Abstract
Prevention of diabetes is crucial to lowering disease incidence, and thus minimizing the individual, familial, and public health burden. The purpose of this review is to gather current information from meta-analyses on dietary and lifestyle practices concerning reduction of risk to develop type 2 diabetes. Low glycemic index dietary patterns reduce both fasting blood glucose and glycated proteins independent of carbohydrate consumption. Diets rich in whole-grain, cereal high fiber products, and non-oil-seed pulses are beneficial. Whereas, frequent meat consumption has been shown to increase risk. Regarding non-alcoholic beverages, 4 cups/day of filtered coffee or tea are associated with a reduced diabetes risk. In contrast, the consumption of alcoholic beverages should not exceed 1-3 drinks/day. Intake of vitamin E, carotenoids, and magnesium can be increased to counteract diabetes risk. Obesity is the most important factor accounting for more than half of new diabetes' cases; even modest weight loss has a favorable effect in preventing the appearance of diabetes. Also, physical exercise with or without diet contributes to a healthier lifestyle, and is important for lowering risk. Finally, there is a positive association between smoking and risk to develop type 2 diabetes. As far as secondary and tertiary prevention is concerned, for persons already diagnosed with diabetes, there is limited evidence of the effectiveness of diet or lifestyle modification on glycemic control, but further studies are necessary.
Keywords: type 2 diabetes, diet, lifestyle, nutrition, physical activity, weight loss, glycemic control, meta-analysis, diabetes prevention
Introduction
Genes and environment can interact to cause chronic diseases such as type 2 diabetes. In epidemic numbers, and the epidemic is expected to continue to grow. This is mainly due to an increasing proportion of aged people in the worldwide population [1]. Other aggravating factors are obesity in general, and increased ratios of abdominal fat distribution [2]. It is considered that more than half (60-90%) of new cases are due to obesity and weight gain [3]. Certainly, unhealthy diet and lifestyle can impose an additional burden on good glycemic control in diabetes patients.
Efforts to prevent diabetes are necessary, as they could make a significant contribution to lowering the rate of new diabetic cases. Apart from the benefits to the individual, they would reduce the, familial, and public health burden caused by the disease. Dietary habits are the personal decisions individuals make when choosing their nutrition. Nutrition therapy is generally recommended for primary, secondary, and tertiary prevention. Primary prevention means intervention before the development of diabetes, secondary prevention refers to the time after diagnosis of diabetes, and tertiary prevention can take place when significant numbers of beta-cells remain after diagnosis. Primary prevention is particularly important in type 2 diabetes, because the time of diagnosis and the severity of the disease course can be influenced beneficially by changing daily lifestyle and dietary practices. However, despite this awareness, there is still no universal dietary approach for diabetes prevention and management.
A multitude of reviews and meta-analyses have been published during the last few years. They summarize present knowledge and quantify differences in effectiveness between different dietary and lifestyle preventive measures. In contrast to biological factors, which have an impact on physical health, lifestyle encompasses modifiable social and behavioral factors [4]. In respect to diabetes, it is commonly limited to exercise, education, and smoking cessation. In this review, we gather and evaluate current information from meta-analyses on dietary and lifestyle practices proposed for reducing the risk of type 2 diabetes.
Materials and methods
English publication meta-analyses between the years 2000 and 2009 were selected through a computer-assisted literature search (i.e., Pubmed). We used combinations of the following key words for computer searches: "diabetes" and "diet" or "lifestyle" and "meta-analysis". Study selection was restricted to meta-analyses to capture most of the available study data, and to standardize and collectively use the results of numerous case-control and prospective studies. The included studies were mainly randomized controlled trials to ensure that the data to be used was of sufficiently high-quality.
In addition, the reference lists of the retrieved articles helped us to find further articles, relevant to the present analysis that were not revealed through the searching procedure. The following information was abstracted according to a fixed protocol: name of the first author and year of publication, sample size, mean age, sex of participants, follow-up duration when available, assay methods and effect measures, and degree of adjustment for potential confounders. For keywords "diabetes" and "diet" and "meta-analysis" 116 papers were retrieved. The publication dates raged from January 2000 to October 2009. For keywords "diabetes" and "lifestyle" and "meta-analysis" 67 papers were retrieved. They had the same publication date range, January 2000 to October 2009.
Of those articles initially identified, 40 were considered relevant to the present study, and these articles were finally included in the present review. To qualify them as relevant, only those in English language, dealing with diabetes type 2 in adults were included. We excluded articles dealing with diabetes prevention aided by pharmaceutical treatments, and those analyzing genetic polymorphism. Articles looking at diabetes care exclusively from a public health perspective, and those dealing with subjects such as obesity in children, colorectal and breast cancer, or renal disease were also rejected from the analysis.
The analysis presented here is structured as follows: the first section analyses food groups and diabetes prevention. We have pooled articles on this topic, because it is easier for individuals to base their decisions on food groups rather than specific ingredients. Subsequently, we looked at micro- and macronutrients and risk of diabetes, and finally we examined dietary patterns in relation to diabetes. The results are presented in Tables 1 and 2.
Table 1. Meta-analyses on lifestyle intervention and exercise to prevent type 2 diabetes.
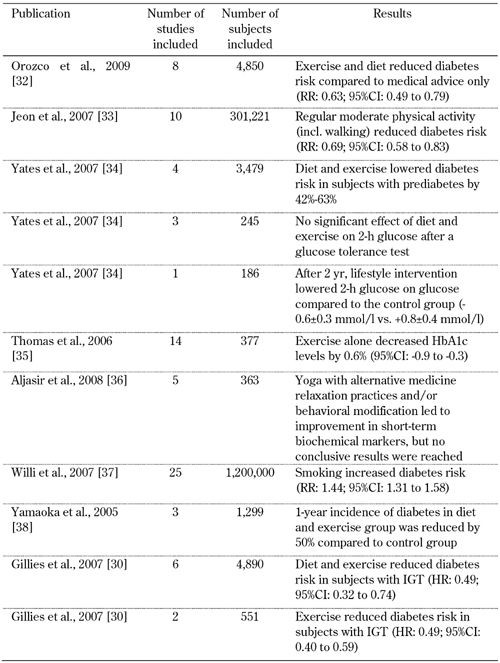
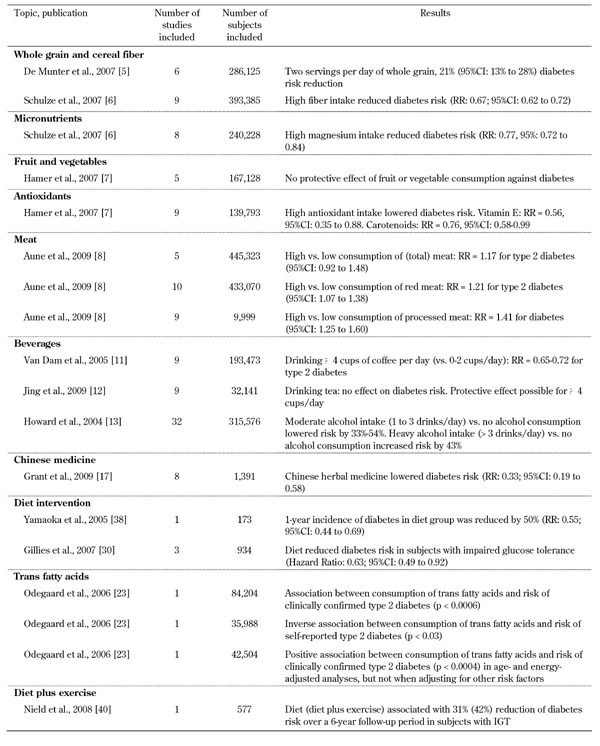
Table 2. Meta-analyses on diet and primary type 2 diabetes prevention.
Food groups and how they influence diabetes
Evidence is accumulating that whole-grain products are beneficial to health, and protect against chronic diseases (mainly cancer and cardiovascular diseases). In a meta-analysis including six cohort studies with 286,125 participants and 10,944 cases, a two-serving-per-day increment in whole grain intake was associated with a 21% reduction in risk for type 2 diabetes (95% CI: 13% to 28%), after adjusting for potential confounders and body mass index [5].
Another large meta-analysis confirmed the above results by showing reduced risk for type 2 diabetes with higher cereal fiber intake (RR for extreme categories: 0.67, 95% CI: 0.62 to 0.72) [6]. Schulze and colleagues have speculated that both insoluble and soluble fibers could play a role in diabetes prevention. In the same paper, the authors found that the intake of fruit (RR: 0.96; 95% CI: 0.88 to 1.04) and vegetable fibers (RR: 1.04; 95% CI: 0.94 to 1.15) did not significantly reduce the risk for type 2 diabetes [6]. Also in accord with these results, a meta-analysis including 167,128 participants and 4,858 incident cases of type 2 diabetes, with a mean follow-up period of 13 years, showed that the consumption of fruit and vegetables provided no protection from type 2 diabetes [7]. Specifically, the relative risk was 1.01 (95% CI: 0.88 to 1.15) for three or more servings of fruit, CI: 0.88 to 1.15) for three or more servings of fruit, and 0.97 (95%CI, 0.86 to 1.10) for three or more servings of vegetables [7].
Frequent meat consumption has been shown to increase the risk of developing type 2 diabetes. This is considered true for other diseases as well. In a meta-analysis of 12 cohort studies, the estimated summary relative risk of diabetes comparing high to low intake was 1.17 for total meat (95% CI: 0.92 to 1.48), 1.21 for red meat (95% CI: 1.07 to 1.38) and 1.41 for processed meat (95% CI: 1.25 to 1.60) [8].
Non-oil-seed pulses (chickpeas, beans, peas, lentils, etc) are another important good protein source. They seem to help with glycemic control, both in diabetic and non-diabetic subjects [9]. In a total of 41 trials, when pulses alone were examined, fasting blood glucose (RR: -0.82; 95% CI: -1.36 to -0.27) and insulin were both lowered (RR: -0.49; 95% CI: -0.93 to -0.04). Furthermore, pulses in the context of low glycemic index diets lowered glycated blood proteins (RR: -0.28; 95% CI: -0.42 to -0.14), while pulses in high-fiber diets lowered both fasting blood glucose (RR: -0.32; 95% CI: -0.49 to -0.15) and glycated blood proteins (RR: -0.27; 95% CI: -0.45 to -0.09) [9].
Fish consumption has also been analyzed for its relation to diabetes. However, fish oil supplementation at an amount of 3 to 18 g/day in diabetics was not found to have any statistically significant effect on fasting glucose and HbA1c [10].
Some beverages have been evaluated for their effect on diabetes prevention. Coffee intake (mainly drip-filtered coffee) was found to have a protective effect. In 9 cohort studies comprising 193,473 participants and 8,394 incident cases of type 2 diabetes, the calculated combined relative risk was 0.65 (95% CI: 0.54 to 0.78) for the highest (≥6 or ≥7 cups per day) and 0.72 (95% CI: 0.62 to 0.83) for the second highest (4-6 cups per day) categories of coffee consumption, when compared with the lowest consumption category (0 to ≤2 cups per day) [11]. Probably due to its antioxidant components, coffee can be a significant contributor to the total antioxidant capacity of the diet needed to reverse oxidative stress. Oxidative stress could otherwise lead to favorable conditions for type 2 diabetes.
Tea consumption was analyzed in a meta-analysis study comprising 9 cohort studies, including 324,141 participants and 11,440 incident cases of type 2 diabetes. The combined adjusted relative risk (RR: 0.96; 95% CI: 0.92 to 1.01) did not show an association with reduced risk for type 2 diabetes. However, in the same review, stratified analysis suggested that tea consumption of ≥4 cups per day may lower the risk of developing type 2 diabetes [12].
It seems that the relationship between alcohol intake and diabetes' incidence is U-shaped. A meta-analysis of 32 studies comprising a time period of 37 years (from 1966 to August 2003) suggested that, moderate consumption (one to 3 drinks/day) is associated with a 33% to 56% lower incidence of diabetes and a 34% to 55% lower incidence of diabetes-related coronary heart disease. In contrast, heavy consumption (>3 drinks/day) was associated with a 43% increased diabetes incidence [13]. Even in diabetics, moderate alcohol consumption did not acutely impair glycemic control [13].
Nuts are rich in mono- and polyunsaturated fats, and vegetable proteins and are generally regarded as part of a healthy diet. However, there seems to be no protective effect associated with the consumption of nuts. Intervention studies on the relation of nuts to type 2 diabetes have not shown any improvement in glycemic indices. However, nuts reduce postprandial oxidative stress [14]. This effect should be further studied.
Micronutrients, supplements and diabetes risk
Chromium is one of the top-selling supplements in the United States. Sometimes it is taken for preventing or alleviating symptoms of diabetes. According to a meta-analysis including 15 reports of randomized controlled trials published in 2002, chromium supplementation does not affect glucose or insulin concentration among non-diabetic subjects. Whereas, studies among diabetics are inconclusive [15].
Magnesium seems to function at the insulin receptor level. Hypomagnesemia has been associated with increased diabetes risk. A recent meta-analysis revealed a significant inverse association (RR for extreme categories, 0.77, 95% CI: 0.72 to 0.84). However, there was a heterogeneity among studies and not all of them showed the same direction of the association [6].
A meta-analysis relating antioxidant intake from vitamins C and E, flavonoids, and carotenoids to diabetes risk included 9 cohort studies, comprising 139,793 participants and 8,813 incident cases of type 2 diabetes. The mean follow-up was 13 years. The pooled relative risk was 0.87 (95% CI: 0.79 to 0.98, p = 0.02) for the highest compared with the lowest antioxidant intake. The protective effect was related to vitamin E intake with RR of 0.56 (95%CI: 0.35 to 0.88, p = 0.01) and total carotenoid intake with RR of 0.76 (95% CI: 0.58 to 0.99, p = 0.04), respectively [7].
In vitro and in vivo studies have shown that cinnamon is an insulin sensitizer, mainly activating insulin receptor kinase. According to a meta-analysis, five prospective randomized controlled trials (n = 282) did not show beneficial effects of cinnamon on HbA1c or fasting blood glucose [16].
Finally, Chinese herbal medicine has received increased attention during recent years. Research was aimed to gain a better understanding of its health potential. In prediabetics (with impaired glucose tolerance, IGT, and/or impaired fasting glucose, IFG), a meta-analysis was performed comprising 16 trials (n = 1,391) using 15 different Chinese herbals in combination with lifestyle modification. It showed that the herbal/lifestyle combination was more effective in returning fasting plasma glucose to normal levels than lifestyle changes alone (RR: 2.07; 95% CI: 1.52 to 2.82). Furthermore, individuals consuming those herbs had lower progression rates to diabetes (RR 0.33; 95% CI: 0.19 to 0.58) [17]. The analyzed studies had several shortcomings and biases, thus high-quality and rigorously evaluated studies are required before definite conclusions can be made.
The role of macronutrients in diets and diabetes
The exact proportion of carbohydrate inclusion in diabetic adults’ diets on a daily basis has been the subject of intense analysis. It has been hypothesized that a restricted-carbohydrate dietary pattern is beneficial for type 2 diabetes. A meta-analysis included 19 randomized studies with 306 patients to investigate the effects of two prescribed diets, a low-fat, high-carbohydrate (LFHC) diet (with a 24%/58% composition in fat/carbohydrate) and a high-fat, low-carbohydrate (HFLC) diet (with a 40%/40% composition in fat/carbohydrate). HbA1c and fasting blood glucose values were found to be similar in the two groups. In contrast, replacing fat with carbohydrate deteriorated insulin resistance, which in turn increased fasting insulin by 8% (p = 0.02) [18].
In another meta-analysis, 56 studies and reviews were evaluated, out of which 13 were selected and finally analyzed (representing 263 patients with type 2 diabetes) [19]. As noted above, it was similarly found that HbA1c, fasting glucose, and some lipid fractions (triglycerides) were improved with low carbohydrate diets (defined as having 45% or less of their total calories from carbohydrates) [19]. High carbohydrate high fiber (HCHF) diets were compared with low carbohydrate or low fiber diets in diabetic individuals. HCHF diets are associated with lower values for fasting, postprandial and average plasma glucose, HbA1c, LDL- and HDL-cholesterol, and triglycerides [20]. Thus, a diet with more than 55% carbohydrates and 25-50 g/day of dietary fibers (15-25 g/1000 kcal) could be advocated. Furthermore, protein intake of 12-16%, and fat intake of <30% (with monounsaturated fat 12-15%) is regarded as beneficial [20]. Generally, a high percentage of unavailable carbohydrates (non-digestible) is associated with lower blood glucose levels and better glycated protein control, which is mainly true for persons with problematic glycemic control [21].
In another class of meta-analyses, embracing 23 randomized controlled trials, 1,075 participants with type 2 diabetes were examined in relation to the beneficial effects of polyunsaturated fatty acids (PUFA). The mean dose of omega-3 PUFA was 3.5 g/d, and the mean treatment duration was 8.9 weeks. It was found that omega-3 supplementation significantly lowered triglycerides and VLDL cholesterol, whereas LDL cholesterol showed a non-significant increase. Glycemic control and fasting insulin were unaffected [22].
Finally, trans-fatty acids (TFA) are generally regarded as potentially harmful when nutritionally available in abundance, as in the diets of western industrialized countries. TFA have been analyzed for their potential to promote insulin resistance and risk to develop type 2 diabetes [23]. Although there is observational and experimental evidence from studies that high intake of TFA may increase the risk for type 2 diabetes, methodological problems and inconsistencies across the studies do not allow a definitive conclusion [23].
The role of dietary patterns
One meta-analysis retrieved by our keyword search dealt with dietary patterns, specifically based on low glycemic index, and their effect on glycemic control [24]. The analysis embraced 11 trials with 402 participants, and assessed randomized controlled trials of at least four weeks duration. The aim of the study was to compare a low glycemic index (GI) (or low glycemic load) diet to a higher GI diet, for subjects with type 2 diabetes having no optimal control of glycemia. It turned out that low GI diet was associated with improved glycemic control [24].
Another meta-analysis comprising 14 studies and 356 subjects with type 1 or type 2 diabetes, examined combined HbA1c and fructosamine data, adjusted for baseline differences. It showed that glycated proteins were reduced by 7.4% (95% CI: 8.8% to 6.0%) by low GI diets as compared to high GI diets [25]. A third meta-analysis included 45 studies with 972 participants. The participants were subdivided into groups of normal, prediabetic, diabetic, and hyperlipidemic subjects, as well as subjects at risk for cardiovascular disease. According to this analysis, it seems that low GI diets reduce both fasting blood glucose and glycated proteins independently of the consumption of available, and unavailable, carbohydrates. The latter are those carbohydrates that are not digested. Given that fat intake changes were minimal, the study showed that low GI diets have a much stronger influence on glycemic control than the nutrition based on unavailable carbohydrates, [21, 26].
Weight loss and blood glucose levels
Obesity seems to worsen metabolic abnormalities resulting in higher risk to develop type 2 diabetes. Weight reduction, even to a small extent, improves glucose biomarkers. A meta-analysis embracing nine studies with 5,168 participants and a follow-up range of 1 to 10 years, examined weight loss effects in prediabetics (IGT and/or IFG) [27]. Modest weight loss through dietary and/or physical activity intervention significantly reduced diabetes incidence in the prediabetic individuals [28]. Studies of diabetic participants resulted in a similar outcome, namely that weight changes corresponded to changes in HbA1c [28-29].
In summary, obesity can increase diabetes risk very substantially, whereas weight loss can lower plasma glucose values just as dramatically. Interestingly, as little as 10% weight loss in obese adults can significantly improve glycemic indices [3].
The importance of lifestyle changes and physical activity in diabetes prevention and management
Lifestyle encompasses modifiable social factors that have an impact on health, whether they be negative or positive. Apart from diet and weight loss, the main lifestyle intervention in the prevention and management of diabetes is to increase physical activity. Physical activity can contribute to lower blood glucose levels and improved insulin resistance. It is suggested that exercise can substitute pharmacological treatment in prediabetics. According to a meta-analysis of 12 studies comprising 6,366 adults, lifestyle interventions reduced the incidence of type 2 diabetes by almost 50%, compared to standard medical advice only (pooled hazard ratio 0.51; 95% CI: 0.44 to 0.60) [30].
Self-management education of diabetics is another useful tool to improve HbA1c, which is an important predictor of glycemic control and later chronic complications of the disease [31]. Studied diabetic individuals were followed up for 4 or more months and reached an HbA1c lowering of 0.26% (95% CI: 0.05 to 0.48) [31].
Randomized controlled trials of six months duration have investigated the effects of combined exercise and diet for preventing type 2 diabetes. 8 trials were included in a meta-analysis. Subjects at risk for type 2 diabetes were subdivided, either into an exercise plus diet arm (2,241 participants), or a standard recommendation arm (2,509 participants). It was found that the combined approach reduced diabetes risk compared to standard recommendations (RR 0.63, 95% CI: 0.49 to 0.79) [32].
The intensity of physical activity was investigated in a systematic review published in 2007, which included 10 prospective cohort studies. These studies analyzed the relation of moderately intensive physical activity on type 2 diabetes risk [33]. The total relative risk of developing type 2 diabetes was 0.69 (95% CI: 0.58 to 0.83) for regular participation in physical activity of moderate intensity compared with being sedentary (i.e. a 30% lower risk). For regular walking (≥2.5 h/wk of brisk walking), the relative risk was 0.70 (95% CI: 0.58 to 0.84) compared with almost no walking. These associations were observed in men and women, in the U.S. and Europe, and remain significant after adjustment for obesity indices [33].
Physical activity as "monotherapy" has rarely been analyzed for its effectiveness in preventing type 2 diabetes in prediabetics (IGT and/or IFG). This is due to the assumption that the positive effects of exercise are not independent of other variables such as weight loss. In a systematic review, 8 controlled trials were re-analyzed to determine the independent effect of exercise on glucose level and risk of type 2 diabetes in people with prediabetes, [34]. In 4 studies, the risk was approximately halved (range 42%-63%). However, the authors speculated that this reduction could be attributed to weight reduction that accompanied the regular physical activity. In the remaining 4 studies, only one reported significant improvement in 2-h plasma glucose [34]. In another meta-study, various types of aerobics, fitness, and progressive training exercise were evaluated, without considering weight loss. The training types, as compared to 'no exercise', seem to improve glycemic control in subjects with type 2 diabetes [35]. In fourteen randomized controlled trials involving 377 participants, ranging from eight weeks to twelve months duration, the exercise intervention significantly decreased HbA1c by 0.6% (95%CI: -0.9 to -0.3). Also, in one of the trials examined, exercise intervention significantly increased insulin response (131 AUC, 95%CI, 20 to 242) [35].
Besides conventional physical activity, complementary methods such as yoga practices have been studied. The hypothesis is that stress management and relaxation could favor glycemic control in patients with type 2 diabetes. A systematic review of randomized controlled trials compared yoga with other types of intervention. Five trials with 363 participants were included [36]. However, the results did not show a clear picture, and cannot be used to recommend or encourage type 2 diabetes patients to practice yoga.
Lifestyle addiction: detrimental role of tobacco smoking in type 2 diabetes
Smoking has an effect on glucose tolerance, possibly by acting on pancreatic beta-cells and insulin secretion [37]. A wide-ranging meta-analysis undertaken in 2007 re-evaluated the effects of cigarette smoking on diabetes [37]. The analysis embraced 25 prospective cohort studies (1.2 million subjects) yielding 45,844 reported incident cases with a diabetes duration of 5-30 years. The study revealed that a dose-response phenomenon was noted between cigarette smoking and the development of type 2 diabetes, with a pooled adjusted relative risk of 1.44 (95% CI: 1.31 to 1.58) [37]. Thus, it seems that active smoking is associated with an increased risk of type 2 diabetes. This finding is quite similar to the well-known relationships between smoking and cardiovascular disease, and various types of cancer.
Lifestyle education
Extensive research is currently under way to test whether lifestyle modifications—through appropriate education, including dietary intervention—can protect adults at high risk of type 2 diabetes. In a meta-analysis of eight studies, with a total duration exceeding 6 months, lifestyle interventions reduced 2-h plasma glucose by 0.84 mmol/l (95% CI, 0.39 to1.29) compared with a conventional treatment group. The 1-year diabetes incidence was approximately halved (RR: 0.55; 95% CI: 0.44 to 0.69) compared with the conventional group [38]. Another meta-analysis of published diabetes prevention trials aimed to define the number needed to treat (NNT) diabetics to prevent or delay one case of diabetes. The NNT for lifestyle interventions (weight reduction, dietary therapy, and physical activity) was estimated at 6.4. Whereas, the NNT for oral diabetes drugs (with follow-up times ranging from 3 to 6 years) was estimated at 10.8 [30]. This result suggests fairly conclusively that lifestyle interventions seem to be more effective in preventing or delaying diabetes than drugs. Other considerations in favor of lifestyle intervention in diabetes and prediabetes, are low costs and personal freedom from drug treatments. Boren and colleagues found that financial constraints are not related to diabetics’ education. They conclude that lifestyle education is a cost-effective intervention [39].
Conclusions
There is a wealth of data to suggest that nutrition therapy is a powerful tool for primary prevention of type 2 diabetes and to minimize associated risk. Additionally, many studies have shown that intensive lifestyle interventions are important in diabetes prevention. These interventions include weight reduction, dietary counseling, and physical activity. The effectiveness of this intervention strategy has been ascertained by comparing it with treatment by antidiabetic drugs and placebo.
Nutrition seems to be a critical element in any prevention strategy. There is ample evidence to show that a diet with a low glycemic index and high in fiber, mainly derived from cereals, is beneficial. Concerning food groups, intake of whole grains, non-oil seed pulses, and fish can be advocated. Data collected for nuts, fruit, and vegetables, produced uncertain and inconclusive results. More research is necessary on this topic, to draw a clear picture. In contrast, there are clear results to show that meat intake should be minimized. Beverages have been identified as important diabetes' risk modifiers. Coffee and tea could be beneficial, even if higher amounts are consumed than the identified beneficial threshold level of 3-4 cups a day. Whereas, alcohol is protective only when consumed in moderate amounts. In excess, it is likely to increase risk. Finally, evidence has been accumulated that micronutrients and supplements could be helpful to prevent or combat diabetes. Chromium, magnesium, vitamin E, carotenoids, cinnamon, all have shown some promising results. Various Chinese herbs have also shown encouraging results, but due to study limitations, further more closely controlled studies are needed to achieve a better evaluation.
We still lack a comprehensive evaluation of dietary modification as the only intervention to prevent type 2 diabetes. Most data assess this question through an examination of changes in glycemic control, changes in morbidity, quality of life or mortality as endpoints [32, 40]. However, some insight has been achieved. Exercise alone is less effective than a combination of exercise and diet. Exercise alone compared with diet modification in persons with prediabetes had no statistically significant effects on diabetes incidence, but the combined intervention including exercise and diet seemed to be very effective. Effective lifestyle interventions should consider setting individual goals. "Lifestyle coaches" can give patients a better understanding of desired interventions through frequent contact with the participants, and also to ensure adherence. Educational materials should be readily available, and supervised activities should be encouraged. Also, the creation of a support network is advised, and could facilitate adherence.
Regular exercise could improve HbA1c values in diabetic subjects at six and twelve months of treatment. Lifestyle changes, such as cessation of smoking and better education of diabetics in managing their disease, could be additional weapons for better glycemic control and reducing the risk of developing diabetes. Despite the clear advantages of dietary and lifestyle interventions, patients encounter obstacles in implementing the interventions in their daily life. It seems that among diabetics there is limited awareness of the effectiveness of diet modification [41-43]. In conclusion, although there are some meta-analyses that are extremely useful in summarizing the present data, there is need for more randomized trials elucidating various aspects in the interaction of diet, lifestyle changes, and type 2 diabetes.
Disclosures (conflict of interests statement): The authors report no conflict of interests.
References
- 1.Diabetes action now. World Health Organization; 2004. [Google Scholar]
- 2.Astrup A. Healthy lifestyles in Europe: prevention of obesity and type II diabetes by diet and physical activity. Public Health Nutr. 2001;4:499–515. doi: 10.1079/phn2001136. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: Review with meta-analysis of clinical studies. J Am Coll Nutr. 2003;22:331–339. doi: 10.1080/07315724.2003.10719316. [DOI] [PubMed] [Google Scholar]
- 4.Hansen E, Easthope G. Lifestyle in medicine. Routledge; Milton Park, Abingdon: 2007. [Google Scholar]
- 5.de Munter JS, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: A prospective cohort study and systematic review. PLoS Med. 2007;4:e261. doi: 10.1371/journal.pmed.0040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H. Fiber and magnesium intake and incidence of type 2 diabetes: A prospective study and meta-analysis. Arch Intern Med. 2007;167:956–965. doi: 10.1001/archinte.167.9.956. [DOI] [PubMed] [Google Scholar]
- 7.Hamer M, Chida Y. Intake of fruit, vegetables, and antioxidants and risk of type 2 diabetes: Systematic review and meta-analysis. J Hypertens. 2007;25:2361–2369. doi: 10.1097/HJH.0b013e3282efc214. [DOI] [PubMed] [Google Scholar]
- 8.Aune D, Ursin G, Veierod MB. Meat consumption and the risk of type 2 diabetes: A systematic review and meta-analysis of cohort studies. Diabetologia. 2009;52:2277–2287. doi: 10.1007/s00125-009-1481-x. [DOI] [PubMed] [Google Scholar]
- 9.Sievenpiper JL, Kendall CW, Esfahani A, Wong JM, Carleton AJ, Jiang HY, Bazinet RP, Vidgen E, Jenkins DJ. Effect of non-oil-seed pulses on glycaemic control: A systematic review and meta-analysis of randomized controlled experimental trials in people with and without diabetes. Diabetologia. 2009;52:1479–1495. doi: 10.1007/s00125-009-1395-7. [DOI] [PubMed] [Google Scholar]
- 10.Farmer A, Montori V, Dinneen S, Clar C. Fish oil in people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2001;2001:CD003205. doi: 10.1002/14651858.CD003205. [DOI] [PubMed] [Google Scholar]
- 11.Van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes: a systematic review. JAMA. 2005;294:97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]
- 12.Jing Y, Han G, Hu Y, Bi Y, Li L, Zhu D. Tea consumption and risk of type 2 diabetes: A meta-analysis of cohort studies. J Gen Intern Med. 2009;24:557–562. doi: 10.1007/s11606-009-0929-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard AA, Arnsten JH, Gourevitch MN. Effect of alcohol consumption on diabetes mellitus: A systematic review. Ann Intern Med. 2004;140:211–219. doi: 10.7326/0003-4819-140-6-200403160-00011. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins DJ, Hu FB, Tapsell LC, Josse AR, Kendall CW. Possible benefit of nuts in type 2 diabetes. J Nutr. 2008;138:1752S–1756S. doi: 10.1093/jn/138.9.1752S. [DOI] [PubMed] [Google Scholar]
- 15.Althuis MD, Jordan NE, Ludington EA, Wittes JT. Glucose and insulin responses to dietary chromium supplements: a meta-analysis. Am J Clin Nutr. 2002;76:148–155. doi: 10.1093/ajcn/76.1.148. [DOI] [PubMed] [Google Scholar]
- 16.Baker WL, Gutierrez-Williams G, White CM, Kluger J, Coleman CI. Effect of cinnamon on glucose control and lipid parameters. Diabetes Care. 2008;31:41–43. doi: 10.2337/dc07-1711. [DOI] [PubMed] [Google Scholar]
- 17.Grant SJ, Bensoussan A, Chang D, Kiat H, Klupp NL, Liu JP, Li X. Chinese herbal medicines for people with impaired glucose tolerance or impaired fasting blood glucose. Cochrane Database Syst Rev. 2009;2009:CD006690. doi: 10.1002/14651858.CD006690.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Sato M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Influence of fat and carbohydrate proportions on the metabolic profile in patients with type 2 diabetes: A meta-analysis. Diabetes Care. 2009;32:959–965. doi: 10.2337/dc08-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirk JK, Graves DE, Craven TE, Lipkin EW, Austin M, Margolis KL. Restricted-carbohydrate diets in patients with type 2 diabetes: a meta-analysis. J Am Diet Assoc. 2008;108:91–100. doi: 10.1016/j.jada.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JW, Randles KM, Kendall CW, Jenkins DJ. Carbohydrate and fiber recommendations for individuals with diabetes: A quantitative assessment and meta-analysis of the evidence. J Am Coll Nutr. 2004;23:5–17. doi: 10.1080/07315724.2004.10719338. [DOI] [PubMed] [Google Scholar]
- 21.Livesey G, Taylor R, Hulshof T, Howlett J. Glycemic response and health--a systematic review and meta-analysis: Relations between dietary glycemic properties and health outcomes. Am J Clin Nutr. 2008;87:258S–268S. doi: 10.1093/ajcn/87.1.258S. [DOI] [PubMed] [Google Scholar]
- 22.Hartweg J, Perera R, Montori V, Dinneen S, Neil HA, Farmer A. Omega-3 polyunsaturated fatty acids (pufa) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008;2008:CD003205. doi: 10.1002/14651858.CD003205.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odegaard AO, Pereira MA. Trans fatty acids, insulin resistance, and type 2 diabetes. Nutr Rev. 2006;64:364–372. doi: 10.1111/j.1753-4887.2006.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 24.Thomas D, Elliott EJ. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev. 2009;2009:CD006296. doi: 10.1002/14651858.CD006296.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003;26:2261–2267. doi: 10.2337/diacare.26.8.2261. [DOI] [PubMed] [Google Scholar]
- 26.Livesey G, Taylor R, Hulshof T, Howlett J. Glycemic response and health--a systematic review and meta-analysis: The database, study characteristics, and macronutrient intakes. Am J Clin Nutr. 2008;87:223S–236S. doi: 10.1093/ajcn/87.1.223S. [DOI] [PubMed] [Google Scholar]
- 27.Norris SL, Zhang X, Avenell A, Gregg E, Schmid CH, Lau J. Long-term non-pharmacological weight loss interventions for adults with prediabetes. Cochrane Database Syst Rev. 2005;2005:CD005270. doi: 10.1002/14651858.CD005270. [DOI] [PubMed] [Google Scholar]
- 28.Norris SL, Zhang X, Avenell A, Gregg E, Brown TJ, Schmid CH, Lau J. Long-term non-pharmacologic weight loss interventions for adults with type 2 diabetes. Cochrane Database Syst Rev. 2005;2005:CD004095. doi: 10.1002/14651858.CD004095.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norris SL, Zhang X, Avenell A, Gregg E, Bowman B, Serdula M, Brown TJ, Schmid CH, Lau J. Long-term effectiveness of lifestyle and behavioral weight loss interventions in adults with type 2 diabetes: a meta-analysis. Am J Med. 2004;117:762–774. doi: 10.1016/j.amjmed.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 30.Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, Khunti K. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ. 2007;334:299–302. doi: 10.1136/bmj.39063.689375.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25:1159–1171. doi: 10.2337/diacare.25.7.1159. [DOI] [PubMed] [Google Scholar]
- 32.Orozco LJ, Buchleitner AM, Gimenez-Perez G, Roque I Figuls M, Richter B, Mauricio D. Exercise or exercise and diet for preventing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008;2008:CD003054. doi: 10.1002/14651858.CD003054.pub3. [DOI] [PubMed] [Google Scholar]
- 33.Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007;30:744–752. doi: 10.2337/dc06-1842. [DOI] [PubMed] [Google Scholar]
- 34.Yates T, Khunti K, Bull F, Gorely T, Davies MJ. The role of physical activity in the management of impaired glucose tolerance: a systematic review. Diabetologia. 2007;50:1116–1126. doi: 10.1007/s00125-007-0638-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;2006:CD002968. doi: 10.1002/14651858.CD002968.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aljasir B, Bryson B, Al-shehri B. Yoga practice for the management of type II diabetes mellitus in adults: a systematic review. Evid Based Complement Alternat Med. 2008 doi: 10.1093/ecam/nen027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: A systematic review and meta-analysis. JAMA. 2007;298:2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 38.Yamaoka K, Tango T. Efficacy of lifestyle education to prevent type 2 diabetes. Diabetes Care. 2005;28:2780–2786. doi: 10.2337/diacare.28.11.2780. [DOI] [PubMed] [Google Scholar]
- 39.Boren SA, Fitzner KA, Panhalkar PS, Specker JE. Costs and benefits associated with diabetes education: A review of the literature. Diabetes Educ. 2009;35:72–96. doi: 10.1177/0145721708326774. [DOI] [PubMed] [Google Scholar]
- 40.Nield L, Summerbell CD, Hooper L, Whittaker V, Moore H. Dietary advice for the prevention of type 2 diabetes mellitus in adults. Cochrane Database Syst Rev. 2008;2008:CD005102. doi: 10.1002/14651858.CD005102.pub2. [DOI] [PubMed] [Google Scholar]
- 41.Moore H, Summerbell C, Hooper L, Cruickshank K, Vyas A, Johnstone P, Ashton V, Kopelman P. Dietary advice for treatment of type 2 diabetes mellitus in adults. Cochrane Database Syst Rev. 2004;2004:CD004097. doi: 10.1002/14651858.CD004097.pub3. [DOI] [PubMed] [Google Scholar]
- 42.van de Laar FA, Akkermans RP, van Binsbergen JJ. Limited evidence for effects of diet for type 2 diabetes from systematic reviews. Eur J Clin Nutr. 2007;61:929–937. doi: 10.1038/sj.ejcn.1602611. [DOI] [PubMed] [Google Scholar]
- 43.Nield L, Moore HJ, Hooper L, Cruickshank JK, Vyas A, Whittaker V, Summerbell CD. Dietary advice for treatment of type 2 diabetes mellitus in adults. Cochrane Database Syst Rev. 2007;2007:CD004097. doi: 10.1002/14651858.CD004097.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]


