Abstract
Cutaneous malignant melanoma remains a therapeutic challenge, and patients with advanced disease have limited survival. Photodynamic therapy (PDT) has been successfully used to treat many malignancies, and it may show promise as an antimelanoma modality. However, high melanin levels in melanomas can adversely affect PDT effectiveness. Herein the extent of melanin contribution to melanoma resistance to PDT was investigated in a set of melanoma cell lines that markedly differ in the levels of pigmentation; 3 new bacteriochlorins successfully overcame the resistance. Cell killing studies determined that bacteriochlorins are superior at (LD50≈0.1 μM) when compared with controls such as the FDA-approved Photofrin (LD50≈10 μM) and clinically tested LuTex (LD50≈1 μM). The melanin content affects PDT effectiveness, but the degree of reduction is significantly lower for bacteriochlorins than for Photofrin. Microscopy reveals that the least effective bacteriochlorin localizes predominantly in lysosomes, while the most effective one preferentially accumulates in mitochondria. Interestingly all bacteriochlorins accumulate in melanosomes, and subsequent illumination leads to melanosomal damage shown by electron microscopy. Fluorescent probes show that the most effective bacteriochlorin produces significantly higher levels of hydroxyl radicals, and this is consistent with the redox properties suggested by molecular-orbital calculations. The best in vitro performing bacteriochlorin was tested in vivo in a mouse melanoma model using spectrally resolved fluorescence imaging and provided significant survival advantage with 20% of cures (P<0.01).—Mroz, P., Huang, Y.-Y., Szokalska, A., Zhiyentayev, T., Janjua, S., Nifli, A.-P., Sherwood, M. E., Ruzié, C., Borbas, K. E., Fan, D., Krayer, M., Balasubramanian, T., Yang, E., Kee, H. L., Kirmaier, C., Diers, J. R., Bocian, D. F., Holten, D., Lindsey, J. S., Hamblin, M. R. Stable synthetic bacteriochlorins overcome the resistance of melanoma to photodynamic therapy.
Keywords: multidrug resistance, melanosomes, electron microscopy
Malignant melanoma is a cancer that arises from melanocytes, the specialized pigmented cells that are found predominantly in the skin. Although melanoma accounts for only 4% of skin cancer cases, it causes 79% of all skin cancer related deaths. If diagnosed early, melanoma can be cured by surgical resection with ∼80% effectiveness for thin lesions. However, once metastases occur, it is largely refractory to existing therapies. The National Comprehensive Cancer Network (1) recommends a plethora of treatments for stage III and local recurrence of melanoma that include intralesional injection of BCG or interferon, and local ablation therapy or radiation therapy. Only interferon α-2b has been shown to have a reproducible benefit (2). Other potential immunotherapies include vaccines or high-dose bolus interleukin-2 alone or in combination with chemotherapy (3). Naylor et al. (4) used topical 5% imiquimod cream and irradiation of skin metastases with a continuous-wave 810 nm laser to widen the response to distant nonirradiated lesions.
Photodynamic therapy (PDT) uses a nontoxic dye molecule or photosensitizer that absorbs a photon of an appropriate wavelength of light to form an excited triplet state (5). The excited molecule can then transfer energy to the (triplet) ground state of molecular oxygen to produce the highly cytotoxic singlet oxygen (type II reaction) or undergo electron transfer (type I reaction) with the ultimate formation of reactive oxygen species. Such species are the superoxide radical anion or hydroxyl radicals that can oxidize important biological molecules such as proteins, lipids, and nucleic acids.
There are 3 main mechanisms that make PDT an effective anticancer procedure: 1) direct tumor killing by the reactive oxygen species, 2) tumor-associated vascular damage, and 3) activation of antitumor immune response. The prevailing view is that all 3 mechanisms are necessary for the optimal tumor damage.
Melanins are the principal surface pigments that play a major role in photoprotection (6, 7). Melanin synthesis is initiated with the enzymatic hydroxylation of the l-tyrosine to l-dihydroxyphenylalanine (l-DOPA) and oxidation of l-DOPA to DOPAquinone. DOPAquinone is subsequently transformed to melanin in a series of reactions accelerated by enzymes and metal cations. Numerous stimuli are able to alter melanogenesis or the production of melanin by cultured melanocytes (8, 9). The type of melanin produced depends on the cellular genotype and environmental factors, resulting in the black pigment eumelanin, the reddish to yellow pigment pheomelanin, or the mixed melanin that contains both components (10). Consequently, melanomas can vary from nonpigmented tumors that have no melanin whatsoever, through moderately pigmented to highly pigmented tumors, and their pigmentation level is proportional to the degree of differentiation and inversely proportional to the growth rate (11, 12).
Bacteriochlorins are tetrapyrrole macrocycles that contain alternating pyrrole and pyrroline (i.e., reduced pyrrole) rings. The macrocycle structure occurs naturally in photosynthetic pigments (bacteriochlorophylls a and b) found in purple photosynthetic bacteria (13). The presence of the reduced rings in the tetrapyrrole macrocycle has a pronounced effect on the absorption spectra. Bacteriochlorins have intense absorption bands in the region of 720–850 nm, allowing for deeper light penetration through tissue and bypassing the melanin absorption.
During the past decade, several naturally occurring or naturally derived bacteriochlorins have been evaluated in PDT applications, and some of them have shown significant in vivo efficacy (14, 15). However, naturally occurring bacteriochlorins have the following drawbacks: limited synthetic malleability, susceptibility to unwanted dehydrogenation, and a requirement for harsh conditions for the modification of functional groups already present in the macrocycle.
To overcome the aforementioned limitations of naturally occurring bacteriochlorins, a de novo synthetic pathway to stable bacteriochlorins has been developed (16). A key design feature of the synthetic bacteriochlorins is a geminal-dimethyl group in each reduced, pyrroline ring that locks in the bacteriochlorin chromophore and precludes dehydrogenation or tautomerization processes. This structural feature dramatically increases the chemical stability and eliminates susceptibility to degradative aerobic oxidation.
In this study, 3 synthetic bacteriochlorins in combination with 730-nm illumination from a diode laser were tested and the results compared with those for the clinically approved photosensitizer Photofrin (630-nm absorption) and with lutetium texaphyrin (LuTex), which is an established near-infrared absorbing photosensitizer (17). These studies utilized a series of human and mouse melanoma cell lines that differ in the pigmentation levels. The selected compounds performed significantly better when compared with Photofrin or LuTex, even in highly pigmented cells and led to a significant survival advantage and 20% of cures in an in vivo model. A preliminary account of this work has been presented at International Photodynamic Association Conference as a proceedings paper (18).
The results presented here suggest that PDT may be a promising therapeutic option to treat melanoma patients and to prevent relapse of the disease. PDT has been shown to have the potential to induce an antitumor immune response capable of destroying well-established tumors as well as distant metastases (19). It is therefore of utmost importance to establish effective PDT treatment regimens of melanoma in order to be able to explore the possible immunological benefits in patients. PDT may never replace surgery for localized melanoma. Nevertheless, it may be an effective treatment option for skin-disseminated tumors, unresectable melanomas, mucosal and ocular tumors, as well as for patients with stage III and IV melanoma.
MATERIALS AND METHODS
Photosensitizers
Bacteriochlorins 1–3 were synthesized as described previously (20,21,22). Photofrin was obtained from QLT (Vancouver, BC, Canada), and lutetium texaphyrin (LuTex) was obtained from Pharmacyclics (Sunnyvale, CA, USA). For in vivo experiments, a cremophor micellar preparation of bacteriochlorin 3 was obtained as follows. Two solutions were prepared, the first containing 1 mg of bacteriochlorin 3 in 1 ml of dry ethanol, and the second containing a solution of cremophor (48 mg) in 1 ml of dry ethanol. In a round-bottomed flask, 200 μl of the solution of bacteriochlorin 3 was mixed with 525 μl of the cremophor solution, and after further addition of 1 ml of dry ethanol to this mixture, the solvent was removed by rotary evaporation at room temperature for 15 min. Then the film was completely dissolved under sterile conditions in 1 ml of sterile 5% dextrose solution (5 distilled water), and used for injections in the mouse PDT studies.
Photophysical measurements and molecular orbital characteristics
Photophysical measurements were preformed as described previously (23). The quantum yield and lifetime measurements utilized Ar-purged solutions (methanol or 2-methyltetrahydrofuran) except that the triplet lifetime for bacteriochlorin 3 was determined using a deoxygenated (by free-pump-thaw) aqueous cremophor micellar solution. Fluorescence yields were determined with respect to 8,8,18,18-tetramethylbacteriochlorin (24) in Ar-purged toluene, for which Φf = 0.125 was established with respect to chlorophyll a in benzene (Φf=0.325; ref. 25) and free-base tetraphenylporphyrin in toluene (Φf=0.090; ref. 26) using Soret and Qx excitation.
Density functional theory calculations were performed with Spartan ’08 for Windows (Wavefunction, Irvine, CA, USA; ref. 27) using the hybrid B3LYP functional and 6-31G* basis set; equilibrium geometries were fully optimized using the default program parameters.
Tumor cells and mice
Mouse B16F10 melanoma cells were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA). Mouse B16-G4F cells were a gift from Dr. Eberle (Basel University Hospital, Basel, Switzerland). Human melanoma cell line C-mel and mouse B16F1 cell line were a gift of Dr. Tsao (Massachusetts General Hospital). B16F10-GFP cells are transduced cells that stably express GFP protein. All cells were cultured in RPMI 1640 medium (Life Technologies, Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Sigma, St. Louis, MO, USA) at 37°C in a 5% CO2 humidified atmosphere in 75 cm2 flasks (BD Falcon, San Jose, CA, USA).
C57BL/6 mice (male, 6 to 8 wk old) were purchased from Charles River Laboratories (Boston, MA, USA). All experiments were carried out according to a protocol approved by the Subcommittee on Research Animal Care (Institutional Animal Care and Use Committee) at Massachusetts General Hospital and were in accord with guidelines from the National Institutes of Health (NIH). Cells (3.5×105) were injected subcutaneously in thigh, and PDT was delivered to a 6-mm tumor on d 9.
Light source
A 730-nm diode laser (Pharmacyclics) was used for bacteriochlorin and LuTex, and a noncoherent light source (Lumacare, Newport Beach, CA, USA) fitted with a light guide containing a bandpass filter (630±10 nm) was used for Photofrin.
In vitro PDT experiments
Cells (5×103/well) were plated in flat-bottomed 96-well plates (Fisher Scientific, Pittsburgh, PA, USA). Cells were allowed to attach for 24 h. Photosensitizers were added at different concentrations to cells in fresh complete medium for 24-h incubation periods. The DMSO concentration in the medium was <0.5%. After incubation, the medium was replaced with 200 μl of fresh medium, and PDT was performed. For experiments where the light dose was varied, fluences of 0 (dark toxicity) to 20 J/cm2 were used, and 4 wells (1 group) were illuminated at one time. Controls entailed cells with no treatment and cells with light alone at the highest fluence or with photosensitizer alone. For experiments where the photosensitizer concentration was varied, a fixed fluence of 10 J/cm2 delivered at the same irradiance was used. Here additional groups of cells were incubated with all the concentrations of photosensitizer but no illumination was used to determine the dark toxicity. At the completion of the illumination, the plates were returned to the incubator for 24 h before initiating further studies. A 4-h MTT colorimetric assay [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was used that measures mitochondrial reductase activity (28). The absorbance for MTT assay was read at 560 nm. The observed toxicity was confirmed by a crystal violet assay (absorbance read at 560 nm) that measures cellular integrity (see Supplemental Fig. S1).
Fluorescence microscopy to determine intracellular localization
B16F10 cells (5×105) were plated on 35-mm dishes in RPMI medium and incubated overnight at 37°C. The next day, 1 μM of bacteriochlorin 1, 2, or 3 in culture medium was added and incubated for 24 h. Cells were washed in PBS, and 5 μg/ml of lysotracker or mitotracker (LysoTracker Green DND-26, MitoTracker Green FM; Molecular Probes, Invitrogen) was added and incubated for 30 min at 37°C. For colocalization of melanosomes, cells were incubated with the appropriate bacteriochlorin for 24 h, washed with PBS, and fixed and permeabilized for 20 min in Cytofix/Cytoperm buffer (BD Biosciences, San Jose, CA, USA). Next, cells were incubated with anti-TRP1 antibody (29; Abcam, Cambridge, MA, USA) for 1 h at room temperature, washed with PBS, and further incubated with FITC-conjugated rat anti-mouse antibody (Sigma) for 20 min. Cells were again washed in PBS, and 5–10 min later they were imaged at a resolution of 1024 × 1024 pixels using a Leica DMR confocal laser fluorescence microscope (Leica Mikroskopie und Systeme, Wetzler, Germany) with excitation by a 488-nm argon laser and emission with either a bandpass filter (525±10 nm) or a 580-nm long-pass filter and an ×63 1.20-NA water-immersion lens. Images were acquired using TCS NT 1.6.551 software (Leica Lasertechnik, Heidelberg, Germany).
Photosensitizer uptake
Cells (5×103/well) were plated in a 96-well plate and incubated overnight at 37°C. The next day, a sample of bacteriochlorin 1, 2, or 3, Photofrin, or LuTex was added at various concentrations and incubated for 24 h in complete medium. After incubation, the medium was removed, 200 μl of 0.1 M NaOH/1% SDS was added, and cells were incubated overnight at 37°C to lyse the cells and dissolve the photosensitizer. Excitation was 402 nm, and fluorescence was measured (730 nm for bacteriochlorin 1, 2, or 3 and LuTex; 632 nm for Photofrin) with a plate reader (Molecular Devices, Sunnyvale, CA, USA). Protein per sample was measured with a bicinchoninic acid protein assay (30). Separate fluorescence calibration curves for each photosensitizer were generated in 0.1 M NaOH/1% SDS and used to determine cellular uptakes in nanomoles of photosensitizer per milligram of cell protein.
Isolation of melanosomes from PDT-treated melanoma cells
A previously described method of melanosomal isolation was utilized (26). Briefly, pigmented B16F10 cells were incubated with bacteriochlorin 2 for 24 h and lysed, and the nuclei and mitochondria were removed by centrifugation. The resultant supernatant was further centrifuged to collect the pigmented melanosomal fraction. The fraction containing isolated melanosomes was imaged and analyzed using the Maestro system (CRI Maestro, Woburn, MA, USA; ref. 27) with excitation at 488 nm and fluorescence collected at >700 nm. The images were then analyzed by applying false-color red using the Maestro software. To measure the uptake of bacteriochlorin 2 in melanosomes, the isolated melanosomes were lysed with 0.1 M NaOH/1% SDS, and the fluorescence was measured using excitation at 402 nm and detection at 730 nm with a plate reader.
Transmission electron microscopy for melanosome damage
B16F10 cells (2.5×105) were plated on 35-mm dishes and incubated overnight at 37°C. The next day, a 1 μM solution of bacteriochlorin 2 was added to the cells, and the cells were incubated for 24 h. On the following day, 2 J/cm2 of 730-nm light was delivered, and the samples were harvested after 0.5, 1, 2, and 4 h. B16F10 PDT-treated cells were fixed overnight at 4°C in 2.5% glutaraldehyde + 2% paraformaldehyde (Karnovsky’s fixative). After the fixative was spun down (1200 rpm) and decanted, 0.1 M cacodylate buffer (pH 7.2) was added to the pelleted cells. After fixation, warm agar (2% in distilled water, heated to boiling and then cooled) was immediately added to each pellet of cells. Once the agar solidified, the cell pellets were then processed routinely as any other tissue for transmission electron microscopy. The cells were postfixed in 2% OsO4 in sodium cacodylate buffer, dehydrated in a graded alcohol series, and embedded in Epon 812 (Tousimis, Rockville, MD, USA). Ultrathin sections were cut on a Reichert-Jung Ultracut E microtome (Reichert, Vienna, Austria), collected on uncoated 200-mesh copper grids, and stained with uranyl acetate and lead citrate. The cells were examined and photographed on a Philips CM10 transmission electron microscope (Philips,Eindhoven, The Netherlands) with an AMT-XR41M 4-megapixel cooled CCD camera (Advanced Microscopy Techniques, Danvers, MA, USA).
Hydroxyl radical and singlet oxygen detection
The 3′-(p-aminophenyl)fluorescein (APF; ref. 31) and singlet oxygen sensor green (SOG; ref. 32; Molecular Probes, Invitrogen) were used to detect hydroxyl radical and singlet oxygen production, respectively. Bacteriochlorin 2 or 3 was added at the final concentration of 5 μM/well in 200 μl of 50% (v/v) acetonitrile and PBS. APF or SOG was added to each well at the final concentration of 10 μM. Four wells formed 1 experimental group. All groups were illuminated simultaneously, and 730-nm light was delivered in sequential doses of 1 J/cm2. After each dose, the probe fluorescence was measured with a fluorescence plate reader (488-nm excitation, 520-nm detection; ref. 31).
Intracellular detection of hydroxyl radical
B16F10 cells (2.5×105) were plated on 35 mm dishes and incubated overnight at 37°C. Next, cells were incubated with 1 μM concentration of bacteriochlorin 2 or 3 for 24 h, and on the next day, 10 μm of APF was added and incubated for 1 h in complete medium at 37°C. After the incubation, the cells were washed with PBS and 10 J/cm2 of 730-nm light was delivered. The cells were imaged immediately after PDT with the confocal microscope (488-nm excitation and 530-nm detection; ref. 28).
PDT and tumor response
C57BL/6 mice (6 to 8 wk old) were purchased from Charles River Laboratories. All experiments were carried out according to a protocol approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital and were in accord with NIH guidelines. Mice were inoculated with 3.5 ×105 B16F10-GFP cells subcutaneously into the depilated right thigh. Two orthogonal dimensions (a and b) of the tumor were measured 2–3 times a week with vernier calipers. Tumor volumes were calculated as follows: volume = 4π/3 × [(a + b)/4]3. When tumors reached a diameter of 5–7 mm. PDT was performed. Mice bearing B16F10-GFP tumors were anesthetized with an intraperitoneal injection of 87.5 mg/kg of ketamine and 12.5 mg/kg xylazine. A cremophor solution of bacteriochlorin 3 (5 mg/kg) was administered intravenously via the supraocular plexus. Control mice received cremophor solution in 5% dextrose only. Fifteen minutes after injection of bacteriochlorin 3, 730-nm laser illumination was performed using a homogeneous spot of 0.9-cm diameter that covered the tumor and a margin of normal tissue. The laser provided power density of 100 mW/cm2, and a fluence of 120 J/cm2 was delivered. The mice were sacrificed when any of the tumor diameters exceeded 1.5 cm.
In vivo fluorescence imaging
B16F10-GFP tumor-bearing mice were anesthetized and subsequently placed in the light-tight chamber of the CRI Maestro in vivo fluorescence imaging system (33, 34). The instrument was set up as follows: images were captured every 10 nm throughout the wavelength range 500–850 nm using a 488-nm excitation filter, an LP 515-nm emission filter, and an exposure time of 100 μs. The focus and stage height were set manually. Mice were imaged immediately after tumor inoculation and on d 7, 9 (after injection of bacteriochlorin 3 and before PDT), 13, 17, 23, and 26. After the fluorescence image acquisition, the image cubes were unmixed (deconvolved) using a spectral library. The spectral library was created by obtaining reference spectra from B16F10-GFP-pelleted cells (green) and a dilute sample of bacteriochlorin 3 in methanol (red).
RESULTS
Photophysical properties
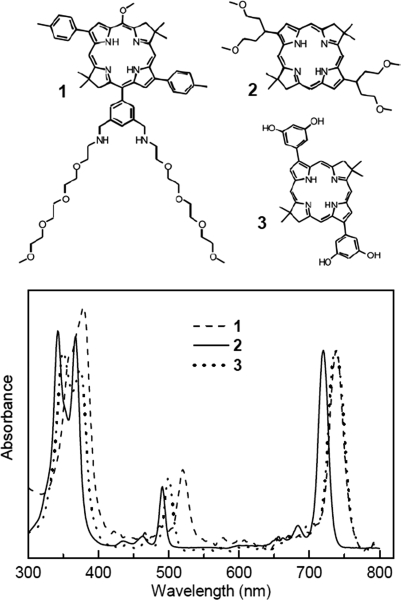
The detailed photophysical properties of the bacteriochlorins 1–3 are given in Supplemental Table S1. The compounds exhibit a narrow range of near-infrared absorption maxima (721–737 nm; Fig. 1), fluorescence yields (0.093–0.18), and lifetimes of the lowest singlet excited state (3.7–4.8 ns). The lifetimes of the lowest triplet excited state are 180–200 μs for bacteriochlorins 2 and 3, ∼55 μs for the analogs of bacteriochlorin 1, and 20 μs for the standard photosensitizer LuTex, all in the absence of molecular oxygen. The triplet lifetimes are reduced to <1 μs in the presence of atmospheric oxygen, indicating facile excited-state quenching. The yields of the triplet excited state (0.41–0.53) are similar to the value of 0.54 for the naturally occurring bacteriopheophytin a (35). These results indicate that any significant differences in phototoxicity of bacteriochlorins 1–3 must derive primarily from sources other than the lifetime and yield of the triplet excited state (from which the reactive oxygen species is produced).
Figure 1.
Molecular structures and absorption spectra (normalized at the Qy band) for bacteriochlorins 1, 2, and 3 in DMSO at room temperature. Spectral characteristics are listed in Supplemental Table S1.
Molecular orbital characteristics
As indicated in Supplemental Table S1, the highest occupied molecular orbital (HOMO) energy becomes more negative along the following series: bacteriochlorin 2 (−4.36 eV) < bacteriochlorin 1 (−4.39 eV) < bacteriochlorin 3 (−4.46 eV). The lowest unoccupied molecular orbital (LUMO) energy becomes more negative along the same series: bacteriochlorin 2 (−2.12) < bacteriochlorin 1 (−2.20) < bacteriochlorin 3 (−2.28). Thus, bacteriochlorin 3 should be harder to oxidize and easier to reduce than bacteriochlorin 1, which in turn should be harder to oxidize and easier to reduce than bacteriochlorin 2. A prior study (36) of a series of zinc chlorins showed excellent linear correlations between the calculated orbital energies and measured redox potentials. These results suggest that the shifts in oxidation and reduction potentials for the bacteriochlorins studied here (i.e., bacteriochlorin 2 vs. 3) are likely to be on the order of 100 mV.
The above values reflect differences in ground-state (S0) properties. The lowest triplet excited state (T1) will be both a more potent oxidizing and reducing agent than S0 by the T1-S0 energy gap. This gap should be the same to within ∼0.05 eV for bacteriochlorins 1–3 due to their similar structure, comparable S1 energies, and comparable LUMO-HOMO energy gaps (Supplemental Table S1). Thus, the trends in the redox characteristics for T1 for bacteriochlorins 1–3 will track those given above for S0. Since bacteriochlorin 3 and not bacteriochlorin 1 is the most potent PDT agent tested, these findings suggest that if a type I (electron-transfer) mechanism is operative, then the reduction of the bacteriochlorin T1 excited state is involved. If bacteriochlorin 1 had been the best photosensitizer, then oxidation (rather than reduction) of the T1 excited state would occur.
Effectiveness of bacteriochlorin-mediated PDT against C-mel melanoma cells
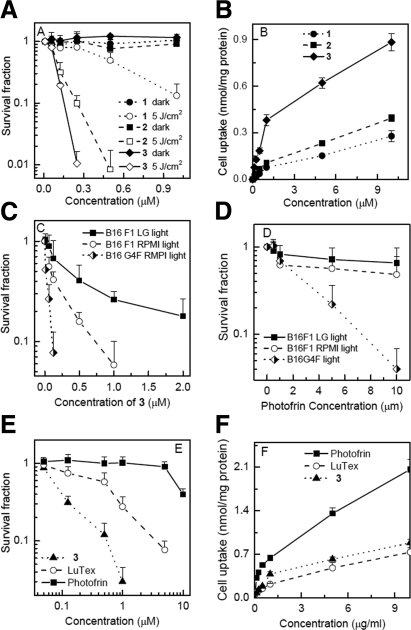
The effectiveness of bacteriochlorins 1, 2, and 3 was tested against the highly pigmented human melanoma cell line C-mel. There was no dark toxicity after 24 h incubation in any case. All compounds tested were effective in producing PDT-induced loss of mitochondrial activity in a dose-dependent and light-dependent manner against C-mel cells (Fig. 2A). The order of effectiveness is bacteriochlorin 3 > 2 > 1. Bacteriochlorin 3 kills over 2 logs of C-mel cells at a remarkably low 0.25 μM and 5 J/cm2 of light, as measured by mitochondrial activity. The order of relative C-mel cell uptake values of bacteriochlorins 1, 2, and 3 after 24 h incubation (Fig. 2B) is bacteriochlorin 3 ≫ bacteriochlorin 2 > bacteriochlorin 1. This trend broadly correlates with the order of PDT effectiveness, except that there is a large difference in PDT effectiveness between bacteriochlorins 2 and 1 and only a small difference in cell uptake.
Figure 2.
A) In vitro PDT effectiveness of bacteriochlorins 1, 2, and 3 in human melanoma cell line, C-mel. B) Cellular uptake of bacteriochlorins 1, 2, and 3 after 24 h incubation with C-mel cells. C, D) Effectiveness of 5 J/cm2 PDT with bacteriochlorin 3 (C) and Photofrin (D) against differently pigmented variants of B16 mouse melanoma cells. E) Comparison of PDT effectiveness on B16F10 melanoma cells mediated by bacteriochlorin 3, LuTex, and Photofrin. F) B16F10 cellular uptake of bacteriochlorin 3, LuTex, and Photofrin.
Effects of melanin content on PDT effectiveness with bacteriochlorin photosensitizers
The effectiveness of the best-performing bacteriochlorin, bacteriochlorin 3, was then tested in 3 variants of the pigmented mouse melanoma cell line B16 that had very different levels of pigmentation. The absorption spectra between 300 and 700 nm showing the melanin levels in these 3 cell lines are shown in Supplemental Fig. S2. B16-G4F lacks the α-MSH receptor and has low melanin levels (OD at 400 nm of 0.055; ref. 37). B16F1 has moderate levels of melanin (OD at 400 nm of 0.5214); however, after growth for 7 d in low glucose-containing medium, the melanin level was greatly increased (B16F1 LG, OD at 400 nm of 1.1841; ref. 38). Figure 2C shows that the extent of killing correlates well with the amount of intracellular melanin pigment: the B16G4F was effectively eradicated after incubation with bacteriochlorin 3 and illumination with 10 J/cm2 of 730-nm light (LD50≈0.1 μM), while the moderately pigmented B16F1 needed somewhat more bacteriochlorin 3 (LD50≈0.2 μM), and even the highly pigmented B16F1 LG was still effectively killed by bacteriochlorin 3 (LD50≈0.5 μM), as measured by mitochondrial activity assay. Cellular uptake values of bacteriochlorin 3 by the different B16 variants showed no significant differences (data not shown), suggesting that differences in PDT killing were solely due to differences in melanin content.
Results were obtained for comparison of the effectiveness of Photofrin-mediated PDT on the same 3 cell lines (Fig. 2D). Photofrin-mediated PDT was much less effective against B16G4F than bacteriochlorin 3, as shown by the higher concentration needed (LD50≈3 μM), but nevertheless significant PDT killing was observed. The effectiveness of Photofrin was dramatically reduced in the case of B16F1 (LD50≈10 μM) and completely abolished in B16F1 LG cells (no LD50).
The effectiveness of bacteriochlorin 3, Photofrin, and LuTex was compared via a photosensitizer dose-variation experiment using a single light fluence (10 J/cm2). Figure 2E shows that the clinically approved Photofrin was hardly able to kill any pigmented B16F10 melanoma cells even at 10 μM (LD50≈10 μM), while LuTex could kill 90% at 5 μM (LD50≈1μM), and the extremely effective bacteriochlorin 3 could kill 98% at only 1 μM (LD50≈0.1 μM). Values for photosensitizer uptake by B16F10 cells (Fig. 2F) show that Photofrin actually had the largest uptake, while the uptake of bacteriochlorin 3 was only slightly higher than LuTex (although the PDT killing was markedly higher). To confirm that the MTT assay correctly reported PDT toxicity, the survival fractions after PDT with bacteriochlorin 3 and Photofrin as determined by the MTT assay were compared with those found using the crystal violet assay that measures cellular integrity (see Supplemental Fig. S1). The toxicity assessed by MTT was somewhat higher than that found with crystal violet but overall the dose response was very similar. Moreover, transmission electron micrographs confirmed complete cellular destruction after PDT with bacteriochlorin 2 (Supplemental Fig. S1).
Intracellular localization of the bacteriochlorins
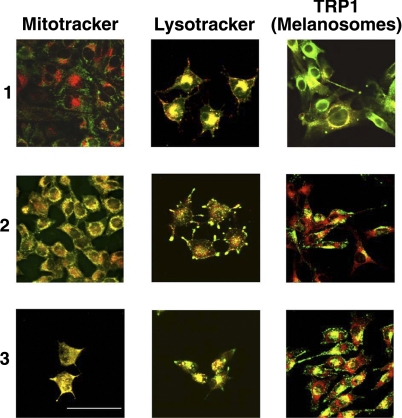
Confocal microscopy using the near-infrared fluorescence of each bacteriochlorin was used to probe intracellular localization. Figure 3 shows that bacteriochlorin 1 has almost exclusively lysosomal localization, while bacteriochlorin 2 localizes in both lysosomes and mitochondria. Interestingly, bacteriochlorin 3 appears to preferentially accumulate in mitochondria (along with lesser localization in lysosomes).
Figure 3.
Fluorescence micrographs of B16F10 cells showing red fluorescence from bacteriochlorin 1, 2, or 3 overlaid with green fluorescence from lysotracker, mitotracker, or FITC-anti-TRP1 antibody that stains melanosomes.
Previously studies have described similarities between lysosomes and melanosomes (39) in that a photosensitizer that targets the former may also target the latter to some degree (40). To test this concept, the antibody against tyrosinase-related protein (TRP-1) was used as a marker of melanosomes (in middle to late stage of development; ref. 29). As can be seen in Fig. 3 (right panel), there is significant colocalization of red and green emission, suggesting that bacteriochlorins 1–3 are effectively accumulated within melanosomes. Cells were coincubated with TRP-1 and Lysotracker in order to determine if the fluorescent markers can differentiate between these organelles. The results showed that TRP-1 specifically stains melanosomes, while Lysotracker stains both lysosomes and melanosomes (Supplemental Fig. S3).
Isolation of melanosomes
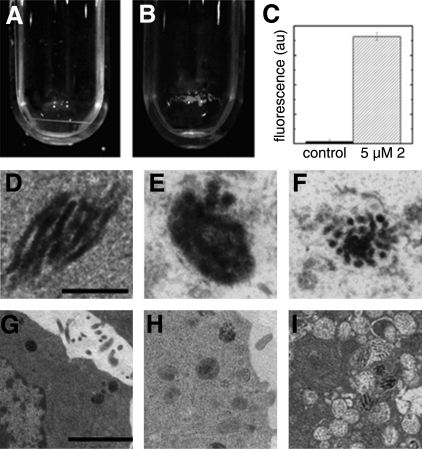
To further confirm the localization of these bacteriochlorins in melanosomes, pigmented B16F10 cells were preincubated with 5 μM bacteriochlorin 2 followed by isolation of melanosomes (41). Figure 4B shows the red fluorescence of bacteriochlorin 2 in the isolated fraction of melanosomes. Figure 4A shows the lack of fluorescence in the control, nontreated cells. Fluorescence quantification shows significant uptake of bacteriochlorin 2 by melanosomes (Fig. 4C).
Figure 4.
A, B) Fluorescence of bacteriochlorin 2 inside isolated melanosomes from pigmented B16F10 cells. A) Melanosomes from control cells. B) Melanosomes containing 5 μM bacteriochlorin 2. C) Fluorescence quantification of bacteriochlorin 2 in melanosomes. D–I) Transmission electron micrographs showing bacteriochlorin-mediated PDT destruction of melanosomes. D, G) Control, non-PDT-treated melanosome. E, H) Melanosomes 0.5 h after PDT. F, I) Melanosomes 1 h after PDT. Scale bars = 100 nm (D–F; ×15,500); 1 μm (G–I; ×1550).
Melanosome destruction by PDT using near-infrared light
The strong colocalization of the bacteriochlorins in melanosomes prompted investigation of whether these intracellular structures could be destroyed by bacteriochlorin PDT. Cells were harvested at different time points after PDT with bacteriochlorin 2, and some damage to melanosomes was observed as early as 0.5 h after PDT compared with the untreated melanosome (Fig. 4E, H). After 1 h of bacteriochlorin-mediated PDT, the complete disaggregation and destruction of melanosomal structures were observed (Fig. 4F, I).
Reactive oxygen species production
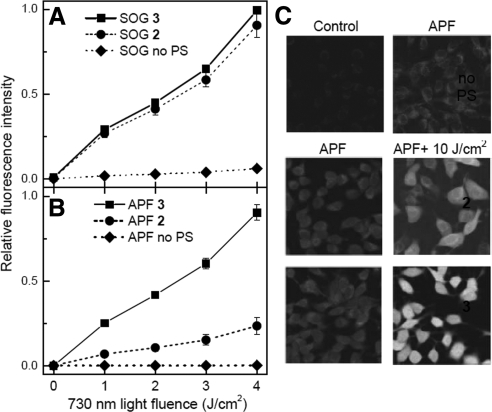
The ability of bacteriochlorins 2 and 3 to produce different reactive oxygen species was monitored using SOG and APF fluorescence probes in order to better understand the relative PDT effectiveness of the 2 compounds. A comparable light- and dose-dependent increase in SOG fluorescence is observed from the 2 bacteriochlorins, indicating similar ability to produce singlet oxygen (Fig. 5A). However, bacteriochlorin 3 shows a much greater increase of APF fluorescence in a light dose-dependent manner than bacteriochlorin 2, indicating that bacteriochlorin 3 is more effective in producing hydroxyl radicals (Fig. 5B).
Figure 5.
A, B) Light (730 nm) dose-dependent increase in fluorescence from 10 μM SOG (A) and APF (B) in solution with 1 μM bacteriochlorin 2 or 3 in 50% acetonitrile and PBS (v/v). C) Fluorescence micrographs of B16F10 cells incubated with 1 μM bacteriochlorin 2 or 3 and 10 μM APF. Cells were either illuminated or not with 10 J/cm2 of 730-nm laser light.
The above-mentioned solution-based studies prompted experiments to examine the production of hydroxyl radicals in a cellular environment. Figure 5C shows that intracellular APF fluorescence is markedly increased in intensity after illumination of B16F10 cells with 730-nm light when bacteriochlorin 3 is present, while cells containing bacteriochlorin 2 did not show as high an increase in APF fluorescence for illuminated vs. nonilluminated samples.
Bacteriochlorin-PDT on pigmented melanoma in mice
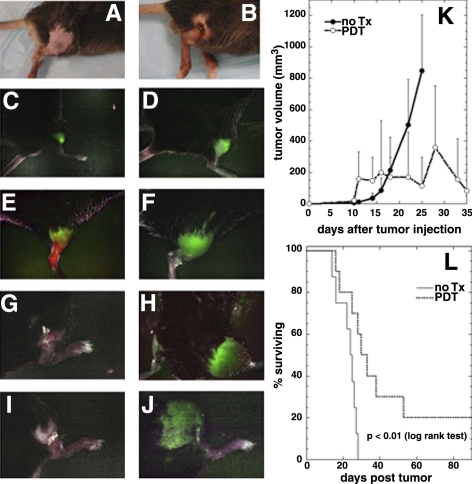
The effects of PDT treatment using near-infrared light were monitored using a Maestro in vivo fluorescence camera system and the B16F10 tumor model that expresses GFP protein. The results are shown in Fig. 6. Figure 6A shows the appearance of an untreated B16F10-GFP tumor, and Fig. 6B shows the appearance of a tumor 4 d after PDT mediated by bacteriochlorin 3. Note the typical black eschar replacing the tumor found after vascular PDT. Figure 6C–J shows a series of fluorescence images of B16F10-GFP tumor treated with PDT. The green signal of GFP from the melanoma is observed shortly after injection (Fig. 6C) and has increased (as the tumor has grown) by d 7 (Fig. 6D). Figure 6E shows a 2-color fluorescence image captured on d 9, 15 min after injection of bacteriochlorin 3; this image combines the near-infrared fluorescence signal from the bacteriochlorin 3 (false colored red) with the B16F10-GFP green signal. In PDT-treated tumors, the GFP signal gradually disappeared (Fig. 6G, d 13) compared with the untreated tumor at the same time point in Fig. 6F. The GFP signal decreased to undetectable levels on d 23 (Fig. 6I), while it steadily increased in nontreated control tumors (Fig. 6H, d 23). It is interesting to note how some of the GFP signal is quenched by black melanin building up in the control tumor and in the skin above it. Some of the PDT-treated tumors that initially had no detectable green fluorescence 3 d after PDT subsequently started to regrow on d 15 and reached a large size on d 26 that led to death of the mice. The signal from GFP also became partially quenched due to melanin buildup (Fig. 6J). Figure 6K shows the mean tumor volumes in both groups of mice over a period of 35 d. PDT produced a local response in B16F10-GFP-treated tumors, as manifested by an initial increase in size due to edema caused by the acute inflammation caused by PDT. Subsequently a marked reduction and stabilization in tumor size until d 25 were observed. However, local tumor regrowth occurred in 80% of treated mice leading to large error bars as mice with recurrent tumors were killed; however, the net result was a difference between the 2 growth curves with 20% of mice exhibiting complete cures. The Kaplan-Meier analysis of the nontreated mice (median survival=24 d) and PDT-treated mice (median survival=32 d) is shown in Fig. 6L. The survival curves were significantly different (P<0.01; log rank test).
Figure 6.
PDT effects of bacteriochlorin 3 on GFP-positive B16F10 melanoma tumors. A) B16F10-GFP subcutaneous tumor on d 7 before PDT treatment. B) B16F10-GFP tumor on d 13 after PDT treatment. C) In vivo fluorescence imaging of B16F10-GFP tumor immediately after inoculation. D) GFP signal from tumor on d 7 before PDT. E) Two-color imaging of bacteriochlorin 3 (red) and B16F10-GFP tumor (green) 15 min postintravenous injection on d 9. F) Control tumor on d 13. G) PDT-treated tumor on d 13. H) Control tumor on d 23. I) PDT-treated tumor on d 23. J) Regrowth of a PDT-treated tumor on d 26. K) Mean tumor volumes of control and PDT-treated B16F10-GFP melanoma tumors. L) Survival analysis of in vivo PDT effectiveness with bacteriochlorin 3 on B16F10-GFP tumors: solid line, no treatment (n=8); dotted line, PDT (n=10). P < 0.01 (log rank test).
DISCUSSION
This report has demonstrated that innovative synthetic stable bacteriochlorins, which absorb light in the near-infrared spectral region, are highly active photosensitizers against melanoma both in vitro and in vivo. There have been suggestions that melanoma is one of the most resistant types of cancer to PDT because photoactivating light is absorbed by intracellular melanin rather than by the photoactive photosensitizer that is localized within the melanoma cells.
The examined synthetic bacteriochlorins when combined with 730 nm light are significantly better at killing both pigmented and nonpigmented melanoma cells compared with the FDA-approved Photofrin and 635-nm light and another near-infrared-absorbing photosensitizer, LuTex. Moreover, the bacteriochlorins examined herein, particularly bacteriochlorins 2 and 3, are effective at significantly lower concentrations (bacteriochlorin<0.5 μM vs. Photofrin>6 μM), thereby reducing side effects.
Regarding the mechanism of PDT for the bacteriochlorins studied herein, in vitro experiments performed with fluorescent probes for singlet oxygen and for hydroxyl radical reveal no difference between bacteriochlorins 2 and 3 in singlet oxygen generation but that 3 produces significantly more hydroxyl radicals than 2. The finding of similar singlet oxygen production for bacteriochlorins 2 and 3 is consistent with the comparable photophysical characteristics (Supplemental Table S1). The finding that bacteriochlorin 3 produces more hydroxyl radicals than bacteriochlorin 2 is consistent with the expected difference in redox properties of the two compounds on the basis of the molecular-orbital calculations. In particular, bacteriochlorin 3 should be easier to reduce (and harder to oxidize) than bacteriochlorin 2 (and bacteriochlorin 1).
A type I mechanism that fits the latter results requires that the triplet excited state of the bacteriochlorin receives an electron from an endogenous substrate that acts as a reducing agent and subsequently passes the electron to O2 leading to formation of the superoxide radical ion and subsequently to hydroxyl radicals (or other reactive species). This mechanism, as opposed to the well-understood type II mechanism (which involves energy transfer from the excited bacteriochlorin triplet to ground state triplet oxygen 3O2 to form reactive singlet oxygen 1O2), is in keeping with prior work on a set of imidazole-substituted porphyrins (23, 28). This type of electron-transfer mechanism has been also proposed as operating during PDT with the palladium bacteriochlorin TOOKAD (42). These considerations suggest that the greater PDT efficacy of bacteriochlorin 3 compared with bacteriochlorin 2 (or bacteriochlorin 1) may derive from enhanced activity of 3 via a type I mechanism that generates hydroxyl radicals (and/or another reactive species), perhaps supplementing singlet oxygen formation via a type II mechanism.
The localization of the bacteriochlorins in melanosomes and subsequent destruction of the latter structures by PDT may have high clinical relevance. It has been reported that melanoma resistance to chemotherapy could be explained by the sequestration and subsequent exocytosis of the cytotoxic drug in melanosomes (43) and that this process also correlated with increased melanogenesis (44). It should be noted, however, that resistance to cytotoxic drugs has been attributed to lysosomes in human ovarian carcinoma cells and that this phenomenon could be explained by similarities between early melanosomes and lysosomes (45, 46).
The influence of the melanin on the effectiveness of PDT was studied here. It was found that the melanin content in various melanoma cells affected PDT effectiveness but the degree of reduction was significantly lower for bacteriochlorins than for Photofrin. In addition to melanin content, other factors that may also be involved in melanoma resistance to PDT include the overexpression of certain multidrug export pumps, such as the ATP-binding cassette transporters (ABCA9, ABCB5, ABCC2, and ABCD1) that are overexpressed in melanoma cells (44, 47). It has been recently reported that certain tetrapyrrole-based photosensitizers such as protoporphyrin IX and pyropheophorbide are substrates of ABCG2 (48). Another reason for melanoma resistance to PDT may be the known ability of melanin to act as an antioxidant and to quench reactive oxygen species. The mechanism of quenching of excited states of positively charged porphyrin molecules bound to melanin was recently determined by femtosecond absorption and picosecond emission spectroscopy (49). Recently, it has been also reported that the increased melanogenesis increases the resistance of melanoma cells to chemo- and immunotherapy. The inhibition of melanogenesis significantly increased the sensitivity of cells to cyclophosphamide treatment (50). Moreover, melanin significantly interfered with the effectiveness of radiation therapy. The inhibition of melanogenesis resulted in a significant enhancement of melanoma cell susceptibility to γ irradiation (51). These findings together with our recent report (52) that cyclophosphamide can potentiate PDT-induced response in nonmelanoma tumors provide strong motivation to seek potential solutions to these resistance mechanisms. Decreasing the melanin levels or application of the near-infrared absorbing photosensitizers presented in this study show considerable promise.
There have been reports of PDT applications for melanoma treatment in both animals and in humans. In animals, both nonpigmented amelanotic cell lines (53) and pigmented melanoma cell lines (54) have been used to grow tumors. Biolo et al. (55) used a liposome-delivered Si(IV)-naphthalocyanine (SiNc) that absorbs at 776 nm, while Woodburn et al. (56) used the lutetium texaphyrin (PCI-0123, LuTex) that absorbs at 732 nm to treat pigmented B16 melanomas in mice. Scherz’s group (15) used the palladium bacteriochlorin (WST11) to treat M2R melanoma xenografts in nude mice. Clinical studies (57) have been carried out in ocular melanomas. There have been a few reports of PDT being tested in cutaneous melanomas in human patients, including a study in which chlorin e6 was injected intravenously at 5 mg/kg and 660-nm light was delivered twice after 1 and 24 h (58).
None of the above-mentioned studies examined the influence of melanin levels on PDT effectiveness. To our knowledge the studies described here represent the first time that the PDT sensitivity of different melanoma cells lines has been shown to correlate with melanin content. Also, this is one of the few studies demonstrating that in vivo PDT of pigmented melanoma B16F10 tumors can lead to permanent cures.
CONCLUSIONS
The lack of development of PDT as a therapy for melanoma is thought to be due to a combination of the ineffectiveness of presently available photosensitizers and to the optical quenching of the activating light by the melanin pigment. The results presented herein show that there is high promise for future clinical application of synthetic bacteriochlorins in PDT of pigmented melanoma.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (R01GM36238 to J.S.L. and R01AI050875 to M.R.H.), a Burroughs-Wellcome fellowship (to M.K.), and the Jimmy V. NCSU Cancer Therapeutics Training Program. P.M. and T.B. were supported by a grant (R41AI072854) from the National Institute of Allergy and Infectious Diseases to NIRvana Pharmaceuticals. P.M. was partly supported by Genzyme-Partners Translational Research Grant. A.S. was supported by the Foundation for Polish Science, the International Union against Cancer, and the European Structural Fund: Mazowieckie Stypendium Doktoranckie. Characterization of the photophysical and redox properties of the bacteriochlorins described herein was initially motivated by solar-energy studies and supported by grants from the Division of Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences of the U.S. Department of Energy to D.F.B. (DE-FG02-05ER15660) and D.H. (DE-FG02-05ER15661). The authors thank Pharmacyclics (Sunnyvale, CA, USA) for the gift of LuTex and loan of 730-nm laser and QLT (Vancouver, BC, Canada) for the gift of Photofrin.
References
- National Comprehensive Cancer Network, Inc Fort Washington, PA, USA: National Comprehensive Cancer Network, Inc.; The NCCN Clinical Practice Guidelines in Oncology™ Melanoma (Version, V. I. 2010) 2009 [Google Scholar]
- Kirkwood J M, Strawderman M H, Ernstoff M S, Smith T J, Borden E C, Blum R H. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- Cummins D L, Cummins J M, Pantle H, Silverman M A, Leonard A L, Chanmugam A. Cutaneous malignant melanoma. Mayo Clin Proc. 2006;81:500–507. doi: 10.4065/81.4.500. [DOI] [PubMed] [Google Scholar]
- Naylor M F, Chen W R, Teague T K, Perry L A, Nordquist R E. In situ photoimmunotherapy: a tumour-directed treatment for melanoma. Br J Dermatol. 2006;155:1287–1292. doi: 10.1111/j.1365-2133.2006.07514.x. [DOI] [PubMed] [Google Scholar]
- Castano A P, Demidova T N, Hamblin M R. Mechanisms in photodynamic therapy: part one–photosensitizers, photochemistry and cellular localization. Photodiagn Photodyn Ther. 2004;1:279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Paus R, Schadendorf D. Melanocytes as “sensory” and regulatory cells in the epidermis. J Theor Biol. 1993;164:103–120. doi: 10.1006/jtbi.1993.1142. [DOI] [PubMed] [Google Scholar]
- Slominski A, Tobin D J, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Carlson A J, Matsuoka L Y, Balch C M, Mihm M C. Malignant melanoma. Arch Pathol Lab Med. 2001;125:1295–1306. doi: 10.5858/2001-125-1295-MM. [DOI] [PubMed] [Google Scholar]
- Riley P A. Melanogenesis and melanoma. Pigment Cell Res. 2003;16:548–552. doi: 10.1034/j.1600-0749.2003.00069.x. [DOI] [PubMed] [Google Scholar]
- Simon J D, Peles D, Wakamatsu K, Ito S. Current challenges in understanding melanogenesis: bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 2009;22:563–579. doi: 10.1111/j.1755-148X.2009.00610.x. [DOI] [PubMed] [Google Scholar]
- Carlson J A, Ross J S, Slominski A, Linette G, Mysliborski J, Hill J, Mihm M., Jr Molecular diagnostics in melanoma. J Am Acad Dermatol. 2005;52:743–775; quiz 775–748. doi: 10.1016/j.jaad.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Suzuki I, Cone R D, Im S, Nordlund J, Abdel-Malek Z A. Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis. Endocrinology. 1996;137:1627–1633. doi: 10.1210/endo.137.5.8612494. [DOI] [PubMed] [Google Scholar]
- Lapouge K, Naveke A, Gall A, Ivancich A, Seguin J, Scheer H, Sturgis J N, Mattioli T A, Robert B. Conformation of bacteriochlorophyll molecules in photosynthetic proteins from purple bacteria. Biochemistry. 1999;38:11115–11121. doi: 10.1021/bi990723z. [DOI] [PubMed] [Google Scholar]
- Trachtenberg J, Weersink R A, Davidson S R, Haider M A, Bogaards A, Gertner M R, Evans A, Scherz A, Savard J, Chin J L, Wilson B C, Elhilali M. Vascular-targeted photodynamic therapy (padoporfin, WST09) for recurrent prostate cancer after failure of external beam radiotherapy: a study of escalating light doses. BJU Int. 2008;102:556–562. doi: 10.1111/j.1464-410X.2008.07753.x. [DOI] [PubMed] [Google Scholar]
- Mazor O, Brandis A, Plaks V, Neumark E, Rosenbach-Belkin V, Salomon Y, Scherz A. WST11, a novel water-soluble bacteriochlorophyll derivative; cellular uptake, pharmacokinetics, biodistribution and vascular-targeted photodynamic activity using melanoma tumors as a model. Photochem Photobiol. 2005;81:342–351. doi: 10.1562/2004-06-14-RA-199. [DOI] [PubMed] [Google Scholar]
- Kim H J, Lindsey J S. De novo synthesis of stable tetrahydroporphyrinic macrocycles: bacteriochlorins and a tetradehydrocorrin. J Org Chem. 2005;70:5475–5486. doi: 10.1021/jo050467y. [DOI] [PubMed] [Google Scholar]
- Yeung P F. Motexafin lutetium (Pharmacyclics) IDrugs. 2001;4:351–359. [PubMed] [Google Scholar]
- Mroz P, Huang Y-Y, Janjua S, Zhiyentayev T, Ruzié C, Borbas K, Fan E D, Krayer M, Balasubramanian T, Kang E, Kee K H, Holten L D, Lindsey S J, Hamblin M R. New stable synthetic bacteriochlorins for photodynamic therapy of melanoma. Proc SPIE. 2009;7380:73802S. [Google Scholar]
- Castano A P, Mroz P, Hamblin M R. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6:535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D, Taniguchi M, Lindsey J S. Regioselective 15-bromination and functionalization of a stable synthetic bacteriochlorin. J Org Chem. 2007;72:5350–5357. doi: 10.1021/jo070785s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbas K E, Ruzie C, Lindsey J S. Swallowtail bacteriochlorins. Lipophilic absorbers for the near-infrared. Org Lett. 2008;10:1931–1934. doi: 10.1021/ol800436u. [DOI] [PubMed] [Google Scholar]
- Ruzié C, Krayer M, Balasubramanian T, Lindsey J S. Tailoring a bacteriochlorin building block with cationic, amphipathic, or lipophilic substituents. J Org Chem. 2008;73:5806–5820. doi: 10.1021/jo800736c. [DOI] [PubMed] [Google Scholar]
- Kee H L, Bhaumik J, Diers J R, Mroz P, Hamblin M R, Bocian D F, Lindsey J S, Holten D. Photophysical characterization of imidazolium-substituted Pd(II), In(III), and Zn(II) porphyrins as photosensitizers for photodynamic therapy. J Photochem Photobiol A. 2008;200:346–355. doi: 10.1016/j.jphotochem.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Cramer D L, Bhise A D, Kee H L, Bocian D F, Holten D, Lindsey J S. Accessing the near-infrared spectral region with stable, synthetic, wavelength-tunable bacteriochlorins. New J Chem. 2008;32:947–958. [Google Scholar]
- Weber G, Teale F W J. Determination of the absolute quantum yield of fluorescent solutions. Trans Faraday Soc. 1957;53:646–655. [Google Scholar]
- Gradyushko A T, Sevchenko A N, Solovyov K N, Tsvirko M P. Energetics of photophysical processes in chlorophyll-like molecules. Photochem Photobiol. 1970;11:387–400. doi: 10.1111/j.1751-1097.1970.tb06011.x. [DOI] [PubMed] [Google Scholar]
- Shao Y, Molnar L F, Jung Y, Kussmann J, Ochsenfeld C, Brown S T, Gilbert A T B, Slipchenko L V, Levchenko S V, O'Neill D P, DiStasio R A, Jr, Lochan R C, Wang T, Beran G J O, Besley N A, Herbert J M, Lin C Y, Van Voorhis T, Chien S H, Sodt A, Steele R P, Rassolov V A, Maslen P E, Korambath P P, Adamson R D, Austin B, Baker J, Byrd E F C, Dachsel H, Doerksen R J, Dreuw A, Dunietz B D, Dutoi A D, Furlani T R, Gwaltney S R, Heyden A, Hirata S, Hsu C-P, Kedziora G, Khalliulin R Z, Klunzinger P, Lee A M, Lee M S, Liang W Z, Lotan I, Nair N, Peters B, Proynov E I, Pieniazek P A, Rhee Y M, Ritchie J, Rosta E, Sherrill C D, Simmonett A C, Subotnik J E, Woodcock H L, III, Zhang W, Bell A T, Chakraborty A K, Chipman D M, Keil F J, Warshel A, Hehre W J, Schaefer H F, Kong J, Krylov A I, Gill P M W, Head-Gordon M. Advances in methods and algorithms in a modern quantum chemistry program package. Phys Chem Chem Phys. 2006;8:3172–3191. doi: 10.1039/b517914a. [DOI] [PubMed] [Google Scholar]
- Mroz P, Bhaumik J, Dogutan D K, Aly Z, Kamal Z, Khalid L, Kee H L, Bocian D F, Holten D, Lindsey J S, Hamblin M R. Imidazole metalloporphyrins as photosensitizers for photodynamic therapy: role of molecular charge, central metal and hydroxyl radical production. Cancer Lett. 2009;282:63–76. doi: 10.1016/j.canlet.2009.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlow S J, Boissy R E, Moran D J, Pifko-Hirst S. Subcellular distribution of tyrosinase and tyrosinase-related protein-1: implications for melanosomal biogenesis. J Invest Dermatol. 1993;100:55–64. doi: 10.1111/1523-1747.ep12354138. [DOI] [PubMed] [Google Scholar]
- Sapan C V, Lundblad R L, Price N C. Colorimetric protein assay techniques. Biotechnol Appl Biochem. 1999;29:99–108. [PubMed] [Google Scholar]
- Price M, Reiners J J, Santiago A M, Kessel D. Monitoring singlet oxygen and hydroxyl radical formation with fluorescent probes during photodynamic therapy. Photochem Photobiol. 2009;85:1177–1181. doi: 10.1111/j.1751-1097.2009.00555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setsukinai K, Urano Y, Kakinuma K, Majima H J, Nagano T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J Biol Chem. 2003;278:3170–3175. doi: 10.1074/jbc.M209264200. [DOI] [PubMed] [Google Scholar]
- Su J, Zhang J, Liu L, Huang Y, Mason R P. Exploring feasibility of multicolored CdTe quantum dots for in vitro and in vivo fluorescent imaging. J Nanosci Nanotechnol. 2008;8:1174–1177. [PubMed] [Google Scholar]
- Chang S K, Rizvi I, Solban N, Hasan T. In vivo optical molecular imaging of vascular endothelial growth factor for monitoring cancer treatment. Clin Cancer Res. 2008;14:4146–4153. doi: 10.1158/1078-0432.CCR-07-4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holten D, Gouterman M, Parson W W, Windsor M W, Rockley M G. Electron transfer from photoexcited singlet and triplet bacteriopheophytin. Photochem Photobiol. 1976;23:415–420. doi: 10.1111/j.1751-1097.1976.tb07275.x. [DOI] [PubMed] [Google Scholar]
- Kee H L, Kirmaier C, Tang Q, Diers J R, Muthiah C, Taniguchi M, Laha J K, Ptaszek M, Lindsey J S, Bocian D F, Holten D. Effects of substituents on synthetic analogs of chlorophylls. Part 2: Redox properties, optical spectra and electronic structure. Photochem Photobiol. 2007;83:1125–1143. doi: 10.1111/j.1751-1097.2007.00151.x. [DOI] [PubMed] [Google Scholar]
- Solca F F, Chluba-de Tapia J, Iwata K, Eberle A N. B16–G4F mouse melanoma cells: an MSH receptor-deficient cell clone. FEBS Lett. 1993;322:177–180. doi: 10.1016/0014-5793(93)81563-f. [DOI] [PubMed] [Google Scholar]
- Nakayasu M, Saeki H, Tohda H, Oikawa A. Effects of sugars on melanogenesis in cultured melanoma cells. J Cell Physiol. 1977;92:49–55. doi: 10.1002/jcp.1040920107. [DOI] [PubMed] [Google Scholar]
- Orlow S J. Melanosomes are specialized members of the lysosomal lineage of organelles. J Invest Dermatol. 1995;105:3–7. doi: 10.1111/1523-1747.ep12312291. [DOI] [PubMed] [Google Scholar]
- Kuroda T S, Itoh T, Fukuda M. Functional analysis of slac2-a/melanophilin as a linker protein between Rab27A and myosin Va in melanosome transport. Methods Enzymol. 2005;403:419–431. doi: 10.1016/S0076-6879(05)03037-5. [DOI] [PubMed] [Google Scholar]
- Hong L, Garguilo J, Anzaldi L, Edwards G S, Nemanich R J, Simon J D. Age-dependent photoionization thresholds of melanosomes and lipofuscin isolated from human retinal pigment epithelium cells. Photochem Photobiol. 2006;82:1475–1481. doi: 10.1562/2006-03-14-RA-846. [DOI] [PubMed] [Google Scholar]
- Ashur I, Goldschmidt R, Pinkas I, Salomon Y, Szewczyk G, Sarna T, Scherz A. Photocatalytic generation of oxygen radicals by the water-soluble bacteriochlorophyll derivative WST11, noncovalently bound to serum albumin. J Phys Chem A. 2009;113:8027–8037. doi: 10.1021/jp900580e. [DOI] [PubMed] [Google Scholar]
- Chen K G, Valencia J C, Lai B, Zhang G, Paterson J K, Rouzaud F, Berens W, Wincovitch S M, Garfield S H, Leapman R D, Hearing V J, Gottesman M M. Melanosomal sequestration of cytotoxic drugs contributes to the intractability of malignant melanomas. Proc Natl Acad Sci U S A. 2006;103:9903–9907. doi: 10.1073/pnas.0600213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K G, Valencia J C, Gillet J P, Hearing V J, Gottesman M M. Involvement of ABC transporters in melanogenesis and the development of multidrug resistance of melanoma. Pigment Cell Melanoma Res. 2009;22:740–749. doi: 10.1111/j.1755-148X.2009.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaei R, Larson B J, Cheng T C, Gibson M A, Otani S, Naerdemann W, Howell S B. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther. 2005;4:1595–1604. doi: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- Safaei R, Katano K, Larson B J, Samimi G, Holzer A K, Naerdemann W, Tomioka M, Goodman M, Howell S B. Intracellular localization and trafficking of fluorescein-labeled cisplatin in human ovarian carcinoma cells. Clin Cancer Res. 2005;11:756–767. [PubMed] [Google Scholar]
- Szakacs G, Gottesman M M. Comparing solid tumors with cell lines: implications for identifying drug resistance genes in cancer. Mol Interv. 2004;4:323–325. doi: 10.1124/mi.4.6.5. [DOI] [PubMed] [Google Scholar]
- Liu W, Baer M R, Bowman M J, Pera P, Zheng X, Morgan J, Pandey R A, Oseroff A R. The tyrosine kinase inhibitor imatinib mesylate enhances the efficacy of photodynamic therapy by inhibiting ABCG2. Clin Cancer Res. 2007;13:2463–2470. doi: 10.1158/1078-0432.CCR-06-1599. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hong L, Wakamatsu K, Ito S, Adhyaru B, Cheng C Y, Bowers C R, Simon J D. Comparison of structural and chemical properties of black and red human hair melanosomes. Photochem Photobiol. 2005;81:135–144. doi: 10.1562/2004-08-03-RA-259.1. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Slominski R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int J Cancer. 2009;124:1470–1477. doi: 10.1002/ijc.24005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozyna A A, VanMiddlesworth L, Slominski A T. Inhibition of melanogenesis as a radiation sensitizer for melanoma therapy. Int J Cancer. 2008;123:1448–1456. doi: 10.1002/ijc.23664. [DOI] [PubMed] [Google Scholar]
- Castano A P, Mroz P, Wu M X, Hamblin M R. Photodynamic therapy plus low-dose cyclophosphamide generates antitumor immunity in a mouse model. Proc Natl Acad Sci U S A. 2008;105:5495–5500. doi: 10.1073/pnas.0709256105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellian M, Richert C, Gamarra F, Goetz A E. Photodynamic eradication of amelanotic melanoma of the hamster with fast acting photosensitizers. Int J Cancer. 1996;65:246–248. doi: 10.1002/(SICI)1097-0215(19960117)65:2<246::AID-IJC19>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Busetti A, Soncin M, Jori G, Rodgers M A. High efficiency of benzoporphyrin derivative in the photodynamic therapy of pigmented malignant melanoma. Br J Cancer. 1999;79:821–824. doi: 10.1038/sj.bjc.6690131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biolo R, Jori G, Soncin M, Rihter B, Kenney M E, Rodgers M A. Effect of photosensitizer delivery system and irradiation parameters on the efficiency of photodynamic therapy of B16 pigmented melanoma in mice. Photochem Photobiol. 1996;63:224–228. doi: 10.1111/j.1751-1097.1996.tb03018.x. [DOI] [PubMed] [Google Scholar]
- Woodburn K W, Fan Q, Kessel D, Luo Y, Young S W. Photodynamic therapy of B16F10 murine melanoma with lutetium texaphyrin. J Invest Dermatol. 1998;110:746–751. doi: 10.1046/j.1523-1747.1998.00182.x. [DOI] [PubMed] [Google Scholar]
- Mennel S, Barbazetto I, Meyer C H, Peter S, Stur M. Ocular photodynamic therapy–standard applications and new indications (part 1). Review of the literature and personal experience. Ophthalmologica. 2007;221:216–226. doi: 10.1159/000101922. [DOI] [PubMed] [Google Scholar]
- Sheleg S V, Zhavrid E A, Khodina T V, Kochubeev G A, Istomin Y P, Chalov V N, Zhuravkin I N. Photodynamic therapy with chlorin e(6) for skin metastases of melanoma. Photodermatol Photoimmunol Photomed. 2004;20:21–26. doi: 10.1111/j.1600-0781.2004.00078.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.