Abstract
The eye lens is an encapsulated avascular organ whose function is to focus light on the retina. Lens comprises a single progenitor cell lineage in multiple states of differentiation. Disruption of lens function leading to protein aggregation and opacity results in age-onset cataract. Cataract is a complex disease involving genetic and environmental factors. Here, we report the development of a new 3-stage system that differentiates human embryonic stem cells (hESCs) into large quantities of lens progenitor-like cells and differentiated 3-dimensional lentoid bodies. Inhibition of BMP signaling by noggin triggered differentiation of hESCs toward neuroectoderm. Subsequent reactivation of BMP and activation of FGF signaling stimulated formation of lens progenitor cells marked by the expression of PAX6 and α-crystallins. The formation of lentoid bodies was most efficient in the presence of FGF2 and Wnt-3a, yielding ∼1000 lentoid bodies/30-mm well. Lentoid bodies expressed and accumulated lens-specific markers including αA-, αB-, β-, and γ-crystallins, filensin, CP49, and MIP/aquaporin 0. Collectively, these studies identify a novel procedure to generate lens cells from hESCs that can be applied for studies of lens differentiation and cataractogenesis using induced pluripotent stem (iPS) cells derived from various cataract patients.—Yang, C., Yang, Y., Brennan, L., Bouhassira, E. E., Kantorow, M., and Cvekl, A. Efficient generation of lens progenitor cells and lentoid bodies from human embryonic stem cells in chemically defined conditions.
Keywords: cataract, crystallins, growth factors, Pax6
Establishment of efficient differentiation procedures to generate lens cells from human embryonic stem cells (hESCs) is an important step for understanding both human embryonic lens development and its diseases. Lens morphogenesis is an excellent system for studying the formation of lens progenitor cells from the naive ectoderm, to identify components of extracellular signaling required in this process, to probe exit from the cell cycle and to elucidate molecular mechanisms underlying lens fiber cell terminal differentiation. Abnormal embryonic lens development is caused by a variety of mutations in genes that encode DNA-binding transcription factors, lens structural proteins, including the crystallins and other important proteins. These mutations lead to congenital cataracts, a group of rare diseases that are often associated with other eye malformations. The major eye disease that is responsible for nearly half of blindness worldwide is age-related cataract. A systematic approach to studying human cataract is hampered by the lack of appropriate animal models and various limitations of human primary lens cultures. Therefore, an efficient in vitro system to direct cell differentiation toward progenitor and mature lens cells from hESCs will aid studies of lens development and mechanisms of cataractogenesis.
To achieve this goal, the experimental strategy should incorporate regulatory processes that recapitulate ontogenic lens development. In vertebrates, lens progenitor cells originate from the preplacodal region (PPR), a common pool of ectodermal cells surrounding the anterior part of the neural plate. In addition to lens, the PPR cells give rise to other cell lineages, including anterior pituitary, olfactory epithelium, and inner ear (1). At the morphological level, lens progenitor cells appear as the thickened disk of surface ectoderm, the lens placode, with underlying optic vesicle (2, 3). Through invagination of the lens placode, a lens vesicle is formed. The posterior cells of the lens vesicle elongate to form the primary fiber cells, whereas the anterior cells differentiate into epithelial cells.
Multiple signal transduction pathways including FGF, TGF-β, and Wnt, have been implicated in lens development. FGF signaling is essential for the establishment of the PPR (1), maintenance of the lens lineage (4), and lens fiber cell differentiation (5, 6). In primary rat lens cultures, low concentration of FGF promoted lens epithelial cell proliferation, moderate concentrations of FGF induced migration, and high concentrations induced fiber cell differentiation (7). It was also found that the concentration of FGF is higher in vitreous than aqueous humor (8). A number of BMP and TGF-β ligands and their transmembrane Ser/Thr kinase receptors are expressed in the lens (9) and are required for lens placode formation (10). Knockout of BMP4 led to severe defects in lens placode induction (11). BMP7-deficient mice showed eye defects that appeared to originate during lens induction (12). In mice, BMP ligand inhibitor noggin and expression of a dominant-negative form of BMP receptor Bmpr1b inhibited primary fiber differentiation (13). Recently, it was reported that BMPs are essential for FGF-induced secondary lens fiber differentiation (14). Wnt and components in the Wnt signaling pathway are expressed in the lens throughout its development (15, 16). Mouse embryos homozygous for a mutation in the lrp6 gene (coding Wnt signaling coreceptor) did not form a normal lens epithelium (16). LRPs are required for Wnt-Fz signaling through the β-catenin pathway, so canonical Wnt signaling is essential for the normal formation of epithelium. GSK-3β activity was decreased and nuclear β-catenin increased in the elongating fiber cells at the equatorial zone of the lens. After FGF priming, Wnt-3a induced cell elongation and the accumulation of β-crystallin (17). Attenuated fiber cell elongation, irregular fiber cell shapes, and down-regulation of Wnt/PCP pathway components were observed when Wnt signaling antagonist Sfrp2 was overexpressed in lens fiber cells of transgenic mice, suggesting a role of Wnt/PCP signaling in organizing lens fiber cell cytoskeleton and lens 3-dimensional architecture (18). In summary, these studies suggested that FGFs, BMP4, BMP7, noggin, and Wnts are excellent factors for use as lens differentiation factors in human ES cell cultures.
A number of studies successfully derived various differentiated cell types using human and mouse ES cells (19). The present study was aimed to induce differentiation of hESCs into lens progenitor cells and to trigger the formation of lentoid bodies in chemically defined conditions. Toward this end, we developed a 3-stage procedure through sequential inhibition and activation of FGF, TGF-β, and Wnt signaling pathways. Large quantities of human lens cells at various developmental stages were obtained, followed by efficient production of lentoid bodies. Molecular analysis of these cells confirmed expression of key lens regulatory and structural genes and opens new research avenue models to study the mechanisms of lens development and cataract formation.
MATERIALS AND METHODS
Antibodies and growth factors
Primary antibodies included Pax6 (Developmental Studies Hybridoma Bank, Iowa City, IA, USA), αA-crystallin (sc-22743; Santa Cruz Biotechnology, Santa Cruz, CA, USA), αB-crystallin (SPA-223; Assay Designs-Stressgen, Ann Arbor, MI, USA), β-crystallin (sc-22745; Santa Cruz Biotechnology), γ-crystallin (sc-22746; Santa Cruz Biotechnology) and MIP/aquaporin 0 (EMD Chemicals, Gibbstown, NJ, USA; Calbiochem 178610). Antibodies against BFSP1/filensin and BFSP2/CP49 were provided by Dr. Roy A. Quinlan (University of Durham, Durham, UK)(20). Cytokines tested included noggin (1967-NG; R&D Systems, Minneapolis, MN, USA), BMP4 (314-BP; R&D Systems), BMP7 (354-BP/CF; R&D Systems) and Wnt-3a (315–20; PeproTech Inc., Rocky Hill, NJ, USA).
Lens proteins
Lenses from a 52-yr-old male, 52-yr-old female and a 26-yr-old male were thawed on ice and homogenized in PBS, pH 7.2. The homogenates were centrifuged at 5000 rpm for 5 min, and the soluble portion of the lens was retained. Equal amounts of soluble lens from each lens homogenates were mixed, and the protein concentration was determined by Bradford assay.
Differentiation of human ES cells
The WA01 (H1) human ES cells (WiCell Research Institute, Madison, WI, USA) were cultured on Matrigel-coated plate in DMEM/F-12 supplemented with 0.05% BSA (A2153; Sigma, St. Louis, MO, USA), 1% nonessential amino aids (Invitrogen, Carlsbad, CA, USA; Gibco 11140-050), 2 mM l-glutamine (25030-081; Gibco), Pen Strep (15140; Gibco), 1× N2 supplements (17502-048; Gibco), 1× B27 supplements (17504-044; Gibco), 62.5 ng/ml bFGF (233-FB/CF; R&D Systems). The culture medium was changed every other day, and the cells were passaged once a week mechanically. Human ES cell differentiation was induced by sequential treatments of individual growth factors or their specific combinations.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis
Total RNAs were isolated using miRNeasy Mini Kit (217004; Qiagen, Valencia, CA, USA). Reverse transcription was performed with Superscript III first-strand kit (Invitrogen, 18080-051). Real-time PCR analysis was carried out using Power SYBR Green PCR Master Mix (4367659; Applied Biosystems, Foster City, CA, USA). GAPDH, B2M, and SDHA were used as the internal controls. Primer sequences for qRT-PCR assays are shown in Table 1.
TABLE 1.
Primer sets for each human gene used in qRT-PCR assays
| Primer, 5′–3′
|
||
|---|---|---|
| Gene | Forward | Reverse |
| B2M | GTGCTCGCGCTACTCTCTCT | TCTCTGCTGGATGACGTGAG |
| BFSP1 | CTCCAGCATCCATTGTGACGA | CGCAGTGTCTCAATCTGCTC |
| BFSP2 | ACGACATCCTTGAGACGATCA | AGTGTTGTGTAACTCCACCCT |
| Brachyury | CCCTATGCTCATCGGAACAA | CAATTGTCATGGGATTGCAG |
| CRYAA | AAGGTGCAGGACGACTTTGT | GTGGAACTCACGGGAAATGT |
| CRYAB | GTTCTTCGGAGAGCACCTGTT | GAGAGTCCAGTGTCAAACCAG |
| CRYBB2 | GCAGGTTCTGTCCTAGTGCAG | CTCTTGGCTGTCCACTTTGAT |
| CRYGC | TGAGCGTCCCAACTACCAAG | GTGGAGGGAACGGATCTCG |
| DLAD | TGGCTGATTCATTCCATCCCT | GTTGGGGTTGCAGACCAAGA |
| FOXA2 | TTCCGGGTCTGAACTGTAAC | GTGCCCTTCCATCTTCACC |
| GAPDH | ATTGCCCTCAACGACCACT | ATGAGGTCCACCACCCTGT |
| MIP | GTGCTGTATAGCGTTACCCCA | CTCGTCGTATGTGGCAAAGAT |
| NANOG | TCCTTGCAAATGTCTTCTGCT | CAGGGCTGTCCTGAATAAGC |
| NEF-H | ACCAGGACCTGCTCAATGTC | TCCGACACTCTTCACCTTCC |
| Nestin | AGAACTCCCGGCTGCAAAC | TCTGGGGTCCTAGGGAATTG |
| OCT4 | GTGGAGGAAGCTGACAACAA | CTGGTTCGCTTTCTCTTTCG |
| PAX4 | CAGGAGGACCAGGGACTACC | GAGCCACTATGGGGAGTGAG |
| PAX6 | TTTGCCCGAGAAAGACTAGC | CATTTGGCCCTTCGATTAGA |
| SDHA | CCCTGTCCTATGTGGACGTT | CACAGTCAGCCTCGTTCAAA |
| SIX3 | CCCACACAAGTAGGCAACTG | GTCCAATGGCCTGGTGCT |
| SOX2 | ATGATGGAGACGGAGCTGAA | GGGCTGTTTTTCTGGTTGC |
Immunocytochemisty
Cell culture was fixed in situ with 4% paraformaldehyde (PFA) fixative for 30 min. After 3 washes with PBS, cells were permeabilized in 0.2% Triton X-100 for 30 min, blocked for 1 h in 10% FCS and 1% BSA in PBS, and incubated overnight at 4°C with primary antibodies in 1% BSA in PBS. The second day, cells were washed 3 times with PBS and incubated with fluorescence-conjugated secondary antibodies and DAPI in 1% BSA in PBS for 1 h at room temperature. Images were taken by inverted Olympus IX81 microscope (Olympus, Tokyo, Japan).
Western immunoblotting
Equal amounts of cell protein lysates were electrophoresed on a 12% SDS-polyacrylamide gel electrophoresis gel and electrophoretically transferred onto a nitrocellulose membrane (162–0122; Bio-Rad, Hercules, CA, USA). Membranes were blocked in Tris-buffered saline with 5% milk and 0.1% Tween. The blots were probed with primary antibodies overnight and revealed with horseradish peroxidase-conjugated secondary antibodies. Anti-Pax6 antibodies were from Covance (PBR-278P; Covance, Princeton, NJ, USA).
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM)
For SEM studies, lentoid cell cultures were fixed in 2.5% glutaraldehyde, 0.1 M sodium cacodylate, 0.2 M sucrose, 5 mM MgCl2, pH 7.4, dehydrated through a graded series of ethanol and critical point dried using liquid carbon dioxide in a Tousimis Samdri 795 Critical Point Drier (Tousimis Research Corp., Rockville, MD, USA). Sputter coat was generated with gold-palladium in a Denton Vacuum Desk-2 Sputter Coater (Denton Vacuum, Cherry Hill, NJ, USA). Examination was carried out in a Jeol JSM6400 scanning electron microscope (Jeol, Peabody, MA, USA), using an accelerating voltage of 10 kV. For TEM studies, the samples were fixed with 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M sodium cacodylate buffer, postfixed with 1% osmium tetraoxide followed by 2% uranyl acetate, dehydrated through a graded series of ethanol, and embedded in LX112 resin (LADD Research Industries, Burlington, VT, USA). Ultrathin sections were cut on a Reichert Ultracut UCT (Leica Microsystems, Wetzlar, Germany), stained with uranyl acetate followed by lead citrate and viewed on a Jeol 1200EX transmission electron microscope at 80 kV.
RESULTS
Differentiation of human ES cells toward lens progenitor cells
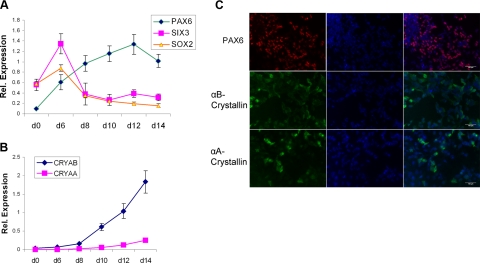
To identify a strategy to generate lens progenitor cells from hESCs, we reasoned that the initial differentiation step should employ those factors that promote ectoderm/neuroectoderm formation. From d 0 through d 6 of the culture, we tested various growth factors, including chordin, follistatin, and noggin and their combinations to induce differentiation of hESC preferentially into ectodermal derivatives. We monitored their differentiation using qRT-PCR followed by immunohistochemistry. We found that a treatment with 100 ng/ml of noggin in N2/B27 medium significantly augmented the expression of neuroectodermal marker PAX6 (Fig. 1A) in agreement with the previous reports (21). More important, PAX6 is also a key regulatory gene of entire lens development (3). In addition, the cells significantly increased expression of SOX2 and SIX3 at d 6 (Fig. 1A). SIX3 and SOX2 are specific markers of the prospective lens placode (22). To further stimulate formation of lens cells, we replaced noggin in the culture with various combinations of FGF2, BMP4, and BMP7, and evaluated expression of PAX6, αA-, and αB-crystallins. We found that a combination of 20 ng/ml BMP4, 20 ng/ml BMP7, and 100 ng/ml FGF2 from d 6 to 14 maintained PAX6 expression (Fig. 1A, C) and sequentially induced expression of αB- and αA-crystallins at the RNA (Fig. 1B) and protein (Fig. 1C) levels. Expression of αB-crystallin commences in the lens placode (23, 24). In contrast, expression of αA-crystallin is initiated later in the cells of the invaginating lens placode/lens pit (25). Using FACS assays, we found that 75 and 65% of the cells expressed PAX6 at d 8 and 14 of the culture, respectively. The number of αA-crystallin-positive cells increased from 10 to 41% (see Supplemental Fig. S1). From these data, we concluded that this two-step procedure generated lens progenitor-like cells that represented a significant portion of all cells in the 2-wk hESC cultures.
Figure 1.
Expression of PAX6, SIX3, SOX2, αA- and αB-crystallins during initial differentiation (d 0 to 14) of human ES cells into lens progenitor-like cells. A) qRT-PCR analysis of lens-lineage specific transcription factors PAX6, SIX3, and SOX2. B) qRT-PCR analysis of “early” αA- and αB-crystallin genes. C) Immunofluorescence detection of αA- and αB-crystallin proteins at d 12.
Formation of lentoid bodies from lens progenitor-like cells
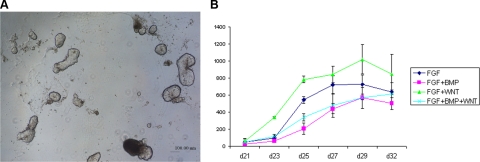
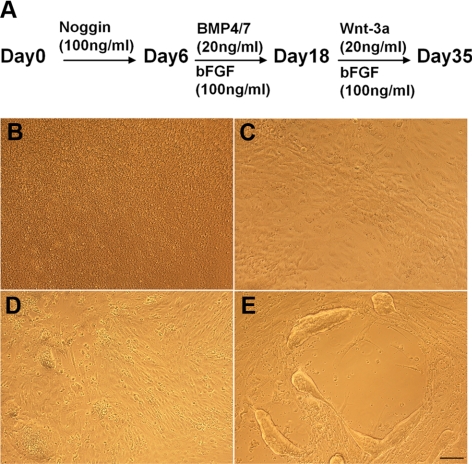
Our next goal was to determine whether these lens progenitor-like cells could further differentiate into 3-dimensional structures, the lentoid bodies. Lentoid bodies are light refractive and transparent biconcave structures that can be formed from primary lens epithelial cultures (26) or from cultured lens epithelial cells (27). In addition to FGF and TGF-β signaling, Wnt signaling is another pathway known to be involved in lens fiber cell differentiation, lens fiber cell cytoskeleton, and lens 3-dimensional architecture as described above. Therefore, from d 18, we studied the effects of all seven possible combinations of FGF2, BMP4, BMP7, and Wnt-3a on lens fiber differentiation and lentoid body formation. From d 21 of the culture, lentoid bodies (see Fig. 2A) started to appear. From d 21 to d 35, the number of lentoid bodies in each group was counted every other day. BMP4, BMP7, and Wnt-3a alone did not promote lentoid body formation (data not shown). In contrast, FGF alone and in combination with other growth factors augmented lentoid body formation as evaluated from d 21 to 32 (Fig. 2B). The lentoid numbers reached peak at d 29. From this time, the lentoid bodies were gradually lost due to their detachment from the surface of the culture dish. We found that the FGF2 and Wnt-3a group generated more lentoid bodies than the other groups at all time points examined. At d 29, we found more than 1000 lentoid bodies/well of 6-well plate in the bFGF/Wnt-3a cultures. The above results suggested that FGF signaling was indispensable for the formation of lentoid bodies, and Wnt-3a had an additional positive effect toward the efficiency of this culture. On the basis of these results, the “optimal” protocol in terms of the total yield of lentoid bodies in the N2/B27 medium and Matrigel-coated wells was a 3-step procedure: 1) treatment of 100 ng/ml Noggin from d 0 to 6; 2) combination of 100 ng/ml bFGF, 20 ng/ml BMP4, and 20 ng/ml BMP7 from d 6 to 18; and 3) combination of 100 ng/ml FGF2 and 20 ng/ml Wnt-3a from d 18 to 35 (Fig. 3A). The morphology of the undifferentiated/differentiated human ES cell cultures for d 0, 6, 18, and 35 is shown in Fig. 3B–E.
Figure 2.
Formation of lentoid bodies from human ES cells. A) Visualization of lentoid body formation by phase contrast microscopy (×40) at d 35. B) Quantitative analysis of lentoid body formation. Analysis was performed from d 21 to 32. Results are shown as means ± sd from 3 independent experiments. Cultures using BMPs, Wnt-3a, and BMPs/Wnt-3a did not stimulate lentoid body formation (data not shown).
Figure 3.
Human ES cell differentiation into lens progenitor-like cells and lentoid bodies. A) Three-step differentiation procedure and “optimized” concentrations of growth factors used. B–E) Morphology of the cells (×100) at d 0 (B), d 6 (C), d 18 (D), and d 35 (E).
Characterization of lens cells and lentoid bodies derived from hESCs
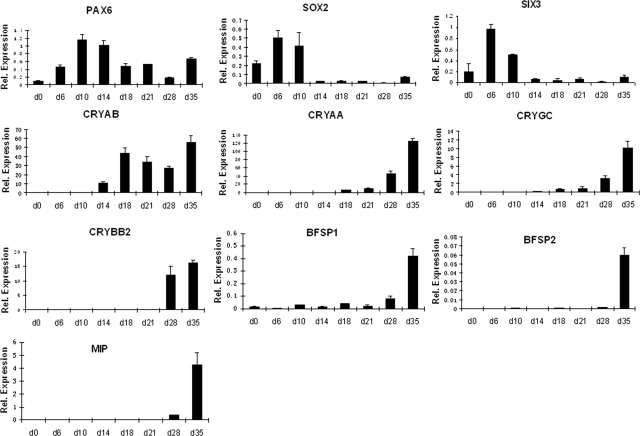
Next, we characterized the entire differentiation procedure from d 0 to 35 followed by examination of the morphological, cellular, and structural properties of the lentoid bodies. First, qRT-PCR analysis of PAX6, SIX3, SOX2, CRYAA, and CRYAB genes described earlier was performed (see Fig. 4). Expression of PAX6 and CRYAB genes was maintained in the system, and expression of CRYAA gene reached its local maximum at d 35 of culture. Quantitatively, αB- and αA-crystallin proteins became detectable from d 8 and 10 (Figs. 1B and 4), respectively. Their expression levels increased as much as 200- and 1800-fold at d 35 compared to d 10, respectively, using the common internal controls (Fig. 4). In contrast, after the initial induction of SIX3 and SOX2 genes at d 6, their expression was attenuated from d 14 toward d 35. Expression profiles of 5 additional genes, CRYGC, CRYBB2, BFSP1/filensin, BFSP2/CP49, and MIP/aquaporin 0, showed their local peak of expression at d 35 of the culture. Expression of stem cell markers NANOG and OCT3/4 decreased in the culture (d 0 to 28) following the noggin treatment (Supplemental Fig. S2), confirming the loss of pluripotency of the hESCs due to their differentiation. Expression of endodermal markers FOXA2 and PAX4 showed increased expression of FOXA2 at d21 and d28. As expected, expression levels of neuroectodermal markers SOX1, nestin, and NEFH were increased in the culture as SOX1 is expressed in differentiating lens fibers. Expression of mesodermal marker brachyury also increased in this system (Supplemental Fig. S2). These data suggest that the nonlens cells generated in the culture are mostly of neuroectodermal and mesodermal origin.
Figure 4.
Quantitative RT-PCR analysis of 10 lens differentiation markers (PAX6, SOX2, SIX3, CRYAB, CRYAA, CRYGC, CRYBB2, BFSP1, BFSP2, and MIP) from d 0 to 35. Results were calculated relative to the average Ct value of B2M, GAPDH, and SDHA genes, as described in Materials and Methods, and are shown as means ± sd from 3 independent experiments.
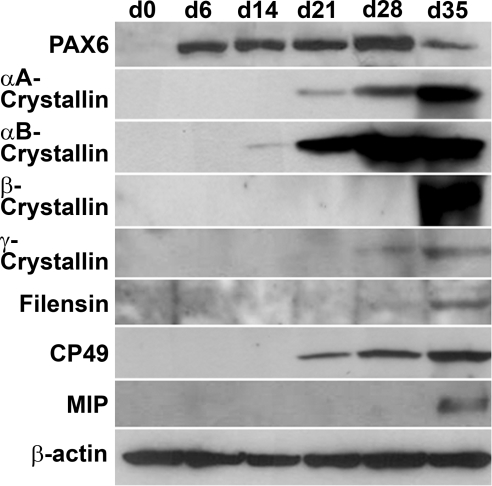
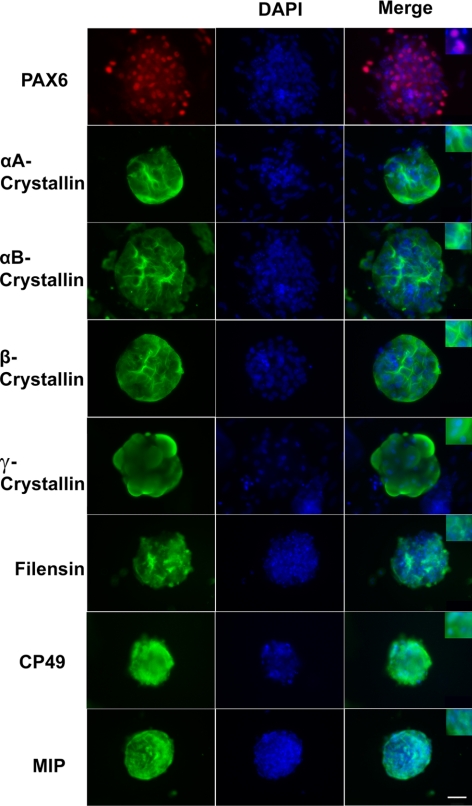
Expression of PAX6, αA-, αB-, β- and γ-crystallins and BFSP1/filensin, BFSP2/CP49, and MIP/aquaporin 0 was further examined by Western immunobloting. These results demonstrated accumulation of all crystallins, lens-specific intermediate beaded filament proteins BFSP1/filensin and BFSP2/CP49, and major intrinsic protein of lens fiber, MIP/aquaporin 0 (Fig. 5). Expression profiles of α-, β-, and γ-crystallins followed their endogenous expression profiles established both for human and mouse lenses (3). Other lens-specific proteins, including BFSP1, BFSP2, and MIP started their accumulation from d 28 and increased significantly at d 35. These results suggest that the lentoid bodies produced in the present in vitro system recapitulate key temporal features of expression of individual lens-specific proteins. Next, immunofluorescence was employed to spatially localize these proteins in lentoid bodies collected at d 35 of the culture as shown in Fig. 6. As expected, significant nuclear signal (Fig. 6, red) was detected for transcription factor PAX6, indicating nuclear localization (see inset in Fig. 6). Staining of α- and β-crystallins showed different distribution of these proteins in the cytoplasm of individual cells within lentoid bodies compared to γ-crystallins (Fig. 6). Together, the expression of α- and β-/γ-crystallins and other specific protein markers of mammalian lens fiber cell formation, including BFSP1, BFSP2, and MIP provides conclusive evidence that the human ES cells grown in culture were differentiated into lens fiber cells that adopted a 3-dimensional lentoid body organization.
Figure 5.
Western blot analysis of protein expression during the differentiation of human ES cells. Specific antibodies were used to detect expression of PAX6, αA-, αB-, β-, and γ-crystallins, filensin, CP49, and MIP/aquaporin 0. Expression of β-actin is shown as a loading control.
Figure 6.
Immunofluorescence localization of proteins in lentoid bodies (d 35). Specific antibodies were used to detect expression of PAX6, αA-, αB-, β-, and γ-crystallins, filensin, CP49, and MIP/aquaporin 0. DAPI counterstaining shows nuclei in lentoid bodies. Scale bar = 100 μm.
Subcellular structure of lentoid bodies
The ultrastructure of lentoid bodies was evaluated through SEM and TEM. Supplemental Fig S3A, B shows SEM of a lentoid body grown on Matrigel-coated surface. The lentoid body showed ellipsoid shape with diameter of ∼200 μm and thickness of ∼30 μm. The surface of the lentoid body was not as smooth as that of the lens. TEM showed that some cultured lentoid cells were elongated and regularly arranged like authentic lens fiber cells, while other cells exhibited epithelial cell morphology. Other cells showed intermediate cell morphology (Supplemental Fig. S3C–F), suggesting that lentoid bodies at d 35 were composed of a mixture of cells at various differentiation stages. As expected, many of the elongated cells showed loss of cytoplasmic organelles. Condensation of nuclei, i.e., the presence of pyknotic lens fiber cell nuclei and partial nuclear degradation were also observed in some cells, which was consistent with the immunofluorescence studies shown in Fig. 6. In some cells within lentoid bodies, we observed the phenomenon of nucleus condensation and disintegration (Supplemental Fig. S3G). In addition, both electron-dense nucleolus (Supplemental Fig. S3F) and fragmented nucleoli (Supplemental Fig. S3G) were found in the individual lens fiber cells. Similar morphological changes of nucleoli were reported during normal lens fiber cell denucleation (28, 29). Denucleation is a hallmark of lens fiber differentiation (30). This process occurs in a restricted region of the lens cortex and results in the formation of a central organelle-free zone (OFZ). We supposed that these lens fiber cells were at the start period of terminal differentiation. To date, the only nuclease implicated directly in this process is an acidic DNase IIβ/DLAD (31, 32). However, at d 35 by qRT-PCR, we only found ∼2-fold increase of DNase IIβ transcripts compared to earlier stages of the differentiation procedure (data not shown). This may be the reason that nuclei were still retained in most of the fiber cells in lentoid bodies.
Comparison of protein expression profiles between lentoid bodies and human lens
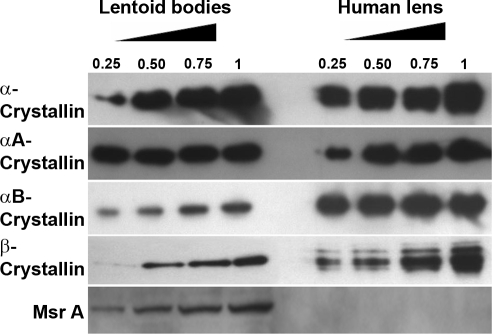
Although the accumulation of crystallins and other lens-specific structural proteins in lentoid bodies derived from human ES cells provides compelling evidence that the in vitro differentiation procedure is specific, we next performed a direct semiquantitative comparison of crystallins and other proteins between the lentoid bodies and human lens. Lens extracts were prepared from three pooled lenses of 26, 52, and 52-yr-old individuals and examined in a range of 0.25 to 1 μg of total lens protein. Five different antibodies specific against α-, αA-, αB-, and β-crystallins and MsrA were used as shown in Fig. 7. The results on α-crystallins showed comparable expression levels of αA-crystallin in both extracts. In contrast, expression of αB- and β-crystallins was lower in extracts derived from lentoid bodies compared to the lens. Finally, we examined expression of methionine sulfoxide reductase A (MsrA), an enzyme that functions in protein repair in lens and many other tissues (33). Lentoid bodies showed expression of MsrA proteins though its expression in the same amounts of whole lens extracts was beyond the detection limit of the assay (Fig. 7).
Figure 7.
Comparative analysis of protein expression in lentoid bodies and human lens. Western blot analysis was performed using α-, αA-, αB-, and β-crystallin and MsrA-specific antibodies and indicated amounts (μg) of total lens protein extracts.
DISCUSSION
The present study demonstrates that a sequential treatment of hESCs with noggin, BMP4/BMP7/FGF2, and FGF2/Wnt-3a induces differentiation toward lens lineage followed by formation of lentoid bodies under chemically defined culture conditions. Selection of these growth factors was based on their properties, i.e., their role in the lens placode formation and stimulation of lens fiber cell differentiation. The formation of lens progenitor cells commenced at around d 10 of the culture, as evidenced by the coexpression of PAX6 and αB-crystallin and initiation of expression of the lens-preferred αA-crystallin. Subsequent treatment of the lens progenitor-like cells with various combinations of BMP4, BMP7, FGF2, and Wnt-3a showed that FGF2 was both essential and sufficient for the formation of lentoid bodies. Use of 100 ng/ml of FGF2 and 20 ng/ml of Wnt-3a yielded nearly 50-fold higher production of lentoid bodies compared to the earlier studies that showed induction of lentoid bodies from primate (cynomologus monkey) ES cells grown on PA6 stromal cell feeder layer in the presence of bFGF (34). Thus, the present procedure adds to a growing list of specialized human cell types and tissues that can be generated from hESCs, including retinal progenitor cells (35), rod and cone photoreceptors (36), and retinal pigment epithelium (36, 37).
The 3-stage protocol established here used the integrative knowledge about FGF, TGF-β, and Wnt signaling pathways, their secreted factors and their antagonists in normal mammalian lens development (1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18, 38). In the default model of neural development, BMPs act as signals of epidermal induction and the inhibition of the BMP signaling in the ectoderm is the hallmark of neural-fate acquisition (39, 40). Thus, during the initial stage of human ES cell differentiation, we blocked BMP signaling by noggin (41). Noggin complexes BMP ligands and prevents them from binding to their corresponding receptors, supporting ectoderm formation (21). Early neuralization subdivides the ectoderm into at least four distinct domains: neural plate, neural crest, preplacodal region (PPR), and primitive epidermis (42). The PPR comprised multiple progenitor cells that give rise to the anterior pituitary, olfactory epithelium, lens, inner ear, and the trigeminal and epibranchial cranial placodes (1). Thus, to promote formation of lens progenitor cells, both BMP4 and BMP7 were used following 6 d of noggin treatment to avert neural cell fate and to stimulate lens cell formation. In addition, we used FGF2 to stimulate FGF signaling that is essential at multiple stages of lens development (38). Lens progenitor-like cell formation was marked by the subsequent activation of expression of PAX6, followed by αB-crystallin, and, finally, by αA-crystallin (Figs. 1, 4 and 5). To induce the formation of lentoid bodies, we tested various combinations of FGF2, BMP4, BMP7, and Wnt-3a. All individual and double combinations without FGF2 did not stimulate lentoid body formation, indicating that FGF2 was necessary for lentoid formation (Fig. 2B). Wnt-3a plus FGFs produced more lentoid bodies compared to FGF2 alone. In contrast, FGF2 plus BMP4/7 generated less lentoid bodies, suggesting opposite roles of Wnt and BMP signaling on the efficiency of lentoid body formation. Although the primary goal of these studies was to establish a lens differentiation procedure, use of antagonists, such as small drugs, inhibitors of signaling pathways implicated in lens development (see below), should provide novel insights into the crosstalk and molecular mechanisms of these pathways during mammalian lens development.
The cellular, ultrastructural, and molecular characterization of lentoid bodies indicates that terminal differentiation of their lens fibers was properly initiated but not completely executed. The initiation is demonstrated by the expression of α-, β-, and γ-crystallins, the incomplete differentiation is indicated by lower amounts of β- and γ-crystallins in lentoid bodies compared to the human lens (Fig. 7, and data not shown). Expression of αB-crystallin was detected prior to the onset of αA-crystallin expression. This temporal aspect of the differentiation procedure is similar to the activation of α-crystallins in mammalian lens (24). However, expression of BFSP1/filensin, BFSP2/CP49, and MIP/aquaporin 0 at d 35 of culture, together with morphological evidence of lens fiber cell elongation, loss of subcellular organelles, including denucleation/karyolysis indicate that lens fiber cell differentiation program has been activated. DNase IIβ is an acidic endonuclease required for lens fiber cell denucleation (30). At d 35, its expression was elevated only 2-fold at the RNA level. However, from d 35 of culture, the lentoid bodies tend to dissociate from their support. Thus, it will be important to establish more selective cell culture conditions, e.g., use of various 3-dimensional matrices containing collagen, fibronectin and laminin, to allow additional differentiation of lentoid bodies past d 35.
In addition to studies of signaling pathways using drug/inhibitors (e.g., FGFR inhibitor PD173074, JNK inhibitor SP600125, MEK inhibitor U0126) that function during embryonic lens development described above, the present differentiation system can be used in combination with RNA interference as a tool to silence specific gene expression at specific stages of the culture. This system can also be used to examine formation of other cell lineages that emerge simultaneously with lens lineage from the embryonic PPR, including progenitors of anterior pituitary, olfactory and inner ear (1, 42).
To achieve this goal, generation of engineered lines with lineage-specific promoters/enhancers driving expression of fluorescent marker proteins (25) will be needed. FACS can be applied to isolate different progenitor cell populations followed by analysis of their transcriptomes (43), epigenomes (44), proteomes (45, 46), and methylomes (47). Similar methods can be used to study cell cycle exit of the lens precursor cells. Finally, the procedure established here should be tested with mouse or any other available ES cell line.
This procedure has a potential to open new research avenues in clinical research to study molecular mechanisms of cataractogenesis. Nuclear reprogramming can be used to generate iPS cells from various cataract patients and the novel differentiation procedure should allow differentiation of these cells into lens cells. Both studies of congenital and age-related cataracts can be envisioned. The iPS cells derived from individuals with mutations in genes encoding transcription factors or lens structural proteins (48) will provide unlimited source of lens cells to study molecular mechanisms in genetically defined systems. Similarly, the lens cells described here can be used to study interactions between environmental factors, such as oxidative stress and response of lens epithelial cells derived via iPS cells (49, 50) from patients with clinically distinct (e.g., early vs. normal onset and cortical vs. nuclear) cataracts.
In summary, the present study shows for the first time that hESCs can be differentiated in vitro to lens progenitor-like cells and that these cells can subsequently be differentiated into lentoid bodies using chemically defined conditions. Analysis of expression of lens lineage regulatory genes PAX6, SIX3, and SOX2, and lens structural proteins including α-, β-, and γ-crystallins, intermediate filament beaded proteins BFSP1/filensin and BFSP2/CP49, and major lens intrinsic protein MIP/aquaporin 0 showed that the major features of mammalian lens differentiation program were recapitulated in the present cell culture system. These findings demonstrate usefulness of this model to study molecular mechanisms of human lens embryonic development, and to study biology of lens cells derived from iPS cells obtained from various cataract patients.
Supplementary Material
Acknowledgments
The authors thank the Ruth L. and David S. Gottesman Institute for Stem Cell and Regenerative Medicine Research for use of their core services. The authors are grateful to Dr. Roy Quinlan for filensin and CP49 antibodies. The authors also thank the West Virginia Eye Bank and Lions Eye Bank of Oregon for providing the human lenses used in this study. This work was supported by National Institutes of Health grants EY012200 (A.C.), EY014237 (A.C.), and EY013022 (M.K.) and the Sisenwein Family Endowment to the Department of Ophthalmology and Visual Sciences of the Albert Einstein College of Medicine. E.E.B. is supported in part by New York State Stem Cell Science (NYSTEM) grants N08S-001 and N08T-006. ESCs were produced by the NSTEM-supported Einstein Human Pluripotent Stem Cell Center (N08S-001).
References
- Streit A. Early development of the cranial sensory nervous system: from a common field to individual placodes. Dev Biol. 2004;276:1–15. doi: 10.1016/j.ydbio.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Lang R A. Pathways regulating lens induction in the mouse. Int J Dev Biol. 2004;48:783–791. doi: 10.1387/ijdb.041903rl. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Duncan M K. Genetic and epigenetic mechanisms of gene regulation during lens development. Prog Retin Eye Res. 2007;26:555–597. doi: 10.1016/j.preteyeres.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yang T, Madakashira B P, Thiels C A, Bechtle C A, Garcia C M, Zhang H, Yu K, Ornitz D M, Beebe D C, Robinson M L. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol. 2008;318:276–288. doi: 10.1016/j.ydbio.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu F J, McAvoy J W. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Robinson M L. An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol. 2006;17:726–740. doi: 10.1016/j.semcdb.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy J W, Chamberlain C G. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development. 1989;107:221–228. doi: 10.1242/dev.107.2.221. [DOI] [PubMed] [Google Scholar]
- Schulz M W, Chamberlain C G, de Iongh R U, McAvoy J W. Acidic and basic FGF in ocular media and lens: implications for lens polarity and growth patterns. Development. 1993;118:117–126. doi: 10.1242/dev.118.1.117. [DOI] [PubMed] [Google Scholar]
- De Iongh R U, Lovicu F J, Overbeek P A, Schneider M D, Joya J, Hardeman E D, McAvoy J W. Requirement for TGFβ receptor signaling during terminal lens fiber differentiation. Development. 2001;128:3995–4010. doi: 10.1242/dev.128.20.3995. [DOI] [PubMed] [Google Scholar]
- Rajagopal R, Huang J, Dattilo L K, Kaartinen V, Mishina Y, Deng C X, Umans L, Zwijsen A, Roberts A B, Beebe D C. The type I BMP receptors, Bmpr1a and Acvr1, activate multiple signaling pathways to regulate lens formation. Dev Biol. 2009;335:305–316. doi: 10.1016/j.ydbio.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Hogan B L. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Hofmann C, Bronckers A L, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808–2820. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- Faber S C, Robinson M L, Makarenkova H P, Lang R A. Bmp signaling is required for development of primary lens fiber cells. Development. 2002;129:3727–3737. doi: 10.1242/dev.129.15.3727. [DOI] [PubMed] [Google Scholar]
- Boswell B A, Overbeek P A, Musil L S. Essential role of BMPs in FGF-induced secondary lens fiber differentiation. Dev Biol. 2008;324:202–212. doi: 10.1016/j.ydbio.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M R, Biggs J J, Schoenwolf G C, Rao M S. Characterization of avian frizzled genes in cranial placode development. Mech Dev. 2000;93:195–200. doi: 10.1016/s0925-4773(00)00263-x. [DOI] [PubMed] [Google Scholar]
- Stump R J, Ang S, Chen Y, von Bahr T, Lovicu F J, Pinson K, de Iongh R U, Yamaguchi T P, Sassoon D A, McAvoy J W. A role for Wnt/β-catenin signaling in lens epithelial differentiation. Dev Biol. 2003;259:48–61. doi: 10.1016/s0012-1606(03)00179-9. [DOI] [PubMed] [Google Scholar]
- Lyu J, Joo C K. Wnt signaling enhances FGF2-triggered lens fiber cell differentiation. Development. 2004;131:1813–1824. doi: 10.1242/dev.01060. [DOI] [PubMed] [Google Scholar]
- Chen Y, Stump R J, Lovicu F J, Shimono A, McAvoy J W. Wnt signaling is required for organization of the lens fiber cell cytoskeleton and development of lens three-dimensional architecture. Dev Biol. 2008;324:161–176. doi: 10.1016/j.ydbio.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- Sandilands A, Prescott A R, Carter J M, Hutcheson A M, Quinlan R A, Richards J, FitzGerald P G. Vimentin and CP49/filensin form distinct networks in the lens which are independently modulated during lens fibre cell differentiation. J Cell Sci. 1995;108:1397–1406. doi: 10.1242/jcs.108.4.1397. [DOI] [PubMed] [Google Scholar]
- Yao S, Chen S, Clark J, Hao E, Beattie G M, Hayek A, Ding S. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A. 2006;103:6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Lagutin O V, Mende M, Streit A, Oliver G. Six3 activation of Pax6 expression is essential for mammalian lens induction and specification. EMBO J. 2006;25:5383–5395. doi: 10.1038/sj.emboj.7601398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes J I, 2nd, Duncan M K, Piatigorsky J. Spatial and temporal activity of the αB-crystallin/small heat shock protein gene promoter in transgenic mice. Dev Dyn. 1996;207:75–88. doi: 10.1002/(SICI)1097-0177(199609)207:1<75::AID-AJA8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Robinson M L, Overbeek P A. Differential expression of αA- and αB-crystallin during murine ocular development. Invest Ophthalmol Vis Sci. 1996;37:2276–2284. [PubMed] [Google Scholar]
- Wolf L, Yang Y, Wawrousek E, Cvekl A. Transcriptional regulation of mouse αA-crystallin gene in a 148kb Cryaa BAC and its derivates. BMC Dev Biol. 2008;8:88. doi: 10.1186/1471-213X-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor M D, McAvoy J W. In vitro generation of functional lens-like structures with relevance to age-related nuclear cataract. Invest Ophthalmol Vis Sci. 2007;48:1245–1252. doi: 10.1167/iovs.06-0949. [DOI] [PubMed] [Google Scholar]
- Wagner L M, Takemoto D J. PKCα and PKCγ overexpression causes lentoid body formation in the N/N 1003A rabbit lens epithelial cell line. Mol Vis. 2001;7:138–144. [PubMed] [Google Scholar]
- Kuwabara T, Imaizumi M. Denucleation process of the lens. Invest Ophthalmol. 1974;13:973–981. [PubMed] [Google Scholar]
- Vrensen G F, Graw J, De Wolf A. Nuclear breakdown during terminal differentiation of primary lens fibres in mice: a transmission electron microscopic study. Exp Eye Res. 1991;52:647–659. doi: 10.1016/0014-4835(91)90017-9. [DOI] [PubMed] [Google Scholar]
- Bassnett S. On the mechanism of organelle degradation in the vertebrate lens. Exp Eye Res. 2009;88:133–139. doi: 10.1016/j.exer.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maria A, Bassnett S. DNase IIβ distribution and activity in the mouse lens. Invest Ophthalmol Vis Sci. 2007;48:5638–5646. doi: 10.1167/iovs.07-0782. [DOI] [PubMed] [Google Scholar]
- Nishimoto S, Kawane K, Watanabe-Fukunaga R, Fukuyama H, Ohsawa Y, Uchiyama Y, Hashida N, Ohguro N, Tano Y, Morimoto T, Fukuda Y, Nagata S. Nuclear cataract caused by a lack of DNA degradation in the mouse eye lens. Nature. 2003;424:1071–1074. doi: 10.1038/nature01895. [DOI] [PubMed] [Google Scholar]
- Brennan L A, Kantorow M. Mitochondrial function and redox control in the aging eye: role of MsrA and other repair systems in cataract and macular degenerations. Exp Eye Res. 2009;88:195–203. doi: 10.1016/j.exer.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooto S, Haruta M, Honda Y, Kawasaki H, Sasai Y, Takahashi M. Induction of the differentiation of lentoids from primate embryonic stem cells. Invest Ophthalmol Vis Sci. 2003;44:2689–2693. doi: 10.1167/iovs.02-1168. [DOI] [PubMed] [Google Scholar]
- Lamba D A, Karl M O, Ware C B, Reh T A. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, Akaike A, Sasai Y, Takahashi M. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26:215–224. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- Idelson M, Alper R, Obolensky A, Ben-Shushan E, Hemo I, Yachimovich-Cohen N, Khaner H, Smith Y, Wiser O, Gropp M, Cohen M A, Even-Ram S, Berman-Zaken Y, Matzrafi L, Rechavi G, Banin E, Reubinoff B. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. 2009;5:396–408. doi: 10.1016/j.stem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Smith A N, Radice G, Lang R A. Which FGF ligands are involved in lens induction? Dev Biol. 2010;337:195–198. doi: 10.1016/j.ydbio.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P A, Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995;376:331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- Munoz-Sanjuan I, Brivanlou A H. Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci. 2002;3:271–280. doi: 10.1038/nrn786. [DOI] [PubMed] [Google Scholar]
- Valenzuela D M, Economides A N, Rojas E, Lamb T M, Nunez L, Jones P, Lp N Y, Espinosa R, 3rd, Brannan C I, Gilbert D J. Identification of mammalian noggin and its expression in the adult nervous system. J Neurosci. 1995;15:6077–6084. doi: 10.1523/JNEUROSCI.15-09-06077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litsiou A, Hanson S, Streit A. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development. 2005;132:4051–4062. doi: 10.1242/dev.01964. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Xin L, Sharov A A, Thomas M, Mowrer G, Meyers E, Piao Y, Mehta S, Yee S, Nakatake Y, Stagg C, Sharova L, Correa-Cerro L S, Bassey U, Hoang H, Kim E, Tapnio R, Qian Y, Dudekula D, Zalzman M, Li M, Falco G, Yang H T, Lee S L, Monti M, Stanghellini I, Islam M N, Nagaraja R, Goldberg I, Wang W, Longo D L, Schlessinger D, Ko M S. Uncovering early response of gene regulatory networks in ESCs by systematic induction of transcription factors. Cell Stem Cell. 2009;5:420–433. doi: 10.1016/j.stem.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golebiewska A, Atkinson S P, Lako M, Armstrong L. Epigenetic landscaping during hESC differentiation to neural cells. Stem Cells. 2009;27:1298–1308. doi: 10.1002/stem.59. [DOI] [PubMed] [Google Scholar]
- Dai B, Rasmussen T P. Global epiproteomic signatures distinguish embryonic stem cells from differentiated cells. Stem Cells. 2007;25:2567–2574. doi: 10.1634/stemcells.2007-0131. [DOI] [PubMed] [Google Scholar]
- Hutchins A P, Robson P. Unraveling the human embryonic stem cell phosphoproteome. Cell Stem Cell. 2009;5:126–128. doi: 10.1016/j.stem.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Lister R, Pelizzola M, Dowen R H, Hawkins R D, Hon G, Tonti-Filippini J, Nery J R, Lee L, Ye Z, Ngo Q M, Edsall L, Antosiewicz-Bourget J, Stewart R, Ruotti V, Millar A H, Thomson J A, Ren B, Ecker J R. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Hejtmancik J F. Genetic origins of cataract. Arch Ophthalmol. 2007;125:165–173. doi: 10.1001/archopht.125.2.165. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik M A, Smuga-Otto K, Antosiewicz-Bourget J, Frane J L, Tian S, Nie J, Jonsdottir G A, Ruotti V, Stewart R, Slukvin I I, Thomson J A. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.