Abstract
Membrane-permeabilizing peptide antibiotics are an underutilized weapon in the battle against drug-resistant microorganisms. This is true, in part, because of the bottleneck caused by the lack of explicit design principles and the paucity of simple high-throughput methods for selection. In this work, we characterize the requirements for broad-spectrum antimicrobial activity by membrane permeabilization and find that different microbial membranes have very different susceptibilities to permeabilization by individual antimicrobial peptides. Broad-spectrum activity requires only that an AMP have at least a small amount of membrane-permeabilizing activity against multiple classes of microbes, a feature that we show to be rare in a peptide library containing many members with species-specific activity. We compare biological and vesicle-based high-throughput strategies for selecting such broad-spectrum AMPs from combinatorial peptide libraries and demonstrate that a simple in vitro, lipid vesicle-based high-throughput screen is the most effective strategy for rapid discovery of novel, broad-spectrum antimicrobial peptides.—Rathinakumar, R., Wimley, W. C. High-throughput discovery of broad- spectrum peptide antibiotics.
Keywords: antimicrobial peptide, combinatorial chemistry, high-throughput screening, membrane permeabilization
In recent decades, morbidity and mortality associated with drug-resistant microbes has risen alarmingly, currently accounting for hundreds of thousands of hospitalizations and tens of thousands of deaths annually in the United States (1, 2) and many more worldwide (3). Recognizing that new approaches to antibiotics are desperately needed, researchers have noted that broad-spectrum, membrane-permeabilizing antimicrobial peptides (AMP) represent a possible new therapy against drug-resistant microbes (4,5,6,7,8,9). However, progress has been slowed by the fact that the fundamental principles of AMP action are not readily understood in classical structure-function terms (10,11,12,13,14), and thus AMPs are difficult to design de novo or to engineer. Here, we explore the principles of broad-spectrum antimicrobial peptide activity and demonstrate a high-throughput approach for identifying candidate peptides.
Antimicrobial peptides are a critical component of the innate immune system of many species, including humans (9, 15). While having extraordinarily diverse structures, they share a composition rich in cationic and hydrophobic amino acids that make them well suited for interacting with microbial cytoplasmic membranes, which typically present an anionic surface to the environment. When microbes are exposed to AMPs, the electrochemical potential of their cytoplasmic membranes can be dissipated in seconds (12) followed by permeation of larger molecules, including dye markers, metabolites and cytosolic proteins, occurring within 1–30 min (12, 16). These events are frequently followed by total destruction of cell morphology (17). Biomembrane permeabilization is often recapitulated in model systems such as lipid bilayer vesicles. However, the correlation between the specific biological activities of AMPs and their model membrane-permeabilizing activity is imperfect. For example, some potent AMPs do not readily permeabilize lipid vesicles or require extremely high concentrations to do so. We have shown that small structural perturbations in AMPs can decouple vesicle-permeabilizing activity from broad-spectrum microbe killing activity (12). Furthermore, some AMPs can readily enter bacterial cytoplasm (18) and kill microbes without extensive biomembrane permeabilization (12) causing some researchers to propose that some AMPs are cell-penetrating peptides with intracellular targets (17, 18). Yet, despite these uncertainties, membrane permeabilization remains the most widely accepted mechanism to explain AMP bioactivity.
To examine the relationships between the biological activity of AMPs and their permeabilization of synthetic and biological membranes, we have used combinatorial chemistry and high-throughput screening (12, 13, 16, 19) together with in vitro and in vivo cross-characterization of selected peptides (12, 16). Here, we focus on a 16,384 member rational library of 9–15 residue peptides, which was designed to contain bioactive AMPs based on their interfacial activity (12). In an in vitro high-throughput screen of 20,000 library members, we previously selected 10 very active peptides based solely on their ability to permeabilize synthetic lipid bilayer vesicles (13) containing 90% zwitterionic phosphatidylcholine and 10% anionic phosphatidylglycerol lipids, a lipid composition that gives the membranes an anionic surface charge like most microbes. In bioactivity assays, the 10 selected peptides sterilize microbe cultures at low micromolar concentrations, including multiple species of gram-positive and gram-negative bacteria, and multiple species of fungi (12). At the same time, the active peptides have only moderate hemolytic or cytotoxic effects on eukaryotic cells (12). Peptides with these properties are potential candidates for more focused testing, formulation, and possible development into antibiotics. Earlier, we used the same vesicle-based screen to select active peptides from a different 26-amino acid combinatorial library. The longer peptides selected from that library also had the same degree of broad-spectrum antimicrobial activity and moderate toxicity as the 9–15 residue peptides described here (16, 19). These results support the idea that this simple in vitro screen specifically selects for peptides that have these highly desirable traits, which are essential for the first stages of peptide antibiotic drug design.
In this work, we use the 9–15 residue library described above and the peptides previously selected from it. First, we explore the nature of broad-spectrum antimicrobial peptide activity using, in parallel, lipid vesicles and living microbes. Second, we compare three high-throughput strategies for selecting broad-spectrum antimicrobial peptides from combinatorial libraries and show that a simple, lipid vesicle-based screen is the most efficient. These findings provide a powerful new tool to circumvent a long-standing barrier to the rapid discovery of novel, broad-spectrum peptide antibiotics.
MATERIALS AND METHODS
Sytox Green membrane permeabilization
Measurements were made as described elsewhere (12, 16). Briefly, 5 μM Sytox Green dye was added to a cell suspension (2×107 bacteria/ml or 5×105 fungus or mammalian cells/ml in PBS) and incubated for 5 min to establish a baseline fluorescence intensity (excitation 485 nm, emission 520 nm). At 5 min, aliquots of peptide in 0.025% acetic were added, and the solution was stirred while fluorescence was monitored. Negative control was buffer only, and positive control was 5 μM of the lytic bee venom peptide melittin. Membrane permeabilization is expressed as a percentage between controls at 40 min.
Liposome permeabilization
Large unilamellar liposomes with entrapped terbium III and external dipicolinic acid (DPA; pyridine-2,6-dicarboxylic acid) were prepared as described previously (13, 19, 20). Vesicles and peptides were mixed, and fluorescence of the terbium/DPA complex was monitored. After 40 min, the intensity was stable because leakage had stopped. Detergent (Triton-X-100) was added to lyse the vesicles and establish the intensity for 100% leakage. Leakage is expressed as the percentage of total at 40 min.
Multiorganism screen
Escherichia coli, Staphylococcus aureus, and Cryptococcus neoformans were grown to midlogorithmic phase and diluted to 103 colony forming units (CFU)/ml with minimal liquid test medium (LTM; 1% of growth broth in PBS). Single library members (∼1 nmol) were synthesized on polystyrene beads using a photolabile linker (12, 19). The linker was photocleaved and the peptides extracted into 10-μl dimethyl sulfoxide in the wells of a 96-well plate. One-third of each peptide solution was transferred into each of three additional 96-well “replica” plates, and buffer was added to bring the peptides into an aqueous solution. Diluted bacterial or fungi cells were added to each of the three plates separately and preincubated at 37°C for 3 h. During this incubation, the wells contain 2.2 μM peptide. Afterward, an equal volume of 2× growth medium was added, and the cultures were allowed to recover at 37°C overnight (for bacteria) or for 2 days (for the fungus). Peptide concentration was 1.1 μM during the recovery phase of the experiment. After recovery, wells were either opaque (OD600 nm>0.5), indicating stationary phase growth, or transparent (OD600 nm<0.02), indicating no growth. Intermediate optical densities were almost never observed. As a negative control, plates with or without nonphotocleaved peptide beads (which will contain nonreleasable peptide) were treated as above. All control plates always contained stationary phase growth of microbes.
Stringent biological screen
E. coli was grown to midlogorithmic phase and diluted to 106 CFU/ml. Single library peptide members were extracted as above into 10 μl dimethyl sulfoxide in the wells of a 96-well plate. Some of the peptide solution was transferred to another plate and diluted with buffer. An E. coli cell suspension (1×106 cells/ml) was added, and the plates were preincubated at 37 C for 3 h. After the initial incubation 2× growth medium was added, and the cultures were allowed to recover overnight. After recovery, wells were either opaque (OD600 nm>0.5), indicating stationary phase growth, or transparent (OD600 nm<0.02), indicating no growth. Intermediate optical densities were almost never observed. Using 2.2 μM peptide and 106 cells/ml in the preincubation phase gave the desired stringency. Three active peptides were observed out of 960 tested. Identical experiments were conducted at these stringencies with S. aureus and Candida albicans, a fungus, but none the tested peptides (n=960) had sterilizing activity for those organisms.
Antimicrobial activity
E. coli, Pseudomonas aeruginosa, S. aureus, C. neoformans, and C. albicans were grown to midlogorithmic phase and diluted to 103 CFU/ml. In sterile 96-well plates, 8 columns were prepared for each peptide in 2/3-fold serial dilution starting at 10 μM. To each well, a cell suspension in minimal medium was added, wells were preincubated at 37°C for up to 3 h, and then 2× concentrated growth medium was added. After overnight recovery at 37°C, wells were either opaque (OD600>0.5), indicating stationary phase growth or they were transparent (OD600<0.02), indicating no growth. Intermediate optical densities were rarely observed. For each experiment, there were a number of sterile wells starting from the highest concentration. The lowest concentration of peptide that sterilized the cells was the minimum sterilizing concentration (MSC). All MSC measurements are the average of 3–5 independent experiments. The addition of rich growth medium to cells first, followed by peptide, gave very similar MSC values. Also, the density of cells used, up to 105 cell/ml, had little effect on the measured MSC values. See Supplemental Table 1 for all measured MSC values.
Statistical analysis
For the selected peptide compositions, binomial probability distributions of the expected number of observations of each residue type or cassette were calculated from the compositions of the peptide libraries and the number of peptides selected. The binomial equation was used to calculate probability distributions from known abundance of each amino acid in the library and the number of peptides selected. Two-sided P values for the observed number of occurrences were calculated by integrating the binomial distribution over all values at least as far from the expected number as the observed number of occurrences.
RESULTS AND DISCUSSION
As described elsewhere (12), the peptide library used in this work has the form {RRG}WOLOLOLOY{GRR}-amide where the terminal basic cassettes are randomly present or absent, and the O residues can be one of the following 8 amino acids: NDTRGAVY. The W, L, and C-terminal Y residues are fixed. The simplified peptide notation used here uses an asterisk for the terminal basic cassette, when present, and identifies only the 4 O residues directly. For example, the peptide RRGWRLVLALAY-amide is referred to as *RVAA.
To assess the requirements and the mechanism of broad-spectrum peptide antibiotic activity, we first characterized in parallel the permeabilization of lipid vesicles and microbial membranes by the peptides previously selected in the vesicle-based screen (VBS) (12). We then compared the activities and mechanism of action of peptides selected by the vesicle-based screen with peptides selected from the same library using two direct biological screens. The two biological screens are a multiorganism screen (MOS), in which library members were screened in parallel against a gram-positive bacteria, a gram-negative bacteria and a fungus; and a stringent biological screen (SBS) in which peptides were screened against a very high concentration of E. coli cells (106 cells/ml) to directly identify the most potent antibacterial peptides in the library.
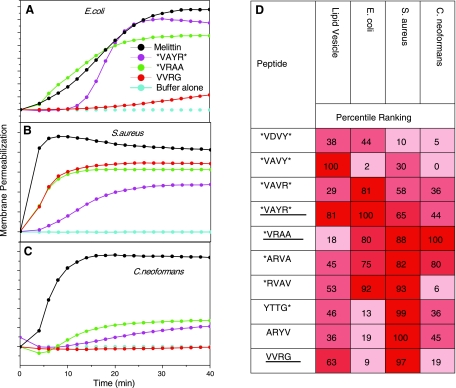
As we have shown in previous work (12), all 10 of the 9–15 residue peptides selected using the vesicle-based screen have sterilizing antimicrobial activity at low micromolar concentration against all the organisms tested. See Supplemental Table 1 for a comprehensive data set on the biological activity of the selected peptides. Most of the peptides also cause leakage through microbial membranes over 5–20 min (Fig. 1A). While most of the 10 peptides have detectible membrane-permeabilizing activity against most microorganisms and against lipid vesicles (13), there is a surprising lack of correlation (Fig. 1B). For example, the 9-residue peptide, which we refer to as VVRG, is very potent toward S. aureus membranes, but it has little activity against E. coli membranes (Fig. 1A, B), while the 12-residue peptide *RVAV has good permeabilizing activity against the two bacteria but barely detectible membrane activity against the fungus in the 40 min after peptide addition (Fig. 1D). The rank orders of membrane-permeabilizing activity of the 10 selected peptides (Fig. 1D) are essentially uncorrelated and are also uncorrelated with the rank order of their antimicrobial potency. An important finding in this experiment is that potent microbicidal activity does not require potent biomembrane-permeabilizing activity. We hypothesize that the broad-spectrum activity of these peptides, and perhaps of most broad-spectrum AMPs, results from a peptide having at least some membrane-permeabilizing interfacial activity (12) against all microbial membranes. We speculate that these AMPs may be translocating across microbial membranes and acting intracellularly. Interestingly, the same phenomenon appears not to be true for mammalian cells: while many of these peptides permeabilize mammalian cells measurably, their toxicity against mammalian cells is low. See Supplemental Table 1.
Figure 1.
Cross characterization of the membrane-permeabilizing activity of vesicle-selected peptides. A–C) Examples of fluorescence of the membrane-impermeant, DNA-binding dye Sytox Green as a measure of biomembrane permeabilization in E. coli (A), S. aureus (B), and C. neoformans (C). Peptides are added at 5 μM to cells, and the fluorescence of Sytox Green is monitored for 40 min. Control for complete permeabilization is 5 μM melittin, a membrane-permeabilizing peptide from bee venom. Experiments for three example peptides in the three different organisms are shown. All permeabilization data are shown in Supplemental Table 1. D) Percentile-ranked permeabilization of vesicles at peptide:lipid ratio of 1:50 or microbes at 5 μM peptide and 107 bacteria/ml or 105 fungi/ml. Permeabilization is measured at 40 min, by which time the leakage had essentially ceased for vesicles and bacteria. Fungal permeabilization was linear and much slower (12). Cells in the table are colored by quartiles for visualization. Underlined peptide names are the example peptides in A–C.
A second key finding is that different microbial membranes have very different susceptibilities to permeabilization by individual peptides, suggesting that broad-spectrum activity requires a specific balance of interfacial activities. Thus, the ability to permeabilize one microbial membrane does not ensure broad-spectrum activity. This point is further supported by the experiments described next. Finally, these results also suggest that lipid bilayer membranes composed of 90% phosphatidylcholine and 10% phosphatidylglycerol, as used in the vesicle-based screen (13, 19), while not matching the actual lipid composition of any particular microbial membrane, effectively represents a “consensus” anionic microbial membrane that can be used in high-throughput screening, engineering, and characterization of AMPs.
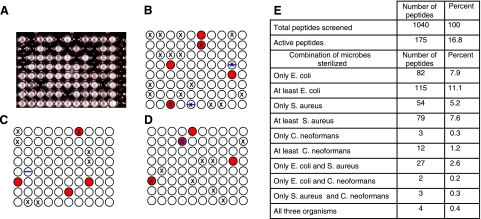
To further explore the nature of broad-spectrum peptide antibiotic activity, we conducted the multiorganism screen on a sample of the library (Fig. 2). Screening against individual species (e.g., Fig. 2A–D) showed that antibacterial activity in the peptide library is at least 200-fold more common than suggested by the vesicle-based screen. At 2.2 μM peptide, ∼17% of the library members have sterilizing antimicrobial activity against ≥1 class of microorganism (Fig. 2E), compared to ∼0.1% of the library that was highly active in the liposome-based in vitro screen (13). While biological activity in the library is surprisingly common, overlapping activity against multiple organisms is rare. For example, 11% of the peptides have activity against E. coli and 8% have activity against S. aureus, but only 3% have activity against both, a number that is not much larger than the expected random overlap of ∼1%. Examination of the data in Fig. 2E reveals mostly weak deviations from purely random overlap among peptides that are active against the multiple species of microorganisms. The strongest correlation was between anti-Cryptococcus activity and antibacterial activity: of the 12 peptides (1.2%) with antifungal activity, 5 also had activity against one of the bacteria, and another 4 had activity against both bacteria as well as the fungus. Of the 1040 sequences tested, 175 microbicidal peptides were observed. Eighty percent of these, or 139 peptides, are active against only a single organism. Only 4 peptides, or 0.4% of those tested, had activity against all three organisms. Thus while species-specific activity is common, the abundance of broad-spectrum peptides by the multiorganism screen (0.4%) is similar to the vesicle-based screen abundance of ∼0.1%. The sequences of three broad-spectrum peptides from this screen are shown in Fig. 3A.
Figure 2.
Multiorganism screen for antimicrobial peptides. As described elsewhere (13, 19), single library members were dissolved in the wells of a 96-well plate, the contents of which were then aliquoted into 3 additional 96-well replica plates. Buffer was added, followed by cells, to give 2.2 μM peptide in each well. After incubation, growth medium was added, and plates were incubated at 37°C overnight (for bacteria) or for 2 days for fungus. A) Representative E. coli plate after overnight incubation. Clear wells were sterilized, while opaque wells have stationary phase growth of microbes. Wells of the 11th column contained all of the components but no library peptides, while the 12th column contained buffer and growth medium but no cells. B–D) Schematic overlay representations of actual 3-plate sets. X indicates that E. coli was sterilized; red circle indicates that S. aureus was sterilized; blue bar indicates that the fungus C. neoformans was sterilized. E) Statistics for the multiorganism screen. Active peptides sterilized at least one microbe. Beads from the 4 broad-spectrum peptides were submitted for sequencing by Edman sequencing. Because of a malfunction, one was lost; thus, 3 complete sequences were obtained.
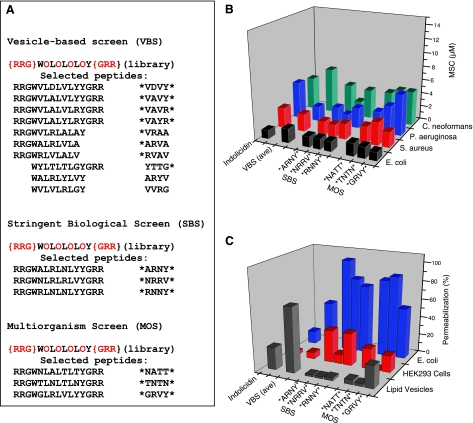
Figure 3.
Biological activity of selected peptides. A) Sequences of all selected peptides, and the library from which they were selected. Our shorthand peptide notation uses an asterisk for the terminal basic cassette and identifies only the 4 varied O residues directly (see text). The 10 vesicle-selected sequences are described elsewhere (12, 13). Three sequences from each of the biological assays were determined and are shown. B) Minimum sterilizing concentration of all peptides against two gram-negative bacteria, E. coli and P. aeruginosa, a gram-positive bacteria, S. aureus, and a fungus, C. neoformans. Indolicidin is a natural bovine neutrophil AMP (22). Data for the 10 vesicle-selected peptides are given as an average (se<0.5) from our published data (12). Individual values are given in Supplemental Table 1. Minimum sterilizing concentrations of peptides were measured in ≥3 independent experiments for each peptide/organism. ses are ±0.5–2 μM. C) Membrane permeabilization assays on lipid vesicles, human cells, and bacteria, as described elsewhere (12, 16). All measurements were repeated ≥3 times and are expressed relative to permeabilization caused by detergent solubilization or lysis with 5 μM of the lytic venom peptide melittin. ses for permeabilization are 3–10%.
The multiorganism screen, Fig. 2, showed that antimicrobial activity is too common to be used as a selection criterion under typical experimental conditions unless tedious simultaneous screening is carried out against multiple organisms. Thus, we developed the stringent biological screen in which we used only E. coli as the test organism. Peptide concentration was decreased, and cell density was increased until high-stringency conditions were reached. Given that treated cultures are allowed to recover overnight (21), even a single surviving cell will multiply detectably and lead to a negative result for antimicrobial activity. Positive activity requires total sterilization. At the highest stringency tested, 2.2 μM peptide and 106 cells/ml, we observed that many library members caused inhibition of cell growth over the first few hours, consistent with the high abundance of active peptides in the library (Fig. 2E). However, for most peptides, the bacterial cultures eventually recovered and approached stationary phase density after overnight incubation in growth medium. Three peptides out of 960 tested (0.3%) caused complete sterilization with no recovery of growth (Fig. 3A). In fact, these 3 wells remained sterile for weeks on a benchtop and had no detectible colony forming units when plated on nutrient agar.
The sequences of the 3 peptides selected by the stringent biological screen along with the 3 selected by the multiorganism and the 10 selected by the vesicle-based screen are shown in Fig. 3A. We noted previously that the vesicle-selected peptides lack a conserved sequence motif, but shared certain composition features, including an overabundance of valine (P=0.0002) (13) and an underabundance of asparagine (P=0.007) suggest that hydrophobic membrane binding is an important factor in vesicle-based selection. In the 6 biologically selected peptides, the opposite is observed; asparagine is the most abundant amino acid observed (P=0.03), and valine is nearly absent (P>0.05 compared to random abundance; P=0.02 compared to vesicle-selected abundance). Furthermore, while vesicle-based selection identified peptides with two, one, or none of the terminal basic cassettes (13), the biologically selected peptides always contained both terminal basic cassettes (P=0.02), suggesting a greater importance for electrostatic interactions in the biological screens. Although we cannot yet explain these observations mechanistically, it is clear that the biological screens are selecting for peptides from a different family compared to the vesicle-selected peptides.
Despite their differences, the antimicrobial activity of the biologically selected peptides is indistinguishable from the vesicle-selected peptides with sterilizing activity against all organisms tested in the low micromolar range (Fig. 3B). Therefore, all three high-throughput screens select equally well for potent, broad-spectrum antimicrobial peptides. However, when the membrane-permeabilizing activity of the selected peptides was compared in lipid vesicles, bacteria, and human cells (Fig. 3C), significant differences are apparent. With one exception, the biologically selected peptides do not permeabilize lipid vesicles. Also, while all selected peptides permeabilize microbial membranes, the biologically selected peptides do so to a greater extent than the vesicle-selected peptides (Fig. 3C and Supplemental Table 1). However, they also have higher average permeabilization of human cells compared to the vesicle-selected peptides (20±13%; n=6 vs. 5.2±3.7%; n=10; P=0.04) and may have higher cytotoxicity also (see Supplemental Table 1).
A novel family of broad-spectrum AMPs was clearly defined by screening samples of the library with the two biological screens. This family would have been mostly undetectable in the vesicle-based screen, which explains why it was not previously observed. While the data suggest that this new family of peptides has potent, broad-spectrum antimicrobial activity, it also has higher mammalian membrane permeabilization and higher toxicity than peptides selected using the vesicle-based screen. Considering that the biologically selected peptides have the same antimicrobial activity as the vesicle-selected peptides, that they have potentially higher toxicity, that the biological screens are much more labor intensive to perform, and that the biological screens are much less amenable to high throughput automation, we conclude that the rapid and simple, vesicle-based high-throughput screen is the most efficient high-throughput screen tested. We envision a high-throughput pipeline strategy for the development of novel, broad-spectrum, antimicrobial peptides which uses the vesicle-based screen to rapidly select candidate membrane-permeabilizing peptides; either from rationally designed libraries or from libraries of modified natural sequences, followed by focused bioactivity, formulation, and toxicity assays to select the most promising lead peptides for further development.
Supplementary Material
Acknowledgments
The authors thank Christopher Bishop for peptide synthesis and purification and William F. Walkenhorst and Paulo F. Almeida for critically reading the manuscript. This work was supported by U.S. National Institutes of Health grant GM060000 and by Louisiana Board of Regents Support Fund grant RC/EEP-05(2007-10).
References
- Klein E, Smith D L, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis. 2007;13:1840–1846. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C A, Murray B E. Antibiotic-resistant bugs in the 21st century—a clinical super-challenge. N Engl J Med. 2009;360:439–443. doi: 10.1056/NEJMp0804651. [DOI] [PubMed] [Google Scholar]
- Pittet D, Allegranzi B, Storr J, Bagheri N S, Dziekan G, Leotsakos A, Donaldson L. Infection control as a major World Health Organization priority for developing countries. J Hosp Infect. 2008;68:285–292. doi: 10.1016/j.jhin.2007.12.013. [DOI] [PubMed] [Google Scholar]
- White S H, Wimley W C, Selsted M E. Structure, function, and membrane integration of defensins. Cur Opinion Struc Biol. 1995;5:521–527. doi: 10.1016/0959-440x(95)80038-7. [DOI] [PubMed] [Google Scholar]
- Hancock R E, Sahl H G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- Yount N Y, Yeaman M R. Multidimensional signatures in antimicrobial peptides. Proc Natl Acad Sci. 2004;101:7363–7368. doi: 10.1073/pnas.0401567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman M R, Yount N Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- Easton D M, Nijnik A, Mayer M L, Hancock R E. Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol. 2009;27:582–590. doi: 10.1016/j.tibtech.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardy J L, Lynn D J, Brinkman F S, Hancock R E. Enabling a systems biology approach to immunology: focus on innate immunity. Trends Immunol. 2009;30:249–262. doi: 10.1016/j.it.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers. 2002;66:236–248. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- Almeida P F, Pokorny A. Mechanisms of antimicrobial, cytolytic, and cell-penetrating peptides: from kinetics to thermodynamics. Biochemistry. 2009;48:8083–8093. doi: 10.1021/bi900914g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinakumar R, Walkenhorst W F, Wimley W C. Broad-spectrum antimicrobial peptides by rational combinatorial design and high-throughput screening: the importance of interfacial activity. J Am Chem Soc. 2009;131:7609–7617. doi: 10.1021/ja8093247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinakumar R, Wimley W C. Biomolecular engineering by combinatorial design and high-throughput screening: small, soluble peptides that permeabilize membranes. J Am Chem Soc. 2008;130:9849–9858. doi: 10.1021/ja8017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechinger B. Rationalizing the membrane interactions of cationic amphipathic antimicrobial peptides by their molecular shape. Curr Opin Colloid Interface Sci. 2009;14:349–355. [Google Scholar]
- Selsted M E, Ouellette A J. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- Rausch J M, Marks J R, Rathinakumar R, Wimley W C. Beta-sheet pore-forming peptides selected from a rational combinatorial library: mechanism of pore formation in lipid vesicles and activity in biological membranes. Biochemistry. 2007;46:12124–12139. doi: 10.1021/bi700978h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura Y, Choda N, Matsuzaki K. Magainin 2 in action: distinct modes of membrane permeabilization in living bacterial and mammalian cells. Biophys J. 2008;95:5757–5765. doi: 10.1529/biophysj.108.133488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong R W, Shchepetov M, Weiser J N, Axelsen P H. Transcriptional profile of the Escherichia coli response to the antimicrobial insect peptide cecropin A. Antimicrob Agents Chemother. 2003;47:1–6. doi: 10.1128/AAC.47.1.1-6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch J M, Marks J R, Wimley W C. Rational combinatorial design of pore-forming beta-sheet peptides. Proc Natl Acad Sci U S A. 2005;102:10511–10515. doi: 10.1073/pnas.0502013102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch J M, Wimley W C. A high-throughput screen for identifying transmembrane pore-forming peptides. Anal Biochem. 2001;293:258–263. doi: 10.1006/abio.2001.5137. [DOI] [PubMed] [Google Scholar]
- Wiegand I, Hilpert K, Hancock R E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- Van Abel R J, Tang Y-Q, Rao V S V, Dobbs C H, Tran D, Barany G, Selsted M E. Synthesis and characterization of indolicidin, a tryptophan-rich antimicrobial peptide from bovine neutrophils. Int J Pept Protein Res. 1995;45:401–409. doi: 10.1111/j.1399-3011.1995.tb01055.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.