Abstract
Cytokine generation by T cells and monocytes was determined for 50 subjects aged 65 yr or older and concurrently studied young subjects individually matched to each old subject for sex, race, and national origin. Highly significant differences between cytokine levels of old and young subjects all were gender specific. For T cells stimulated with anti-CD3 plus anti-CD28 antibodies, mean ratios of IFN-γ generation for healthy old to young subjects were 0.22 for men (P<0.001; n=15) and 3.35 for women (P<0.001; n=13), and those of IL-17 were 0.30 for men (P<0.001) and no difference for women. CD8 T cells were the source of high IFN-γ in healthy old women. For old men with an inflammatory or immune disease (n=10), mean old to young ratios of T-cell-generated IFN-γ and IL-17 increased with disease severity up to 5.78 and 2.97 (both P<0.01), respectively, without changes for old women with similar diseases (n=12). For differentiated LPS-stimulated monocytes, old to young ratios of TNF-α and IL-6 generation were high only in women with immune or inflammatory disease (2.38, P<0.05 and 1.62, P<0.01, respectively), whereas ratios of IFN-γ-evoked IP-10 chemokine were low in all groups. Alterations in immune cytokine profiles with aging show significant gender specificity.—Goetzl, E. J., Huang, M.-C., Kon, J., Patel, K., Schwartz, J. B., Fast, K., Ferrucci, L., Madara, K., Taub, D. D., Longo, D. L. Gender specificity of altered human immune cytokine profiles in aging.
Keywords: T cells, monocytes, immunodeficiency, autoimmunity
Immunosenescence, a term descriptive of deterioration of human immunity with aging, encompasses both diminished protective immunity and enhanced immune inflammation (1, 2). Of the myriad alterations in immune cells and proteins observed with aging, the most profound have been in T-cell subsets, antigen recognition repertoires, and effector functions (3,4,5,6,7). T-cell generation of a diverse series of immune cytokines, which reflects T-cell subset representation and their separate responses to different types of stimulation, is a sensitive profile of adaptive cellular immune function. Although several investigators have found significant differences between levels of cytokines produced by T cells of old and young human subjects, such findings have been difficult to interpret or even contradictory. Results of studies of human T cells and of mixed mononuclear leukocytes (MLs) have consistently shown diminished generation of IL-2 and, as a result, decreased expression of IL-2 receptors in old as compared to young subjects (3, 8, 9). In contrast, the levels of generation of IFN-γ, another T-cell-derived cytokine that is critical for both innate and adaptive immune responses, were found to be unchanged, decreased, or increased in old as compared to young subjects (9,10,11,12,13,14). Four of 5 investigations that quantified cytokine generation by MLs stimulated with a mitogenic lectin showed lower levels of IFN-γ from the old subjects as compared to young subjects, and one showed the same levels of IFN-γ from MLs of both groups (13). In one of the 4 investigations, a different type of stimulation of MLs with a phorbol ester and ionophore produced identical levels of IFN-γ for old and young subjects (11). In the only investigation in which gender was considered, the diminished levels of generation of IFN-γ by MLs of old women and old men were identical (10). Only one investigation assessed age-related differences in generation of cytokines by purified CD4 and CD8 T cells stimulated with anti-CD3 plus anti-CD28 antibodies, that showed higher levels of IFN-γ from both CD4 and CD8 T cells of old, as contrasted with young subjects (14). Studies that have evaluated ML or T-cell generation of IL-4 have yielded no differences or slight decreases in generation of IL-4 with aging (9, 11, 12, 14).
None of the published studies has employed otherwise completely matched young controls for the old subjects, systematically considered effects of gender, applied more than one immune stimulus or examined effects of immune or inflammatory diseases on T-cell generation of cytokines. In the study described here, we have paired old subjects with concurrently evaluated young subjects matched individually for gender, race, and national origin, activated isolated T cells and differentiated monocytes with two different types of immune stimuli, and extended the investigation to old subjects with mild or severe immune and inflammatory states.
MATERIALS AND METHODS
Study design and subject selection
Fifty subjects aged 65 yr or older (termed old) were studied with concurrent young controls of ages 21 to 40 yr, who were matched with each old subject for identical sex, race, and national origin. Subjects were recruited from the Ambulatory Faculty Practice of the University of California, San Francisco Medical Center, the Jewish Home of San Francisco, and the Clinical Research Unit of the National Institute on Aging at Harbor Hospital in Baltimore, MD. The study procedures and potential risks were described to every participant before they signed an informed consent approved with the study protocol by Committees for Human Research at the three institutions. Each old participant was assigned to one of 3 groups (Table 1). Group I participants were healthy, without any infections or immune/inflammatory diseases currently or during the preceding 2 yr. Group IIA participants presented with one therapeutically-responsive infection of one organ system, such as sinusitis, bronchitis, bladder infection, or skin abscess and/or a single limited inflammatory or immune condition, such as active osteoarthritis, skin rash, thyroiditis, recently treated cancer, lymphopenia to levels no lower than 20% below the lower limit of normal, or hypogamma globulinemia not requiring regular replacement therapy. Group IIB participants presented with severe persistent infections and/or an immunodeficiency such as lymphopenia to levels below 20% of the lower limit of normal or hypo-gamma globulinemia requiring regular replacement therapy, and/or an autoimmune condition such as rheumatoid arthritis, multiple sclerosis, or inflammatory parenchymal lung disease.
TABLE 1.
Characteristics of the study populations
| Group | Number | Racial distribution
|
Age (mean±sd) | Medical conditions | ||
|---|---|---|---|---|---|---|
| Ca | A-A | As | ||||
| I | No inflammatory or infectious problems | |||||
| Male | 15 | 11 | 3 | 1 | 72.4 ± 4.3 | |
| Female | 13 | 11 | 1 | 1 | 75.6 ± 7.9 | |
| IIA | ||||||
| Male | 5 | 5 | 0 | 0 | 70.6 ± 5.7 | I(2), L(3), A(2), C(2), H(1) |
| Female | 6 | 4 | 1 | 1 | 74.2 ± 5.8 | I(4), L(1), A(3), T(1) |
| IIB | ||||||
| Male | 5 | 4 | 1 | 0 | 69.2 ± 3.7 | I(4), IL(3), L(1), H(2) |
| Female | 6 | 6 | 0 | 0 | 69.7 ± 4.3 | I(3), Rh(4), L(2), T(1) |
Group I, healthy; group IIA, mild infections and/or inflammatory disease; group IIB, autoimmunity and/or severe infections; Ca, Caucasian; A-A, African-American; As, Asian; I, infections; L, lymphopenia; A, inflammatory osteoarthritis; C, recent cancer; H, hypogammaglobulinemia; T, thyroiditis; IL, inflammatory lung disease; Rh, multiple sclerosis, rheumatoid arthritis, and/or uveitis.
No young subject (all young subjects were healthy) or healthy old subject (group I) was receiving any immunoactive drug or hormone. Sixty milliliters of venous blood was taken into a heparinized syringe once from each old subject and healthy young control.
Isolation, stimulation, and flow cytometric analyses of monocytes and T cells
Heparinized blood diluted 1:1 with Ca2+- and Mg2+-free Dulbecco’s balanced salt solution was centrifuged on cushions of Ficoll-Paque (GE Healthcare Lifesciences, Pittsburgh, PA, USA) to purify mixed ML, as described previously (15). Monocytes were isolated from ML by anti-human CD14 immunomagnetic bead positive selection on columns in a magnetic field (Miltenyi Biotec, Auburn, CA, USA). Suspensions of 106 monocytes/ml of RPMI 1640 medium with 10% FBS, 100 U/ml of penicillin and 50 μg/ml of streptomycin (complete RPMI) were incubated for 24 h with 104 IU/ml of human recombinant IFN-α 2A (ProSpec-Tany TechnoGene Ltd., Rehovot, Israel) to induce differentiation (16). Aliquots of 5 × 105 conditioned monocytes in 0.5 ml of complete RPMI 1640 then were stimulated with 100 ng/ml of LPS (E. coli 0111:B4, Sigma, St. Louis, MO, USA) or 2 and 20 ng/ml of human recombinant IFN-γ (Peprotech, Rocky Hill, NJ, USA) with removal of portions of supernatant at 6 h and 24 h.
T cells were isolated from ML by immunomagnetic negative selection (human T cell isolation kit II; Miltenyi). T-cell purity and the relative representation of CD4, CD8, CD161, CD4+FOXP3+, and CD3+CD4−8− subsets were delineated by flow cytometry (17); PE-anti-human CD161 (clone HP-3G10) was from Biolegend (San Diego, CA, USA). Intracellular FOXP3 was detected with anti-FOXP3-FITC (clone PCH101) using the eBioscience (San Diego, CA, USA) protocol for staining. Replicate aliquots of 5 × 105 T cells/0.5 ml of complete RPMI were added to 48-well plates and incubated without and with 1 or 3 μg each of adherent anti-human CD3 plus anti-human CD28 antibodies or IL-12 plus IL-18 at 5 ng/ml and 50 ng/ml, respectively (18).
Cytokine ELISAs
The concentrations of IL-6, TNF-α, and IP-10 in macrophage supernatants were determined by ELISAs (eBioscience, San Diego, CA, USA). T-cell supernatant media were analyzed by a panel of ELISA kits from eBioscience. Macrophage migration inhibitory factor (MIF) was quantified with an ELISA kit from Ray Biotech (Norcross, GA, USA). Color intensity of each well was determined in an ELISA plate reader (MRX Revelation, Dynex Technologies, Chantilly, VA, USA).
Data analyses
The statistical significance of each difference between levels of surface markers and cytokines was determined by the Mann-Whitney nonparametric test. Some groups of numerical data were normally distributed or were representative of a normally distributed set, as assessed by a D statistic from the Kolmogorov-Smirnov test. For normally distributed groups of data, statistical significance also was determined by the 2-sample t test with Welch’s correction, and the level of significance was similar to that found with the nonparametric assessment.
RESULTS
Study design and subject characteristics
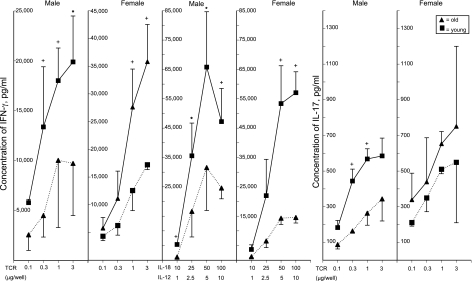
Each old subject was evaluated concurrently with a young subject matched for sex, race, and national origin to control for any temporal alterations in T-cell isolation or immune assays. The usefulness of this design was examined initially by incubating T cells from 3 old-young pairs of healthy (group 1) men and women with a range of concentrations of each principal stimulus (Fig. 1). At the higher concentrations of both T-cell antigen receptor (TCR)-directed and IL-18 + IL-12 stimuli, T cells of young men generated significantly greater levels of IFN-γ than those of old men. In contrast, TCR-stimulated T cells of old women generated significantly greater levels of IFN-γ than those of young women (Fig. 1). When stimulated by the higher concentrations of IL-18 + IL-12, however, T cells of old women generated significantly lower levels of IFN-γ than those of young women, with responses similar to those of old-young pairs of healthy men (Fig. 1). The higher levels of TCR immune stimulation elicited significantly more IL-17 generation by T cells of young men than old men, whereas there were no significant differences between the levels generated by TCR-stimulated T cells of old women and young women (Fig. 1). Accordingly, subsequent studies were performed with 1 μg/well each of anti-CD3 + anti-CD28 antibodies and 50 ng/ml of IL-18 + 5 ng/ml of IL-12, as these evoked similar levels of cytokine generation by T cells of young women and men, and revealed significant gender- and stimulus-specific differences in cytokine generation between T cells of old and young subjects.
Figure 1.
Dependence of T-cell generation of IFN-γ and IL-17 on the stimulating concentrations of anti-human CD3 + anti-human CD28 antibodies (TCR; μg/well) and of human IL-12 + IL-18 (ng/ml). Points and bars depict mean ± sd results of studies of T cells from 3 old and 3 young healthy (group I) female or male subjects. Significance of differences in values between young and old subjects after 3 d of incubation was calculated by a 2-sample t test. +P < 0.05; *P < 0.01.
Cytokine generation by T cells of young women and young men showed no significant differences in the total study population. After 3 d of incubation, T cells of young women produced mean ± sd levels of IFN-γ that were 17,370 ± 6133 and 47,540 ± 28,193 pg/ml with TCR-directed and IL-12 + IL-18 stimulation, respectively, and levels of IL-17 that were 1105 ± 512 pg/ml with TCR-directed stimulation. Parallel studies of cytokine generation by T cells from young men showed levels that were statistically indistinguishable from those of T cells of young women at 21,465 ± 6,929 pg of IFN-γ/ml, 36, 770 ± 12,830 pg of IFN-γ/ml, and 888 ± 191 pg of IL-17/ml, respectively.
The population of healthy old subjects studied consisted of 15 men and 13 women (Table 1). The group of old subjects with different degrees of increased susceptibility to infection, immune deficiency, and/or inflammatory disease included 10 men and 12 women. Of the healthy subjects, 8 of the men and 7 of the women were living independently in their community, and the others were in assisted-living facilities. Racial distribution was representative of the urban study areas, with a predominance of Caucasians and fewer African-Americans and Asians. Mean ages of the groups ranged from 69 to 76 yr, with no significant differences between mean ages of the men and women in any subgroup or the total study population. No subject was taking any hormone or other medication at a dose known to alter immune or inflammatory responses.
Age-related differences in sizes of T-cell subsets
T cells isolated from all subjects were >98% CD3 positive. The mean percentage of total T cells expressing CD4 was higher, and the mean percentage expressing CD8 was lower for healthy old subjects than young subjects, with considerable overlap of the two populations (Table 2). Flow cytometric analyses of T-cell subsets from most of the old group IIA subjects showed mean ± sd percentages of 69 ± 9.8 CD4 and 26 ± 8.4 CD8 for group IIA (7 of 11 subjects) and 71 ± 11 CD4 and 25 ± 10 CD8 for group IIB (8 of 11 subjects). These percentages of CD4 and CD8 T cells for groups IIA and IIB are statistically indistinguishable from those for group I subjects (Table 2). It is known that the most significant differences between T cells of old and young subjects are in minor functionally relevant subsets (7, 19). Here, the percentage of T cells with properties of the recently characterized CD4−8− immunoregulatory subset was very significantly lower in healthy old than young subjects (20). A very preliminary analysis of MIF secretion by anti-CD3 + anti-CD28 antibody-stimulated CD4−8− T cells of two old-young pairs of healthy subjects showed mean generation of 1575 and 2632 pg/106 cells, respectively, after 12 h of incubation. Thus, the level of function of CD4−8− T cells, as well as their blood concentration may be lower in immunosenescence. An equally significant, but opposite, difference was seen for CD4+FOXP3+ Treg cells, the levels of which are higher in healthy old than young subjects. In contrast, the CD161 subset of T cells, which includes developmental precursors of most human Th17 cells (21), showed an identical level in old and young healthy subjects (Table 2). The differences in CD4, CD8, and CD4−8− subsets observed for the total group were similar for the female and male substituent groups.
TABLE 2.
Differences in T-cell subsets between healthy old subjects and young control subjects
| T-cell subset | old subjects | young controls |
|---|---|---|
| CD4 | 72 ± 9.2* | 63 ± 7.4 |
| CD8 | 24 ± 8.4* | 32 ± 7.6 |
| CD161 | 52 ± 16 | 52 ± 17 |
| CD3+4−8− | 2.4 ± 0.6** | 4.7 ± 1.8 |
| CD4+FOXP3+a | 6.0 ± 0.3** | 4.0 ± 0.3 |
Values are the means ± sd of the results of analyses of T cells from 20 healthy old subjects (9 male, 11 female) and their concurrently studied young controls. Values are expressed as the percentage of total CD3+ T cells. aAnalyses encompassed only 15 of the subject pairs (9 males, 6 females).
P < 0.01,
P < 0.001 vs. controls; 2-sample t test.
Differences in cytokine generation by T cells as a function of age and immune status
Stimulation of T cells from healthy subjects with anti-CD3 + anti-CD28 antibodies yielded very significantly lower levels of IFN-γ for old males than young males and very significantly higher levels of IFN-γ for old females than young females (Fig. 2). Stimulation of the same sets of T cells by IL-12 + IL-18 evoked generation of very significantly lower levels of IFN-γ for old males than young males and moderately lower levels of IFN-γ for old females than young females. The different results for TCR-directed vs. IL-12 + IL-18 stimulation of T cells from female subjects is one example of gender-related stimulus-specificity. For anti-CD3 + anti-CD28 antibody stimulation of IL-17 generation by T cells, the levels for old males again were very significantly lower than for young males after 2 and 3 d, but there was no difference between the levels for old and young females (Fig. 2). Most previous studies have shown lower levels of generation of IL-2 by T cells of healthy old subjects than young subjects (8, 9, 22). IL-2 generation by TCR-stimulated T cells of this group of old subjects also was lower than that by TCR-stimulated T cells of young control subjects after 3 d, with respective old/young mean ± sd ratios of 0.50 ± 0.48 (P<0.001 by the Mann-Whitney test) for men and 0.66 ± 0.58 (P<0.05) for women. To assess temporal reproducibility of TCR-stimulated generation of cytokines, two studies were performed at intervals of 6 to 8 wk with T cells from 3 healthy old women and 3 healthy old men. For the women, mean ± sd concentrations of IFN-γ and IL-17 were 31,365 ± 19,494 and 355 ± 134 pg/ml, respectively, in the first study and 32,332 ± 16,915 and 408 ± 209 pg/ml, respectively, in the second study. For the men, concentrations of IFN-γ and IL-17 were 3330 ± 2142 and 168 ± 131 pg/ml, respectively, in the first study and 3041 ± 1751 and 151 ± 94 pg/ml, respectively, in the second study. There were no significant differences between any of the first and second sets of cytokine levels, as determined by a paired t test.
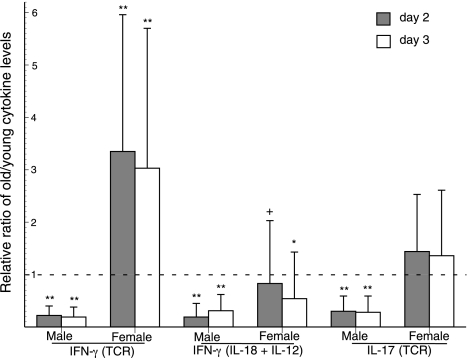
Figure 2.
Ratios of generation of IFN-γ and IL-17 by T cells from old healthy (group I) and young subjects. Stimuli were 1 μg/well each of anti-human CD3 + anti-human CD28 antibodies (TCR) and 50 ng/ml of IL-18 + 5 ng/ml of IL-12. Columns and bars depict mean ± sd values for supernates harvested at 2 d (hatched columns) and 3 d (open columns). Statistical significance of increases and decreases from the level of 1 (no difference between young and old) was calculated by the Mann-Whitney nonparametric test. +P < 0.05; *P < 0.01; **P < 0.001.
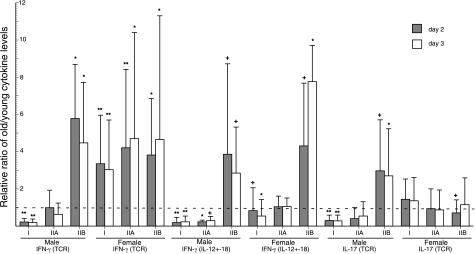
The effects of inflammation and immune deficiency on T-cell production of IFN-γ and IL-17 revealed further substantial gender-related differences. The ratios of generation of IFN-γ by TCR-stimulated and IL-12 + IL-18-stimulated T cells of old males to young male controls rose from very significantly low in healthy group I subjects to similar levels when the old males had minor immune deficiency, inflammation, and/or infections (IIA) (Fig. 3). The same ratios of old to young male T-cell generation of IFN-γ were very significantly higher when the old males had serious immune deficiency, inflammation, and/or infections (group IIB). In contrast, the level of generation of IFN-γ by TCR-stimulated T cells of healthy group I old females was already very significantly higher than that of young female controls, and the ratios did not increase further when the old females had minor or serious immune, inflammatory, and/or infectious conditions. In contrast, when T cells were stimulated with IL-12 + IL-18, the same pattern of IFN-γ generation was observed for old females as old males (Fig. 3). The old to young IFN-γ ratios for female subject pairs increased progressively from slightly low for group I healthy old females to no difference for old females in group IIA to significantly higher than that of young controls for old females in group IIB. The level of generation of IL-17 by TCR-stimulated T cells of old men also rose from very significantly lower than that of young controls for group I to the same level as controls for group IIA to significantly higher than that of young controls for group IIB just as had IFN-γ generation for the same groups of subjects (Fig. 3). In contrast, none of the levels of generation of IL-17 by TCR-stimulated T cells of old women were substantially different from those of young female controls.
Figure 3.
Relationships between immune-inflammatory status and the old-young ratios of generation of IFN-γ and IL-17 by T cells of subjects in groups I, IIA, and IIB. Stimuli, conditions, meanings of each type of column and bar, and statistical method and symbols denoting statistical significance of increases above and decreases below a ratio of 1 (no difference between age groups) are the same as in Fig. 2.
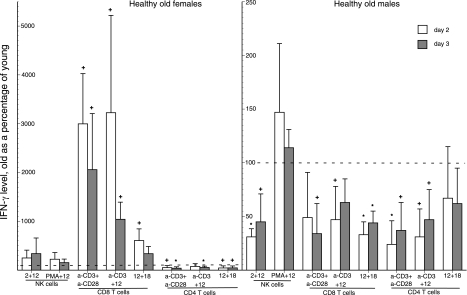
Differences in generation of IFN-γ by T-cell subsets and NK/NKT cells of healthy old women and men
NK/NKT cells, CD8 T cells, and CD4 T cells were isolated from ML for studies designed to delineate the sources of elevated levels of IFN-γ generation in healthy old women and the basis for depressed levels of IFN-γ in healthy old men. Stimulation of CD8 T cells from healthy old women with anti-CD3 + anti-CD28 antibodies or anti-CD3 antibody + IL-12 evoked a level of generation of IFN-γ that was strikingly elevated relative to that from identically stimulated CD8 T cells of control young women. The generation of IFN-γ elicited by IL-12 + IL-18 stimulation of CD8 T cells from healthy old women was only modestly higher than that from CD8 T cells of young women (Fig. 4). In contrast, incubation of CD4 T cells from healthy old women with the same three sets of stimuli elicited generation of IFN-γ that was significantly lower than that from CD4 T cells of young women. There were no differences in IFN-γ generation when NK/NKT cells from old women and young women were incubated with their preferential stimuli (Fig. 4). Thus, CD8 T cells are the principal source of elevated levels of IFN-γ generation in healthy old women compared to young women after TCR-dependent stimulation of mixed populations of T cells. In contrast, the slightly higher levels of IFN-γ from IL-12 + IL-18-stimulated CD8 T cells do not compensate adequately for the reduced levels of IFN-γ from IL-12 + IL-18-stimulated CD4 T cells resulting in the slightly lower levels of IFN-γ for old women than young women in studies of cytokine-stimulated mixed populations of T cells (Fig. 3).
Figure 4.
Immune cellular bases for aging-associated alterations in generation of IFN-γ by immune cells of healthy old subjects. Columns and bars depict mean ± sd results of studies of NK/NKT cells and T cells from 3 healthy (group I) old female or male subjects and 3 young healthy female or male control subjects, with harvesting of supernates on d 2 (open bars) and d 3 (cross-hatched bars). Stimuli were 1 μg/well of anti-human CD3 + anti-human CD28 antibodies or combinations of 1 μg/well of anti-human CD3 antibody, 50 ng/ml of IL-18, 5 ng/ml of IL-2 or IL-12, and 50 ng/ml of phorbol myristate acetate (PMA). Significance of increases above and decreases below the 100% level, where values for young and old subjects would be the same, was calculated by a Mann-Whitney nonparametric test. +P < 0.05; *P < 0.01.
When the same immune cell subsets from healthy old men were incubated with the same stimuli as for old women, IFN-γ generation was depressed significantly relative to the levels for CD8 T cells of young men with all three stimuli (Fig. 4). The ratios of IFN-γ generation by CD4 T cells of healthy old men to that of young men were low after stimulation by TCR-directed antibodies, but not IL-12 + IL-18. The same ratios of IFN-γ generation by NK/NKT cells were low when stimulation was by IL-2 + IL-12, but not PMA + IL-12. Thus the very depressed levels of IFN-γ generated by TCR-stimulated T cells of healthy old men relative to those of young men were attributable to lower levels from CD4 and CD8 T cells, whereas the depressed levels of IFN-γ elicited by IL-12 + IL-18 stimulation of T cells from healthy old men are predominantly attributable to lower levels from CD8 T cells.
Differences in cytokine generation by macrophages as a function of age and immune status
As for T-cell cytokine generation, the levels of cytokines generated by differentiated monocytes of young women and young men were not significantly different based on analyses of the results from the total study population. Monocytes of young women produced mean ± sd levels of IL-6 and IP-10 chemokines that were 16,698 ± 4626 and 5844 ± 3,780 pg/ml when stimulated for 24 h with LPS and 20 ng/ml of IFN-γ, respectively, and a mean level of TNF-α that was 2505 ± 1604 pg/ml after 6 h of stimulation with LPS. Parallel studies of cytokines generated by monocytes from young men showed levels that were statistically indistinguishable from those produced by monocytes of young women at 13,429 ± 2077 pg IL-6/ml, 7253 ± 5763 pg IP-10/ml, and 2515 ± 2406 pg TNF-α/ml, respectively.
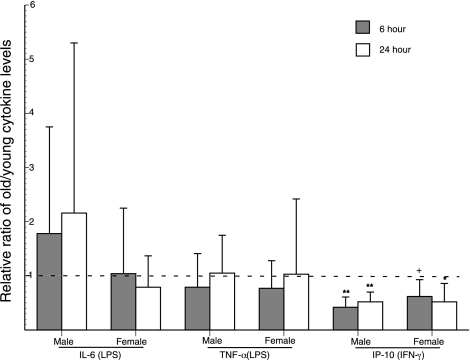
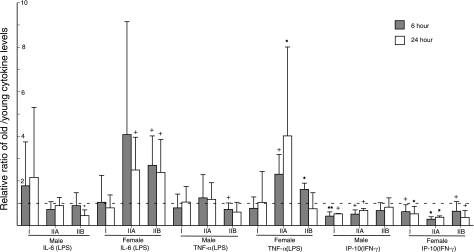
Stimulation of differentiated monocytes from healthy subjects with LPS for 6 h and 24 h yielded statistically indistinguishable levels of IL-6 and of TNF-α for old males as compared to young males and for old females as compared to young females (Fig. 5). In contrast, stimulation of monocytes from healthy subjects with IFN-γ for 6 h and 24 h yielded very significantly lower levels of IP-10 for old male subjects than young male subjects and lower levels of IP-10 of moderate significance for old female subjects as compared to young female subjects (Fig. 5). The immune and inflammatory state of subjects influenced the levels of cytokine generation by their differentiated monocytes as it had for T-cell cytokines. LPS-evoked levels of generation of IL-6 and TNF-α by monocytes from old men were significantly depressed relative to those by monocytes from young men at one of two time-points only for group IIB (Fig. 6). In contrast, LPS-evoked levels of generation of IL-6 and TNF-α by monocytes from old women were significantly elevated relative to those by monocytes from young women at one or both time-points for groups IIA and IIB (Fig. 6). IFN-γ-elicited levels of generation of IP-10 by monocytes from old women were significantly reduced relative to those by monocytes from young women at both time points for subject groups IIA and IIB. In contrast, for old men the levels of IP-10 attained by IFN-γ-stimulated monocytes were significantly reduced relative to those by monocytes from young men at both time points for subject group IIA, but not IIB (Fig. 6). Thus gender-specific differences were apparent for monocyte generation of IL-6 and TNF-α only in immune and inflammatory states with respective increases for old women and decreases for old men. In contrast, monocyte generation of IP-10 chemokine was decreased in both old men and women.
Figure 5.
Ratios of generation of IL-6, TNF-α, and IP-10 chemokine by differentiated monocytes from healthy old (group I) and young subjects. Columns and bars depict mean ± sd values for supernates harvested at 6 h (hatched columns) and 24 h (open columns). Statistical significance of increases above and decreases below the level of 1 (no difference between young and old) was calculated by the Mann-Whitney nonparametric test. +P < 0.05; *P < 0.01; **P < 0.001.
Figure 6.
Relationships between immune-inflammatory status and the old-young ratio of generation of IL-6, TNF-α, and IP-10 by differentiated monocytes of subjects in groups I, IIA, and IIB. Stimuli, conditions, and meanings of each type of column, bar, and symbol denoting statistical significance of increases above and decreases below the ratio of 1 (no difference between young and old) were the same as in Fig. 5.
DISCUSSION
The present results provide new evidence for altered immunity in human aging, which includes major changes in functional constituents of the T-cell constellation and highly significant differences in T-cell cytokine generation between old and young subjects. The recently characterized immunoregulatory CD4−8− subset of T cells alters immune responses principally by enhancing generation of IFN-γ and IL-17 after stimulation of mixed T cells by anti-CD3 + anti-CD28 antibodies and by IL-12 + IL-18 (20). Current flow-cytometric data document for the first time that the levels of CD4−8− T cells are very significantly lower in T-cell populations from old than young healthy subjects (Table 2). Conflicting assessments of the relative percentages of CD4+25+ Treg cells in old as compared to young subjects have resulted from other investigations (23,24,25). In our set of healthy old-young subject pairs, the old subjects had significantly more CD4+25+ Treg cells than the young subjects (Table 2). Functional analyses have shown consistently that the suppressive activities of CD4+25+ Treg cells are greater for old than young populations of T cells (6, 26). Lower immunostimulatory activity of the CD4−8− subset of T cells and greater suppressive effects of CD4+25+ Treg cells in aging predict that production of IFN-γ and IL-17 by stimulated T cells of old subjects would be lower than for those of young subjects. However, the results of subsequent investigations are more complex, implicate many other immune variables, and reveal that gender is a critical primary determinant of changes in T-cell cytokine generation with aging.
The generation of IFN-γ and IL-17 by stimulated T cells was much lower for healthy old than young men (Fig. 2). In the same studies, generation of IFN-γ and IL-17 by stimulated T cells was much higher or minimally different for healthy old women as contrasted with young women, respectively. Gender specificity of differences in generation of IFN-γ and IL-17 initially suggested potential gender-dependent changes in expression of T-cell immunoactive surface proteins, such as CD28. Lower levels of surface expression of CD28 have been described for some subsets of T cells in old subjects, but there is no evidence for gender-related differences in altered expression of CD28 or related membrane proteins (7). It was of interest to find that CD8 T cells were the major source of increased IFN-γ generation by T cells of old women and the predominant site of decreased IFN-γ generation by T cells of old men, irrespective of the type of stimulus (Fig. 4). Expanded clones of CD8+28− T cells in aged humans have been shown to produce IFN-γ and not Th2 cytokines, but no gender selectivity was identified (5).
Estrogens may be the principal determinant of gender differences in the altered cytokine generation by T cells in aging. Incubation of mouse thymocytes and splenic lymphocytes with estrogen significantly enhanced mitogen-induced expansion of IFN-γ-producing cells and the level of generation of IFN-γ, but not IL-4, by a transcriptional mechanism (27, 28). Long-term treatment of mice with estrogens also selectively enhanced lymphocyte production of IFN-γ ex vivo. Similarly, low concentrations of estradiol, but not progesterone or high levels of estradiol, augmented generation of IFN-γ by mitogen-stimulated human mononuclear leukocytes in whole blood (29). In mouse splenocytes, that bear estrogen receptors, estradiol increased transcriptional activity of the IFN-γ promoter and enhanced IFN-γ mRNA expression elicited by mitogen activation (30). Thus, the remaining low concentrations of estrogen in old women and consequent up-regulation of IFN-γ promoter activity is the most likely explanation for higher levels of production of IFN-γ by their T cells. There is no information currently available to suggest that estrogens regulate IL-17 generation. For monocytes induced to differentiate into macrophages, the only significant differences in cytokine generation attributable to aging in healthy female and male subjects were significantly lower levels of IP-10 chemokine elicited by IFN-γ, but there is no known involvement of estrogens (Fig. 5).
The effects of altered immune and inflammatory states on cytokine generation also were clear-cut for both T cells and monocytes of old subjects. When T cell-derived cytokine levels were lower for old than young subjects, as for IFN-γ elicited by both types of stimuli in old men and by IL-12 + IL-18 in old women, and for IL-17 in old men, significant increases were seen in relation to the severity of immune deficiency or inflammatory or autoimmune disease (Fig. 3). The requirement that no subject be receiving immunoactive drugs or hormones resulted in small study clusters and the need to consider effects of immune, inflammatory, and infectious diseases in groups based on severity. Although subject numbers are too low for meaningful analyses of further subdivided groups in most instances, it is of interest to ask whether there is a greater influence of an immune disorder with inflammation or an immune disorder with excessive infections. For generation of IFN-γ by anti-CD3 + anti-CD28 antibody-stimulated T cells after 2 d of incubation, mean ratios of old/young male pairs with severe disease were 5.8 when immune-inflammatory conditions predominated in the old subject (n=2) and 5.4 with principally host defense-infection problems (n=3). For the same data sets from old/young female pairs with severe disease, mean ratios were 2.7 when immune-inflammatory conditions predominated in the old subject (n=4) and 5.4 with principally host defense-infection problems (n=2). This suggests that perhaps host defense-infection problems are stronger drivers of increased IFN-γ production than immune-inflammatory conditions in old women, but not old men.
For monocyte-derived IL-6 and TNF-α, where levels for healthy subjects were no different in the old than young groups, significant increases were observed only in old women compared to young women in association with any immune or inflammatory disease (Figs. 5 and 6). IP-10 levels remained significantly lower in old than young subjects despite immune or inflammatory disease of any severity, except for normalization in the old men with severe immunity-related diseases. The initial relevance of these findings to immunotherapy for conditions with inappropriately robust inflammation mediated by immune cytokines is that gender as well as disease state will determine the array of T cell and macrophage cytokines that are potential targets.
The decreased generation of IFN-γ and IL-17 by total T cells of old men after challenge with either TCR-dependent or -independent stimuli may be due to known deficiencies of senescent T cells, including diminished responsiveness of some subsets to antigenic stimulation, increased suppressive effects of Treg cells, and decreased enhancing activity of CD4−8− T cells, and/or enhanced susceptibility to activation-induced apoptosis. However, the very significantly heightened generation of IFN-γ by TCR-stimulated T cells of old women is not readily explained by known characteristics of senescent T cells.
The impact of altered tissue concentrations of IFN-γ in immunosenescence may be apparent in both immune and nonimmune physiological systems. Numerous innate and adaptive immune effector responses are dependent directly and indirectly on IFN-γ (31,32,33). IFN-γ also mediates diverse immune regulatory events, that encompass control of development and activation of CD4+25+ Treg cells in antitumor responses to CD8 T-cell suppression of delayed-type hypersensitivity (34, 35). In some immune pathways, diminished responsiveness to IFN-γ in aging may reduce undesirable immune and inflammatory effects. For example, the lower levels of production of IP-10 inflammatory chemokine by IFN-γ-stimulated monocytes of all old male and female subjects (Figs. 5 and 6) could limit the possibly deleterious inflammatory consequences of elevated production of IFN-γ by T cells of old women (Figs. 123). Similarly, broad nonimmune roles of IFN-γ include regulation of cardiac repair responses after myocardial infarction and attenuation of insulin signaling and lipid storage in adipocytes (36, 37). The influences of gender on all aspects of generation and effects of immune cytokines in normal adaptive immune responses, autoimmune diseases, and responses to immunotherapeutic measures must now be considered carefully.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Aging, a grant from the Kenneth Rainin Foundation, and National Institutes of Health RO-1 grant HL31809. E.J.G., M.C.H., J.B.S., D.D.T., and D.L.L. designed research; E.J.G., J.B.S., K.F., L.F., and K.M. evaluated and selected subjects; E.J.G., M.C.H., J.K., and K.P. performed laboratory research; E.J.G., M.C.H., J.B.S., and D.L.L. analyzed data and wrote the paper. The authors are grateful to Judith H. Goetzl for preparation of the figures and tables.
References
- Gupta S, Agrawal A, Agrawal S, Su H, Gollapudi S. A paradox of immunodeficiency and inflammation in human aging: lessons learned from apoptosis. Immun Ageing. 2006;3:5. doi: 10.1186/1742-4933-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G. Immunosenescence comes of age. Symposium on Aging Research in Immunology: The Impact of Genomics. EMBO Rep. 2007;8:220–223. doi: 10.1038/sj.embor.7400922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea I M, Stewart M, Campbell P, Alexander H D, Crockard A D, Morris T C. Changes in lymphocyte subsets, interleukin 2, and soluble interleukin 2 receptor in old and very old age. Gerontology. 1996;42:69–78. doi: 10.1159/000213775. [DOI] [PubMed] [Google Scholar]
- Wack A, Cossarizza A, Heltai S, Barbieri D, D'Addato S, Fransceschi C, Dellabona P, Casorati G. Age-related modifications of the human αβ T cell repertoire due to different clonal expansions in the CD4+ and CD8+ subsets. Int Immunol. 1998;10:1281–1288. doi: 10.1093/intimm/10.9.1281. [DOI] [PubMed] [Google Scholar]
- Saurwein-Teissl M, Lung T L, Marx F, Gschosser C, Asch E, Blasko I, Parson W, Bock G, Schonitzer D, Trannoy E, Grubeck-Loebenstein B. Lack of antibody production following immunization in old age: association with CD8+CD28− T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- Lages C S, Suffia I, Velilla P A, Huang B, Warshaw G, Hildeman D A, Belkaid Y, Chougnet C. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181:1835–1848. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng N P, Akbar A N, Goronzy J. CD28− T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S, Kozak R, Durante M, Weksler M E. Immunological studies of aging. Decreased production of and response to T cell growth factor by lymphocytes from aged humans. J Clin Invest. 1981;67:937–942. doi: 10.1172/JCI110143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso C, Candore G, Cigna D, DiLorenzo G, Sireci G, Dieli F, Salerno A. Cytokine production pathway in the elderly. Immunol Res. 1996;15:84–90. doi: 10.1007/BF02918286. [DOI] [PubMed] [Google Scholar]
- Abb J, Abb H, Deinhardt F. Age-related decline of human interferon alpha and interferon gamma production. Blut. 1984;48:285–289. doi: 10.1007/BF00320399. [DOI] [PubMed] [Google Scholar]
- Al-Rayes H, Pachas W, Mirza N, Ahern D J, Geha R S, Vercelli D. IgE regulation and lymphokine patterns in aging humans. J Allergy Clin Immunol. 1992;90:630–636. doi: 10.1016/0091-6749(92)90136-p. [DOI] [PubMed] [Google Scholar]
- Candore G, Di Lorenzo G, Melluso M, Cigna D, Colucci A T, Modica M A, Caruso C. γ-Interferon, interleukin-4 and interleukin-6 in vitro production in old subjects. Autoimmunity. 1993;16:275–280. doi: 10.3109/08916939309014646. [DOI] [PubMed] [Google Scholar]
- Fagiolo U, Cossarizza A, Scala E, Fanales-Belasio E, Ortolani C, Cozzi E, Monti D, Franceschi C, Paganelli R. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23:2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- Yen C J, Lin S L, Huang K T, Lin R H. Age-associated changes in interferon-gamma and interleukin-4 secretion by purified human CD4+ and CD8+ T cells. J Biomed Sci. 2000;7:317–321. doi: 10.1007/BF02253251. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Voice J K, Kong Y, Goetzl E J. Altered expression and functional profile of lysophosphatidic acid receptors in mitogen-activated human blood T lymphocytes. FASEB J. 2000;14:2387–2389. doi: 10.1096/fj.00-0492fje. [DOI] [PubMed] [Google Scholar]
- Gerlini G, Mariotti G, Chiarugi A, Di Gennaro P, Caporale R, Parenti A, Cavone L, Tun-Kyi A, Prignano F, Saccardi R, Borgognoni L, Pimpinelli N. Induction of CD83+CD14+ nondendritic antigen-presenting cells by exposure of monocytes to IFN-α. J Immunol. 2008;181:2999–3008. doi: 10.4049/jimmunol.181.5.2999. [DOI] [PubMed] [Google Scholar]
- Dixit V D, Yang H, Sun Y, Weeraratna A T, Youm Y H, Smith R G, Taub D D. Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007;117:2778–2790. doi: 10.1172/JCI30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef M J, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M, Tanimoto T, Torigoe K, Fujii M, Ikeda M, Fukuda S, Kurimoto M. Interferon-γ-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-γ production. Eur J Immunol. 1996;26:1647–1651. doi: 10.1002/eji.1830260736. [DOI] [PubMed] [Google Scholar]
- Posnett D N, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy”. J Exp Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M C, Patel K, Taub D D, Longo D L, Goetzl E J. Human CD4−8− T cells are a distinctive immunoregulatory subset. [E-pub ahead of print] FASEB J. 2010 doi: 10.1096/fj.09-153148. doi: 10.1096/fj.09-153148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, Berrino L, Fambrini M, Caproni M, Tonelli F, Lazzeri E, Parronchi P, Liotta F, Maggi E, Romagnani S, Annunziato F. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossarizza A, Monti D, Bersani F, Paganelli R, Montagnani G, Cadossi R, Cantini M, Franceschi C. Extremely low frequency pulsed electromagnetic fields increase interleukin-2 (IL-2) utilization and IL-2 receptor expression in mitogen-stimulated human lymphocytes from old subjects. FEBS Lett. 1989;248:141–144. doi: 10.1016/0014-5793(89)80449-1. [DOI] [PubMed] [Google Scholar]
- Tsaknaridis L, Spencer L, Culbertson N, Hicks K, LaTocha D, Chou Y K, Whitham R H, Bakke A, Jones R E, Offner H, Bourdette D N, Vandenbark A A. Functional assay for human CD4+CD25+ Treg cells reveals an age-dependent loss of suppressive activity. J Neurosci Res. 2003;74:296–308. doi: 10.1002/jnr.10766. [DOI] [PubMed] [Google Scholar]
- Gregg R, Smith C M, Clark F J, Dunnion D, Khan N, Chakraverty R, Nayak L, Moss P A. The number of human peripheral blood CD4+ CD25 high regulatory T cells increases with age. Clin Exp Immunol. 2005;140:540–546. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryl E, Witkowski J M. Decreased proliferative capability of CD4+ cells of elderly people is associated with faster loss of activation-related antigens and accumulation of regulatory T cells. Exp Gerontol. 2004;39:587–595. doi: 10.1016/j.exger.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Vukmanovic-Stejic M, Zhang Y, Cook J E, Fletcher J M, McQuaid A, Masters J E, Rustin M H, Taams L S, Beverley P C, Macallan D C, Akbar A N. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116:2423–2433. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpuzoglu-Sahin E, Zhi-Jun Y, Lengi A, Sriranganathan N, Ansar Ahmed S. Effects of long-term estrogen treatment on IFN-γ, IL-2 and IL-4 gene expression and protein synthesis in spleen and thymus of normal C57BL/6 mice. Cytokine. 2001;14:208–217. doi: 10.1006/cyto.2001.0876. [DOI] [PubMed] [Google Scholar]
- Nakaya M, Tachibana H, Yamada K. Effect of estrogens on the interferon-γ producing cell population of mouse splenocytes. Biosci Biotechnol Biochem. 2006;70:47–53. doi: 10.1271/bbb.70.47. [DOI] [PubMed] [Google Scholar]
- Matalka K Z. The effect of estradiol, but not progesterone, on the production of cytokines in stimulated whole blood, is concentration-dependent. Neuro Endocrinol Lett. 2003;24:185–191. [PubMed] [Google Scholar]
- Fox H S, Bond B L, Parslow T G. Estrogen regulates the IFN-γ promoter. J Immunol. 1991;146:4362–4367. [PubMed] [Google Scholar]
- O'Garra A, Murphy K. Role of cytokines in development of Th1 and Th2 cells. Chem Immunol. 1996;63:1–13. doi: 10.1159/000319475. [DOI] [PubMed] [Google Scholar]
- Taub D D, Turcovski-Corrales S M, Key M L, Longo D L, Murphy W J. Chemokines and T lymphocyte activation: I. Beta chemokines costimulate human T lymphocyte activation in vitro. J Immunol. 1996;156:2095–2103. [PubMed] [Google Scholar]
- Lu B, Ebensperger C, Dembic Z, Wang Y, Kvatyuk M, Lu T, Coffman R L, Pestka S, Rothman P B. Targeted disruption of the interferon-γ receptor 2 gene results in severe immune defects in mice. Proc Natl Acad Sci U S A. 1998;95:8233–8238. doi: 10.1073/pnas.95.14.8233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa H, Kato T, Tawara I, Ikeda H, Kuribayashi K, Allen P M, Schreiber R D, Old L J, Shiku H. IFN-γ controls the generation/activation of CD4+ CD25+ regulatory T cells in antitumor immune response. J Immunol. 2005;175:4433–4440. doi: 10.4049/jimmunol.175.7.4433. [DOI] [PubMed] [Google Scholar]
- Cone R E, Li X, Sharafieh R, O'Rourke J, Vella A T. The suppression of delayed-type hypersensitivity by CD8+ regulatory T cells requires interferon-gamma. Immunology. 2007;120:112–119. doi: 10.1111/j.1365-2567.2006.02486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujak M, Dobaczewski M, Gonzalez-Quesada C, Xia Y, Leucker T, Zymek P, Veeranna V, Tager A M, Luster A D, Frangogiannis N G. Induction of the CXC chemokine interferon-γ-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res. 2009;105:973–983. doi: 10.1161/CIRCRESAHA.109.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillicuddy F C, Chiquoine E H, Hinkle C C, Kim R J, Shah R, Roche H M, Smyth E M, Reilly M P. Interferon gamma attenuates insulin signaling, lipid storage, and differentiation in human adipocytes via activation of the JAK/STAT pathway. J Biol Chem. 2009;284:31936–31944. doi: 10.1074/jbc.M109.061655. [DOI] [PMC free article] [PubMed] [Google Scholar]