Abstract
The diaphragm muscles in vivo are subjected to mechanical forces both in the direction of the muscle fibers and in the direction transverse to the fibers. However, the effect of directional mechanical forces in skeletal muscle gene regulation is completely unknown. Here, we identified that stretch in the longitudinal and transverse directions to the diaphragm muscle fibers up-regulated Ankrd2 gene expression by two distinct signaling pathways in wild-type (WT) and mdm, a mouse model of muscular dystrophy with early-onset of progressive muscle-wasting. Stretch in the longitudinal direction activated both NF-κB and AP-1 transcription factors, whereas stretch in the transverse direction activated only AP-1 transcription factor. Interestingly, longitudinal stretch activated Ankrd2 promoter only by NF-κB, whereas transverse stretch activated Ankrd2 promoter by AP-1. Moreover, we found that longitudinal stretch activated Akt, which up-regulated Ankrd2 expression through NF-κB. However, transverse stretch activated Ras-GTP, Raf-1, and Erk1/2 proteins, which up-regulated Ankrd2 expression through AP-1. Surprisingly, the stretch-activated NF-κB and AP-1 signaling pathways was not involved in Ankrd2 regulation at the basal level, which was high in the mdm mouse diaphragm. Taken together, our data show the anisotropic regulation of Ankrd2 gene expression in the diaphragm muscles of WT and mdm mice via two distinct mechanosensitive signaling pathways.—Mohamed, J. S., Lopez, M. A., Cox, G. A., Boriek, A. M. Anisotropic regulation of Ankrd2 gene expression in skeletal muscle by mechanical stretch.
Keywords: diaphragm, NF-κB, AP-1, mdm mice, mechanotransduction
Mechanotransduction is the fundamental mechanism by which mechanical stress acting through a cell initiates intracellular signaling pathways. All adhesion-dependent cells, especially skeletal muscle cells, are sensitive and routinely subjected to mechanical forces (1, 2). Force transmission in skeletal muscles varies greatly with muscle fiber architecture (3, 4). In vivo skeletal muscles of either the hindlimb or forelimb are essentially mechanically loaded along the muscle fiber direction, whereas in diaphragm, its membrane is mechanically loaded with transdiaphragmatic pressure. Therefore, diaphragm muscles are subjected to mechanical forces not only in the direction of the muscle fibers but also in the direction transverse to the fibers (5). It is therefore important to determine how mechanosensitive signaling pathways are activated in the diaphragm muscle fibers in response to longitudinal and transverse directional forces.
Recently, it has been shown that titin and titin-interacting proteins are involved in the sensing of mechanical forces during muscle function (6,7,8). Titin is a giant cytoskeleton protein that extends over half a sarcomere, and it maintains temporal and spatial assembly of the contractile filaments during muscle action (9). Mutations in titin gene cause titinopathy, a muscular dystrophy, in humans (10, 11). A complex rearrangement and LINE insertion into the N2A region of the mouse titin gene leads to muscular dystrophy with myositis (mdm), an early-onset autosomal recessive disease. The mdm mouse model is a pertinent clinical phenotype because it exhibits a clinical course similar to that of Duchenne’s muscular dystrophy (DMD), in which the mdm mouse has an early onset, rapidly progressive muscle-wasting disease that ends in premature death likely due to respiratory insufficiency. Moreover, recently, we have shown the contractile and passive mechanical aberrations in the diaphragm muscle of mdm mice (12). The titin mdm mutation leads to aberrant splicing of four critical skeletal muscle-specific exons encoding 83 amino acids in the N2A region of the I band. The central I band of titin interacts with many proteins including the stretch responsive gene Ankyrin repeat protein2 (Ankrd2), a member of three muscle ankyrin repeat proteins (MARPs).
Ankrd2 was discovered from human esophageal carcinoma cell line, and its expression is restricted mostly to skeletal muscle (13). Recently, it has been shown that Ankrd2 can interact with 3 transcription factors, YB1, PML, and p53, as well as with the Z-disc protein telethonin (14), which suggests that Aknrd2 might have a role in cellular signaling pathways. In addition, Ankrd2 protein is localized mostly in the nucleus in proliferating myoblasts, but as the myoblasts differentiate into multinucleated myotubes, Ankrd2 accumulates in the cytoplasm (15). It has been shown that Ankrd2 is regulated by the muscle-specific transcription factor MyoD (16). Furthermore, overexpression of Ankrd2 in C2C12 myoblasts significantly affects myogenic differentiation with the down-regulation of MyoD, Myogenin, and their target gene Myh1, as well as the induction of cell cycle arrest and p53-dependent apoptotic pathway (15), which suggests that Ankrd2 has a major role in skeletal muscle formation as a myogenic regulator.
Expression of Ankrd2 is apparently induced in response to various forms of muscle stress. For example, Ankrd2 is up-regulated in muscles after chronic immobilization in a stretched position (17) and in denervated muscles (18). It has been shown that Ankrd2 mRNA levels are elevated a few hours after exercise (19, 20). Ankrd2 is highly responsive to mechanical forces both in vivo and in vitro (21). Furthermore, many studies have also shown that Ankrd2 is highly responsive to muscle plasticity (22,23,24). These lines of evidence suggest that Ankrd2 is an important protein that plays essential roles in skeletal muscle under stress conditions. However, the molecular mechanism that regulates Ankrd2 gene expression in skeletal muscle is completely unknown.
The present study aimed to investigate whether stretch can alter Ankrd2 gene regulation in the diaphragm of wild-type (WT) mice and whether such alteration is dependent on the direction of stretch. The present study also focused on determining whether mutation in the titin gene could dysregulate Ankrd2 gene expression in mdm mouse diaphragm in response to stretch. We found that stretch anisotropically regulates Ankrd2 gene expression in WT mouse diaphragm. Stretch in the longitudinal direction up-regulates Ankrd2 expression through Akt-NF-κB-dependent signaling pathway. In contrast, stretch in the transverse direction activates AP-1 through GTP-Ras-dependent Raf-1-Erk1/2 signaling pathway that up-regulates Ankrd2 expression. Surprisingly, the stretch-induced Ankrd2 regulation in mdm mouse diaphragm was similar to that of WT mouse diaphragm. However, the basal Ankrd2 expression in mdm mouse diaphragm is high and is independent of the NF-κB and AP-1 signaling pathways.
MATERIALS AND METHODS
Materials
The following inhibitors were obtained from Calbiochem (La Jolla, CA, USA): Akt inhibitor IV (Akt inhibitor IV is a cell-permeable benzimidazole compound that inhibits Akt phosphorylation by targeting the ATP binding site of a kinase upstream of AKT and downstream of PI3K), IKK inhibitor BAY 11-7082, Raf-1 inhibitor GW5074, MEK1/2 inhibitor U0126, and JNK inhibitor SP600125. The p50 and p65 inhibitor peptides (decoy) were obtained from Imgenex (San Diego, CA, USA). Normal and phospho-specific antibodies specific for p50, p65, c-Jun, c-Fos, Akt, IKK, IκBα, Erk1/2, and Raf-1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Ras-GTPase activation kit was obtained from Millipore (Temecula, CA, USA).
Mice and ex vivo mechanical loading of diaphragm muscle
The C57BL/6JWT and B6.B6C3Fe-Ttnmdm−J/Cx strain mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA). Mice were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Institutional Animal Care and Use Committee of Baylor College of Medicine approved the animal protocols. Tissue preparation and ex vivo stretch were conducted as explained earlier (12, 25). In all experiments, we used 2-wk-old WT and mdm mice diaphragms. Each experiment was repeated ≥3 times in different mice (n≥3). Briefly, stretch was applied to the entire costal muscles of the left hemidiaphragm (the right hemidiaphragm was treated as unstretched control) by passively stretching the muscle in the longitudinal or transverse direction to the muscle fibers with a passive tension of ∼0.4 N/cm. This application was equivalent to a passive stress of ∼11 N/cm2. Mechanical stretch of nearly 50% from the unstressed state was achieved in the normal diaphragm muscle. It is important to recognize that unstressed length or length of excised muscle is the shortest muscle length and is equivalent to diaphragm length at lung volume of total lung capacity. Optimal length is 125% of this unstressed length. Therefore, our stretch of 50% placed the diaphragm at a length equivalent to 120% of optimal muscle length. In each of these two protocols, the muscle was held in the stretched state for 15 min and processed for analysis. For Ankrd2 mRNA and protein estimation, the diaphragms were kept in the muscle bath for 30 min after the stretch protocol.
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from diaphragm muscle using RNeasy min kit (Qiagen, Valencia, CA, USA) and treated with Turbo DNase I (Ambion, Austin, TX, USA) according to the manufacturer’s instructions. First-strand cDNA was synthesized from 1 μg total RNA by reverse transcription in a 20-μl reaction using SuperScript III RT first-strand cDNA synthesis kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s directions. All PCR assays were performed in a 25-μl reaction mixture containing 1 μl of diluted (1:5) cDNA and 200 nM of each primer using PCR master mix (Promega, Madison, WI. USA). The temperature cycle profile for the PCR reactions was 94°C for 2 min, 35 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min followed by 72°C for 5 min. GAPDH (glyceraldehyde 3-phosphate dehydrogenase) primers were used as internal control. Specificity of the PCR products was confirmed by 2% agarose gel electrophoresis and direct DNA sequencing method. Real-time PCR (qPCR) was performed in an Mx3005P qPCR system using Brilliant SYBR green qPCR Master Mixt (Stratagene, La Jolla, CA, USA) according to the manufacturer’s procedures. The temperature cycle profile for the real-time PCR reactions was 95°C for 10 min and 40 cycles of 95°C for 30 s, 60°C for 1 min, and 72°C for 1 min. Melting curve analysis was also included at one cycle of 95°C for 1 min, 55°C for 30 s, and 95°C for 30 s to verify the specificity of the amplified PCR products. The amount of mRNA transcripts (2−ΔCT) was estimated by the comparative CT (ΔCT) method and normalized to an endogenous reference (GAPDH) relative to a calibrator. The following sets of primer were used in the PCRs: 5′-GCCTCGGGGTTCAGAGTCCT-3′ and 5′-GATCTCACGTCGCAGGTCCA-3′ for Ankrd2; 5′-TGTTCCTACCCCCAATGTGT-3′ and 5′-CCTGCTTCACCACCTTCTTG-3′ for GAPDH; 5′-GTTTCTGCAAGCCACAGGGC-3′ and 5′-AACAGATGGACAGGTTCTGT-3′for Ankrd2 promoter.
Western blot
Cell lysates were isolated by using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce, Rockford, IL, USA) according to the manufacturer’s instructions. Proteins (80 μg) were resolved by SDS-PAGE and transferred to nitrocellulose membrane. Membrane was blocked with 5% fat-free milk for 1 h and probed with normal or phospho-specific primary antibody (as indicated in the Western blot pictures). Antibody binding was detected with a peroxidase-conjucated anti-rabbit or anti-mouse IgG and chemiluminescence (Pierce). α-Tubulin antibody served as an internal control.
Quantification of Ras GTPase protein
Measurement of GTP-bound Ras proteins was performed using Ras activation assay kit (Upstate Biotechnology, Lake Placid, NY, USA) following the manufacturer’s directions. Briefly, diaphragm muscles were homogenized in 1× ice-cold assay/lysis buffer (125 mM, pH 7.5; 750 mM NaCl; 5% Nonidet P-40; 50 mM MgCl2; 5 mM EDTA; and 10% glycerol), incubated for 15 min on ice and centrifuged at 14,000 g for 10 min. The supernatant from each sample (500 μl) was adjusted to 1 ml with 1× assay/lysis buffer and mixed with 40 μl Raf1 RBD agarose bead slurry followed by incubation at 4°C for 1 h with gentle agitation. The agarose beads were pelleted by centrifugation at 14,000 g for 10 s, washed 3 times with 500 μl 1× assay/lysis buffer, and resuspended in 40 μl 2× reducing SDS-PAGE sample buffer. The precipitated GTP-bound Ras proteins and total Ras in lysate were detected by immunoblot analysis using a mice monoclonal anti-Ras antibody.
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed to determine the binding activity of AP-1 or NF-κB on the mice Ankrd2 promoter. Briefly, diaphragms were cross-linked by adding 10 μl 37% formaldehyde/ml medium and incubated for 15 min at RT, followed by the addition of glycine to a final concentration of 125 mM. Cells were then washed twice with phosphate-buffered saline (PBS) and pelletized in PBS containing 1× protease inhibitor by centrifugation at 700 g at 4°C for 5 min. The cell pellets were lysed in 1 ml of lysis buffer (1% SDS, 10 mM EDTA, and 50 mM Tris, pH 8.1) and sonicated on ice to shear DNA to an average length of 200- to 1000-bp fragments. The sheared DNA were precleared by centrifugation at 10,000 g at 4°C for 10 min and split into 100-μl aliquots. To each of the 100-μl aliquots, 900 μl dilution buffer (0.01% SDS; 1.1% Triton X-100; 1.2 mM EDTA; 16.7 mM Tris-HCl, pH 8.1; and 167 mM NaCl) and 60 μl protein G agarose were added and incubated at 4°C for 1 h with rotation. The Protein G Agarose was pelleted by centrifugation at 3000 g 4°C for 1 min, and the supernatant was collected, followed by incubation with either 5 μg of affinity-purified anti-c-Fos, c-Jun, p50, p65, or normal IgG (negative control) rabbit polyclonal antibodies at 4°C for overnight with rotation (before incubation, 10 μl supernatant was removed as input and stored at 4°C). The protein-DNA complexes were then collected by addition of 60 μl protein G agarose and incubated at 4°C for 1 h, followed by 1 min centrifugation at 5000 g. The pellets were washed in 1 ml each of the following cold wash buffers in the following order: low-salt buffer (1% SDS; 1% Triton X-100; 2 mM EDTA; 20 mM Tris-HCl, pH 8.1; and 150 mM NaCl), high-salt buffer (1% SDS; 1% Triton X-100; 2 mM EDTA; 20 mM Tris-HCl, pH 8.1; and 500 mM NaCl), LiCl buffer [0.25 M LiCl, 1% IGEPAL-CA630, 1% deoxycholic acid (sodium salt), 1 mM EDTA, and 10 mM Tris-HCl, pH 8.1] and TE buffer (10 mM Tris-HCl and 1 mM EDTA, pH 8.0). During every wash, the suspension was incubated for 5 min with rotation and centrifuged at 5000 g for 1 min, and the supernatant was discarded. The protein-DNA complex was eluted by resuspending the pellet with 200 μl elution buffer (10 μl 20% SDS, 20 μl 1 M NaHCO3, and 170 μl sterile distilled water). Crosslinks of the protein-DNA complexes were reversed by addition of 8 μl 5 mM NaCl, incubating at 65°C for 5 h, and addition of 1 μl RNase A followed by incubation at 37°C for 30 min. The DNA was analyzed by PCR using Ankrd2 primers encompassing the Ankrd2 promoter region bearing the AP-1 or NF-κB transcription factor binding sequence and separated on 1% agarose gel. PCR amplification was also performed from an input sample that represents 1% of the total input chromatin.
Statistical analysis
The results are expressed as means ± se of ≥3 independent experiments. The comparison among different groups was performed by 1-way ANOVA followed by Bonferroni test. Paired data were evaluated by Student’s t test. P < 0.05 was considered statistically significant.
RESULTS
Stretch in the longitudinal or transverse direction to muscle fiber of the diaphragm up-regulates Ankrd2 expression
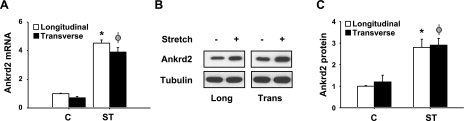
Since Ankrd2 is a stretch-responsive gene in skeletal muscle, we hypothesized that stretch in the longitudinal or transverse direction to the diaphragm muscle fibers alters Ankrd2 gene expression. To test this hypothesis, we applied a static stretch either longitudinally or transversely to the muscle fibers in the WT mouse diaphragms for 15 min. Unstretched diaphragms were treated as controls. Total RNA and protein were isolated from the diaphragms and subjected to RT-PCR and Western blot analyses to determine Ankrd2 expression level. Our data showed that longitudinal or transverse stretch significantly increased Ankrd2 mRNA and protein levels (Fig. 1A, B). qPCR analysis showed that stretch by both directions increased Ankrd2 mRNA levels to 4.5-fold (Fig. 1A). The densitometry analysis showed that stretch in longitudinal or transverse direction increased Ankrd2 protein levels to ∼2.8-fold (Fig. 1C). Tubulin, a control for Ankrd2 protein, was relatively unchanged by stretch. These data indicate that Ankrd2 is a mechanosensitive gene and that its expression increases in response to both longitudinal and transverse directional stretches in the diaphragms of WT mouse.
Figure 1.
Longitudinal and transverse directional stretches up-regulate Ankrd2 mRNA and protein expressions in the mouse diaphragm. Diaphragms were excised and stretched in ex vivo either longitudinally or transversely to the myofibers for 15 min or maintained under unstretched conditions. A) Total RNA (1 μg) prepared from each diaphragm was used to synthesize cDNA, and equal amounts of cDNA from each sample were subjected to real-time qPCR assays to estimate Ankrd2 and GAPDH (as a normalizer) mRNA levels. The level of Ankrd2 mRNA was presented relative to that of the GAPDH mRNA level. B) Whole-cell lysates prepared from each diaphragm (85 μg/sample) were resolved by SDS-PAGE gel and immunoblotted with Ankrd2 or tubulin (loading control) antibody to show Ankrd2 and tubulin protein expressions. C) Relative level of Ankrd2 protein was determined by densitometry of the Western blot image and compared to that in control. Values represent the mean ± sem of three independent experiments. C, control; ST, stretch. Values represent the mean ± se of 3 independent experiments. *P < 0.05 vs. control. Gel pictures are representative of 3 independent experiments (n=3).
Stretch differentially regulates Ankrd2 promoter
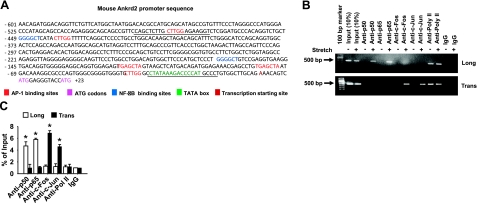
To determine the specific transcription factor by which stretch up-regulates Ankrd2 expression, we analyzed the Ankrd2 promoter sequence using the public software PATCH (available at http://www.gene-regulation. com/cgi-bin/pub/programs/patch/bin/patch.cgi). A scan of the 600-bp genomic sequence located upstream of the transcription initiation site in Ankrd2 gene identified many putative transcription factor binding sites, including 5 AP-1 and 2 NF-κB consensus binding sites (Fig. 2A). We have shown previously that stretch activates AP-1 and NF-κB in mouse diaphragm (25,26,27). These findings provided a rationale to identify whether Ankrd2 is a transcriptional target of AP-1 and/or NF-κB. We performed ChIP assays to test whether these transcription factors can bind directly to the promoter region of Ankrd2. As shown in Fig. 2B, stretch in the longitudinal direction recruited the NF-κB heterodimer p50 and p65 proteins to the Ankrd2 promoter. In contrast, stretch in the transverse direction recruited the AP-1 heterodimer c-Fos and c-Jun proteins to Ankrd2 promoter (Fig. 2B). Moreover, the qPCR results showed that the recruitment of c-Fos was significantly higher than c-Jun and showed no significant difference between the recruitments of p50 and p65 proteins to the Ankrd2 promoter (Fig. 2C). These results suggest that stretch regulates the Ankrd2 promoter through the activation of AP-1 and NF-κB in WT mouse diaphragm. The recruitment of AP-1 and NF-κB to Ankrd2 promoter is dependent on the direction of stretch.
Figure 2.
Stretch activates Ankrd2 promoter through AP-1 and NF-κB transcription factors. A) Sequence of Ankrd2 promoter shows putative binding sites for AP-1 (red) and NF-κB (blue) transcription factors followed by a TATA box (green), transcription initiation site (bold black) and two ATG start codons (indigo). Underscores represent primer sequences used in ChIP assays. B) Diaphragm of WT mouse was excised and stretched either longitudinally or transversely to the myofibers for 15 min or maintained under unstretched conditions. Immediately after stretch, the diaphragms were cross-linked with formaldehyde and processed for ChIP assays using c-Fos, c-Jun, p50, p65, RNA polymerase II (positive control), or nonspecific antibody (negative control). Equal amount of resulting DNA (100 ng) from the ChIP assays were subjected to PCR analyses with primers specific for Ankrd2 or GAPDH promoter and resolved in 2% agarose gel to show the binding activity of AP-1 or NF-κB on the Ankrd2 promoter. The fraction of chromatin used in the ChIP is shown (10% input). C) Equal amounts of DNA from the above samples were subjected to real-time qPCR assays to estimate Ankrd2 or GAPDH promoter DNA levels. The ΔCT value of Ankrd2 DNA level was calibrated against that of the input DNA level to obtain the ΔΔCT value. A 1.0-fold change in site occupancy indicates no difference in the global transcription or IP efficiency. Values represent the mean ± se of 3 independent experiments. *P < 0.05 for corresponding stretch and nonstretch samples. Gel pictures are representative of 3 independent experiments (n=3).
Stretch activates Akt and NF-kB in mouse diaphragm
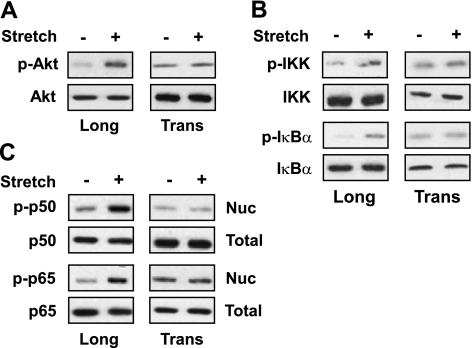
It has been shown that longitudinal stretch activates Akt, IKK, and Nf-κB of diaphragm muscle (28, 29). IKK is a well-known upstream signaling protein in the classical NF-κB signaling pathway in which degradation of IκB by IKK activates NF-κB (30, 31). To determine whether the stretch-induced activation of NFκB is mediated through Akt, we stretched mouse diaphragm for 15 min, and total or nuclear protein was isolated for Western blot assays. Stretch in the longitudinal direction phosphorylated Akt, IKK, and IκBα proteins (Fig. 3A, B). In contrast, stretch in the transverse direction did not phosphorylate any of these proteins. Next, we determined whether stretch could induce NF-κB phosphorylation and subsequent nuclear translocation. We performed Western blots using proteins from the above experiments (Fig. 3A, B). Stretch in the longitudinal direction of the muscle fiber induced the phosphorylation of both p50 and p65 proteins and subsequent translocation to the nucleus (Fig. 3C). In contrast, transverse stretch did not induce the phosphorylation of p50 and p65 proteins. These results indicate that Akt-NF-κB signaling pathway is only mechanosensitive in response to longitudinal stretch of the diaphragm.
Figure 3.
Longitudinal stretch phosphorylates Akt, IKK, IκBα, p50, and p65 in the mouse diaphragm. Diaphragms were stretched for 15 min either longitudinally or transversely to the direction of the muscle fibers. Cytoplasmic and nuclear lysates prepared from each diaphragm were resolved, 85 μg for each sample, by SDS-PAGE gel and immunoblotted with ether normal or phospho-specific Akt (A), IKK and IκBα (B), p50 and p65 (C), or tubulin (loading control) antibody. Gel pictures are representative of 3 independent experiments (n=3).
The stretch-activated Akt-NF-κB signaling pathway regulates Ankrd2 expression
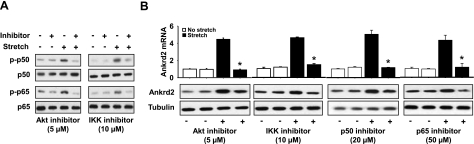
To determine whether the stretch-induced Akt-NF-κB signaling pathway can regulate Ankrd2 expression in the diaphragms of WT mouse, we preincubated the diaphragms in a muscle bath treated with any of the following inhibitors: Akt inhibitor IV, BAY 11–7082 (IKK inhibitor), p50 inhibitor peptide, or p65 inhibitor peptide for 30 min followed by transverse stretch. Total RNA and protein were isolated and used in qRT-PCR and Western blot assays, respectively. As shown in Fig. 4A, Akt inhibitor blocked the phosphorylation of p50 and p65 proteins and their subsequent nuclear translocation in response to stretch, which suggests that Akt is an upstream signaling protein of NF-κB. The IKK inhibitor BAY 11-7082 also blocked the phosphorylation of p50 and p65 proteins and their subsequent nuclear translocation (Fig. 4A). Moreover, the inhibitors of Akt, IKK, p50, and p65 significantly decreased the stretch-induced Ankrd2 mRNA and protein expressions (Fig. 4B). These results provide experimental evidence demonstrating that Akt-NF-κB signaling pathway regulates the longitudinal stretch-induced Ankrd2 expression in the WT mouse diaphragm.
Figure 4.
Longitudinal stretch regulates Ankrd2 through Akt dependent NF-κB signaling pathway. Diaphragms of WT mice were preincubated with or without 5 μM Akt inhibitor IV, 10 μM IKK inhibitor BAY 11-7082, 20 μM p50 inhibitor peptide, or 50 μM p65 inhibitor peptide for 30 min. After preincubation, the diaphragms were subjected to stretch longitudinally to the myofibers for 15 min or maintained under unstretched conditions. A) Cytoplasmic and nuclear lysates prepared from each diaphragm (85 μg/sample) were resolved by SDS-PAGE gel and immunoblotted with ether normal or phospho-specific p50 or p65 antibody. Gel pictures are representative of 3 independent experiments (n=3). B) Top panels: total RNA (1 μg) prepared from each diaphragm was subjected to cDNA synthesis, and equal amounts of cDNA from the above samples were subjected to real-time qPCR assays to estimate Ankrd2 and GAPDH (as a normalizer) mRNA levels. Level of Ankrd2 mRNA is presented relative to that of GAPDH mRNA level. Values represent the mean ± se of 3 independent experiments. *P < 0.05 vs. corresponding stretch sample. Bottom panels: cytoplasmic and nuclear lysates prepared from each diaphragm (85 μg/sample) were resolved by SDS-PAGE gel and immunoblotted with ether Ankrd2 or tubulin (loading control) antibody. Gel pictures are representative of 3 independent experiments (n=3).
Both longitudinal and transverse stretches activate Ras, Raf-1, Erk1/2, and AP-1 in mouse diaphragm
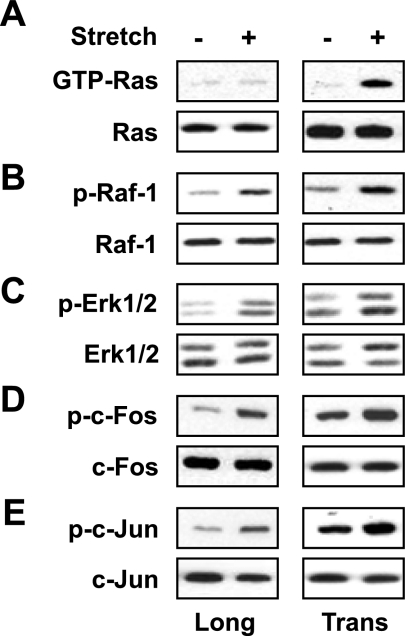
Previously, we have shown that stretch either in longitudinal or transverse direction activates Raf-1, Erk-1/2, and AP-1 in mouse diaphragm muscle (25). To assess the activations of Raf-1, Erk-1/2 and AP-1 diaphragms were stretched longitudinally or transversely to the muscle fiber for 15 min. Total or nuclear protein was isolated and used in the Western blot assays. As shown in Fig. 5A, stretch only in the transverse direction activated Ras-GTP protein; whereas stretch in both longitudinal and transverse directions activated Raf-1, ERK1/2, c-Fos, and c-Jun proteins (Fig. 5B–E). These results demonstrate that the activation of Ras-GTP is dependent on the direction of stretch, whereas the activation of Raf-1, Erk1/2, and AP-1 is independent of the direction of stretch.
Figure 5.
Both longitudinal and transverse stretches phosphorylates Raf-1, Erk1/2, c-Fos, and c-jun in the diaphragm muscles. Diaphragms of WT mice were stretched for 15 min longitudinally or transversely to the myofibers. A–C) Cytoplasmic lysates prepared from each diaphragm were either used to determine GTP bound Ras protein (A) or resolved (85 μg/sample) by SDS-PAGE gel and immunoblotted with ether normal or phospho-specific Raf-1 (B) or Erk1/2 antibody (C). D, E) Nuclear lysates prepared from each diaphragm (85 μg/sample) were resolved by SDS-PAGE gel and immunoblotted with ether normal or phospho-specific c-Fos (D) or c-Jun antibody (E). Tubulin antibody was used as a loading control. Gel pictures are representative of 3 independent experiments (n=3).
Ras-, Raf-1-, Erk1/2-, and AP-1-dependent signaling pathway regulates Ankrd2 expression in response to transverse but not longitudinal stretch
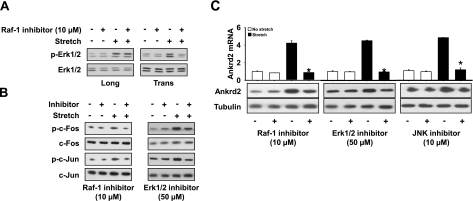
First, we sought to determine whether Raf-1 could regulate Erk1/2, c-Fos, and c-Jun phosphorylations in response to stretch. We used the commercially available Raf-1 inhibitor GW5074. GW5074 is reported to be highly selective for Raf-1 compared with a panel of related kinases (32). Diaphragms were preincubated in GW5074 for 30 min, followed by stretch either longitudinally or transversely to the muscle fibers. Total RNA and protein were isolated from the diaphragms immediately after the end of the stretch protocol and used in RT-PCR and Western blot, respectively. As shown in Fig. 6A, B, Raf-1 inhibitor reduced Erk1/2, c-Fos, and c-Jun phosphorylations in response to longitudinal or transverse stretch, which suggests that Raf-1 is an upstream target of Erk1/2. Second, we sought to determine whether Erk1/2 could be involved in the phosphorylation of c-Fos and c-Jun. We used the Erk1/2 inhibitor UO126. Diaphragms were preincubated in UO126 for 30 min and stretched longitudinally or transversely. Total or nuclear protein and RNA were isolated and used in Western blot and RT-PCR, respectively. The phosphorylation of c-Fos and c-Jun by stretch was blocked by Erk1/2 inhibitor (Fig. 6B), which suggests that the stretch-induced c-Fos and c-Jun phosphorylation is mediated through Erk1/2. Next, we sought to determine whether Raf-1 is necessary for Ankrd2 expression. We used the RNA and proteins isolated from the above Raf-1 (Fig. 6A) inhibitor experiments. As shown in Fig. 6C, blocking of Raf-1 by GW5074 decreased Ankrd2 expression, which was increased by transverse stretch. These data suggest that Raf-1 regulates Ankrd2 expression in response to transverse stretch. Finally, we sought to determine whether the Raf-1-dependent Erk1/2, c-Fos, and c-Jun can regulate Ankrd2 expression (Fig. 6B). To determine the involvement of c-Jun in the regulation of Ankrd2 expression, we used the JNK inhibitor SP600125. As shown in Fig. 6C, the inhibitors of Erk1/2 and c-Jun attenuated Ankrd2 mRNA levels in response to transverse stretch. A similar trend was also observed for Ankrd2 protein expression (Fig. 6C). Taken together, our results provide experimental evidence demonstrating that stretch in the transverse direction up-regulates Ankrd2 expression through a GTP-Ras-Raf-1-Erk1/2-AP-1-dependent signaling pathway.
Figure 6.
Transverse but not longitudinal stretch-induced Ankrd2 is regulated by Raf-1 dependent AP-1 signaling pathway in the diaphragm. Diaphragms of WT mice were preincubated with or without 10 μM Raf-1 inhibitor GW5074, 50 μM Erk1/2 inhibitor U0126, or 10 μM JNK inhibitor II SP-60025 for 30 min. After preincubation, the diaphragms were subjected to stretch transversely to the myofibers for 15 min or maintained under unstretched conditions. Cytoplasmic and nuclear lysates prepared from each diaphragm (85 μg/sample) were resolved by SDS-PAGE gel and immunoblotted with ether normal or phospho-specific Erk1/2 (A), c-Fos, c-Jun (B), or Ankrd2 (C) antidbody. Tubulin antibody was used as a loading control. Gel pictures are representative of 3 independent experiments (n=3). Total RNA (1 μg) prepared from each diaphragm was subjected to cDNA synthesis, and equal amounts of cDNA from the above samples were subjected to real-time qPCR assays to estimate Ankrd2 and GAPDH (as a normalizer) mRNA levels (C). Level of Ankrd2 mRNA is presented relative to that of GAPDH mRNA level. Values represent the mean ± se of 3 independent experiments. *P < 0.05 vs. corresponding nonstretch sample.
Mechanoregulation of Ankrd2 expression in the mdm mouse diaphragm is similar to that of WT mouse diaphragm
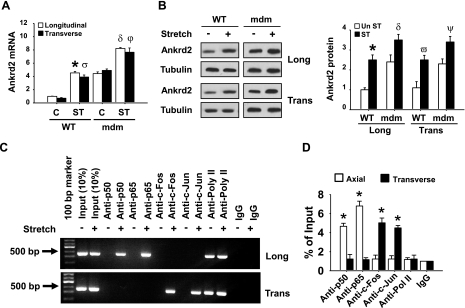
To determine whether stretch can induce Ankrd2 gene expression in the mdm mouse diaphragm, we stretched mdm diaphragms either longitudinally or transversely to the muscle fibers for 15 min. Unstretched diaphragms were treated as controls. Total RNA and protein were isolated and subjected to RT-PCR and Western blot analyses to observe Ankrd2 gene expression. As shown in Fig. 7A, B, stretching of mdm diaphragm muscle in the longitudinal or transverse direction increased Ankrd2 mRNA and protein levels. qPCR analysis showed that stretch significantly increased Ankrd2 mRNA levels by nearly 8-fold (Fig. 7A). Tubulin, a control for Ankrd2 protein, was relatively unchanged by stretch. Moreover, the basal Ankrd2 mRNA levels were relatively higher in the mdm mouse diaphragm (∼4-fold), which was lower in WT mouse diaphragm (Fig. 7A). These results indicate that, like WT mouse diaphragm, Ankrd2 is a mechanosensitive gene and that its expression increases in response to both longitudinal and transverse directional stretches in mdm mouse diaphragm.
Figure 7.
Longitudinal and transverse directional stretches up-regulate Ankrd2 mRNA and protein expressions through the activation of Ankrd2 promoter in the mdm mouse diaphragm. Diaphragms of mdm mice were excised and stretched in ex vivo longitudinally or transversely to the myofibers for 15 min or maintained under unstretched conditions. A) Estimation of Ankrd2 mRNA by qPCR as described in Fig. 1A. Level of Ankrd2 mRNA is presented relative to that of GAPDH mRNA level (normalizer). B) Whole-cell lysates prepared from each diaphragm (85 μg/sample) were resolved by SDS-PAGE gel and immunoblotted with Ankrd2 or tubulin (loading control) antibody to show Ankrd2 and tubulin protein expressions. In another experiment, immediately after stretch, the diaphragms were cross-linked with formaldehyde and processed for ChIP assays as described in Fig. 2B. C) Agarose gel pictures show the binding activity of AP-1 or NF-κB on the Ankrd2 promoter. The fraction of chromatin used in the ChIP is shown (1% input). D) Equal amounts of DNA from the above samples were subjected to real-time qPCR assays to estimate Ankrd2 or GAPDH promoter DNA levels. Values represent the mean ± se of 3 independent experiments. *P < 0.05 for corresponding stretch and nonstretch samples. Greek letters on bars indicate significant difference to respective control. Gel pictures are representative of 3 independent experiments (n=3).
Next, we sought to determine whether the NF-κB or AP-1 signaling pathway that regulates Ankrd2 expression in WT mouse diaphragm may be involved in the regulation of Ankrd2 expression in the mdm mouse diaphragm. We performed ChIP assays to test whether these transcription factors bind directly to the Ankrd2 promoter. As shown in Fig. 7C, stretch in the longitudinal direction recruited p50 and p65 proteins to the Ankrd2 promoter. In contrast, stretch in the transverse direction recruited c-Fos and c-Jun proteins to the Ankrd2 promoter (Fig. 7C). Moreover, in the mdm mouse diaphragm, the recruitment of p65 was significantly higher than p50, and no significant difference was found between c-Fos and c-Jun protein recruitment to the Ankrd2 promoter (Fig. 7D). These results suggest that stretch regulates the Ankrd2 promoter through the activation of the transcription factors AP-1 and NF-κB in mdm mouse diaphragm. This differential regulation of Ankrd2 promoter by AP-1 and NF-κB is dependent on the direction of stretch.
DISCUSSION
In the present study, we uncovered anisotropic mechanosensitive regulation of Ankrd2 gene expression in skeletal muscles of WT and mdm mouse diaphragm. Our data demonstrated that both longitudinal and transverse stretches regulate Ankrd2 gene expression by two distinct signaling pathways in the WT and mdm mouse diaphragms. In particular, stretch in the longitudinal direction to the diaphragm muscle fibers activated Akt-dependent NF-κB signaling pathway, which increased Ankrd2 expression. This signaling pathway was inactive in response to transverse stretch, whereas stretch in the transverse direction activated Raf-1-dependent AP-1 signaling pathway via Erk1/2 that increased Ankrd2 expression. Furthermore, transverse stretch, but not longitudinal stretch, activated small GTP-Ras protein, which induced the Raf-1 dependent AP-1 signaling pathway. In addition, none of the above stretch-induced signaling pathways regulated the basal level of Ankrd2 expression, which was high in the mdm mouse diaphragm. This suggests the involvement of other signaling pathways in the regulation of Ankrd2 basal level in the mdm mouse diaphragm.
Mechanical stretch in skeletal muscle can be transmitted longitudinally as well as in the transverse plane of the cell (33). Biaxial loading is the most obvious feature of the mechanical environment of the diaphragm, in which muscle fibers experience both transverse and longitudinal loads during each respiratory cycle (34, 35). In this study, our experiments applied mechanical stress in either longitudinal or transverse directions to the muscle fibers, with the magnitude of the mechanical stress along the muscle fibers is essentially the same as that applied in the transverse direction to the muscle fibers. Stress-strain relationship data from our previous studies clearly demonstrate that muscle compliance is small and extensibility less in the transverse direction when compared with those in the direction of the muscle fibers (5, 35, 36). Also, the resistance to mechanical stretch is greater in the transverse direction of the muscle fibers than in the direction of the fibers (5). In the present study, the higher activations of Raf-1, Erk1/2, and AP-1 in response to transverse stretch are correlated with greater transverse muscle stiffness compared with longitudinal stiffness. Furthermore, the small Rho GTPase protein GTP-Ras, an immediate upstream target of Raf-1, was activated only by transverse stretch rather than longitudinal stretch, which suggests specificity in the mechanosensing ability of this signaling protein. Taken together, these results suggest that activation of signaling proteins by mechanosignal transduction in the diaphragm muscle is dependent on the direction of mechanical force that applied to the diaphragm muscle fibers.
The use of specific inhibitors has been a powerful tool in delineation of the downstream signaling in response to numerous stimuli. Use of inhibitors to block specific signaling protein in the diaphragm muscle has been validated in our laboratory (25, 26, 29, 37). The specificity of each inhibitor was determined by detecting phosphorylation of the corresponding signaling protein (data not shown) in the diaphragm. In addition, we used vehicle in which the inhibitor was dissolved (either water or DMSO) as a control. In this study, we identified the anisotropic regulation of Ankrd2 expression by two different stretch-induced signaling pathways using specific inhibitors. Our previous findings show that stretching of diaphragm in the longitudinal or transverse direction activates two distinct MAP kinase signal transduction pathways (25). In particular, both longitudinal and transverse stretches activate Raf-1-dependent AP-1 signaling pathways through the activation of Erk1/2. Although the activation of Erk1/2 by either form of stretches, the PI-3K and PKC were involved in the activation of Erk1/2 in response to longitudinal stretch, whereas PKA was involved in the activation of Erk1/2 in response to transverse stretch. This study was limited to signaling proteins, whereas our current study was extended to gene level. It is important to note that this is the first study to demonstrate regulation of gene expression by mechanosignal transduction pathway in the intact diaphragm muscles. More precisely, stretching diaphragm in the longitudinal direction activated Akt-dependent NF-κB signaling pathway. However, stretching the diaphragm either longitudinally or transversely activated Raf-1 dependent AP-1 signaling pathway through Erk1/2. This finding is consistent with our previous study (25). Surprisingly, the Raf-1 dependent AP-1 signaling pathway up-regulated Ankrd2 expression only in response to transverse stretch. This differential regulation is due to the activation of GTP-Ras. GTP-Ras was activated only in response to transverse stretch, and the activated GTP-Ras phosphorylated its immediate downstream target, Raf-1. Moreover, in our previous study, Raf-1 was activated by PTK through PKA in response to transverse stretch (25). In the present study, although Raf-1 was activated by PTK and PKA (data not shown), this pathway did not regulate Ankrd2 expression. In other words, transverse stretch-induced Ankrd2 up-regulation in the diaphragm is mediated through the GTP-Ras-activated Raf-1 signaling pathway rather than the PTK-activated Raf-1 pathway. Many lines of evidence show that Raf is one of the best-characterized target proteins of Ras and that the binding of active Ras to Raf triggers the activation of the Erk-MAPK pathway, which participates in a variety of cellular, physiological, and pathological responses (38,39,40,41,42,43). Taken together, our data provide experimental evidence demonstrating that stretch transverse to the diaphragm muscle fibers activates GTP-Ras, which then up-regulates Ankrd2 expression through its immediate downstream target Raf-1-dependent Erk1/2-AP-1 signaling pathway. Furthermore, we noticed that no dysregulation in the stretch-induced Ankrd2 expression in mdm mouse diaphragm. This finding is most likely due to the age of the mdm mice that we used in this study. Muscular dystrophy in mdm mice starts after 2 wk and peaks at 6 wk. In our previous study, we showed that histopathological signs in the mdm diaphragm were largely absent at 2 wk when compared to the 6-wk-old mdm diaphragm (12). Therefore, our experiments using 2-wk-old mdm mice before onset of the disease may explain the similarity in the regulation of Ankrd2 between WT and mdm mouse diaphragm in response to stretch.
Ankrd2 is localized on both the I-band of the sarcomere and nucleus, providing a link between mechanical stretch and gene expression (21). Moreover, up-regulation of Ankrd2 in diseased skeletal muscle and in normal skeletal muscle after injury or exercise suggests a role in stress-response pathways that lead to muscle hypertrophy (14, 21). We reported previously that mdm mouse diaphragm was mechanically dysfunctional and that there were histopathological signs of muscular dystrophy, including centrally located nuclei, increased variation in myofiber diameter, hypertrophy, and fatty or connective tissue infiltration (12). Consistent with these studies, our present data showed that the basal level of Ankrd2 was up-regulated in the mdm mouse diaphragm, which suggests the pathological role of Ankrd2 in this mouse model. This finding is in agreement with a previous study in which Ankrd2 was shown to be regulated by the bHLH transcription factor MyoD in C2C12 cells during differentiation (16). Moreover, recent data showed that enforced expression of Ankrd2 inhibited myogenesis in C2C12 cells (15). Unpublished data from our laboratory also showed that myoblasts isolated from mdm mouse failed to differentiate into myotubes and that siRNA-mediated knockdown of Ankrd2 reverse this effect. These findings suggest that Ankrd2 has a major role in the skeletal muscle dysfunction via its involvement in the inhibition of myogenesis and/or the induction of hypertrophy in mdm mice.
We reported previously that longitudinal stretch activates Akt and NF-κB in diaphragm muscle (26). In this study, we determined that longitudinal stretch activates NF-κB via Akt, which suggests an interconnection between signaling pathways and sharing of substrates among multiple enzymes (44, 45). Classical NF-κB activity is initiated in response to extracellular signals, which trigger the activation of the IKK complex, composed of IKKα, IKKβ, and IKKγ, or NEMO (30, 31). The activated IKK complex phosphorylates IκB bound to NF-κB, which leads to IκB ubiquitination and subsequent proteasomal degradation. Released NF-κB, most commonly found as a p65/p50 heterodimer, is then free to translocate to the nucleus where it binds and regulates the expression of its target genes (46, 47). In this study, the activation of NF-κB, IκBα, and IKK by stretch suggested that NF-κB activation is preceded by the activation of IKK via the subsequent activation of IκBα protein. This finding was supported further by the IKK inhibitor experiments, in which the inhibition of IKK blocked the stretch-induced activation of NF-κB. Since the activation of NF-κB was associated with the activation of IκB, these results suggest that stretch activates NF-κB via the canonical IκB phosphorylation-degradation cycle pathway that requires activation of the IKKα. In addition, we used both anti-p50 and anti-p65 antibodies to identify the specific homo- or heterodimer of activated NF-κB complex in the nuclear extracts of diaphragm muscles, and the result showed that the active NF-κB complex contains mainly the heterodimers of p50 and p65. Furthermore, using anti-p50 and anti-p65 antibodies in the ChIP assay, we confirmed the stretch-induced activity of p50 and p65 proteins and their binding ability to the Ankrd2 promoter. This finding is consistent with our previous findings in which longitudinal stretch of the diaphragm muscle activates p50 and p65 proteins (26).
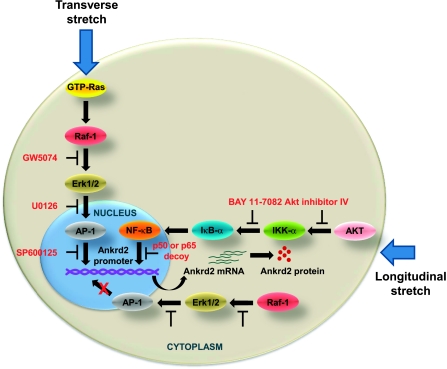
In summary, our study reveals that stretch anisotropically regulates Ankrd2 gene expression by two independent signaling pathways (Fig. 8). This anisotropic signaling property of the diaphragm is due to the sensing ability of signaling molecules to forces. Moreover, higher activation of the basal level Ankrd2 in the mdm mouse diaphragm suggests that this protein may have a critical role in skeletal muscle dysfunction in this model of muscular dystrophy.
Figure 8.
Schematic representation of anisotropic regulation of Ankrd2 in the diaphragm muscle. Potential mechanism of activation of two distinct signaling pathways and their involvement in the transcription of Ankrd2 gene. Longitudinal stretch of the diaphragm up-regulates Ankrd2 gene expression via the activation of Akt-dependent NF-κB signaling pathway. Despite activation of Raf-1-dependent AP-1 signaling pathway via the activation of Erk1/2 by either form of stretch, only the transverse stretch-activated Raf-1-dependent AP-1 pathway regulates Ankrd2 gene expression by GTP-Ras, which is activated only by transverse stretch.
Acknowledgments
National Heart, Lung and Blood Institute grant HL-63134 and National Science Foundation grant CBET-0650686 supported this work.
References
- Goldspink G, Scutt A, Loughna P T, Wells D J, Jaenicke T, Gerlach G F. Gene expression in skeletal muscle in response to stretch and force generation. Am J Physiol. 1992;262:R356–R363. doi: 10.1152/ajpregu.1992.262.3.R356. [DOI] [PubMed] [Google Scholar]
- Roy R R, Baldwin K M, Edgerton V R. The plasticity of skeletal muscle: effects of neuromuscular activity. Exerc Sport Sci Rev. 1991;19:269–312. [PubMed] [Google Scholar]
- Boriek A M, Ortize J, Zhu D. Fiber architecture of canine abdominal muscles. J Appl Physiol. 2002;92:725–735. doi: 10.1152/jappl.2002.92.2.725. [DOI] [PubMed] [Google Scholar]
- Boriek A M, Zhu D, Zeller M, Rodarte J R. Inferences on force transmission from muscle fiber architecture of the canine diaphragm. Am J Physiol. 2001;280:R156–165. doi: 10.1152/ajpregu.2001.280.1.R156. [DOI] [PubMed] [Google Scholar]
- Boriek A M, Capetanaki Y, Hwang W, Officer T, Badshah M, Rodarte J, Tidball J G. Desmin integrates the three-dimensional mechanical properties of muscles. Am J Physiol Cell Physiol. 2001;280:C46–C52. doi: 10.1152/ajpcell.2001.280.1.C46. [DOI] [PubMed] [Google Scholar]
- Granzier H L, Labeit S. The giant protein titin: A major player in myocardial mechanics, signaling, and disease. Circ Res. 2004;94:284–295. doi: 10.1161/01.RES.0000117769.88862.F8. [DOI] [PubMed] [Google Scholar]
- Lange S, Xiang F, Yakovenko A, Vihola A, Hackman P, Rostkova E, Kristensen J, Brandmeier B, Franzen G, Hedberg B, Gunnarsson L G, Hughes S M, Marchand S, Sejersen T, Richard I, Edstrom L, Ehler E, Udd B, Gautel M. The kinase domain of titin controls muscle gene expression and protein turnover. Science. 2005;308:1599–1603. doi: 10.1126/science.1110463. [DOI] [PubMed] [Google Scholar]
- Puchner E M, Alexandrovich A, Kho A L, Hensen U, Schäfer L V, Brandmeier B, Gräter F, Grubmüller H, Gaub H E, Gautel M. Mechanoenzymatics of titin kinase. Proc Natl Acad Sci U S A. 2008;105:13385–13390. doi: 10.1073/pnas.0805034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tskhovrebova L, Trinick J. Titin: properties and family relationships. Nat Rev Mol Cell Biol. 2003;4:679–689. doi: 10.1038/nrm1198. [DOI] [PubMed] [Google Scholar]
- Hackman P, Vihola A, Haravuori H, Marchand S, Sarparanta J, de Seze J, Labeit S, Witt C, Peltonen L, Richard I, Udd B. Tibial muscular dystrophy is a titinopathy caused by mutations in TTN, the gene encoding the giant skeletal-muscle protein titin. Am J Hum Gen. 2002;71:492–500. doi: 10.1086/342380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haravuori H, Vihola A, Straub V, Auranen M, Richard I, Marchand S, Voit T, Labeit S, Somer H, Peltonen L, Beckmann J S, Udd B. Secondary calpain3 deficiency in 2q-linked muscular dystrophy: titin is the candidate gene. Neurology. 2001;56:869–877. doi: 10.1212/wnl.56.7.869. [DOI] [PubMed] [Google Scholar]
- Lopez M A, Pardo P S, Cox G A, Boriek A M. Early mechanical dysfunction of the diaphragm in the muscular dystrophy with myositis (Ttnmdm) model. Am J Physiol Cell Physiol. 2008;295:C1092–C1102. doi: 10.1152/ajpcell.16.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama M, Tsukamoto Y, Fujiwara M, Kondo G, Nakada C, Baba T, Ishiguro N, Miyazaki A, Nakamura K, Hori N, Sato K, Shomori K, Takeuchi K, Satoh H, Mori S, Ito H. Identification of a novel human ankyrin-repeated protein homologous to CARP. Biochem Biophys Res Commun. 2001;285:713–723. doi: 10.1006/bbrc.2001.5216. [DOI] [PubMed] [Google Scholar]
- Kojic S, Medeot E, Guccione E, Krmac H, Zara I, Martinelli V, Valle G, Faulkner G. The Ankrd2 protein, a link between the sarcomere and the nucleus in skeletal muscle. J Mol Biol. 2004;339:313–325. doi: 10.1016/j.jmb.2004.03.071. [DOI] [PubMed] [Google Scholar]
- Bean C, Facchinello N, Faulkner G, Lanfranchi G. The effects of Ankrd2 alteration indicate its involvement in cell cycle regulation during muscle differentiation. Biochim Biophys Acta—Mol Cell Res. 2008;1783:1023–1035. doi: 10.1016/j.bbamcr.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Bean C, Salamon M, Raffaello A, Campanaro S, Pallavicini A, Lanfranchi G. The Ankrd2, Cdkn1c and calcyclin genes are under the control of MyoD during myogenic differentiation. J Mol Biol. 2005;349:349–366. doi: 10.1016/j.jmb.2005.03.063. [DOI] [PubMed] [Google Scholar]
- Kemp T J, Sadusky T J, Saltisi F, Carey N, Moss J, Yang S Y, Sassoon D A, Goldspink G, Coulton G R. Identification of Ankrd2, a novel skeletal muscle gene coding for a stretch-responsive ankyrin-repeat protein. Genomics. 2000;66:229–241. doi: 10.1006/geno.2000.6213. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Senda T, Nakano T, Nakada C, Hida T, Ishiguro N, Kondo G, Baba T, Sato K, Osaki M, Mori S, Ito H, Moriyama M. Arpp, a new homolog of carp, is preferentially expressed in type 1 skeletal muscle fibers and is markedly induced by denervation. Lab Invest. 2002;85:645–655. doi: 10.1038/labinvest.3780459. [DOI] [PubMed] [Google Scholar]
- Ishiguro N, Baba T, Ishida T, Takeuchi K, Osaki M, Araki N, Okada E, Takahashi S, Saito M, Watanabe M, Nakada C, Tsukamoto Y, Sato K, Ito K, Fukayama M, Mori S, Ito H, Moriyama M. Carp, a cardiac ankyrin-repeated protein, and its new homologue, Arpp, are differentially expressed in heart, skeletal muscle, and rhabdomyosarcomas. Am J Pathol. 2002;160:1767–1778. doi: 10.1016/S0002-9440(10)61123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehti T, Kalliokoski R, Komulainen J. Repeated bout effect on the cytoskeletal proteins titin, desmin, and dystrophin in rat skeletal muscle. J Muscle Res Cell Motil. 2007;28:39–47. doi: 10.1007/s10974-007-9102-0. [DOI] [PubMed] [Google Scholar]
- Miller M K, Bang M-L, Witt C C, Labeit D, Trombitas C, Watanabe K, Granzier H, McElhinny A S, Gregorio C C, Labeit S. The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules. J Mol Biol. 2003;333:951–964. doi: 10.1016/j.jmb.2003.09.012. [DOI] [PubMed] [Google Scholar]
- McKoy G, Hou Y, Yang S Y, Vega Avelaira D, Degens H, Goldspink G, Coulton G R. Expression of Ankrd2 in fast and slow muscles and its response to stretch are consistent with a role in slow muscle function. J Appl Physiol. 2005;98:2337–2343. doi: 10.1152/japplphysiol.01046.2004. [DOI] [PubMed] [Google Scholar]
- Barash I A, Mathew L, Ryan A F, Chen J, Lieber R L. Rapid muscle-specific gene expression changes after a single bout of eccentric contractions in the mouse. Am J Physiol Cell Physiol. 2004;286:C355–C364. doi: 10.1152/ajpcell.00211.2003. [DOI] [PubMed] [Google Scholar]
- Hentzen E R, Lahey M, Peters D, Mathew L, Barash I A, Fridén J, Lieber R L. Stress-dependent and -independent expression of the myogenic regulatory factors and the MARP genes after eccentric contractions in rats. J Physiol. 2006;570:157–167. doi: 10.1113/jphysiol.2005.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Chaudhry I, Reid M B, Boriek A M. Distinct signaling pathways are activated in response to mechanical stress applied axially and transversely to skeletal muscle fibers. J Biol Chem. 2002;277:46493–46503. doi: 10.1074/jbc.M203654200. [DOI] [PubMed] [Google Scholar]
- Kumar A, Boriek A M. Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: a possible role in Duchenne muscular dystrophy. FASEB J. 2003;17:386–396. doi: 10.1096/fj.02-0542com. [DOI] [PubMed] [Google Scholar]
- Kumar A, Takada Y, Boriek A M, Aggarwal B B. Nuclear factor-kappa B: its role in health and disease. J Mol Med. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- Charu D, Harish C, Jon E W, Ashok K. Regulation of phosphatidylinositol 3-kinase (PI3K)/Akt and nuclear factor-kappa B signaling pathways in dystrophin-deficient skeletal muscle in response to mechanical stretch. J Cell Physiol. 2006;208:575–585. doi: 10.1002/jcp.20696. [DOI] [PubMed] [Google Scholar]
- Pardo P S, Lopez M A, Boriek A M. FOXO transcription factors are mechanosensitive and their regulation is altered with aging in the respiratory pump. Am J Physiol Cell Physiol. 2008;294:C1056–C1066. doi: 10.1152/ajpcell.00270.2007. [DOI] [PubMed] [Google Scholar]
- Baldwin A S. The NF-κB and IκB proteins: New discoveries and insights. Ann Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Baeuerle P A, Henkel T. Function and activation of NF-kappaB in the immune system. Ann Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Lackey K, Cory M, Davis R, Frye S V, Harris P A, Hunter R N, Jung D K, McDonald O B, McNutt R W, Peel M R, Rutkowske R D, Veal J M, Wood E R. The discovery of potent cRaf1 kinase inhibitors. Bioorg Med Chem Lett. 2000;10:223–226. doi: 10.1016/s0960-894x(99)00668-x. [DOI] [PubMed] [Google Scholar]
- Boriek A M, Miller C C, Iii, Rodarte J R. Muscle fiber architecture of the dog diaphragm. J Appl Physiol. 1998;84:318–326. doi: 10.1152/jappl.1998.84.1.318. [DOI] [PubMed] [Google Scholar]
- Margulies S S, Lei G T, Farkas G A, Rodarte J R. Finite-element analysis of stress in the canine diaphragm. J Appl Physiol. 1994;76:2070–2075. doi: 10.1152/jappl.1994.76.5.2070. [DOI] [PubMed] [Google Scholar]
- Boriek A M, Kelly N G, Rodarte J R, Wilson T A. Biaxial constitutive relations for the passive canine diaphragm. J Appl Physiol. 2000;89:2187–2190. doi: 10.1152/jappl.2000.89.6.2187. [DOI] [PubMed] [Google Scholar]
- Patel N D, Jannapureddy S R, Hwang W, Chaudhry I, Boriek A M. Altered muscle force and stiffness of skeletal muscles in alpha -sarcoglycan-deficient mice. Am J Physiol Cell Physiol. 2003;284:C962–C968. doi: 10.1152/ajpcell.00326.2002. [DOI] [PubMed] [Google Scholar]
- Kumar A, Khandelwal N, Malya R, Reid M B, Boriek A M. Loss of dystrophin causes aberrant mechanotransduction in skeletal muscle fibers. FASEB J. 2004;18:102–113. doi: 10.1096/fj.03-0453com. [DOI] [PubMed] [Google Scholar]
- Stokoe D, McCormick F. Activation of c-Raf-1 by Ras and Src through different mechanisms: activation in vivo and in vitro. EMBO J. 1997;16:2384–2396. doi: 10.1093/emboj/16.9.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer H J, Weber M J. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu B E, Karandikar M, Berman K, Cobb M H. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Schramek H. MAP kinases: from intracellular signals to physiology and disease. News Physiol Sci. 2002;17:62–67. doi: 10.1152/nips.01365.2001. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Takano K, Yoshida A, Katada F, Sun P, Takenawa T, Andoh T, Endo T. DA-Raf1, a competent intrinsic dominant-negative antagonist of the Ras-ERK pathway, is required for myogenic differentiation. J Cell Biol. 2007;177:781–793. doi: 10.1083/jcb.200703195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb M H, Goldsmith E J. How MAP kinases are regulated. J Biol Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- Cobb M H, Goldsmith E J. Dimerization in MAP-kinase signaling. Trends Biochem Sci. 2000;25:7–9. doi: 10.1016/s0968-0004(99)01508-x. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Bonizzi G, Karin M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]