Abstract
Sirtuin1 (SIRT1) deacetylase levels are decreased in chronic inflammatory conditions and aging where oxidative stress occurs. We determined the mechanism of SIRT1 redox post-translational modifications leading to its degradation. Human lung epithelial cells exposed to hydrogen peroxide (150–250 μM), aldehyde-acrolein (10–30 μM), and cigarette smoke extract (CSE; 0.1–1.5%) in the presence of intracellular glutathione-modulating agents at 1–24 h, and oxidative post-translational modifications were assayed in cells, as well as in lungs of mice lacking and overexpressing glutaredoxin-1 (Glrx1), and wild-type (WT) mice in response to cigarette smoke (CS). CSE and aldehydes dose and time dependently decreased SIRT1 protein levels, with EC50 of 1% for CSE and 30 μM for acrolein at 6 h, and >80% inhibition at 24 h with CSE, which was regulated by modulation of intracellular thiol status of the cells. CS decreased the lung levels of SIRT1 in WT mice, which was enhanced by deficiency of Glrx1 and prevented by overexpression of Glrx1. Oxidants, aldehydes, and CS induced carbonyl modifications on SIRT1 on cysteine residues concomitant with decreased SIRT1 activity. Proteomics studies revealed alkylation of cysteine residue on SIRT1. Our data suggest that oxidants/aldehydes covalently modify SIRT1, decreasing enzymatic activity and marking the protein for proteasomal degradation, which has implications in inflammatory conditions.—Caito, S., Rajendrasozhan, S., Cook, S., Chung, S., Yao, H., Friedman, A. E., Brookes, P. S., Rahman, I. SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress.
Keywords: sirtuins, aldehydes, epithelium, cigarette smoke, inflammation
Sirtuins, class III histone deacetylases (HDACs), seem to have evolved to respond to a variety of stresses and are emerging as key antiaging molecules and regulators in many diseases, including type II diabetes, cardiovascular disease, Alzheimer’s disease, and chronic obstructive pulmonary disease (COPD), as well as aging (1,2,3,4,5,6,7). SIRT1, which is homologous to yeast (Saccharomyces cerevisiae) silent information regulator 2 (Sir2), is the most extensively studied sirtuin, has been shown to be the key molecule responsible for extending life span in response to caloric restriction (8) and is involved in silencing the chromatin of the mating locus (2, 9). SIRT1 can deacetylate histones, but it can also suppress inflammation through the deacetylation of the RelA/p65 subunit of NF-κB (10), suppress apoptosis through the deacetylation of p53, Ku70, and FOXO3a (3, 11, 12), and inhibit senescence through the deacetylation of FOXO3a, FOXO4, and p53 (2, 13), which are hallmarks of several chronic inflammatory diseases.
Oxidative stress has been shown to contribute to a variety of chronic inflammatory diseases (14, 15). During oxidative stress, reversible and irreversible modifications on proteins are formed as a result of direct oxidation of amino acid side chains or addition of reactive intermediates from the oxidation of other cellular components on cysteine, histidine, and lysine residues. Modifications, such as formation of disulfide bonds, S-nitrosylation, and S-glutathionylation can easily be reversed by thioredoxin and glutaredoxin (Glrx) enzymes (16). However, irreversible covalent modifications, such as carbonylation and tyrosine nitration, can lead to loss of protein function, protein aggregation folding, or degradation (16).
The biochemical regulation of SIRT1 seems to be complex (17). SIRT1 has been shown to reduce cellular oxidative stress burden through deacetylation of FOXO3a, leading to an up-regulation of catalase and manganese superoxide dismutase (SOD) (1) SIRT1 also regulates aging and oxidative stress in the heart (18), and oxidative stress leads to a redistribution of SIRT1 on chromatin (19). We have recently shown that the levels of SIRT1 are decreased in vitro in macrophages in response to cigarette smoke extract (CSE), as well as in lungs of patients with COPD (20, 21). However, the mechanism of this down-regulation by CSE and its reactive components (oxidants and aldehydes) in lung cells is not known. On the basis of the above observations, we hypothesized that cigarette smoke (CS)-mediated oxidative stress decreases SIRT1 protein level and activity by a redox-dependent mechanism; causing irreversible oxidative post-translational modifications on SIRT1 leading to inactivation and decreased protein level in lung epithelial cells in vitro and in mouse lungs in vivo. We tested this hypothesis by altering the redox status of human bronchial and airway epithelial cells and in lungs of mice exposed to CS. We further studied the posttranslational modifications of SIRT1 by carbonyls in lung epithelial cells, as well as in mice-deficient and overexpressing Glrx1, a key enzyme, which reverses protein S-glutathionylation and is involved in maintaining the redox status of proteins.
MATERIALS AND METHODS
Reagents
Unless otherwise stated, all biochemical reagents used in this study were purchased from Sigma Chemicals (St. Louis, MO, USA). Antibodies used to detect SIRT1 include mouse-specific SIRT1 (catalog no. 2028; Cell Signaling, Danvers, MA, USA), human SIRT1 (2310; Cell Signaling) for Western blot analysis, and human SIRT1 (7343; Abcam, Cambridge, MA, USA) for immunocytochemistry and immunoprecipitation.
Mouse exposure
Adult male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME, USA) and Glrx1-knockout (Glrx1-KO) and Glrx1-transgenic (Glrx1-Tg) mice overexpressing Glrx1 only in lung alveolar epithelial cells (obtained from Dr. Ye-Shih Ho, Wayne State University, Detroit, MI, USA; ref. 22) were housed in the Inhalation Core Facility at the University of Rochester for 1 wk of acclimatization before CS exposure. All animal procedures were approved by the Committee on Animal Research of the University of Rochester. Eight- to 10-wk-old mice (6–8 mice/group) were used for CS exposure for 3 d. Briefly, mice were housed in an individual wire cage compartment, which was placed inside an aerated plastic box, which was connected to the smoke source. Research-grade cigarettes (2R4F; University of Kentucky, Lexington, KY; 9.7 mg tar and 0.85 mg nicotine/cigarette) were used to generate smoke, and mice were exposed according to the U.S. Federal Trade Commission protocol (1 puff/min of 2 s duration and 35 ml volume) using an automatic cigarette-smoking machine (Baumgartner-Jaeger CSM2072i; CH Technologies, Westwood, NJ, USA). Mainstream CS was diluted with filtered air and smoke exposure [300 mg/m3 total particulate matter (TPM) of air in chamber corresponding to human consumption of 1–1.5 packs/d] was monitored in real time with an aerosol monitor (MicroDust Pro, Casella CEL, Bedford, UK), verified daily by gravimetric sampling (21, 23, 24). Chamber atmosphere was also monitored for TPM by adjustment of the flow rate of the diluted medical air (24). The exposure regimen consisted of two 1-h CS exposures 1 h apart for 3 consecutive days, with the mice sacrificed 24 h after the last exposure.
Protein extraction from lung tissue
Mice were injected with pentobarbiturate (100 mg/kg body weight, i.p.; Abbott Laboratories, Chevy Chase, MD, USA) and sacrificed by exsanguination. The lungs were removed en bloc, and the left lungs were lavaged 3 times with 0.5 ml of 0.9% NaCl, while the right lung lobe was frozen for immunoblot analysis. One hundred milligrams of right lung lobe was mechanically homogenized in 0.5 ml of ice-cold RIPA buffer supplemented with a protease inhibitor cocktail (leupeptin, aprotinin, pepstatin, and PMSF), and then placed on ice for 45 min to allow for total cell lysis to occur. The homogenate was centrifuged at 13,000 rpm in a bench-top centrifuge for 25 min at 4°C for removal of cellular debris. The supernatant was then transferred to a fresh 1.7-ml Eppendorf tube and used as whole lysate.
Cell culture
The human bronchial epithelial cell line BEAS-2B was grown in DMEM-Ham’s F12 50:50 mixture (DMEM-F12; Mediatech, Manassas, VA, USA) supplemented with 5% FBS, 15 mM HEPES, 100 μg/ml penicillin, and 100 U/ml streptomycin. Human small airway epithelial cells (SAECs) derived from a single healthy donor were purchased from Lonza (formerly Cambrex, Walkersville, MD, USA) along with growth medium [Small Airway Epithelial Cell Growth Medium (SAGM)] bullet kit supplemented with 52 μg bovine pituitary extract, 0.5 ng/ml human recombinant epidermal growth factor (EGF), 0.5 μg/ml epinephrine, 10 μg/ml transferrin, 5 μg/ml insulin, 0.1 ng/ml retinoic acid (RA), 6.5 ng/ml triiodothyronine, 50 μg/ml gentamicin/amphotericin-B (GA-1000), and 50 μg/ml fatty acid-free BSA. Cells were cultured at 37°C in a humidified atmosphere containing 7.5% CO2.
Preparation of aqueous CSE
Ten percent CSE was prepared by bubbling smoke from one 2R4F research-grade cigarette into 10 ml of culture medium at a rate of 1 cigarette/2 min, as described previously (21, 25,26,27), using a modification of the method described by Carp and Janoff (28). The CSE was adjusted to pH 7.4 and was sterile-filtered through a 0.45-μm filter (25-mm Acrodisc; Pall Corporation, Ann Arbor, MI, USA). The CSE preparation was standardized by monitoring the absorbance at 320 nm (OD=0.75±0.05). The spectral variations observed between different CSE preparations at λ320 were minimal. CSE was freshly prepared for each experiment and diluted with culture medium containing 1% FBS immediately before use. Control medium was prepared by bubbling air through 10 ml of culture medium supplemented with 1% FBS, adjusting pH to 7.4, and sterile filtering as described for 10% CSE.
Treatments
BEAS-2B cells were seeded at a density of 1 × 106 cells/well (final volume 2 ml with medium) and grown to ∼80–90% confluency in 6-well plates containing DMEM-F12 medium with 1% FBS. The cells were treated with CSE (0.1–1.5%) or H2O2 (150, 250, or 500 μM) for 6 or 24 h in the presence or absence of buthionine sulfoximine (BSO; 100 μM) pretreatment for 18 h or with N-acetyl-l-cysteine (NAC; 2 mM) for 2 h at 37°C with 7.5% CO2. Cells were also treated with polyethylene glycol (PEG)-conjugated SOD (200 U/ml), catalase (200 U/ml), or a mixture of both (200 U/ml each) for 30 min prior to treatment with CSE for 6 h. Cells were treated for 1 or 6 h with KO2 (1 μM), a superoxide anion generator, prepared in dry dimethyl sulfoxide (DMSO), as described previously (29). At the end of treatment, the cells were washed with cold sterile Ca2+/Mg2+-free PBS and lysed using RIPA buffer supplemented with a protease inhibitor cocktail (leupeptin, aprotinin, pepstatin, and PMSF).
Immunoblot analysis
Protein levels were measured using the bicinchoninic acid kit (Pierce, Rockford, IL, USA). Linear regression was used to determine the protein concentration of the samples. Twenty micrograms of BEAS-2B whole-cell extract, prepared as described above, was subjected to electrophoresis on 7.5% PAGE gels and transferred onto nitrocellulose membranes (Amersham, Arlington Heights, IL, USA). The nitrocellulose membrane was blocked with 5% BSA and subsequently incubated with rabbit polyclonal anti-human SIRT1 antibody (1:1000 dilution; Cell Signaling). After 3 washing steps (10 min each), the levels of protein were detected using goat anti-rabbit antibody (1:20,000 dilution) linked to horseradish peroxidase (Dako, Santa Barbara, CA, USA), and bound complexes were detected using enhanced chemiluminescence method (ECL; Jackson Immunology Research, West Grove, PA, USA).
Covalent modification of SIRT1 by carbonylation
SIRT1 was immunoprecipitated using whole-cell extracts; SIRT1 antibody (1:80 dilution; Abcam) was added to 100 μg of protein in a final volume of 400 μl and incubated for 1 h. Protein-A/G agarose beads (20 μl) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were added to each sample and left overnight at 4°C on a rotator (Barnstead Thermolyne, Dubuque, IA, USA). The samples were then centrifuged at 13,000 rpm at 4°C for 5 min. The supernatant was discarded, and the beads were washed 3 times and then resuspended in 50 μl lysis buffer. For immunoblots, 100 μg of the immunoprecipitated SIRT1 agarose bead suspension was added to 5× sample loading buffer, boiled, and resolved using SDS-PAGE as described above. Agarose beads alone were used as negative control. To determine the carbonylation of SIRT1, blots were probed first with anti-SIRT1 antibody (Abcam). After stripping, membranes were equilibrated with 20% (v/v) methanol, 80% Tris-buffered saline for 5 min, then incubated with 0.5 mM 2,4-dinitrophenylhydrazine (DNP) for 30 min at room temperature. The membranes were washed and then incubated overnight in anti-DNP antibody, as described previously (30).
Biotin-switch assay
Modifications on cysteine residues were measured by maleimide-PEO2-biotin labeling, as described previously (31). After immunoprecipitation of SIRT1, SIRT1 conjugated to the agarose beads were diluted in PBS and 1 μl of maleimide-PEO2-biotin (Pierce) was added for 2 h rocking at room temperature in the dark. Samples were then boiled and electrophoresed on a polyacrylamide gel. After transferring the gel, the nitrocellulose membrane was blocked with 5% BSA, streptavidin conjugated to horseradish peroxidase was added for 1 h, and visualized using ECL.
MALDI-TOF/TOF MS spectra of SIRT1 modified by reactive aldehydes
Recombinant human SIRT1 (30 μg) (Enzo Life Sciences, Plymouth Meeting, PA, USA; cat no. SE-239, 82 kDa, 747 aa, which was produced from human cDNA and expressed in E. coli and sequence identical to that at GenBank accession no. NM012238) was modified with 4-hydroxy-2-nonenal (4-HNE; 30 μM) for 18 h at 37°C, which was then digested with trypsin gold solution (Promega, Madison, WI, USA) in ammonium bicarbonate (50 mM) in 10% acetonitrile (pH 8) for 18 h at 37°C. The protein was not reduced during the digestion; no dithiothreitol was used. Peptides were extracted using 50% acetonitrile, 5% formic acid, and analyzed by MALDI TOF/TOF mass spectrometry (AutoflexIII TOF/TOF MALDI mass spectrometer; Bruker Daltonics, Billerica, MA, USA). Data were analyzed using Mascot 2.1.04 (Matrix Science, London, UK).
SIRT1 activity assay
SIRT1 activity was assayed using a deacetylase colorimetric activity assay kit (Enzo Life Sciences), according to the manufacturer’s instructions. Briefly, SIRT1 was immunoprecipitated as described above from whole-cell extracts treated with CSE (0.1%–1.5%) or H2O2 (150 μM) for 6 or 24 h. Cells were also treated with resveratrol (5 μM) or sirtinol (10 μM), a well-characterized activator and inhibitor of SIRT1, respectively. After the final washing, Color de Lys substrate reagent and NAD+ was added to the SIRT1-conjugated beads and incubated for 80 min at 37°C. The substrate-SIRT1 mixture was then placed on a 96-well plate, and the Color de Lys developer reagent was added to the wells for 20 min at 37°C. The plate was then read at 405 nm using a spectrophotometer (Model 680 microplate reader, Bio-Rad, Hercules, CA, USA).
Immunofluorescence
BEAS-2B cells were grown on 8-well chamber slides (1×104 cells/well), treated with H2O2 (150 μM) or CSE (0.5–1.5%) in the presence or absence of NAC (2 mM), and then fixed in 4% paraformaldehyde for 10 min. The cells were then permeabilized for 10 min in 0.3% Triton X-100 in PBS, and blocked for 1 h using 10% normal goat serum in PBS. Samples were incubated with antibodies specific for SIRT1 in a humidified chamber overnight. The primary antibody was detected with Alexa Fluor 594 goat anti-rabbit secondary antibody (Invitrogen, Carlsbad, CA, USA). Nuclei were stained with 1 μg/ml Hoechst 33342 for 1 min. Samples without primary antibodies were used as negative controls. The coverslips were mounted onto the slides using VectaShield (Vector Laboratories, Burlingame, CA, USA) and viewed under a Nikon TE2000-E microscope (Nikon, Tokyo, Japan).
Statistical analysis
Results are shown as means ± se. Statistical significance was calculated using 1-way ANOVA by StatView; values of P > 0.05 were considered nonsignificant.
RESULTS
SIRT1 protein levels and activity are dose and time dependently decreased by CS-mediated oxidative stress and aldehyde in both primary and transformed cells
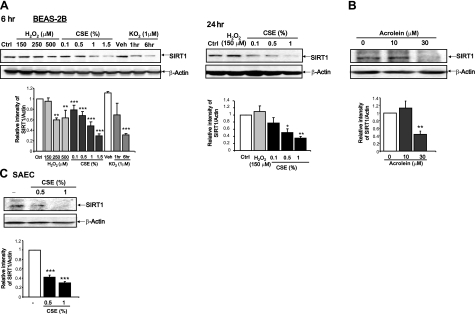
CS is shown to decrease SIRT1 protein levels and activity in lungs of rats and humans (20, 21, 32) and in macrophages in vitro (20, 21), but the mechanism of CS-induced oxidative modifications of SIRT1 is not known. We investigated the mechanism of reduction of SIRT1 by CS in both primary and transformed epithelial cell lines. Whole-cell extracts of BEAS-2B cells showed reduced SIRT1 levels in response to increasing concentrations of CSE from 6 to 24 h. Prior to 6 h, there was no reduction in SIRT1 levels (data not shown). As a control for oxidative stress, KO2, which generates superoxide anion, and H2O2 were used. H2O2 (150 μM) did not significantly decrease SIRT1 levels at either 6 or 24 h (Fig. 1A). Treatment with higher concentrations of H2O2 (250 and 500 μM) for 6 h resulted in significant loss of SIRT1; however, there was an increasing level of cytotoxicity associated with these treatments (data not shown). Cells treated with KO2 (1 μM) showed a time-dependent loss of SIRT1 levels (Fig. 1A). As cigarettes contain between 1.92 mg and 3.14 mg of reactive carbonyl compounds, SIRT1 levels were examined in response to the reactive aldehyde acrolein, a component of CS (394 μM of acrolein and up to 500 μg acrolein/cigarette, which is ∼39.4 μM in 1% of CSE) (33,34,35,36). Whole-cell extracts of BEAS-2B cells treated with acrolein (10 or 30 μM) showed a dose-dependent loss of SIRT1 protein, with EC50 of 30 μM at 6 h, with > 80% inhibition at 24 h (Fig. 1B), suggesting that aldehyde/carbonyl stress may lead to decreased SIRT1 levels. To compare the findings of the transformed cell lines, human primary epithelial cells were examined for SIRT1 levels in response to CS. Treatment of human SAECs with CSE (0.5% and 1%) for 4 h resulted in a dose-dependent decrease in SIRT1 levels in whole-cell extracts (Fig. 1C). CSE (0.1% to 1.5%) was equivalent to ∼5 to 50 μM of aldehydes present in CS (34,35,36).
Figure 1.
Aldehyde and CS lower SIRT1 levels in human lung epithelial cells. A) BEAS-2B cells were treated with varying concentrations of CSE (0.1–1.5%) or H2O2 (150, 250, or 500 μM) for 6 or 24 h. Cells were also treated for 1 or 6 h with either KO2 (1 μM) or dry DMSO vehicle (Veh). Ctrl, untreated control. B) BEAS-2B cells were treated with acrolein (10 or 30 μM) for 6 h. C) SAECs were treated with CSE (0.5 and 1%) for 4 h. Whole-cell extracts from treated cells were used for immunoblot analysis for SIRT1. β-Actin was used as a housekeeping loading control. Blots are representative of ≥3 separate experiments (n=3). *P < 0.05, **P < 0.01, ***P < 0.001 vs. controls.
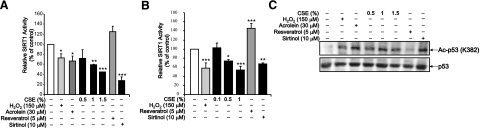
SIRT1 activity was assayed to determine whether CSE reduced SIRT1 deacetylase activity in lung epithelial cells. SIRT1 activity was reduced in a dose-dependent manner at 6 and 24 h by CSE (Fig. 2A, B). H2O2 (150 μM) caused a reduction in SIRT1 activity without a reduction in protein level at both 6 and 24 h (Fig. 2A, B). Acrolein with EC50 of 30 μM also decreased SIRT1 activity significantly at 6 h treatment (Fig. 2A, B). As a second measure of SIRT1 activity, levels of acetylated p53, a target of SIRT1, was measured in BEAS-2B cells treated with CSE, H2O2, acrolein, resveratrol, or sirtinol for 6 h. CSE, H2O2, acrolein, and sirtinol increased acetylation on lysine 382 residue on p53, whereas untreated cells and resveratrol treated cells had low levels of acetylated p53 (Fig. 2C). These data confirm that SIRT1 activity is inhibited by carbonyl and oxidative stress.
Figure 2.
Oxidants, aldehyde, and CSE dose and time dependently decrease SIRT1 deacetylase activity. A, B) BEAS-2B cells were treated with varying concentrations of CSE (0.1, 0.5, 1, 1.5%), H2O2 (150 μM), and acrolein (30 μM) for 6 h (A) or 24 h (B). Cells were also treated with sirtinol (10 μM) and resveratrol (5 μM), a known inhibitor and activator of SIRT1, respectively. SIRT1 was immunoprecipitated from whole-cell extracts and used for the SIRT1 deacetylase activity assay. C) Cell extracts were also used for immunoblot for acetylated p53 (Lys 382) and p53 as a second measure of SIRT1 activity. Blots are representative of ≥3 separate experiments (n=3). *P < 0.05, **P < 0.01, ***P < 0.001 vs. controls.
SIRT1 is degraded by the proteasome in response to CSE
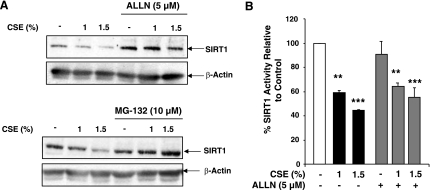
The proteasome is the major route of protein degradation in the cell; therefore, it was examined whether CS decreased SIRT1 levels through proteasomal degradation. BEAS-2B cells were pretreated with the proteasome inhibitors N-Acetyl-Leu-Leu-Nle-CHO (ALLN; 5 μM) and MG-132 (10 μM) for 30 min, washed with PBS, and treated for 6 h with CSE (1 and 1.5%). Treatment with ALLN increased SIRT1 levels over untreated control cells, perhaps because of inhibiting basal protein turnover (Fig. 3A). Cells treated with both ALLN and CSE had significantly higher levels of SIRT1 above both the untreated control cells and cells treated with CSE. Similar findings were observed in cells treated with MG-132 and CSE (Fig. 3A). It was next investigated whether the increased protein levels due to proteasome inhibitor treatment resulted in increased SIRT1 enzymatic activity. BEAS-2B cells treated with ALLN alone had no significant difference in SIRT1 activity as compared to untreated cells (Fig. 3B); however, pretreatment with ALLN did not prevent CSE-induced loss of SIRT1 activity. Furthermore, the activity of SIRT1 in the ALLN and CSE-treated cells was not statistically different from the cells treated with CSE alone. Since inhibition of the proteasome led to increased SIRT1 protein levels, but not altered enzymatic activity, these data suggest that SIRT1 is inactive prior to degradation in response to CSE treatment. Phosphorylation is an important post-translational modification that plays a key role in marking proteins for degradation. BEAS-2B cells treated with increasing doses of CSE (0.5–1.5%) for 1 h showed levels of phosphorylation on serine 47 residue above those of untreated control and H2O2 (150 μM)-treated cells (data not shown). No phosphorylation was detected on serine 27 residue basally or in response to CSE (data not shown).
Figure 3.
SIRT1 is degraded by the proteasome in response to CSE. A) BEAS-2B cells were treated with the proteasome inhibitor N-Acetyl-Leu-Leu-Nle-CHO (ALLN; 5 μM) or MG-132 (10 μM) for 30-min pretreatment prior to a 6-h CSE (1 and 1.5%) treatment. Whole-cell extracts were analyzed by Western blot (representative of 3 separate experiments). β-Actin was used as a loading control. B) SIRT1 was immunoprecipitated from whole-cell extracts from A and used for the SIRT1 deacetylase activity assay. **P < 0.01, ***P < 0.001 vs. control.
Reduction of SIRT1 is dependent on intracellular thiol status
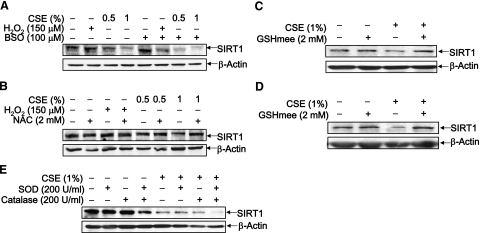
CSE contains and generates a variety of oxidants/free radicals and electrophilic compounds in aqueous solution (33, 37). Hence, the effect of key oxidants and electrophilic aldehydes on altering SIRT1 function was determined, particularly the effect of altering the thiol status of lung epithelial cells on SIRT1 protein level and activity in response to CSE. First, the BEAS-2B cells’ glutathione (GSH) levels were depleted by the addition of BSO (100 μM) 18 h prior to a 24-h treatment with CSE (0.5 and 1%) or H2O2 (150 μM). Pretreatment with BSO resulted in further loss of SIRT1 in response to CSE treatment; SIRT1 levels were significantly lower in cells treated with BSO and CSE (0.5%) than in cells treated with CSE (0.5%) alone (P<0.01) (Fig. 4A). It was interesting to note that BSO-treated cells did not have significantly lower SIRT1 levels than untreated control cells. These data suggest that an oxidative environment may prime cells for SIRT1 loss, however, does not lead to SIRT1 loss in the absence of an environmental insult.
Figure 4.
Modulation of cellular thiol level regulates SIRT1 protein level. A) BEAS-2B cells were treated with BSO (100 μM) to deplete GSH levels. BSO was pretreated 18 h before a 24-h treatment with CSE (0.5 or 1%) or H2O2 (150 μM). B) BEAS-2B cells were treated with NAC (2 mM) for 2 h prior to a 24-h treatment with CSE (0.5 or 1%) or H2O2 (150 μM). C, D) BEAS-2B cells (C) and SAECs (D) were treated with glutathione monoethyl ester (GSHmee; 2 mM) for 2 h, washed off with sterile PBS, and treated with CSE (1%) for 6 h. E) PEG-conjugated SOD (200 U/ml) and catalase (200 U/ml) were given to BEAS-2B cells alone or in combination for 30 min prior to being washed off with PBS, followed by a 6-h treatment with CSE (1%). Whole-cell extracts were analyzed by immunoblot. Gels are representative of 3 separate experiments (n=3). β-Actin was used as a loading control.
It was next determined whether increasing the GSH levels with NAC (2 mM), and thus increasing the lung cells’ thiol levels (38), could prevent CSE-mediated SIRT1 loss. BEAS-2B cells pretreated with NAC for 2 h before a 24-h treatment with CSE (0.5 and 1%) had significantly higher levels of SIRT1 than cells that were treated with CSE alone (P<0.001) (Fig. 4B). Levels of SIRT1 in cells treated with both NAC and CSE were also significantly higher than control levels, suggesting that intracellular thiols may be able to prevent CSE-dependent loss of SIRT1. BEAS-2B cells and SAEC were also treated with glutathione monoethyl ester (GSHmee; 2 mM), a cell-permeable form of GSH, for 2 h prior to CSE (1%) treatment for 6 h to determine whether directly increasing GSH levels could restore SIRT1 protein. Pretreatment with GSHmee also prevented CSE-induced SIRT1 loss at this shorter time point (Fig. 4C, D). Altogether, these data suggest that intracellular thiol levels play an important role in down-regulation of SIRT1 protein level in response to CS.
To determine whether other ROS scavengers could also prevent CSE-mediated SIRT1 loss, BEAS-2B cells pretreated with either PEG-conjugated SOD (200 U/ml), catalase (200 U/ml), or a combination of both (200 U/ml of each) for 30 min prior to a 6-h treatment with 1% CSE. Surprisingly, the combination of these antioxidant enzymes with CSE did not restore SIRT1 levels as NAC had done (Fig. 4E). These data further suggest that the thiol status of the cell is important for maintaining SIRT1 protein levels in response to CS.
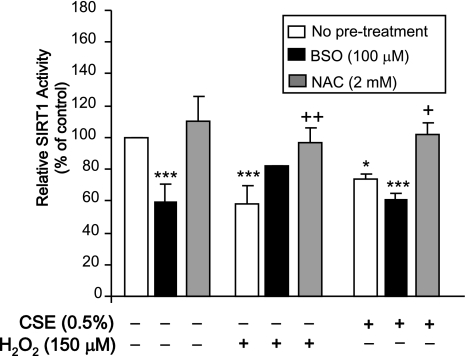
It was next determined whether these changes on SIRT1 protein level had any effect on its enzymatic activity. BEAS-2B cells treated with CSE (0.5%) for 24 h in the presence or absence of an 18-h pretreatment with BSO or a 2-h pretreatment with NAC were assayed for SIRT1 activity. BSO treatment alone lowered SIRT1 activity (P<0.001), whereas NAC had no effect on SIRT1 activity (Fig. 5). H2O2 reduced SIRT1 activity (P<0.001), which was prevented by NAC pretreatment (P<0.01). BSO pretreatment had no significant effect on SIRT1 activity in combination with H2O2. Similarly, CSE significantly lowered SIRT1 activity (P<0.05), which was prevented by pretreatment with NAC (P<0.05). Interestingly, BSO in combination with CSE did not further decrease SIRT1 activity compared to CSE alone. These data suggest that the thiol status of the cell is important not only for maintaining SIRT1 protein level in response to smoke, but also its activity.
Figure 5.
SIRT1 activity is modulated by intracellular thiol status. BEAS-2B cells were treated with CSE (0.5%) and H2O2 (150 μM) for 24 h following an 18 h pretreatment with BSO (100 μM) or a 2 h pretreatment with NAC (2 mM). SIRT1 was immunoprecipitated from whole-cell extracts and used for the SIRT1 deacetylase assay. *P < 0.05, ***P < 0.001 vs. control; +P < 0.05, ++P < 0.01 vs. respective H2O2 or CSE treatments (n=3).
Oxidative stress causes nucleocytoplasmic shuttling of SIRT1 by a redox-dependent mechanism
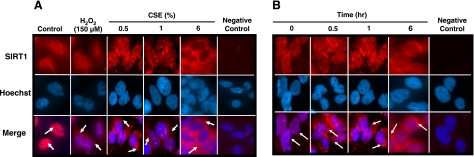
It is known that in response to antimycin A, a mitochondrial complex III inhibitor, SIRT1, is shuttled out of the nucleus and has decreased deacetylase function (39). It was then investigated whether CSE-mediated oxidants/free radicals could cause nucleocytoplasmic shuttling of SIRT1 in BEAS-2B cells. The BEAS-2B cells were treated with increasing concentrations of CSE (0.5–1.5%) or H2O2 (150 μM) for 1 h. Cells were then stained for SIRT1 by immunofluorescence. Nuclei were stained with Hoechst 33342 dye. CSE caused SIRT1 to be dose-dependently shuttled out of the nucleus (Fig. 6A). H2O2 treatment also leads to shuttling of SIRT1 out of the nucleus. It was next examined whether CSE time-dependently shuttled SIRT1 out of the nucleus. BEAS-2B cells were treated with CSE (0.5%) for 30 min, 1 h and 6 h, and stained for SIRT1. SIRT1 was present exclusively in the nucleus prior to CSE exposure. SIRT1 was shuttled out of the nucleus starting at 30 min and continued to 6 h (Fig. 6B). At 6 h, there was also a decrease in the intensity of staining for SIRT1, which may be due to loss of SIRT1 protein seen at this time.
Figure 6.
SIRT1 is dose- and time-dependently shuttled out of the nucleus by oxidants. A) BEAS-2B cells were treated with CSE (0.5–1.5%) or H2O2 (150 μM) for 1 h. B) BEAS-2B cells were treated with CSE (0.5%) for 30 min, 1 h, and 6 h. Cells were fixed with 4% paraformaldehyde and used for immunostaining. SIRT1 was visualized by using goat anti-rabbit secondary antibody conjugated to Alexa Fluor 594 dye. Nuclei were stained with Hoechst 33342 dye. Untreated cells that received Hoechst 33342 and secondary antibody but no SIRT1 primary antibody was used as a negative control. Arrows denote SIRT1. Images represent 3 separate experiments (n=3).
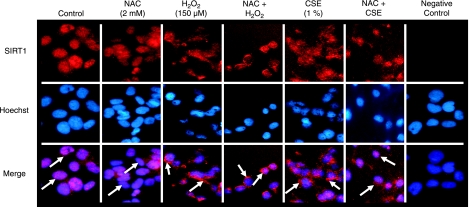
To determine whether CSE-induced shuttling of SIRT1 is redox regulated, BEAS-2B cells were treated with NAC (2 mM) for 2 h followed by CSE (1%) or H2O2 (150 μM) for 1 h before immunostaining. Both untreated cells and cells treated with NAC alone showed nuclear localization of SIRT1, whereas CSE and H2O2 induced nucleocytoplasmic shuttling of SIRT1 (Fig. 7). Pretreatment of NAC did not entirely prevent nucleocytoplasmic shuttling of SIRT1 in response to both CSE and H2O2; however, it did significantly decrease the amount of SIRT1 localized to the cytoplasm. These data suggest that oxidative stress-induced shuttling of SIRT1 is redox dependent in lung epithelial cells.
Figure 7.
Nucleocytoplasmic shuttling of SIRT1 by oxidative stress is reversed by NAC. BEAS-2B cells were treated with NAC (2 mM) for 2 h prior to a 1-h treatment with either CSE (1%) or H2O2 (150 μM). Cells were fixed with 4% paraformaldehyde and used for immunostaining. SIRT1 was visualized by using goat anti-rabbit secondary antibody conjugated to Alexa Fluor 594 dye. Nuclei were stained with Hoechst 33342 dye. Untreated cells that received Hoechst 33342 and secondary antibody but no SIRT1 primary antibody was used as a negative control. Arrows denote SIRT1. Images represent 3 separate experiments (n=3).
Oxidant/aldehyde-induced post-translational modification of SIRT1 is altered by redox status
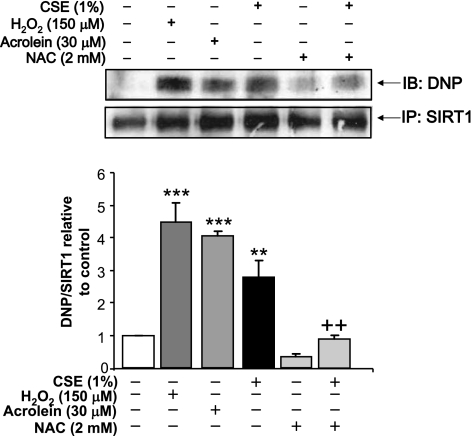
To investigate the possible reason for reduction in SIRT1 protein levels with CSE treatments and a restoration of protein levels by pretreatments of NAC or GSHmee prior to CSE, oxidative post-translational modifications on SIRT1 were investigated. CSE can induce lipid peroxidation and lead to carbonylation of histidine, lysine, and cysteine residues of proteins by a Michael addition reaction, which is irreversible (covalent modification) (14, 33). Previously, we have shown that 4-HNE adducts form on SIRT1 in lungs from smokers and COPD patients and MonoMac6 cells treated with CSE for 4 h (20). Here, we investigated whether these oxidative/electrophilic modifications occur at an early time point (1 h) before SIRT1 is down-regulated by CSE. SIRT1 was immunoprecipitated from BEAS-2B cells treated with either CSE (1%), H2O2 (150 μM), or acrolein (30 μM) for 1 h. Total carbonyl modification on SIRT1 was measured by derivitizing the immunoprecipitated SIRT1 with 2,4-dinitrophenyl hydrazine (DNPH). There were relatively low levels of carbonylation on SIRT1 in untreated cells; however, H2O2, acrolein and CSE lead to increased carbonylation (Fig. 8). Pretreatment with NAC led to a significant reduction in levels of carbonylation adducts in CSE-treated cells (P<0.01). This suggests that carbonylation of SIRT1 in response to CSE occurs early (1 h) and is followed by the reduction in SIRT1 protein levels or enzymatic activity (6 to 24 h).
Figure 8.
Oxidative stress and CSE induce carbonylation on SIRT1. SIRT1 was immunoprecipitated (IP) from whole-cell extracts of BEAS-2B cells treated for 1 h with acrolein (30 μM), H2O2 (150 μM), and CSE (1%) in the presence or absence of a 2-h pretreatment with NAC (2 mM). Equal amount (100 μg) of immunoprecipitated SIRT1 protein was used for immunoblotting (IB). Carbonylation was detected by first derivitizing the samples with DNPH and immunoblotting with an anti-DNP antibody. Blot is representative of ≥3 separate experiments **P < 0.01, ***P < 0.001 vs. control; ++P < 0.01 vs. CSE.
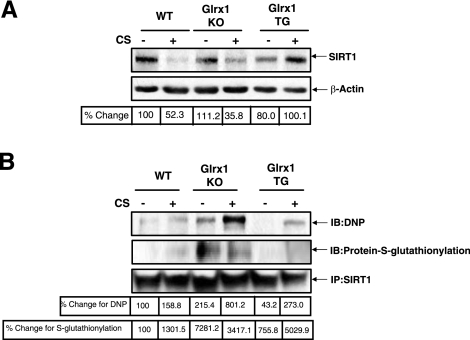
It was also investigated whether there was increased total carbonylation in vivo in lungs of mice exposed to CS. SIRT1 was significantly decreased in mouse whole lung extracts from mice exposed to CS (P<0.05) (Fig. 9A). Mice exposed to CS had increased carbonylation on SIRT1 as compared to air-exposed mice, suggesting carbonylation is an important post-translational modification to SIRT1 that occurs in vivo (Fig. 9B). To further investigate the role of carbonyl modifications on SIRT1 levels, mice lacking Glrx1 were exposed to CS. These mice are unable to repair/reverse S-glutathionylation, a reversible modification on proteins and thus are susceptible to oxidative stress. The level of SIRT1 was dramatically reduced in response to CS in the lungs of Glrx1-KO mice as compared to air-exposed Glrx1-KO mice (Fig. 9A). In contrast, mice overexpressing Glrx1 (Glrx1-Tg) showed no reduction in SIRT1 levels in response to CS compared to WT mice (Fig. 9A). SIRT1 was then immunoprecipitated from WT, Glrx1-KO, and Glrx1-Tg mice, and protein carbonylation and glutathionylation levels were measured. CS increased S-glutathionylation on SIRT1, which was augmented in the Glrx1-KO mice exposed to CS (Fig. 9B). There was also increased S-glutathionylation on SIRT1 in lungs of air-exposed Glrx1-KO mice. S-glutathionylation was not seen in lungs of Glrx1-Tg mice exposed to either air or CS. Carbonylation on SIRT1 was increased in both air- and CS-exposed Glrx1-KO mice compared to their counterpart WT mice. Glrx1-Tg mice exposed to air had no carbonylation present on SIRT1 in the lung, particularly lung epithelial cells, as these mice express Glrx1 only in airway epithelial cells, while CS-exposed transgenic mice had carbonylation on SIRT1 similar to the WT CS-exposed mice (Fig. 9B). Taken together, these data suggest that covalent modifications on SIRT1 affect the SIRT1 protein level in response to CS.
Figure 9.
Reversible covalent modification on SIRT1 by CS does not affect total SIRT1 levels. A) Whole-lung extracts were blotted for SIRT1 levels from WT C57BL/6J, Glrx1-KO, and Glrx1-Tg mice exposed to air or CS (300 mg/m3 TPM) for 3 d and sacrificed 24 h after last exposure, as described in Materials and Methods. B) Whole lung cell extract (100 μg) was immunoprecipitated and S-glutathionylated, and total carbonylated SIRT1 levels were detected by immunoblot with anti-GSH and anti-DNP antibodies. Blots are representative of 3 separate experiments (n=3).
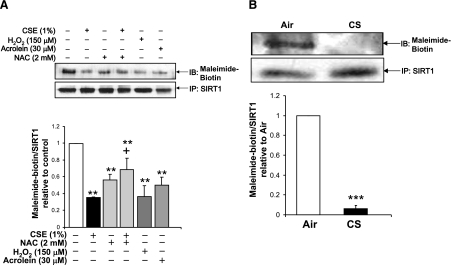
It was next determined whether the CSE-induced carbonylation was on cysteine residues on SIRT1 in BEAS-2B cells and mouse lung and whether there was an effect on altering the intracellular thiol levels on the cysteine modifications. SIRT1 was immunoprecipitated from whole-cell extracts of BEAS-2B cells exposed to CSE for 6 h in the presence or absence of a 2-h pretreatment with NAC. Free cysteine residues were labeled with maleimide-PEO2-biotin. Treatment with CSE to BEAS-2B cells led to decreased maleimide-PEO2-biotin labeling of cysteine residues on SIRT1 compared to control (Fig. 10A). Treatment with either NAC alone did not affect the maleimide-PEO2-biotin labeling; however, the combination of NAC and CSE resulted in labeling that was similar to control cells, suggesting that NAC pretreatment prevented CSE from modifying free cysteine residues on SIRT1. Immunoprecipitation of SIRT1 from WT mouse lung extracts also revealed that there were decreased free cysteine residues on SIRT1 with CS exposure, as compared to air-exposed mice (Fig. 10B).
Figure 10.
Altering cell thiol status affects post-translational modifications on cysteine residues of SIRT1 in response to aldehydes and oxidants. SIRT1 was immunoprecipitated (IP) from whole-cell extracts of BEAS-2B cells treated for 6 h with acrolein (30 μM), H2O2 (150 μM), and CSE (1%) in the presence or absence of a 2-h pretreatment with NAC (2 mM) (A) or wild type C57BL/6J mice exposed to air or CS (B). Free cysteine residues were labeled using maleimide-PEO2-biotin and visualized using immunoblot with streptavidin conjugated to horseradish peroxidase. Blot is representative of three separate experiments (n=3). **P < 0.01, ***P < 0.001 vs. controls; +P < 0.05 vs. CSE (1%).
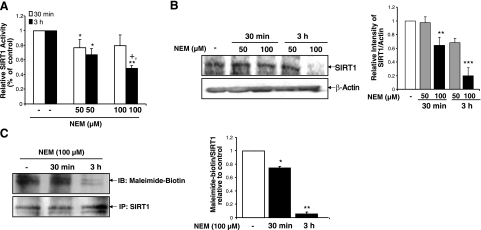
It was next determined whether inducing alkylation of free cysteines on SIRT1 would lead to decreased activity or protein levels. BEAS-2B cells were treated with the alkylating agent N-ethylmaleimide (NEM; 50 or 100 μM) for 30 min or 3 h, and then examined for SIRT1 levels and activity. Alkylation of SIRT1 by NEM led to decreased enzymatic activity at both time points and concentrations (Fig. 11A). Interestingly, 50 μM NEM only slightly decreased SIRT1 levels only at 3 h, whereas 100 μM NEM treatment decreased SIRT1 levels at both 30 min and 3 h (Fig. 11B). To determine whether there was increased labeling of SIRT1 with the longer incubations with NEM, SIRT1 was immunoprecipitated from BEAS-2B cells and labeled with maleimide-PEO2-biotin (Fig. 11C). As the treatment time of NEM increased, more cysteines were alkylated, giving a faint signal at 3 h compared to untreated cells. These data suggest that alkylation of SIRT1 on cysteine residues can directly decrease SIRT1 activity and protein levels.
Figure 11.
Cysteine alkylation decreases SIRT1 protein levels and enzymatic activity. A) BEAS-2B cells were treated with NEM (50 or 100 μM) for 30 min or 3 h, and SIRT1 was immunoprecipitated from whole-cell extracts for measurement of deacetylase activity. B) Extracts were also used for immunoblot for SIRT1 protein levels. β-Actin was used as a loading control. C) Nonalkylated/free cysteine residues on immunoprecipitated SIRT1 were labeled using maleimide-PEO2-biotin and visualized using immunoblot with streptavidin conjugated to horseradish peroxidase. Blots are representative of 3 separate experiments (n=3). *P < 0.05, **P < 0.01, ***P < 0.001 vs. controls; +P < 0.05 vs. NEM (50 μM) at 3 h.
MALDI-TOF/TOF MS spectra of SIRT1 modified by reactive aldehydes
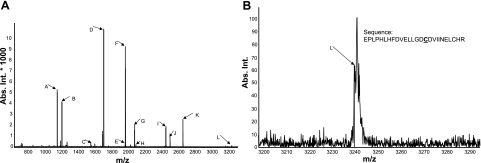
To further confirm carbonylation/alkylation on SIRT1 in response to carbonyl stress, recombinant human SIRT1 was modified with 4-HNE and analyzed by MALDI TOF/TOF mass spectrometry. MS/MS spectra were collected by the lift method in positive mode to fragment parent peaks, and fragment sequences were compared to human SIRT1 using MASCOT version 2.1.04 (40, 41). As the peptides were extracted under acidic conditions, the only possible modification sites observed would be on cysteine residues since modifications on arginines, lysines, and histidines are acid labile. We found carbonylation/alkylation of cysteine residues between aa 467 and 492 on SIRT1 with 4-HNE (Table 1 and Fig. 12), suggesting that environmental reactive aldehydes are able to directly modify SIRT1 by post-translational modification.
TABLE 1.
Peptides identified by MALDI TOF/TOF-MS from SIRT1 modified By 4-HNE
| Mass (Da) | Sequence position | Sequence | Peak |
|---|---|---|---|
| 1144.817 | 385–394 | GDIFNQVVPR | A |
| 1200.815 | 66–77 | GCPGAAAAALWR 2:Carbamidom ethyl (C) | B |
| 1547.042 | 637–649 | LDGNQYLFLPPNR | C |
| 1703.201 | 636–649 | RLDGNQYLFLPPNR | D |
| 1961.387 | 256–274 | IIVLTGAGVSVSCGIPDFR 13:Carbamidom ethyl (C) | E |
| 1963.323 | 342–358 | NYTQNIDTLEQVAGIQR | F |
| 2073.405 | 182–199 | IGPYTFVQQHLMIGTDPR | G |
| 2089.480 | 255–274 | KIIVLTGAGVSVSCGGIPDFR 14:Carbamidom ethyl (C) | H |
| 2448.817 | 445–466 | VRPVALIPSSIPHEVPQILINR | I |
| 2499.581 | 283–303 | LAVDFPDLPDPQAMFDIEYFR | J |
| 2653.705 | 520–543 | ELAYLSELPPTPLHVSEDSSSPER | K |
| 3239.535 | 467–492 | EPLPHLHFDVELLGDCDVIINELCHR 4-HNE (C), Carbamidom ethyl (C), on either residue 16 or 24 | L |
Figure 12.
MALDI-TOF/TOF MS spectra of SIRT1 modified by reactive aldehydes. A) Recombinant human SIRT1 (30 μg) was modified with 4-HNE (30 μM) for 18 h at 37°C and analyzed by MALDI TOF/TOF mass spectrometry (AutoflexIII TOF/TOF MALDI mass spectrometer; Bruker Daltonics). Data were analyzed using Mascot 2.1.04. B) Spectra of the sequence (residues 467–492) containing a cysteine residue at 482 modified by 4-HNE; modified cysteine is denoted by bold and underscored font.
DISCUSSION
Epithelial cells are important cells in initiating and perpetuating the inflammatory response to CS by the recruitment of inflammatory cells to the lung through the release of cytokines and chemokines. We and others have recently shown that the levels of SIRT1 are decreased in lungs of smokers and patients with COPD and in macrophages, leading to increased proinflammatory cytokine release (20, 21, 32). Herein, the redox mechanism, by which oxidants and aldehydes decrease SIRT1 protein levels, was examined in human BEAS-2B cells, human SAECs, and mice exposed to CS-induced oxidative stress. It was first determined that CSE dose and time dependently decreased SIRT1 protein levels in lung epithelial cells. Both treatments of superoxide anion-generating molecule, KO2, and the reactive aldehyde, acrolein, showed reduction in SIRT1 levels, suggesting that loss of SIRT1 may be due to direct effects of ROS and carbonyl components of CS. Recently, it has been shown that products of ethanol metabolism reduce both SIRT1 levels and activity in RKC1 macrophage cell line (42). One metabolite tested was the reactive aldehyde acetaldehyde, which is also present in CS (34, 35). Our data show that there was no loss of SIRT1 with noncytotoxic doses of H2O2. Both H2O2 and KO2 induce oxidative stress by producing ROS; however, they had different effects on SIRT1 levels. This may be explained by the exposure conditions; treatment with H2O2 is a bolus, whereas KO2 has a more sustained generation of superoxide anion (43), perhaps allowing for SIRT1 decrease. Interestingly, both CS and H2O2 decreased SIRT1 protein levels in endothelial cells, fibroblasts, and neuronal cells, which may be explained by different responses to oxidative stress by different cell types (44,45,46,47). Accompanying the reduction in SIRT1 protein, there was a dose- and time-dependent reduction of SIRT1 activity in response to CSE, acrolein, and H2O2 highlighting the role of oxidants and carbonyls in reduction of SIRT1 in BEAS-2B cells.
SIRT1 is involved in redox-dependent cellular processes, such as the redox-dependent development of neuronal cells, apoptosis, or senescence (39, 46,47,48,49,50). Induction of SIRT1 has been shown to reverse oxidant-mediated repression of complement H factor expression, a risk factor for age-related macular degeneration (51). To determine the contribution of intracellular thiol status on CSE-induced loss of SIRT1, GSH levels were modulated in BEAS-2B cells using BSO; an inhibitor of GSH biosynthesis, GSHmee; and NAC—the precursors of intracellular GSH. The oxidized environment caused by BSO pretreatment enhanced CSE-induced loss of SIRT1 protein level and activity, whereas the reduced environment created by GSHmee or NAC pretreatment prevented the loss of SIRT1 protein and activity in response to CS. It was found that BSO only lowered SIRT1 levels in response to CSE in BEAS-2B cells, but not alone. This discrepancy may be due to a difference in cell lines and cell types. Surprisingly, treatment of BEAS-2B cells with KO2, a superoxide anion generator, resulted in decreased SIRT1 levels, but pretreatment of cells with SOD and catalase could not prevent CSE-induced loss of SIRT1 protein. This finding suggests that the thiol pool is important in the regulation of SIRT1 protein level in response to CS and that CSE-induced loss of SIRT1 may be due to generation of reactive intermediates, like lipid peroxidation byproducts, rather than direct ROS damage. An alternate explanation may be that modulating intracellular GSH levels has a more profound effect on the cells’ redox state than the two antioxidant enzymes due to the fact that GSH is so abundant in cells (52). It is also important to consider that oxidative stress decreases the GSH levels of cells, altering the GSH:GSSG ratio, and that addition of thiols can restore this imbalance (38).
SIRT1 has been shown to change its cellular localization in response to various stimuli, such as developmental stimuli (39, 50, 53). Here, we observed that CSE induced a time- and dose-dependent shuttling of SIRT1 from the nucleus in BEAS-2B cells. In corroboration with previous reports, we have also found that H2O2 treatment leads to cytoplasmic localization of SIRT1 (50). Since CSE induces loss of SIRT1, our data suggest that nucleocytoplasmic shuttling of SIRT1 may facilitate degradation in the proteasome in the cytoplasm, although H2O2 also induces shuttling of SIRT1 but does not lead to SIRT1 protein loss. Recent studies have established that cytoplasmic localized SIRT1 occurs during apoptosis; however, no cell death was observed under the CSE and H2O2 (150 μM) treatment conditions performed in this study. Whether cytoplasmic localization of SIRT1 contributes to senescence, both of which can be induced by CS and H2O2 (47, 54, 55), remains to be determined. It was also observed that shuttling of SIRT1 by oxidative stress could be reduced by pretreatment of cells with NAC, suggesting that nucleocytoplasmic shuttling is mediated by a redox-sensitive mechanism. Phosphorylation by a redox-sensitive kinase may explain this observation. Oxidative stress has been shown to activate redox-sensitive kinases, such as I-κB kinase (IKK), phosphoinositide 3-kinase (PI-3K), and extracellular signal-related kinase 1/2 (ERK1/2) (56, 57). PI-3K has been shown to be involved in the nuclear localization of SIRT1, as the use of PI-3K inhibitors leads to cytoplasmic localization of SIRT1 (39), while phosphorylation by CK2 increases SIRT1 activity (58, 59). Phosphorylation is a key determiner of cellular localization for several proteins, such as FOXO3 transcription factor (60, 61), and since SIRT1 has several phosphorylation sites of unknown function (58, 62), it is speculated that phosphorylation may contribute to CS-induced nucleocytoplasmic shuttling of SIRT1.
Oxidants present in CS are known to cause peroxidation of sensitive biomolecules, such as DNA, lipids, and proteins. Carbonylation of proteins results from reactive α-β-unsaturated aldehydes reacting with cysteine, histidine, and lysine residues by Michael addition. It was found that CS, H2O2, and acrolein all increased carbonylation on SIRT1 both in vitro in BEAS-2B cells and in vivo in mouse lung. Furthermore, these modifications occurred before the loss of SIRT1 protein level and activity in BEAS-2B cells. These carbonyl adducts were decreased in cells pretreated with NAC prior to CSE exposure, which is in agreement with previous reports that NAC can prevent lipid peroxidation (63). Although carbonylation occurs on specific amino acid residues, several proteins may be affected by oxidative stress/reactive carbonyls. Whether there are protein targets of CS-induced carbonylation that are activated or inhibited and subsequently affect SIRT1 remains to be determined.
Oxidative stress can induce sulfenic acid formation on cysteine residues of proteins, which can oxidize to a more stable acid or react with thiols to produce disulfides or GSH to form adducts, termed glutathionylation (64). Glutathionylation can be reversed by the enzyme Glrx1. It was investigated whether this reversible oxidative modification occurred on SIRT1 in response to CS. Genetic ablation of Glrx1 leads to increased SIRT1 loss, while overexpressing Glrx1 was protective. Glrx1 is a cytosolic protein (64), which implies that its action on SIRT1, a predominantly nuclear protein, may occur post-translationally before SIRT1 is shuttled to the nucleus or once SIRT1 is shuttled out of the nucleus in response to oxidative stress. Further investigation is needed to explore this relationship. Levels of glutathionylation and carbonylation on SIRT1 were increased in lungs of air- and CS-exposed Glrx1-KO mice, while transgenic mice showed the same amount of carbonylation as wild-type mice, but no effect on glutathionylation, suggesting that carbonylation and glutathionylation on SIRT1 occur independently. Currently, it is not known whether glutathionylation or carbonylation occurs first and if these two modifications have similar effects on SIRT1.
Since cysteine, lysine, and histidine residues are potential targets for oxidative adduct formation, it was investigated whether cysteines on SIRT1 were the targets of CSE-induced modifications. Free cysteines were evaluated using a biotin switch assay; free cysteines were strongly labeled in untreated cells using maleimide-PEO2-biotin; however, acrolein, H2O2, and CSE greatly decreased this labeling, suggesting that it had modified these residues. NAC given before CSE could prevent the modification of cysteines. The modification of cysteine residues was also seen in vivo in whole-cell extracts from mouse lungs exposed to CS. Cysteines do not play a role in the enzymatic functioning of SIRT1, however, there are key cysteine residues located in the sirtuin fold domain, which are responsible for the coordination of a structural Zn atom (17, 65). This domain along with a Rossmann fold domain, are believed to form a cleft for NAD+ and substrate binding (17). It is possible that CS modifies cysteine residues, causing a loss of SIRT1’s function by altering this binding cleft.
Oxidative post-translational modifications to proteins are deleterious: often resulting in inactivation, loss of function, and/or degradation (15, 66). It was evident that carbonyl modifications were increased on SIRT1 in response to acrolein, H2O2, and CSE, and that only acrolein and CSE decreased SIRT1 protein levels, the question remained what was the functional consequence of the carbonylation on cysteine residues. To determine whether cysteine alkylation resulted in loss of SIRT1 protein level and/or activity, BEAS-2B cells were treated with NEM. NEM caused a dose-dependent loss of SIRT1 activity, most likely because of the alkylation of the critical cysteines in the NAD+ binding pocket. There was also a dose- and time-dependent loss of SIRT1 protein in response to NEM, suggesting that loss of SIRT1 can occur by modifying cysteine residues. MALDI TOF/TOF mass spectrometry further confirmed that cysteine residues are able to be alkylated/carbonylated by reactive aldehydes on a SIRT1, finding a cysteine residue between aa 467 and 492 that was carbonylated. The possibility of carbonylation of other electrophilic target amino acids, such as histidine and lysine, cannot be ruled out as the MALDI TOF/TOF analysis was performed under acid-labile conditions, where any modifications on lysine and histidine would be removed. In addition, the endogenous alkylation or carbonylation target of SIRT1 in response to oxidative/carbonyl stress in vivo may vary since in our study, we used recombinant SIRT1 for proteomic studies.
Oxidatively modified proteins may be degraded by the proteasome by ubiquitin-independent or -dependent mechanisms (14). Indeed, proteasomal degradation of SIRT1 in response to CS was observed in this study. Furthermore, SIRT1, which was accumulated when the proteasome was inhibited had lower activity than untreated cells, suggesting SIRT1 is inactivated by CS before degradation. Oxidative and carbonyl stresses and thiol modulation may cause a multitude of biomolecular/bioenergetic effects contributing to loss of SIRT1 in response to CSE, and whether any specific pathway is involved has yet to be determined. There are many signals for protein degradation (67). It is hypothesized that oxidative modifications, such as carbonylation, lead to an unstable hydrophobic conformation and directly lead to degradation by the 20S proteasome (68). This may be the case with NEM treatments, as levels of phosphorylated or ubiquitinated SIRT1 were not investigated. Recently, ubiquitin-independent proteasome degradation of SIRT1 has been observed in response to ionizing radiation in articular chondrocytes (48). However, in CS-induced loss of SIRT1, the possible role of phosphorylation-ubiquitination pathway cannot be ignored as oxidatively modified proteins like alcohol dehydrogenase are degraded by the ubiquitin-dependent 26S proteasome (69). Ubiquitin-dependent proteasomal degradation has been observed in the diaphragm of patients with COPD (70), and our laboratory has shown increased ubiquitin-dependent degradation of HDAC2 in response to CSE (71), suggesting a possible role for ubiquitin-dependent proteasomal degradation of SIRT1 in response to CS. Recent research has identified several sites of phosphorylation on SIRT1 (58, 62, 72, 73) and shown that phosphorylation of serine 27 residue of SIRT1 is important in protein stability in cancer (74). We have observed phosphorylation on serine 47 residue; however, whether this residue is involved in proteasomal degradation of SIRT1 by CS remains to be determined. Phosphorylation is a reversible modification, but oxidative/covalent carbonyl modifications are irreversible; therefore, it will be important to determine the order of events that occur prior to SIRT1 degradation in response to CS. We propose that electrophilic compounds present in CS/CSE would covalently modify SIRT1 along with phosphorylation, leading to its degradation, as seen in the present study and reported earlier in lungs of smokers and patients with COPD (20, 31).
Overall, our data show that SIRT1 is oxidatively down-regulated by CS/aldehydes, leading to post-translational modification, inactivation, and protein loss via the proteasome. Further inducing oxidative stress with the combination of GSH depletion and CSE resulted in greater loss of SIRT1 protein. Thiol-replenishing agents could prevent CSE-induced modification of SIRT1, leading to increased SIRT1 protein levels when compared to CSE-treated cells; however, the antioxidant enzymes SOD and catalase could not prevent SIRT1 protein loss. CS is a complex mixture of free radicals, electrophilic compounds, and oxidants (33), and the oxidative/carbonyl stress it induced had different effects on SIRT1 than that of H2O2; which decreased SIRT1 activity but had no effect on protein level in BEAS-2B cells. This suggests that there are multiple pathways and events occurring during CS exposure that affect SIRT1 functioning. SIRT1 was shown to be carbonylated and glutathionylated in vivo in mice exposed to CS and that these modifications could be attenuated by increasing intracellular thiols by NAC in vitro and in vivo in mice overexpressing Glrx1, an enzyme that repairs glutathionylated proteins. Our findings suggest that oxidant/carbonyl stress-mediated reduction of SIRT1 will lead to loss of its control of acetylation of its target proteins p53, RelA/p65, and FOXO1/3, and hence this will enhance inflammatory, senescence and apoptosis responses (hallmarks of various chronic inflammatory diseases). There has been recent development of SIRT1 activators for the potential use in the treatment of Type 2 diabetes and other chronic inflammatory diseases (75,76,77). However, it remains to be determined whether these agents are beneficial in chronic inflammatory conditions or in treating diseases, like COPD, where there is a decrease in SIRT1 protein level. Our findings advance the emerging field of research on SIRT1 regulation by suggesting that a simple activation of SIRT1 by pharmacological agents may not be effective since SIRT1 is covalently modified in oxidative/inflammatory conditions. We propose that reversal of oxidative post-translationally modified SIRT1 may be an avenue before effective therapeutic strategies can be designed for chronic inflammatory diseases. Nevertheless, our data not only demonstrate the basic understanding of oxidant/carbonyl-mediated reduction of SIRT1 and redox regulation/modifications of SIRT1 but also have widespread implications in understanding the pathogenesis of various chronic inflammatory diseases where oxidative/carbonyl stress occur, leading to inflammation-aging (inflammaging).
Acknowledgments
This work was supported by U.S. National Institutes of Health grants 1R01-HL092842, R01-HL085613, and 1R01HL097751-01; National Institute of Environmental Health Sciences Center grant ES01247; and Toxicology Training Program grant T32-ES07026. The authors thank Dr. Se-Ran Yang for her technical assistance. The authors also thank Dr. Ye-Shih Ho (Institute of Environmental Health Sciences and Department of Biochemistry and Molecular Biology, Wayne State University, Detroit, MI, USA) for providing Glrx1-KO and Glrx1-Tg mice. None of the authors have any ethical conflicts of interest for publication of this manuscript.
References
- Elliott P J, Jirousek M. Sirtuins: novel targets for metabolic disease. Curr Opin Investig Drugs. 2008;9:371–378. [PubMed] [Google Scholar]
- Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal C H, Dipp M A, Guarente L. A therapeutic role for sirtuins in diseases of aging? Trends Biochem Sci. 2007;32:555–560. doi: 10.1016/j.tibs.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhou Y, Mueller-Steiner S, Chen L F, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-κB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- Vogelmeier C, Bals R. Chronic obstructive pulmonary disease and premature aging. Am J Respir Crit Care Med. 2007;175:1217–1218. doi: 10.1164/rccm.200703-513ED. [DOI] [PubMed] [Google Scholar]
- Tuder R M. Aging and cigarette smoke: fueling the fire. Am J Respir Crit Care Med. 2006;174:490–491. doi: 10.1164/rccm.200607-924ED. [DOI] [PubMed] [Google Scholar]
- Karrasch S, Holz O, Jorres R A. Aging and induced senescence as factors in the pathogenesis of lung emphysema. Respir Med. 2008;102:1215–1230. doi: 10.1016/j.rmed.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Cohen H Y, Miller C, Bitterman K J, Wall N R, Hekking B, Kessler B, Howitz K T, Gorospe M, de Cabo R, Sinclair D A. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Zhang T, Kraus W L. SIRT1-dependent regulation of chromatin and transcription: Linking NAD(+) metabolism and signaling to the control of cellular functions. [E-pub ahead of print] Biochim Biophys Acta. 2009 doi: 10.1016/j.bbapap.2009.10.022. doi: 10.1016/j.bbapap.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung F, Hoberg J E, Ramsey C S, Keller M D, Jones D R, Frye R A, Mayo M W. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Sweeney L B, Sturgill J F, Chua K F, Greer P L, Lin Y, Tran H, Ross S E, Mostoslavsky R, Cohen H Y, Hu L S, Cheng H L, Jedrychowski M P, Gygi S P, Sinclair D A, Alt F W, Greenberg M E. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Luo J, Nikolaev A Y, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- Salminen A, Ojala J, Huuskonen J, Kauppinen A, Suuronen T, Kaarniranta K. Interaction of aging-associated signaling cascades: inhibition of NF-kappaB signaling by longevity factors FoxOs and SIRT1. Cell Mol Life Sci. 2008;65:1049–1058. doi: 10.1007/s00018-008-7461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsrud P A, Xie H, Griffin T J, Bernlohr D A. Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem. 2008;283:21837–21841. doi: 10.1074/jbc.R700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystrom T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005;24:1311–1317. doi: 10.1038/sj.emboj.7600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi P, Bonetto V. Redox proteomics: identification of oxidatively modified proteins. Proteomics. 2003;3:1145–1153. doi: 10.1002/pmic.200300435. [DOI] [PubMed] [Google Scholar]
- Sauve A A, Wolberger C, Schramm V L, Boeke J D. The biochemistry of sirtuins. Annu Rev Biochem. 2006;75:435–465. doi: 10.1146/annurev.biochem.74.082803.133500. [DOI] [PubMed] [Google Scholar]
- Alcendor R R, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner S F, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park S K, Hartlerode A, Stegmuller J, Hafner A, Loerch P, Wright S M, Mills K D, Bonni A, Yankner B A, Scully R, Prolla T A, Alt F W, Sinclair D A. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 2008;135:907–918. doi: 10.1016/j.cell.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendrasozhan S, Yang S R, Kinnula V L, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:861–870. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S R, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-κB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol. 2007;292:L567–L576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- Ho Y S, Xiong Y, Ho D S, Gao J, Chua B H, Pai H, Mieyal J J. Targeted disruption of the glutaredoxin 1 gene does not sensitize adult mice to tissue injury induced by ischemia/reperfusion and hyperoxia. Free Radic Biol Med. 2007;43:1299–1312. doi: 10.1016/j.freeradbiomed.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher T H, Maggirwar S B, Baglole C J, Lakatos H F, Gasiewicz T A, Phipps R P, Sime P J. Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-κB component RelB. Am J Pathol. 2007;170:855–864. doi: 10.2353/ajpath.2007.060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Edirisinghe I, Rajendrasozhan S, Yang S R, Caito S, Adenuga D, Rahman I. Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1174–L1186. doi: 10.1152/ajplung.00439.2007. [DOI] [PubMed] [Google Scholar]
- Caito S, Yang S R, Kode A, Edirisinghe I, Rajendrasozhan S, Phipps R P, Rahman I. Rosiglitazone and 15-deoxy-Delta12,14-prostaglandin J2, PPARγ agonists, differentially regulate cigarette smoke-mediated pro-inflammatory cytokine release in monocytes/macrophages. Antioxid Redox Signal. 2008;10:253–260. doi: 10.1089/ars.2007.1889. [DOI] [PubMed] [Google Scholar]
- Kode A, Yang S R, Rahman I. Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells. Respir Res. 2006;7:132–151. doi: 10.1186/1465-9921-7-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edirisinghe I, Yang S R, Yao H, Rajendrasozhan S, Caito S, Adenuga D, Wong C, Rahman A, Phipps R P, Jin Z G, Rahman I. VEGFR-2 inhibition augments cigarette smoke-induced oxidative stress and inflammatory responses leading to endothelial dysfunction. FASEB J. 2008;22:2297–2310. doi: 10.1096/fj.07-099481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H, Janoff A. Possible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis. 1978;118:617–621. doi: 10.1164/arrd.1978.118.3.617. [DOI] [PubMed] [Google Scholar]
- Reiter C D, Teng R J, Beckman J S. Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. J Biol Chem. 2000;275:32460–32466. doi: 10.1074/jbc.M910433199. [DOI] [PubMed] [Google Scholar]
- Murtaza I, Wang H X, Feng X, Alenina N, Bader M, Prabhakar B S, Li P F. Down-regulation of catalase and oxidative modification of protein kinase CK2 lead to the failure of apoptosis repressor with caspase recruitment domain to inhibit cardiomyocyte hypertrophy. J Biol Chem. 2008;283:5996–6004. doi: 10.1074/jbc.M706466200. [DOI] [PubMed] [Google Scholar]
- Ying J, Clavreul N, Sethuraman M, Adachi T, Cohen R A. Thiol oxidation in signaling and response to stress: detection and quantification of physiological and pathophysiological thiol modifications. Free Radic Biol Med. 2007;43:1099–1108. doi: 10.1016/j.freeradbiomed.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamaru Y, Vuppusetty C, Wada H, Milne J C, Ito M, Rossios C, Elliot M, Hogg J, Kharitonov S, Goto H, Bemis J E, Elliott P, Barnes P J, Ito K. A protein deacetylase SIRT1 is a negative regulator of metalloproteinase-9. FASEB J. 2009;23:2810–2819. doi: 10.1096/fj.08-125468. [DOI] [PubMed] [Google Scholar]
- Church D F, Pryor W A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserich J P, van der Vliet A, Handelman G J, Halliwell B, Cross C E. Dietary antioxidants and cigarette smoke-induced biomolecular damage: a complex interaction. Am J Clin Nutr. 1995;62:1490S–1500S. doi: 10.1093/ajcn/62.6.1490S. [DOI] [PubMed] [Google Scholar]
- Fujioka K, Shibamoto T. Determination of toxic carbonyl compounds in cigarette smoke. Environ Toxicol. 2006;21:47–54. doi: 10.1002/tox.20153. [DOI] [PubMed] [Google Scholar]
- Smith C J, Hansch C. The relative toxicity of compounds in mainstream cigarette smoke condensate. Food Chem Toxicol. 2000;38:637–646. doi: 10.1016/s0278-6915(00)00051-x. [DOI] [PubMed] [Google Scholar]
- Kodama M, Kaneko M, Aida M, Inoue F, Nakayama T, Akimoto H. Free radical chemistry of cigarette smoke and its implication in human cancer. Anticancer Res. 1997;17:433–437. [PubMed] [Google Scholar]
- Mulier B, Rahman I, Watchorn T, Donaldson K, MacNee W, Jeffery P K. Hydrogen peroxide-induced epithelial injury: the protective role of intracellular nonprotein thiols (NPSH) Eur Respir J. 1998;11:384–391. doi: 10.1183/09031936.98.11020384. [DOI] [PubMed] [Google Scholar]
- Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282:6823–6832. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- Sayre L M, Lin D, Yuan Q, Zhu X, Tang X. Protein adducts generated from products of lipid oxidation: focus on HNE and one. Drug Metab Rev. 2006;38:651–675. doi: 10.1080/03602530600959508. [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Tomas H, Havlis J, Olsen J V, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2006;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- Shen Z, Ajmo J M, Rogers C Q, Liang X, Le L, Murr M M, Peng Y, You M. Role of SIRT1 in regulation of LPS- or two ethanol metabolites-induced TNFα production in cultured macrophage cell lines. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1047–G1053. doi: 10.1152/ajpgi.00016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. Spectrophotometric study of spontaneous disproportionation of superoxide anion radical and sensitive direct assay for superoxide dismutase. J Biol Chem. 1976;251:7504–7507. [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Podlutsky A, Kaminski P M, Wolin M S, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota H, Eto M, Kano M R, Ogawa S, Iijima K, Akishita M, Ouchi Y. Cilostazol inhibits oxidative stress-induced premature senescence via upregulation of Sirt1 in human endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:1634–1639. doi: 10.1161/ATVBAHA.108.164368. [DOI] [PubMed] [Google Scholar]
- Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schroter F, Ninnemann O, Siegert E, Bendix I, Brustle O, Nitsch R, Zipp F, Aktas O. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol. 2008;10:385–394. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- Furukawa A, Tada-Oikawa S, Kawanishi S, Oikawa S. H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion. Cell Physiol Biochem. 2007;20:45–54. doi: 10.1159/000104152. [DOI] [PubMed] [Google Scholar]
- Hong E H, Lee S J, Kim J S, Lee K H, Um H D, Kim J H, Kim S J, Kim J I, Hwang S G. Ionizing radiation induces cellular senescence of articular chondrocytes via negative regulation of SIRT1 by p38 kinase. J Biol Chem. 2010;285:1283–1295. doi: 10.1074/jbc.M109.058628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratelli M, Goodwin L O, Orom U A, Lombardi S, Tonelli R, Mengozzi M, Ghezzi P. Gene expression profiling reveals a signaling role of glutathione in redox regulation. Proc Natl Acad Sci U S A. 2005;102:13998–14003. doi: 10.1073/pnas.0504398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Yan T, Ge X, Sun C, Shi X, Zhai Q. Cytoplasm-localized SIRT1 enhances apoptosis. J Cell Physiol. 2007;213:88–97. doi: 10.1002/jcp.21091. [DOI] [PubMed] [Google Scholar]
- Wu Z, Lauer T W, Sick A, Hackett S F, Campochiaro P A. Oxidative stress modulates complement factor H expression in retinal pigmented epithelial cells by acetylation of FOXO3. J Biol Chem. 2007;282:22414–22425. doi: 10.1074/jbc.M702321200. [DOI] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Lung glutathione and oxidative stress: implications in cigarette smoke-induced airway disease. Am J Physiol Lung Physiol. 1999;277:L1067–L1088. doi: 10.1152/ajplung.1999.277.6.L1067. [DOI] [PubMed] [Google Scholar]
- Hisahara S, Chiba S, Matsumoto H, Tanno M, Yagi H, Shimohama S, Sato M, Horio Y. Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc Natl Acad Sci U S A. 2008;105:15599–15604. doi: 10.1073/pnas.0800612105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med. 2006;174:886–893. doi: 10.1164/rccm.200509-1374OC. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Aoshiba K, Nagai A. Cigarette smoke induces senescence in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2004;31:643–649. doi: 10.1165/rcmb.2003-0290OC. [DOI] [PubMed] [Google Scholar]
- Mercer B A, Kolesnikova N, Sonett J, D'Armiento J. Extracellular regulated kinase/mitogen activated protein kinase is up-regulated in pulmonary emphysema and mediates matrix metalloproteinase-1 induction by cigarette smoke. J Biol Chem. 2004;279:17690–17696. doi: 10.1074/jbc.M313842200. [DOI] [PubMed] [Google Scholar]
- Pantano C, Reynaert N L, van der Vliet A, Janssen-Heininger Y M. Redox-sensitive kinases of the nuclear factor-κB signaling pathway. Antioxid Redox Signal. 2006;8:1791–1806. doi: 10.1089/ars.2006.8.1791. [DOI] [PubMed] [Google Scholar]
- Zschoernig B, Mahlknecht U. Carboxy-terminal phosphorylation of SIRT1 by protein kinase CK2. Biochem Biophys Res Commun. 2009;381:372–377. doi: 10.1016/j.bbrc.2009.02.085. [DOI] [PubMed] [Google Scholar]
- Kang H, Jung J W, Kim M K, Chung J H. CK2 is the regulator of SIRT1 substrate-binding affinity, deacetylase activity and cellular response to DNA-damage. PLoS One. 2009;4:e6611. doi: 10.1371/journal.pone.0006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Heide L P, Hoekman M F, Smidt M P. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380:297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon I K, Jans D A. Regulation of nuclear transport: central role in development and transformation? Traffic. 2005;6:173–186. doi: 10.1111/j.1600-0854.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Maier B, Koclega K D, Chruszcz M, Gluba W, Stukenberg P T, Minor W, Scrable H. Phosphorylation regulates SIRT1 function. PLoS ONE. 2008;3:e4020. doi: 10.1371/journal.pone.0004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitekus M J, Li N, Zhang M, Wang M, Horwitz M A, Nelson S K, Horwitz L D, Brechun N, Diaz-Sanchez D, Nel A E. Thiol antioxidants inhibit the adjuvant effects of aerosolized diesel exhaust particles in a murine model for ovalbumin sensitization. J Immunol. 2002;168:2560–2567. doi: 10.4049/jimmunol.168.5.2560. [DOI] [PubMed] [Google Scholar]
- Gallogly M M, Mieyal J J. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol. 2007;7:381–391. doi: 10.1016/j.coph.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Sanders B D, Jackson B, Marmorstein R. Structural basis for sirtuin function: What we know and what we don’t. [E-pub ahead of print] Biochim Biophys Acta. 2010 doi: 10.1016/j.bbapap.2009.09.009. doi: 10.1016/ j.bbapap.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen D R, Doorn J A. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Ravid T, Hochstrasser M. Diversity of degradation signals in the ubiquitin-proteasome system. Nat Rev Mol Cell Biol. 2008;9:679–690. doi: 10.1038/nrm2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grune T, Merker K, Sandig G, Davies K J. Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem Biophys Res Commun. 2003;305:709–718. doi: 10.1016/s0006-291x(03)00809-x. [DOI] [PubMed] [Google Scholar]
- Carbone D L, Doorn J A, Petersen D R. 4-Hydroxynonenal regulates 26S proteasomal degradation of alcohol dehydrogenase. Free Radic Biol Med. 2004;37:1430–1439. doi: 10.1016/j.freeradbiomed.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Ottenheijm C A, Heunks L M, Li Y P, Jin B, Minnaard R, van Hees H W, Dekhuijzen P N. Activation of the ubiquitin-proteasome pathway in the diaphragm in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174:997–1002. doi: 10.1164/rccm.200605-721OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adenuga D, Yao H, March T H, Seagrave J, Rahman I. Histone deacetylase 2 is phosphorylated, ubiquitinated, and degraded by cigarette smoke. Am J Respir Cell Mol Biol. 2009;40:464–473. doi: 10.1165/rcmb.2008-0255OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beausoleil S A, Jedrychowski M, Schwartz D, Elias J E, Villen J, Li J, Cohn M A, Cantley L C, Gygi S P. Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc Natl Acad Sci U S A. 2004;101:12130–12135. doi: 10.1073/pnas.0404720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J V, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Ford J, Ahmed S, Allison S, Jiang M, Milner J. JNK2-dependent regulation of SIRT1 protein stability. Cell Cycle. 2008;7:3091–3097. doi: 10.4161/cc.7.19.6799. [DOI] [PubMed] [Google Scholar]
- Milne J C, Lambert P D, Schenk S, Carney D P, Smith J J, Gagne D J, Jin L, Boss O, Perni R B, Vu C B, Bemis J E, Xie R, Disch J S, Ng P Y, Nunes J J, Lynch A V, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair D A, Olefsky J M, Jirousek M R, Elliott P J, Westphal C H. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur J A. Biochemical effects of SIRT1 activators. [E-pub ahead of print] Biochim Biophys Acta. 2009 doi: 10.1016/j.bbapap.2009.10.025. doi: 10.1016/j.bbapap.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavu S, Boss O, Elliott P J, Lambert P D. Sirtuins–novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7:841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]