependent, it is likely that this pleiotropic signal also affected the developmental program of oral teeth. Our model therefore provides an explanation for oral tooth loss in the lineage leading to the cypriniforms. Indeed, the lack of expression of aldh1a2 and aldh1a3 indicates that the anterior region of the embryo and mostly the oral region are devoid of RA (14). This model is interesting to consider in light of recent results showing that the signaling pathways implicated in tooth induction in the oral and pharyngeal regions are largely identical (6, 11).
Cypriniforms were unlikely to lose oral teeth due to structural mutations in the gene repertoire required for tooth formation in general, as pleiotropic defects elsewhere would have been deleterious. Our empirically deduced scenario, in which tooth development acquires RA dependency, thus provides an alternative and plausible hypothesis that provides a functional link between the loss of oral teeth and the gain of RA regulation in pharyngeal teeth in cypriniforms. This situation is reminiscent to the diastema, a region devoid of teeth between the incisor and molar fields, found in rodents (50). It has been observed that the expression of natural inhibitors of the FGF pathway, encoded by the sprouty genes, in the diastema prevents freshly induced tooth buds to further engage in the tooth developmental program (51). We believe the situation in zebrafish to be rather different, as no indication of tooth formation is apparent in the oral region, which suggests a blockade at a very early step (6); and the obligatory inclusion of an additional signaling pathway into the tooth developmental program that is otherwise inactive in the oral region may explain the loss of oral teeth.
Supplementary Material
Acknowledgments
This work was supported by an ARC fellowship (to Y.G.), funding from the Deutsche Forschungsgemeinschaft (to G.B.), funding from the ENS Lyon, the ANR program and the French Ministry of Research (to V.L.), an ANR-Neuro (to S.R.), INRA, CNRS, the ANR Grant Choregnet, the Marine Genomics Center of Excellence, the FP6 STREP Plurigenes (to J.-S.J.), and NIH 5F32DE015029 and P20RR016463 (to W.R.J.), and a NSF IOS-0446720 (to D.W.S.). The authors thank Pierre Germain (Centre de Biochimie Structurale, Montpellier, France) for his kind gift of the RARa and RARg selective agonists and antagonists. Thanks to Matthieu Simion and Laurent Legendre for expert animal care in Gif.
References
- Smith M M, Fraser G J, Mitsiadis T A. Dental lamina as source of odontogenic stem cells: evolutionary origins and developmental control of tooth generation in gnathostomes. J Exp Zoolog B Mol Dev Evol. 2009;312B:260–280. doi: 10.1002/jez.b.21272. [DOI] [PubMed] [Google Scholar]
- Miletich I, Sharpe P T. Neural crest contribution to mammalian tooth formation. Birth Defects Res C Embryo Today. 2004;72:200–212. doi: 10.1002/bdrc.20012. [DOI] [PubMed] [Google Scholar]
- Mitsiadis T A, Smith M M. How do genes make teeth to order through development? J Exp Zoolog B Mol Dev Evol. 2006;306:177–182. doi: 10.1002/jez.b.21104. [DOI] [PubMed] [Google Scholar]
- Tompkins K. Molecular mechanisms of cytodifferentiation in mammalian tooth development. Connect Tissue Res. 2006;47:111–118. doi: 10.1080/03008200600727756. [DOI] [PubMed] [Google Scholar]
- Stock D W. Zebrafish dentition in comparative context. J Exp Zoolog B Mol Dev Evol. 2007;308:523–549. doi: 10.1002/jez.b.21187. [DOI] [PubMed] [Google Scholar]
- Stock D W, Jackman W R, Trapani J. Developmental genetic mechanisms of evolutionary tooth loss in cypriniform fishes. Development. 2006;133:3127–3137. doi: 10.1242/dev.02459. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Van der heyden C, Sire J Y. Early development of the zebrafish (Danio rerio) pharyngeal dentition (Teleostei, Cyprinidae) Anat Embryol (Berl) 1998;198:289–305. doi: 10.1007/s004290050185. [DOI] [PubMed] [Google Scholar]
- Van der Heyden C, Huysseune A, Sire J Y. Development and fine structure of pharyngeal replacement teeth in juvenile zebrafish (Danio rerio) (Teleostei, Cyprinidae) Cell Tissue Res. 2000;302:205–219. doi: 10.1007/s004410000180. [DOI] [PubMed] [Google Scholar]
- Schilling T F, Piotrowski T, Grandel H, Brand M, Heisenberg C P, Jiang Y J, Beuchle D, Hammerschmidt M, Kane D A, Mullins M C, van Eeden F J, Kelsh R N, Furutani-Seiki M, Granato M, Haffter P, Odenthal J, Warga R M, Trowe T, Nusslein-Volhard C. Jaw and branchial arch mutants in zebrafish I: branchial arches. Development. 1996;123:329–344. doi: 10.1242/dev.123.1.329. [DOI] [PubMed] [Google Scholar]
- Jackman W R, Draper B W, Stock D W. Fgf signaling is required for zebrafish tooth development. Dev Biol. 2004;274:139–157. doi: 10.1016/j.ydbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Debiais-Thibaud M, Borday-Birraux V, Germon I, Bourrat F, Metcalfe C J, Casane D, Laurenti P. Development of oral and pharyngeal teeth in the medaka (Oryzias latipes): comparison of morphology and expression of eve1 gene. J Exp Zoolog B Mol Dev Evol. 2007;308:693–708. doi: 10.1002/jez.b.21183. [DOI] [PubMed] [Google Scholar]
- Nelson J S. Hoboken, NJ, USA: John Wiley & Sons; Fishes of the World. (4th Ed.) 2006 [Google Scholar]
- Hernandez R E, Rikhof H A, Bachmann R, Moens C B. vhnf1 integrates global RA patterning and local FGF signals to direct posterior hindbrain development in zebrafish. Development. 2004;131:4511–4520. doi: 10.1242/dev.01297. [DOI] [PubMed] [Google Scholar]
- White R J, Nie Q, Lander A D, Schilling T F. Complex regulation of cyp26a1 creates a robust retinoic acid gradient in the zebrafish embryo. PLoS Biol. 2007;5:e304. doi: 10.1371/journal.pbio.0050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu I O, Duester G. Retinoic-acid signalling in node ectoderm and posterior neural plate directs left-right patterning of somitic mesoderm. Nat Cell Biol. 2006;8:271–277. doi: 10.1038/ncb1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamade A, Deries M, Begemann G, Bally-Cuif L, Genet C, Sabatier F, Bonnieu A, Cousin X. Retinoic acid activates myogenesis in vivo through Fgf8 signalling. Dev Biol. 2006;289:127–140. doi: 10.1016/j.ydbio.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Ribes V, Wang Z, Dolle P, Niederreither K. Retinaldehyde dehydrogenase 2 (RALDH2)-mediated retinoic acid synthesis regulates early mouse embryonic forebrain development by controlling FGF and sonic hedgehog signaling. Development. 2006;133:351–361. doi: 10.1242/dev.02204. [DOI] [PubMed] [Google Scholar]
- Mark M, Ghyselinck N B, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- Germain P, Chambon P, Eichele G, Evans R M, Lazar M A, Leid M, De Lera A R, Lotan R, Mangelsdorf D J, Gronemeyer H. International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol Rev. 2006;58:712–725. doi: 10.1124/pr.58.4.4. [DOI] [PubMed] [Google Scholar]
- Morriss-Kay G M, Ward S J. Retinoids and mammalian development. Int Rev Cytol. 1999;188:73–131. doi: 10.1016/s0074-7696(08)61566-1. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Begemann G, Schilling T F, Rauch G J, Geisler R, Ingham P W. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development. 2001;128:3081–3094. doi: 10.1242/dev.128.16.3081. [DOI] [PubMed] [Google Scholar]
- Grandel H, Lun K, Rauch G J, Rhinn M, Piotrowski T, Houart C, Sordino P, Kuchler A M, Schulte-Merker S, Geisler R, Holder N, Wilson S W, Brand M. Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development. 2002;129:2851–2865. doi: 10.1242/dev.129.12.2851. [DOI] [PubMed] [Google Scholar]
- Kopinke D, Sasine J, Swift J, Stephens W Z, Piotrowski T. Retinoic acid is required for endodermal pouch morphogenesis and not for pharyngeal endoderm specification. Dev Dyn. 2006;235:2695–2709. doi: 10.1002/dvdy.20905. [DOI] [PubMed] [Google Scholar]
- Iwamatsu T. Stages of normal development in the medaka Oryzias latipes. Mech Dev. 2004;121:605–618. doi: 10.1016/j.mod.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Menuet A, Alunni A, Joly J S, Jeffery W R, Retaux S. Expanded expression of sonic hedgehog in Astyanax cavefish: multiple consequences on forebrain development and evolution. Development. 2007;134:845–855. doi: 10.1242/dev.02780. [DOI] [PubMed] [Google Scholar]
- Kimmel C B, Ballard W W, Kimmel S R, Ullmann B, Schilling T F. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Halloran M C, Sato-Maeda M, Warren J T, Su F, Lele Z, Krone P H, Kuwada J Y, Shoji W. Laser-induced gene expression in specific cells of transgenic zebrafish. Development. 2000;127:1953–1960. doi: 10.1242/dev.127.9.1953. [DOI] [PubMed] [Google Scholar]
- Akimenko M A, Ekker M, Wegner J, Lin W, Westerfield M. Combinatorial expression of three zebrafish genes related to distal-less: part of a homeobox gene code for the head. J Neurosci. 1994;14:3475–3486. doi: 10.1523/JNEUROSCI.14-06-03475.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenti P, Thaeron C, Allizard F, Huysseune A, Sire J Y. Cellular expression of eve1 suggests its requirement for the differentiation of the ameloblasts and for the initiation and morphogenesis of the first tooth in the zebrafish (Danio rerio) Dev Dyn. 2004;230:727–733. doi: 10.1002/dvdy.20080. [DOI] [PubMed] [Google Scholar]
- Fisher S, Halpern M E. Patterning the zebrafish axial skeleton requires early chordin function. Nat Genet. 1999;23:442–446. doi: 10.1038/70557. [DOI] [PubMed] [Google Scholar]
- Borday-Birraux V, Van der Heyden C, Debiais-Thibaud M, Verreijdt L, Stock D W, Huysseune A, Sire J Y. Expression of Dlx genes during the development of the zebrafish pharyngeal dentition: evolutionary implications. Evol Dev. 2006;8:130–141. doi: 10.1111/j.1525-142X.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- Begemann G, Marx M, Mebus K, Meyer A, Bastmeyer M. Beyond the neckless phenotype: influence of reduced retinoic acid signaling on motor neuron development in the zebrafish hindbrain. Dev Biol. 2004;271:119–129. doi: 10.1016/j.ydbio.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Liang D, Zhang M, Bao J, Zhang L, Xu X, Gao X, Zhao Q. Expressions of Raldh3 and Raldh4 during zebrafish early development. Gene Expr Patterns. 2008;8:248–253. doi: 10.1016/j.gep.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Pittlik S, Domingues S, Meyer A, Begemann G. Expression of zebrafish aldh1a3 (raldh3) and absence of aldh1a1 in teleosts. Gene Expr Patterns. 2008;8:141–147. doi: 10.1016/j.gep.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Canestro C, Postlethwait J H, Gonzalez-Duarte R, Albalat R. Is retinoic acid genetic machinery a chordate innovation? Evol Dev. 2006;8:394–406. doi: 10.1111/j.1525-142X.2006.00113.x. [DOI] [PubMed] [Google Scholar]
- Gibert Y, Gajewski A, Meyer A, Begemann G. Induction and prepatterning of the zebrafish pectoral fin bud requires axial retinoic acid signaling. Development. 2006;133:2649–2659. doi: 10.1242/dev.02438. [DOI] [PubMed] [Google Scholar]
- Ahn D G, Kourakis M J, Rohde L A, Silver L M, Ho R K. T-box gene tbx5 is essential for formation of the pectoral limb bud. Nature. 2002;417:754–758. doi: 10.1038/nature00814. [DOI] [PubMed] [Google Scholar]
- Bertrand S, Thisse B, Tavares R, Sachs L, Chaumot A, Bardet P L, Escriva H, Duffraisse M, Marchand O, Safi R, Thisse C, Laudet V. Unexpected novel relational links uncovered by extensive developmental profiling of nuclear receptor expression. PLoS Genet. 2007;3:e188. doi: 10.1371/journal.pgen.0030188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale L A, Tallafuss A, Yan Y L, Dudley L, Eisen J S, Postlethwait J H. Characterization of the retinoic acid receptor genes raraa, rarab and rarg during zebrafish development. Gene Expr Patterns. 2006;6:546–555. doi: 10.1016/j.modgep.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Escriva H, Bertrand S, Germain P, Robinson-Rechavi M, Umbhauer M, Cartry J, Duffraisse M, Holland L, Gronemeyer H, Laudet V. Neofunctionalization in vertebrates: the example of retinoic acid receptors. PLoS Genet. 2006;2:e102. doi: 10.1371/journal.pgen.0020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lera A R, Bourguet W, Altucci L, Gronemeyer H. Design of selective nuclear receptor modulators: RAR and RXR as a case study. Nat Rev Drug Discov. 2007;6:811–820. doi: 10.1038/nrd2398. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- Linville A, Radtke K, Waxman J S, Yelon D, Schilling T F. Combinatorial roles for zebrafish retinoic acid receptors in the hindbrain, limbs and pharyngeal arches. Dev Biol. 2009;325:60–70. doi: 10.1016/j.ydbio.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furthauer M, Reifers F, Brand M, Thisse B, Thisse C. sprouty4 acts in vivo as a feedback-induced antagonist of FGF signaling in zebrafish. Development. 2001;128:2175–2186. doi: 10.1242/dev.128.12.2175. [DOI] [PubMed] [Google Scholar]
- Mandler M, Neubuser A. FGF signaling is necessary for the specification of the odontogenic mesenchyme. Dev Biol. 2001;240:548–559. doi: 10.1006/dbio.2001.0490. [DOI] [PubMed] [Google Scholar]
- Debiais-Thibaud M, Germon I, Laurenti P, Casane D, Borday-Birraux V. Low divergence in Dlx gene expression between dentitions of the medaka (Oryzias latipes) versus high level of expression shuffling in osteichtyans. Evol Dev. 2008;10:464–476. doi: 10.1111/j.1525-142X.2008.00257.x. [DOI] [PubMed] [Google Scholar]
- Escriva H, Holland N D, Gronemeyer H, Laudet V, Holland L Z. The retinoic acid signaling pathway regulates anterior/posterior patterning in the nerve cord and pharynx of amphioxus, a chordate lacking neural crest. Development. 2002;129:2905–2916. doi: 10.1242/dev.129.12.2905. [DOI] [PubMed] [Google Scholar]
- Matt N, Ghyselinck N B, Wendling O, Chambon P, Mark M. Retinoic acid-induced developmental defects are mediated by RARbeta/RXR heterodimers in the pharyngeal endoderm. Development. 2003;130:2083–2093. doi: 10.1242/dev.00428. [DOI] [PubMed] [Google Scholar]
- Peterkova R, Lesot H, Peterka M. Phylogenetic memory of developing mammalian dentition. J Exp Zoolog B Mol Dev Evol. 2006;306:234–250. doi: 10.1002/jez.b.21093. [DOI] [PubMed] [Google Scholar]
- Klein O D, Minowada G, Peterkova R, Kangas A, Yu B D, Lesot H, Peterka M, Jernvall J, Martin G R. Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Dev Cell. 2006;11:181–190. doi: 10.1016/j.devcel.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.