Abstract
Identifying factors that accelerate the aging process can provide important therapeutic targets for slowing down this process. Misregulation of phosphate homeostasis has been noted in various skeletal, cardiac, and renal diseases, but the exact role of phosphate toxicity in mammalian aging is not clearly defined. Phosphate is widely distributed in the body and is involved in cell signaling, energy metabolism, nucleic acid synthesis, and the maintenance of acid-base balance by urinary buffering. In this study, we used an in vivo genetic approach to determine the role of phosphate toxicity in mammalian aging. Klotho-knockout mice (klotho−/−) have a short life span and show numerous physical, biochemical, and morphological features consistent with premature aging, including kyphosis, uncoordinated movement, hypogonadism, infertility, severe skeletal muscle wasting, emphysema, and osteopenia, as well as generalized atrophy of the skin, intestine, thymus, and spleen. Molecular and biochemical analyses suggest that increased renal activity of sodium-phosphate cotransporters (NaPi2a) leads to severe hyperphosphatemia in klotho−/− mice. Genetically reducing serum phosphate levels in klotho−/− mice by generating a NaPi2a and klotho double-knockout (NaPi2a−/−/klotho−/−) strain resulted in amelioration of premature aging-like features. The NaPi2a−/−/klotho−/− double-knockout mice regained reproductive ability, recovered their body weight, reduced their organ atrophy, and suppressed ectopic calcifications, with the resulting effect being prolonged survival. More important, when hyperphosphatemia was induced in NaPi2a−/−/klotho−/− mice by feeding with a high-phosphate diet, premature aging-like features reappeared, clearly suggesting that phosphate toxicity is the main cause of premature aging in klotho−/− mice. The results of our dietary and genetic manipulation studies provide in vivo evidence for phosphate toxicity accelerating the aging process and suggest a novel role for phosphate in mammalian aging.—Ohnishi, M., Razzaque, M. S. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging.
Keywords: klotho, NaPi2a, survival, fertility, emphysema, calcification
Aging is a complex biological process controlled and influenced by numerous genetic, humoral, and environmental factors (1,2,3). For instance, DNA damage through oxidative stress, among others, is thought to be an important contributing factor in aging and has been extensively studied in mammalian systems (4,5,6,7,8,9,10,11). Recent in vivo studies, however, have identified additional factors that can significantly affect the overall deterioration of physiological functions for various organ systems and thus accelerate mammalian aging. Phosphorus is a widely distributed mineral ion in the body that is an essential component of cell signaling, energy metabolism, and nucleic acid synthesis (12). In this study, we examined the effects of phosphate toxicity in mammalian aging. We used hyperphosphatemic klotho-knockout mice as an in vivo model to determine the effects of phosphate toxicity on mammalian aging.
Systemic regulation of phosphate homeostasis is a complex process, usually maintained by a delicate coordination between the intestine and the kidney (13,14,15). After intestinal absorption, circulating phosphorus is taken up by cells that need it, accumulates in the bone matrix protein, and enters the kidney. About 95% of filtered phosphate is reabsorbed in the proximal tubules of the kidneys. Phosphate from the proximal tubular lumen moves across tubular epithelial cells and effluxes at the basolateral membrane to enter blood vessels. The identification of specific phosphate transporters in the intestine and kidney has enhanced our understanding of phosphate metabolism. Sodium-phosphate (NaPi) cotransporters that are located in the intestine and the kidney play an important role in maintaining physiological phosphate balance (16). NaPi2b in the intestine aids in phosphate absorption, while the NaPi2a and NaPi2c transporters, in a sodium-dependent manner, assist in reabsorption of filtered phosphate in the kidney according to the needs of the body. The klotho-knockout mice develop severe hyperphosphatemia by 3 wk of age and remain hyperphosphatemic throughout their life span due to increased renal activity of the NaPi system (17,18,19).
Genetic inactivation of klotho in mice results in a phenotype resembling human aging, including kyphosis, muscle wasting, infertility, atherosclerosis, ectopic calcifications, generalized tissue atrophy, and an extremely shortened life span (20, 21). To determine whether phosphate toxicity can accelerate the aging-like features in klotho-knockout mice, we genetically reduced serum phosphate levels in klotho-knockout mice by generating NaPi2a; klotho double-knockout (NaPi2a−/−/klotho−/−; DKO) mice. Our results suggest that phosphate toxicity accelerates the mammalian aging process and that reducing the phosphate burden can delay the aging.
MATERIALS AND METHODS
Generation of NaPi2a−/−/klotho−/− mice
We interbred heterozygous klotho mutants (Lexicon Genetics; Mutant Mouse Regional Resource Centers, University of California, Davis, CA, USA) with heterozygous NaPi2a mutants to obtain compound heterozygous animals, which were then interbred to generate the desired double-homozygous mutants (NaPi2a−/−/klotho−/−; refs. 22, 23). Routine PCR was used to genotype the mice as detailed earlier (24). All studies performed were approved by the Harvard Medical School Institutional Animal Care and Use Committee (Boston, MA, USA).
Animal feeding
Wild-type, klotho−/−, and klotho−/−/NaPi2a−/− double-mutant mice were housed in a barrier facility and maintained under specific pathogen-free conditions during the entire study period. After weaning, at 3 wk of age, mice were divided into 2 groups: wild-type, klotho−/−, and klotho−/−/NaPi2a−/− mice continued to receive a normal phosphate diet (NPD: 0.6%) ad libitum, while an experimental group of klotho−/−/NaPi2a−/− mice received a high-phosphate diet (HPD: 1.2%) from 3 wk onwards for the rest of their lives. Mice were provided with food purchased from LabDiet/TestDiet (St. Louis, MO, USA). The energy in the NPD (kcal/g) was provided by protein at 23.1%, with fat and carbohydrate contributing 21.6 and 55.1%, respectively. The energy distribution in the HPD (kcal/g) was very similar, with protein providing 23.2%, fat supplying 22.1%, and carbohydrate contributing 54.7%.
Gross phenotype and body weight
The total body weight of wild-type, klotho−/−, and NaPi2a−/−/klotho−/− mice fed with either NPD or HPD was measured every week, starting at 3 wk of age until 20 wk of age. The maximum survival of klotho−/− mice and NaPi2a−/−/klotho−/− mice fed with HPD was ∼15 wk.
Measurement of serum phosphate and calcium
Blood was obtained by cheek pouch bleeding of wild-type, klotho−/−, and NaPi2a−/−/klotho−/− mice fed with either NPD or HPD. Serum was isolated by centrifugation at 3000 g for 10 min and stored at −80°C. Serum phosphorus and calcium were determined by colorimetric measurements using the Stanbio Phosphorus Liqui-UV Test and Calcium (Arsenazo) LiquiColor Test, respectively (Stanbio Laboratory, Boerne, TX, USA).
Measurement of serum creatinine and 1,25-dehydroxyvitamin D
Serum creatinine levels were determined using the Stanbio creatinine kits (Stanbio Laboratory), as recommended by the manufacturer. The levels of 1,25-dehydroxyvitamin D were measured in serum obtained from wild-type, klotho−/−, and NaPi2a−/−/klotho−/− mice fed with either NPD or HPD using a kit purchased from IDS (Fountain Hills, AZ, USA).
Histological analyses
Soft tissues obtained from wild-type, klotho−/−, and NaPi2a−/−/klotho−/− mice fed with either NPD or HPD at 9–12 wk were fixed with 4% paraformaldehyde and 10% buffered formalin or Carnoy’s solution and were subsequently embedded in paraffin. Four- to 6-μm paraffin sections of various tissues were mounted on SuperFrost Plus glass slides. Sections were then stained with hematoxylin and eosin and von Kossa. Histological samples were observed by light microscopy (25, 26).
Calcification analyses
To determine the effects of hyperphosphatemia on ectopic calcification, sections were prepared from heart, lung, and kidney of wild-type, klotho−/−, and NaPi2a−/−/klotho−/− mice fed with either NPD or HPD and were stained with von Kossa to visualize mineralized tissues by light microscopy. The von Kossa staining procedure is detailed in an earlier publication (27).
Immunohistochemical staining
Immunostaining was performed as described previously (26, 28). Briefly, kidneys obtained from wild-type and klotho−/− mice were fixed in 10% formalin and embedded in paraffin. Paraffin sections were deparaffinized and incubated in blocking solution for 30 min and then incubated overnight with polyclonal anti-NaPi2a antibody (dilution 1:100) at 4°C. Slides were washed with PBS and incubated with secondary antibody (dilution, 1:100) for 30 min. After a PBS wash, coverslips were placed on slides using mounting medium. The expression of NaPi2a was visualized using a bright-field microscope. Rabbit serum in place of primary antibody was used as a negative control. Kidney sections prepared from NaPi2a−/− mice and incubated with NaPi2a antibody did not show any staining.
TUNEL staining
The extent of apoptosis in wild-type, klotho−/−, and NaPi2a−/−/klotho−/− mice fed with either NPD or HPD was determined by TUNEL assays using a commercial kit (Roche Diagnostics, Indianapolis, IN, USA) according to the manufacturer’s instructions (29, 30). Briefly, deparaffinized sections of various soft tissues, including lung, muscle, and kidney, were treated with Proteinase K for 15–30 min at 37°C. After the slides were rinsed with PBS, they were treated with the TUNEL reaction mixture for 60 min at 37°C in a humidified chamber. Slides were washed with PBS and incubated for another 30 min with peroxidase solution. TUNEL-positive cells were visualized following incubation of the sections in the diaminobenzidine substrate. As a negative control, the TUNEL reaction mixture was substituted by a control solution provided in the kit. The numbers of TUNEL-positive cells were randomly counted for at least 5 fields per millimeter squared area of different tissues.
Statistical analysis
Statistically significant differences between groups were evaluated either by the Student’s t test or the Mann-Whitney U test for a comparison between 2 groups. All values are expressed as means ± se. A value of P < 0.05 was considered to be statistically significant. All analyses were performed using Microsoft Excel (Microsoft, Redmond, WA, USA).
RESULTS
NaPi2a and phosphate toxicity in klotho−/− mice
Compared with wild-type mice, klotho−/− mice showed increased expression of NaPi2a protein in the luminal side of the proximal tubules by immunohistochemistry (Supplemental Fig. 1). Increased expression of NaPi2a was associated with markedly increased serum phosphate levels in klotho−/− mice. To determine the pathological consequences of phosphate toxicity for mammalian aging, we genetically reduced serum phosphate levels in klotho−/− mice by generating NaPi2a−/−/klotho−/− double mutants.
Induction of phosphate toxicity in NaPi2a−/−/klotho−/− mice
To determine whether phosphate toxicity in klotho−/− mice accelerates aging-like phenotypes, we generated a new mouse model with reduced serum phosphate levels by interbreeding NaPi2a and klotho mutants. Consistent with our earlier observations, the NaPi2a−/−/klotho−/− double mutants were viable and larger in size than the klotho−/− mice (Fig. 1). At birth, NaPi2a−/−/klotho−/−-mutant mice were indistinguishable from their littermates. At 3 wk of age, NaPi2a−/−/klotho−/− double mutants (12.4±0.2 g) were smaller than wild type (16±0.4 g) but larger in size to klotho−/− mice (10.4±0.3 g; Fig. 1).
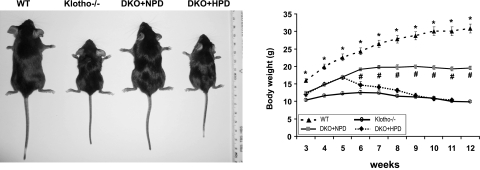
Figure 1.
Gross phenotypes of mice. Gross features of wild-type (WT) mice, klotho−/− mice, NaPi2a−/−/klotho−/− mice fed with a normal-phosphate diet (DKO+NPD), and NaPi2a−/−/klotho−/− mice fed with a high-phosphate diet (DKO+HPD) at ∼9 wk of age (left panel). Body weight curves (right panel) for the 4 different categories show that DKO+HPD mice (n=7) are significantly smaller than DKO+NPD mice (n=46). When compared with WT mice (n=22), hyperphosphatemic klotho−/− mice (n=23) are significantly smaller. Note that DKO+NPD mice are larger than klotho−/− mice, suggesting that reducing serum phosphate levels in klotho−/− mice can help to increase body weight in DKO+NPD mice. *P < 0.0001, WT vs. klotho−/− mice; #P < 0.001, DKO+NPD vs. DKO+HPD.
At 6–9 wk of age, NaPi2a−/−/klotho−/− double-mutant mice fed with NPD were still smaller than their wild-type littermates (NaPi2a−/−/klotho−/−: 19.9±0.5 g vs. wild type: 28.8±1.4 g at 9 wk) and larger in size than the klotho−/− mice (klotho−/−: 11.3±0.4 g at 9 wk). However, at 9 wk the average body weight of NaPi2a−/−/klotho−/− double mutants fed with HPD was 11.7 ± 0.6 g (Fig. 1).
Estimation of phosphate toxicity in NaPi2a−/−/klotho−/− mice
Serum phosphate and calcium levels were measured in 3-, 6- and 9-wk-old wild-type, klotho−/−, and NaPi2a−/−/klotho−/−mice fed with either NPD or HPD. The double mutants fed with NPD were hypophosphatemic by 6 wk of age (7.3±0.2 mg/dl) compared with wild-type mice (8.5±0.6 mg/dl) of similar age. The high serum phosphate levels in NaPi2a−/−/klotho−/− mice fed with HPD (11.8±0.6 mg/dl) were similar to those seen in age-matched klotho−/− mice (12.1±0.6 mg/dl) at 6 wk of age but different from those of NaPi2a−/−/klotho−/− mice fed with NPD. Similar patterns of serum phosphate levels were also observed in 9-wk-old mice (Fig. 2). Collectively, these findings suggest that inactivating NaPi2a function in klotho mice reverts hyperphosphatemia to hypophosphatemia.
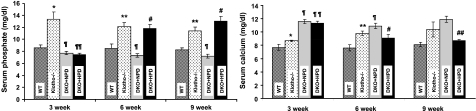
Figure 2.
Serum phosphate and calcium levels. Serum phosphate and calcium levels in WT, klotho−/−, DKO+NPD, and DKO+HPD mice at 3, 6, and 9 wk of age. Serum phosphate (left panel) and calcium (right panel) levels are higher in klotho−/− mice when compared with WT mice at 3, 6, and 9 wk of age. In contrast to klotho−/− mice, serum phosphate levels are markedly reduced in DKO+NPD mice. Serum phosphate levels are significantly increased in DKO+HPD mice. Increased serum calcium levels are observed in normal-phosphate diet-fed klotho−/− and DKO+NPD mice compared with WT controls, but levels are slightly lower in DKO+HPD mice. *P < 0.05, **P < 0.01 vs. WT; ¶P < 0.01, ¶¶P < 0.001 vs. klotho−/−; #P < 0.05, ##P < 0.001 vs. DKO+NPD.
We also measured serum calcium levels in the various genotypes. Compared with wild-type mice (7.6±0.4 mg/dl at 6 wk), klotho−/− mice showed elevated serum calcium levels (9.8±0.3 mg/dl at 6 wk) at all measured time points, and a similar pattern was also noted in NaPi2a−/−/klotho−/− mice fed either with NPD (10.9±0.4 mg/dl at 6 wk) or HPD (9.1±0.5 mg/dl at 6 wk; Fig. 2). Serum calcium levels in NPD-fed NaPi2a−/−/klotho−/− mice, however, were slightly higher than in HPD-fed NaPi2a−/−/klotho−/− mice.
Phosphate toxicity and premature aging-like phenotypes
In contrast to the normal phenotype of heterozygous klotho-mutant mice, homozygous klotho mutants displayed numerous features resembling premature aging from 3 wk onwards, including visible growth retardation, sluggish movement, infertility, and premature death between 7 and 15 wk. Hyperphosphatemic klotho−/− mice were easily recognized by their small size and marked kyphosis. The genital organs in both sexes of klotho−/− mice were severely atrophic as observed by macroscopic and microscopic examinations (Supplemental Fig. 2); this severe hypogonadism in both sexes results in infertility, which is a major consequence of aging in both humans and experimental animals (6, 31). The crossbreeding between homozygous klotho mice or between double-homozygous NaPi2a/klotho mice fed with HPD did not produce any offspring, despite an extended period of mating. Interestingly, when serum phosphate levels were reduced for klotho−/− mice, the NaPi2a−/−/klotho−/− double-mutant mice fed with NPD recovered body weight, regained fertility, and most important, survived longer. Neither NaPi2a−/−/klotho−/− mice fed with HPD nor klotho−/− mice survived past 15 wk, while all wild-type mice and NaPi2a−/−/klotho−/− mice fed with NPD survived beyond 20 wk (Fig. 3). The rescue of premature aging-like phenotypes of NaPi2a−/−/klotho−/− mice fed with NPD can be reversed by feeding NaPi2a−/−/klotho−/− double-mutant mice with HPD, clearly suggesting that phosphate toxicity is driving the premature aging-like features in klotho-mutant mice.
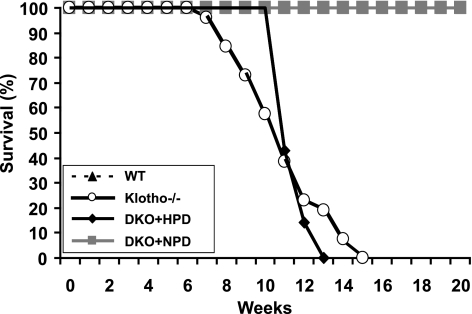
Figure 3.
Survival curve. Survival curves for WT (n=17), klotho−/− (n=23), DKO+NPD (n=15), and DKO+HPD mice (n=7). Note that the survival of DKO+HPD mice is much lower than for DKO+NPD mice and is similar to hyperphosphatemic klotho−/− mice. Most DKO+HPD mice and klotho−/− mice die by 15 wk of age, while no WT and DKO+NPD mice had died by the end of the 20-wk observation period.
Effects of phosphate toxicity on lung, intestine, and skin
Hyperphosphatemic klotho−/− mice showed typical features of emphysema in the lungs (Fig. 4), which appeared as early as 6 wk of age and are consistent with similar emphysematous changes documented in aged populations (32). Reducing serum phosphate levels in klotho−/− mice suppressed emphysematous changes in the NaPi2a−/−/klotho−/− double-mutant mice. However, feeding NaPi2a−/−/klotho−/− double mutants with HPD induced severe emphysema, clearly suggesting a role for phosphate toxicity in this lung pathology.
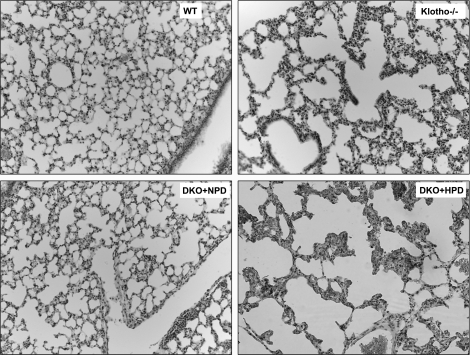
Figure 4.
Histological features of lung tissue. Hematoxylin and eosin-stained sections of lungs from 9- to 11-wk-old WT, klotho−/−, DKO+NPD, and DKO+HPD mice. Compared with WT mice, there is marked expansion of alveolar spaces (emphysema) in klotho−/− mice. Such pulmonary emphysematous changes are reduced in DKO+NPD mice and reappear in DKO+HPD. Lungs are fixed in formalin for at least 24 h and then processed in the paraffin before sectioning and staining (view ×10).
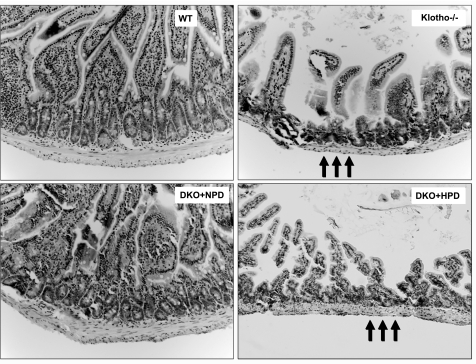
Reduction in villus height of the intestinal mucosa and reduced mucosal surface area are pathologies usually noted in aged populations (33). Compared with wild-type mice, klotho−/− mice showed focal areas of intestinal mucosal atrophy with thin muscular layers (Fig. 5). Such intestinal lesions are reduced in hypophosphatemic NaPi2a−/−/klotho−/− double-mutant mice (fed with NPD) and reappear in hyperphosphatemic NaPi2a−/−/klotho−/− double mutants (fed with HPD), implicating a pathological role for phosphate toxicity in intestinal atrophy.
Figure 5.
Histological features of intestine. Hematoxylin and eosin-stained sections of intestines from WT, klotho−/−, DKO+NPD, and DKO+HPD mice. Compared with WT mice, there is marked atrophy of the intestinal wall (arrows) in klotho−/− mice. These focal atrophic changes of the intestine are improved in DKO+NPD mice and reappear in DKO+HPD mice, suggesting that reducing serum phosphate levels in the klotho−/− mice can help to restore intestinal anomalies (intestine view ×20).
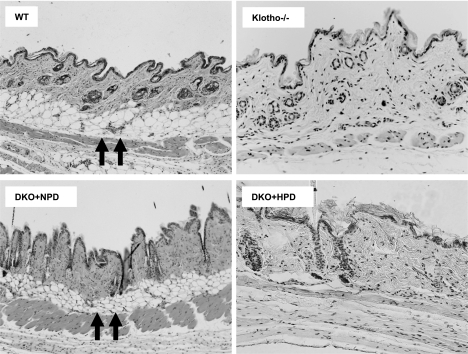
Hairs are sparser in the skin of the klotho−/− mice than in control littermates. A reduced number of hair follicles with markedly reduced dermal and epidermal thickness as well as barely detectable subcutaneous fat were consistently noted in hyperphosphatemic klotho−/− mice (Fig. 6). When serum phosphate levels were reduced in klotho−/− mice, there were obvious improvements in skin structure in the NaPi2a−/−/klotho−/− double mutants, with clearly apparent subcutaneous fat layers. The rescue of skin phenotypes for hypophosphatemic NaPi2a−/−/klotho−/− mice (fed with NPD) was reversed in hyperphosphatemic NaPi2a−/−/klotho−/− double-mutant mice (fed with HPD). The abnormal changes in lung (Fig. 4), intestine (Fig. 5), and skin (Fig. 6) for hyperphosphatemic klotho−/− mice were dramatically ameliorated and rescued by genetically lowering serum phosphate levels in hypophosphatemic NaPi2a−/−/klotho−/− double mutants, suggesting a pathological role for phosphate toxicity in soft tissue injury (Table 1). In addition, severe atrophy of the thymus and spleen (Supplemental Fig. 3) was noted in hyperphosphatemic klotho−/− mice when compared with controls. Again, such atrophy of the thymus and spleen was suppressed in hypophosphatemic NaPi2a−/−/klotho−/− mice (fed with NPD) and reappeared in hyperphosphatemic NaPi2a−/−/klotho−/−double mutants (fed with HPD).
Figure 6.
Histological features of skin tissues. Hematoxylin and eosin-stained sections of skin from 9- to 11-wk-old WT, klotho−/−, DKO+NPD, and DKO+HPD mice. Compared with WT mice, there is marked atrophy of the skin in klotho−/− mice. Subcutaneous fat tissue layer (arrows) seen in wild-type skin is mostly absent in klotho homozygous mutant mice. Such focal atrophic changes are improved in DKO+NPD mice and reappear in DKO+HPD mice, suggesting a role for phosphate toxicity in skin atrophy (view ×10 for WT and DKO+NPD; ×20 for klotho−/− and DKO+HPD).
TABLE 1.
Phenotypes of various mutant mice compared with those of wild-type mice
| Phenotype | Wild-type (NPD) | klotho−/− (NPD) | klotho−/−/NaPi2a−/− (NPD) | klotho−/−/NaPi2a−/− (HPD) |
|---|---|---|---|---|
| Biochemical changes | ||||
| Serum phosphate | Normal | High | Low/normal | High |
| Serum calcium | Normal | High (M) | High (M) | High (S) |
| Gross appearance | ||||
| Body weight | Normal | Reduced (M) | Reduced (S) | Reduced (M) |
| Growth retardation | Absent | Present (M) | Present (S) | Present (M) |
| Kyphosis | Absent | Present | Absent | Present |
| Generalized atrophy | ||||
| Spleen atrophy | Absent | Present | Absent | Present |
| Muscle atrophy | Absent | Present | Absent | Present |
| Skin atrophy | Absent | Present | Absent | Present |
| Intestinal atrophy | Absent | Present | Absent | Present |
| Gonadal atrophy | Absent | Present | Absent | Present |
| Morphological changes | ||||
| Vascular calcifications | Absent | Present | Absent | Present |
| Ectopic calcifications | Absent | Present | Absent | Present |
| Emphysema | Absent | Present (D) | Present (F) | Present (D) |
| Overall affect | ||||
| Physical activity | Normal | Sluggish | Normal | Sluggish |
| Fertility | Normal | Lost | Normal | Lost |
| Lifespan | Normal | Short | Normal | Short |
Note that the phenotypes of the NPD-fed klotho−/− mice and klotho−/−/NaPi2a−/− double-knockout mice are consistent with our earlier reported observations (23). M, markedly; S, slightly; D, diffuse; F, focal.
Phosphate toxicity and ectopic calcification
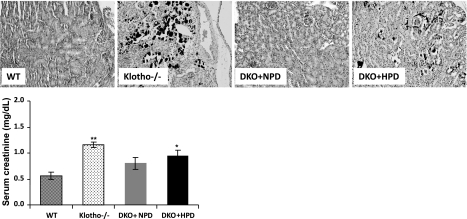
We examined calcification in the various murine genotypes by von Kossa staining. Consistent with our earlier observations, we detected extensive vascular and soft tissue calcification in the aorta, lung (Supplemental Fig. 4), kidney (Fig. 7), and other organs in klotho−/− mice. The extensive calcification noted in klotho−/− mice was absent in NaPi2a−/−/klotho−/− mice fed with NPD but reappeared in NaPi2a−/−/klotho−/− mice fed with HPD, suggesting a crucial role for phosphate in vascular and soft tissue calcification.
Figure 7.
Ectopic calcification of the kidney. Kidney sections prepared from WT, klotho−/−, DKO+NPD, and DKO+HPD mice, showing extensive calcification (black staining) in kidneys of hyperphosphatemic klotho−/− mice. Inactivation of NaPi2a in klotho−/− mice reduces this calcification in DKO+NPD fed mice. However, ectopic calcification reappears in DKO+HPD-fed mice, implicating phosphate toxicity in such ectopic biomineralization (von Kossa staining; ×20). The renal damage by calcification is also appearent from the serum creatinine levels of the mutant mice (bottom panel). Note that compared with the WT mice (n=4), serum creatinine level is significantly increased in the klotho−/− mice (n=4), and DKO+HPD (n=4). No such significant elevation is noted in DKO+NPD mice (n=4). *P < 0.05, **P < 0.01 vs. WT.
Phosphate toxicity and apoptosis
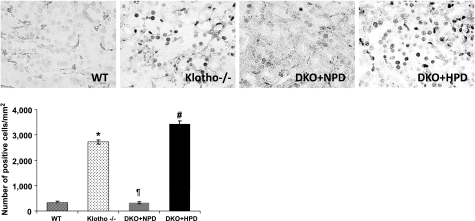
To elucidate how phosphate toxicity induces generalized tissue anomalies, we used TUNEL staining to determine the numbers of apoptotic cells in various tissues obtained from wild-type, klotho−/−, and NaPi2a−/−/klotho−/− mice fed with either NPD or HPD. There is an increase in the number of apoptotic cells in kidneys from hyperphosphatemic klotho−/− mice (Fig. 8). The number of apoptotic cells was significantly reduced in hypophosphatemic NaPi2a−/−/klotho−/− double mutants (fed with NPD) and markedly increased in hyperphosphatemic NaPi2a−/−/klotho−/− double mutants (fed with HPD). Similar pattern of apoptosis is also noted in lung and skeletal muscle. Impairment of cellular homeostasis following an increased rate of apoptosis caused by phosphate toxicity may contribute to generalized tissue anomalies.
Figure 8.
Apoptotic cells in the kidney. Detection of TUNEL-positive cells in kidney sections prepared from WT, klotho−/−, DKO+NPD, and DKO+HPD mice. Kidney sections prepared from klotho−/− and DKO+HPD mice showing increased numbers of apoptotic cells when compared with kidneys of WT and DKO+NPD mice. Note that, compared with the hyperphosphatemic klotho−/− mice, the hypophosphatemic NaPi2a−/−/klotho−/− mice (fed with NPD) have reduced apoptotic cell death, while inducing hyperphosphatemia in NaPi2a−/−/klotho−/− double mutants (fed with HPD) increases apoptotic cell death, which is similar to klotho−/− mice (view ×60). *P < 0.001 vs. WT; ¶P < 0.001 vs. klotho−/−; #P < 0.001 vs. DKO+NPD.
DISCUSSION
We present here a novel role for phosphate in accelerating the mammalian aging process and reducing survival. Our results show that genetically ablated klotho mice develop extensive premature aging-like features, which include but are not limited to loss of body weight, kyphosis, hypogonadism, infertility, generalized tissue atrophy, and reduced life span. These all bear similarity to phenotypes observed during human aging. Despite the fact that expression of the klotho gene is very restricted and is mainly found in the kidney, parathyroid gland, and pituitary gland (34, 35), klotho-knockout mice show systemic premature aging-like phenotypes (20, 21, 36). Generalized involvement of tissues and organs outside klotho-expressing tissues in knockout mice suggests that these premature aging-like features are likely to be non-cell autonomous. It is possible that dysregulation of the physiological balance caused by loss of klotho function induces systemic aging-like features. In fact, the extensive age-associated phenotypes in klotho-knockout mice can be suppressed by genetically reducing serum phosphate levels in NaPi2a−/−/klotho−/− DKO mice, thus extending survival. More important, when serum phosphate levels are increased in NaPi2a−/−/klotho−/− DKO mice by feeding with an HPD, the premature aging-like features reappear in the DKO mice similarly as in klotho single mutants. These results clearly suggest that phosphate toxicity is the main cause of premature aging-like features in klotho knockouts. Of relevance, compared with the wild-type controls, the serum 1,25-dehydroxyvitamin D levels were higher in klotho−/− mice and in NaPi2a−/−/klotho−/− mice fed either with an HPD or NPD (Supplemental Fig. 5); these results suggest that rescue of premature aging-like features in NaPi2a−/−/klotho−/− mice fed with an NPD and reappearance in NaPi2a−/−/klotho−/− mice fed with an HPD are independent of vitamin D activities. Moreover, the rescue of premature aging-like features of klotho−/−mice by inactivating vitamin D activities (in 1a-hydroxylase−/−/klotho−/− DKO mice) is due to reduced serum phosphate levels in double-mutant mice (37). A similar pattern of rescue is also noted in fibroblast growth factor 23 (Fgf23)-knockout mice without vitamin D activity (in 1a-hydroxylase−/−/Fgf23−/− DKO mice), as we reported in our earlier publications (29, 36, 38).
Klotho mutants show severe hyperphosphatemia leading to widespread tissue atrophy in spleen, skeletal muscle, intestine, and skin. Interestingly, reducing serum phosphate levels in klotho knockouts by genetic ablation of the NaPi2a gene rescues tissue atrophy in NaPi2a−/−/klotho−/− DKO mice. However, feeding NaPi2a−/−/klotho−/− DKO mice with an HPD results in severe tissue atrophy, suggesting that the generalized tissue atrophy in klotho mutants is partly caused by phosphate toxicity. The klotho-knockout mice have severe muscle wasting with reduced fat tissue. Lowering serum phosphate levels from klotho-knockout mice not only reduced muscle wasting but also helped gain fat tissues; whether such improvements in the muscle and the fat tissues result in better survival of NaPi2a−/−/klotho−/− DKO mice will need additional studies.
Consistent with our earlier observations, hyperphosphatemic klotho-knockout mice show typical features of emphysema in the lungs, which mostly disappear in the NPD-fed NaPi2a−/−/klotho−/− DKO mice (23). However, emphysema in the lungs reappears in NaPi2a−/−/klotho−/− DKO mice fed with an HPD, implicating phosphate toxicity in lung damage. Of relevance to this observation, emphysematous changes have also been documented in aged populations (32).
The genital organs of both sexes of hyperphosphatemic klotho-knockout mice are severely atrophic as observed by macroscopic and microscopic examinations; this severe hypogonadism in both sexes results in infertility, which is a major consequence of aging in both humans and experimental animals (6, 31). The klotho-knockout mice with reduced serum phosphate levels regained fertility, as evidenced in the NaPi2a−/−/klotho−/− DKO mice. More important, the NaPi2a−/−/klotho−/− DKO mice lost their fertility when fed with an HPD, clearly suggesting that phosphate toxicity can affect fertility and thereby influence the aging process.
The mechanism by which phosphate toxicity accelerates the aging process requires further study. It is likely that high phosphate exerts its cytotoxic effects to compromise the physiological functions of various organ systems. We found that high phosphate can induce an increased rate of apoptosis in various organs and that this apoptosis is suppressed by lowering serum phosphate levels in the NaPi2a−/−/klotho−/− DKO mice. It is likely that continued cell deletion through apoptosis and due to phosphate toxicity leads to generalized tissue atrophy as well as reduced organ function and viability, thereby accelerating the aging-like process as noted in our mouse models. In a similar line of observation, extensive renal calcification with increased numbers of apoptotic cells in the kidneys of the hyperphosphatemic mutant mice is associated with increased serum levels of creatinine (Fig. 7); such impairment of renal function may contribute to the reduced survival of klotho-knockout mice and NaPi2a−/−/klotho−/− mice fed with an HPD (26, 39).
CONCLUSIONS
The results of our in vivo genetic and dietary manipulation studies provide evidence for a novel role of phosphate in mammalian aging. Our results showing that genetically reducing serum phosphate levels in klotho-knockout mice suppresses the aging-like phenotypes in NaPi2a−/−/klotho−/− DKO mice and that increasing serum phosphate levels in NaPi2a−/−/klotho−/− DKO mice accelerates the aging-like phenotype to reduce longevity support a role for phosphate toxicity in regulating the aging process. One of the limitations of the generated NaPi2a−/−/klotho−/− DKO mice is that loss of NaPi2a function can be gradually compensated by other phosphate transporters, including NaPi2c, which is particularly apparent in the aged DKO mice. Aged NaPi2a−/−/klotho−/− DKO mice have relatively higher serum phosphate levels and that may influence survival of these mice, again suggesting a role of phosphate in aging and survival. Finally, NaPi2a−/−/klotho−/− DKO mice provide an important in vivo tool to study the effects of various dietary components on aging. Our understanding of intrinsic factors that accelerate aging may provide better insights into the mechanisms by which extrinsic factors influence mammalian senescence.
Supplementary Material
Acknowledgments
Parts of this research were supported by National Institute of Diabetes and Digestive and Kidney Disease grant R01-DK-077276 (to M.S.R.).
References
- Dolle M E, Giese H, Hopkins C L, Martus H J, Hausdorff J M, Vijg J. Rapid accumulation of genome rearrangements in liver but not in brain of old mice. Nat Genet. 1997;17:431–434. doi: 10.1038/ng1297-431. [DOI] [PubMed] [Google Scholar]
- Kirkwood T B, Austad S N. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- De Boer J, Andressoo J O, de Wit J, Huijmans J, Beems R B, van Steeg H, Weeda G, van der Horst G T, van Leeuwen W, Themmen A P, Meradji M, Hoeijmakers J H. Premature aging in mice deficient in DNA repair and transcription. Science. 2002;296:1276–1279. doi: 10.1126/science.1070174. [DOI] [PubMed] [Google Scholar]
- Tarry-Adkins J L, Chen J H, Smith N S, Jones R H, Cherif H, Ozanne S E. Poor maternal nutrition followed by accelerated postnatal growth leads to telomere shortening and increased markers of cell senescence in rat islets. FASEB J. 2009;23:1521–1528. doi: 10.1096/fj.08-122796. [DOI] [PubMed] [Google Scholar]
- Kujoth G C, Hiona A, Pugh T D, Someya S, Panzer K, Wohlgemuth S E, Hofer T, Seo A Y, Sullivan R, Jobling W A, Morrow J D, Van Remmen H, Sedivy J M, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla T A. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink J N, Rovio A T, Bruder C E, Bohlooly Y M, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs H T, Larsson N G. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- Jang Y C, Lustgarten M S, Liu Y, Muller F L, Bhattacharya A, Liang H, Salmon A B, Brooks S V, Larkin L, Hayworth C R, Richardson A, Van Remmen H. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. [E-pub ahead of print] FASEB J. 2009 doi: 10.1096/fj.09-146308. doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon A B, Perez V I, Bokov A, Jernigan A, Kim G, Zhao H, Levine R L, Richardson A. Lack of methionine sulfoxide reductase A in mice increases sensitivity to oxidative stress but does not diminish life span. FASEB J. 2009;23:3601–3608. doi: 10.1096/fj.08-127415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M G, Sarkar D, Klopp R, Morrow J D, Weindruch R, Prolla T A. Age-related impairment of the transcriptional responses to oxidative stress in the mouse heart. Physiol Genomics. 2003;13:119–127. doi: 10.1152/physiolgenomics.00172.2002. [DOI] [PubMed] [Google Scholar]
- Radak Z, Chung H Y, Naito H, Takahashi R, Jung K J, Kim H J, Goto S. Age-associated increase in oxidative stress and nuclear factor kappaB activation are attenuated in rat liver by regular exercise. FASEB J. 2004;18:749–750. doi: 10.1096/fj.03-0509fje. [DOI] [PubMed] [Google Scholar]
- Judge S, Jang Y M, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J. 2005;19:419–421. doi: 10.1096/fj.04-2622fje. [DOI] [PubMed] [Google Scholar]
- Gaasbeek A, Meinders A E. Hypophosphatemia: an update on its etiology and treatment. Am J Med. 2005;118:1094–1101. doi: 10.1016/j.amjmed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Razzaque M S. FGF23-mediated regulation of systemic phosphate homeostasis: is Klotho an essential player? Am J Physiol Renal Physiol. 2009;296:F470–476. doi: 10.1152/ajprenal.90538.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque M S. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5:611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque M S, Lanske B. The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. J Endocrinol. 2007;194:1–10. doi: 10.1677/JOE-07-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenhouse H S. Regulation of phosphorus homeostasis by the type iia na/phosphate cotransporter. Annu Rev Nutr. 2005;25:197–214. doi: 10.1146/annurev.nutr.25.050304.092642. [DOI] [PubMed] [Google Scholar]
- Nakatani T, Bara S, Ohnishi M, Densmore M J, Taguchi T, Goetz R, Mohammadi M, Lanske B, Razzaque M S. In vivo genetic evidence of klotho-dependent functions of FGF23 in regulation of systemic phosphate homeostasis. FASEB J. 2009;23:433–441. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa H, Yamanaka S, Ohno Y, Onitsuka A, Shiozawa K, Aranami F, Furutani J, Tomoe Y, Ito M, Kuwahata M, Imura A, Nabeshima Y, Miyamoto K. Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am J Physiol Renal Physiol. 2007;292:F769–F779. doi: 10.1152/ajprenal.00248.2006. [DOI] [PubMed] [Google Scholar]
- Nakatani T, Ohnishi M, Razzaque M S. Inactivation of klotho function induces hyperphosphatemia even in presence of high serum fibroblast growth factor 23 levels in a genetically engineered hypophosphatemic (Hyp) mouse model. FASEB J. 2009;23:3702–3711. doi: 10.1096/fj.08-123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanske B, Razzaque M S. Premature aging in klotho mutant mice: cause or consequence? Ageing Res Rev. 2007;6:73–79. doi: 10.1016/j.arr.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima Y I. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Beck L, Karaplis A C, Amizuka N, Hewson A S, Ozawa H, Tenenhouse H S. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci U S A. 1998;95:5372–5377. doi: 10.1073/pnas.95.9.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi M, Nakatani T, Lanske B, Razzaque M S. In vivo genetic evidence for suppressing vascular and soft-tissue calcification through the reduction of serum phosphate levels, even in the presence of high serum calcium and 1,25-Dihydroxyvitamin D levels. Circ Cardiovasc Genet. 2009;2:583–590. doi: 10.1161/CIRCGENETICS.108.847814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitara D, Kim S, Razzaque M S, Bergwitz C, Taguchi T, Schuler C, Erben R G, Lanske B. Genetic evidence of serum phosphate-independent functions of FGF-23 on bone. PLoS Genet. 2008;4:e1000154. doi: 10.1371/journal.pgen.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque M S, Kumatori A, Harada T, Taguchi T. Coexpression of collagens and collagen-binding heat shock protein 47 in human diabetic nephropathy and IgA nephropathy. Nephron. 1998;80:434–443. doi: 10.1159/000045217. [DOI] [PubMed] [Google Scholar]
- Zha Y, Le V T, Higami Y, Shimokawa I, Taguchi T, Razzaque M S. Life-long suppression of growth hormone-insulin-like growth factor I activity in genetically altered rats could prevent age-related renal damage. Endocrinology. 2006;147:5690–5698. doi: 10.1210/en.2006-0302. [DOI] [PubMed] [Google Scholar]
- Razzaque M S, Soegiarto D W, Chang D, Long F, Lanske B. Conditional deletion of Indian hedgehog from collagen type 2alpha1-expressing cells results in abnormal endochondral bone formation. J Pathol. 2005;207:453–461. doi: 10.1002/path.1870. [DOI] [PubMed] [Google Scholar]
- Razzaque M S, Taguchi T. Collagen-binding heat shock protein (HSP) 47 expression in anti-thymocyte serum (ATS)-induced glomerulonephritis. J Pathol. 1997;183:24–29. doi: 10.1002/(SICI)1096-9896(199709)183:1<24::AID-PATH1106>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Razzaque M S, Sitara D, Taguchi T, St-Arnaud R, Lanske B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin-D mediated process. FASEB J. 2006;20:720–722. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque M S, Koji T, Kumatori A, Taguchi T. Cisplatin-induced apoptosis in human proximal tubular epithelial cells is associated with the activation of the Fas/Fas ligand system. Histochem Cell Biol. 1999;111:359–365. doi: 10.1007/s004180050368. [DOI] [PubMed] [Google Scholar]
- Rauser C L, Mueller L D, Rose M R. Aging, fertility, and immortality. Exp Gerontol. 2003;38:27–33. doi: 10.1016/s0531-5565(02)00148-1. [DOI] [PubMed] [Google Scholar]
- Martin C J, Chihara S, Chang D B. A comparative study of the mechanical properties in aging alveolar wall. Am Rev Respir Dis. 1977;115:981–988. doi: 10.1164/arrd.1977.115.6.981. [DOI] [PubMed] [Google Scholar]
- Evers B M, Townsend C M, Jr, Thompson J C. Organ physiology of aging. Surg Clin North Am. 1994;74:23–39. doi: 10.1016/s0039-6109(16)46226-2. [DOI] [PubMed] [Google Scholar]
- Kato Y, Arakawa E, Kinoshita S, Shirai A, Furuya A, Yamano K, Nakamura K, Iida A, Anazawa H, Koh N, Iwano A, Imura A, Fujimori T, Kuro-o M, Hanai N, Takeshige K, Nabeshima Y. Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun. 2000;267:597–602. doi: 10.1006/bbrc.1999.2009. [DOI] [PubMed] [Google Scholar]
- Li S A, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- Razzaque M S, Lanske B. Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol Med. 2006;12:298–305. doi: 10.1016/j.molmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Ohnishi M, Nakatani T, Lanske B, Razzaque M S. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int. 2009;75:1166–1172. doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque M S, St-Arnaud R, Taguchi T, Lanske B. FGF-23, vitamin D and calcification: the unholy triad. Nephrol Dial Transplant. 2005;20:2032–2035. doi: 10.1093/ndt/gfh991. [DOI] [PubMed] [Google Scholar]
- Razzaque M S. Does renal ageing affect survival? Ageing Res Rev. 2007;6:211–222. doi: 10.1016/j.arr.2007.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.