Abstract
Cholyl-2,4-3H-glycine-1-14C was administered orally to eight healthy subjects with indwelling nasoduodenal tubes. The distribution of radioactivity among bile acids and the specific activity of cholylglycine were determined in bile collected at intervals for 7 days. 3H and 14C were measured in stool. 14C in breath was calculated from interval 14CO2 specific activity determinations.
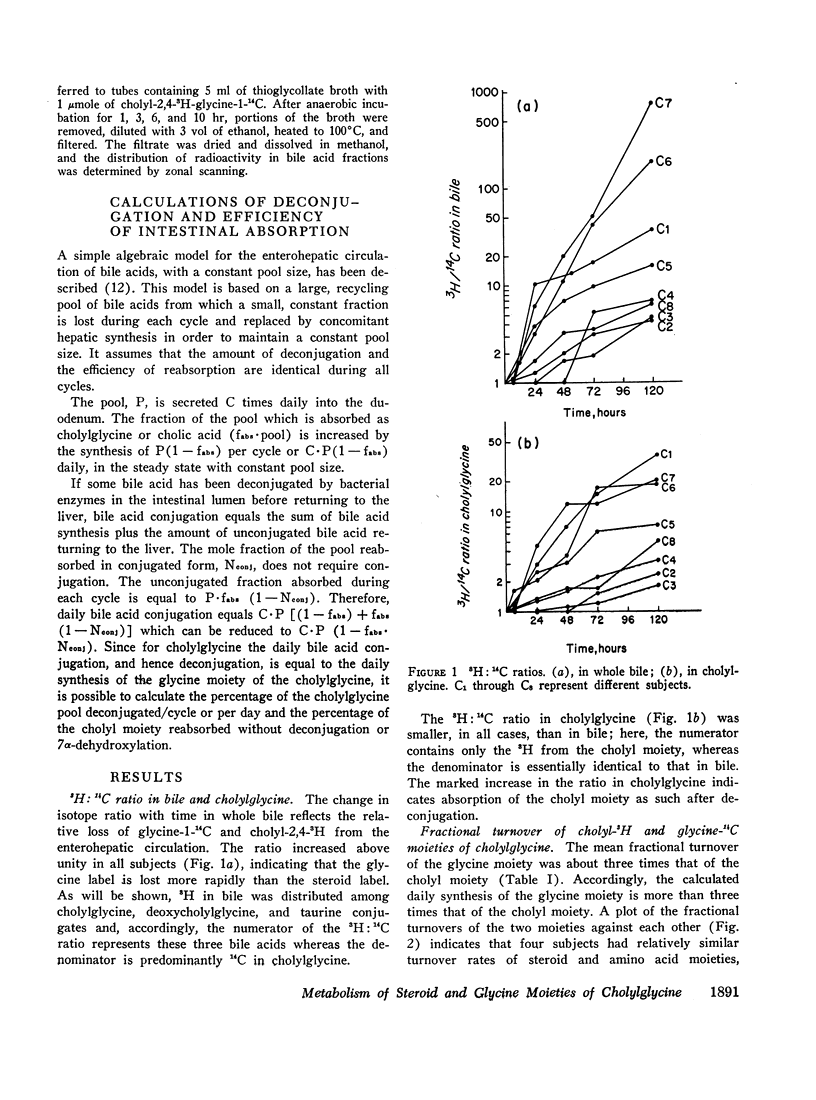
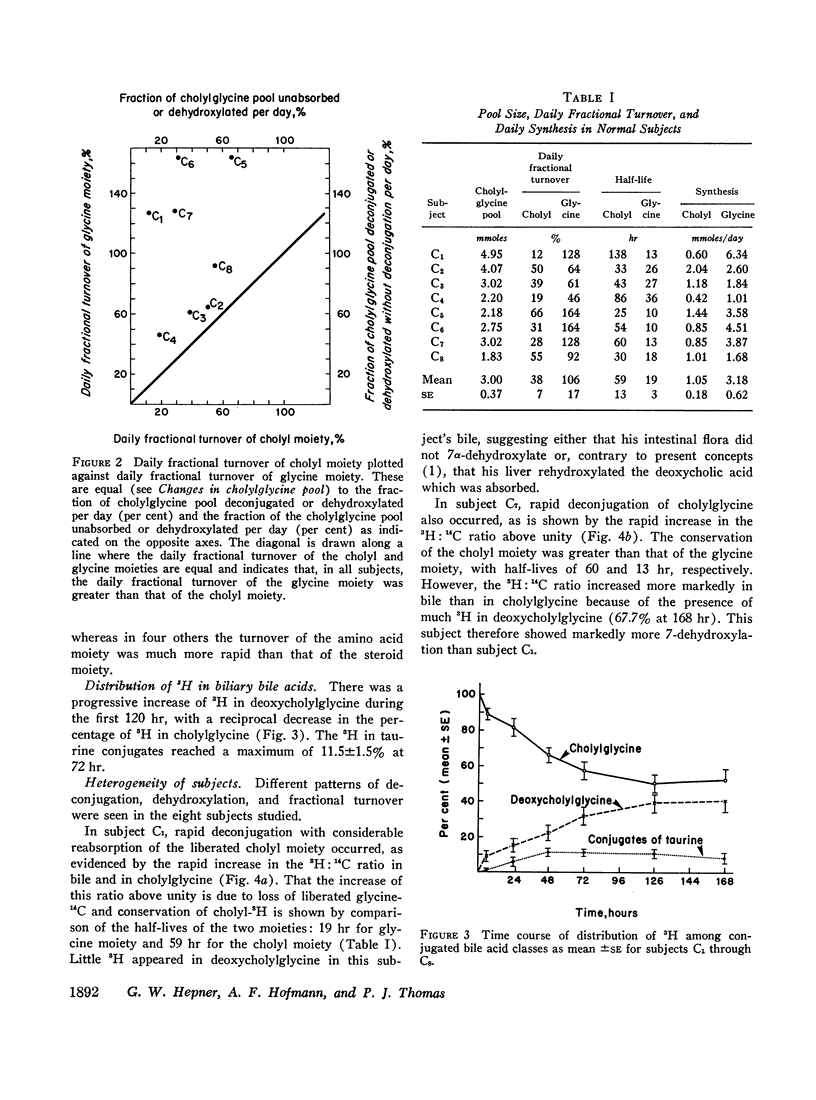
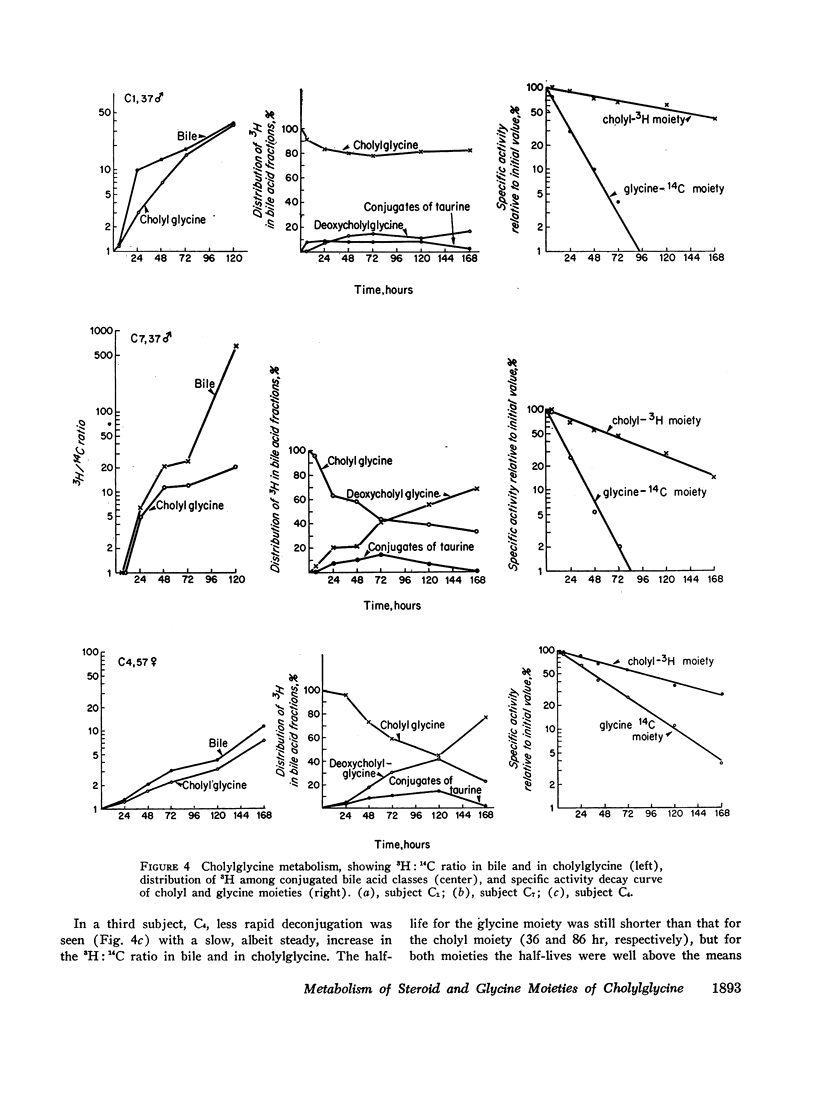
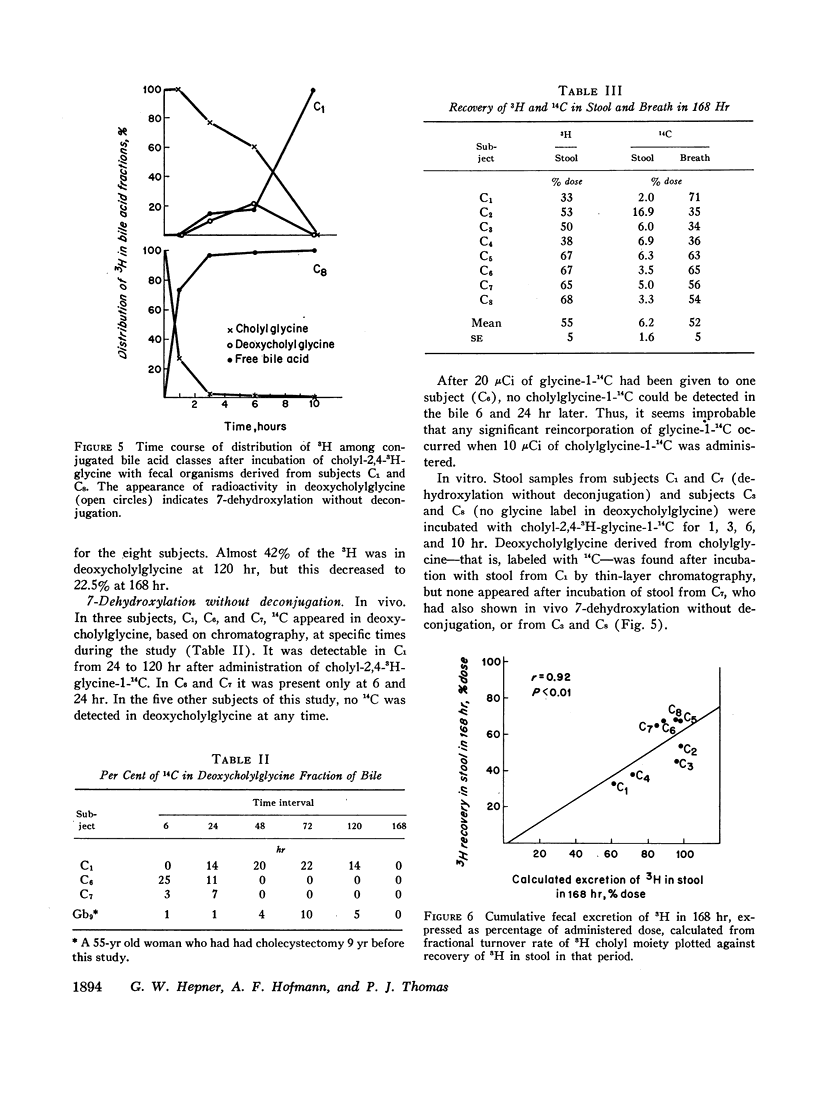
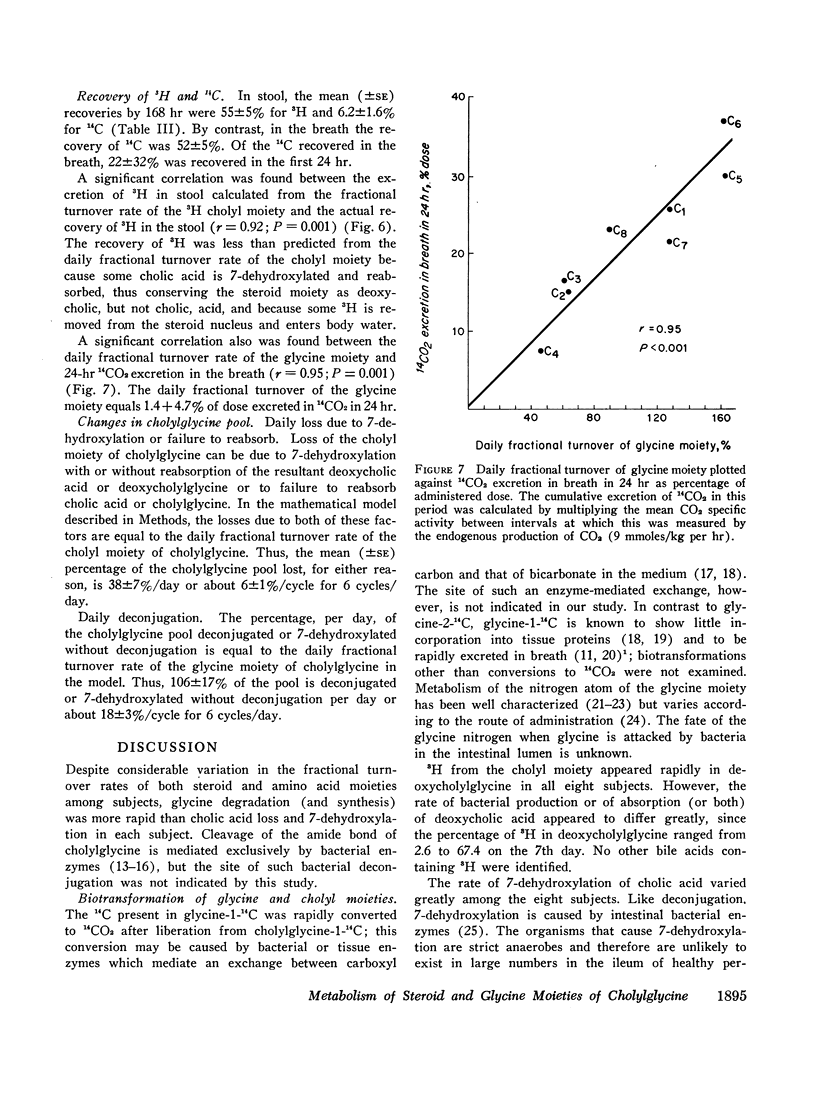
The daily fractional turnover of the glycine moiety (mean ±SE, 106±17%) was three times greater than that of the cholyl moiety (38±7%). On the basis of certain assumptions, it was calculated that about 18% of the cholylglycine pool was deconjugated per enterohepatic cycle. The extent of deconjugation appeared to be unrelated to the efficiency of absorption of the cholyl moiety, which averaged 90-95% per enterohepatic cycle. 14C was recovered predominantly in breath (52±5% of administered dose), and 24 hr 14CO2 excretion correlated highly (r = 0.95) with daily fractional turnover of the glycine moiety. 3H excretion occurred predominantly in feces, and the rate correlated highly (r = 0.92) with the daily fractional turnover of the cholyl moiety. Deoxycholylglycine became labeled with 3H rapidly, indicating the occurrence of bacterial 7-dehydroxylation of the cholyl moiety and absorption of deoxycholic acid. This biotransformation occurred in all eight subjects but varied in degree and was unrelated to the degree of deconjugation. Since ingested glycine-1-14C was not incorporated into bile acid glycine, appearance of 14C in deoxycholylglycine (observed in three of eight subjects) indicated that 7-dehydroxylation of cholylglycine can occur without deconjugation. Dehydroxylation was also observed in vitro when fecal homogenates were incubated with cholylglycine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Admirand W. H., Earnest D. L., Williams H. E. Hyperoxaluria and bowel disease. Trans Assoc Am Physicians. 1971;84:307–312. [PubMed] [Google Scholar]
- Ando T., Nyhan W. L., Gerritsen T., Gong L., Heiner D. C., Bray P. F. Metabolism of glycine in the nonketotic form of hyperglycinemia. Pediatr Res. 1968 Jul;2(4):254–263. doi: 10.1203/00006450-196807000-00004. [DOI] [PubMed] [Google Scholar]
- Aries V., Hill M. J. Degradation of steroids by intestinal bacteria. I. Deconjugation of bile salts. Biochim Biophys Acta. 1970 May 5;202(3):526–534. doi: 10.1016/0005-2760(70)90123-2. [DOI] [PubMed] [Google Scholar]
- Aries V., Hill M. J. Degradation of steroids by intestinal bacteria. II. Enzymes catalysing the oxidoreduction of the 3 alpha-, 7 alpha- and 12 alpha-hydroxyl groups in cholic acid, and the dehydroxylation of the 7-hydroxyl group. Biochim Biophys Acta. 1970 May 5;202(3):535–543. doi: 10.1016/0005-2760(70)90124-4. [DOI] [PubMed] [Google Scholar]
- CHOITZ H. C., KURRIE D., GAEBLER O. H. UTILIZATION OF GLYCINE NITROGEN AT VARIOUS LEVELS OF GLYCINE INTAKE. J Nutr. 1963 Aug;80:365–369. doi: 10.1093/jn/80.4.365. [DOI] [PubMed] [Google Scholar]
- DANIELSSON H. PRESENT STATUS OF RESEARCH ON CATABOLISM AND EXCRETION OF CHOLESTEROL. Adv Lipid Res. 1963;1:335–385. doi: 10.1016/b978-1-4831-9937-5.50015-6. [DOI] [PubMed] [Google Scholar]
- Dowling R. H., Rose G. A., Sutor D. J. Hyperoxaluria and renal calculi in ileal disease. Lancet. 1971 May 29;1(7709):1103–1106. doi: 10.1016/s0140-6736(71)91840-x. [DOI] [PubMed] [Google Scholar]
- Fromm H., Hofmann A. F. Breath test for altered bile-acid metabolism. Lancet. 1971 Sep 18;2(7725):621–625. doi: 10.1016/s0140-6736(71)80068-5. [DOI] [PubMed] [Google Scholar]
- Gaebler O. H., Choitz H. C. Comparative utilization of glycine-N15 administered orally or parenterally. Metabolism. 1969 May;18(5):416–421. doi: 10.1016/0026-0495(69)90070-5. [DOI] [PubMed] [Google Scholar]
- Garbutt J. T., Wilkins R. M., Lack L., Tyor M. P. Bacterial modification of taurocholate during enterohepatic recirculation in normal man and patients with small intestinal disease. Gastroenterology. 1970 Oct;59(4):553–566. [PubMed] [Google Scholar]
- Gorbach S. L. Intestinal microflora. Gastroenterology. 1971 Jun;60(6):1110–1129. [PubMed] [Google Scholar]
- Heaton K. W., Austad W. I., Lack L., Tyor M. P. Enterohepatic circulation of C14-labeled bile salts in disorders of the distal small bowel. Gastroenterology. 1968 Jul;55(1):5–16. [PubMed] [Google Scholar]
- Hofmann A. F. A physicochemical approach to the intraluminal phase of fat absorption. Gastroenterology. 1966 Jan;50(1):56–64. [PubMed] [Google Scholar]
- Hofmann A. F., Szczepanik P. A., Klein P. D. Rapid preparation of tritium-labeled bile acids by enolic exchange on basic alumina containing tritiated water. J Lipid Res. 1968 Nov;9(6):707–713. [PubMed] [Google Scholar]
- Kaihara S., Wagner H. N., Jr Measurement of intestinal fat absorption with carbon-14 labeled tracers. J Lab Clin Med. 1968 Mar;71(3):400–411. [PubMed] [Google Scholar]
- Klein S. M., Sagers R. D. Glycine metabolism. I. Properties of the system catalyzing the exchange of bicarbonate with the carboxyl group of glycine in Peptococcus glycinophilus. J Biol Chem. 1966 Jan 10;241(1):197–205. [PubMed] [Google Scholar]
- LINDSTEDT S. The turnover of cholic acid in man: bile acids and steroids. Acta Physiol Scand. 1957 Sep 17;40(1):1–9. doi: 10.1111/j.1748-1716.1957.tb01473.x. [DOI] [PubMed] [Google Scholar]
- McLeod G. M., Wiggins H. S. Bile-salts in small intestinal contents after ileal resection and in other malabsorption syndromes. Lancet. 1968 Apr 27;1(7548):873–876. doi: 10.1016/s0140-6736(68)90235-3. [DOI] [PubMed] [Google Scholar]
- NORMAN A., GRUBB R. Hydrolysis of conjugated bile acids by Clostridia and enterococci; bile acids and steroids 25. Acta Pathol Microbiol Scand. 1955;36(6):537–547. doi: 10.1111/j.1699-0463.1955.tb04651.x. [DOI] [PubMed] [Google Scholar]
- NORMAN A., WIDSTROEM O. A. HYDROLYSIS OF CONJUGATED BILE ACIDS BY EXTRACELLULAR ENZYMES PRESENT IN RAT INTESTINAL CONTENTS. Proc Soc Exp Biol Med. 1964 Nov;117:442–444. doi: 10.3181/00379727-117-29603. [DOI] [PubMed] [Google Scholar]
- Nilsson S., Scherstén T. Importance of bile acids for phospholipid secretion into human hepatic bile. Gastroenterology. 1969 Nov;57(5):525–532. [PubMed] [Google Scholar]
- Norman A. Metabolism of glycocholic acid in man. Scand J Gastroenterol. 1970;5(3):231–236. [PubMed] [Google Scholar]
- PLAYOUST M. R., ISSELBACHER K. J. STUDIES ON THE TRANSPORT AND METABOLISM OF CONJUGATED BILE SALTS BY INTESTINAL MUCOSA. J Clin Invest. 1964 Mar;43:467–476. doi: 10.1172/JCI104932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHERT D. A., AMBERG R., WILSON M. Metabolism of glycine by avian liver. J Biol Chem. 1962 Jan;237:99–103. [PubMed] [Google Scholar]
- STEMPFEL R. S., Jr, SIDBURY J. B., Jr STUDIES WITH THE HYDROXYSTEROID DEHYDROGENASES. I. A SIMPLIFIED METHOD FOR THE ENZYMATIC ESTIMATION OF 3- AND 17-HYDROXYSTEROIDS. J Clin Endocrinol Metab. 1964 Apr;24:367–374. doi: 10.1210/jcem-24-4-367. [DOI] [PubMed] [Google Scholar]
- Sherr H. P., Sasaki Y., Newman A., Banwell J. G., Wagner H. N., Jr, Hendrix T. R. Detection of bacterial deconjugation of bile salts by a convenient breath-analysis technic. N Engl J Med. 1971 Sep 16;285(12):656–661. doi: 10.1056/NEJM197109162851204. [DOI] [PubMed] [Google Scholar]
- Snyder F., Kimble H. An automatic zonal scraper and sample collector for radioassay of thin-layer chromatograms. Anal Biochem. 1965 Jun;11(3):510–518. doi: 10.1016/0003-2697(65)90069-2. [DOI] [PubMed] [Google Scholar]
- Tada K., Narisawa K., Yoshida T., Konno T., Yokoyama Y. Hyperglycinemia: a defect in glycine cleavage reaction. Tohoku J Exp Med. 1969 Jul;98(3):289–296. doi: 10.1620/tjem.98.289. [DOI] [PubMed] [Google Scholar]
- Vlahcevic Z. R., Miller J. R., Farrar J. T., Swell L. Kinetics and pool size of primary bile acids in man. Gastroenterology. 1971 Jul;61(1):85–90. [PubMed] [Google Scholar]
- Winchell H. S., Stahelin H., Kusubov N., Slanger B., Fish M., Pollycove M., Lawrence J. H. Kinetics of CO2-HCO3 minus in normal adult males. J Nucl Med. 1970 Dec;11(12):711–715. [PubMed] [Google Scholar]
- Yesair D. W., Himmelfarb P. Hydrolysis of conjugated bile acids by cell-free extracts from aerobic bacteria. Appl Microbiol. 1970 Feb;19(2):295–300. doi: 10.1128/am.19.2.295-300.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


