Abstract
Chenodeoxycholyl-2,4-3H-glycine-1-14C and deoxycholyl-2,4-3H-glycine-1-14C were synthesized and administered orally to 10 healthy subjects. Distribution of radioactivity among bile acids and specific activity of steroid and amino acid moieties were determined in bile samples. 3H and 14C were measured in feces. 14C in breath was calculated from interval 14CO2 specific activity determinations.
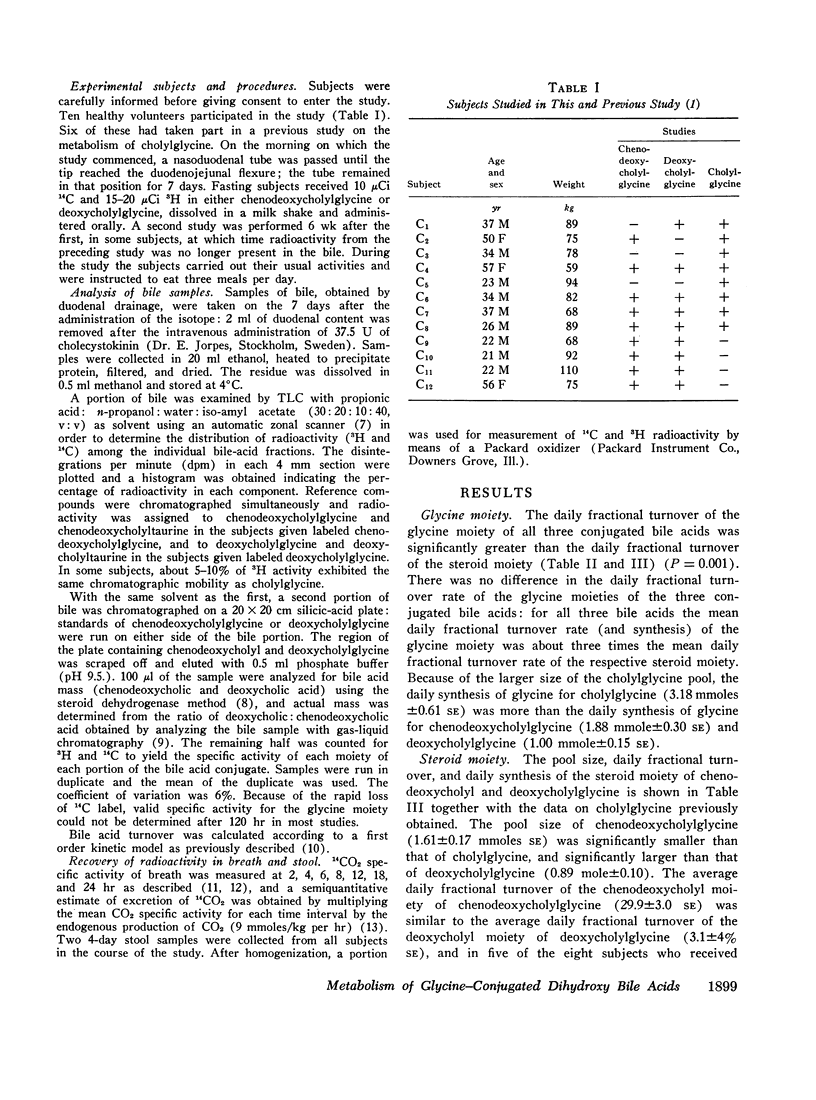
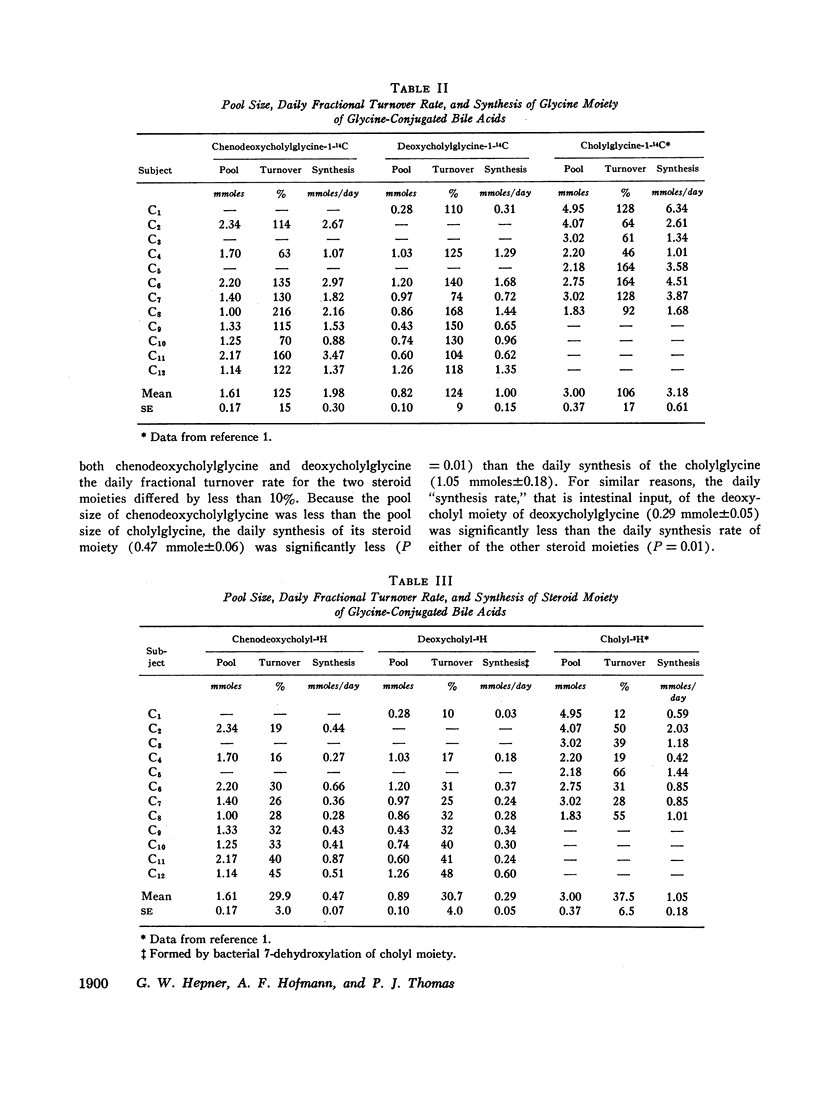
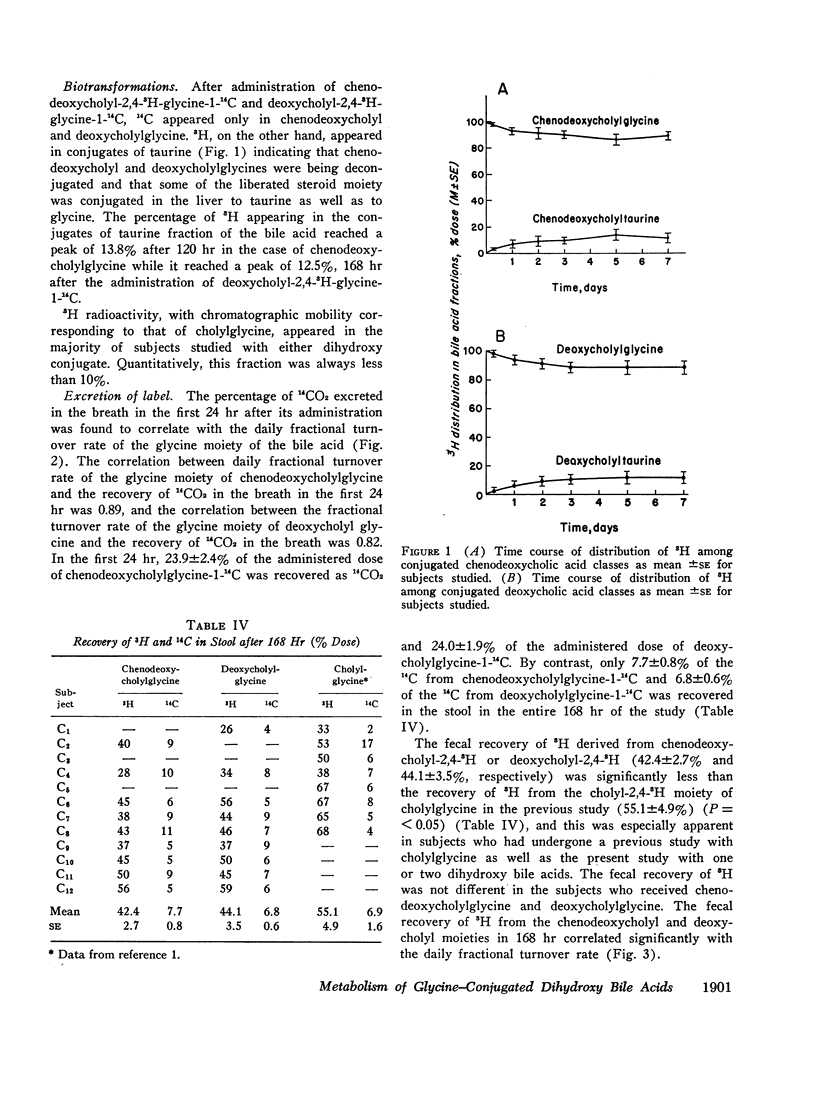
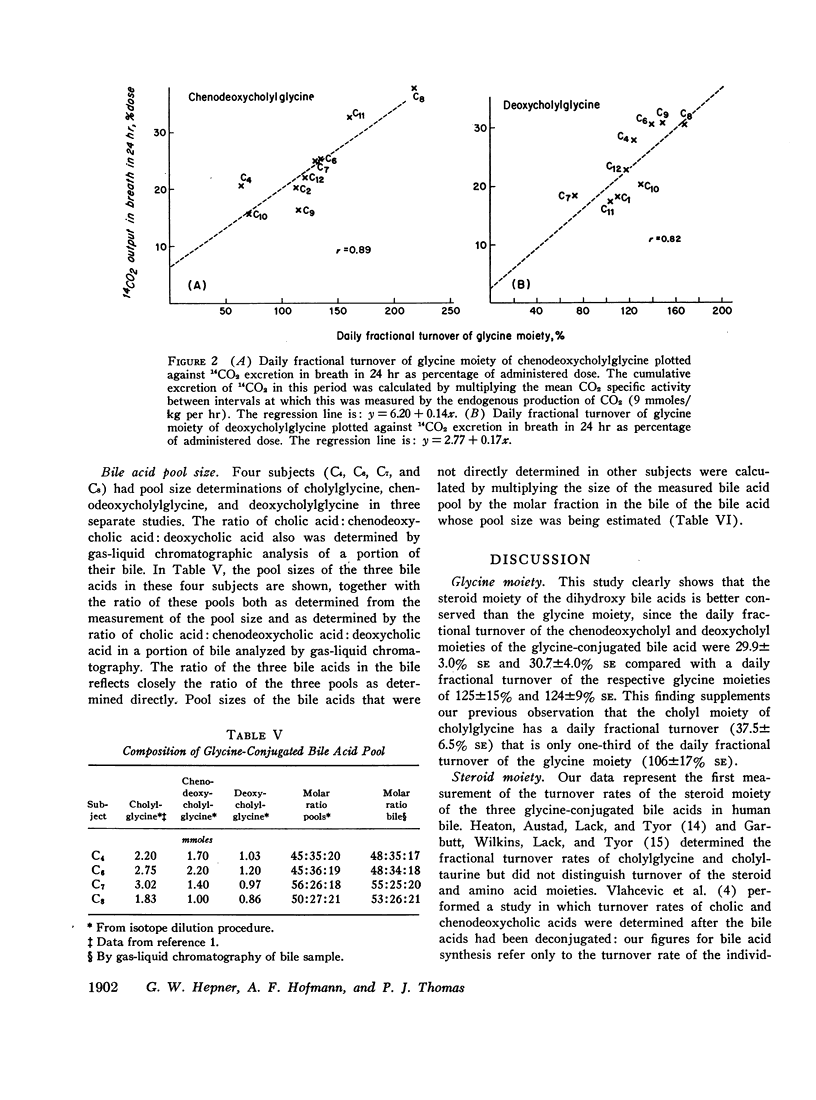
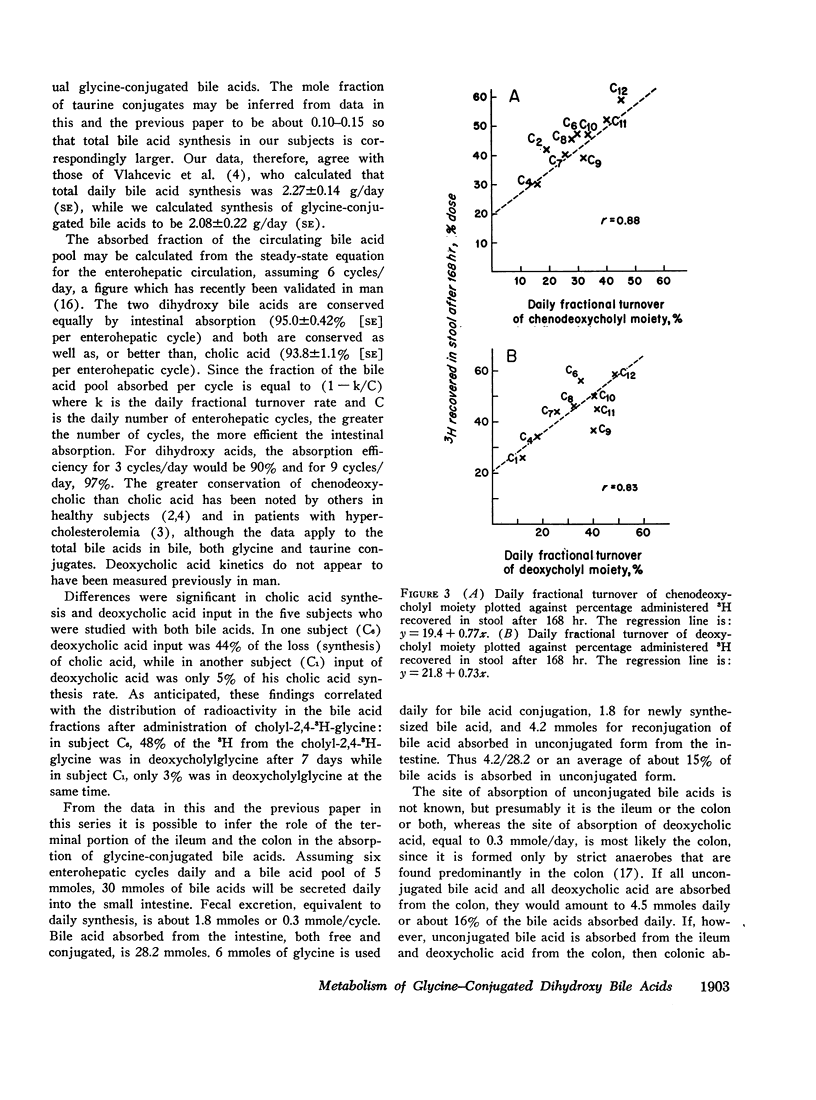
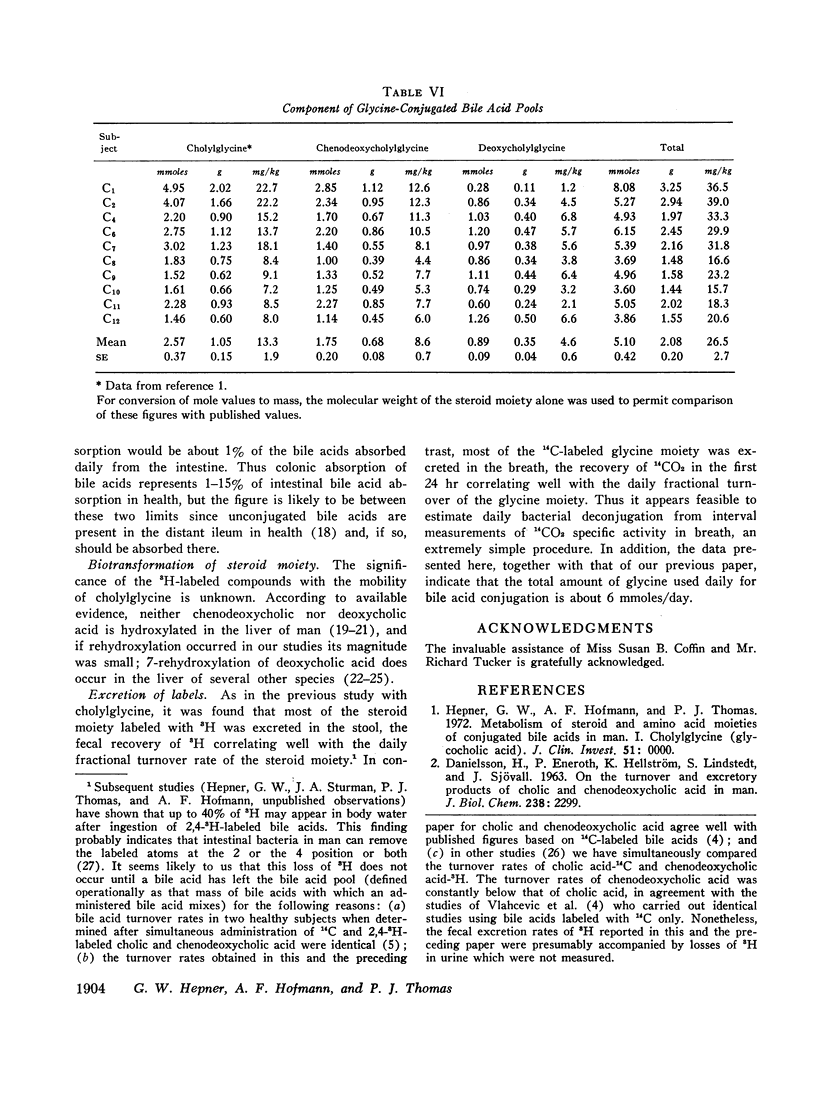
The daily fractional turnover of the glycine moiety of chenodeoxycholyl and deoxycholylglycines was more than three times that of the steroid moiety. Pool size of chenodeoxycholylglycine was about twice that of deoxycholylglycine, but similar fractional turnover rates of steroid and amino acid moieties suggested that intestinal absorption of the two conjugated bile acids was equally efficient (about 95%). The amount of unlabeled deoxycholic acid (newly formed by bacterial 7α-dehydroxylation) absorbed from the intestine approximated 30% of the cholic acid that was lost. 3H radioactivity remained predominantly in administered bile acid implying that, normally, secondary bile acids derived from chenodeoxycholic acid are not appreciably absorbed from the intestine and that deoxycholic acid is not hydroxylated by the liver.
Approximately 25% of administered 14C was recovered in the breath in the first 24 hr and less than 8% in the feces in 8 days; 14CO2 excretion correlated highly with fractional turnover of the glycine moiety. 3H appeared predominantly in feces, and the rate of excretion correlated highly with the fractional turnover of the steroid moiety of bile acids. From the results in this paper plus previous measurements on the metabolism of cholylglycine, we calculated that about 6 mmoles/day of glycine is used for bile acid conjugation in health.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGSTROM S., DANIELSSON H., KAZUNO T. Bile acids and steroids. 98. The metabolism of bile acids in python and constrictor snakes. J Biol Chem. 1960 Apr;235:983–988. [PubMed] [Google Scholar]
- BERGSTROM S., ROTTENBERG M., SJOVALL J. Uber den Stoffwechsel der Cholsäure und Desoxycholsäure in der Ratte. Hoppe Seylers Z Physiol Chem. 1953;295:278–285. doi: 10.1515/bchm2.1953.295.1.278. [DOI] [PubMed] [Google Scholar]
- DANIELSSON H., ENEROTH P., HELLSTROM K., LINDSTEDT S., SJOVALL J. On the turnover and excretory products of cholic and chenodeoxycholic acid in man. J Biol Chem. 1963 Jul;238:2299–2304. [PubMed] [Google Scholar]
- Fromm H., Hofmann A. F. Breath test for altered bile-acid metabolism. Lancet. 1971 Sep 18;2(7725):621–625. doi: 10.1016/s0140-6736(71)80068-5. [DOI] [PubMed] [Google Scholar]
- Garbutt J. T., Wilkins R. M., Lack L., Tyor M. P. Bacterial modification of taurocholate during enterohepatic recirculation in normal man and patients with small intestinal disease. Gastroenterology. 1970 Oct;59(4):553–566. [PubMed] [Google Scholar]
- Gorbach S. L. Intestinal microflora. Gastroenterology. 1971 Jun;60(6):1110–1129. [PubMed] [Google Scholar]
- Heaton K. W., Austad W. I., Lack L., Tyor M. P. Enterohepatic circulation of C14-labeled bile salts in disorders of the distal small bowel. Gastroenterology. 1968 Jul;55(1):5–16. [PubMed] [Google Scholar]
- Hofmann A. F., Szczepanik P. A., Klein P. D. Rapid preparation of tritium-labeled bile acids by enolic exchange on basic alumina containing tritiated water. J Lipid Res. 1968 Nov;9(6):707–713. [PubMed] [Google Scholar]
- LINDSTEDT S. The turnover of cholic acid in man: bile acids and steroids. Acta Physiol Scand. 1957 Sep 17;40(1):1–9. doi: 10.1111/j.1748-1716.1957.tb01473.x. [DOI] [PubMed] [Google Scholar]
- MAHOWALD T. A., MATSCHINER J. T., HSIA S. L., RICHTER R., DOISY E. A., Jr, ELLIOTT W. H., DOISY E. A. Bile acids. II. Metabolism of deoxycholic acid-24-C14 and chenodeoxycholic acid-24-C14 in the rat. J Biol Chem. 1957 Apr;225(2):781–793. [PubMed] [Google Scholar]
- NORMAN A., PALMER R. H. METABOLITES OF LITHOCHOLIC ACID-24-C-14 IN HUMAN BILE AND FECES. J Lab Clin Med. 1964 Jun;63:986–1001. [PubMed] [Google Scholar]
- NORMAN A., SHORB M. S. In vitro formation of deoxycholic and lithocholic acid by human intestinal microorganisms. Proc Soc Exp Biol Med. 1962 Jul;110:552–555. doi: 10.3181/00379727-110-27577. [DOI] [PubMed] [Google Scholar]
- Roovers J., Evrard E., Vanderhaeghe H. An improved method for measuring human blood bile acids. Clin Chim Acta. 1968 Mar;19(3):449–457. doi: 10.1016/0009-8981(68)90272-6. [DOI] [PubMed] [Google Scholar]
- STEMPFEL R. S., Jr, SIDBURY J. B., Jr STUDIES WITH THE HYDROXYSTEROID DEHYDROGENASES. I. A SIMPLIFIED METHOD FOR THE ENZYMATIC ESTIMATION OF 3- AND 17-HYDROXYSTEROIDS. J Clin Endocrinol Metab. 1964 Apr;24:367–374. doi: 10.1210/jcem-24-4-367. [DOI] [PubMed] [Google Scholar]
- Sherr H. P., Sasaki Y., Newman A., Banwell J. G., Wagner H. N., Jr, Hendrix T. R. Detection of bacterial deconjugation of bile salts by a convenient breath-analysis technic. N Engl J Med. 1971 Sep 16;285(12):656–661. doi: 10.1056/NEJM197109162851204. [DOI] [PubMed] [Google Scholar]
- Snyder F., Kimble H. An automatic zonal scraper and sample collector for radioassay of thin-layer chromatograms. Anal Biochem. 1965 Jun;11(3):510–518. doi: 10.1016/0003-2697(65)90069-2. [DOI] [PubMed] [Google Scholar]
- Vlahcevic Z. R., Miller J. R., Farrar J. T., Swell L. Kinetics and pool size of primary bile acids in man. Gastroenterology. 1971 Jul;61(1):85–90. [PubMed] [Google Scholar]
- Winchell H. S., Stahelin H., Kusubov N., Slanger B., Fish M., Pollycove M., Lawrence J. H. Kinetics of CO2-HCO3 minus in normal adult males. J Nucl Med. 1970 Dec;11(12):711–715. [PubMed] [Google Scholar]
- Wollenweber J., Kottke B. A., Owen C. A., Jr Pool size and turnover of bile acids in six hypercholesteremic patients with and without administration of nicotinic acid. J Lab Clin Med. 1967 Apr;69(4):584–593. [PubMed] [Google Scholar]


